Abstract
Simple Summary
Parkinson’s disease is a neurodegenerative disease of the central nervous system, characterized by movement problems and accompanied by behavioral changes such as depression and anxiety. It is a multifactorial condition that is affected by genetic alterations and environmental factors that progressively lead to the death of specialized neurons. This systematic review discusses the attractive hypothesis that gut intestinal dysbiosis is an initial step of a process that leads to Parkinson’s. Gut microbiota alterations and their metabolites can cause intestinal inflammation, but they can also alter gut-brain communication and the brain barrier. This can lead to brain inflammation and the deterioration of brain cells, a process called neurodegeneration. Understanding the role of gut microbiota in the progression of Parkinson’s could be a key element for research toward therapeutic approaches that could delay and even cure the disease.
Abstract
Parkinson’s disease is a progressive neurodegenerative disorder with motor, physical and behavioral symptoms that can have a profound impact on the patient’s quality of life. Most cases are idiopathic, and the exact mechanism of the disease’s cause is unknown. The current hypothesis focuses on the gut-brain axis and states that gut microbiota dysbiosis can trigger inflammation and advances the development of Parkinson’s disease. This systematic review presents the current knowledge of gut microbiota analysis and inflammation based on selected studies on Parkinson’s patients and experimental animal models. Changes in gut microbiota correlate with Parkinson’s disease, but only a few studies have considered inflammatory modulators as important triggers of the disease. Nevertheless, it is evident that proinflammatory cytokines and chemokines are induced in the gut, the circulation, and the brain before the development of the disease’s neurological symptoms and exacerbate the disease. Increased levels of tumor necrosis factor, interleukin-1β, interleukin-6, interleukin-17A and interferon-γ can correlate with altered gut microbiota. Instead, treatment of gut dysbiosis is accompanied by reduced levels of inflammatory mediators in specific tissues, such as the colon, brain and serum and/or cerebrospinal fluid. Deciphering the role of the immune responses and the mechanisms of the PD-associated gut microbiota will assist the interpretation of the pathogenesis of Parkinson’s and will elucidate appropriate therapeutic strategies.
Keywords: Parkinson’s disease, cytokines, chemokines, gut, microbiota
1. Introduction
Parkinson’s disease (PD) is a slow-progressing neurodegenerative disease with high prevalence, currently affecting over 6 million people worldwide [1]. PD is mainly characterized by the impairment of motor function, as well as by a variety of nonmotor manifestations, including hyposmia, dysautonomia (e.g., orthostatic hypotension.), neurocognitive impairment, and sleep disturbances, all of which can have detrimental impacts on the patient’s quality of life [2,3,4]. There are currently no therapeutic approaches that can delay the progression of PD, only symptomatic treatments [5,6]. Sporadic forms of PD are thought to result from complex interactions between different genetic and environmental factors [7]. Up to 15% of patients with PD have genetic alterations [8]. Although these genetic variants frequently result in familial PD, illness penetrance varies greatly across mutation carriers, indicating that additional genetic modifiers or non-genetic environmental factors may also have an impact on the development of the disease. From a pathological perspective, PD is mainly characterized by alpha-synuclein misfolding, which results in the amyloid formation of insoluble aggregates (i.e., Lewy bodies and neuritis) in neurons, as well as glia, followed by subsequent neurodegeneration [8,9,10]. There is increasing evidence that gut-brain communication contributes to the development and progression of PD and involves the role of a-synuclein signaling [11,12,13]. Gut microbiota is considered an important mediator in communication that influences brain development and function [14]. In healthy individuals, the crosstalk between gut microbiota and their host is typically mutually beneficial. However, the fact that environmental factors impact the structure and function of microbiota in the alteration of their numbers and/or species could advance the development of certain diseases, a state known as dysbiosis. [15,16,17,18]. Many studies support that gut dysbiosis, which leads to a leaky gut, an alteration in the blood–brain barrier and neuroinflammation, is prevalent in PD [19,20,21,22,23,24,25]. Additionally, there is experimental evidence that gut microbiota induces a-synuclein synthesis [26]. The first study states that gut lumen (Escherichia coli) releases the extracellular amyloid protein Curli [27], which in rats could induce the disposition of a-synuclein in both the gut and the brain [28]. Subsequent studies have revealed that the presence of Curli in the gut can modulate a-synuclein aggregation [29,30,31]. Similarly, the lipopolysaccharide (LPS) expressed on the plasma membrane of Gram-negative bacteria has been shown to induce a-synuclein aggregation and provides another explanation of how microbiota contributes to PD [32,33,34,35]. Therefore, the role of gut microbiota in PD represents a significant area of research as they are potential therapeutic targets for the development of new strategies to tackle this disease [36].
Intestinal microbiota is also known to induce inflammation in conditions like dysbiosis, a topic that has been discussed extensively in other studies but not in relation to PD [37]. Studies on both human PD and animal models note that both innate and adaptive immune responses are affected during the disease [38,39]. Research on human donors has focused on peripheral inflammation, mainly due to the nature of the disease and the availability of samples. Increased levels of proinflammatory cytokines and chemokines, such as interleukin-6 (IL-6), tumor necrosis factor (TNF), interleukin-1β (IL-1β), chemokine (C-C motif) ligand 5 (CCL5) and interleukin-2 (IL-2) have been detected in serum, cerebrospinal fluid (CSF) and the brains of PD patients [40,41,42,43,44]. A recent hypothesis indicates that PD-related peripheral inflammation is a result of intestinal barrier deterioration, which causes systemic exposure to bacterial products such as LPS [45]. Increased levels of LPS and decreased LPS-binding protein (LBP) in the blood and plasma of PD patients further support this hypothesis [46,47]. The role of LPS in the progression of PD led to the development of various animal experimental models, through which mechanistic and drug discovery studies related to the disease will be performed [46].
This review highlights the role of gut microbiota dysbiosis and consequent inflammation in the contribution of the gut-brain axis in Parkinson’s disease. We will discuss the current knowledge of gut microbiota and inflammatory mediators, cytokines and chemokines, as well as their contribution to the advancement of PD. The usage of experimental models and the application of advanced methodologies to human PD research will facilitate our understanding of the interplay between these factors. This will further help us to design strategies that will prevent peripheral inflammation and neuroinflammation, therefore inhibiting neurodegeneration in PD patients.
