Abstract
Neurodegenerative diseases (NDDs) are among the 10 causes of death worldwide. The effects of NDDs, including irreversible motor impairments, have an impact not only on patients themselves but also on their families and social environments. One strategy to mitigate the pain of NDDs is to early identify and remotely monitor related motor impairments using wearable devices. Technological progress has contributed to reducing the hardware complexity of mobile devices while simultaneously improving their efficiency in terms of data collection and processing and energy consumption. However, perhaps the greatest challenges of current mobile devices are to successfully manage the security and privacy of patient medical data and maintain reasonable costs with respect to the traditional patient consultation scheme. In this work, we conclude: (1) Falls are most monitored for Parkinson’s disease, while tremors predominate in epilepsy and Alzheimer’s disease. These findings will provide guidance for wearable device manufacturers to strengthen areas of opportunity that need to be addressed, and (2) Of the total universe of commercial wearables devices that are available on the market, only a few have FDA approval, which means that there is a large number of devices that do not safeguard the integrity of the users who use them.
Keywords: neurodegenerative diseases, monitoring, sensors, wearables
1. Introduction
Neurodegenerative diseases (NDDs) affect the central nervous system as they destroy the neuronal cells found there. Some of these diseases are epilepsy, Parkinson’s disease (PD), and Alzheimer’s disease (AD), among others. In turn, neuronal deterioration affects different organs and activities, such as balance, speech, breathing, and heart function. NDDs can also cause cognitive deficits, dementia, and behavioral disorders [1]. In 2019, the World Health Organization (WHO) reported that 55% of the 55.4 million deaths that occurred worldwide were identified in the ten leading causes of death [2,3]. Currently, seven of the ten leading causes of death are non-communicable diseases. This number constitutes a considerable increase if compared to 2000, when non-communicable diseases were four of the top 10 causes of death. Nowadays, NDDs such as AD are already among the 10 leading causes of death worldwide and have ranked third in both the Americas and Europe since 2019 [3,4].
Around 50 million people worldwide are affected by NDDs, of which more than half live in countries considered low- and middle-income. Approximately 10 million new cases are registered annually, and it is estimated that between 5% and 8% of the general population aged 60 or over will suffer from some NDDs [3], and by 2030 and 2050, respectively, 78 and 139 million patients are expected to have some NDDs [5]. There is no cure or reversal of the progressive evolution of NDDs; however, there is research on new treatments that can be offered to support and improve the lives of patients, as well as their caregivers and relatives [6].
The relationship between cognitive impairment and mobility has been widely studied and recognized [7]. The different NDDs share symptoms of progressive cognitive deterioration, which also impacts the motor functions of the patient, such as walking gait and balance; therefore, continuous monitoring of these motor functions helps to diagnose and assess the severity of neurological disorders. This has motivated technological development (Apps, wearable devices, among others) in order to provide relevant and precise metrics on the motor functions of patients with some NDDs [8].
A wearable device is any electronic device capable of processing and storing information, which has been incorporated into the clothing or accessories that a person uses on their body on a daily basis; for example, watches, bracelets, glasses, caps, headbands, shirts, and shoes, among others [9]. Among the sectors where it is common to use wearable devices are industrial, military, and health, and because its operation is based on acquiring and processing data to make a decision based on it, it is to be expected that they have an operating system built into the microcomputer found incorporated in the wearable device [10]. The Android operating system is recognized to be used in more than 70% of smartphones worldwide and is easy to use [11]. This open-source operating system based on the Linux kernel offers great flexibility due to its customization properties, making it a dominant mobile operating system. It is increasingly common to find Android smartphones, wearable devices and portable devices with healthcare applications installed [12]. As an example of the above, there are commercial wearable devices that work together with devices with the Android Operating System (for example, a cell phone) to generate a user database [13]. A wearable device must have the characteristic of being imperceptible to the user; that is to say, to interfere as little as possible in the activities carried out daily and at the same time to be efficient and precise in the information provided by the user [14]. Currently, the manufacture of wearable devices that have this characteristic is under development; as it is, the technology is based on tattoos, smart textiles, and biosensors [15]. Wearable devices have made it possible to monitor aspects of health outside of a clinical setting, favorably impacting clinical care and self-monitoring of symptoms and, therefore, the health of the patient in general. This has also benefited patients who live in remote regions or far from specialized clinics. Remote monitoring has been shown to be useful in recording behaviors that occur sporadically outside of a clinical setting or in patients exhibiting time-varying behaviors or presenting particular symptoms in the clinic compared to home [16].
Common Motor Impairments Caused by NDDs
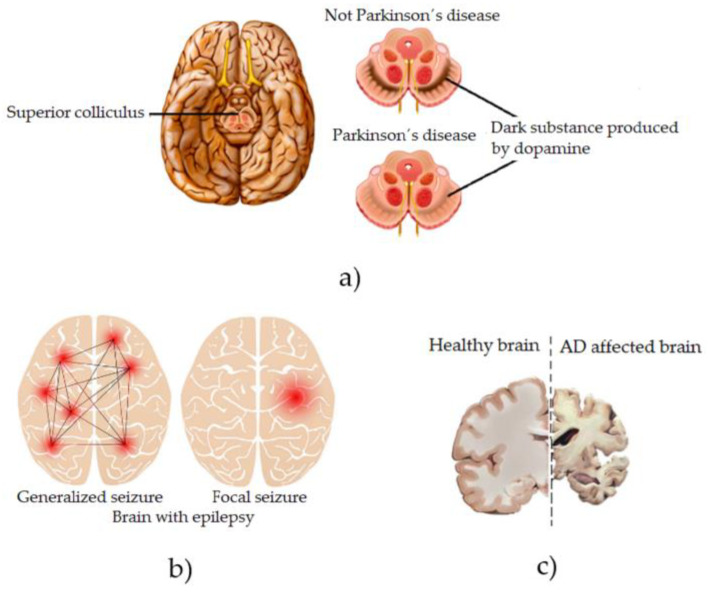
NDDs gradually kill the patient’s motor neurons, thus affecting essential muscle activities such as talking, walking, breathing, and swallowing [17]. They cause gradual muscle weakness, uncontrollable tics, muscle or limb rigidity, and spasms on the knee and ankles, finally causing the patient to lose their ability to voluntarily control their movements [18]. In patients diagnosed with PD, specific areas of their brain deteriorate, and significant changes occur in their brain chemistry, for example, a lack of dopamine [19], which is graphically illustrated in Figure 1a. As the PD progresses, the symptoms that appear in the patient are uncontrollable tremors, slow movements in their movements (bradykinesia), voice and speech disorders, changes in posture and balance, and muscle rigidity, among others [20]. As the disease progresses, all of these symptoms often expand and intensify throughout the patient’s body, causing depression and dementia-like symptoms [21]. Patients diagnosed with Epilepsy (Neurological Disorder) are characterized by having recurrent and unpredictable seizures when the normal electrical signaling between neurons in their brain is disrupted [22], which is graphically illustrated in Figure 1b. Among the symptoms found in this disease are temporary confusion and, therefore, episodes of absences, rigidity in the muscles of the whole body producing uncontrollable spasmodic movements mainly in the arms and legs, and psychological affectations, such as fear or anxiety [23]. In the event that the disease is not controlled under medical surveillance and its progression is allowed to advance, the patient’s brain suffers neurological alterations over time, irreversibly affecting their motor and cognitive skills [24]. Patients diagnosed with AD have a brain disorder that slowly destroys memory and the ability to think and, over time, ceases to be independent when performing daily activities [25], which is graphically illustrated in Figure 1c. Among the symptoms of this disease are cognitive impairment, temporary and spatial disorientation, and difficulty in expressing oneself, among others [26]. The havoc in the patient’s body as the disease progresses is reflected in leaving him practically unable to perform any physical or cognitive activity considered essential in any person [27].
Figure 1.
Graphic representation of the effects on the brain of some NDDs. (a) Parkinson´s disease, (b) Epilepsy and (c) Alzheimer disease.
Examining NDDs through wearable technology involves monitoring related motor impairments, such as tremors, falls, and seizures. In the following paragraphs, we summarize the NDD-related motor impairments that are monitored via wearable devices. Then, in Figure 2, we illustrate the parts of the human body that are commonly associated with such devices.
Figure 2.
Types of wearable devices and the impairments that they are typically used to detect.
Tremors: They appear when the person is at rest, regularly and rhythmically, disappearing when they change posture or make movements. At early stages, tremors commonly occur on one side of the upper body (often one hand). This motor symptom, similar to the rest, evolves over time: (1) it first manifests itself in the upper limbs (although it can occur in the lower limbs, lip, chin, or tongue) and on one side of the body in a specific area (commonly the hand). (2) It progresses through the extremity and usually passes to the lower area, foot, and leg, on the affected side. Finally, (3) it evolves on both sides of the body. The voice and head are rarely affected. The severity of the tremors also depends on the time of the day. Fatigue, anxiety, and concentration make them more intense, while during sleep hours, they disappear. This affectation affects not only the patient but also their family, work, and/or social environments [28]. Technological advancement has led to the development of motion sensors that are wireless and wearable. Accelerometer, gyroscopic, and electromyography (EMG) are just some of the sensing technologies used to record patient tremor data [29].
Falls: The most common symptoms of NDDs include rigidity, bradykinesia, and posture, which contribute to the patient’s risk of falling. Rigidity means little flexibility in the movement of the neck and trunk, which results in a loss of balance, thus increasing the likelihood of a patient falling. A person’s center of gravity is below the navel, and the legs form the base of support. Hence, problems with the center of gravity contribute to a fall [30]. Loss of balance might occur while doing a regular activity, such as standing up, bending over, walking, turning the head, avoiding obstacles, or talking. Falls can also occur due to impaired postural reflexes, irregular changes in posture, and episodes of sudden paralysis while walking. Other risk factors in falls are the patient’s vision and depth perception problems [31]. The technologies used to detect falls include motion sensors, sound sensors, and video cameras. They collect data on the patient’s movement activity. Then, using algorithms, they analyze and determine a patient’s activity level to accurately detect a fall. Usually, motion and sound sensors are installed in fixed locations; however, some researchers have proposed their use in the form of a wearable device to monitor patient movement patterns [32].
Seizures are uncontrolled and sudden electrical disturbance in the brain that manifest as changes in patient behavior, motor movements, and levels of consciousness. Having more than one seizure in a 24-h period with no apparent cause is considered epilepsy. There are several types of seizures, with different symptoms and levels of severity, which vary depending on the region of the brain in which they start and end [33]. The duration of a seizure is commonly 30 to 120 s. If the seizure lasts more than five minutes, it is considered a medical emergency and causes the person to fall over, shake, and be unaware of what is going on around them [34]. Accelerometry is widely used to detect seizures that are expressed in movements or alter normal movement patterns. Three-dimensional accelerometers, algorithms based on Hidden Markov Models (HMM), and Bayesian analysis are just some of the proposals to optimally detect seizures in patients [35].
