Abstract
In searching the Staphylococcus aureus genome, we found several homologs to SarA. One of these genes, sarT, codes for a basic protein with 118 residues and a predicted molecular size of 16,096 Da. Northern blot analysis revealed that the expression of sarT was repressed by sarA and agr. An insertion sarT mutant generated in S. aureus RN6390 and 8325-4 backgrounds revealed minimal effect on the expression of sarR and sarA. The RNAIII level was notably increased in the sarT mutant, particularly in postexponential-phase cells, while the augmentative effect on RNAII was less. SarT repressed the expression of α-hemolysin, as determined by Northern blotting, Western blotting, and a rabbit erythrocyte hemolytic assay. This repression was relieved upon complementation. Similar to agr and sarA mutants, which predictably displayed a reduction in hla expression, the agr sarT mutant exhibited a lower level of hla transcription than the sarT mutant. In contrast, hla transcription was enhanced in the sarA sarT mutant compared with the single sarA mutant. Collectively, these results indicated that the sarA locus, contrary to the regulatory action of agr, induced α-hemolysin production by repressing sarT, a repressor of hla transcription.
Staphylococcus aureus is an important human pathogen. Within its arsenal are genes coding for virulence proteins with activities ranging from quorum sensing, tissue colonization, and immune evasion to tissue destruction (39). Superimposed upon these virulence genes is a network of regulatory genes (global regulatory network) that allow exquisite and precise coordination of protein expression during different stages of infection (4, 11, 13, 17, 38). Presumably, the regulatory network permits the bacteria to respond to environmental cues and hence allows the pathogen to thrive in diverse host microenvironments, e.g., blood, heart, lung, kidney, and spleen (39).
During growth in vitro, S. aureus expresses a number of cell wall-associated adhesions (fibronectin and fibrinogen binding proteins) that are believed to support adherence and colonization of host tissues (9). In transition to the postexponential phase, the expression of adhesion proteins is repressed, while the synthesis of exoproteins with enzymatic activity (e.g., hemolysins, toxins, proteases, and lipase) predominates. By virtue of their proteolytic enzyme activities (e.g., V8 protease) as well as direct toxin effects on host cells (e.g., α-toxin), these exoproteins likely facilitate dissemination of the organism in vivo (39).
Postexponential protein expression in S. aureus is controlled by global regulatory systems such as sarA and agr (4, 17, 27). The sarA locus encodes a 372-bp open reading frame with three upstream promoters (P2, P3, and P1) that initiate overlapping transcripts, each coding for the 14.5-kDa SarA protein (6, 33). The sarA P1 and P2 promoters, most active during the exponential phase, are SigA dependent, while the P3 promoter is primarily active during the postexponential phase and is SigB dependent (5, 33). Phenotypically, the sarA locus activates the synthesis of fibronectin and fibrinogen binding proteins (for adhesion), as well as that of α-, β-, and δ-hemolysins (for tissue spread) (40). Protein-DNA binding studies revealed that SarA binds to a 29-bp recognition sequence within the P2-P3 interpromoter region of agr (16, 36), thus playing a role in activating agr transcription. As confirmation, a sarA mutant also displayed reduced levels of RNAII and RNAIII transcription of agr when compared to the parental strain in vitro (12).
The agr locus, a well-described pleiotropic regulator of exoproteins synthesis in S. aureus (25, 27, 40), comprises two divergent transcripts: RNAII, which encodes agrDBCA, and RNAIII, encoding hld. AgrC and AgrA are thought to be the sensor and activator of a two-component regulatory system. AgrB and AgrD participate in the synthesis of a cyclic octapeptide, which acts as a quorum-sensing molecule (25). The secreted octapeptide activates the transmembrane sensor AgrC (30), leading to phosphorylation of AgrC and a second step phosphorylation of AgrA, the activator. Phosphorylated AgrA has been postulated to bind to the agr promoter region to activate RNAII and RNAIII promoters, leading to the expression of RNAIII, the regulatory molecule that is responsible for the agr phenotype (induction of exoproteins and repression of fibrinogen, fibronectin binding proteins, and protein A).
While mutations in sarA and agr have been shown to reduce virulence in several animal model studies, these mutations did not render the bacteria avirulent (1, 8, 22, 24), suggesting that other regulatory factors may be at work. With the partial release of the S. aureus genome, additional genes with homology to sarA could be identified. For example, sarR encodes a 115-residue protein that represses SarA expression during the postexponential phase, presumably by down-modulating sarA P1 transcription (32). In contrast, SarS (also called SarH1) acts downstream of sarA and agr to activate the transcription of spa (protein A) (13, 42). An additional regulatory gene, rot, has been defined as a repressor of alpha-toxin synthesis (35).
In searching the S. aureus genome (at www.TIGR.org), we found an additional gene with homology to sarA. We report here this sarA homolog, designated sarT, the expression of which is negatively controlled by sarA and agr. SarT represses the expression of hla. Surprisingly, RNAIII of the agr locus was induced in a sarT mutant. Additional transcriptional analysis with sarA sarT and agr sarT double mutants disclosed that sarA, but not agr, activates the synthesis of α-hemolysin by repressing sarT expression.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
The bacterial strains and plasmids used to generate the data in this study are listed in Table 1. Phage 80α (37) was used as a transducing phage. Escherichia coli strains were grown in Luria-Bertani medium (31). S. aureus strains were maintained with tryptic soy medium (Difco) and grown in CYGP or 03GL medium (37). Erythromycin (5 μg/ml), chloramphenicol (34 μg/ml for E. coli and 10 μg/ml for S. aureus), tetracycline (5 μg/ml), ampicillin (50 μg/ml), and kanamycin (50 μg/ml) were used for selection of transformants and transductants.
TABLE 1.