2. Materials and Methods
2.1. Information Sources and Search Strategy
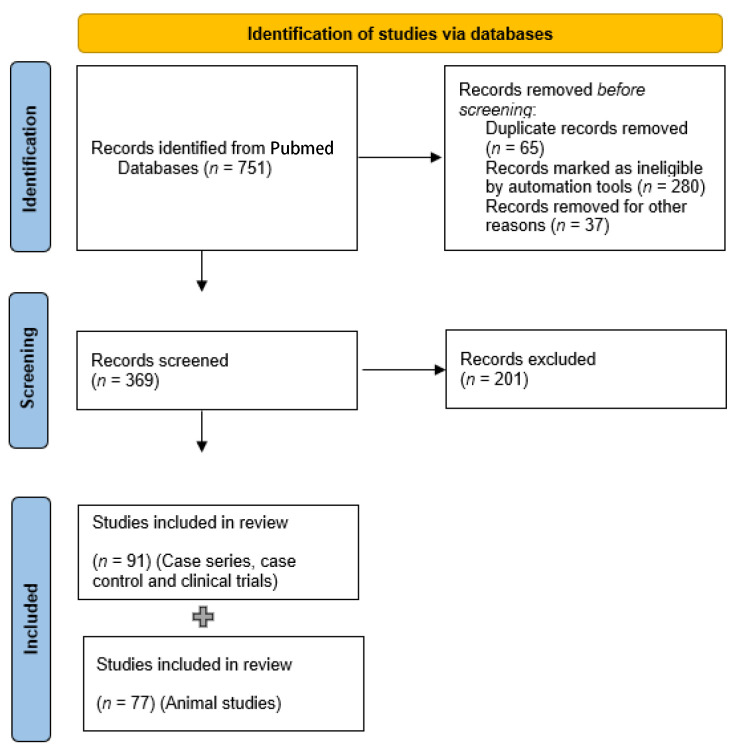
In this systematic review, studies on the association of gut microbiota, inflammatory mediators and PD were searched in PubMed from inception to June 2022. The search terms were as follows: (Parkinson OR Parkinson’s disease OR Parkinson’s syndrome) AND (gut intestine flora) OR (microbiota) OR (microbiome) OR (flora) OR (gut microflora) OR (appendix) OR (vermiform appendix). References in the articles were assessed to retrieve additional potentially relevant studies. There were no language restrictions. The PRISMA guidelines were followed for this review [48], and the flow diagram is shown in Figure 1.
Figure 1.
Flow diagram describing the process of identification, screening and final selection of the studies used in this review.
2.2. Assessment for Eligibility
Studies were included if they met the following criteria: (a) studies that had a cross-sectional, cohort, or case–control design; (b) studies that recruited subjects who met the PD diagnostic criteria; (c) studies in which microbiota was studied with the following tests: the GBT (Glucose Hydrogen Breath Test), LBT (Lactulose Hydrogen Breath Test) or JAC (Jejunal Aspirated Culture); (d) studies that compared the association of PD and microbiota; (e) animal studies; (f) studies with full texts available; and (g) studies that included measurement of cytokines and chemokines. The exclusion criteria were as follows: case reports, review articles, and letters. The manuscripts were assessed in duplicates by a team (AA, PD, SP).
3. Results
A total of 751 potentially eligible articles were identified based on the described search strategy. Of all the extracted articles, 382 were excluded because they were duplicates, reviews, meta-analyses, or irrelevant studies. Finally, 369 studies were screened again, and we included in the analysis 91 case series, case–control studies and clinical trials and 77 animal studies. In total, four out of the human studies and 33 out of the animal studies had included approaches for cytokine and chemokine measurement.
3.1. Metagenomics
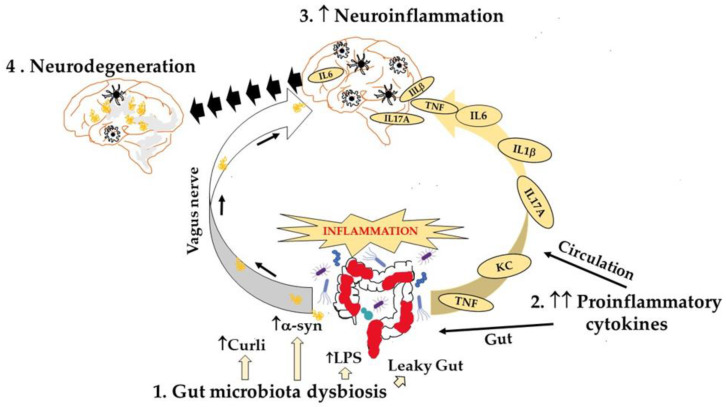
PD has received a great deal of research attention in the field of metagenomics and the role of gut microbiota in the pathogenesis of PD [49]. The first metagenomic investigation was published by Scheperjans et al. in 2015, and it revealed a decline in Prevotellaceae in feces from PD patients compared to healthy donors, as well as a positive link between Enterobacteriaceae abundance and the motor phenotype, specifically postural instability, and gait deviations [50]. A year later, another study discovered changes in these bacterial families, where Enterobacteriaceae were more prevalent than Prevotellaceae [51]. It is noteworthy to mention that another study showed a correlation between increased intestinal permeability and the presence of E. coli, a bacterial species that is assigned to the family Enterobacteriaceae [52]. Also, the phylum Bacteriodetes was significantly reduced in the feces of patients with PD [51]. The same PD fecal samples had lower levels of short chain fatty acids (SCFAs), e.g., acetate, propionate and butyrate, an important mediator for the maintenance of a healthy intestinal barrier [51,53]. In a recent review, Castillo-Alvarez and Marzo-Sola discuss studies by research groups that demonstrated a relationship between PD and specific gut microbiota compositions [54]. These results show that some microbes that produce SCFAs that have anti-inflammatory effects are more prevalent in feces from control donors than in patients with PD, while other microorganisms with a more proinflammatory profile are more prevalent in patients with PD. The gut microbiome has been reported to correlate with the disease symptoms since the first metagenomic study; Barichella et al. also showed that differences in taxa abundances were associated with the disease duration and affected the clinical profile of the disease [55]. A metagenomic shotgun analysis, where fecal microbiomes of early stage, L-DOPA-naïve PD patients compared to healthy controls, revealed differences in colonic microbiota with the total virus abundance decreased in PD participants [56]. A brief summary of gut microbiota dysbiosis’s impact on gut and brain function and communication is presented in Figure 2.
Figure 2.
Gut microbiota dysbiosis leads to neurodegeneration. Dysbiosis in the gut can cause (a) inflammation with increased levels of proinflammatory cytokines locally, in circulation, and in brain, (b) induction of a-synuclein aggregates in the gut that via the vagus nerve promote further aggregation in the brain where a-synuclein activates microglia and astrocytes and (c) neuroinflammation that over time leads to neurodegeneration.
3.2. Cytokines and Chemokines
It has been recently recognized that there is a low-grade inflammation in the gut in PD. Gene expression studies showed upregulation of proinflammatory cytokines and chemokines in gut tissue from PD donors compared to healthy controls [57,58]. Specifically, Perez-Pardo et al. performed microarray analysis on human colonic biopsies and observed higher pro-inflammatory milieu in the tissues of PD patients, including, among others, upregulation of the IFNG, IL1B, IL17A and IL8 genes [57]. In the same study, the authors clearly demonstrated the contribution of Toll-like receptor 4 (TLR-4) signaling in gut inflammation by showing that the TLR4 knockout mice were resistant to many of the PD-like consequences of rotenone-induced phenotype. Similarly, Devos et al. observed elevated mRNA expression levels of TNF, IFNG, IL6 and IL1B in the ascending colon of PD patients [58]. Higher levels of IL-1α, IL-1β, IL-8 and C-reactive protein (CRP) have been found in stool homogenates from PD patients using a multiplexed immunoassay [59]. According to this study, the differences in those inflammatory mediators were not dependent on subject age or disease duration. Finally, increased numbers of T cells have been identified in colon tissue from PD patients compared to healthy individuals [57]. These findings support the hypothesis that gut inflammation could impact PD pathophysiology. In the following sections, the findings from human and experimental animal studies will be discussed separately.
