Key Points
Question
What are the risk factors associated with symptomatic SARS-CoV-2 and rhinovirus infection in King County, Washington, from June 2020 to July 2022?
Findings
In this case control study with a test-negative design of 23 498 participants, reporting close contact with a SARS-CoV-2 case was the strongest risk factor associated with a positive SARS-CoV-2 test, while young age was associated with a positive rhinovirus test. Sociodemographic disparities were present for both SARS-CoV-2 and rhinovirus.
Meaning
These findings suggest that monitoring risk factors associated with respiratory pathogen test positivity remains important to identify at-risk populations in the post–SARS-CoV-2 pandemic period.
This case-control study evaluates how risk factors and symptoms associated with SARS-CoV-2 test positivity have changed over the course of the pandemic among individuals in King County, Washington, and compares these with the risk factors associated with rhinovirus test positivity.
Abstract
Importance
Few US studies have reexamined risk factors for SARS-CoV-2 positivity in the context of widespread vaccination and new variants or considered risk factors for cocirculating endemic viruses, such as rhinovirus.
Objectives
To evaluate how risk factors and symptoms associated with SARS-CoV-2 test positivity changed over the course of the pandemic and to compare these with the risk factors associated with rhinovirus test positivity.
Design, Setting, and Participants
This case-control study used a test-negative design with multivariable logistic regression to assess associations between SARS-CoV-2 and rhinovirus test positivity and self-reported demographic and symptom variables over a 25-month period. The study was conducted among symptomatic individuals of all ages enrolled in a cross-sectional community surveillance study in King County, Washington, from June 2020 to July 2022.
Exposures
Self-reported data for 15 demographic and health behavior variables and 16 symptoms.
Main Outcomes and Measures
Reverse transcription–polymerase chain reaction–confirmed SARS-CoV-2 or rhinovirus infection.
Results
Analyses included data from 23 498 individuals. The median (IQR) age of participants was 34.33 (22.42-45.08) years, 13 878 (59.06%) were female, 4018 (17.10%) identified as Asian, 654 (2.78%) identified as Black, and 2193 (9.33%) identified as Hispanic. Close contact with an individual with SARS-CoV-2 (adjusted odds ratio [aOR], 3.89; 95% CI, 3.34-4.57) and loss of smell or taste (aOR, 3.49; 95% CI, 2.77-4.41) were the variables most associated with SARS-CoV-2 test positivity, but both attenuated during the Omicron period. Contact with a vaccinated individual with SARS-CoV-2 (aOR, 2.03; 95% CI, 1.56-2.79) was associated with lower odds of testing positive than contact with an unvaccinated individual with SARS-CoV-2 (aOR, 4.04; 95% CI, 2.39-7.23). Sore throat was associated with Omicron infection (aOR, 2.27; 95% CI, 1.68-3.20) but not Delta infection. Vaccine effectiveness for participants fully vaccinated with a booster dose was 93% (95% CI, 73%-100%) for Delta, but not significant for Omicron. Variables associated with rhinovirus test positivity included being younger than 12 years (aOR, 3.92; 95% CI, 3.42-4.51) and experiencing a runny or stuffy nose (aOR, 4.58; 95% CI, 4.07-5.21). Black race, residing in south King County, and households with 5 or more people were significantly associated with both SARS-CoV-2 and rhinovirus test positivity.
Conclusions and Relevance
In this case-control study of 23 498 symptomatic individuals, estimated risk factors and symptoms associated with SARS-CoV-2 infection changed over time. There was a shift in reported symptoms between the Delta and Omicron variants as well as reductions in the protection provided by vaccines. Racial and sociodemographic disparities persisted in the third year of SARS-CoV-2 circulation and were also present in rhinovirus infection. Trends in testing behavior and availability may influence these results.
Introduction
Studies from the United Kingdom have found that risk factors and symptoms associated with SARS-CoV-2 infection have fluctuated over the course of the pandemic and should be reassessed periodically to guide control strategies.1,2,3 In the United States, early studies identified contact with a case and community and workplace exposures as important risk factors,4,5,6 while many studies also noted the disproportionate impact on Black, Hispanic, and socioeconomically disadvantaged communities.7,8,9,10,11,12,13,14 Few studies in the United States have reexamined these risk factors in the context of widespread vaccination and circulation of new variants.
A feature of the SARS-CoV-2 pandemic has been substantial reduction in the circulation of endemic respiratory pathogens because of nonpharmaceutical interventions (NPIs), with rhinovirus as a notable exception.15,16,17,18 After an initial decline in activity during the spring 2020 lockdown period, rhinovirus quickly rebounded to circulate at prepandemic levels and was the only pathogen to substantially cocirculate with SARS-CoV-2 during the first year of the pandemic.19,20,21,22 Rhinovirus is a common pathogen that typically causes symptomatic upper respiratory tract disease in children and adults23,24,25,26 and is generally thought to have a mild disease course. As a result, rhinovirus is relatively poorly studied, and risk factors for infection remain unclear.
In this study, we evaluate risk factors and symptoms associated with SARS-CoV-2 test positivity among children and adults participating in the greater Seattle Coronavirus Assessment Network (SCAN) study over a 25-month pandemic period encompassing the circulation of new variants and rising immunity from natural infection and vaccination. We examine characteristics associated with all SARS-CoV-2 infections and compare risk factors for Delta and Omicron infections separately. We contrast our findings with risk factors associated with rhinovirus test positivity during the same study period when other endemic respiratory pathogens, such as influenza, were largely absent.
Methods
Study Design
SCAN was designed as a cross-sectional surveillance study using online community recruitment to monitor the incidence of SARS-CoV-2 and other respiratory pathogens in the greater Seattle area from June 10, 2020, to July 27, 2022. Participants were not hospitalized at the time of enrollment, but were largely symptomatic (>90%) and oversampled in areas with fewer testing sites. Recruitment criteria appear in the eMethods in Supplement 1.
Enrolled participants received a free testing kit delivered to their home for self-collection of a nasal swab.27 Samples were tested by polymerase chain reaction (PCR) for the presence of SARS-CoV-2 and 24 other respiratory pathogens (eFigure 1 in Supplement 1), including rhinovirus (distinct from other enteroviruses). Presumed Delta and Omicron variants were identified using S-gene target failure criteria. The eMethods and eTable 1 in Supplement 1 describe the study design, inclusion criteria, and laboratory methods. This case-control study was approved by the University of Washington institutional review board. All participants provided informed consent at enrollment. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for case-control studies.
Variable Definitions
To assess risk factors for infection with SARS-CoV-2 and rhinovirus, we used a test-negative design.28,29,30 Participants who tested positive are referred to as cases, while those who tested negative serve as the control group. The dependent variables in regression models were either SARS-CoV-2 or rhinovirus positivity. Not all samples were tested for non–SARS-CoV-2 pathogens. Therefore, in the main analysis we defined a SARS-CoV-2 case participant as any participant with a positive SARS-CoV-2 result (including coinfections) and a SARS-CoV-2 control participant as any participant with a negative SARS-CoV-2 result. In sensitivity analysis we restricted the sample to those tested for other pathogens and excluded coinfections (eMethods in Supplement 1). A similar logic was applied to presumed Delta and Omicron cases, with control participants drawn from a comparable period as case participants. Rhinovirus case participants were defined as anyone with a positive rhinovirus result, excluding coinfections with SARS-CoV-2. Rhinovirus control participants included participants who had a negative rhinovirus result, after excluding those positive for SARS-CoV-2.