Bacterial strains and plasmids used for this study
| Strain or plasmid | Comment | Reference or source |
|---|---|---|
| S. aureus | ||
| RN4220 | Mutant strain of 8325-4 that accepts foreign DNA | 37 |
| RN6390 | agr+ laboratory strain related to 8325-4, maintains hemolytic pattern when propagated on sheep erythrocytes | 37 |
| 38 | ||
| 8325-4 | Prophage-cured strain of NCTC8325 harboring an 11-bp deletion in rsbU which regulates sigB activity by activating RsbV, a factor that competitively binds to the anti-sigma factor RsbW | 37 |
| 21 | ||
| COL | Methicillin-resistant laboratory strain | 37 |
| DB | Clinical blood isolate previously used in adhesion and endocarditis studies | 9 |
| Newman | Laboratory strain | 34 |
| S. epidermidis | From the Ultrecht University Hospital | |
| S. haemolyticus | From the Ultrecht University Hospital | |
| S. saprophyticus | From the Ultrecht University Hospital | |
| ALC133 | RN 6112; RN6390 with agrA::ermC | 40 |
| RN6911 | agr mutant of RN6390 (Δagr::tetM) | 38 |
| ALC135 | agr sarA double mutant of RN6390 | 2 |
| ALC1342 | sarA deletion mutant in which sarA (nt 586–1107) has been replaced by ermC | 13 |
| ALC1905 | sarT mutant of RN6390 (sarT::ermC) | This study |
| ALC2031 | RN8325-4 with a sarA::kan mutation | 4 |
| ALC2050 | sarA sarT mutant of 8325-4 | This study |
| ALC2056 | agr sarT mutant of RN6390 | This study |
| ALC2057 | RN6390 with a sarA::kan mutation | 13 |
| ALC2060 | sarT mutant of 8325-4 (sarT::ermC) | This study |
| ALC2063 | RN4220 with pALC2047 | This study |
| ALC2071 | ALC1905 with pALC2047 | This study |
| ALC2072 | ALC2050 with pALC2047 | This study |
| ALC2075 | ALC2060 with pALC2047 | This study |
| ALC2076 | ALC2056 with pALC2047 | This study |
| ALC2122 | sarA sarT mutant of RN6390 | This study |
| ALC2150 | ALC2122 with pALC2047 | This study |
| E. coli | ||
| XL1 Blue | General-purpose host strain for cloning | 31 |
| InvαF′ | Host strain for the TA cloning vector (pCRII) | Invitrogen |
| BL21(DE3)pLysS | Host strain for expression vector pET14b | Novagen |
| ALC1904 | BL21(DE3)pLysS containing pET14b::sarT | This study |
| Plasmids | ||
| pUC18 | E. coli cloning vector | 31 |
| pCL52.2 | Temperature-sensitive E. coli-S. aureus shuttle vector | 29 |
| pSK236 | Shuttle vector containing pUC19 cloned into the HindIII site of pC194 | 19 |
| pCR2.1 | E. coli PCR cloning vector | Invitrogen |
| pET14b | Expression vector | Novagen |
| pALC1894 | pUC18 with a 3.2-kb fragment containing the sarT coding region | This study |
| pALC1896 | pUC18 with a 4.4-kb fragment containing the sarT::ermC mutation | This study |
| pALC1898 | pCL52.2 with a 4.4-kb fragment containing the sarT::ermC mutation ligated at the BamHI site | This study |
| pALC1904 | pET14b with the sarT coding region at the XhoI-BamHI site | This study |
| pALC2046 | pCR2::sarT | This study |
| pALC2047 | pSK236::sarT | This study |
| pALC1742 | pSK236 (gfpuvr with agr P2 promoter) | This study |
| pALC1743 | PSK236 (gfpuvr with agr P3 promoter) | 26 |
DNA isolation.
Chromosomal DNA was isolated from overnight broth cultures of S. aureus by lysostaphin lysis and phenol extraction as described elsewhere (11). Plasmid DNA was isolated from E. coli strains by using a Qiagen plasmid mini kit. Plasmid DNA was extracted from S. aureus strains by a modification of the Qiagen plasmid mini kit in which cells collected from overnight culture were resuspended in the Qiagen P1 buffer with lysostaphin (100 μg/ml) and incubated at 37°C for 1 h.
Southern blot hybridization.
Restriction endonuclease-digested staphylococcal chromosomal DNA was resolved by overnight electrophoresis at 20 V in 0.8% agarose as described elsewhere (31). The DNA was transferred to Hybond-N+ nylon membrane by alkaline blotting (Amersham, Pharmacia Biotech UK). Target genes were detected by hybridization with gel-purified DNA probes radiolabeled with [α32P]dCTP (Amersham, Pharmacia Biotech) using a Ready-To-Go labeling kit (Amersham, Pharmacia Biotech) or Random Primer kit (Roche).
Cloning sarT and generating a sarT mutant.
To clone the sarT gene, primers based on flanking sequences (TIGR S. aureus contig 8076 [COL], nucleotides [nt] 1417 to 4061) were synthesized. A 3.2-kb fragment was amplified by PCR from S. aureus RN6390 chromosomal DNA with primers 1003 and 1004 (Table 2), digested with BamHI, ligated into the BamHI site of pUC18 (to make pALC1894), and transformed into E. coli XL1 Blue. Plasmids extracted from ampicillin-resistant colonies were screened for sarT fragment insertion by restriction endonuclease mapping and confirmed by DNA sequencing. To generate a sarT mutant, ermC (20) was ligated into a blunted Ndel site within the putative sarT coding region (nt 3093 to 3098). The resultant 4.4-kb sarT::ermC BamHI fragment was confirmed by DNA sequencing, gel purified, ligated into the temperature-sensitive shuttle plasmid pCL52.2 (to yield pALC1898), and electroporated into RN4220 as previously described (37) to generate transformants. Putative transformants were confirmed by restriction mapping. Electrocompetent RN6390 was subsequently transformed with pALC1898 isolated from RN4220 (11, 43). Colonies isolated at 30°C and resistant to erythromycin and tetracycline were screened for the presence of plasmid by restriction mapping.
TABLE 2.
Primers used for this study
| Primer | Primer sequence | Comment |
|---|---|---|
| 1003 | 5′-ACGGGGATCCTTATGACGTTGGAGAAAA | Upstream of sarT, BamHI site added (underlined) |
| 1004 | 5′-AGCGGGGATCCCAAGTTTTACCAGCATA | Downstream reverse primer, sarT |
| 1005 | 5′-GTAAGGGATGAACTCGAGATGAATGATT | Start of sarT (bold), added XhoI site (underlined) |
| 1006 | 5′-ACGGGGATCCAAAAATACATTTAACTGC | Reverse primer, downstream of sarT, BamHI site added (underlined) |
| 1013 | 5′-ATGGTCTATTTCAATGGCAGTTAC-3′ | Internal reverse primer, ermC |
| 1017 | 5′-GATGCGATTGAACGTATGAATAATGAT-3′ | Upstream of primer 1003 |
| 1035 | 5′-GCGAATTCTACCGGTCCTTTCTTATCTCT | Downstream of sarT coding for complete transcript, EcoRI site added (underlined) |
| 1036 | 5′-GCGAATTCCAGATTGTTTGTAAAGTATGT | Upstream sarT complete transcript, EcoRI site added |
RN6390 harboring pALC1898 was grown in 03GL broth with erythromycin (5 μg/ml) at 30°C, diluted 1:1,000 in fresh medium, and propagated through several cycles of alternating 30 and 42°C as described elsewhere (3). Erythromycin-resistant, tetracycline-sensitive colonies, representing possible double-crossover events, were selected (11) and screened for ermC insertion into sarT by Southern blotting, PCR, and sequencing of the PCR fragment containing the junctional fragment. One putative sarT mutant (ALC1905) was selected for further study.