3.2.1. Human Studies
Only four of the selected studies had a combination of data about gut microbiota and cytokine or chemokine levels in PD patients. Lin et al. measured fecal microbiota in healthy and PD donors, as well as inflammatory mediators in serum, combining two different cohorts [60]. Among all the cytokines, only IFNγ and TNF plasma levels correlated with the altered gut microbiota. In a clinical trial in which samples were taken before and after treatment, it was found that berberine hydrochloride reduced both gut dysbiosis and serum levels of IL-6, IL-1β and TNF [61]. In a report by Aho et al., stool SCFAs and stool and plasma inflammatory mediators were measured and analyzed in relation to the disease (healthy versus PD donors) and gut microbiota [62]. Samples from PD patients had reduced SCFAs and showed signs of inflammation in correlation to the microbiota and disease onset, but they were not reflected in the systemic inflammatory profile. Lastly, a very recent study showed the beneficial effect of probiotics on PD patients using the M-SHIME® system, a short-term culture of human fecal samples [48,63,64]. The results revealed changes in the microbial community, in specific SCFAs levels (acetate, propionate and butyrate) and lactate, which is the product of carbohydrate fermentation and key to good health. Moreover, colonic extracts from healthy and PD donors were tested in an in vitro setting and induced various cytokines and chemokines to a different degree [64].
3.2.2. Experimental Animal Studies
Most of the animal studies were based on the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) model, which was established almost 40 years ago [65], followed by 6-OHDA (6-hydroxydopamine) [66] and rotenone-induced models [67]. Much fewer studies involved transgenic mice, such as the human α-synuclein overexpressing models [68,69]. The experimental approaches and the outcomes of the cytokine/chemokine measurements are described in Table 1. It is clear that gut microbiota dysbiosis correlates with inflammation in the gut and brain, as well as in the periphery, as reflected by plasma and serum data. Among the known inflammatory cytokines, there are differences in TNF, IL-6, IL-1β, IFN-γ and IL-17A, and in some cases, in the anti-inflammatory cytokine IL-10, which can be translated as a host response to dampen the inflammatory environment. Among the approaches to prevent the progress of PD and reduce tissue inflammation, one can see the use of antibiotics. In such a study, Koutzoumis et al. showed that 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in rats was ameliorated by chronic treatment with a cocktail of broad-spectrum antibiotics (neomycin, vancomycin, bacitracin and pimarcin) [70]. The treatment led to a reduction of TNF and IL-1β levels in the striatum, as observed by real-time PCR. Also, oral administration of probiotics such as Lactobacillus plantarum reshaped the gut microbiota and caused an increase of the anti-inflammatory cytokine IL-10 and reduction of the proinflammatory cytokines TNF, IL-6 and IL-1β in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD model in mice [71]. The use of TNF inhibitors in an MPTP-treated rhesus macaque monkey model showed that blocking of soluble TNF may have been associated with attenuated inflammation in biofluids, such as serum and CSF [72]. Lastly, fecal microbiota transplantation (FMT) has been utilized as a therapeutic approach in experimental animal models, such as an MPTP-induced mouse model [73]. In that study, Sun et al. showed that FMT not only affected the gut microbiota but had a remarkable effect on the brain, including a reduction of activation of microglia and astrocytes and TNF levels in the substantia nigra [73]. Similar studies using the FMT approach are presented in Table 1 below.
Table 1.
Tissue-specific cytokine levels in various experimental animal models of PD and in vitro approaches.
| Chemically-Induced PD Models | ||||
| Experimental Model | Treatment | Tissues | Cytokine Chemokine | Ref. |
| MPTP-induced | FMTS | B, C | ↓TNF | [73] |
| FMT | B | ↓TNF | [74] | |
| FMD | B | ↑TNF, IL-1β, IL-17A | [75] | |
| B, C | ↓TNF, IL-6 | [76] | ||
| Polymannuronic acid | S | ↓IL-6, IL-10 | [77] | |
| TNF inhibitor | CSF, P | ↓TNF, IL-6 | [72] | |
| Casein | B, I | ↑IL-22, IFN-γ | [78] | |
| Ceftriaxone | S | ↓TNF, IL-6, IL-1β | [79] | |
| Ceftriaxone | I | ↑TNF | [80] | |
| ↑TNF, IL-6, IL-1β, | ||||
| IL-10, IFN-γ, IL-17A, | ||||
| IL-17F, IL-22 | ||||
| Chicoric acid | S, B, C | ↓TNF, IL-6, IL-1β | [81] | |
| Cord blood plasma | B, G | ↑TNF | [82] | |
| Vancomycin | C | ↓TNF | [83] | |
| Engineered bacterium | B | ↓TNF, IL-6, IL-1β | [84] | |
| UC-MSCs | S, C | ↑TNF, IL-6 | [85] | |
| Lactobacillus plantarum | S | ↓TNF, IL-6, IL-1β | [71] | |
| ↑IL-10 | ||||
| Lactobacillus plantarum | B, S | ↓TNF, IL-6, IL-1β | [86] | |
| Rotenone-induced | FMT FMT FMT FLZ |
B, C, S S B B, C |
↓TNF, IL-6, IL-1β ↑TNF, IL-6 ↓TNF, IL-1β ↑TNF, IL-6, IL-1β |
[87] [88] [89] [90] |
| 6-OHDA | Antibiotics | B | ↓TNF, IL-6, IL-1β | [70] |
| hUBC+P | B, G | ↓TNF | [91] | |
| Helicobacter suis | P, S | ↑ IL-1β, KC | [92] | |
| ⇔ IL-6, IL-10, TNF, IL17A | ||||
| TPG | S | ↓TNF, IL-2, IL-1β, IL-4, IL-6 | [93] | |
| L-DOPA | S | ↑TNF | [94] | |
| Transgenic Animal Models | ||||
| Experimental Model | Tissues | Cytokine/Chemokine | Ref. | |
| AAV-α-synuclein | I | ⇔ TNF, IL-6, IL-1β | [95] | |
| MitoPark | I, C | ↑TNF | [96] | |
| Proteus mirabilis | C | ↑TNF | [97] | |
| (LRRK2*R1441G)135Cjli/J mice |
S B |
↑IL-17A ↑TNF, IL-1β |
[98] | |
| Thy1-αSyn mice | S | ↑TNF, IL-1b, IL-6, IL-10 | [99] | |
| SNCA-TG mice | F C B |
↓IFN-γ, IL-12p70,↑MCP-1 IL-8 signalling, TH-1 responses, ↓TNF, IL-6 |
[69] [68] |
|
| In Vitro | ||||
| Cell line | Treatment | Cytokine/Chemokine | Ref. | |
| Caco-2 | With PD, human colonic extracts | ↑TNF, CXCL10, ↓IL-6, IL-10 | [64] | |
Abbreviations: FMT: fecal microbiota transplantation, FMD: fasting-mimicking diet, 6-OHDA: 6-hydroxydopamine, TPG: Tianqi Pingchan Granule, DSS: Dextran Sodium Sulfate, MCP-1: Monocyte chemoattractant protein-1, B: brain, C: colon, I: ileum, G: gut, S: serum, P: plasma, CSF: cerebrospinal fluid, F: feces, UC-MSCs: umbilical cord mesenchymal stem cells.