Independent variables missing more than 5% of data were excluded, and for the remaining variables, complete cases were used. Five core sociodemographic variables were included in all models: age, sex, race and ethnicity (Asian [non-Hispanic], Black [non-Hispanic], Hispanic [any race], White [non-Hispanic], and other [includes American Indian or Alaska Native, Native Hawaiian and other Pacific Islander, other, and ≥2 races]), county region (based on Public Use Microdata Area of residence), and social and economic risk index (SERI), a local risk indicator based on census-tract of residence. SERI was developed by Public Health Seattle and King County to describe socioeconomic inequalities and identify communities at increased risk for COVID-19. Details appear in the eMethods in Supplement 1 and a report by Public Health Seattle and King County.31 We included 10 additional demographic and health behavior variables, including close contact with a case (<6 feet away for ≥10 minutes), and indicator variables for 16 symptoms and number of symptoms (≤3 or >3). See eMethods, eFigure 2, and eFigure 3 in Supplement 1 for full list and associations between independent variables. All demographic, health behavior, and symptom variables were based on self-report. We used a categorical variable for time-period: wild-type variant (June 10, 2020, to January 31, 2021), pre-Omicron variants (February 1 to December 11, 2021), Omicron variants (December 12, 2021, to July 27, 2022). For the SARS-CoV-2 model, we included the log of the weekly reported SARS-CoV-2 cases in the county as an external measure of community incidence. For the rhinovirus model, we included the weekly percentage positive of rhinovirus tests from the Pacific Northwest Respiratory Virus Epidemiology Data.32
Statistical Analysis
Following prior work,1,7,8,10,33 we used univariate and multivariable logistic regression to infer associations between SARS-CoV-2 test positivity and the independent variables, and we used forward and backward stepwise Akaike information criterion (AIC) for variable selection. To explore time trends, we tested interactions between each variable and time period (eMethods in Supplement 1). Confidence intervals were based on nonparametric bootstrap (1000 simulations). We used the same approach with the rhinovirus model, but with SARS-CoV-2–specific variables excluded (ie, contact with a SARS-CoV-2 case, prior SARS-CoV-2 infections, and COVID-19 vaccination). Sensitivity analyses based on choice of model structure and controls are provided in the supplement (eMethods, eTable 2, eFigure 4, and eFigure 6 in Supplement 1). Statistical analyses were performed in R version 4.2.1 (R Group for Statistical Computing). All hypothesis tests were 2-sided. We considered P < .05 to be statistically significant.
Results
Study Population
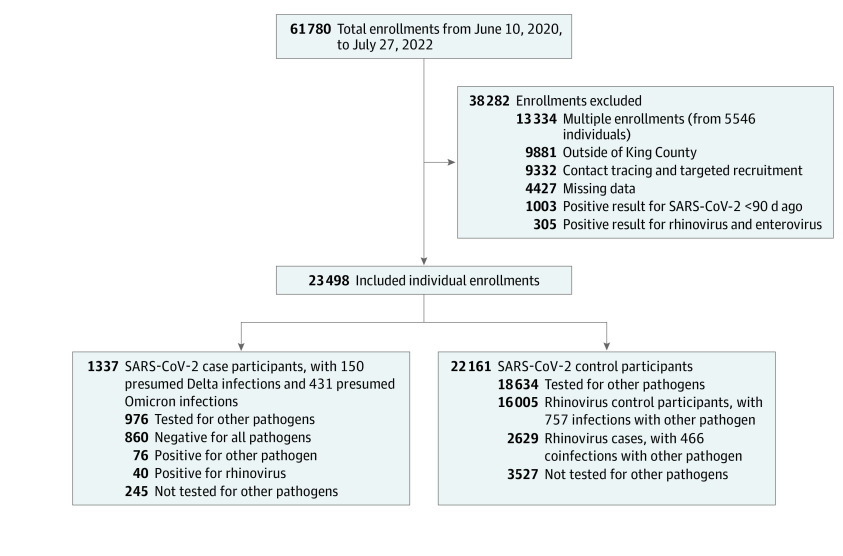
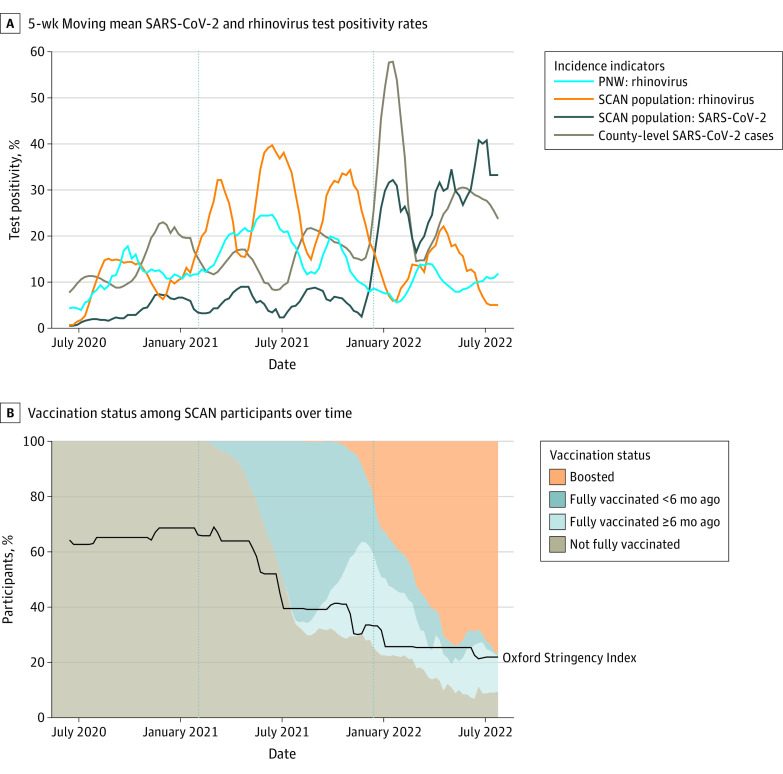
Analyses included data from 23 498 individuals (Figure 1). The median (IQR) age of participants was 34.33 (22.42-45.08) years, 13 878 (59.06%) of participants were female, 4018 (17.10%) identified as Asian, 654 (2.78%) identified as Black, 2193 (9.33%) identified as Hispanic, 7379 (31.40%) resided in south King County, and 6512 (27.71%) resided in a high-risk census tract based on SERI (Table 1). There were 1337 individuals (5.69%) with SARS-CoV-2 positivity, including 40 coinfections with rhinovirus, and 2629 participants (11.19%) with rhinovirus positivity. The percentage of participants positive for SARS-CoV-2 was highest during the Omicron variants period, while the percentage positive for rhinovirus was highest during the pre-Omicron variants period (Figure 2A).34 The percentage of participants vaccinated against COVID-19 increased over the study period as NPIs gradually relaxed (Figure 2B). Among 3829 participants reporting close contact with a confirmed case, social contacts were the most common (1926 [50.38%]), followed by household (1171 [30.58%]) and workplace (873 [22.80%]) contacts, and 141 participants reported more than 1 type of contact.
