Key Points
Question
Does a weight loss and exercise program in community settings lead to improvement in knee pain in patients with osteoarthritis and overweight or obesity?
Findings
This randomized clinical trial included 823 patients with knee osteoarthritis and overweight or obesity treated with diet and exercise vs an attention control. After 18 months, the adjusted mean difference in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score (range, 0-20) was −0.6, a difference that met statistical significance.
Meaning
In patients with knee osteoarthritis and overweight or obesity, an 18-month program of weight loss and exercise based in community settings, compared with an attention control group, led to a small difference in knee pain of uncertain clinical importance.
Abstract
Importance
Some weight loss and exercise programs that have been successful in academic center–based trials have not been evaluated in community settings.
Objective
To determine whether adaptation of a diet and exercise intervention to community settings resulted in a statistically significant reduction in pain, compared with an attention control group, at 18-month follow-up.
Design, Setting, and Participants
Assessor-blinded randomized clinical trial conducted in community settings in urban and rural counties in North Carolina. Patients were men and women aged 50 years or older with knee osteoarthritis and overweight or obesity (body mass index ≥27). Enrollment (N = 823) occurred between May 2016 and August 2019, with follow-up ending in April 2021.
Interventions
Patients were randomly assigned to either a diet and exercise intervention (n = 414) or an attention control (n = 409) group for 18 months.
Main Outcomes and Measures
The primary outcome was the between-group difference in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) knee pain score (range, 0 [none] to 20 [severe]; minimum clinically important difference, 1.6) over 18 months, tested using a repeated-measures mixed linear model with adjustments for covariates. There were 7 secondary outcomes including body weight.
Results
Among the 823 randomized patients (mean age, 64.6 years; 637 [77%] women), 658 (80%) completed the trial. At 18-month follow-up, the adjusted mean WOMAC pain score was 5.0 in the diet and exercise group (n = 329) compared with 5.5 in the attention control group (n = 316) (adjusted difference, −0.6; 95% CI, −1.0 to −0.1; P = .02). Of 7 secondary outcomes, 5 were significantly better in the intervention group compared with control. The mean change in unadjusted 18-month body weight for patients with available data was −7.7 kg (8%) in the diet and exercise group (n = 289) and −1.7 kg (2%) in the attention control group (n = 273) (mean difference, −6.0 kg; 95% CI, −7.3 kg to −4.7 kg). There were 169 serious adverse events; none were definitely related to the study. There were 729 adverse events; 32 (4%) were definitely related to the study, including 10 body injuries (9 in diet and exercise; 1 in attention control), 7 muscle strains (6 in diet and exercise; 1 in attention control), and 6 trip/fall events (all 6 in diet and exercise).
Conclusions and Relevance
Among patients with knee osteoarthritis and overweight or obesity, diet and exercise compared with an attention control led to a statistically significant but small difference in knee pain over 18 months. The magnitude of the difference in pain between groups is of uncertain clinical importance.
Trial Registration
ClinicalTrials.gov Identifier: NCT02577549
This randomized clinical trial assesses the effect of a diet and exercise intervention vs an attention control condition on knee pain at 18-month follow-up among individuals aged 50 years or older with knee osteoarthritis and overweight or obesity.
Introduction
Osteoarthritis (OA) is the most common and persistent cause of mobility dependence and disability.1 Between 1990 and 2013, OA affected approximately 240 million people worldwide.2 On average, patients with OA live with symptoms for about 26.1 years.3 Optimal care for OA is limited by low rates of prescription of nonpharmacologic interventions, low patient health literacy, and barriers to lifestyle modifications.1,4
Obesity is a modifiable risk factor for knee OA symptoms, and weight loss is a safe, effective nonpharmacologic intervention to improve clinical outcomes.5,6 Clinical guidelines for knee OA encourage diet and exercise to relieve pain and improve function.7,8 These interventions, shown to be efficacious under highly controlled conditions, have not been rigorously tested in community-based settings.5,6,9 The objective of this study was to determine whether a diet and exercise intervention that was found to be effective when administered in an academic center resulted in a statistically significant reduction in OA pain when administered in community settings, compared with an attention control group.
Methods
Study Design
The Weight-Loss and Exercise for Communities With Arthritis in North Carolina (WE-CAN) trial was an assessor-blinded, 3-center (Forsyth County, Haywood County, and Johnston County, North Carolina), randomized clinical trial with 2 parallel groups followed up for 18 months. The coordinating center was located at Wake Forest University and Wake Forest Health Sciences in Forsyth County. A data and safety monitoring board, appointed by the study sponsor (the National Institute of Arthritis and Musculoskeletal and Skin Diseases), provided trial oversight. The institutional review board of Wake Forest Health Sciences approved this protocol (No. 33618). The study protocol was also reviewed and approved by the human subjects committees of the University of North Carolina at Chapel Hill (Supplement 1). Patients provided written informed consent. The trial design has been published previously.10
Patients
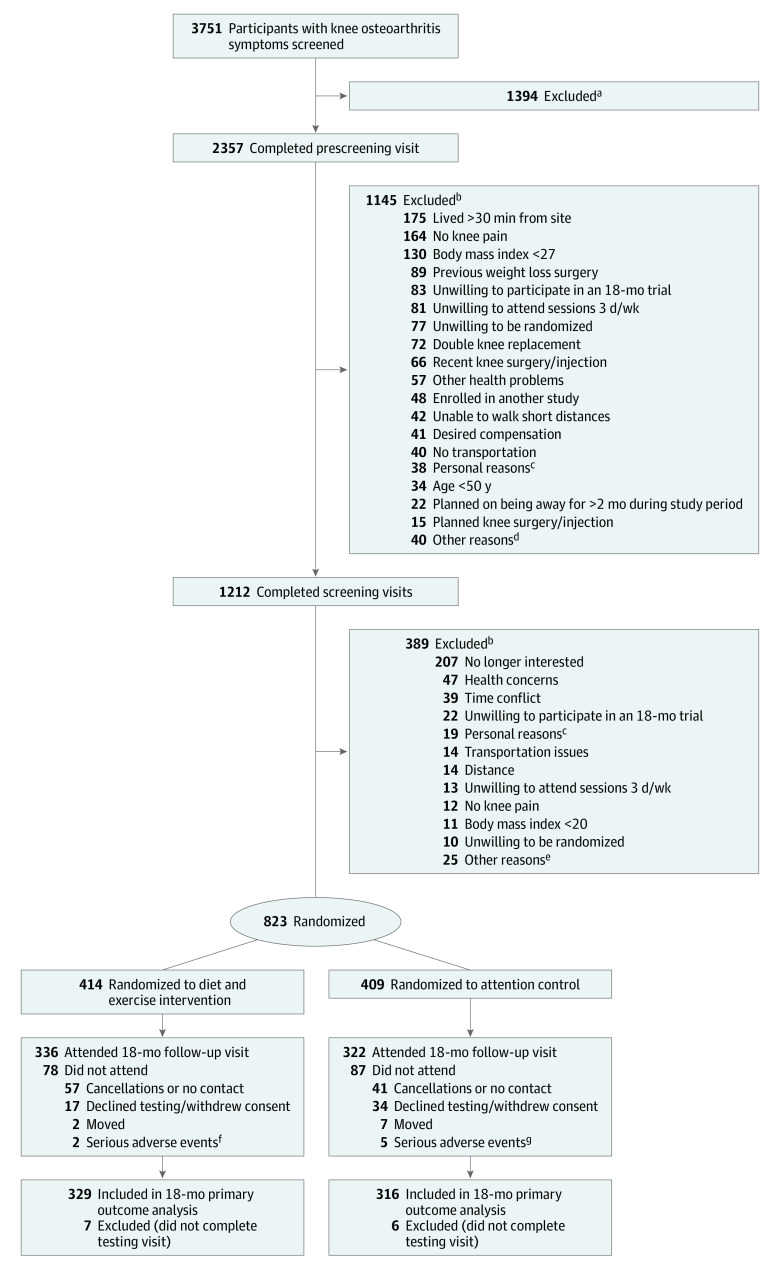