The rest of this document is organized into the following sections: (1) objective and justification of this research; (2) explained the methods; (3) research questions, the search strategy, the selection process of primary studies and data extraction; (4) present the results of the review; (5) discussion of the results; and (6) conclusions.
2. Research Goal and Need for Literature Review
The literature reports a substantial number of scientific contributions in the management, follow-up, and detection of NDDs, including monitoring technologies, patient behavior analysis, and technology development. Researchers such as Kristoffersson et al. [36], Mughal et al. [37], Sica et al. [38], Celik et al. [39], Cicirelli et al. [8], Albán-Cadena et al. [40], Storm et al. [41], Zampogna et al. [42], Rehman et al. [43], Godoi et al. [44], Chirra et al. [45], Brognara et al. [46], Rovini et al. [47], Díaz et al. [48], Porciuncula et al. [49], Silva de Lima et al. [50], Vienne et al. [51], Wang et al. [52], Giggins et al. [53], Hubble et al. [54], Oung et al. [55], and Steins et al. [56] based on wearable sensors and machine learning (ML) models, proposed optimizing the monitoring and analysis of physical activity in patients with PD, to assess the quality of gait and balance maintained by the patient in question. In all of these cases, relevant data have been obtained to establish rehabilitation routines, the prognosis of falls, and monitoring disease progression.
In their work, Stavropoulos et al. [57] and Pasluosta et al. [58] evaluated the opportunities and challenges of wearable sensors with access to the Internet of Things (IoT) and applied them to the care of patients with Parkinson’s disease. Researchers Tăuţan et al. [59], Ghannam et al. [60], Buckley et al. [61], and Belić et al. [62] implemented Artificial Intelligence (AI) and evaluated the opportunities and challenges through ML techniques and Big Data in wearable devices for the care of patients with PD. In turn, Sivathamboo et al. [63], Olsen et al. [64], Rukasha et al. [65], Simblett et al. [66], Johansson et al. [67], and Ryvlin et al. [68] evaluated the challenges faced by wearable sensors for the care of patients with epilepsy. Finally, Naganur et al. [69], Ong et al. [70], Cullen et al. [71], Nielsen et al. [72], Beniczky et al. [73], Shum et al. [74], Ó Breasail et al. [75], de la Fuente García et al. [76], Machado et al. [77], Gillani et al. [78], Anderson et al. [79], Bruno et al. [80], Vinals et al. [81], Kurada et al. [82], Klimova et al. [83], and Ienca et al. [84] comparatively evaluated a series of wearable devices that use AI to monitor motor impairments caused by AD. The purpose of the review was to identify gaps and research opportunities to better care for patients with AD.
The four underlying differences of our research, if compared to the literature reviews previously mentioned, can be summarized as follows: First, our review focuses on currently available commercial and non-commercial wearable devices for monitoring NDD-related motor impairments. Second, we classify these devices according to their characteristics. Third, we identify both NDD-related motor impairments and the sensors used for monitoring these impairments. Finally, we discuss the Food and Drug Administration (FDA) status of each of the reviewed wearable technologies. The focus of this paper is NDD-related motor impairments. In this sense, our four main objectives are (1) to identify currently available commercial and non-commercial wearables for monitoring NDD-related motor impairments, (2) to list the characteristics of such devices, (3) to rate the top most used devices for monitoring motor impairment in patients with NDDs, and (4) to determine the FDA status of such devices.
3. Methods
This review examines the quantitative and qualitative aspects of research on wearable devices (Commercial and Non-commercial) for monitoring the motor affectations caused by neurodegenerative diseases. We follow the three stages of the methodology proposed by Brereton et al. [85]. It is considered a reference method to carry out systematic reviews of the literature. The first stage of this methodology is planning, in which the need to carry out a review of the literature is determined, research questions are formulated, and the protocol to be followed is reviewed. In the second stage, the search strategy is determined, the primary studies are selected, and the data of interest are extracted and synthesized. In the final stage, the results are obtained, their validity is verified, and conclusions are made.
3.1. Research Questions and Motivations
Four research questions were posed to achieve the objectives of this review. Table 1 shows the five research questions.
Table 1.
Research Questions.
| Research Question (RQ) | Question | Motivation |
|---|---|---|
| 1 | Which are commercial wearables for monitoring NDD-related motor impairments currently available? | To identify the main commercial wearables for monitoring NDD-related motor impairments currently available. |
| 2 | Which are the top most used commercial wearables for monitoring motor impairment in patients with NDDs? | To identify the top most used commercial wearables for monitoring motor impairment in patients with NDDs. |
| 3 | Which are the technical characteristics of non-commercial wearables for monitoring NDD-related motor impairments currently available? | To identify the technical characteristics of non-commercial wearables for monitoring NDD-related motor impairments currently available. |
| 4 | Which are the FDA status of commercial wearables for monitoring NDD-related motor impairments currently available? | To determine if the patient can confidently use the commercial wearables for monitoring NDD-related motor impairments currently available. |
| 5 | What are the gaps in the monitoring of NDDs using commercial wearables devices? How are these gaps covered by non-commercial wearables devices? | To identify the areas of opportunity that must be strengthened in commercial wearables devices. |
3.2. Search Strategy
The following five digital libraries were used to search for the primary studies: JMIR, Springer Link, Science Direct, Annuals Reviews, and Wiley Online. Google Scholar search engine was used to expand the results obtained. The libraries were selected based on their prestige and popularity with the research community, they all provide access to a wealth of digital literature, and they are peer-reviewed studies. The search was conducted based on keywords. The search period used was from 2012 to mid-2022 (The last 10 years). Table 2 shows the keywords used in this review.
Table 2.
Keywords and related concepts used in this literature review.
| Area | Keywords | Related Concepts |
|---|---|---|
| Neurodegenerative Disease | Motor impairments | Falls |
| Mental health | Wearables and sensors devices | Tremor |
| Seizures | ||
| Parkinson | ||
| Epileptic | ||
| Alzheimer |
From a combination of the keywords shown in Table 2, search strings were formed using the “AND” and “OR” connectors as follows: ((wearables devices) OR (Sensors)) AND ((Neurodegenerative diseases) OR (mental diseases)) between the years 2012 and 2022. Figure 3 graphically shows the relevant search results beginning in 2005, and the top five results were: 687 JMIR, 381 Springer Link, 329 Science Direct, 151 Annuals Reviews, 114 Wiley Online, 343 among others, such as BioMed Central, Google Scholar, IOP Science, Hindawi, IEEE Xplore, AHA Journals, MDPI, Clinicals Trials and JACC.
Figure 3.
Research papers by digital libraries.
3.3. Selection of Primary Studies
This research is a review of the wearable devices used for monitoring NDD-related motor impairments. The review follows the PRISMA guidelines [86] to ensure the correct organization and clarity of our findings.
3.3.1. Collection of Sources
We initially obtained our search results from 2005 sources of interest across multiple databases. After refining the search to comprise research from 2010 and onwards, we discarded 236 results and left 1769 potential sources for review.
3.3.2. Inclusion and Exclusion Criteria
Inclusion criteria: The literature related to the topic of monitoring NDD-related motor impairments were published from 2010 to 2022, and the particular topics related to (1) wearables sensor devices and (2) commercial and non-commercial wearables were considered. Exclusion criteria: The literature that was discarded was for (1) manuscripts written in languages different than English, (2) letters and reports, (3) conference and symposium proceedings, (4) non-primary studies, and (5) non-peer-reviewed sources.
3.3.3. Information Sources
Table 3 shows the sources of information where the search was carried out based on the related term (Category).
Table 3.
Table 3 shows the sources of information used.
| Category | |
|---|---|
| Information Technologies | Healthcare |
| Google Scholar, Hindawi, IEEE Xplore, IOP science, JACC, MDPI, Nature, Science Direct, Springer Link, and Wiley Online Library. | AHA Journals, Annual Reviews, BioMed Central, Clinical Trials, JMIR, and PubMed |
3.3.4. Search Strategy
We combined the research keywords with Boolean connectors (AND/OR) to limit the number of results obtained in the search. The search keywords were extracted from the research questions. Below we list the intermediate search phrases used to find the terms that were used in subsequent queries:
Main motor impairments related to NDDs.
Wearable devices used to monitor NDD-related impairments.
Commercial and non-commercial wearable devices for monitoring NDDs.
Commercial sensors used in wearable devices.
FDA status of commercial wearable devices.
From the list of queries above, new terms were found that were relevant to this study.
3.3.5. Selection Process
Four experts in the field (SMEs) participated in this selection process. Figure 4 shows the flowchart of our PRISMA-based methodology, which shows the process of how the 50 articles were obtained that meet the inclusion criteria set forth above. The 50 selected articles were downloaded in their entirety from the following databases: MDPI (11), Science Direct (11), Google Scholar (8), PubMed (8), IEEE Xplore (5), Biomed central (2), Springer Link (2), Wiley Online Library (2), and ACM (1).
Figure 4.
PRISMA flow diagram for the literature search.
3.3.6. Data Collection and Analysis
From the 50 selected articles, relevant information for this study was collected and organized in structured tables. Four SMEs supervised the analysis to extract relevant and interesting information, such as motor impairment, brand and model of wearable device, operational features, used sensor(s), and FDA status.
3.4. Data Extraction
We extracted two types of data in each of the selected studies: (1) bibliographic; for example, title, authors, objectives, and repository and (2) content, with which the research questions raised in this review were answered. The following section presents the results obtained from the studies considered.
4. Results
This review analyzes commercial and non-commercial wearable devices used for monitoring NDD-related motor impairments. The commercial wearables considered in this review comprise both pre-sale and commercially available devices. Prototype and investigational wearable devices were categorized as non-commercial. In total, we found 22 commercial and 28 non-commercial wearable devices for monitoring NDD-related motor impairments.
4.1. RQ1. Which Are Commercial Wearables for Monitoring NDD-Related Motor Impairments Currently Available?
Table 4 summarizes our findings in this review regarding commercially available wearable devices. Practically the majority of the wearable devices found are aimed at monitoring the motor deficiencies caused by NDDs during the daily activities of the patient; however, not all of these devices have been approved by regulatory agencies, such as the US Food and Drug Administration (FDA), which regulates the sale of such wearable devices and provides guarantees to consumers that they are safe and effective in their intended use.
Table 4.