Figure 1. Total Enrollments, Excluded Enrollments, and Final Sample Size.
Table 1. Demographic Characteristics of Seattle Coronavirus Assessment Network Participants, June 10, 2020, to July 27, 2022.
| Characteristic | Participants, No. (%) | |||
|---|---|---|---|---|
| Wild-type variant period, 6/10/2020 to 1/31/2021 (n = 17 079) | Pre-Omicron variants, 2/1/2021 to 12/11/2021 (n = 4785) | Omicron variants, 12/12/2021 to 7/27/2022 (n = 1634) | Overall, 6/10/2020 to 7/27/20 22 (N = 23 498) | |
| Test results | ||||
| SARS-CoV-2 casesa | 579 (3.39) | 283 (5.91) | 475 (29.07) | 1337 (5.69) |
| Presumed Delta | 0 | 142 (2.97) | 8 (0.49) | 150 (0.64) |
| Presumed Omicron | 0 | 0 | 431 (26.38) | 431 (1.83) |
| Rhinovirus casesb | 1311 (7.68) | 1169 (24.43) | 149 (9.12) | 2629 (11.19) |
| Other pathogen positivec | 402 (2.35) | 248 (5.18) | 107 (6.55) | 757 (3.22) |
| Pan-negative | 11 416 (66.84) | 2985 (62.38) | 846 (51.77) | 15 247 (64.89) |
| Not tested for other pathogens | 3580 (20.96) | 109 (2.28) | 83 (5.08) | 3772 (16.05) |
| Age, y | ||||
| Median (IQR) | 35.08 (23.50-45.83) | 32.00 (16.25- 42.33) | 34.75 (23.25-45.08) | 34.33 (22.42-45.08) |
| 12-50 | 11 857 (69.42) | 3102 (64.83) | 1147 (70.20) | 16 106 (68.54) |
| <12 | 1961 (11.48) | 985 (20.59) | 187 (11.44) | 3133 (13.33) |
| ≥50 | 3261 (19.09) | 698 (14.59) | 300 (18.36) | 4259 (18.12) |
| Sex | ||||
| Female | 9978 (58.42) | 2879 (60.17) | 1021 (62.48) | 13 878 (59.06) |
| Male | 7101 (41.58) | 1906 (39.83) | 613 (37.52) | 9620 (40.94) |
| Race and ethnicity | ||||
| Asian, non-Hispanic | 2791 (16.34) | 832 (17.39) | 395 (24.17) | 4018 (17.10) |
| Black, non-Hispanic | 405 (2.37) | 196 (4.10) | 53 (3.24) | 654 (2.78) |
| Hispanic, any race | 1575 (9.22) | 457 (9.55) | 161 (9.85) | 2193 (9.33) |
| White, non-Hispanic | 10 918 (63.93) | 2824 (59.02) | 871 (53.30) | 14 613 (62.19) |
| Otherd | 1390 (8.14) | 476 (9.95) | 154 (9.42) | 2020 (8.60) |
| County region | ||||
| North | 12 191 (71.38) | 2992 (62.53) | 936 (57.28) | 16 119 (68.60) |
| South | 4888 (28.62) | 1793 (37.47) | 698 (42.72) | 7379 (31.40) |
| SERI | ||||
| Low | 6915 (40.49) | 1760 (36.78) | 494 (30.23) | 9169 (39.02) |
| Moderate | 5773 (33.80) | 1511 (31.58) | 533 (32.62) | 7817 (33.27) |
| High | 4391 (25.71) | 1514 (31.64) | 607 (37.15) | 6512 (27.71) |
| Vaccination status | ||||
| Not fully vaccinated | 17 079 (100) | 2719 (56.82) | 298 (18.24) | 20 096 (85.52) |
| Fully vaccinated >6 mo ago | 0 | 413 (8.63) | 335 (20.50) | 748 (3.18) |
| Fully vaccinated <6 mo ago | 0 | 1567 (32.75) | 204 (12.48) | 1771 (7.54) |
| Boosted | 0 | 86 (1.80) | 797 (48.78) | 883 (3.76) |
| Household size | ||||
| <5 people | 14 162 (82.92) | 3931 (82.15) | 1362 (83.35) | 19 455 (82.79) |
| ≥5 people | 2917 (17.08) | 854 (17.85) | 272 (16.65) | 4043 (17.21) |
| Prior test history | ||||
| Prior positive test >90 d ago | 80 (0.47) | 121 (2.53) | 91 (5.57) | 292 (1.24) |
| No prior testing | 6619 (38.76) | 3461 (72.33) | 1231 (75.34) | 11 311 (48.14) |
| Symptoms, median (IQR), No. | 3.0 (1.0-5.0) | 3.0 (2.0-6.0) | 4.0 (2.0-7.0) | 3.0 (2.0-5.0) |
Abbreviation: SERI, social and economic risk index.
Includes coinfections with other pathogens.
Includes coinfections with other pathogens, excluding SARS-CoV-2.
Not including coinfections with SARS-CoV-2 or rhinovirus.
Includes American Indian or Alaska Native, Native Hawaiian and other Pacific Islander, other, and 2 or more races.
Figure 2. Trends in Viral Circulation, Nonpharmaceutical Interventions, and Vaccination.
A, The 5-week moving average of the percentage of samples positive for SARS-CoV-2 and rhinovirus in the Seattle Coronavirus Assessment Network (SCAN) study population over time, the square root of the reported SARS-CoV-2 cases in King County divided by 3 (for easier visualization), and the percentage of positive rhinovirus tests in the Pacific Northwest (PNW) surveillance system. Vertical dashed lines separate time-periods (wild-type variant, pre-Omicron variants, Omicron variants). B, The percentage of SCAN study participants by vaccination status (not fully vaccinated includes unvaccinated or received an incomplete primary schedule) and the weekly average of the Oxford Stringency Index in Washington State,34 which reflects the strength of interventions on a scale of 0% to 100%. Dashed lines separate time-periods (wild-type variant, pre-Omicron variants, Omicron variants).