4. Discussion
This review discusses current findings regarding the contribution of gut microbiota dysbiosis and inflammation to Parkinson’s disease. Experimental animal studies have evidenced that PD is presented with gut dysbiosis and correlates with abnormal levels of inflammatory mediators in the periphery and central nervous system. In recent years, much research activity has been focused on the gut-brain axis, the bidirectional network between the gut and the brain, which involves the vagus nerve, immune factors, neuroendocrine pathways, and microbial metabolites [100]. The fact that PD is the most prevalent movement disorder and the second most prevalent neurodegenerative disease after Alzheimer’s has inevitably attracted much attention. An increasing number of studies feature observations that support the hypothesis that the pathogenesis of PD derives from the gut and that there is a correlation between PD and gastrointestinal diseases [101]. For example, one study observes Lewy pathology in the enteric nervous system before the onset of motor symptoms [102,103]. Additionally, some human studies demonstrate that the microbial populations in the gut of PD patients differ from the ones of healthy individuals [104,105,106]. Moreover, a metatranscriptomic analysis of the appendix microbiome, which is a lymphoid tissue that has been linked to a risk for PD development [107], reveals a microbial dysbiosis in PD patients, including an upregulation of bacteria that is responsible for secondary bile acid synthesis [108]. Changes in microbial populations inevitably affect the type and number of microbial metabolites, such as SCFAs, which play an important role in gut physiology and gut-brain communication [109,110,111]. A recent study exhibits a reduction in fecal SCFAs, but an increase in plasma SCFAs is observed in patients with PD, which correlates with specific gut microbiota changes and the clinical severity of PD [112]. Importantly, the gut microenvironment may be an element that modifies how PD symptoms develop in hereditary variants. Numerous rodent models of PD have been used to explore the intricate interactions between genetic risk factors in the host and environmental variables, particularly concerning gut microbes and gut metabolites. Genome-wide association studies (GWAS) have identified numerous genetic loci that increase their susceptibility to sporadic PD [113]. Among them, they have identified a number of genes linked to intestinal inflammation and gut microbial regulation, including TLR1, TLR2, TLR4 and MUC2, the gene that encodes a component of the mucosal layer that protects the intestinal epithelial barrier [112]. The risk of inflammatory bowel disease (IBD) increases by some identified genetic factors that cause sporadic PD. For instance, NOD2 is recognized to be a powerful indicator of IBD and to interact with LRRK2 [114,115,116]. Gut dysbiosis has been shown to induce gastrointestinal inflammation and increase proinflammatory cytokines, including TNF, IFN-γ, IL-6 and IL-1β [116]. Since the first report by Devos et al. [58] that linked intestinal inflammation and PD, chronic gut inflammation and impaired intestinal barrier integrity have been observed in human PD patients and mouse models of PD [117]. Figure 2 summarizes the effect of gut microbiota dysbiosis, the induction of local and systemic inflammation, and the consequences that lead to neuroinflammation and PD.
There are several limitations in studies related to neurodegenerative diseases like PD. Firstly, the available experimental animal models are characterized by inconsistencies and, in some cases, the lack of Lewy body inclusions [118]. Yet, knowledge of the interplay between gut microbiota and intestinal and/or systematic inflammation is even more limited in humans, as it lacks evidence that correlates gut microbiota changes with disease severity or gender variability. Further studies are needed in order to demonstrate the role of fungi and viruses in the microbiome and to validate the hypothesis that microbial alterations observed in patients with Parkinson’s disease are a cause rather than an effect of the disease.
5. Conclusions
Parkinson’s disease is a complex, multifactorial disease regulated by both genetic and environmental factors. Gut microbiome is in constant interaction with the host, affecting the function of the intestinal epithelium, immune cells, and neurons, therefore regulating gut–brain interaction. In this review, we discuss the hypothesis that gut microbiota dysbiosis and its metabolites can provoke intestinal inflammation as well as dysregulation of gut-brain communication and the brain barrier, gradually leading to neuroinflammation and neurodegeneration. Further studies will enable novel approaches to the prevention and treatment of neurological diseases.
Author Contributions
Conceptualization, G.X. and S.P.; methodology, G.X. and C.M., data curation, C.M. and D.M.; writing—original draft preparation, S.P.; writing—review and editing, A.A., P.N., G.X. and S.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dorsey E.R., Constantinescu R., Thompson J.P., Biglan K.M., Holloway R.G., Kieburtz K., Marshall F.J., Ravina B.M., Schifitto G., Siderowf A., et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 2.Schapira A.H.V., Chaudhuri K.R., Jenner P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017;18:509. doi: 10.1038/nrn.2017.91. [DOI] [PubMed] [Google Scholar]
- 3.Jankovic J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 4.Sveinbjornsdottir S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016;139((Suppl. 1)):318–324. doi: 10.1111/jnc.13691. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong M.J., Okun M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 6.Jankovic J., Tan E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry. 2020;91:795–808. doi: 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- 7.Yamada-Fowler N., Soderkvist P. Coffee, Genetic Variants, and Parkinson’s Disease: Gene-Environment Interactions. J. Caffeine Res. 2015;5:3–10. doi: 10.1089/jcr.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng H., Wang P., Jankovic J. The genetics of Parkinson disease. Ageing Res. Rev. 2018;42:72–85. doi: 10.1016/j.arr.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Domingo A., Klein C. Genetics of Parkinson disease. Handb. Clin. Neurol. 2018;147:211–227. doi: 10.1016/B978-0-444-63233-3.00014-2. [DOI] [PubMed] [Google Scholar]