Commercial Wearable Devices for Monitoring NDD-Related Motor Impairments.
| Motor Disability | Device Type | Device Brand | Device Model | Monitoring Features | Sensors Used | FDA Status/Year/AP (AP: Accuracy Percentage) |
Android Compatibility |
|---|---|---|---|---|---|---|---|
| Falls | Smartwatch | Apple | Watch Series 7 [87] |
Detect user idleness for about a minute. It begins a 30-s countdown while tapping you on the wrist and sounding an alert. | Blood oxygen, electric HR, optical HR, GPS, compass, microphone, altimeter, and horn. | Approved/2018 /98%/(Only for ECG) | No |
| Smartwatch | Watchseniors | Plus 4 G [88] |
Detect falls, trajectories, and user location. | Blood pressure sensor, temperature sensor, and heart rate sensor | Unknown | Yes | |
| Smart Phone | Freeus | FallSafety [89] |
Detect user falls with automatic generation of alerts to the emergency systems. | Information not available | Unknown | Yes | |
| Belt | Fallskip | TF11-MP005-ES [90] |
Assess the risk of falling in older adults. | IMU (Inertial Measurement Unit) | Unknown/Unknow/75% | No | |
| Pendant pager | Neki | Nock Senior [91] |
Fall detector with GPS locator monitorable with its application on the cell phone. | Fall detector sensor | Unknown | Yes | |
| Static voice-enabled device | Amazon | Alexa together [92] |
Virtual assistant equipped with functions that help the user in case of falls. | Speech processor | Unknown | Yes | |
| Wristband | MyNotifi® | MYNOTIFI FALL DETECTION SYSTEM [93] |
Detect falls and alert the user’s family and friends if a fall occurs. | AI sensor | Class I (Exempt)/2020/73% | Yes | |
| Vision system in Bedside | VIRTUSENSETM | VSTOne [94] |
Predict a crash around 30–65 s before it occurs. | AI and LiDAR sensors |
Unknown/Unknown/98% | No | |
| Tremor | Smartwatch | Microsoft | Emma Watch [95] |
Reduce tremors associated with Parkinson’s disease. | Movement is regulated by a sensorimotor feedback loop involving the perception of movement and position of the body. | Unknown | No |
| Smartwatch | Detekt | PKG watch (The Parkinson’s KinetiGraph) [96] |
Analyze user movements throughout the day and output a graph that allows the doctor to analyze and compare user movement speed and overall user capability to move throughout the day. | Gyroscopic stabilization | 510(k) Cleared/2014/96% | No | |
| Smartwatch | Parkinson Smartwatch |
Parkinson Smartwatch [97] |
User (i.e., patient) manually records information on their perception of well-being during the day (for example, after taking their medication). The information that is recorded on the device is sent to the cloud, where it is stored. The user and their doctor have online access to the graphs of the data recorded from anywhere in the world using a computer, tablet, or mobile phone. | Information not available | Unknown | No | |
| Glove | Steadiwear | Steadi-Two [98] |
Reduce tremor magnitude using two magnets to control a disk that moves in the opposite direction of the tremor. | The technology is based on a seismic design and works similarly to a see-saw in a park. The disk, which is controlled by magnets, responds to the tremor by providing an equal and opposite force, lowering its magnitude. | Class I/2021/80% | No | |
| Handheld Therapeutic device |
VILIM | VILIM ball [99] |
Reduces the hand tremors of the patient while performing daily tasks. | Embedded algorithm that analyzes tremors and adapts to each patient’s symptoms individually. | Unknown | Yes | |
| Hand Tremor Device |
Five Microns® | Tremelo [100] |
Reduces intermediate-degree tremors in the arms and hands by 85 to 90%. | Non-invasive and mechanical (no electricity: no batteries) device relying on vibration absorbers and tuned mass damper. | Class 1/Unknown/90% | No | |
| Spoon | GYENNO | GYENNO SPOON [101] |
Stabilize unwanted tremors by 85% to stop intentional hand movement and help people with PD or tremors to eat more easily. | Intelligent rehabilitation robotics, Intelligent high-speed servo control system, algorithm technology of unmanned aerial vehicles, and self-adapted ML. | Unknown | Yes | |
| Wristband | Parkinson’s KinetiGraph (PKG) |
PKGTM [102] |
Collect data on motor disabilities and other complications caused by PD (e.g., slowness of movement, tremor, stiffness). | Sensors that monitor the wearer’s activity and buzzes for medication reminders. | Cleared/Unknown/Unknown | No | |
| Wristband | The Cala Trio therapy |
Cala Trio™ [103] |
Deliver electrical stimulation—also known as neuromodulation—to the nerves in the effective wrist. The stimulation disrupts the tremor network in the brain and delivers meaningful tremor reduction in the affected hand. | Information not available | Cleared Class II /2020/68% | No | |
| Seizures | Bed | EpiUSA | Emfit Movement Monitor [104] |
Nightly monitoring of a person’s movements to alert their caregivers if necessary. | Motion sensor installed in a pad, which is installed under the patient’s mattress. | Unknown | No |
| Bed | MedPage | BMA-01 [105] |
Detect certain types of movements (e.g., muscular spasms) that people make while sleeping using a movement-sensing alarm. | Sophisticated software algorithms that continually analyze the signals produced by a special sensor positioned under the bed mattress, even a memory foam type. | Unknown | No | |
| Bed | Epi USA | Emfit [106] |
Detect most movements, including light movements in patients with epileptic disease. The company states that this product is also suitable for small children. | Flexible and durable bed sensor, a bedside monitor, a bed clip, and a wall bracket. The movement monitor detects movement over a preset amount of time and triggers an alarm if a person moves more than it expects. | Unknown | No | |
| Bracelet | Empatica | Embrace 2 [107] |
Detect seizures | Information not available | FDA cleared/2018/98% | Yes | |
| Video monitor | SAMi | SAMi-3 [108] |
Process and record patient movements in real-time. Send alerts to caregivers in case of patient seizures. | Information not available | Unknown | Yes |
4.2. RQ2. Which Are the Top Most Used Commercial Wearables for Monitoring Motor Impairment in Patients with NDDs?
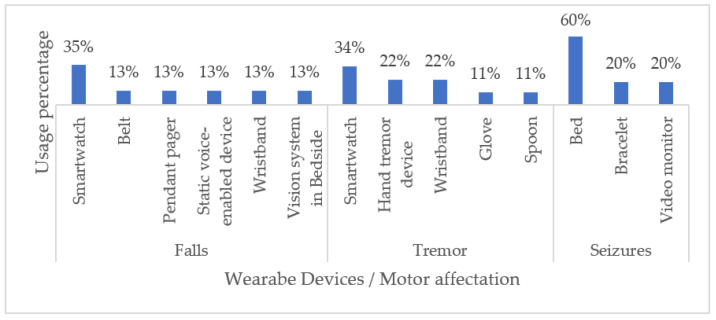
Table 4 summarizes our findings regarding the most important characteristics of each device reviewed, including its FDA status. Most of the commercially available wearable devices used for monitoring NDD-related motor impairments are smartwatches and beds (60%). Even though smartwatches have been best implemented to monitor tremors and seizures (35% and 34%, respectively), the work continues to improve the performance of other wearable devices. In this sense, fabrics and tattoos have also emerged as non-invasive alternatives for monitoring patients during daily activities. Figure 5 illustrates our classification of wearable devices with respect to the NDD-related motor impairments that they commonly monitor.
Figure 5.
Classification of Commercial Wearable Devices by NDD-Related Motor Disabilities.
As Figure 6 illustrates, the smartwatch is the most used to monitor NDD-related motor disabilities. Current scientific and technological progress has made it possible for companies to manufacture increasingly compact wearable devices that can be easily worn on the body while promising favorable and convenient results in terms of health monitoring. However, much better performance in monitoring and patient care remains a challenge for such devices.
Figure 6.
Classification of Commercial Wearable Devices for Monitoring NDD-Related Motor Impairments.
4.3. RQ3. Which Are Technical Characteristics of Non-Commercial Wearables for Monitoring NDD-Related Motor Impairments Currently Available?
In this review, non-commercial wearable devices comprised prototype devices and devices developed solely for research purposes. Table 5 summarizes our findings in terms of the following aspects:
Year of publication of the research;
Aimed NDD;
Type of wearable device;
Brief research description;
Sensors or technology used;
Real-time device monitoring capability.
Table 5.
Non-Commercial / Research Wearables for Monitoring NDD-Related Motor Impairments.
| NDD Type | Device Type | Research Description | Sensors or Technology Used | Real-Time Monitoring |
|---|---|---|---|---|
| Parkinson | Bracelet (2017) | Use wearable sensors to quantify doses in patients with PD to address motor affections such as tremors, bradykinesia, and dyskinesia [109]. | Wrist and ankle motion sensors | Yes |
| Parkinson | Bracelet (2018) | Evaluate a fall prediction test using body sensors in patients with PD [110]. | Inertial sensor and software system (Kinesis QTUG™, Kinesis Health Technologies, Dublin, Ireland) | Yes |
| Parkinson | Bracelet and Belt (2021) | Evaluate motor disabilities in patients with PD [111]. | Accelerometer | Yes |
| Parkinson | Shoe accessory (2020) | Using a 3D accelerometer, they validated a pair of pressure insoles in shoes to detect walking problems in patients with PD [112]. | Accelerometer 3D and pressure insoles | Yes |
| Parkinson | Armband | Propose a wearable device for the diagnosis of motor affections such as rigidity, tremor, and bradykinesia in patients with PD [113]. | Sensor system composed of a force sensor, three inertial measurement units (IMUs), and four custom mechanomyography (MMG) sensors | Yes |
| Parkinson | Bracelet and Belt (2021) | Evaluate the data obtained from a group of patients with PD by means of wearable sensors to quantify the severity of symptoms in the extremities of the patients [114]. | Accelerometer | Yes |
| Parkinson | Wrist (2020) | Validate a mechatronic wearable device that seeks to mitigate wrist stiffness in patients affected by PD [115]. | One actuated joint and four passive revolute joints with a high overall intrinsic back drivability. | Yes |
| Parkinson | Shoe accessory and Belt (2022) | Monitor and evaluate gait in patients with PD through a portable physiograph [116]. | Pressure sensors, electromyography (EMG) sensors, and accelerometers. | Yes |
| Parkinson | Device on the back (2022) |
Evaluate the performance of a device to monitor and improve postural alignment, balance, and gait in patients with PD [117]. | Device Up Right Go | Yes |
| Parkinson | Device on the neck and back (2019) |
Propose a method to estimate stooped posture through sensors (i.e., accelerometers) mounted on the patient’s neck or upper back [118]. | Accelerometer | Yes |
| Epileptic | Bracelet (2021) | Evaluate the performance of the bracelet in detecting seizures through algorithms implemented with ML using multisignal biosensors worn on the patient’s wrist and ankle [119]. | Wrist- and ankle-worn multisignal biosensors in conjunction with machine learning algorithms (MLAs) | No |
| Epileptic | Chest patch (2019) | Evaluate seizure detection through heart rate variability using a portable electrocardiography device [120]. | Portable Electrocardiogram (ECG) in conjunction with algorithms implemented in LabView | No |
| Epileptic | Wristband (2019) | Evaluate a portable system based on accelerometry to detect tonic–clonic seizures [121]. | Inertial sensors | No |
| Epileptic | Bracelet (2022) | Propose an automated method based on machine learning to classify seizures [122]. | Accelerometer and gyroscope | Yes |
| Epileptic | Bracelet (2022) | Develop a system to detect seizures (epileptic / non-epileptic) using wearable sensors [123]. | Electroencephalography (EEG), Electromyography (EMG), and ECG |
Yes |