Risk Factors and Symptoms Associated With SARS-CoV-2 Infection
In multivariable logistic regression, close contact with a case had the strongest association with a positive SARS-CoV-2 test result (adjusted odds ratio [aOR], 3.89; 95% CI, 3.34-4.57) (Figure 3A; eTable 3 in Supplement 1), but this association dropped to 1.75 (95% CI, 0.97-3.25) during the Omicron variants period (eTable 4 in Supplement 1). In a subanalysis accounting for vaccination status of the contact from October 2021 to July 2022, we found that participants reporting contact with a vaccinated person with SARS-CoV-2 were less likely to test positive than participants reporting contact with an unvaccinated person with SARS-CoV-2 (aOR, 2.03 [95% CI, 1.56-2.76] vs 4.04 [95% CI, 2.39-7.23]) (Table 2). However, there was no significant difference in the risk of infection after exposure to an asymptomatic or symptomatic case (Table 2). Black (aOR, 2.03; 95% CI, 1.46-2.77) and Hispanic (aOR, 2.07; 95% CI, 1.73-2.50) participants as well as those living in high-risk SERI census tracts (aOR, 1.49, 95%CI 1.19-1.88), households with 5 or more people (aOR, 1.40; 95% CI, 1.20-1.63), or reporting travel (eg, international travel: aOR, 2.30; 95% CI, 1.38-3.49) in the last 14 days had higher odds of testing positive than White participants, those living in low-risk SERI census tracts, households with fewer than 5 people, and no travel history. Participants living in south King County and children younger than 12 years had higher odds of testing positive than participants from north King County or participants aged 12 to 50 years during the wild-type variant period, but this was not statistically significant during later periods (eTable 4 in Supplement 1). In a sensitivity analysis using control participants who tested negative for all pathogens (pan-negative), the interaction between time period and age lost significance, and the risk associated with younger age was higher than when the control group included participants positive for other pathogens (eFigure 4 in Supplement 1). Participants who were fully vaccinated within the last 6 months or had received a booster dose, self-reported a previous positive test more than 90 days ago, or reported attending or working at a school in the past 14 days were all significantly less likely to test positive than those who were not fully vaccinated, had never tested positive, or did not attend or work at a school. Among symptoms, loss of smell or taste (aOR, 3.49; 95% CI, 2.77-4.41), cough (aOR, 2.51; 95% CI, 2.17-2.97), and fever (aOR, 2.37; 95% CI, 1.99-2.82) were the most associated with test positivity (eFigure 5 in Supplement 1).
Figure 3. Risk Factors and Symptoms Associated With SARS-CoV-2 and Rhinovirus Infection.
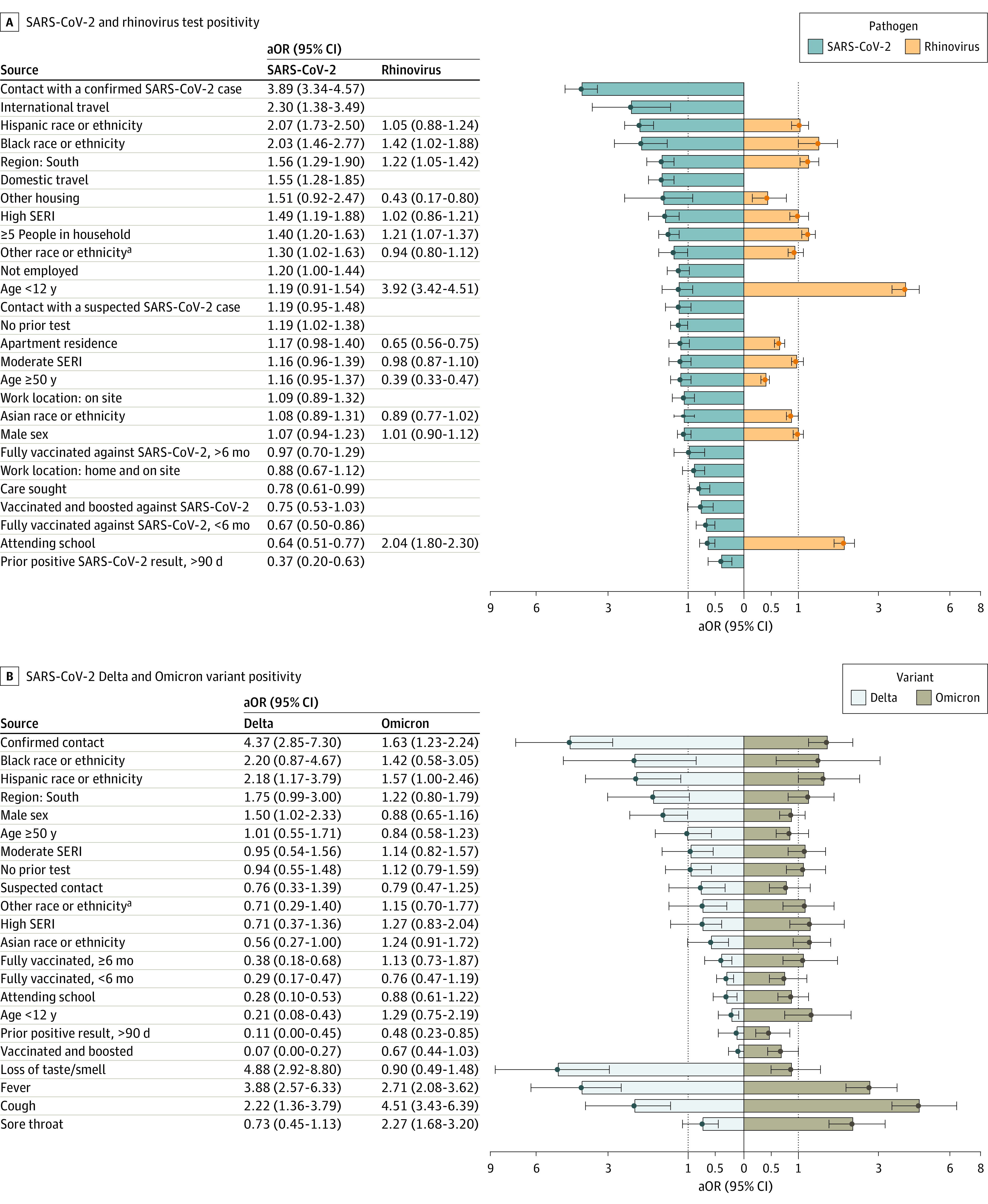
A, Adjusted odds ratios (aORs) and 95% CIs for risk factors associated with SARS-CoV-2 and rhinovirus positivity. Odds ratios are ordered from highest to lowest for SARS-CoV-2. Missing bars indicate the variable was not included in the model a priori or dropped during model selection. Bars touching the dashed vertical line are not statistically significant. B, Separate models were run for the risk of test positivity with Delta and Omicron.
aOther race and ethnicity includes American Indian or Alaska Native, Native Hawaiian and other Pacific Islander, other, and 2 or more races.
Table 2. Odds Ratios Associated With a Positive SARS-CoV-2 Test for Participants Reporting Contact With an Individual With SARS-CoV-2 in the Past 2 Weeks Based on Vaccination Status and Reported Symptoms of the Contact.
| Date | Participants, No. | OR (95% CI) | P valueb | OR (95% CI) | P valuec | ||
|---|---|---|---|---|---|---|---|
| Contact with vaccinated casea | Contact with unvaccinated casea | Contact with asymptomatic casea | Contact with symptomatic casea | ||||
| October 2021 –July 2022d,e | 2355 | 2.03 (1.56-2.76) | 4.04 (2.39-7.23) | .04 | 1.80 (1.23-2.67) | 2.78 (2.07-3.91) | .15 |
| December 2021– July 2022f,g | 1589 | 1.68 (1.26-2.31) | 2.77 (1.54-5.31) | .33 | 1.44 (0.95-2.23) | 2.16 (1.55-3.04) | .27 |
Reference group is participants not reporting contact with a case.
P value for comparison between unvaccinated contact and vaccinated contact using Tukey honest significant difference test.