Patients were ambulatory, community-dwelling men and women with either overweight or obesity (body mass index ≥27; calculated as weight in kilograms divided by height in meters squared) who met the American College of Rheumatology clinical criteria for knee OA, which include age of 50 years or older and knee pain on most days of the week with at least 2 of the following: stiffness for less than 30 min/d, crepitus, bony tenderness, bony enlargement, no palpable warmth.11 Study staff trained by study physicians performed the knee examinations. Exclusion criteria included symptomatic coronary artery disease, type 1 diabetes, active cancer (except nonmelanoma skin cancer), body mass index of less than 27, or not meeting American College of Rheumatology clinical criteria for knee OA. Enrollment occurred between May 2016 and August 2019; follow-up ended in April 2021 (Figure 1). Race and ethnicity were documented to help readers evaluate the similarity of this sample to those of other research studies and clinical settings and assess generalizability of results. These variables were obtained by self-report using fixed-category questions with options for write-in answers.
Figure 1. Participant Flow Through the WE-CAN Trial.
aPersons with time conflicts, persons who stated they were not interested, and persons who did not return screening telephone calls.
bParticipants may have been ineligible for more than 1 reason.
cParticipants who said they were unable to participate due to personal circumstances in their lives.
dOther reasons included change in activities due to injury (n = 7), type 1 diabetes (n = 7), staff believed participant was unable or unwilling to change dietary and exercise behaviors (n = 6), contacted study after recruitment closed (n = 5), spouse enrolled in study (n = 4), financial constraints (n = 3), previously enrolled (n = 3), blind (n = 2), unable to speak English (n = 1), community advisory board member (n = 1), and location of center inconvenient (n = 1).
eOther reasons included desire for compensation (n = 6), staff believed participant was unable or unwilling to change dietary and exercise behaviors (n = 5), planned knee surgery/injection (n = 5), planned on being away for more than 2 months during study period (n = 4), enrolled in another study (n = 3), spouse enrolled in study (n = 1), and double knee replacement (n = 1).
fSerious adverse events included cancer (n = 1) and severe cardiovascular complications/hospitalization (n = 1).
gSerious adverse events included cancer (n = 3), motor vehicle collision (n = 1), and cerebral edema (n = 1).
Randomization Procedures
Eligible patients were assigned to either diet and exercise or attention control using a stratified random permuted-block randomization scheme. Strata were defined by pretrial body mass index values (27.0-34.9, 35.0-44.9, or ≥45), sex, and clinic site, with random block sizes of 2, 4, or 6. All patients were analyzed according to their randomization assignment.
Interventions
The trial was conducted in established community facilities in urban (Forsyth County, 800 people per square mile) and rural (Haywood County, 100 people per square mile; Johnston County, 200 people per square mile) counties in North Carolina.12 The community centers where interventions were conducted included a medical mall comprising a variety of outpatient practices, recreation center, rural hospital-affiliated community fitness center, YMCA, local gymnasium, church recreation facility, and community healthy lifestyle program. Interventionists were hired within each county and surrounding area, had at least a bachelor’s degree in a health-related field, and were subsequently trained by experienced coordinating center staff who tailored the instruction to the local facilities.
Patients selected from study alternatives for a calorie-restricted diet plan, which consisted of distribution of low-calorie recipes for a reduced-calorie diet and use of meal replacements to supplement low-calorie meals and foods of the patient’s choice. A meal replacement provided to participants (Lean Shakes, GNC) consisted of a nutritional powder that could be mixed with water or other healthy liquids and was 1 option to replace 1 or 2 traditional meals per day for the first 6 months and could replace 1 meal per day during months 7 to 18. The initial diet was designed to attain an energy intake deficit of 800 to 1000 kcal/d from the estimated energy expenditure (predicted resting metabolism calculated using the Owen equation13 × 1.2 activity factor). An average of 200 kcal/d was expended with exercise for a total imbalance of at least 1000 kcal/d. The lowest intake goal was 1100 kcal for women and 1200 kcal for men. The calorie distribution goal was 15% to 20% protein with at least 1.2 g of protein per kilogram of ideal body weight, less than 30% fat, less than 10% saturated fatty acids, and 45% to 60% carbohydrates.14 The weight loss goal was at least 10% of baseline body weight as recommended by the National Institutes of Health for adults with overweight or obesity.15
The diet intervention staff conducted group and individual sessions. The number of contacts per month consisted of 4 in-person visits for months 0 to 6 (2 group and 2 individual visits), 2 in-person visits for months 7 to 12 (1 group and 1 individual visit), and 1 visit for months 13 to 18 (patient choice of in person or remote). Content emphasized nutrition and behavioral strategies to attain the weight loss goal and was grounded in a social cognitive framework.16
The exercise component consisted of 60-minute sessions 3 days per week for 18 months in a group setting at one of the designated community facilities. Prescribed exercise consisted of aerobic walking (15 minutes), resistance training (20 minutes), a second walking phase (15 minutes), and cooldown (10 minutes). The protocol was consistent with the American College of Sports Medicine guidelines for exercise for older adults.17
The attention control group was modeled after previous control groups5,18,19 and provided social interaction and evidence-based nutrition and health education delivered in five 1-hour, in-person group meetings at months 1, 3, 6, 9, and 15. Information was also provided in paper packets and in individual telephone sessions every other month.