| Epileptic | Electrodes (2022) | Monitor patients with epilepsy disease to propose effective strategies for seizure detection [124]. | EEG, ECG, and accelerometer |
Yes |
| Epileptic | Wrist-Worn (2018) |
Develop a wireless monitoring system (with an accelerometer as a sensor) worn on the patient’s wrist for seizure detection [125]. | Accelerometer | Yes |
| Epileptic | Wrist and ankle Bracelet (2022) |
Investigate the effects of anticonvulsant medications monitored by a wearable device in patients with epilepsy [126]. | Body temperature sensor, optical, infrared sensors, and a 3D accelerometer and gyroscope. | Yes |
| Epileptic | Diadem (2022) | Evaluate the accuracy of absence seizure detection using an electroencephalographic wearable device [127]. | EEG | No |
| Epileptic | Wrist-Worn and Electrodes (2017) |
Develop a wearable system that detects seizures and alerts patient caregivers [128]. | EEG, gyroscope, 3D accelerometer, optical, infrared sensors, and body temperature. | No |
| Alzheimer | Ankle Bracelet (2018) |
Evaluate an algorithm to monitor and record gait movements in patients with AD [129]. | Accelerometer and gyroscope |
No |
| Alzheimer | Electrodes (2022) |
Develop and evaluate a multiclass classification system for AD based on a commercial EEG acquisition system that uses sixteen channels [130]. | EEG | No |
| Alzheimer | Wrist and ankle Bracelet (2019) |
Review wearable devices that monitor and control posture and gait in patients with dementia [131]. | Accelerometer and gyroscope | No |
| Alzheimer | Video camera (2018) |
Develop a platform to support patients suffering from impaired facial perception with an assistive intelligence device [132]. | Algorithm- Facial Perception Model | No |
| Alzheimer | Wrist band (2015) |
They developed a localization band targeted at people suffering from memory diseases [133]. | GPS and global system for mobile (GSM) communication | Yes |
| Alzheimer | Wrist band (2018) |
Determine whether characteristics extracted from arterial pulse waves (PWs) measured by wearable sensors could be useful for stratifying patients at risk of AD [134]. | Photoplethysmography (PPG) | No |
| Alzheimer | Feet mounted (2014) |
Develop gait and balance analysis algorithms for the diagnosis of patients with AD [135]. | Inertial sensor | No |
| Alzheimer | Belt (2016) |
Investigate ML classifiers applied in postural control in patients with AD [136]. | Multiple Layer Perceptrons (MLPs), accelerometer, and gyroscope |
No |
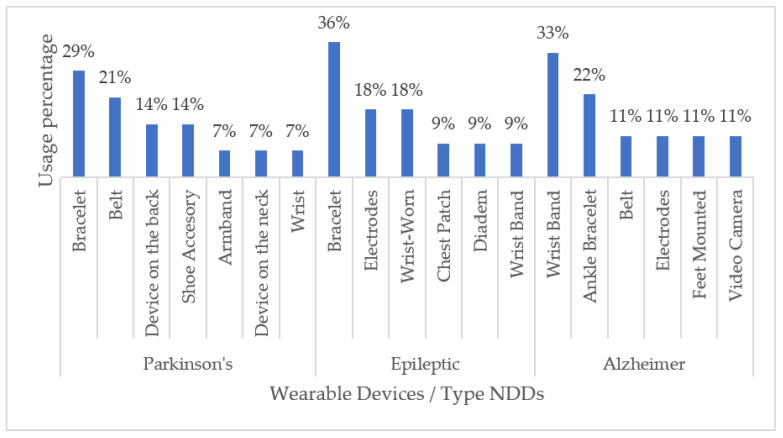
With respect to commercial handheld devices, non-commercial ones are commonly developed for a particular NDD. Overall, we found that devices to be worn on the wrist, such as bracelets, are the most used for monitoring motor disabilities related to Parkinson’s disease (29%), Alzheimer’s disease (33%), and epilepsy (36%). Generally, wrist wearable devices are chosen as the first option with respect to other options of wearable devices due to a good relationship between the comfort-accuracy of said device. Figure 7 shows a graphical summary of our findings.
Figure 7.
Non-Commercial Wearable Devices for Monitoring NDD-Related Impairments.
Real-time monitoring of patients with conditions caused by NDD allows health professionals and patients themselves to obtain timely information on their health status during daily activities. Table 6 summarizes the findings of this review with regard to non-commercial wearables.
Table 6.
Non-Commercial Wearables with Real-Time Monitoring Capabilities.
| NDD Type | Real-Time Monitoring | Distribution |
|---|---|---|
| Alzheimer’s disease | Yes | 13% |
| No | 87% | |
| Epilepsy | Yes | 50% |
| No | 50% | |
| Parkinson’s disease | Yes | 100% |
| No | 0% |
Table 7 summarizes our findings regarding the technologies implemented in non-commercial wearables to monitor NDD-related disabilities. As can be seen, the technological implementation based on accelerometers and AI (through ML techniques) is the most used. Accelerometers are implemented in bracelets or wristbands, thus implying once more that the comfort–accuracy ratio of such devices contributes to their popularity.
Table 7.
Technology in Non-Commercial Wearables for Motoring NDD-Related Motor Impairments.
| NDD Type | Sensor/Technology Used | Usage Percentage |
|---|---|---|
| Parkinson’s disease | Accelerometer | 30% |
| Mechanical joint | 14% | |
| Pressure sensors | 14% | |
| MMG sensors | 7% | |
| EMG | 7% | |
| Force sensor | 7% | |
| Inertial sensor | 7% | |
| Software system | 7% | |
| Wrist and ankle motion | 7% | |
| Epilepsy | Accelerometer | 21% |
| EEG | 21% | |
| Body temperature sensor | 11% | |
| ECG | 11% | |
| Gyroscope | 11% | |
| Biosensors with Machine Learning Algorithms (MLAs) |
5% | |
| EMG | 5% | |
| Inertial sensors | 5% | |
| Infrared sensors | 5% | |
| Optical sensors | 5% | |
| Alzheimer’s disease | Accelerometer | 20% |
| Gyroscope | 20% | |
| MLAs | 20% | |
| EEG | 10% | |
| GPS and GSM | 10% | |
| Inertial sensor | 10% | |
| PPG | 10% |
4.4. RQ4. Which Are the FDA Status of Commercial Wearables for Monitoring NDD-Related Motor Impairments Currently Available?
We considered five statuses or categories to classify the reviewed commercial wearable devices: (1) Approved, which means that the benefits of the wearable device outweigh the known risks for the intended use. (2) Class I, the device is not intended for use in supporting or sustaining life or to be of substantial importance in preventing impairment to human health but may not present a potentially unreasonable risk of illness or injury. (3) 510 (K) Exempt means that the FDA is requesting notification, along with evidence, that the medical device intended to be marketed is safe and effective prior to a company commercializing its product. (4) Cleared, which means that the manufacturer can demonstrate that their product is substantially equivalent to another (similar) legally marketed device that already has FDA clearance or approval; and finally, (5) Unknown, which means that we did not find any information on the FDA status of a particular device. In this sense, it is worth mentioning that some manufacturers do not publicly disclose FDA-related information about their products. Our findings revealed that 32% of the wearable devices have some degree of FDA approval, while for the remaining 68%, we did not find any related information. Figure 8 and Table 8 summarize these results.
Figure 8.
FDA Status of Commercial Wearables Used for Monitoring NDD-Related Motor Impairments.
Table 8.
NDD-Related Motor Impairments Monitored by Commercial Wearable Devices.
| Motor Disabilities | FDA Devices | Non-FDA Devices |
|---|---|---|
| Falls | 38% | 62% |
| Tremor | 33% | 67% |
| Seizures | 20% | 80% |
4.5. RQ5. What Are the Gaps in the Monitoring of NDDs Using Commercial Wearables Devices? And How Are These Gaps Covered by the Non-Commercial Wearables Devices?
Based on the information presented in Table 3, it can be concluded that the gap that commercial wearable devices have to cover can be summarized in two ways: (1) Improving the accuracy of the information collected from the user in any environment in which the user; that is to say, that the information that is collected from the user must be in an environment of the normal activity of the latter; that is, when the user is sleeping, walking, running, in the office, among others since from the aforementioned data it will be possible to obtain relevant results of the user’s health and (2) Build commercial wearables devices that are imperceptible to the user. The reason that most of the devices in the form of watches and bracelets are found on the market is because that technology offers a high relationship between comfort–accuracy of the data obtained from the patient compared to traditional visits to a health center. Connecting to a device to passively monitor any sign of the patient’s health does not guarantee relevant results. The Research Centers are public, private, or mixed organizations dedicated to the generation of knowledge to solve the needs of society in general, including health. Currently, there are research centers where non-commercial wearables devices are developed based on technology in the form of tattoos, smart wearables, and biosensors, among others, with which the gap that currently exists in commercial wearables devices is expected to decrease.
This review provides valuable information on wearable devices for monitoring motor impairments suffered by patients with NDDs. First, our findings revealed that commercial wearable technologies generally focus on measuring falls, tremors, and seizures via smartwatches. As for non-commercial wearables, bracelets and wristbands are the most popular and usually rely on an accelerometer for monitoring. Both commercial and non-commercial wearable devices are significantly more used on the patient’s wrist because wrist-worn devices offer a significantly good comfort-accuracy ratio. The ideal wearable device is one that makes a precise measurement of the monitoring performed and that, in turn, is transparent to the user and/or patient. That is, it is a wearable device that does not interfere with the patient’s day-to-day activities. In this sense, tattoos, rings, glasses, and clothing (among others) have emerged as wearable trends that are almost completely imperceptible to the patient. Moreover, thanks to the implementation of AI in the development of these devices, technology based on video monitoring promises to be encouraging in optimizing the monitoring of NDD-related motor impairments since it will not be necessary to wear any device to monitor our health.
5. Discussion
5.1. Challenges and Trends
Current commercial wearable devices are aimed at monitoring factors such as physical activity, heart rate, caloric intake, diet, reading texts, answering calls, and sending notifications. However, as their main critical aspect, NDDs are difficult to diagnose at their early stages, which implies that, despite their great usefulness in the monitoring and follow-up of some chronic-degenerative diseases, current wearable devices are not specifically intended to support NDD care and/or treatment. In this sense, the following challenges must be addressed in the monitoring of NDDs and NDD-related impairments:
Research new risk factors (biomarkers and/or biometric factors) for NDDs.
Optimize monitoring and measuring algorithms.
Develop non-invasive and transparent technology for users.
Optimize the connectivity of the data transmitted/received by these devices through wireless networks and personal area networks (PANs).
Develop devices that optimally manage power consumption and rely on alternative sources of energy, such as solar energy.
Guarantee the security and privacy of patient data.
In recent years, the medical and scientific communities have thrived in identifying biomarkers (i.e., biologically measurable parameters) that help timely and accurately indicate the development of an NDD. Ideally, a biomarker should be easily accessible, highly sensitive and specific, and must correlate with high levels of NDD progression. Advances in biomarker research have given rise to wearable devices that can form part of the body. An example of this is implantable, wearable technology, which consists of devices such as adhesive patches or electronic chips that are implanted in the human body through surgery so that they become part of the user permanently.