P value for comparison between symptomatic contact and asymptomatic contact using Tukey honest significant difference test.
Adjusted for age, sex, race and ethnicity, region, social and economic risk index, cough, sore throat, fever, loss of smell or taste, attending or working at school, prior positive test, vaccination status of participant, log of cases reported in community, and dominant variant.
Includes all cases within date range (Delta, Omicron, and undetermined).
Adjusted for age, sex, race and ethnicity, region, social and economic risk index, cough, sore throat, fever, loss of smell or taste, attending or working at school, prior positive test, vaccination status of participant, and log of cases reported in community.
Includes presumed Omicron cases only.
Differences Between Delta and Omicron Variants
We found several notable differences in analysis comparing the Delta and Omicron variants (Figure 3B). Reporting contact with a case was associated with higher odds of testing positive for Delta (aOR, 4.37; 95% CI, 2.85-7.30) compared with Omicron (aOR, 1.63; 95% CI, 1.23-2.24). Similarly, loss of smell and taste was associated with an OR of 4.88 (95% CI 2.29-8.80) for Delta, but was not significant for Omicron, while sore throat was associated with Omicron infection (aOR, 2.27; 95% CI, 1.68-3.20) but not Delta. Being fully vaccinated was associated with a vaccine effectiveness (VE) of 71% against symptomatic infection with Delta in the first six months (aOR, 0.29; 95% CI, 0.17-0.47), and 62% 6 months after vaccination (aOR, 0.38; 95% CI, 0.18-0.68). An additional booster dose increased VE to 93% against Delta (aOR, 0.07; 95% CI, 0.00-0.27). An additional booster increased protection against Omicron relative to being fully vaccinated alone; however, neither were statistically significant. A prior infection provided significant protection against Omicron (aOR, 0.48; 95% CI, 0.23-0.85) and Delta (aOR, 0.11, 95% CI, 0.00-0.45).
Risk Factors and Symptoms Associated With Rhinovirus Infection
There were fewer variables associated with rhinovirus test positivity than SARS-CoV-2 (Figure 3A; eTable 3 in Supplement 1). The greatest risk factor was being younger than 12 years (aOR, 3.92; 95% CI, 3.42-4.51). Similar to SARS-CoV-2, we found a higher odds of rhinovirus positivity among Black participants and those living in south King County or in a household with 5 or more people. In contrast with SARS-CoV-2, attending or working at a school was associated with an increased odds of rhinovirus positivity (aOR, 2.04; 95% CI, 1.80-2.30). Runny or stuffy nose (aOR, 4.58; 95% CI, 4.07-5.21) and sore throat (aOR, 2.05; 95% CI, 1.85-2.30) were the symptoms most associated with rhinovirus test positivity (eFigure 5 in Supplement 1). Additional results are provided in eFigures 6 and 7 in Supplement 1). The percentage positive for rhinovirus was anticorrelated with the percentage positive for SARS-CoV-2 infection in our sample, with 10 to 12 weeks separating the peaks of each virus (eFigure 8 in Supplement 1).
Discussion
We have presented risk factors and symptoms associated with SARS-CoV-2 infection, considered how these associations have changed over a 25-month period, and compared against characteristics associated with rhinovirus test positivity. We found that many characteristics strongly associated with SARS-CoV-2 early in the pandemic attenuated or disappeared during the Omicron variants period. Several sociodemographic characteristics associated with SARS-CoV-2 positivity persisted over time, but geographic disparities weakened. Vaccination and prior infection offered considerably more protection against the Delta variant than the Omicron variant. In contrast to SARS-CoV-2, younger age and attending or working at a school were important risk factors for rhinovirus.
In line with prior studies,4,6 risk of SARS-CoV-2 test positivity was higher for participants reporting close contact with a person with SARS-CoV-2 compared with those reporting no contacts. However, the observed attenuation of this association over time, particularly during the period when Omicron variants predominated, is surprising. It is possible that higher transmissibility of the Omicron variant35,36,37,38 and reduced NPI stringency could have resulted in many asymptomatic, presymptomatic, or unknown contacts with individuals with SARS-CoV-2,39 driving estimates toward the null. Inability to link cases has important implications for contact tracing efforts, and as of February 2022, the Centers for Disease Control and Prevention no longer recommends universal contact tracing or case investigation for COVID-19.40 Alternatively, high levels of vaccination during the Omicron period may have lessened the risk of onward transmission. Other studies have found a lower risk of onward transmission from vaccinated individuals with SARS-CoV-2 compared with unvaccinated individuals,41,42,43 possibly due to a lower viral load among breakthrough cases.44,45 Similarly, we found that contact with a vaccinated individual lowered the odds of testing positive compared with contact with an unvaccinated individual. Although vaccines are less effective against Omicron infection compared with earlier variants,46,47 the extent of primary vaccination and booster coverage in our study population was much higher during the period of Omicron circulation than earlier periods. Modeling studies early in the pandemic suggested that higher vaccine coverage with lower vaccine efficacy might reduce SARS-CoV-2 cases more than lower coverage with a higher efficacy vaccine.48 Importantly, there is evidence that vaccines remain effective against severe outcomes with Omicron,49 but SCAN data are not appropriate to study severe infections.
Our VE estimates for Delta and Omicron are consistent with other studies,46,47,50,51 showing waning protection against symptomatic infection with the Delta variant after 6 months and restoration of protection to greater than 90% following an additional booster dose. We found substantially less protection from vaccination and self-reported prior infection against Omicron, consistent with the immune escape feature of Omicron. We did not account for time since prior infection or time since receipt of booster dose but acknowledge this could affect results due to both waning immunity and trends in variant circulation. There were also notable differences in the reported symptoms between Delta and Omicron. Loss of smell and taste, the early hallmark of SARS-CoV-2 infection, was not significantly associated with Omicron infection, while individuals with Omicron were more likely to report a sore throat, in line with other studies.2,3
We found higher odds of testing positive for SARS-CoV-2 among Black and Hispanic participants and participants from south King County and high-risk census tracts as measured by SERI. Many studies have documented the disproportionate toll of COVID-19 on minority racial and ethnic groups and economically disadvantaged communities,7,8,9,10,11,12,13,14 and some have shown that excess mortality in these populations is largely driven by a higher incidence of infection stemming from disparities in exposure risks.52,53,54 Limited opportunities to work from home or socially distance have been proposed as possible mechanisms driving racial disparities in SARS-CoV-2 exposure.55 We found that onsite work was associated with SARS-CoV-2 infection in univariate analysis, while the association with household size remained in the adjusted model and persisted in all time-periods. We did not have data for other variables that may influence disparities in exposure risk, such as household crowding (people per room), household income, and access to health care, transportation, or personal protective equipment, but acknowledge these could be important variables to explain the higher odds of infection among Black and Hispanic participants in our study. Moreover, many sociodemographic variables are interrelated, and reconstruction of the causal pathways that affect infection and the total contribution of each variable in our study population would require different approaches than used here. We also note that SERI is a localized measure, developed specifically to identify communities at increased risk for COVID-19 and may not apply in other settings or to other health outcomes.