- 10.Klein C., Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klann E.M., Dissanayake U., Gurrala A., Farrer M., Shukla A.W., Ramirez-Zamora A., Mai V., Vedam-Mai V. The Gut-Brain Axis and Its Relation to Parkinson’s Disease: A Review. Front. Aging Neurosci. 2021;13:782082. doi: 10.3389/fnagi.2021.782082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braak H., Rub U., Gai W.P., Del Tredici K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 13.Hawkes C.H., Del Tredici K., Braak H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang D., Zhao D., Ali Shah S.Z., Wu W., Lai M., Zhang X., Li J., Guan Z., Zhao H., Li W., et al. The Role of the Gut Microbiota in the Pathogenesis of Parkinson’s Disease. Front. Neurol. 2019;10:1155. doi: 10.3389/fneur.2019.01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommer F., Backhed F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 16.Yang D., Zhao D., Shah S.Z.A., Wu W., Lai M., Zhang X., Li J., Guan Z., Zhao H., Li W., et al. Implications of gut microbiota dysbiosis and metabolic changes in prion disease. Neurobiol. Dis. 2020;135:104704. doi: 10.1016/j.nbd.2019.104704. [DOI] [PubMed] [Google Scholar]
- 17.Cheng L., Qi C., Zhuang H., Fu T., Zhang X. gutMDisorder: A comprehensive database for dysbiosis of the gut microbiota in disorders and interventions. Nucleic Acids Res. 2020;48:D554–D560. doi: 10.1093/nar/gkz843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alshehri D., Saadah O., Mosli M., Edris S., Alhindi R., Bahieldin A. Dysbiosis of gut microbiota in inflammatory bowel disease: Current therapies and potential for microbiota-modulating therapeutic approaches. Bosn. J. Basic Med. Sci. 2021;21:270–283. doi: 10.17305/bjbms.2020.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danau A., Dumitrescu L., Lefter A., Tulba D., Popescu B.O. Small Intestinal Bacterial Overgrowth as Potential Therapeutic Target in Parkinson’s Disease. Int. J. Mol. Sci. 2021;22:11663. doi: 10.3390/ijms222111663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romano S., Savva G.M., Bedarf J.R., Charles I.G., Hildebrand F., Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7:27. doi: 10.1038/s41531-021-00156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong S., Sun M., He C., Cheng H. Brain-gut-microbiota axis in Parkinson’s disease: A historical review and future perspective. Brain Res. Bull. 2022;183:84–93. doi: 10.1016/j.brainresbull.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Voigt R.M., Wang Z., Brown J.M., Engen P.A., Naqib A., Goetz C.G., Hall D.A., Verhagen Metman L., Shaikh M., Forsyth C.B., et al. Gut microbial metabolites in Parkinson’s disease: Association with lifestyle, disease characteristics, and treatment status. Neurobiol. Dis. 2022;170:105780. doi: 10.1016/j.nbd.2022.105780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Q.Q., Haikal C., Li W., Li J.Y. Gut Inflammation in Association With Pathogenesis of Parkinson’s Disease. Front. Mol. Neurosci. 2019;12:218. doi: 10.3389/fnmol.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun M.F., Shen Y.Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018;45:53–61. doi: 10.1016/j.arr.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Li Z., Lu G., Luo E., Wu B., Li Z., Guo J., Xia Z., Zheng C., Su Q., Zeng Y., et al. Oral, Nasal, and Gut Microbiota in Parkinson’s Disease. Neuroscience. 2022;480:65–78. doi: 10.1016/j.neuroscience.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald E., Murphy S., Martinson H.A. Alpha-Synuclein Pathology and the Role of the Microbiota in Parkinson’s Disease. Front. Neurosci. 2019;13:369. doi: 10.3389/fnins.2019.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnhart M.M., Chapman M.R. Curli biogenesis and function. Annu. Rev. Microbiol. 2006;60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S.G., Stribinskis V., Rane M.J., Demuth D.R., Gozal E., Roberts A.M., Jagadapillai R., Liu R., Choe K., Shivakumar B., et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Sci. Rep. 2016;6:34477. doi: 10.1038/srep34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chorell E., Andersson E., Evans M.L., Jain N., Gotheson A., Aden J., Chapman M.R., Almqvist F., Wittung-Stafshede P. Bacterial Chaperones CsgE and CsgC Differentially Modulate Human alpha-Synuclein Amyloid Formation via Transient Contacts. PLoS ONE. 2015;10:e0140194. doi: 10.1371/journal.pone.0140194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans M.L., Chorell E., Taylor J.D., Aden J., Gotheson A., Li F., Koch M., Sefer L., Matthews S.J., Wittung-Stafshede P., et al. The bacterial curli system possesses a potent and selective inhibitor of amyloid formation. Mol. Cell. 2015;57:445–455. doi: 10.1016/j.molcel.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherny I., Rockah L., Levy-Nissenbaum O., Gophna U., Ron E.Z., Gazit E. The formation of Escherichia coli curli amyloid fibrils is mediated by prion-like peptide repeats. J. Mol. Biol. 2005;352:245–252. doi: 10.1016/j.jmb.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Bussell R.J., Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J. Mol. Biol. 2003;329:763–778. doi: 10.1016/S0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 33.Qin L., Wu X., Block M.L., Liu Y., Breese G.R., Hong J.S., Knapp D.J., Crews F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim C., Lv G., Lee J.S., Jung B.C., Masuda-Suzukake M., Hong C.S., Valera E., Lee H.J., Paik S.R., Hasegawa M., et al. Exposure to bacterial endotoxin generates a distinct strain of alpha-synuclein fibril. Sci. Rep. 2016;6:30891. doi: 10.1038/srep30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhattacharyya D., Mohite G.M., Krishnamoorthy J., Gayen N., Mehra S., Navalkar A., Kotler S.A., Ratha B.N., Ghosh A., Kumar R., et al. Lipopolysaccharide from Gut Microbiota Modulates alpha-Synuclein Aggregation and Alters Its Biological Function. ACS Chem. Neurosci. 2019;10:2229–2236. doi: 10.1021/acschemneuro.8b00733. [DOI] [PubMed] [Google Scholar]
- 36.Zhu M., Liu X., Ye Y., Yan X., Cheng Y., Zhao L., Chen F., Ling Z. Gut Microbiota: A Novel Therapeutic Target for Parkinson’s Disease. Front. Immunol. 2022;13:937555. doi: 10.3389/fimmu.2022.937555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schonhoff A.M., Williams G.P., Wallen Z.D., Standaert D.G., Harms A.S. Innate and adaptive immune responses in Parkinson’s disease. Prog. Brain Res. 2020;252:169–216. doi: 10.1016/bs.pbr.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kannarkat G.T., Boss J.M., Tansey M.G. The role of innate and adaptive immunity in Parkinson’s disease. J. Parkinsons Dis. 2013;3:493–514. doi: 10.3233/JPD-130250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagatsu T., Mogi M., Ichinose H., Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J. Neural Transm. Suppl. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 41.Chen X., Hu Y., Cao Z., Liu Q., Cheng Y. Cerebrospinal Fluid Inflammatory Cytokine Aberrations in Alzheimer’s Disease, Parkinson’s Disease and Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Front. Immunol. 2018;9:2122. doi: 10.3389/fimmu.2018.02122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H., O’Reilly E.J., Schwarzschild M.A., Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am. J. Epidemiol. 2008;167:90–95. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- 43.Qin X.Y., Zhang S.P., Cao C., Loh Y.P., Cheng Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 2016;73:1316–1324. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- 44.Bessler H., Djaldetti R., Salman H., Bergman M., Djaldetti M. IL-1 beta, IL-2, IL-6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson’s disease. Biomed. Pharmacother. 1999;53:141–145. doi: 10.1016/S0753-3322(99)80079-1. [DOI] [PubMed] [Google Scholar]