Trends in NDDs research already provide solutions in the form of wearable devices that collect biometric data by recording data on patient behavior and habits (e.g., when typing, scrolling or moving fingers) to timely identify and treat neuromotor or neurodegenerative diseases, such as PD or AD. Additionally, current technologies for monitoring NDD-related impairments (motor and cognitive) include Augmented Reality (AR), audio systems, e-skin devices, e-textiles, ingestible and insertable technologies, neural interfaces, and chemical biosensors. AR-based wearable devices can provide additional information that cannot normally be seen with the naked eye. Other areas of AR include tourism, art exhibitions, and manufacturing. Regarding audio systems, conventional (wired and wireless) speakers-headphones and high-quality equalizers can be integrated as part of the AR system to improve patient immersion. As for e-Skin or nanopatches, their technological development is similar to “artificial skin”, with the same mechanical properties as human skin. This attribute significantly increases the detection area of wearable devices that use this type of sensor. The use of this technology is not limited to humans, as it also extends to robotic systems, with the intention of providing them with near-human perception abilities to be able to use them, for example, during surgical interventions.
Unlike the e-Skin concept, e-Textiles or smart fabrics cover a significant part of the body. In this case, sensors, circuits, or input/output devices are integrated into the fabric that is part of the patient’s daily-use garments. As for ingestible and insertable technologies, they are inserted into the human body or administered as medicine in a capsule form. They comprise sensors, microprocessors, and controllers, among others, that collect, process, and send biomedical signals of patient health data. Ingestible and insertable wearable technology is considered the future in the monitoring and diagnosis of diseases. Neural interfaces aim at supporting the care of patients with complex medical conditions via an interpretation of brain function to diagnose and monitor the disease.
Chemical biosensors such as saliva, tears, and sweat are biological fluids with multiple physiologically relevant chemical components. Saliva is a complex biofluid with numerous components that permeate blood through transcellular or paracellular pathways. Saliva analysis is a non-invasive option to analyze blood and monitor the emotional, hormonal, nutritional, and metabolic state of the human body. On the other hand, the concentration levels of glucose, lactate, or neurotransmitters in tears are of vital importance for health monitoring. Finally, sweat is commonly used for both monitoring fluid and electrolyte loss and managing certain diseases. In this sense, it is possible to monitor non-invasively and in real-time relevant aspects in patients with NDDs. Finally, the reality of wearables in 2022 is marked by the predominance of smart bracelets and smartwatches. As time goes by, these devices are likely to pave the way for other wearable technologies (e.g., smart glasses, smart chips and cybernetic implants) with greater monitoring capabilities.
5.2. Emerging Solutions
The current technological revolution is driven by digitization and, above all, the development and application of AI in multiple fields. AI technologies can learn and act in response to the environment, which simulates a “mental” process that allows machines to make decisions and perform tasks that are originally human or that people are not even capable of doing on their own. AI has great potential to improve NDD detection and monitoring thanks to intelligent algorithms that are capable of recognizing and analyzing unstructured data to convert them into relevant and useful information. Additionally, as a branch of AI, ML enables pattern recognition and/or the ability to continuously learn and make predictions based on data to make adjustments automatically.
The implementation of AI altogether with ML is present in different areas of the scientific panorama, such as medicine. Hence, the synergy between ML and AI has allowed for faster and more accurate diagnosis of existing diseases, including cancer, dementia, and Alzheimer’s disease, which is a disease that modifies the brain and causes alterations that affect memory, understanding, judgment, behavior, and functional activity.
AD is one of the greatest health challenges that health systems face around the world, both because of the impact it has on patients and their families and because of the high resources needed to treat it. Addressing NDDs with ML and AI algorithms has made it possible to identify the first markers of AD with more than 99% accuracy. In fact, by evaluating the brain scans of older adults, algorithms can detect subtle changes that often occur before diagnosis, allowing doctors to offer early treatment to high-risk individuals.
5.3. Limitations
This review has four main limitations: (1) We did not consider scenarios or comparative clinical studies that assess the quality of life of patients when using multiple portable devices, (2) mobile applications associated with the reviewed devices were not analyzed, (3) studies on consumer acceptance of wearable devices were not reviewed, and (4) the medical-grade accuracy and reliability of the reviewed wearable devices could not be established due to limited information on their FDA status.
6. Conclusions
The wearable devices that are used for the remote monitoring of NDD-related motor impairments include built-in sensors and can be used during daily activities. Our findings revealed that falls are most monitored for PD, whereas tremor monitoring is predominant in wearable devices for epilepsy and AD management. Only wearable devices with suitable, well-calibrated, and coordinated sensors can provide relevant medical monitoring data. The use of a certain type of wearable device over another largely depends on the condition to be monitored; however, in general, the most frequently cited wearable devices in the literature include smartwatches and wristbands. Additionally, this review discusses the medical-grade accuracy and reliability of the cited wearable devices by listing their FDA status. In this regard, we found that 67% of the cited devices held an unknown FDA status, 28% were partially approved, and only 5% were fully FDA authorized.
Overall, NDDs cause two types of impairments: cognitive and motor. The scope of this research is limited to wearable devices monitoring only NDD-related motor impairments. In this sense, our main findings can be summarized as follows: commercial wearable devices mainly monitor falls, tremors, and seizures. Additionally, 35% of these devices are wrist-worn devices in the form of either a smartwatch or a band. Finally, only 33% of the reviewed commercial wearables hold an FDA approval status (approved, partially approved, authorized). Regarding non-commercial wearable devices, wrist-worn are also ranked as the most common due to their good comfort–accuracy ratio. Finally, thanks to the implementation of AI in mobile device software development, monitoring technologies based on video monitoring promise to be encouraging in terms of optimizing the monitoring capabilities of current wearable devices, thus implying that in the future, patients may not need to wear any type of device to still be able to monitor aspects of their health.
Acknowledgments
This work was supported by Mexico’s National Technological Institute (TecNM) and sponsored by both Mexico’s National Council of Science and Technology (CONACYT) and the Secretariat of Public Education (SEP) through the PRODEP project (Programa para el Desarrollo Profesional Docente).
Author Contributions
Conceptualization, G.P.-A., G.A.-H. and J.L.S.-C.; data curation, J.L.S.-C. and L.N.S.-M.; formal analysis, L.N.S.-M. and J.L.S.-C.; funding acquisition, G.A.-H., J.L.S.-C. and G.P.-A.; investigation G.P.-A. and G.A.-H.; methodology, G.P.-A. and J.L.S.-C.; project administration, G.A.-H.; resources, G.A.-H.; software, G.P.-A. and L.N.S.-M.; supervision, J.L.S.-C. and G.P.-A.; validation, G.P.-A., L.N.S.-M., G.A.-H. and J.L.S.-C.; visualization, J.L.S.-C. and L.N.S.-M.; writing—original draft, G.P.-A. and L.N.S.-M.; writing—review and editing, G.P.-A., G.A.-H. and J.L.S.-C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Council of Science and Technology (CONACYT) and the Public Secretariat of Education (SEP) through the Sectorial Fund of Research for Education, grant number A1-S-51808 and the project 52–2016: “Application of Big Data and Semantic Web techniques to Develop Intelligent Systems”, a postdoctoral grant, and a doctoral grant. This research was funded by the National Council of Science and Technology (CONACYT) for the scholarship awarded by participating in the call for POST-DOCTORAL STAYS FOR MEXICO MODE 1, application number 839433, to develop the project titled “Self-care of health in chronic degenerative diseases using machine learning techniques and the Internet of things”. Additionally. this research was funded by Council for Scientific Research and Technological Development in Veracruz (COVEICYDET), grant number 12 1806.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gugliandolo G., Campobello G., Capra P.P., Marino S., Bramanti A., di Lorenzo G., Donato N. A movement-tremors recorder for patients of neurodegenerative diseases. IEEE Trans. Instrum. Meas. 2019;68:1451–1457. doi: 10.1109/TIM.2019.2900141. [DOI] [Google Scholar]
- 2.World Health Organization (WHO) [(accessed on 10 July 2022)]. Available online: https://www.who.int.
- 3.World Health Organization . World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals. WHO; Geneva, Switzerland: 2022. [Google Scholar]
- 4.Castelpietra G., Knudsen A.K.S., Agardh E.E., Armocida B., Beghi M., Iburg K.M., Monasta L. The burden of mental disorders, substance use disorders and self-harm among young people in Europe, 1990–2019: Findings from the Global Burden of Disease Study 2019. Lancet Reg. Health-Eur. 2022;16:100341. doi: 10.1016/j.lanepe.2022.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019? [(accessed on 10 July 2022)]. Available online: https://www.who.int/es/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000–2019.
- 6.Dementia. [(accessed on 10 July 2022)]. Available online: https://www.who.int/es/news-room/fact-sheets/detail/dementia.