Patients were queried monthly regarding adverse events and encouraged to report adverse events to their interventionist soon after they occurred. Due to the COVID-19 pandemic, beginning March 16, 2020, all intervention sessions were changed to virtual, and data collection was limited to questionnaires that were either completed online, via telephone, or by regular mail. Patients had the option of returning to the center once restrictions were lifted on August 31, 2020. Adherence, defined as the number of sessions completed divided by the number scheduled, was promoted by interventionists using standard techniques developed in a social cognitive framework.16
Measurement and Procedures
Primary Outcome
The primary outcome was the between-group difference in self-reported knee pain at 18-month follow-up using the Likert version of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale,20,21 which assesses knee pain over the last 48 hours.22 The total score ranges from 0 to 20 (higher scores indicate greater pain). The pain categories on a 0- to 20-point scale are 0 (no pain), 2 to 8 (mild pain), ≥8 to 14 (moderate pain), and ≥14 to 20 (severe pain).23 The estimated minimum clinically important difference (MCID) between groups using the half-SD formula was 1.6.24
Secondary Outcomes
Prespecified secondary outcomes at 18 months included the WOMAC function score, which assesses the degree of difficulty with activities of daily living in the last 48 hours (total score range, 0-68; higher scores indicate poorer function; MCID = 6).24,25 Six-minute walk distance assessed the maximum distance a participant could walk along a standardized walkway in 6 minutes (minimum clinically important improvement [MCII] within group, 26-55 m).26 The Short Form 36 measures health-related quality of life using 2 broad summary scores, physical health and mental health, scaled from 0 (worst) to 100 (best), with an MCID of 5 for both scales.27,28 Body weight, height, body mass index, and waist circumference were measured using standard techniques. Waist circumference thresholds for increased health risk are greater than 88 cm for women and greater than 102 cm for men (MCII = −7.5 cm).24,29 Patients recorded pain medication use by questionnaire adapted from the Atherosclerosis Risk in Communities (ARIC) study.30 Secondary outcomes not included in this analysis were blood pressure, self-efficacy, dietary intake, timed stair ascent and descent, Short Physical Performance Battery,31 Knee Injury and Osteoarthritis Outcome Score,32 Intermittent and Constant Osteoarthritis Pain questionnaire,33 and Montreal Cognitive Assessment.34
Post Hoc Outcomes
Post hoc outcomes at 18 months included physical activity level (Physical Activity Scale for the Elderly [PASE])35; number of depressive symptoms Center for Epidemiologic Studies Depression Scale (CES-D)36; the relative risk of achieving a clinically meaningful change in pain of 2 points on the Likert WOMAC pain scale37; the percentage in each group at 18-month follow-up who achieved at least 10% weight loss, achieved at least 5% weight loss, and weighed less compared with baseline; and a comparison of 18-month outcomes including and excluding patients enrolled in the trial when COVID-19 restrictions were instituted.
Sample Size and Power Calculations
The improvement in pain in a control group from a highly controlled efficacy trial with a similar population was 1.2 points (17%).5 Considering that the current trial had fewer exclusion criteria and was anticipated to have more disability than the population in the previous trial,5 a conservative anticipated pain reduction for the control group was estimated at 10% (0.73 units from a baseline mean of 7.3 on a 0- to 20-point scale).5 An estimated 25% within-group improvement from baseline (1.8 units) in the diet and exercise group would result in a 15% (1.0 units) between-group difference. A total sample of 820 (410 per group) participants provided 94% statistical power to detect between-group differences in pain of at least 15% or 1.0 units at 18 months using a 2-sided .05 significance level with 80% retention (2-sample t test; nQuery Advisor38). This sample size was estimated to detect an effect size of 0.23 at 85% power for secondary outcomes with relevant detectable treatment differences. Power was determined based on absolute effects.
Statistical Analysis
Absolute and relative weight changes were compared by group using t tests. The primary hypothesis that the diet and exercise group would have a reduced WOMAC pain score at 18 months compared with the attention control group was tested using a 2-tailed significance level of .05 using contrast statements from a repeated-measures mixed linear model with time (6, 12, and 18 months), randomization group (diet and exercise vs attention control), and the interaction, with additional adjustment for baseline values, body mass index, county, and sex using SAS version 9.4 (SAS Institute Inc). Similarly, repeated-measures analysis used mixed linear models to analyze prespecified secondary outcomes of body weight, waist circumference, WOMAC function score, 6-minute walk distance, pain medication use, and Short Form 36 physical and mental subscales. Each outcome was modeled separately with adjustment for baseline body mass index, sex, county (site), and baseline values of the outcome, and 18-month effectiveness tested based on a 2-tailed significance level of .05. Analysis of outcomes included patients for whom data were available at 1 or more follow-up time points. To account for missing data, prespecified sensitivity analyses were conducted for all patients using multiple imputation methods for missing observations based on baseline data and, when available, follow-up data assuming missing data were missing at random. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. The half-SD formula was used as a proxy to calculate MCII and MCID values when empirical values were not available.24
Post Hoc Analyses
Comparisons between participants who completed the trial and those who did not complete the trial were performed in post hoc analyses to identify factors associated with study dropout. Repeated-measures Poisson regression was used to determine the effect of the intervention, compared with attention control, on the outcome of a WOMAC pain score reduction of 2.0 points. This criterion was recommended by the Osteoarthritis Research Society International as a moderate intervention response on a 0- to 20-point scale, a relative improvement of 15% to 35%.37 To assess whether COVID-19 influenced the treatment effect, analyses were repeated using data points collected before COVID-19 restrictions were implemented on March 16, 2020. Additional post hoc outcomes (PASE, CES-D) were analyzed with mixed models similar to the prespecified secondary outcomes, adjusting for the baseline values of each outcome. Post hoc risk and relative risk for achieving clinically meaningful (2-unit decrease) change in pain were modeled using a generalized estimating equation model with contrasts for group comparisons at 6- and 18-month follow-up, adjusted for baseline pain scores. Scatterplots were created depicting the relationship between weight loss and change in pain for each group. Primary and secondary analyses were repeated using intervention site as a random effect.