To generate ALC2031, a sarT mutant of 8325-4, an 80α phage lysate of ALC1905 was used to infect S. aureus strain 8325-4 as previously described (11, 43). The sarA sarT double mutants derived from strains RN6390 and 8325-4 (ALC2122 and ALC2050) were generated by transducing ALC2057 and ALC2031, respectively, with an 80α phage lysate of the sarT mutant (ALC1905). An 80α phage lysate of RN6911, an agr mutant of RN6390 (ALC134), was used to infect the sarT mutant (ALC1905) to yield the agr sarT double mutant (ALC2056). To confirm the genotypes, DNA extracts of putative transductants were digested with restriction enzymes and screened by Southern blot hybridization for the presence of ermC genes and a shift in the size of the restriction digest fragment hybridizing with a sarT-specific probe. Interruption of the desired gene was also confirmed by PCR followed by sequencing of the PCR fragment.
Complementation.
The sarT transcript as derived from the sarT mRNA on a Northern blot was estimated to be ∼800 nt long. In examining the sarT sequence (Fig. 1B), a putative transcriptional termination signal could be identified. Based on these data, we amplified by PCR an 1,196-bp fragment with genomic DNA from RN6390, using primers 1035 and 1036 (nt 2469 to 3665). The PCR fragment was ligated into pCR2.1 and transformed into E. coli InvαF′ (Invitrogen) to generate pALC2046. The correct insert was confirmed by DNA sequencing. The inserted fragment in pALC2046 was then cleaved with EcoRI, ligated into pSK236, and transformed into E. coli XL1 Blue. RN4220 was electroporated with the recombinant plasmid containing sarT (37, 41), and transformants selected on tryptic soy agar with chloramphenicol. Recombinant plasmid was purified from RN4220 transformants and electroporated into the RN6390 mutants ALC1905 (sarT mutant), ALC 2122 (sarA sarT mutant), and ALC2056 (agr sarT mutant) and the 8325-4 mutant strains ALC2060 (sarT mutant of 8325-4) and ALC2050 (sarA sarT mutant of 8325-4). Putative transformants containing the plasmid were verified by restriction mapping. The presence of a sarT transcript in the transformants was confirmed by Northern blots.
FIG. 1.
Amino acid sequences for the SarA family of proteins. (A) Comparison of SarT with SarA, SarR (32), and SarS (13, 42). Con, consensus (shaded with black or gray). SarS, a 250-residue protein, has two 125-residue SarA-like modules; the C-terminal half (SarS2, 126 to 250 amino acids) shows homology with the N-terminal half (SarS1) and with other SarA homologs. (B) Promoter and termination regions of sarT. The putative −35 and −10 promoter recognition sites are underlined. The ribosomal binding site 7 bp upstream of the predicted translation start is underlined, and typical start (ATG) and termination (TAA) codons are bold. The putative terminator region consists of a T-rich region containing two potential base-paired stem-loop sequences (underlined).
RNA analysis.
To minimize variations from environmental factors, all of the strains in an experimental set were grown up within the same week, in the same incubator, using the same batch lot of CYGP broth. Results were obtained from at least two complete experimental sets, using RNA from cells grown and extracted at different times. In brief, overnight cultures were diluted to an optical density at 650 nm (OD650) of 0.1 (using an 18-mm borosilicate glass tube) in CYGP broth with appropriate antibiotics and grown at 37°C with shaking. At exponential (OD650 = 0.7), late exponential (OD650 = 1.1), and postexponential (OD650 = 1.7) phases, RNA was extracted with a reciprocating shaking device (BIO 101, Vista, Calif.) and precipitated with 2-propanol as previously described (14, 28) and then resuspended in 0.5% sodium dodecyl sulfate (SDS); the RNA concentration was determined by absorbance at 260 nm.
Twenty micrograms of total RNA was electrophoresed through a 1.5% agarose–0.66 M formaldehyde gel in morpholine propane sulfonic acid and blotted onto Hybond-N+ membranes as previously described (12). Prior to blotting, the gel was viewed under UV light to ensure that equivalent amounts of ethidium bromide-stained rRNA bands were present for each sample. After blotting, the gel was viewed again under UV light to confirm complete RNA transfer.
Gel-purified DNA probes were radiolabeled with [α32P]dCTP as described above for the detection of specific transcripts (sarR, sarT, sarA, hla, agrRNAII, and agr RNAIII). Blots were hybridized under high-stringency conditions, washed, and autoradiographed with Kodak X-Omat film.
RNAII and RNAIII promoter activation.
Plasmids pALC1742 and pALC1743, derivatives of shuttle plasmid pSK236 (26) containing the green fluorescent protein (GFPuvr) gene under the control of the agr P2 and P3 promoters, respectively, were electroporated into S. aureus strains ALC1905 (sarT mutant), ALC 2057 (sarA mutant), ALC2122 (sarA sarT mutant), and ALC2056 (agr sarT mutant). The resulting strains harboring the plasmids were grown with shaking in tryptic soy broth at 37°C. Aliquots were removed to microtiter plates, and the cell density (OD650) and degree of fluorescence were read hourly for 10 h in an FL600 fluorescence microplate reader (BioTek Instruments, Winooski, V.). Promoter activation was plotted as the ratio of fluorescence/optical density versus optical density, using the average values from triplicate readings.
Phenotypic characterization.
Extracellular proteins were precipitated from supernatants of overnight cultures with trichloroacetic acid as described previously (10, 40). Proteins were separated by electrophoresis on SDS–12% polyacrylamide gels (44) and electroblotted onto nitrocellulose (Osmonics, Westborough, Mass.). The blots were blocked overnight in blocking buffer (0.1 M Tris–0.5 M NaCl [pH 8.2] with 2% bovine serum albumin and 1% Tween 20) and probed with sheep antibody specific for α-hemolysin (1:2,000 dilution) (Toxin Technology, Sarasota, Fla.). Antibody binding was detected with alkaline phosphatase-labeled secondary antibody (Jackson ImmunoResearch Laboratories) and nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate substrate (Sigma) as described previously (6). Band intensities for the Northern blots were determined by densitometric scanning using SigmaGel software (Jandel Scientific, San Rafael, Calif.), with the data presented as integrated area units.
Hemolysin assays.