- 7.Kiper P., Richard M., Stefanutti F., Pierson-Poinsignon R., Cacciante L., Perin C., Meroni R. Combined Motor and Cognitive Rehabilitation: The Impact on Motor Performance in Patients with Mild Cognitive Impairment. Systematic Review and Meta-Analysis. J. Pers. Med. 2022;12:276. doi: 10.3390/jpm12020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicirelli G., Impedovo D., Dentamaro V., Marani R., Pirlo G., D’Orazio T. Human gait analysis in neurodegenerative diseases: A review. IEEE J. Biomed. Health Inform. 2021;26:229–242. doi: 10.1109/JBHI.2021.3092875. [DOI] [PubMed] [Google Scholar]
- 9.Seneviratne S., Hu Y., Nguyen T., Lan G., Khalifa S., Thilakarathna K., Seneviratne A. A survey of wearable devices and challenges. IEEE Commun. Surv. Tutor. 2017;19:2573–2620. doi: 10.1109/COMST.2017.2731979. [DOI] [Google Scholar]
- 10.Feng L., Jing-Long H., Ji Q.I., Zhang Y., Jia-Luo Y., Wen-Peng L., Bo-Wei L. Research and application progress of intelligent wearable devices. Chin. J. Anal. Chem. 2021;49:159–171. [Google Scholar]
- 11.Syaifudin Y.W., Funabiki N., Kuribayashi M., Kao W.C. A proposal of Advanced Widgets learning topic for interactive application in Android programming learning assistance system. SN Comput. Sci. 2021;2:1–13. doi: 10.1007/s42979-021-00580-1. [DOI] [Google Scholar]
- 12.Sarkar A., Goyal A., Hicks D., Sarkar D., Hazra S. Android application development: A brief overview of android platforms and evolution of security systems; Proceedings of the 2019 Third International Conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC); Palladam, India. 12–14 December 2019; Piscataway, NJ, USA: IEEE; 2019. pp. 73–79. [Google Scholar]
- 13.Haghi M., Thurow K., Stoll R. Wearable devices in medical internet of things: Scientific research and commercially available devices. Healthc. Inform. Res. 2017;23:4–15. doi: 10.4258/hir.2017.23.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Won P., Kim K.K., Kim H., Park J.J., Ha I., Shin J., Ko S.H. Transparent soft actuators/sensors and camouflage skins for imperceptible soft robotics. Adv. Mater. 2021;33:2002397. doi: 10.1002/adma.202002397. [DOI] [PubMed] [Google Scholar]
- 15.Anwer A.H., Khan N., Ansari M.Z., Baek S.S., Yi H., Kim S., Jeong C. Recent advances in touch sensors for flexible wearable devices. Sensors. 2022;22:4460. doi: 10.3390/s22124460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talitckii A., Kovalenko E., Anikina A., Zimniakova O., Semenov M., Bril E., Somov A. Avoiding misdiagnosis of Parkinson’s disease with the use of wearable sensors and artificial intelligence. IEEE Sens. J. 2020;21:3738–3747. doi: 10.1109/JSEN.2020.3027564. [DOI] [Google Scholar]
- 17.Hathaliya J.J., Modi H., Gupta R., Tanwar S., Sharma P., Sharma R. Parkinson and essential tremor classification to identify the patient’s risk based on tremor severity. Comput. Electr. Eng. 2022;101:107946. doi: 10.1016/j.compeleceng.2022.107946. [DOI] [Google Scholar]
- 18.Ma C., Li D., Pan L., Li X., Yin C., Li A., Zong R. Quantitative assessment of essential tremor based on machine learning methods using wearable device. Biomed. Signal Process. Control. 2022;71:103244. doi: 10.1016/j.bspc.2021.103244. [DOI] [Google Scholar]
- 19.Balestrino R., Schapira A.H.V. Parkinson disease. Eur. J. Neurol. 2020;27:27–42. doi: 10.1111/ene.14108. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong M.J., Okun M.S. Diagnosis and treatment of Parkinson disease: A review. Jama. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 21.Blauwendraat C., Nalls M.A., Singleton A.B. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020;19:170–178. doi: 10.1016/S1474-4422(19)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thijs R.D., Surges R., O’Brien T.J., Sander J.W. Epilepsy in adults. Lancet. 2019;393:689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 23.Walsh S., Donnan J., Fortin Y., Sikora L., Morrissey A., Collins K., MacDonald D. A systematic review of the risks factors associated with the onset and natural progression of epilepsy. Neurotoxicology. 2017;61:64–77. doi: 10.1016/j.neuro.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Pitkänen A., Löscher W., Vezzani A., Becker A.J., Simonato M., Lukasiuk K., Beck H. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 2016;15:843–856. doi: 10.1016/S1474-4422(16)00112-5. [DOI] [PubMed] [Google Scholar]
- 25.Lane C.A., Hardy J., Schott J.M. Alzheimer’s disease. Eur. J. Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 26.Knopman D.S., Amieva H., Petersen R.C., Chételat G., Holtzman D.M., Hyman B.T., Jones D.T. Alzheimer disease. Nat. Rev. Dis. Prim. 2021;7:33. doi: 10.1038/s41572-021-00269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uwishema O., Mahmoud A., Sun J., Correia I.F.S., Bejjani N., Alwan M., Dost B. Is Alzheimer’s disease an infectious neurological disease? A review of the literature. Brain Behav. 2022;12:e2728. doi: 10.1002/brb3.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigas G., Tzallas A.T., Tsipouras M.G., Bougia P., Tripoliti E.E., Baga D., Konitsiotis S. Assessment of tremor activity in the Parkinson’s disease using a set of wearable sensors. IEEE Trans. Inf. Technol. Biomed. 2012;16:478–487. doi: 10.1109/TITB.2011.2182616. [DOI] [PubMed] [Google Scholar]
- 29.Ali S.M., Arjunan S.P., Peters J., Perju-Dumbrava L., Ding C., Eller M., Kumar D.K. Wearable sensors during drawing tasks to measure the severity of essential tremor. Sci. Rep. 2022;12:5242. doi: 10.1038/s41598-022-08922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernando E., Fraser M., Hendriksen J., Kim C.H., Muir-Hunter S.W. Risk factors associated with falls in older adults with dementia: A systematic review. Physiother. Can. 2017;69:161–170. doi: 10.3138/ptc.2016-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brims L., Oliver K. Effectiveness of assistive technology in improving the safety of people with dementia: A systematic review and meta-analysis. Aging Ment. Health. 2019;23:942–951. doi: 10.1080/13607863.2018.1455805. [DOI] [PubMed] [Google Scholar]
- 32.Wu X., Zheng Y., Chu C.H., Cheng L., Kim J. Applying deep learning technology for automatic fall detection using mobile sensors. Biomed. Signal Process. Control. 2022;72:103355. doi: 10.1016/j.bspc.2021.103355. [DOI] [Google Scholar]
- 33.Chowdhury M.E., Khandakar A., Alzoubi K., Mohammed A., Taha S., Omar A., Reaz M.B.I. Biomedical Signals Based Computer-Aided Diagnosis for Neurological Disorders. Springer; Cham, Switzerland: 2022. Wearable Real-Time Epileptic Seizure Detection and Warning System; pp. 233–265. [Google Scholar]
- 34.Vieluf S., Reinsberger C., El Atrache R., Jackson M., Schubach S., Ufongene C., Meisel C. Autonomic nervous system changes detected with peripheral sensors in the setting of epileptic seizures. Sci. Rep. 2020;10:11560. doi: 10.1038/s41598-020-68434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen F., Chen I., Zafar M., Sinha S.R., Hu X. Seizures detection using multimodal signals: A scoping review. Physiol. Meas. 2022;43:07TR01. doi: 10.1088/1361-6579/ac7a8d. [DOI] [PubMed] [Google Scholar]
- 36.Kristoffersson A., Lindén M. A Systematic Review of Wearable Sensors for Monitoring Physical Activity. Sensors. 2022;22:573. doi: 10.3390/s22020573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mughal H., Javed A.R., Rizwan M., Almadhor A.S., Kryvinska N. Parkinson’s Disease Management Via Wearable Sensors: A Systematic Review. IEEE Access. 2022;10:35219–35237. doi: 10.1109/ACCESS.2022.3162844. [DOI] [Google Scholar]
- 38.Sica M., Tedesco S., Crowe C., Kenny L., Moore K., Timmons S., Komaris D.S. Continuous home monitoring of Parkinson’s disease using inertial sensors: A systematic review. PLoS ONE. 2021;16:e0246528. doi: 10.1371/journal.pone.0246528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celik Y., Stuart S., Woo W.L., Godfrey A. Gait analysis in neurological populations: Progression in the use of wearables. Med. Eng. Phys. 2021;87:9–29. doi: 10.1016/j.medengphy.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Albán-Cadena A.C., Villalba-Meneses F., Pila-Varela K.O., Moreno-Calvo A., Villalba-Meneses C.P., Almeida-Galárraga D.A. Wearable sensors in the diagnosis and study of Parkinson’s disease symptoms: A systematic review. J. Med. Eng. Technol. 2021;45:532–545. doi: 10.1080/03091902.2021.1922528. [DOI] [PubMed] [Google Scholar]
- 41.Storm F.A., Cesareo A., Reni G., Biffi E. Wearable inertial sensors to assess gait during the 6-minute walk test: A systematic review. Sensors. 2020;20:2660. doi: 10.3390/s20092660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zampogna A., Mileti I., Palermo E., Celletti C., Paoloni M., Manoni A., Suppa A. Fifteen years of wireless sensors for balance assessment in neurological disorders. Sensors. 2020;20:3247. doi: 10.3390/s20113247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rehman R.Z.U., Zhou Y., del Din S., Alcock L., Hansen C., Guan Y., Lamoth C.J. Gait analysis with wearables can accurately classify fallers from non-fallers: A step toward better management of neurological disorders. Sensors. 2020;20:6992. doi: 10.3390/s20236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godoi B.B., Amorim G.D., Quiroga D.G., Holanda V.M., Júlio T., Tournier M.B. Parkinson’s disease and wearable devices, new perspectives for a public health issue: An integrative literature review. Rev. Assoc. Médica Bras. 2019;65:1413–1420. doi: 10.1590/1806-9282.65.11.1413. [DOI] [PubMed] [Google Scholar]
- 45.Chirra M., Marsili L., Wattley L., Sokol L.L., Keeling E., Maule S., Merola A. Telemedicine in neurological disorders: Opportunities and challenges. Telemed. e-Health. 2019;25:541–550. doi: 10.1089/tmj.2018.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brognara L., Palumbo P., Grimm B., Palmerini L. Assessing gait in Parkinson’s disease using wearable motion sensors: A systematic review. Diseases. 2019;7:18. doi: 10.3390/diseases7010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rovini E., Maremmani C., Cavallo F. Automated systems based on wearable sensors for the management of Parkinson’s disease at home: A systematic review. Telemed. e-Health. 2019;25:167–183. doi: 10.1089/tmj.2018.0035. [DOI] [PubMed] [Google Scholar]
- 48.Díaz S., Stephenson J.B., Labrador M.A. Use of wearable sensor technology in gait, balance, and range of motion analysis. Appl. Sci. 2019;10:234. doi: 10.3390/app10010234. [DOI] [Google Scholar]