Results
Study Cohort
Among the 823 patients randomized (mean age, 64.6 years; 77% women), 658 (80%) attended the 18-month visit (579 in person, 79 virtually) (Figure 1). Baseline characteristics by randomization group are shown in Table 1 (demographic data limited to patients with available follow-up data are available in eTable 1 in Supplement 2). During the first 6 months, patients in the diet and exercise group had an adherence rate of 84%. The overall intervention adherence rate was 72%. Adherence rates for the attention control group were 89% at 6 months and 78% overall. At 6 months, 36% of the diet and exercise group used meal replacements between 5 and 7 days per week; at 12 months, 29% used meal replacements; and by 18 months, 26% used meal replacements (eTable 7 in Supplement 2).
Table 1. Baseline Demographic and Clinical Characteristicsa.
| Characteristics | Diet and exercise (n = 414) | Attention control (n = 409) |
|---|---|---|
| Age, mean (SD), y | 64.5 (7.8) | 64.7 (7.8) |
| Weight, mean (SD), kg | 100.7 (20.6) | 101.1 (22.0) |
| Height, mean (SD), m | 1.65 (0.09) | 1.66 (0.09) |
| Body mass index, mean (SD)b | 36.7 (6.5) | 36.9 (7.2) |
| Sex, No. (%) | ||
| Female | 320 (77.3) | 317 (77.5) |
| Male | 94 (22.7) | 92 (22.5) |
| Race, No. (%) | ||
| American Indian | 1 (0.2) | 1 (0.2) |
| Asian | 2 (0.5) | 1 (0.2) |
| Black | 111 (26.8) | 116 (28.4) |
| White | 289 (69.8) | 281 (68.7) |
| Other race or more than 1 racec | 11 (2.6) | 10 (2.4) |
| Hispanic ethnicity, No./total (%)d | 7/414 (1.7) | 3/404 (0.7) |
| Annual household income, $, No./total (%) | ||
| <20 000 | 54/395 (13.7) | 61/398 (15.3) |
| 20 000-34 999 | 64/395 (16.2) | 69/398 (17.3) |
| 35 000-49 999 | 67/395 (17.0) | 63/398 (15.8) |
| 50 000-74 999 | 93/395 (23.5) | 90/398 (22.6) |
| 75 000-99 999 | 52/395 (13.2) | 52/398 (13.1) |
| ≥100 000 | 65/395 (16.5) | 63/398 (15.8) |
| Education, No./total (%) | ||
| Less than high school | 13/412 (3.2) | 8/407 (2.0) |
| High school or equivalent | 47/412 (11.4) | 51/407 (12.5) |
| Some college | 164/412 (39.8) | 162/407 (39.8) |
| College degree | 115/412 (27.9) | 118/407 (29.0) |
| Graduate degree | 73/412 (17.7) | 68/407 (16.7) |
| Comorbid illnesse | ||
| Obesity (body mass index ≥30) | 365 (88) | 359 (88) |
| Hypertension | 276 (67) | 280 (69) |
| Arthritis in other joints | 229 (55) | 221 (54) |
| Diabetes | 74 (18) | 91 (22) |
| Cardiovascular disease | 43 (10) | 39 (10) |
| WOMAC score, mean (SD)f | ||
| Pain | 7.5 (3.2) | 7.5 (3.3) |
| Function | 26.0 (11.6) | 25.9 (12.0) |
eTable 1 in Supplement 2 contains the same categories restricted to patients for whom 18-month outcome data were available.
Calculated as weight in kilograms divided by height in meters squared.
Other categories included self-identified Egyptian (n = 1), Hispanic ethnicity with no race defined (n = 8), and Semitic (n = 1).
Reported on a self-administered demographics questionnaire.
Reported on a self-administered health history questionnaire as conditions diagnosed by a health care professional. With comorbid illnesses that could exclude patients from participation, final approval or denial for participation was provided after patient evaluation by a study physician.
The Western Ontario and McMaster Universities Osteoarthritic Index (WOMAC) consists of a self-administered questionnaire including 5 questions on pain and 17 questions on physical function. The scale for each question ranges from 0 (no symptoms) to 4 (extreme symptoms). Composite scores for pain range from 0 to 20 and for function from 0 to 68. Pain cut points on a 0- to 20-point scale are as follows: 2 to 8, mild; >8 to 14, moderate; and >14 to 20, severe (transformed from a 0- to 10-point scale reported in Kapstad et al23).