The spent supernatant from overnight cultures was assayed for α-hemolysin production using 4% defibrinated rabbit blood in triplicate in a microtiter assay as previously described (18). The positive control for lysis was 1% SDS. Titers were expressed as the reciprocal of the highest dilution showing 50% of the mean of the value for SDS hemolysis after 2 h of incubation at 37°C.
RESULTS
In searching for SarA homologs in the S. aureus genome, we found three homologous proteins, SarR, SarS (also called SarH1), and SarT (Fig. 1A). SarR is a 113-residue protein that binds to the sarA promoter to down-modulate SarA expression (32). SarS, a 250-residue protein that is identical to SarH1 recently reported by Tegmark et al. (42), is normally repressed by sarA and agr (13). Contrary to SarA, SarS is an activator of protein A synthesis (13). An additional putative regulator, SarT, was also identified by its homology with SarA in the S. aureus genome database (TIGR contig 8076). A six-frame translation of the sequence revealed a putative protein of 118 amino acids (Fig. 1A). Lying 7 bp upstream of the predicted translation start is a ribosomal binding site, followed by typical initiation (ATG) and termination (TAA) codons (Fig. 1B). The SarT protein has a predicted molecular mass of 16,096 Da, a high percentage of charged residues (43%), and homology with SarA (35%) and SarR (20%).
The gene was expressed by cloning the putative sarT coding region (primers 1005 and 1006) into pET14b, a His-tag (Invitrogen) expression vector. After induction with isopropyl-β-d-thiogalactopyranoside and purification on a nickel affinity column, we isolated a protein of ∼16 kDa after thrombin digestion. This protein, upon N-terminal microsequencing, showed agreement with the nine N-terminal amino acids of the predicted sequence (data not shown).
Characterizing the sarT gene in staphylococcal strains and in sarA and arg mutants.
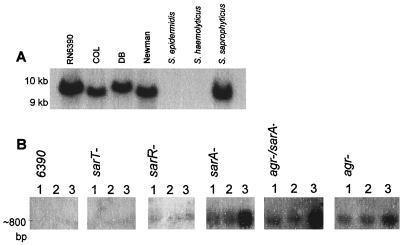
Previous studies (32) have shown that sarR, a gene homologous with sarA, was present in S. aureus and S. saprophyticus but not in S. epidermidis or S. haemolyticus when hybridized under high-stringency conditions. To determine the distribution of sarT in staphylococci, a 0.4-kb fragment encompassing the putative sarT gene was used to probe genomic DNA from several staphylcoccal species. The sarT probe hybridized with S. aureus strains COL, RN6390, Newman, and DB and S. saprophyticus, but not with S. epidermidis or S. haemolyticus, on a Southern blot of HindIII-digested genomic DNA (Fig. 2A). As with sarR (32), the failure of the sarT probe to hybridize with S. epidermidis or S. haemolyticus genomic DNA may be a result of either the absence of sarT or genetic divergence.
FIG. 2.
sarT genes and expression. (A) Southern blot. Genomic DNAs extracted from a collection of S. aureus and other staphlyococcal species were digested with HindIII (expected fragment size, 9,470 bp) and probed with a 0.4-kb fragment encompassing the putative sarT. The sarT probe did not hybridize with chromosomal DNA from S. epidermidis or S. haemolyticus. (B) Northern blots to determine if the sarT message is influenced by the sarA/agr regulatory system. RNA extracted from wild-type (RN6390) and mutant strains of S. aureus was probed with 32P-labeled sarT at exponential phase (OD650 = 0.7) (lane 1), late exponential phase (OD650 = 1.1) (lane 2), and postexponential phase (OD650 = 1.7) (lane 3).
The sarT message (Fig. 2B) was found to be ∼800 bp long when calculated from a plot of relative migration distance versus RNA size markers in a sarA mutant (Fig. 2B, fourth panel from the left). As a putative transcriptional termination signal was found downstream of the stop codon (Fig. 1B), we surmise that the sarT transcript is likely monocistronic.
Northern blots of wild-type, agr, sarA, and agr sarA mutant strains of RN6390 were probed with a 32P-labeled sarT fragment to ascertain sarT expression in these genetic backgrounds (Fig. 2B). In sarA, agr, and sarA agr mutants of RN6390, expression of sarT was significantly higher than in the parental strain at all time points during growth. Notably, sarT expression in these mutants was maximal during the postexponential phase (OD650 = 1.7), at a time when the secretion of exoproteins is generally the highest in the parental strain. In addition, the expression of sarT was higher in the sarA and agr sarA double mutants than the agr mutant. These data suggest that sarT transcription is repressed by sarA and agr, particularly in the postexponential phase.
Construction of sarT and sarA sarT and agr sarT double mutants.
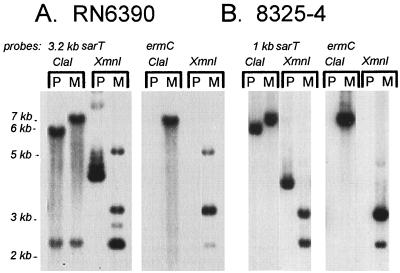
Since sarT has homology with sarA and other genes in the SarA family, we surmise that SarT may participate as an additional regulator downstream of sarA and agr in the regulatory cascade. To address this possibility, we generated the sarT mutant ALC1905 by transforming RN6390, a prototypic S. aureus strain, with a temperature-sensitive plasmid (pALC1898) that contained an ermC cassette within the sarT coding region, and selecting recombinants by antibiotic sensitivity. Successful generation of the sarT mutant in S. aureus was confirmed by probing Southern blots of ClaI or XmnI chromosomal digests with 32P-labeled fragments of ermC and sarT (Fig. 3A).
FIG. 3.
Southern blot of restriction digests of genomic DNA to demonstrate a change in the band size of the putative insertion mutation relative to the parental type, indicative of insertion of ermC into sarT. P, parental; M, sarT mutant. ClaI cleaves sarT but not ermC; the 3.2-kb sarT DNA probe hybridizes with a 5.6- and a 2.2-kb fragment, while the 1-kb sarT DNA probe hybridizes only with the 5.6-kb fragment. Insertion of ermC increases the larger fragment to 6.9 kb. There is a single XmnI site within ermC. XmnI yields a 4.0-kb fragment encompassing sarT. With the ermC insert, expected fragment sizes are 2.2 and 3.1 kb. The 2.6- and 5-kb fragments seen in panel A are consistent with incomplete enzyme digestion.
Northern blotting revealed that there was no detectable sarT message in the mutant strain (ALC1905) (Fig. 2B). For additional confirmation, we generated a PCR fragment by using a primer specific for ermC (primer 1013) and another primer from the chromosomal region outside the original sarT construct (primer 1017). The size of the PCR fragment as well as direct sequencing of the PCR fragment confirmed that a double-crossover event had taken place between the plasmid and the chromosome.