- 49.Porciuncula F., Roto A.V., Kumar D., Davis I., Roy S., Walsh C.J., Awad L.N. Wearable movement sensors for rehabilitation: A focused review of technological and clinical advances. Pm&r. 2018;10:S220–S232. doi: 10.1016/j.pmrj.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva de Lima A.L., Evers L.J., Hahn T., Bataille L., Hamilton J.L., Little M.A., Faber M.J. Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: A systematic review. J. Neurol. 2017;264:1642–1654. doi: 10.1007/s00415-017-8424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vienne A., Barrois R.P., Buffat S., Ricard D., Vidal P.P. Inertial sensors to assess gait quality in patients with neurological disorders: A systematic review of technical and analytical challenges. Front. Psychol. 2017;8:817. doi: 10.3389/fpsyg.2017.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Q., Markopoulos P., Yu B., Chen W., Timmermans A. Interactive wearable systems for upper body rehabilitation: A systematic review. J. Neuroeng. Rehabil. 2017;14:20. doi: 10.1186/s12984-017-0229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giggins O.M., Clay I., Walsh L. Physical activity monitoring in patients with neurological disorders: A review of novel body-worn devices. Digit. Biomark. 2017;1:14–42. doi: 10.1159/000477384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hubble R.P., Naughton G.A., Silburn P.A., Cole M.H. Wearable sensor use for assessing standing balance and walking stability in people with Parkinson’s disease: A systematic review. PLoS ONE. 2015;10:e0123705. doi: 10.1371/journal.pone.0123705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oung Q.W., Hariharan M., Basah S.N., Yaacob S., Sarillee M., Lee H.L. Use of technological tools for Parkinson’s disease early detection: A review; Proceedings of the 2014 IEEE International Conference on Control System, Computing and Engineering (ICCSCE 2014); Penang, Malaysia. 28–30 November 2014; Piscataway, NJ, USA: IEEE; 2014. pp. 343–348. [Google Scholar]
- 56.Steins D., Dawes H., Esser P., Collett J. Wearable accelerometry-based technology capable of assessing functional activities in neurological populations in community settings: A systematic review. J. Neuroeng. Rehabil. 2014;11:36. doi: 10.1186/1743-0003-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stavropoulos T.G., Papastergiou A., Mpaltadoros L., Nikolopoulos S., Kompatsiaris I. IoT wearable sensors and devices in elderly care: A literature review. Sensors. 2020;20:2826. doi: 10.3390/s20102826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pasluosta C.F., Gassner H., Winkler J., Klucken J., Eskofier B.M. An emerging era in the management of Parkinson’s disease: Wearable technologies and the internet of things. IEEE J. Biomed. Health Inform. 2015;19:1873–1881. doi: 10.1109/JBHI.2015.2461555. [DOI] [PubMed] [Google Scholar]
- 59.Tăuţan A.M., Ionescu B., Santarnecchi E. Artificial intelligence in neurodegenerative diseases: A review of available tools with a focus on machine learning techniques. Artif. Intell. Med. 2021;117:102081. doi: 10.1016/j.artmed.2021.102081. [DOI] [PubMed] [Google Scholar]
- 60.Ghannam R., Curia G., Brante G., Fan H., Heidari H. Wearable electronics for neurological applications: A review of undergraduate engineering programmes; Proceedings of the 2020 Transnational Engineering Education Using Technology (TREET); Glasgow, UK. 31 July 2020; Piscataway, NJ, USA: IEEE; 2020. pp. 1–4. [Google Scholar]
- 61.Buckley C., Alcock L., McArdle R., Rehman R.Z.U., del Din S., Mazzà C., Rochester L. The role of movement analysis in diagnosing and monitoring neurodegenerative conditions: Insights from gait and postural control. Brain Sci. 2019;9:34. doi: 10.3390/brainsci9020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belić M., Bobić V., Badža M., Šolaja N., Đurić-Jovičić M., Kostić V.S. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease—A review. Clin. Neurol. Neurosurg. 2019;184:105442. doi: 10.1016/j.clineuro.2019.105442. [DOI] [PubMed] [Google Scholar]
- 63.Sivathamboo S., Nhu D., Piccenna L., Yang A., Antonic-Baker A., Vishwanath S., Kwan P. Preferences and User Experiences of Wearable Devices in Epilepsy: A Systematic Review and Mixed-Methods Synthesis. Neurology. 2022;99:e1380–e1392. doi: 10.1212/WNL.0000000000200794. [DOI] [PubMed] [Google Scholar]
- 64.Olsen L.S., Nielsen J.M., Simonÿ C., Kjær T.W., Beck M. Wearables in real life: A qualitative study of experiences of people with epilepsy who use home seizure monitoring devices. Epilepsy Behav. 2021;125:108398. doi: 10.1016/j.yebeh.2021.108398. [DOI] [PubMed] [Google Scholar]
- 65.Rukasha T., Woolley S.I., Kyriacou T., Collins T. Evaluation of wearable electronics for epilepsy: A systematic review. Electronics. 2020;9:968. doi: 10.3390/electronics9060968. [DOI] [Google Scholar]
- 66.Simblett S.K., Biondi A., Bruno E., Ballard D., Stoneman A., Lees S., Wykes T. Patients’ experience of wearing multimodal sensor devices intended to detect epileptic seizures: A qualitative analysis. Epilepsy Behav. 2020;102:106717. doi: 10.1016/j.yebeh.2019.106717. [DOI] [PubMed] [Google Scholar]
- 67.Johansson D., Malmgren K., Alt Murphy M. Wearable sensors for clinical applications in epilepsy, Parkinson’s disease, and stroke: A mixed-methods systematic review. J. Neurol. 2018;265:1740–1752. doi: 10.1007/s00415-018-8786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryvlin P., Ciumas C., Wisniewski I., Beniczky S. Wearable devices for sudden unexpected death in epilepsy prevention. Epilepsia. 2018;59:61–66. doi: 10.1111/epi.14054. [DOI] [PubMed] [Google Scholar]
- 69.Naganur V., Sivathamboo S., Chen Z., Kusmakar S., Antonic-Baker A., O’Brien T.J., Kwan P. Automated seizure detection with non-invasive wearable devices: A systematic review and meta-analysis. Epilepsia. 2022;63:1930–1941. doi: 10.1111/epi.17297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ong J.S., Wong S.N., Arulsamy A., Watterson J.L., Shaikh M. Medical Technology: A Systematic Review on Medical Devices Utilized for Epilepsy Prediction and Management. Curr. Neuropharmacol. 2022;20:950–964. doi: 10.2174/1570159X19666211108153001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cullen A., Mazhar M.K.A., Smith M.D., Lithander F.E., Ó Breasail M., Henderson E.J. Wearable and Portable GPS Solutions for Monitoring Mobility in Dementia: A Systematic Review. Sensors. 2022;22:3336. doi: 10.3390/s22093336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nielsen J.M., Rades D., Kjaer T.W. Wearable electroencephalography for ultra-long-term seizure monitoring: A systematic review and future prospects. Expert Rev. Med. Devices. 2021;18((Suppl. 1)):57–67. doi: 10.1080/17434440.2021.2012152. [DOI] [PubMed] [Google Scholar]
- 73.Beniczky S., Wiebe S., Jeppesen J., Tatum W.O., Brazdil M., Wang Y., Ryvlin P. Automated seizure detection using wearable devices: A clinical practice guideline of the International League Against Epilepsy and the International Federation of Clinical Neurophysiology. Clin. Neurophysiol. 2021;132:1173–1184. doi: 10.1016/j.clinph.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 74.Shum J., Friedman D. Commercially available seizure detection devices: A systematic review. J. Neurol. Sci. 2021;428:117611. doi: 10.1016/j.jns.2021.117611. [DOI] [PubMed] [Google Scholar]
- 75.Breasail M.Ó., Biswas B., Smith M.D., Mazhar M.K.A., Tenison E., Cullen A., Henderson E.J. Wearable GPS and Accelerometer Technologies for Monitoring Mobility and Physical Activity in Neurodegenerative Disorders: A Systematic Review. Sensors. 2021;21:8261. doi: 10.3390/s21248261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De la Fuente García S., Ritchie C.W., Luz S. Artificial intelligence, speech, and language processing approaches to monitoring Alzheimer’s disease: A systematic review. J. Alzheimer’s Dis. 2022;78:1547–1574. doi: 10.3233/JAD-200888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Machado S.D., Barbosa J.L.V. Technologies applied in the care of patients with Alzheimer’s disease: A systematic review; Proceedings of the Brazilian Symposium on Multimedia and the Web; New York, NY, USA. 7–11 November 2022; pp. 29–32. [Google Scholar]
- 78.Gillani N., Arslan T. Intelligent sensing technologies for the diagnosis, monitoring and therapy of alzheimer’s disease: A systematic review. Sensors. 2021;21:4249. doi: 10.3390/s21124249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson M.S., Homdee N., Bankole A., Alam R., Mitchell B., Hayes J., Lach J. Behavioral interventions for Alzheimer’s management using technology: Home-based monitoring. Curr. Geriatr. Rep. 2020;9:90–100. doi: 10.1007/s13670-020-00312-y. [DOI] [Google Scholar]
- 80.Bruno E., Biondi A., Böttcher S., Lees S., Schulze-Bonhage A., Richardson M.P., RADAR-CNS Consortium Day and night comfort and stability on the body of four wearable devices for seizure detection: A direct user-experience. Epilepsy Behav. 2020;112:107478. doi: 10.1016/j.yebeh.2020.107478. [DOI] [PubMed] [Google Scholar]
- 81.Vinals L., Akinola T., Sarri G. Pnd93 what good are digital technologies in Alzheimer’s disease research?—A systematic literature review. Value Health. 2019;22:S754. doi: 10.1016/j.jval.2019.09.1863. [DOI] [Google Scholar]
- 82.Kurada A.V., Srinivasan T., Hammond S., Ulate-Campos A., Bidwell J. Seizure detection devices for use in antiseizure medication clinical trials: A systematic review. Seizure. 2019;66:61–69. doi: 10.1016/j.seizure.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Klimova B., Valis M., Kuca K. Exploring assistive technology as a potential beneficial intervention tool for people with Alzheimer’s disease—A systematic review. Neuropsychiatr. Dis. Treat. 2018;14:3151. doi: 10.2147/NDT.S181849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ienca M., Fabrice J., Elger B., Caon M., Scoccia Pappagallo A., Kressig R.W., Wangmo T. Intelligent assistive technology for Alzheimer’s disease and other dementias: A systematic review. J. Alzheimer’s Dis. 2017;56:1301–1340. doi: 10.3233/JAD-161037. [DOI] [PubMed] [Google Scholar]
- 85.Brereton P., Kitchenham B.A., Budgen D., Turner M., Khalil M. Lessons from applying the systematic literature review process within the software engineering domain. J. Syst. Softw. 2017;80:571–583. doi: 10.1016/j.jss.2006.07.009. [DOI] [Google Scholar]
- 86.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021;10:372. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Apple Watch Series 7—Apple. [(accessed on 15 May 2022)]. Available online: https://www.apple.com/apple-watch-series-7/
- 88.Watchseniors—Smartwatch GPS Para La Tercera Edad. [(accessed on 15 May 2022)]. Available online: https://watchseniors.com.
- 89.FallSafety Home—iPhone, Android, & Apple Watch Fall Detection. [(accessed on 14 May 2022)]. Available online: https://fallsafetyapp.com/fallsafety-home.
- 90.FallSkip Scientific Basis—Technology for Fall Risk Evaluation in Older Adults. [(accessed on 16 May 2022)]. Available online: https://fallskip.com/en/fallskip-scientific-basis/
- 91.Neki—Solutions and GPS Devices for the Security of Your Family. [(accessed on 16 May 2022)]. Available online: https://www.nekiglobal.com.
- 92.Alexa Together. [(accessed on 14 May 2022)]. Available online: https://www.amazon.com/Alexa-Together/b?ie=UTF8&node=21390531011.
- 93.MyNotifi. [(accessed on 29 March 2022)]. Available online: https://www.mynotifi.com.
- 94.VirtuSense Technologies|Fall Prevention and RPM with AI. [(accessed on 17 May 2022)]. Available online: https://www.virtusense.ai.
- 95.Emma Watch: A Device to Ease Tremors. [(accessed on 29 March 2022)]. Available online: https://parkinsonsdisease.net/news/emma-watch-wearable-device-tremors.
- 96.How RPM Works|Global Kinetics. [(accessed on 29 March 2022)]. Available online: https://pkgcare.com/healthcare-professionals/how-it-works/
- 97.Parkinson Smartwatch—Parkinson Smartwatch. [(accessed on 11 May 2022)]. Available online: https://parkinsonsmartwatch.com/en/
- 98.Essential Tremor Glove|Hand Tremor Devices—Steadiwear Inc. [(accessed on 12 May 2022)]. Available online: https://steadiwear.com/?_atid=KHPdPvqWHDNFddV1UcSTePmtuyiIbl.