Primary Outcome
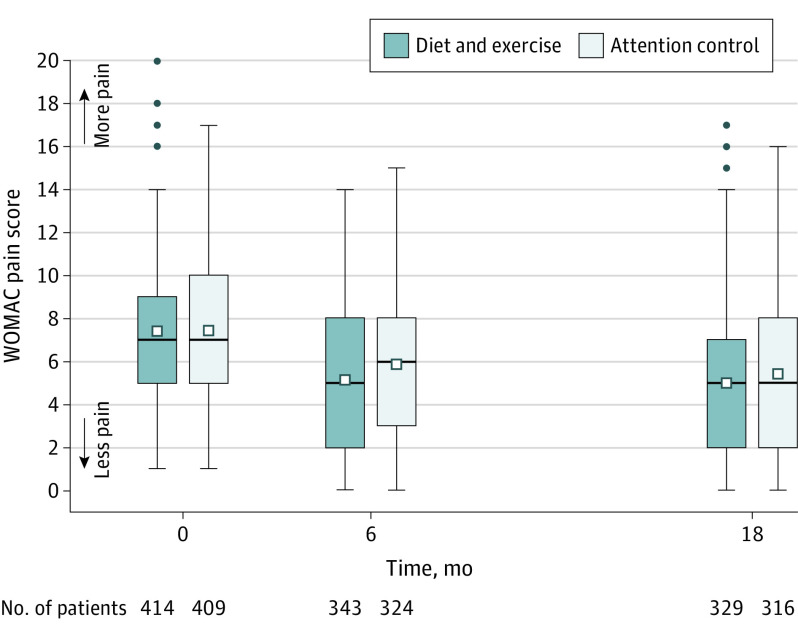
At 18-month follow-up, the adjusted mean WOMAC pain score was 5.0 in the diet and exercise group vs 5.5 in the attention control group (adjusted difference, −0.6; 95% CI, −1.0 to −0.1; P = .02) (Table 2 and Figure 2).
Table 2. Primary, Prespecified Secondary, and Post Hoc Outcomes for Patients for Whom Outcome Data Were Available at 6 Months and/or 18 Months.
| Outcome measures | Diet and exercise | Attention control | Adjusted mean difference (95% CI) | P valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (SD) | 18 Months | Baseline (SD) | 18 Months | |||||||
| Adjusted mean (95% CI) | Unadjusted mean (SD) | Absolute change (95% CI) | Adjusted mean (95% CI) | Unadjusted mean (SD) | Absolute change (95% CI) | |||||
| Primary outcome | ||||||||||
| WOMAC pain scoreb | 7.4 (3.1) [n = 377] | 5.0 (4.6-5.4) [n = 329] | 5.0 (3.8) [n = 329] | −2.4 (−2.8 to −2.1) | 7.4 (3.1) [n = 316] | 5.5 (5.1-5.9) [n = 316] | 5.5 (3.7) [n = 316] | −1.8 (−2.2 to −1.4) | −0.6 (−1.0 to −0.1) | .02 |
| Secondary outcomes | ||||||||||
| Body weight, kg | 99.6 (19.3) [n = 377] | 91.4 (90.2-92.6) [n = 289] | 89.8 (17.2) [n = 289] | −7.7 (−8.7 to −6.7) | 100.8 (22.2) [n = 343] | 97.4 (96.2-98.7) [n = 273] | 98.4 (21.7) [n = 273] | −1.7 (−2.5 to −0.8) | −6.0 (−7.3 to −4.7) | <.001 |
| Waist circumference, cmc | 113 (14) [n = 376] | 105 (104-107) [n = 212] | 104 (14) [n = 212] | −9 (−10 to −8) | 115 (16) [n = 342] | 111 (109-113) [n = 214] | 111 (16) [n = 214] | −4 (−5 to −3) | −5 (−7 to −4) | <.001 |
| WOMAC function scored | 25.5 (11.5) [n = 377] | 16.5 (15.3-17.7) [n = 325] | 16.6 (13.1) [n = 325] | −9.2 (−10.5 to −7.9) | 25.5 (11.4) [n = 343] | 19.8 (18.6-21.0) [n = 311] | 19.5 (12.6) [n = 311] | −5.5 (−6.9 to −4.1) | −3.3 (−4.9 to −1.7) | <.001 |
| Six-minute walk distance, me | 373 (90) [n = 375] | 419 (411-428) [n = 247] | 427 (103) [n = 247] | 41 (32 to 51) | 371 (95) [n = 341] | 376 (368-385) [n = 219] | 375 (97) [n = 219] | −4 (−12 to 4) | 43 (31 to 55) | <.001 |
| Pain medication use | 8.2 (5.0) [n = 377] | 8.3 (7.9-8.8) [n = 231] | 8.3 (5.0) [n = 231] | −0.1 (−0.5 to 0.3) | 8.4 (5.0) [n = 343] | 8.3 (7.8-8.8) [n = 228] | 8.3 (5.0) [n = 228] | −0.4 (−0.8 to 0.0) | 0.0 (−0.5 to 0.5) | .90 |
| Short Form 36 score (range, 0-100)f | ||||||||||
| Physical subscale | 34.3 (9.2) [n = 377] | 41.4 (40.2-42.6) [n = 321] | 41.2 (11.1) [n = 321] | 6.7 (5.6 to 7.7) | 35.9 (9.1) [n = 343] | 37.6 (36.3-38.8) [n = 306] | 38.4 (10.6) [n = 306] | 2.1 (1.2 to 3.1) | 3.8 (2.5 to 5.2) | <.001 |
| Mental subscale | 55.4 (9.8) [n = 377] | 55.3 (54.3-56.2) [n = 321] | 55.2 (9.1) [n = 321] | 0.1 (−0.9 to 1.1) | 55.0 (9.7) [n = 343] | 54.1 (53.1-55.0) [n = 306] | 54.0 (11.3) [n = 306] | −1.0 (−2.4 to −0.4) | 1.2 (−0.1 to 2.5) | .07 |
| Post hoc outcomes | ||||||||||
| PASE scoreg | 116 (60) [n = 375] | 139 (133-146) [n = 311] | 138 (69) [n = 311] | 23 (15 to 30) | 112 (58) [n = 339] | 117 (111-124) [n = 284] | 114 (53) [n = 284] | 1 (−6 to 8) | 22 (13 to 31) | <.001 |
| CES-D scoreh | 5.2 (4.8) [n = 374] | 3.9 (3.2-4.7) [n = 243] | 3.8 (4.2) [n = 243] | −1.1 (−1.7 to −0.6) | 5.5 (5.2) [n = 341] | 4.6 (3.9-5.3) [n = 226] | 5.0 (5.7) [n = 226] | −0.4 (−1.0 to 0.3) | −0.6 (−1.3 to 0.0) | .07 |
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale; MCID, minimum clinically important difference; MCII, minimum clinically important improvement; PASE, Physical Activity Scale for the Elderly; WOMAC, Western Ontario and McMaster Universities Osteoarthritic Index.