- 45.Brown G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019;16:180. doi: 10.1186/s12974-019-1564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal G.D., Shaikh M., Forsyth C.B., Ouyang B., Keshavarzian A., Shannon K.M. Abnormal lipopolysaccharide binding protein as marker of gastrointestinal inflammation in Parkinson disease. Front. Neurosci. 2015;9:306. doi: 10.3389/fnins.2015.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasegawa S., Goto S., Tsuji H., Okuno T., Asahara T., Nomoto K., Shibata A., Fujisawa Y., Minato T., Okamoto A., et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE. 2015;10:e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aardoom M.A., Veereman G., de Ridder L. A Review on the Use of Anti-TNF in Children and Adolescents with Inflammatory Bowel Disease. Int. J. Mol. Sci. 2019;20:2529. doi: 10.3390/ijms20102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheperjans F., Aho V., Pereira P.A., Koskinen K., Paulin L., Pekkonen E., Haapaniemi E., Kaakkola S., Eerola-Rautio J., Pohja M., et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 51.Unger M.M., Spiegel J., Dillmann K.U., Grundmann D., Philippeit H., Burmann J., Faßbender K., Schwiertz A., Schäfer K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 52.Forsyth C.B., Shannon K.M., Kordower J.H., Voigt R.M., Shaikh M., Jaglin J.A., Estes J.D., Dodiya H.B., Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karunaratne T.B., Okereke C., Seamon M., Purohit S., Wakade C., Sharma A. Niacin and Butyrate: Nutraceuticals Targeting Dysbiosis and Intestinal Permeability in Parkinson’s Disease. Nutrients. 2020;13:28. doi: 10.3390/nu13010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castillo-Alvarez F., Marzo-Sola M.E. Role of the gut microbiota in the development of various neurological diseases. Neurologia. 2022;37:492–498. doi: 10.1016/j.nrleng.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 55.Barichella M., Severgnini M., Cilia R., Cassani E., Bolliri C., Caronni S., Ferri V., Cancello R., Ceccarani C., Faierman S., et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019;34:396–405. doi: 10.1002/mds.27581. [DOI] [PubMed] [Google Scholar]
- 56.Bedarf J.R., Hildebrand F., Coelho L.P., Sunagawa S., Bahram M., Goeser F., Bork P., Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med. 2017;9:39. doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Pardo P., Dodiya H.B., Engen P.A., Forsyth C.B., Huschens A.M., Shaikh M., Voigt R.M., Naqib A., Green S.J., Kordower J.H., et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: A translational study from men to mice. Gut. 2019;68:829–843. doi: 10.1136/gutjnl-2018-316844. [DOI] [PubMed] [Google Scholar]
- 58.Devos D., Lebouvier T., Lardeux B., Biraud M., Rouaud T., Pouclet H., Coron E., Bruley des Varannes S., Naveilhan P., Nguyen J.M., et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Houser M.C., Chang J., Factor S.A., Molho E.S., Zabetian C.P., Hill-Burns E.M., Payami H., Hertzberg V.S., Tansey M.G. Stool Immune Profiles Evince Gastrointestinal Inflammation in Parkinson’s Disease. Mov. Disord. 2018;33:793–804. doi: 10.1002/mds.27326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin C.H., Chen C.C., Chiang H.L., Liou J.M., Chang C.M., Lu T.P., Chuang E.Y., Tai Y.C., Cheng C., Lin H.Y., et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 2019;16:129. doi: 10.1186/s12974-019-1528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J., Meng P., Zhang J., He M. Effect of Berberine Hydrochloride on the Diversity of Intestinal Flora in Parkinson’s Disease Patients. Contrast Media Mol. Imaging. 2022;2022:8381870. doi: 10.1155/2022/8381870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aho V.T.E., Houser M.C., Pereira P.A.B., Chang J., Rudi K., Paulin L., Hertzberg V., Auvinen P., Tansey M.G., Scheperjans F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021;16:6. doi: 10.1186/s13024-021-00427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van den Abbeele P., Grootaert C., Marzorati M., Possemiers S., Verstraete W., Gerard P., Rabot S., Bruneau A., El Aidy S., Derrien M., et al. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl. Environ. Microbiol. 2010;76:5237–5246. doi: 10.1128/AEM.00759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghyselinck J., Verstrepen L., Moens F., Van Den Abbeele P., Bruggeman A., Said J., Smith B., Barker L.A., Jordan C., Leta V., et al. Influence of probiotic bacteria on gut microbiota composition and gut wall function in an in-vitro model in patients with Parkinson’s disease. Int. J. Pharm. X. 2021;3:100087. doi: 10.1016/j.ijpx.2021.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langston J.W., Langston E.B., Irwin I. MPTP-induced parkinsonism in human and non-human primates—Clinical and experimental aspects. Acta Neurol. Scand. Suppl. 1984;100:49–54. [PubMed] [Google Scholar]
- 66.Mendez J.S., Finn B.W. Use of 6-hydroxydopamine to create lesions in catecholamine neurons in rats. J. Neurosurg. 1975;42:166–173. doi: 10.3171/jns.1975.42.2.0166. [DOI] [PubMed] [Google Scholar]
- 67.Betarbet R., Sherer T.B., MacKenzie G., Garcia-Osuna M., Panov A.V., Greenamyre J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 68.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh Y., El-Hadidi M., Admard J., Wassouf Z., Schulze-Hentrich J.M., Kohlhofer U., Quintanilla-Martinez L., Huson D., Riess O., Casadei N. Enriched Environmental Conditions Modify the Gut Microbiome Composition and Fecal Markers of Inflammation in Parkinson’s Disease. Front. Neurosci. 2019;13:1032. doi: 10.3389/fnins.2019.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koutzoumis D.N., Vergara M., Pino J., Buddendorff J., Khoshbouei H., Mandel R.J., Torres G.E. Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Exp. Neurol. 2020;325:113159. doi: 10.1016/j.expneurol.2019.113159. [DOI] [PubMed] [Google Scholar]
- 71.Wang L., Zhao Z., Zhao L., Zhao Y., Yang G., Wang C., Gao L., Niu C., Li S. Lactobacillus plantarum DP189 Reduces alpha-SYN Aggravation in MPTP-Induced Parkinson’s Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut Microbiota Disorder. J. Agric. Food Chem. 2022;70:1163–1173. doi: 10.1021/acs.jafc.1c07711. [DOI] [PubMed] [Google Scholar]