We recognized the possibility that the observed phenotypes might be strain dependent. To evaluate this, we generated another sarT mutant in strain 8325-4 by using an 80α lysate of ALC1905. Putative transductants were confirmed by Southern blot analysis (Fig. 3B) with ermC and sarT probes as described. The results from the RN6390 mutant strains were compared with results in corresponding 8325-4 mutant strains.
We also generated additional mutant strains harboring sarA sarT and agr sarT mutations to explore the effect of sarT on the sarA and agr mutant phenotypes. Accordingly, an 80α lysate of RN6911 (Δagr::tetM) was used to transduce the agr mutation into ALC1905 to generate an agr sarT double mutant. Similarly, a sarA sarT mutant was constructed by transducing ALC2057 (RN6390 with a sarA::kan mutation), and ALC2031 (8325-4 with a sarA::kan mutation) with an 80α lysate of ALC1905. To ensure that the observed effect of the above strains was attributable to the sarT mutation, the double mutant was also complemented with a recombinant shuttle plasmid (pSK236) carrying a 1.2-kb sarT fragment (pALC2047).
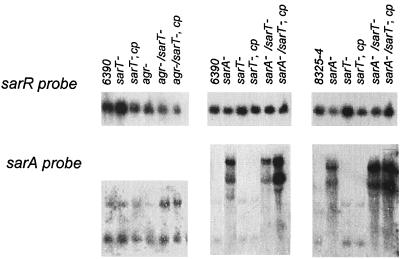
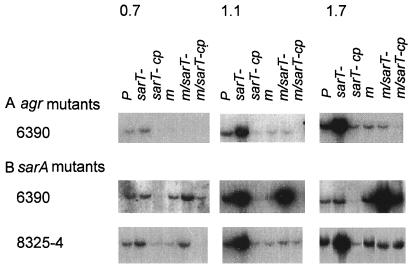
To ascertain the effect of the sarT mutation on the transcription of sarR and sarA, Northern blots of the wild-type strains (RN6390 and 8325-4), sarT mutants and complemented strains were probed with gel-purified 32P-labeled DNA fragments of sarR and sarA (Fig. 4). In blots probed with sarR, the sarT mutant strains showed a very slight increase in sarR transcription that appeared to be reversed by complementation in both RN6390 and 8325-4 backgrounds (Fig. 4). Interestingly, the sarA transcript level (i.e., P1, P3, and P2 transcripts) was not significantly altered among any of the sarT mutants or complemented strains compared with parental strains. Notably in strains RN6390 and 8325-4, the insertion of the kanamycin cassette (kan) within the sarA gene led to sarA transcripts of higher molecular size (Fig. 4). However, these altered transcripts did not result in synthesis of SarA, the sarA regulatory molecule, as determined by probing an immunoblot of cell extracts of sarA::kan strains with anti-SarA antibody (data not shown). Additionally, mutation in agr in a sarT mutant also did not markedly modify sarA transcription. Collectively, the data indicate that the effect of the sarT mutant on the expression of sarA is minimal and that sarT likely lies downstream of sarA.
FIG. 4.
Northern blots of RNA extracted from S. aureus strains at the postexponential phase of growth (OD650 = 1.7) and probed with sarR or sarA. cp, complemented with sarT in trans. The sarA mutant strains express an RNA message that is larger than the wild-type message, but it is apparently not translated, since SarA protein cannot be detected by a monoclonal antibody on a Western blot of whole-cell extracts.
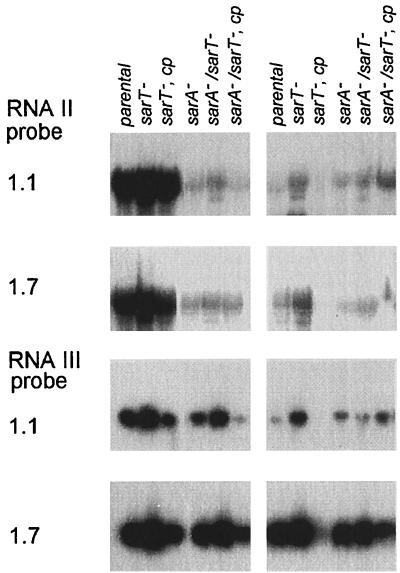
Northern blots (Fig. 5) showed that the RNAII levels were slightly higher in the sarT mutant than with the parental strains (RN6390 and 8325-4) but returned to near parental levels with sarT complementation. In contrast, the agr RNAIII message increased markedly in sarT mutants and was reduced to near parental levels in complemented sarT mutant strains in both RN6390 and 8325-4 backgrounds (Fig. 5). Thus, despite the repressive effect of sarA and agr on sarT expression, these data suggested that sarT might have a significant down-modulating effect on RNAIII transcription, while the effect on RNAII is much less.
FIG. 5.
Effects of sarT mutation on expression of RNAII and RNAIII. Northern blots of RNA extracted from S. aureus strains RN6390 (left) and 8325-4 (right) at late exponential (OD650 = 1.1) and postexponential (OD650 = 1.7) phases of growth were hybridized with agr RNAII or agr RNAIII probes. cp, complemented with sarT in trans.
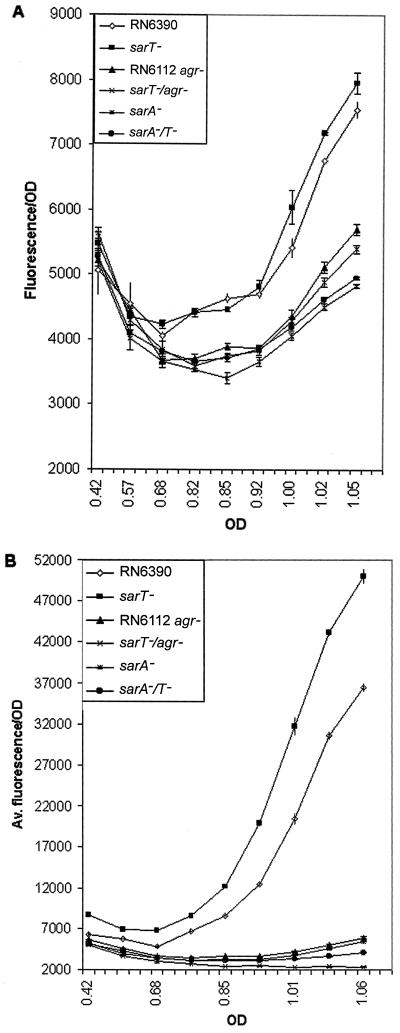
To further confirm the differential effects of the sarT mutation on agr expression, RN6390 and the isogenic sarT mutant strains were transformed with shuttle plasmid pSK236, harboring the GFPuvr gene driven by the RNAII or RNAIII promoter (pALC1742 or pALC1743, respectively). Levels of GFP expression in the mutants paralleled the RNA blot data (Fig. 6). With this assay, RNAII expression levels were slightly increased and RNAIII levels were significantly elevated when sarT was inactivated by mutation, particularly in postexponential-phase cells (Fig. 6). The sarA sarT, agr, and agr sarT mutants expressed GFP reporter activities for RNAII and RNAIII promoters at levels comparable to those for the sarA mutant (Fig. 6).