All model-adjusted estimates were created from a mixed linear model fit with treatment group, visit number, and treatment-by-visit interaction, adjusted for baseline body mass index, clinic site, sex, and baseline values of the outcome. Contrast statements were used to produce visit-specific means, mean differences, and P values.
Range, 0 to 20 (0, no pain; 2 to 8, mild; >8 to 14, moderate; and >14 to 20, severe); between-group MCID = 1.6, calculated as half the SD.37
Waist circumference thresholds (women, >88 cm; men, >102 cm) for increased health risk.29 MCID = −7.5 cm.24,37
Range, 0 to 68 (higher scores indicate poorer function); between-group MCID = 6.25
The maximum distance a participant could walk along a standardized walkway in 6 minutes. Within-group MCII, 26 m to 55 m.26
Health-related quality-of-life physical and mental health summary scores; range, 0 (worst) to 100 (best); within-group MCII = 5 for both physical and mental subscales.28
Score range, 0 to 400 (higher scores indicate more activity; MCII = 17-25).39
Score range, 0 to 30 (score of ≥10 indicates presence of significant depressive symptoms); MCID = 30, calculated as half the SD.36
Figure 2. Unadjusted Mean WOMAC Pain Scores for Patients for Whom Follow-up Data Were Available at 6 Months and/or 18 Months.
WOMAC indicates Western Ontario and McMaster Universities Osteoarthritis Index (range, 0 [no pain] to 20 [severe pain]). Box plots are shown for the primary outcome of pain, in which the middle lines represent median values; squares, mean values; and boxes, IQRs. Whiskers extend to the most extreme observed values within 1.5 times the IQR of the nearer quartile, and dots represent observed values outside the range. Unadjusted means are for the diet and exercise and attention control groups across the 18-month intervention period. The within-group minimum clinically important improvement at 18 months is a 20% improvement from baseline or 1.5 points. The between-group minimum clinically important difference is 1.6 points using the half-SD formula.24
Secondary Outcomes
Between baseline and 18-month follow-up, mean body weight changed by −7.7 kg (−8%) in the diet and exercise group and −1.7 kg (−2%) in the attention control group (difference in absolute change, −6.0 kg; −7.3 kg to −4.7 kg) (Table 2; eFigure 1 in Supplement 2). At 18-month follow-up, mean waist circumference was 105 cm in the diet and exercise group compared with 111 cm in the attention control group (adjusted difference, −5 cm; 95% CI, −7 cm to −4 cm; P < .001).
At 18-month follow-up, the mean WOMAC function score was significantly better in the diet and exercise group compared with the attention control group (mean score, 16.5 vs 19.8; adjusted difference, −3.3; 95% CI, −4.9 to −1.7; P < .001) (Table 2). At 18-month follow-up, the mean 6-minute walk distance was 419 m in the diet and exercise group and 376 m in the attention control group (adjusted difference, 43 m; 95% CI, 31-55 m; P < .001), and Short Form 36 physical subscale mean scores were 41.4 in the diet and exercise group compared with 37.6 in the attention control group (adjusted difference, 3.8; 95% CI, 2.5-5.2; P < .001).
Prespecified Sensitivity Analyses
Multiple imputation analyses yielded similar results for the primary and prespecified secondary outcomes (eTable 4 in Supplement 2).
Adverse Events
Seventy serious adverse events occurred in the diet and exercise group compared with 99 serious adverse events in the attention control group. None were designated as definitely related to the study. A total of 428 nonserious adverse events occurred in the diet and exercise group and 301 occurred in the attention control group. Of these, 32 (4%) were determined to be definitely related to the study. The most common adverse events related to the study consisted of 10 body injuries (9 in diet and exercise; 1 in attention control), 7 muscle strains (6 in diet and exercise; 1 in attention control), 6 trip/fall events (all in diet and exercise), and 6 leg/knee pain events (all in diet and exercise). There were 44 knee replacement surgeries (21 in diet and exercise; 23 in attention control) and 119 intra-articular knee injections (44 in diet and exercise; 75 in attention control) (eTable 5, A and B, in Supplement 2).
Post Hoc Outcomes
Post hoc analysis using site as a random effect produced similar results (eTable 3 in Supplement 2).
The number of patients in each group who achieved a clinically meaningful improvement in the WOMAC pain score of 2 or more points was 198 (60.2%) in the diet and exercise group and 157 (49.7%) in the attention control group (risk ratio, 1.20; 95% CI, 1.04-1.38; P = .01) (Table 3); the diet and exercise group was 20% more likely to attain a moderate 2-point improvement in pain compared with the attention control group, a relative within-group improvement of 15% to 35%.37 The weight loss goal of 10% of baseline body weight at 18-month follow-up was achieved by 32% of participants in the diet and exercise group. Among participants in the diet and exercise group, 60% lost at least 5% of body weight, and 87% weighed less at 18-month follow-up compared with baseline. Among the attention control group, at 18-month follow-up, 9% lost at least 10% of body weight, 23% lost at least 5% of body weight, and 58% weighed less compared with baseline. At 18-month follow-up, the mean PASE physical activity level was significantly greater in the diet and exercise group compared to attention control (mean score, 139 vs 117; adjusted difference, 22; 95% CI, 13-31; P < .001). Comparison of primary and secondary outcomes at 18 months with vs without patients who were actively enrolled when COVID-19 restrictions were instituted showed results similar to the primary analyses (eTable 6 in Supplement 2). For the diet and exercise group, the correlation between weight loss and pain reduction was R = 0.25 (P < .001). For the attention control group, the correlation between weight loss and pain reduction was R = 0.05 (P = .40) (eFigures 2 and 3 in Supplement 2). In multiple imputation analysis, in which missing data were replaced with an estimated value based on available information, the primary and secondary outcome results were similar to the complete case analysis (eTable 2 in Supplement 2).