- 72.Joers V., Masilamoni G., Kempf D., Weiss A.R., Rotterman T.M., Murray B., Yalcin-Cakmakli G., Voll R.J., Goodman M.M., Howell L., et al. Microglia, inflammation and gut microbiota responses in a progressive monkey model of Parkinson’s disease: A case series. Neurobiol. Dis. 2020;144:105027. doi: 10.1016/j.nbd.2020.105027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun M.F., Zhu Y.L., Zhou Z.L., Jia X.B., Xu Y.D., Yang Q., Cui C., Shen Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav. Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Zhong Z., Chen W., Gao H., Che N., Xu M., Yang L., Zhang Y., Ye M. Fecal Microbiota Transplantation Exerts a Protective Role in MPTP-Induced Parkinson’s Disease via the TLR4/PI3K/AKT/NF-kappaB Pathway Stimulated by alpha-Synuclein. Neurochem. Res. 2021;46:3050–3058. doi: 10.1007/s11064-021-03411-0. [DOI] [PubMed] [Google Scholar]
- 75.Zhou Z.L., Jia X.B., Sun M.F., Zhu Y.L., Qiao C.M., Zhang B.P., Zhao L.P., Yang Q., Cui C., Chen X., et al. Neuroprotection of Fasting Mimicking Diet on MPTP-Induced Parkinson’s Disease Mice via Gut Microbiota and Metabolites. Neurotherapeutics. 2019;16:741–760. doi: 10.1007/s13311-019-00719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lai F., Jiang R., Xie W., Liu X., Tang Y., Xiao H., Gao J., Jia Y., Bai Q. Intestinal Pathology and Gut Microbiota Alterations in a Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Mouse Model of Parkinson’s Disease. Neurochem. Res. 2018;43:1986–1999. doi: 10.1007/s11064-018-2620-x. [DOI] [PubMed] [Google Scholar]
- 77.Dong X.L., Wang X., Liu F., Liu X., Du Z.R., Li R.W., Xue C.H., Wong K.H., Wong W.T., Zhao Q., et al. Polymannuronic acid prevents dopaminergic neuronal loss via brain-gut-microbiota axis in Parkinson’s disease model. Int. J. Biol. Macromol. 2020;164:994–1005. doi: 10.1016/j.ijbiomac.2020.07.180. [DOI] [PubMed] [Google Scholar]
- 78.Liu X., Liu S., Tang Y., Pu Z., Xiao H., Gao J., Yin Q., Jia Y., Ba Q. Intragastric Administration of Casein Leads to Nigrostriatal Disease Progressed Accompanied with Persistent Nigrostriatal-Intestinal Inflammation Activited and Intestinal Microbiota-Metabolic Disorders Induced in MPTP Mouse Model of Parkinson’s Disease. Neurochem. Res. 2021;46:1514–1539. doi: 10.1007/s11064-021-03293-2. [DOI] [PubMed] [Google Scholar]
- 79.Zhou X., Lu J., Wei K., Wei J., Tian P., Yue M., Wang Y., Hong D., Li F., Wang B., et al. Neuroprotective Effect of Ceftriaxone on MPTP-Induced Parkinson’s Disease Mouse Model by Regulating Inflammation and Intestinal Microbiota. Oxid. Med. Cell Longev. 2021;2021:9424582. doi: 10.1155/2021/9424582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie W., Gao J., Jiang R., Liu X., Lai F., Tang Y., Xiao H., Jia Y., Bai Q. Twice subacute MPTP administrations induced time-dependent dopaminergic neurodegeneration and inflammation in midbrain and ileum, as well as gut microbiota disorders in PD mice. Neurotoxicology. 2020;76:200–212. doi: 10.1016/j.neuro.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Wang N., Feng B.N., Hu B., Cheng Y.L., Guo Y.H., Qian H. Neuroprotection of chicoric acid in a mouse model of Parkinson’s disease involves gut microbiota and TLR4 signaling pathway. Food Funct. 2022;13:2019–2032. doi: 10.1039/D1FO02216D. [DOI] [PubMed] [Google Scholar]
- 82.Lee J.Y., Tuazon J.P., Ehrhart J., Sanberg P.R., Borlongan C.V. Gutting the brain of inflammation: A key role of gut microbiome in human umbilical cord blood plasma therapy in Parkinson’s disease model. J. Cell Mol. Med. 2019;23:5466–5474. doi: 10.1111/jcmm.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cui C., Hong H., Shi Y., Zhou Y., Qiao C.M., Zhao W.J., Zhao L.P., Wu J., Quan W., Niu G.Y., et al. Vancomycin Pretreatment on MPTP-Induced Parkinson’s Disease Mice Exerts Neuroprotection by Suppressing Inflammation Both in Brain and Gut. J. Neuroimmune Pharmacol. 2022:1–18. doi: 10.1007/s11481-021-10047-y. [DOI] [PubMed] [Google Scholar]
- 84.Fang X., Tian P., Zhao X., Jiang C., Chen T. Neuroprotective effects of an engineered commensal bacterium in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine Parkinson disease mouse model via producing glucagon-like peptide-1. J. Neurochem. 2019;150:441–452. doi: 10.1111/jnc.14694. [DOI] [PubMed] [Google Scholar]
- 85.Sun Z., Gu P., Xu H., Zhao W., Zhou Y., Zhou L., Zhang Z., Wang W., Han R., Chai X., et al. Human Umbilical Cord Mesenchymal Stem Cells Improve Locomotor Function in Parkinson’s Disease Mouse Model Through Regulating Intestinal Microorganisms. Front. Cell Dev. Biol. 2021;9:808905. doi: 10.3389/fcell.2021.808905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liao J.F., Cheng Y.F., You S.T., Kuo W.C., Huang C.W., Chiou J.J., Hsu C.C., Hsieh-Li H.M., Wang S., Tsai Y.C. Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse models of Parkinson’s disease. Brain Behav. Immun. 2020;90:26–46. doi: 10.1016/j.bbi.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 87.Zhao Z., Ning J., Bao X.Q., Shang M., Ma J., Li G., Zhang D. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome. 2021;9:226. doi: 10.1186/s40168-021-01107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang X., Qian Y., Xu S., Song Y., Xiao Q. Longitudinal Analysis of Fecal Microbiome and Pathologic Processes in a Rotenone Induced Mice Model of Parkinson’s Disease. Front. Aging Neurosci. 2017;9:441. doi: 10.3389/fnagi.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang T., Wang T., Chen X., Zhao Z., Chen Z. Gut microbiota relieves inflammation in the substantia nigra of chronic Parkinson’s disease by protecting the function of dopamine neurons. Exp. Ther. Med. 2022;23:52. doi: 10.3892/etm.2021.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Z., Li F., Ning J., Peng R., Shang J., Liu H., Shang M., Bao X.Q., Zhang D. Novel compound FLZ alleviates rotenone-induced PD mouse model by suppressing TLR4/MyD88/NF-kappaB pathway through microbiota-gut-brain axis. Acta Pharm. Sin. B. 2021;11:2859–2879. doi: 10.1016/j.apsb.2021.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee J.Y., Tuazon J.P., Corey S., Bonsack B., Acosta S., Ehrhart J., Sanberg P.R., Borlongan C.V. A Gutsy Move for Cell-Based Regenerative Medicine in Parkinson’s Disease: Targeting the Gut Microbiome to Sequester Inflammation and Neurotoxicity. Stem Cell Rev. Rep. 2019;15:690–702. doi: 10.1007/s12015-019-09906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berlamont H., Bruggeman A., Bauwens E., Vandendriessche C., Clarebout E., Xie J., De Bruyckere S., Van Imschoot G., Van Wonterghem E., Ducatelle R., et al. Gastric Helicobacter suis Infection Partially Protects against Neurotoxicity in A 6-OHDA Parkinson’s Disease Mouse Model. Int. J. Mol. Sci. 2021;22:11328. doi: 10.3390/ijms222111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Z., Zhao J., Yang S., Zhang Y., Song L., Wu N., Liu Z. Network Pharmacology and Absolute Bacterial Quantification-Combined Approach to Explore the Mechanism of Tianqi Pingchan Granule Against 6-OHDA-Induced Parkinson’s Disease in Rats. Front. Nutr. 2022;9:836500. doi: 10.3389/fnut.2022.836500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsao S.P., Nurrahma B.A., Kumar R., Wu C.H., Yeh T.H., Chiu C.C., Lee Y.P., Liao Y.C., Huang C.H., Yeh Y.T., et al. Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson’s Disease Rats. Antioxidants. 2021;10:1823. doi: 10.3390/antiox10111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Donovan S.M., Crowley E.K., Brown J.R., O’Sullivan O., O’Leary O.F., Timmons S., Nolan Y.M., Clarke D.J., Hyland N.P., Joyce S.A., et al. Nigral overexpression of alpha-synuclein in a rat Parkinson’s disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol. Motil. 2020;32:e13726. doi: 10.1111/nmo.13726. [DOI] [PubMed] [Google Scholar]