FIG. 6.
GFP expression driven by the agr RNAII (A) and agr RNAIII (B) promoters.
Characterization of the sarT mutant phenotype.
The sarA agr global regulatory network has been shown to activate the expression of a number of exoproteins with toxin and enzymatic activities (e.g., hemolysins, toxins, proteases, and lipase) during the postexponential phase. As a putative regulatory component downstream of sarA and possibly agr in the regulatory cascade, we hypothesize that sarT could function as an intermediary to repress exoprotein synthesis, particularly in light of the observation that sarT transcription was elevated in sarA and agr mutants and that sarT was maximally expressed during the postexponential phase.
To ascertain the effect of sarT on the expression of α-hemolysin, an important extracellular virulence determinant of S. aureus, we probed the parental, mutant, and complemented strains for hla expression on Northern blots (Fig. 7). Remarkably, the level of message for hla was higher in sarT mutants than in parental strains for both RN6390 and 8325-4 (Fig. 7, P versus sarT- lanes). However, upon complementation, the level of hla expression was reduced to very low levels (sarT- cp lane in each panel), presumably due to enhanced repression from increased sarT gene dosage. As predicted from the agr phenotype, hla transcription was markedly diminished in the agr mutant (Fig. 7A, lane m). Contrary to the sarT mutant, which displayed augmented hla transcription relative to the parental strain, the agr sarT double mutant did not display a higher level of hla transcription than the agr single mutant (Fig. 7A, lane m/sarT-), thus implying that sarT is not the primary intermediary target of agr that mediates enhanced hla expression.
FIG. 7.
Comparison of hla expression in agr (A) and sarA (B) mutants. Shown are Northern blots of RNA extracted from S. aureus strains at mid-exponential (OD650 = 0.7), late exponential (1.1), and postexponential (1.7) phases of growth. P, parental strain, either 6390 or 8325-4; m, mutation, either agr or sarA; cp, complemented with sarT in trans. The various sarA and sarT mutants show that hla transcription is repressed by sarT. However, hla expression is also influence by the strain background.
Contrary to the agr sarT mutant, hla transcription was enhanced in the sarA sarT mutant compared with the sarA single mutant (Fig. 7B, lane m/sarT- versus lane m). Complementation of the sarA sarT mutant with a recombinant shuttle plasmid carrying sarT repressed hla expression to a certain extent, but not always to parental levels, particularly during the postexponential phase (Fig. 7B, lane m/sarT-cp). This finding would be consistent with the presence of a SarA-independent activator of hla that can overcome the suppressive effect of sarT on hla transcription.
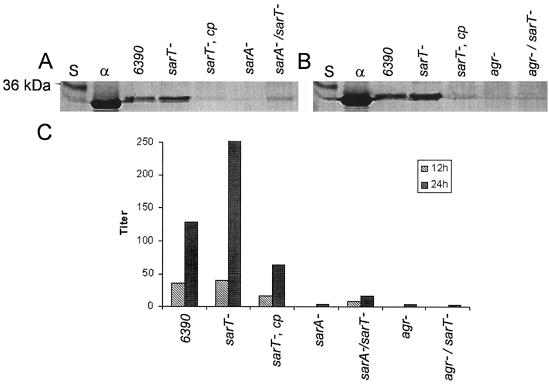
Western blot analysis.
Western blots of extracellular protein from wild-type, mutant, and complemented mutant strains were probed with sheep antibody specific for α-hemolysin (Fig. 8A and B). α-Hemolysin, normally expressed maximally during the postexponential phase, was produced in higher quantities in the sarT mutant and returned to a very low level in the sarT complemented strains (Fig. 8A and B). Although the sarA mutant expressed very little α-hemolysin, the sarA sarT double mutant exhibited detectable levels of α-hemolysin production (Fig. 8A). In contrast, the agr sarT mutant did not produce a detectable level of α-hemolysin (Fig. 8B). The titers from the rabbit erythrocyte hemolysis assay (Fig. 8C) are comparable with the Western blot results with respect to relative activity levels for various strains. The 24-h broths showed an increase in α-hemolysin relative to the 12-h broths, possibly due to accumulation of α-hemolysin with time.
FIG. 8.
Western blot and α-hemolysin assay of sarT mutants. (A and B) Extracellular protein probed with sheep polyclonal antibody to α-hemolysin; (C) α-hemolysin-induced rabbit erythrocyte hemolysis assay. S, protein molecular weight standards; α, α-hemolysin control; cp, complemented with sarT in trans: (A) RN6390 parental, 452 integrated area units determined by densitometric scanning (IAU); sarT, 600 IAU; sarA/sarT, 83 IAU; sarA mutant, undetectable level. (B) RN6390 parental, 207 IAU; sarT, 765 IAU. Titers are expressed as the reciprocal of the highest dilution showing 50% hemolysis.
DISCUSSION
The sarA agr regulatory system is a major controlling element for the expression of a number of virulence determinants during the growth cycle (4, 10, 17). In addition to modulating the expression of a number of cell wall proteins (e.g., fibronectin binding proteins) during the exponential phase, the sarA/agr regulatory system also plays a major role in regulating toxin synthesis (e.g., α-hemolysin) during the postexponential phase. Because of the complexity and the growth phase dependency of the sarA/agr regulatory system, it has been speculated that other regulatory elements may be involved in the precise downstream control of virulence determinants during the transition from one growth phase to another.
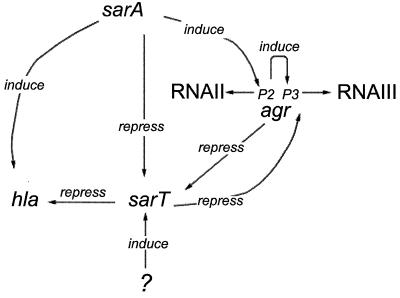
Synthesis of α-hemolysin occurs primarily in transition from late exponential phase to postexponential phase. This suggests a requirement for the activation of additional genes or the suppression of preexisting repressor gene products. sarT, discovered by virtue of its homology to sarA, appears to be an intermediary gene that functions downstream of sarA. Evidence from our data (Fig. 2) indicates that sarT is induced during the exponential-postexponential transition and that sarA acts as a major sarT repressor, since sarT levels are significantly elevated in sarA mutants. As sarR and sarA expression was not significantly altered in sarT mutants relative to the parental strain, sarT is likely downstream of sarA in the regulatory cascade (Fig. 9).