We found that geographic differences between north and south King County (more and less affluent populations, respectively) gradually diminished. Mixing between north and south King County increased after stay-at-home orders were lifted in June 2020, which may have homogenized risk of exposure over time, particularly with the introduction of the highly transmissible Omicron variant. Overall, the attenuation of geographic and sociodemographic disparities in the risk of infection would be expected during SARS-CoV-2’s transition to endemicity, where early infection of high-exposure groups confers immunity to later epidemic waves, with few eventually escaping infection. Although prior infection offered less protection against Omicron than earlier variants, test positivity was still lower among individuals with previous infection.
Odds of SARS-CoV-2 infection in children younger than 12 years were slightly higher than for participants aged 12 to 50 years in the wild-type variant period but not in the later periods, despite low levels of vaccination in children even after they became eligible. We cannot rule out that changes in sampling over the study period could affect these results; however, restricting analysis to pan-negative controls removed this decreasing trend. This suggests that the rebound of rhinovirus later in the pandemic may have affected the propensity for children to test positive for SARS-CoV-2 in our study. Accounting for virus cocirculation and potential interactions between pathogens will be an important aspect of ascertaining risk for respiratory infections in the postpandemic period. Furthermore, working at or attending school in the 2 weeks prior to enrolling in SCAN was associated with lower odds of SARS-CoV-2 test positivity, particularly during the pre-Omicron variants period, which coincides with the start of the school year in fall 2021. In general, studies have shown that NPIs and frequent testing can reduce the occurrence of outbreaks in schools.56,57 However, when strong interventions are in place in the work or school environment, protective associations can reflect reverse causality, a phenomenon also reported with onsite work in the United Kingdom.1 Children and staff allowed to attend school have to be free of symptoms and lack recent contact with a SARS-CoV-2 case and are thus unlikely to test positive.
In contrast to SARS-CoV-2, younger age and school attendance were highly associated with rhinovirus infection. The persistent circulation of rhinovirus in children throughout the SARS-CoV-2 pandemic, despite declines in other pathogens, has been widely observed.18,19,20,21 Accordingly, we detected more rhinovirus than SARS-CoV-2 infections but very few other viral pathogens. Other studies have found evidence of viral interaction between SARS-CoV-2 and rhinovirus on individual-level data58 and cellular-level data,59 which may explain the anticorrelation in circulation we observed at the population level. Few studies have considered risk factors beyond age for rhinovirus infection. Rhinovirus infection was more likely in Black participants and participants living in south King County or households with 5 or more people. This was less pronounced than the differences observed for SARS-CoV-2 infection but suggests that similar sociodemographic inequalities also modulate the burden of endemic respiratory pathogens.53 Examining sociodemographic risk factors for other endemic pathogens, and how they may interact with SARS-CoV-2 circulation, is an important consideration for future analyses.
Strengths and Limitations
A strength of the test-negative design is that it inherently accounts for health care–seeking behavior in both case and control participants, but as a result, it may produce estimates that are not generalizable to populations with different care-seeking behavior.60 Accordingly, our study participants were not a representative sample of the King County population. Eligibility criteria, testing demand, and the composition of the study participants changed over the course of the study (Table 1; eMethods in Supplement 1). Participation in less-affluent south King County increased over time because of targeted efforts to broaden access to testing. This may affect the associations we identified, particularly when considering time trends. Furthermore, due to our online enrollment scheme, our participants were disproportionately female and middle-aged. Other internet-based surveillance studies have noted similar patterns.61 This limited our ability to examine narrower age groupings to detect risk factors in young children and older adults. Another concern is that differences in testing behaviors could create spurious associations between SARS-CoV-2 infection and sex62; however, we did not find this in our data.
All data were based on self-report, and while we expect that misclassification is minimal, we acknowledge that this may affect our results. Misclassification could be quite important for prior infection since testing propensity was low in the early phase of the pandemic. Additionally, we relied on unsupervised, self-collection of nasal swabs to determine test positivity, which could result in misclassification of our outcome variables. However, prior work supports this as a viable strategy for studying respiratory pathogens in the community.27 Furthermore, both SARS-CoV-2 and rhinovirus can be detected by PCR for several weeks to months after acute infection,63,64 and our study may have included persistent infections for which the collected data on health behaviors may be less relevant. Our study included mostly symptomatic individuals, which should limit this issue,65,66 but some misclassification is still possible.
Conclusions
This case-control study identified several important changes in the risk factors and symptoms associated with SARS-CoV-2 test positivity. Reported symptoms shifted between the Delta and Omicron variants, while vaccines and previous infections offered reduced protection against later symptomatic infections. High levels of SARS-CoV-2 circulation during the Omicron variants period weakened the association between test positivity and known contact with a case, which has important implications for contact tracing. Importantly, racial disparities identified early in the pandemic have persisted and also appeared to affect common pathogens like rhinovirus. Continued efforts to understand the drivers of respiratory virus infections in the postpandemic period remain important to improve targeted interventions.
eMethods.
eFigure 1. Samples Collected Over Time
eTable 1. Comparison of Participants Who Enrolled in SCAN Multiple Times vs Participants Who Enrolled Only Once
eTable 2. Comparison of the Odds Ratios and Selected Variables Under Different Regression Approaches
eFigure 2. Associations Between Demographic Variables
eFigure 3. Correlation Matrix for Categorical Variables Using Cramer’s V
eFigure 4. Sensitivity Analysis for Risk Factors and Symptoms Associated With SARS-CoV-2 Positivity Using Pan-Negative Control Participants and Excluding Asymptomatic Participants
eFigure 5. Symptoms Associated With Rhinovirus and SARS-CoV-2 Positivity
eFigure 6. Sensitivity Analysis for Risk Factors and Symptoms Associated With Rhinovirus Positivity Using Pan-Negative Control Participants and Excluding Asymptomatic Participants
eTable 3. Unadjusted Odds Ratios for SARS-CoV-2 and Rhinovirus Test Positivity
eTable 4. Adjusted Odds Ratios for SARS-CoV-2 Test Positivity by Time Period for Variables With Significant Interactions With Time Period
eFigure 7. Adjusted Odds Ratios for Rhinovirus Test Positivity for Each Time Period for Variables With Significant Interactions With Time Period
eFigure 8. Cross-Correlation Between Rhinovirus and SARS-CoV-2 Test Positivity in the SCAN Study
eReferences.