- 96.Ghaisas S., Langley M.R., Palanisamy B.N., Dutta S., Narayanaswamy K., Plummer P.J., Sarkar S., Ay M., Jin H., Anantharam V., et al. MitoPark transgenic mouse model recapitulates the gastrointestinal dysfunction and gut-microbiome changes of Parkinson’s disease. Neurotoxicology. 2019;75:186–199. doi: 10.1016/j.neuro.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi J.G., Kim N., Ju I.G., Eo H., Lim S.M., Jang S.E., Kim D.H., Oh M.S. Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci. Rep. 2018;8:1275. doi: 10.1038/s41598-018-19646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng Y.K., Wu Q.L., Peng Y.W., Liang F.Y., You H.J., Feng Y.W., Li G., Li X.J., Liu S.H., Li Y.C., et al. Oral P. gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice. J. Neuroinflamm. 2020;17:347. doi: 10.1186/s12974-020-02027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jackson A., Engen P.A., Forsyth C.B., Shaikh M., Naqib A., Wilber S., Frausto D.M., Raeisi S., Green S.J., Bradaric B.D., et al. Intestinal Barrier Dysfunction in the Absence of Systemic Inflammation Fails to Exacerbate Motor Dysfunction and Brain Pathology in a Mouse Model of Parkinson’s Disease. Front. Neurol. 2022;13:882628. doi: 10.3389/fneur.2022.882628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sandhu K.V., Sherwin E., Schellekens H., Stanton C., Dinan T.G., Cryan J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017;179:223–244. doi: 10.1016/j.trsl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 101.Zeng J., Wang X., Pan F., Mao Z. The relationship between Parkinson’s disease and gastrointestinal diseases. Front. Aging Neurosci. 2022;14:955919. doi: 10.3389/fnagi.2022.955919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim S., Kwon S.H., Kam T.I., Panicker N., Karuppagounder S.S., Lee S., Lee J.H., Kim W.R., Kook M., Foss C.A., et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron. 2019;103:627–641. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stokholm M.G., Danielsen E.H., Hamilton-Dutoit S.J., Borghammer P. Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann. Neurol. 2016;79:940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- 104.Boertien J.M., Pereira P.A.B., Aho V.T.E., Scheperjans F. Increasing Comparability and Utility of Gut Microbiome Studies in Parkinson’s Disease: A Systematic Review. J. Parkinsons Dis. 2019;9((Suppl. 2)):S297–S312. doi: 10.3233/JPD-191711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bullich C., Keshavarzian A., Garssen J., Kraneveld A., Perez-Pardo P. Gut Vibes in Parkinson’s Disease: The Microbiota-Gut-Brain Axis. Mov. Disord. Clin. Pract. 2019;6:639–651. doi: 10.1002/mdc3.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nuzum N.D., Loughman A., Szymlek-Gay E.A., Hendy A., Teo W.P., Macpherson H. Gut microbiota differences between healthy older adults and individuals with Parkinson’s disease: A systematic review. Neurosci. Biobehav. Rev. 2020;112:227–241. doi: 10.1016/j.neubiorev.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 107.Killinger B.A., Madaj Z., Sikora J.W., Rey N., Haas A.J., Vepa Y., Lindqvist D., Chen H., Thomas P.M., Brundin P., et al. The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci. Transl. Med. 2018;10:eaar5280. doi: 10.1126/scitranslmed.aar5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li P., Killinger B.A., Ensink E., Beddows I., Yilmaz A., Lubben N., Lamp J., Schilthuis M., Vega I.E., Woltjer R., et al. Gut Microbiota Dysbiosis Is Associated with Elevated Bile Acids in Parkinson’s Disease. Metabolites. 2021;11:29. doi: 10.3390/metabo11010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 110.Yao J., Chen Y., Xu M. The critical role of short-chain fatty acids in health and disease: A subtle focus on cardiovascular disease-NLRP3 inflammasome-angiogenesis axis. Clin. Immunol. 2022;238:109013. doi: 10.1016/j.clim.2022.109013. [DOI] [PubMed] [Google Scholar]
- 111.Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 112.Chen S.J., Chen C.C., Liao H.Y., Lin Y.T., Wu Y.W., Liou J.M., Wu M.S., Kuo C.H., Lin C.H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids With Gut Microbiota and Clinical Severity in Patients With Parkinson Disease. Neurology. 2022;98:e848–e858. doi: 10.1212/WNL.0000000000013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smeland O.B., Shadrin A., Bahrami S., Broce I., Tesli M., Frei O., Wirgenes K.V., O’Connell K.S., Krull F., Bettella F., et al. Genome-wide Association Analysis of Parkinson’s Disease and Schizophrenia Reveals Shared Genetic Architecture and Identifies Novel Risk Loci. Biol. Psychiatry. 2021;89:227–235. doi: 10.1016/j.biopsych.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gorecki A.M., Bakeberg M.C., Theunissen F., Kenna J.E., Hoes M.E., Pfaff A.L., Akkari P.A., Dunlop S.A., Kõks S., Mastaglia F.L., et al. Single Nucleotide Polymorphisms Associated With Gut Homeostasis Influence Risk and Age-at-Onset of Parkinson’s Disease. Front. Aging Neurosci. 2020;12:603849. doi: 10.3389/fnagi.2020.603849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma Q., An X., Li Z., Zhang H., Huang W., Cai L., Hu P., Lin Q., Tzeng C.M. P268S in NOD2 associates with susceptibility to Parkinson’s disease in Chinese population. Behav. Brain Funct. 2013;9:19. doi: 10.1186/1744-9081-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rocha J.D., Schlossmacher M.G., Philpott D.J. LRRK2 and Nod2 promote lysozyme sorting in Paneth cells. Nat. Immunol. 2015;16:898–900. doi: 10.1038/ni.3255. [DOI] [PubMed] [Google Scholar]
- 117.Lee H.S., Lobbestael E., Vermeire S., Sabino J., Cleynen I. Inflammatory bowel disease and Parkinson’s disease: Common pathophysiological links. Gut. 2021;70:408–417. doi: 10.1136/gutjnl-2020-322429. [DOI] [PubMed] [Google Scholar]
- 118.Neurath M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019;20:970–979. doi: 10.1038/s41590-019-0415-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.