FIG. 9.
Regulation of sarT. The most probable interaction of the sarA/agr/sarT regulatory network as it is currently understood. SarA induces the P2 promoter of RNAII, which in turn induces expression of RNAIII (15). SarA and agr both act to repress expression of sarT; SarT in turn appears to repress expression of agr RNAIII and hla. An unknown element induces sarT, and sarA induces hla via an agr-independent pathway (7). It is possible that hla induction by SarA is via SarT.
Although sarT is repressed by agr (Fig. 2), our data also indicated that sarT significantly down-modulates the expression of RNAIII of the agr locus. This effect on RNAIII was reversible upon complementation. It is possible that sarT also has a slight effect on agr RNAII, since levels were slightly elevated in sarT mutants. This effect is sarA independent, since there are no major differences in RNAII and RNAIII expression levels in the sarA and sarA sarT mutants.
Based on the finding that sarT may be a repressor of hla expression, it is logical to assume that repression of sarT by both sarA and agr may activate hla expression. However, our data clearly demonstrated that only sarA activates hla transcription by repressing sarT, since a sarA sarT double mutant was able to augment hla expression to a level higher than that in the sarA mutant. In contrast, the agr locus did not utilize this pathway because hla expression in the agr sarT double mutant remained depressed to a level similar to that of the agr mutant. Collectively, these data indicate that sarA likely activates hla expression by repressing sarT.
The effect of sarT on hla expression is complex. While a sarT mutation resulted in an increase in hla transcription, the mutant also exhibited an increase in RNAIII transcription, as verified by Northern blot and transcriptional fusion data (Fig. 5 and 6). This finding for sarT thus hinted at the complexity of hla regulation by the sarA locus. In the presence of an intact sarA, the expression of sarT is repressed, leading to elevated hla transcription. However, the relative contribution of the effect of SarT on the expression of hla, as mediated via RNAIII, versus that which occurs as a result of direct interaction of sarT with the hla promoter is not clear. In addition, we have previously reported that SarA, the major sarA regulatory molecule, can up-regulate hla expression via both RNAIII-independent and RNAIII-dependent pathways. With the RNAIII-independent pathway, SarA binds directly to a recognition sequence in the hla promoter to activate transcription (17). With the RNAIII-dependent pathway, SarA binds to the conserved sequence upstream of the agr promoter to stimulate RNAII and RNAIII transcription (37) and possibly transcription and translation of hla (36). Collectively, these data hint at the complexity of the pathways by which hla expression is activated.
Our data also seems to suggest complex interactions between sarT and agr (Fig 9). On one hand, we recognize that the transcription of sarT is increased in agr mutants. On the other hand, RNAIII expression is also increased in a sarT mutant. Thus, there appears to be an inverse relationship (or possibly a negative feedback loop) between the presence of sarT and the expression of RNAIII. This putative feedback loop may conceivably lie downstream of sarA. This mode of regulatory hierarchy may explain (i) increased hla transcription in the sarA sarT double mutant by virtue of increasing RNAIII expression (Fig. 7B, lane 5; Fig. 5, lane 5) and (ii) a failure to increase hla transcription in an agr sarT double mutant compared with the agr single mutant (Fig. 7A, lane 5 versus lane 4).
Although sarA likely mediates hla expression by repressing sarT, RNA complementation data disclosed that the regulation of hla by the sarA locus, particularly during the postexponential phase, likely involves additional regulatory factors. This notion is supported by the observation that complementation of the sarA sarT mutant with sarT in trans could suppress hla expression in the mutant strain only during the exponential phase (OD650 of 0.7 and 1.1) but not during the postexponential phase (OD650 = 1.7) (Fig. 7B and C). Additionally, RNAIII repression in a complemented sarA sarT mutant was highly successful during exponential growth but not postexponentially (Fig. 5). These data are consistent with the observation of Vandenesch et al. (45) that a separate postexponential phase signal other than agr is also needed for activating hla transcription.
The large number of regulatory proteins recently described in S. aureus as a result of genomic advances (4, 11, 23), coupled with the elucidation of their regulatory controls on target genes, suggests that virulence gene regulation in S. aureus entails a complex network of regulatory genes. Some of these gene products (e.g., SigB and SarR) control the expression of SarA, while others such as SarH1 (also called SarS), Rot, and SarT may act as intermediaries between the regulatory elements (sarA/agr) and target genes (e.g., hla and spa). Clearly, additional regulatory factors will be discovered as the S. aureus genome is completed.
ACKNOWLEDGMENTS
This work was supported in part by PHS grant A107519-14 and NIH grants AI43968 and AI37142.
We thank Stephen A. Bobin of the molecular biology core facility for assistance and sequencing advice, Simon Foster for sharing PC1839 (sarA mutant with a sarA::kan mutation), and Willem van Wamel for providing S. epidermidis, S. haemolyticus, and S. saprophyticus. Access to the genomic data at TIGR and at the University of Oklahoma genome center is gratefully acknowledged.