Table 3. Post Hoc Analysis of the Relative Risk of Achieving a Clinically Meaningful Change in WOMAC Pain Score.
| Follow-up time | No./total (%) with significant pain reduction of ≥2 pointsa | Absolute risk difference, % (95% CI) | Risk ratio (95% CI)b | P valuec | |
|---|---|---|---|---|---|
| Diet and exercise | Attention control | ||||
| 6 mo | 181/343 (52.8) | 145/324 (44.8) | 8 (0.45-15.6) | 1.17 (1.00-1.35) | .045 |
| 18 mo | 198/329 (60.2) | 157/316 (49.7) | 10.5 (2.9-18.1) | 1.20 (1.04-1.38) | .01 |
Abbreviation: WOMAC, Western Ontario and McMaster Universities Osteoarthritic Index.
The minimum clinically important difference for pain is 1.6 (calculated as half the SD) on a 0- to 20-point scale.24 This post hoc analysis is at the individual patient level, in which only whole numbers are possible. The 2-point criterion was recommended by the Osteoarthritis Research Society International as a moderate intervention response.37
Relative risk of achieving a clinically meaningful change in pain, defined as a decrease in pain of 2 points or more on the Likert WOMAC pain scale (range, 0-20). Estimated by a generalized estimating equation model fit with contrasts for group comparisons at 6-and 18- month follow-up adjusted for baseline pain scores. The risk (ie, benefit) of achieving a significant decrease in pain increases from 6- to 18-month follow-up and is significantly greater in the diet and exercise group compared with the attention control group.
Test of the risk ratio under the null hypothesis that the risk ratio = 1.0.
Discussion
Among patients with knee OA and overweight or obesity, diet and exercise, compared with an attention control group, led to a statistically significant but small difference in knee pain over 18 months. The magnitude of the difference in pain between groups is of uncertain clinical importance.37
The attention control group was designed to determine whether the diet and exercise intervention provided benefit to patients beyond the benefit of the attention provided to the diet and exercise group. Previous control groups in knee OA behavioral clinical trials reported improvements in pain that varied between 1% and 33%.5,18,40 The 24% reduction in adjusted 18-month pain in the attention control group may relate to the natural history of knee OA or might suggest that interacting with patients in a social environment had a positive effect on clinical outcomes. Moderate (5%) to intensive (10%) weight loss in patients with knee OA and obesity achieved in previous academic center–based efficacy trials showed benefits on clinical and mechanistic outcomes such as knee pain, plasma interleukin 6 levels, and knee joint compressive forces, with a dose-response association.6,41 The 7.7-kg (8%) weight loss combined with a 9-cm reduction in waist circumference in the diet and exercise group has the potential for health benefits for older adults with knee OA.29
Limitations
This study has several limitations. First, at baseline, participants who did not complete the trial had more pain and higher weight and were younger than those who completed the trial. Second, there was no radiographic or magnetic resonance imaging confirmation of knee OA. Third, the option of using nutritional supplements as meal replacements at no cost to participants and the availability of exercise facilities at no cost to the patients in the intervention group limited the generalizability of the results and feasibility of the intervention outside the clinical trial.
Conclusions
Among patients with knee osteoarthritis and overweight or obesity, diet and exercise compared with an attention control led to a statistically significant but small difference in knee pain over 18 months. The magnitude of the difference in pain is of uncertain clinical importance.
Trial Protocol
eTable 1. Baseline Demographic and Clinical Characteristics for Patients for Whom Outcome Data Were Available
eTable 2. Comparison of Baseline Values of Completers Versus Non-Completers (No Follow-up Data)
eTable 3. Primary and Pre-Specified Secondary Outcomes Using Site as a Random Effect
eTable 4. Between Group Differences at 18-Month Follow-up for Primary and Secondary Outcomes Using Multiple Imputation
eTable 5A. Adverse Events Definitely Related to the Study by Group Assignment
eTable 5B. Knee Replacement and Intraarticular Knee Injections by Group Assignment
eTable 6. Comparison of 18-Month Outcomes With Versus Without Participants That Were Active During COVID
eTable 7. Number and Percent of Meal Replacement Use for the Diet and Exercise and Control Groups
eFigure 1. Unadjusted Mean (95% CI) Body Weight Across the 18-Month Intervention Period for the Diet and Exercise and Control Groups
eFigure 2. Change in Weight Versus Change in Pain for the Diet and Exercise Group
eFigure 3. Change in Weight Versus Change in Pain for the Control Group
Data Sharing Statement
References
- 1.Brand CA, Ackerman IN, Bohensky MA, Bennell KL. Chronic disease management: a review of current performance across quality of care domains and opportunities for improving osteoarthritis care. Rheum Dis Clin North Am. 2013;39(1):123-143. doi: 10.1016/j.rdc.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Barber RM, Bell B, et al. ; Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743-800. doi: 10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins JE, Deshpande BR, Katz JN, Losina E. Race- and sex-specific incidence rates and predictors of total knee arthroplasty: seven-year data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2016;68(7):965-973. doi: 10.1002/acr.22771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basedow M, Esterman A. Assessing appropriateness of osteoarthritis care using quality indicators: a systematic review. J Eval Clin Pract. 2015;21(5):782-789. doi: 10.1111/jep.12402 [DOI] [PubMed] [Google Scholar]
- 5.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501-1510. doi: 10.1002/art.20256 [DOI] [PubMed] [Google Scholar]
- 6.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263-1273. doi: 10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-162. doi: 10.1016/j.joca.2007.12.013 [DOI] [PubMed] [Google Scholar]
- 8.Fernandes L, Hagen KB, Bijlsma JW, et al. ; European League Against Rheumatism (EULAR) . EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125-1135. doi: 10.1136/annrheumdis-2012-202745 [DOI] [PubMed] [Google Scholar]
- 9.Aaboe J, Bliddal H, Messier SP, Alkjær T, Henriksen M. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis Cartilage. 2011;19(7):822-828. doi: 10.1016/j.joca.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 10.Messier SP, Callahan LF, Beavers DP, et al. Weight-Loss and Exercise for Communities With Arthritis in North Carolina (WE-CAN): design and rationale of a pragmatic, assessor-blinded, randomized controlled trial. BMC Musculoskelet Disord. 2017;18(1):91. doi: 10.1186/s12891-017-1441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman R, Asch E, Bloch D, et al. ; Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association . Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039-1049. doi: 10.1002/art.1780290816 [DOI] [PubMed] [Google Scholar]