Data Sharing Statement
References
- 1.Pritchard E, Jones J, Vihta KD, et al. ; COVID-19 Infection Survey Team . Monitoring populations at increased risk for SARS-CoV-2 infection in the community using population-level demographic and behavioural surveillance. Lancet Reg Health Eur. 2022;13:100282. doi: 10.1016/j.lanepe.2021.100282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618-1624. doi: 10.1016/S0140-6736(22)00327-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitaker M, Elliott J, Bodinier B, Barclay W. Variant-specific symptoms of COVID-19 among 1,542,510 people in England. medRxiv. Preprint posted online May 23, 2022. doi: 10.1101/2022.05.21.22275368 [DOI] [PMC free article] [PubMed]
- 4.Fisher KA, Tenforde MW, Feldstein LR, et al. ; IVY Network Investigators; CDC COVID-19 Response Team . Community and close contact exposures associated with COVID-19 among symptomatic adults ≥18 years in 11 outpatient health care facilities—United States, July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1258-1264. doi: 10.15585/mmwr.mm6936a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher KA, Olson SM, Tenforde MW, et al. ; IVY Network Investigators; CDC COVID-19 Response Team . Telework before illness onset among symptomatic adults aged ≥18 years with and without COVID-19 in 11 outpatient health care facilities—United States, July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1648-1653. doi: 10.15585/mmwr.mm6944a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenforde MW, Fisher KA, Patel MM. Identifying COVID-19 risk through observational studies to inform control measures. JAMA. 2021;325(14):1464-1465. doi: 10.1001/jama.2021.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo CH, Nguyen LH, Drew DA, et al. ; COPE Consortium . Race, ethnicity, community-level socioeconomic factors, and risk of COVID-19 in the United States and the United Kingdom. EClinicalMedicine. 2021;38:101029. doi: 10.1016/j.eclinm.2021.101029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vahidy FS, Nicolas JC, Meeks JR, et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. 2020;10(8):e039849. doi: 10.1136/bmjopen-2020-039849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figueroa JF, Wadhera RK, Lee D, Yeh RW, Sommers BD. Community-level factors associated with racial and ethnic disparities in COVID-19 rates in Massachusetts. Health Aff (Millwood). 2020;39(11):1984-1992. doi: 10.1377/hlthaff.2020.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mannheim J, Konda S, Logan LK. Racial, ethnic and socioeconomic disparities in SARS-CoV-2 infection amongst children. Paediatr Perinat Epidemiol. 2021;2022:1-10. doi: 10.1111/ppe.12865 [DOI] [PubMed] [Google Scholar]
- 11.Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72(4):703-706. doi: 10.1093/cid/ciaa815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore JT, Ricaldi JN, Rose CE, et al. ; COVID-19 State, Tribal, Local, and Territorial Response Team . Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5-18, 2020—22 states, February-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(33):1122-1126. https://pubmed.ncbi.nlm.nih.gov/32817602/. doi: 10.15585/mmwr.mm6933e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dyke ME, Mendoza MCB, Li W, et al. Racial and ethnic disparities in COVID-19 incidence by age, sex, and period among persons aged <25 years—16 U.S. jurisdictions, January 1-December 31, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(11):382-388. doi: 10.15585/mmwr.mm7011e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rozenfeld Y, Beam J, Maier H, et al. A model of disparities: risk factors associated with COVID-19 infection. Int J Equity Health. 2020;19(1):126. doi: 10.1186/s12939-020-01242-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic—United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70(29):1013-1019. doi: 10.15585/mmwr.mm7029a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased influenza activity during the COVID-19 pandemic—United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1305-1309. doi: 10.15585/mmwr.mm6937a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhteg K, Amadi A, Forman M, Mostafa HH. Circulation of non–SARS-CoV-2 respiratory pathogens and coinfection with SARS-CoV-2 amid the COVID-19 pandemic. Open Forum Infect Dis. 2021;9(3):ofab618. doi: 10.1093/ofid/ofab618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groves HE, Piché-Renaud PP, Peci A, et al. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: a population-based study. Lancet Reg Health Am. 2021;1:100015. doi: 10.1016/j.lana.2021.100015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh DY, Buda S, Biere B, et al. Trends in respiratory virus circulation following COVID-19-targeted nonpharmaceutical interventions in Germany, January - September 2020: analysis of national surveillance data. Lancet Reg Health Eur. 2021;6:100112. doi: 10.1016/j.lanepe.2021.100112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuitunen I, Artama M, Haapanen M, Renko M. Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions: a nationwide register study in Finland. J Med Virol. 2021;93(10):6063-6067. doi: 10.1002/jmv.27180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole S, Brendish NJ, Tanner AR, Clark TW. Physical distancing in schools for SARS-CoV-2 and the resurgence of rhinovirus. Lancet Respir Med. 2020;8(12):e92-e93. doi: 10.1016/S2213-2600(20)30502-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitanovski S, Horemheb-Rubio G, Adams O, et al. ; Respiratory Virus Network . Rhinovirus prevalence as indicator for efficacy of measures against SARS-CoV-2. BMC Public Health. 2021;21(1):1178. doi: 10.1186/s12889-021-11178-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szilagyi PG, Blumkin A, Treanor JJ, et al. Incidence and viral aetiologies of acute respiratory illnesses (ARIs) in the United States: a population-based study. Epidemiol Infect. 2016;144(10):2077-2086. doi: 10.1017/S0950268816000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. doi: 10.1056/NEJMoa1500245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. doi: 10.1056/NEJMoa1405870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs SE, Lamson DM, St George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev. 2013;26(1):135-162. doi: 10.1128/CMR.00077-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim AE, Brandstetter E, Wilcox N, et al. Evaluating specimen quality and results from a community-wide, home-based respiratory surveillance study. J Clin Microbiol. 2021;59(5):e02934-20. doi: 10.1128/JCM.02934-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandenbroucke JP, Brickley EB, Vandenbroucke-Grauls CMJE, Pearce N. The evolving usefulness of the test-negative design in studying risk factors for COVID-19. Epidemiology. 2021;33(2)e7-e8. doi: 10.1097/EDE.0000000000001438 [DOI] [PubMed] [Google Scholar]
- 29.Vandenbroucke JP, Pearce N. Test-negative designs: Differences and commonalities with other case-control studies with “other patient” controls. Epidemiology. 2019;30(6):838-844. doi: 10.1097/EDE.0000000000001088 [DOI] [PubMed] [Google Scholar]
- 30.Vandenbroucke JP, Brickley EB, Vandenbroucke-Grauls CMJE, Pearce N. A test-negative design with additional population controls can be used to rapidly study causes of the SARS-COV-2 epidemic. Epidemiology. 2020;31(6):836-843. doi: 10.1097/EDE.0000000000001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Public Health Seattle & King County . Social & economic inequalities in COVID-19 testing and outcomes in King County census tracts. July 16, 2021. Accessed November 1, 2022. https://kingcounty.gov/depts/health/covid-19/data/~/media/depts/health/communicable-diseases/documents/C19/king-county-seri-technical-report.ashx
- 32.University of Washington. Pacific Northwest respiratory viral epidemiology data. Accessed July 21, 2022. http://depts.washington.edu/uwviro/respiratory-viral-epidemiology-data/
- 33.Jacob JT, Baker JM, Fridkin SK, et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open. 2021;4(3):e211283. doi: 10.1001/jamanetworkopen.2021.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.University of Oxford. COVID-19 government response tracker. Accessed November 1, 2022. https://www.bsg.ox.ac.uk/research/covid-19-government-response-tracker