- 99.Certified Medical Class 2a Hand Therapeutic Tremor Device—Vilim Ball. [(accessed on 13 May 2022)]. Available online: https://www.vilim.lt/en/1630-2/
- 100.Hand Tremor Device for Essential Tremor by Five Microns. [(accessed on 29 March 2022)]. Available online: https://fivemicrons.com/?gclid=Cj0KCQjw3IqSBhCoARIsAMBkTb1P2xnOz9ViAqYXGuwTL2OS_Tn0JFzimtP_OOjcN0VQ20E_HkBD0B4aAmF6EALw_wcB.
- 101.Gyenno Spoon. [(accessed on 29 March 2022)]. Available online: https://www.gyenno.com/spoon-en.
- 102.Parkinson’s KinetiGraph (PKG)|Dementech Neurosciences. [(accessed on 11 May 2022)]. Available online: https://dementech.com/parkinsons-kinetigraph-pkg/
- 103.Essential Tremor Bracelet—Wrist Device for Tremors—Cala Trio. [(accessed on 12 May 2022)]. Available online: https://calatrio.com.
- 104.Movement Monitor|Epi USA. [(accessed on 29 March 2022)]. Available online: https://www.epiusa.net/movement-monitor.
- 105.Commercial Care Systems. [(accessed on 29 March 2022)]. Available online: https://www.medpage-ltd.com/epilepsy/epilepsy-commercial-care-systems.
- 106.EpiUSA Emfit Movement Monitor Epilepsy Seizure Alarm, United States and Canada. [(accessed on 29 March 2022)]. Available online: https://www.epiusa.net/
- 107.Embrace2 Seizure Monitoring|Smarter Epilepsy Management|Embrace Watch|Empatica. [(accessed on 29 March 2022)]. Available online: https://www.empatica.com/en-eu/embrace2/
- 108.Standard SAMi-3 Kit (US and Canada) — SAMi: The Sleep Activity Monitor. [(accessed on 29 March 2022)]. Available online: https://www.samialert.com.
- 109.Pulliam C.L., Heldman D.A., Brokaw E.B., Mera T.O., Mari Z.K., Burack M.A. Continuous assessment of levodopa response in Parkinson’s disease using wearable motion sensors. IEEE Trans. Biomed. Eng. 2017;65:159–164. doi: 10.1109/TBME.2017.2697764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Greene B.R., Caulfield B., Lamichhane D., Bond W., Svendsen J., Zurski C., Pratt D. Longitudinal assessment of falls in patients with Parkinson’s disease using inertial sensors and the Timed Up and Go test. J. Rehabil. Assist. Technol. Eng. 2018;5:2055668317750811. doi: 10.1177/2055668317750811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Daneault J.F., Vergara-Diaz G., Parisi F., Admati C., Alfonso C., Bertoli M., Bonato P. Accelerometer data collected with a minimum set of wearable sensors from subjects with Parkinson’s disease. Sci. Data. 2021;8:48. doi: 10.1038/s41597-021-00830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marcante A., di Marco R., Gentile G., Pellicano C., Assogna F., Pontieri F.E., Antonini A. Foot pressure wearable sensors for freezing of gait detection in Parkinson’s disease. Sensors. 2022;21:128. doi: 10.3390/s21010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huo W., Angeles P., Tai Y.F., Pavese N., Wilson S., Hu M.T., Vaidyanathan R. A heterogeneous sensing suite for multisymptom quantification of Parkinson’s disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2020;28:1397–1406. doi: 10.1109/TNSRE.2020.2978197. [DOI] [PubMed] [Google Scholar]
- 114.Vergara-Diaz G., Daneault J.F., Parisi F., Admati C., Alfonso C., Bertoli M., Bonato P. Limb and trunk accelerometer data collected with wearable sensors from subjects with Parkinson’s disease. Sci. Data. 2021;8:47. doi: 10.1038/s41597-021-00831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raiano L., di Pino G., di Biase L., Tombini M., Tagliamonte N.L., Formica D. PDMeter: A Wrist Wearable Device for an at-home Assessment of the Parkinson’s Disease Rigidity. IEEE Trans. Neural Syst. Rehabil. Eng. 2020;28:1325–1333. doi: 10.1109/TNSRE.2020.2987020. [DOI] [PubMed] [Google Scholar]
- 116.Ileșan R.R., Cordoș C.G., Mihăilă L.I., Fleșar R., Popescu A.S., Perju-Dumbravă L., Faragó P. Proof of Concept in Artificial-Intelligence-Based Wearable Gait Monitoring for Parkinson’s Disease Management Optimization. Biosensors. 2020;12:189. doi: 10.3390/bios12040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stuart S., Godfrey A., Mancini M. Staying UpRight in Parkinson’s disease: A pilot study of a novel wearable postural intervention. Gait Posture. 2022;91:86–93. doi: 10.1016/j.gaitpost.2021.09.202. [DOI] [PubMed] [Google Scholar]
- 118.Dang Q.K., Seo H.G., Pham D.D., Chee Y. Wearable sensor based stooped posture estimation in simulated Parkinson’s disease gaits. Sensors. 2019;19:223. doi: 10.3390/s19020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang J., El Atrache R., Yu S., Asif U., Jackson M., Roy S., Loddenkemper T. Seizure detection using wearable sensors and machine learning: Setting a benchmark. Epilepsia. 2021;62:1807–1819. doi: 10.1111/epi.16967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jeppesen J., Fuglsang-Frederiksen A., Johansen P., Christensen J., Wüstenhagen S., Tankisi H., Beniczky S. Seizure detection based on heart rate variability using a wearable electrocardiography device. Epilepsia. 2019;60:2105–2113. doi: 10.1111/epi.16343. [DOI] [PubMed] [Google Scholar]
- 121.Johansson D., Ohlsson F., Krýsl D., Rydenhag B., Czarnecki M., Gustafsson N., Malmgren K. Tonic-clonic seizure detection using accelerometry-based wearable sensors: A prospective, video-EEG controlled study. Seizure. 2019;65:48–54. doi: 10.1016/j.seizure.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 122.Dong C., Ye T., Long X., Aarts R.M., van Dijk J.P., Shang C., Wang Y. A Two-Layer Ensemble Method for Detecting Epileptic Seizures Using a Self-Annotation Bracelet with Motor Sensors. IEEE Trans. Instrum. Meas. 2022;71:164. doi: 10.1109/TIM.2022.3173270. [DOI] [Google Scholar]
- 123.Ertuğrul Ö.F., Sönmez Y., Sezgin N., Eşref A.K.I.L. Assessment of Epileptic Seizures and Non-Epileptic Seizures via Wearable Sensors and Priori Detection of Epileptic Seizures. Balk. J. Electr. Comput. Eng. 2022;10:150–155. doi: 10.17694/bajece.1054818. [DOI] [Google Scholar]
- 124.Nielsen J.M., Zibrandtsen I.C., Masulli P., Sørensen T.L., Andersen T.S., Kjær T.W. Towards a wearable multi-modal seizure detection system in epilepsy: A pilot study. Clin. Neurophysiol. 2022;136:40–48. doi: 10.1016/j.clinph.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 125.Kusmakar S., Karmakar C.K., Yan B., O’Brien T.J., Muthuganapathy R., Palaniswami M. Automated detection of convulsive seizures using a single wrist-worn accelerometer device. IEEE Trans. Biomed. Eng. Submitt. 2019;66:421–432. doi: 10.1109/TBME.2018.2845865. [DOI] [PubMed] [Google Scholar]
- 126.Halimeh M., Yang Y., Sheehan T., Vieluf S., Jackson M., Loddenkemper T., Meisel C. Wearable device assessments of antiseizure medication effects on diurnal patterns of electrodermal activity, heart rate, and heart rate variability. Epilepsy Behav. 2022;129:108635. doi: 10.1016/j.yebeh.2022.108635. [DOI] [PubMed] [Google Scholar]
- 127.Japaridze G., Loeckx D., Buckinx T., Armand Larsen S., Proost R., Jansen K., Beniczky S. Automated detection of absence seizures using a wearable electroencephalographic device: A phase 3 validation study and feasibility of automated behavioral testing. Epilepsia. 2022:1–7. doi: 10.1111/epi.17200. [DOI] [PubMed] [Google Scholar]
- 128.Cogan D., Birjandtalab J., Nourani M., Harvey J., Nagaraddi V. Multi-biosignal analysis for epileptic seizure monitoring. Int. J. Neural Syst. 2017;27:1650031. doi: 10.1142/S0129065716500313. [DOI] [PubMed] [Google Scholar]
- 129.Varatharajan R., Manogaran G., Priyan M.K., Sundarasekar R. Wearable sensor devices for early detection of Alzheimer disease using dynamic time warping algorithm. Clust. Comput. 2018;21:681–690. doi: 10.1007/s10586-017-0977-2. [DOI] [Google Scholar]
- 130.Perez-Valero E., Lopez-Gordo M.Á., Gutiérrez C.M., Carrera-Muñoz I., Vílchez-Carrillo R.M. A Self-driven Approach for Multi-class Discrimination in Alzheimer’s Disease Based on Wearable EEG. Comput. Methods Programs Biomed. 2022;220:106841. doi: 10.1016/j.cmpb.2022.106841. [DOI] [PubMed] [Google Scholar]
- 131.Godfrey A., Brodie M., van Schooten K.S., Nouredanesh M., Stuart S., Robinson L. Inertial wearables as pragmatic tools in dementia. Maturitas. 2019;127:12–17. doi: 10.1016/j.maturitas.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 132.Roopaei M., Rad P., Prevost J.J. A wearable IoT with complex artificial perception embedding for Alzheimer patients; Proceedings of the 2018 World Automation Congress (WAC); Stevenson, WA, USA. 3–6 June 2018; Piscataway, NJ, USA: IEEE; 2018. pp. 1–6. [Google Scholar]
- 133.Goel I., Kumar D. Design and implementation of android based wearable smart locator band for people with autism, dementia, and Alzheimer. Adv. Electron. 2015;2015:8. doi: 10.1155/2015/140762. [DOI] [Google Scholar]
- 134.Pontoriero A.D., Charlton P.H., Alastruey J. Alzheimer’s disease: A step towards prognosis using smart wearables. Proceedings. 2019;4:8. doi: 10.3390/ecsa-5-05742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsu Y.L., Chung P.C., Wang W.H., Pai M.C., Wang C.Y., Lin C.W., Wang J.S. Gait and balance analysis for patients with Alzheimer’s disease using an inertial-sensor-based wearable instrument. IEEE J. Biomed. Health Inform. 2014;18:1822–1830. doi: 10.1109/JBHI.2014.2325413. [DOI] [PubMed] [Google Scholar]
- 136.Costa L., Gago M.F., Yelshyna D., Ferreira J., David Silva H., Rocha L., Bicho E. Application of machine learning in postural control kinematics for the diagnosis of Alzheimer’s disease. Comput. Intell. Neurosci. 2016;2016:3891253. doi: 10.1155/2016/3891253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.