REFERENCES
- 1.Abdelnour A, Arvidson S, Bremell T, Rydén C, Tarkowski A. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun. 1993;61:3879–3885. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth M C, Cheung A L, Hatter K L, Jett B D, Callegan M C, Gilmore M S. Staphylococcal accessory regulatory (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect Immun. 1997;65:1550–1556. doi: 10.1128/iai.65.4.1550-1556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilli A, Portnoy D A, Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990;172:3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan P F, Foster S J. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J Bacteriol. 1998;180:6232–6241. doi: 10.1128/jb.180.23.6232-6241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan P F, Foster S J, Ingham E, Clements M O. The Staphylococcus aureus alternative sigma factor ςB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung A L, Bayer M G, Heinrichs J H. sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J Bacteriol. 1997;179:3963–3971. doi: 10.1128/jb.179.12.3963-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A L, Chien Y T, Bayer A S. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect Immun. 1999;67:1331–1337. doi: 10.1128/iai.67.3.1331-1337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung A L, Eberhardt K J, Chung E, Yeaman M R, Sullam P M, Ramos M, Bayer A S. Diminished virulence of a sar/agr mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Investig. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung A L, Fischetti V. The role of fibrinogen in staphylococcal adherence to catheters in vitro. J Infect Dis. 1990;161:1177–1186. doi: 10.1093/infdis/161.6.1177. [DOI] [PubMed] [Google Scholar]
- 10.Cheung A L, Fischetti V. Variation in the expression of cell wall proteins of Staphylococcus aureus grown on solid and liquid media. Infect Immun. 1988;56:1061–1065. doi: 10.1128/iai.56.5.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung A L, Koomey J M, Butler C A, Projan S J, Fischetti V A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung A L, Projan S J. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J Bacteriol. 1994;176:4168–4172. doi: 10.1128/jb.176.13.4168-4172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung A L, Schmidt K, Bateman B, Manna A C. SarS, a SarA homolog repressible by agr, is an activator of protein A synthesis in Staphylococcus aureus. Infect Immun. 2001;69:2448–2455. doi: 10.1128/IAI.69.4.2448-2455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung A L, Wolz C, Yeaman M R, Bayer A S. Insertional inactivation of a chromosomal locus that modulates expression of potential virulence deteminants in Staphylococcus aureus. J Bacteriol. 1995;177:3220–3226. doi: 10.1128/jb.177.11.3220-3226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien Y-T, Cheung A L. Molecular interactions between two global regulators, sar and agr, in Staphylococcus aureus. J Biol Chem. 1998;273:2645–2652. doi: 10.1074/jbc.273.5.2645. [DOI] [PubMed] [Google Scholar]
- 16.Chien Y-T, Manna A C, Cheung A L. SarA level is a determinant of agr activation in Staphylococcus aureus. Mol Microbiol. 1998;30:991–1001. doi: 10.1046/j.1365-2958.1998.01126.x. [DOI] [PubMed] [Google Scholar]
- 17.Chien Y-T, Manna A C, Projan S J, Cheung A L. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J Biol Chem. 1999;274:37169–37176. doi: 10.1074/jbc.274.52.37169. [DOI] [PubMed] [Google Scholar]
- 18.Fluckiger U, Wolz C, Cheung A L. Characterization of a sar homolog of Staphylococcus epidermidis. Infect Immun. 1998;66:2871–2878. doi: 10.1128/iai.66.6.2871-2878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaskill M E, Khan S A. Regulation of the enterotoxin B gene in Staphylococcus aureus. J Biol Chem. 1988;263:6276–6280. [PubMed] [Google Scholar]
- 20.Gennaro M L, Novick R P. An enhancer of DNA replication. J Bacteriol. 1988;170:5709–5717. doi: 10.1128/jb.170.12.5709-5717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gertz S, Englemann S, Schmid R, Ohlsen K, Hacker J, Hecker M. Regulation of sigmaB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol Gen Genet. 1999;261:558–566. doi: 10.1007/s004380051001. [DOI] [PubMed] [Google Scholar]
- 22.Gillaspy A F, Hickmon S G, Skinner R A, Thomas J R, Nelson C L, Smeltzer M A. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun. 1995;63:3373–3380. doi: 10.1128/iai.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giraudo A T, Cheung A L, Nagel R. The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Arch Microbiol. 1997;168:53–58. doi: 10.1007/s002030050469. [DOI] [PubMed] [Google Scholar]
- 24.Giraudo A T, Rampone H, Calzolari A, Nagel R. Phenotypic characterization and virulence of a sae−agr− mutant of Staphylococcus aureus. Can J Microbiol. 1995;42:120–123. doi: 10.1139/m96-019. [DOI] [PubMed] [Google Scholar]
- 25.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khal B C, Goulian M, v. Wamel W, Herrmann M, Simon S M, Kaplan G, Peters G, Cheung A L. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect Immun. 2000;68:5385–5392. doi: 10.1128/iai.68.9.5385-5392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornblum J, Kreiswirth B, Projan S J, Ross H, Novick R P. agr. a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 373–402. [Google Scholar]
- 28.Kornblum J, Projan S J, Moghazeh S L, Novick R P. A rapid method to quantitate non-labeled RNA species in bacterial cells. Gene. 1988;63:75–85. doi: 10.1016/0378-1119(88)90547-1. [DOI] [PubMed] [Google Scholar]
- 29.Lee C Y. Cloning of genes affecting capsule expression in Staphylococcus aureus strain M. Mol Microbiol. 1992;6:1515–1522. doi: 10.1111/j.1365-2958.1992.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 30.Lina G, Jarraud S, Ji G, Greenland T, Pedraza A, Etienne J, Novick R P, Vandenesch F. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol Microbiol. 1998;28:655–662. doi: 10.1046/j.1365-2958.1998.00830.x. [DOI] [PubMed] [Google Scholar]
- 31.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Manna A, Cheung A L. Characterization of sarR, a modulator of sar expression in Staphylococcus aureus. Infect Immun. 2001;69:885–896. doi: 10.1128/IAI.69.2.885-896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manna A C, Bayer M G, Cheung A L. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J Bacteriol. 1998;180:3828–3836. doi: 10.1128/jb.180.15.3828-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDevitt D, Francois P, Vandaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 35.McNamara P J, Milligan-Monroe K C, Khalili S, Proctor R A. Identification, cloning and initial characterization of rot, a locus encoding a regulator of virulence factor expression in Staphylococcus aureus. J Bacteriol. 2000;182:3197–3203. doi: 10.1128/jb.182.11.3197-3203.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morfeldt E, Tegmark K, Arvidson S. Transcriptional control of the agr-dependent virulence gene regulator, RNAIII, in Staphylococcus aureus. Mol Microbiol. 1996;21:1227–1237. doi: 10.1046/j.1365-2958.1996.751447.x. [DOI] [PubMed] [Google Scholar]
- 37.Novick R P. The Staphylococcus as a molecular genetic system. In: Novick R P, editor. Molecular biology of the Staphylococcus. New York, N.Y: VCH Publishers; 1990. pp. 1–40. [Google Scholar]
- 38.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Projan S J, Novick R P. The molecular basis of pathogenicity. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingston; 1997. pp. 55–81. [Google Scholar]
- 40.Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick R P. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 41.Schenk S, Laddaga R A. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett. 1992;73:133–138. doi: 10.1016/0378-1097(92)90596-g. [DOI] [PubMed] [Google Scholar]
- 42.Tegmark K, Karlsson A, Arvidson S. Identification and characterization of SarH1, a new global regulator of virulence gene expression in Staphylococcus aureus. Mol Microbiol. 2000;37:398–409. doi: 10.1046/j.1365-2958.2000.02003.x. [DOI] [PubMed] [Google Scholar]
- 43.Thomas W D, Jr, Archer G L. Identification and cloning of the conjugative transfer region of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1989;171:684–691. doi: 10.1128/jb.171.2.684-691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandenesch F, Kornblum J, Novick R P. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J Bacteriol. 1991;173:6313–6320. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]