- 35.Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457-466.e4. doi: 10.1016/j.cell.2021.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaguza C, Coppi A, Earnest R, et al. Rapid emergence of SARS-CoV-2 Omicron variant is associated with an infection advantage over Delta in vaccinated persons. Med (N Y). 2022;3(5):325-334.e4. doi: 10.1016/j.medj.2022.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott P, Eales O, Steyn N, et al. Twin peaks: the Omicron SARS-CoV-2 BA.1 and BA.2 epidemics in England. Science. 2022;376(6600):eabq4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Rocklöv J. The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta. J Travel Med. 2022;29(3):1-4. doi: 10.1093/jtm/taac037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joung SY, Ebinger JE, Sun N, et al. Awareness of SARS-CoV-2 Omicron variant infection among adults with recent COVID-19 seropositivity. JAMA Netw Open. 2022;5(8):e2227241. doi: 10.1001/jamanetworkopen.2022.27241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Centers for Disease Control and Prevention . Prioritizing case investigation and contact tracing for COVID-19. Updated February 28, 2022. Accessed July 27, 2022. https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/prioritization.html
- 41.Eyre DW, Taylor D, Purver M, et al. Effect of COVID-19 vaccination on transmission of Alpha and Delta variants. N Engl J Med. 2022;386(8):744-756. doi: 10.1056/NEJMoa2116597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyngse FP, Kirkeby CT, Denwood M, et al. Transmission of SARS-CoV-2 Omicron VOC subvariants BA. 1 and BA. 2: evidence from Danish households. medRxiv. Preprint posted online January 30, 2022. doi: 10.1101/2022.01.28.22270044 [DOI]
- 43.Prunas O, Warren JL, Crawford FW, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. Science. 2022;375(6585):1151-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Relative infectiousness of SARS-CoV-2 vaccine breakthrough infections, reinfections, and primary infections. Nat Commun. 2022;13(1):532. doi: 10.1038/s41467-022-28199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puhach O, Adea K, Hulo N, et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med. 2022;28(7):1491-1500. doi: 10.1038/s41591-022-01816-0 [DOI] [PubMed] [Google Scholar]
- 46.Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532-1546. doi: 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA. 2022;327(7):639-651. doi: 10.1001/jama.2022.0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel MD, Rosenstrom E, Ivy JS, et al. Association of simulated COVID-19 vaccination and nonpharmaceutical interventions with infections, hospitalizations, and mortality. JAMA Netw Open. 2021;4(6):e2110782. doi: 10.1001/jamanetworkopen.2021.10782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nyberg T, Ferguson NM, Nash SG, et al. ; COVID-19 Genomics UK (COG-UK) consortium . Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi: 10.1016/S0140-6736(22)00462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407-1416. doi: 10.1016/S0140-6736(21)02183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health Am. 2022;9:100198. doi: 10.1016/j.lana.2022.100198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths a systematic review. Ann Intern Med. 2021;174(3):362-373. doi: 10.7326/M20-6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muñoz-Price LS, Nattinger AB, Rivera F, et al. Racial disparities in incidence and outcomes among patients with COVID-19. JAMA Netw Open. 2020;3(9):e2021892. doi: 10.1001/jamanetworkopen.2020.21892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holden TM, Simon MA, Arnold DT, Halloway V, Gerardin J. Structural racism and COVID-19 response: higher risk of exposure drives disparate COVID-19 deaths among Black and Hispanic/Latinx residents of Illinois, USA. BMC Public Health. 2022;22(1):312. doi: 10.1186/s12889-022-12698-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466-2467. doi: 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nemoto N, Dhillon S, Fink S, et al. Evaluation of test to stay strategy on secondary and tertiary transmission of SARS-CoV-2 in K-12 schools—Lake County, Illinois, August 9-October 29, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):1778-1781. doi: 10.15585/mmwr.mm705152e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harris-McCoy K, Lee VC, Munna C, Kim AA. Evaluation of a test to stay strategy in transitional kindergarten through grade 12 schools—Los Angeles County, California, August 16-October 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):1773-1777. doi: 10.15585/mmwr.mm705152e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burstein R, Althouse BM, Adler A, et al. Interactions among 17 respiratory pathogens: a cross-sectional study using clinical and community surveillance data. medRxiv. Preprint posted online February 6, 2022. doi: 10.1101/2022.02.04.22270474 [DOI]
- 59.Dee K, Goldfarb DM, Haney J, et al. Human rhinovirus infection blocks severe acute respiratory syndrome coronavirus 2 replication within the respiratory epithelium: implications for COVID-19 epidemiology. J Infect Dis. 2021;224(1):31-38. doi: 10.1093/infdis/jiab147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dean NE, Hogan JW, Schnitzer ME. COVID-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385(15):1431-1433. doi: 10.1056/NEJMe2113151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baltrusaitis K, Santillana M, Crawley AW, Chunara R, Smolinski M, Brownstein JS. Determinants of participants’ follow-up and characterization of representativeness in flu near you, a participatory disease surveillance system. JMIR Public Health Surveill. 2017;3(2):e18. doi: 10.2196/publichealth.7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Accorsi EK, Qiu X, Rumpler E, et al. How to detect and reduce potential sources of biases in studies of SARS-CoV-2 and COVID-19. Eur J Epidemiol. 2021;36(2):179-196. doi: 10.1007/s10654-021-00727-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sundell N, Andersson L-M, Brittain-Long R, et al. PCR detection of respiratory pathogens in asymptomatic and symptomatic adults. J Clin Microbiol. 2019;57(1):e00716-e00718. doi: 10.1128/JCM.00716-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249-2251. doi: 10.1001/jama.2020.8259 [DOI] [PubMed] [Google Scholar]
- 65.Wright PF, Deatly AM, Karron RA, et al. Comparison of results of detection of rhinovirus by PCR and viral culture in human nasal wash specimens from subjects with and without clinical symptoms of respiratory illness. J Clin Microbiol. 2007;45(7):2126-2129. doi: 10.1128/JCM.02553-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loeffelholz MJ, Trujillo R, Pyles RB, et al. Duration of rhinovirus shedding in the upper respiratory tract in the first year of life. Pediatrics. 2014;134(6):1144-1150. doi: 10.1542/peds.2014-2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Samples Collected Over Time
eTable 1. Comparison of Participants Who Enrolled in SCAN Multiple Times vs Participants Who Enrolled Only Once
eTable 2. Comparison of the Odds Ratios and Selected Variables Under Different Regression Approaches
eFigure 2. Associations Between Demographic Variables
eFigure 3. Correlation Matrix for Categorical Variables Using Cramer’s V
eFigure 4. Sensitivity Analysis for Risk Factors and Symptoms Associated With SARS-CoV-2 Positivity Using Pan-Negative Control Participants and Excluding Asymptomatic Participants
eFigure 5. Symptoms Associated With Rhinovirus and SARS-CoV-2 Positivity
eFigure 6. Sensitivity Analysis for Risk Factors and Symptoms Associated With Rhinovirus Positivity Using Pan-Negative Control Participants and Excluding Asymptomatic Participants
eTable 3. Unadjusted Odds Ratios for SARS-CoV-2 and Rhinovirus Test Positivity
eTable 4. Adjusted Odds Ratios for SARS-CoV-2 Test Positivity by Time Period for Variables With Significant Interactions With Time Period
eFigure 7. Adjusted Odds Ratios for Rhinovirus Test Positivity for Each Time Period for Variables With Significant Interactions With Time Period
eFigure 8. Cross-Correlation Between Rhinovirus and SARS-CoV-2 Test Positivity in the SCAN Study
eReferences.
Data Sharing Statement