- 12.US Department of Agriculture Economic Research Service . What is rural? Accessed May 2022. https://www.ers.usda.gov/topics/rural-economy-population/rural-classifications/what-is-rural/
- 13.Owen OE, Kavle E, Owen RS, et al. A reappraisal of caloric requirements in healthy women. Am J Clin Nutr. 1986;44(1):1-19. doi: 10.1093/ajcn/44.1.1 [DOI] [PubMed] [Google Scholar]
- 14.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289(14):1799-1804. doi: 10.1001/jama.289.14.1799 [DOI] [PubMed] [Google Scholar]
- 15.National Heart, Lung, and Blood Institute. Aim for a healthy weight: key recommendations. 2015. Accessed November 21, 2022. https://www.nhlbi.nih.gov/health/educational/lose_wt/recommen.htm
- 16.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191-215. doi: 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- 17.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. ; American College of Sports Medicine . American College of Sports Medicine position Stand: exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-1530. doi: 10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- 18.Messier SP, Mihalko SL, Beavers DP, et al. Effect of high-intensity strength training on knee pain and knee joint compressive forces among adults with knee osteoarthritis: the START randomized clinical trial. JAMA. 2021;325(7):646-657. doi: 10.1001/jama.2021.0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettinger WH Jr, Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis: the Fitness Arthritis and Seniors Trial (FAST). JAMA. 1997;277(1):25-31. doi: 10.1001/jama.1997.03540250033028 [DOI] [PubMed] [Google Scholar]
- 20.Bellamy N. Outcome measurement in osteoarthritis clinical trials. J Rheumatol Suppl. 1995;43:49-51. [PubMed] [Google Scholar]
- 21.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes following total hip or knee arthroplasty in osteoarthritis. J Orthop Rheumatol. 1988;1:95-108. [PubMed] [Google Scholar]
- 22.Griffiths G, Bellamy N, Kean WF, Campbell J, Gerecz-Simon E. A study of the time frame dependency of responses to the WOMAC osteoarthritis index. Inflammopharmacology. 1993;2(1):85-87. doi: 10.1007/BF02663745 [DOI] [Google Scholar]
- 23.Kapstad H, Hanestad BR, Langeland N, Rustøen T, Stavem K. Cutpoints for mild, moderate and severe pain in patients with osteoarthritis of the hip or knee ready for joint replacement surgery. BMC Musculoskelet Disord. 2008;9:55. doi: 10.1186/1471-2474-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582-592. doi: 10.1097/01.MLR.0000062554.74615.4C [DOI] [PubMed] [Google Scholar]
- 25.Clement ND, Bardgett M, Weir D, Holland J, Gerrand C, Deehan DJ. What is the minimum clinically important difference for the WOMAC index after TKA? Clin Orthop Relat Res. 2018;476(10):2005-2014. doi: 10.1097/CORR.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naylor JM, Mills K, Buhagiar M, Fortunato R, Wright R. Minimal important improvement thresholds for the six-minute walk test in a knee arthroplasty cohort: triangulation of anchor- and distribution-based methods. BMC Musculoskelet Disord. 2016;17(1):390. doi: 10.1186/s12891-016-1249-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 28.Ogura K, Yakoub MA, Christ AB, et al. What are the minimum clinically important differences in SF-36 scores in patients with orthopaedic oncologic conditions? Clin Orthop Relat Res. 2020;478(9):2148-2158. doi: 10.1097/CORR.0000000000001341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16(3):177-189. doi: 10.1038/s41574-019-0310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 31.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94. doi: 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 32.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi: 10.2519/jospt.1998.28.2.88 [DOI] [PubMed] [Google Scholar]
- 33.Hawker GA, Davis AM, French MR, et al. Development and preliminary psychometric testing of a new OA pain measure—an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16(4):409-414. doi: 10.1016/j.joca.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 35.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The Physical Activity Scale for the Elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52(7):643-651. doi: 10.1016/S0895-4356(99)00049-9 [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, O’Brien N, Forrest JI, et al. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One. 2012;7(7):e40793. doi: 10.1371/journal.pone.0040793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dougados M, Leclaire P, van der Heijde D, Bloch DA, Bellamy N, Altman RD. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage. 2000;8(6):395-403. doi: 10.1053/joca.2000.0361 [DOI] [PubMed] [Google Scholar]
- 38.Sample Size and Power Calculation. Statistical Solutions Ltd; 2017. [Google Scholar]
- 39.Granger CL, Parry SM, Denehy L. The self-reported Physical Activity Scale for the Elderly (PASE) is a valid and clinically applicable measure in lung cancer. Support Care Cancer. 2015;23(11):3211-3218. doi: 10.1007/s00520-015-2707-8 [DOI] [PubMed] [Google Scholar]
- 40.Bliddal H, Leeds AR, Stigsgaard L, Astrup A, Christensen R. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann Rheum Dis. 2011;70(10):1798-1803. doi: 10.1136/ard.2010.142018 [DOI] [PubMed] [Google Scholar]
- 41.Messier SP, Resnik AE, Beavers DP, et al. Intentional weight loss for overweight and obese patients with knee osteoarthritis: is more better? Arthritis Care Res (Hoboken). 2018;70:1569-1575. doi: 10.1002/acr.23608 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Demographic and Clinical Characteristics for Patients for Whom Outcome Data Were Available
eTable 2. Comparison of Baseline Values of Completers Versus Non-Completers (No Follow-up Data)
eTable 3. Primary and Pre-Specified Secondary Outcomes Using Site as a Random Effect
eTable 4. Between Group Differences at 18-Month Follow-up for Primary and Secondary Outcomes Using Multiple Imputation
eTable 5A. Adverse Events Definitely Related to the Study by Group Assignment
eTable 5B. Knee Replacement and Intraarticular Knee Injections by Group Assignment
eTable 6. Comparison of 18-Month Outcomes With Versus Without Participants That Were Active During COVID
eTable 7. Number and Percent of Meal Replacement Use for the Diet and Exercise and Control Groups
eFigure 1. Unadjusted Mean (95% CI) Body Weight Across the 18-Month Intervention Period for the Diet and Exercise and Control Groups
eFigure 2. Change in Weight Versus Change in Pain for the Diet and Exercise Group
eFigure 3. Change in Weight Versus Change in Pain for the Control Group
Data Sharing Statement