Abstract
Background:
The feasibility and effectiveness of delaying surgery to transfer patients with acute type A aortic dissection – a catastrophic disease that requires prompt intervention – to higher-volume aortic surgery hospitals is unknown. We investigated the hypothesis that regionalizing care at high-volume hospitals for acute type A aortic dissections will lower mortality. We further decomposed this hypothesis into subparts – investigating the isolated effect of transfer and the isolated effect of receiving care at a high-volume vs. a low-volume facility.
Methods:
We compared the operative mortality and long-term survival between 16,886 Medicare beneficiaries diagnosed with an acute type A aortic dissection between 1999 and 2014 who: 1) were transferred vs. not transferred; 2) underwent surgery at high- vs. low-volume hospitals; and 3) were rerouted vs. not rerouted to a high-volume hospital for treatment. We used a preference-based instrumental variable design to address unmeasured confounding and matching to separate the effect of transfer from volume.
Results:
Between 1999 and 2014, 40.5% of patients with an acute type A aortic dissection were transferred, and 51.9% received surgery at a high-volume hospital. Interfacility transfer was not associated with a change in operative mortality (risk difference −0.69%, 95% CI −2.7% to 1.35%) or long-term mortality. Despite delaying surgery, a regionalization policy that transfers patients to high-volume hospitals was associated with a 7.2% (95% CI 4.1% to 10.3%) absolute risk reduction in operative mortality; this association persisted in the long term (HR 0.81, 95% CI 0.75 to 0.87). The median distance needed to reroute each patient to a high-volume hospital was 50.1 miles (IQR 12.4 to 105.4 miles).
Conclusions:
Operative and long-term mortality were substantially reduced in patients with acute type A aortic dissection who were rerouted to high-volume hospitals. Policy-makers should evaluate the feasibility and benefits of regionalizing the surgical treatment of acute type A aortic dissection in the United States.
Keywords: aortic dissection, epidemiology, surgery, interfacility transfer, instrumental variable
INTRODUCTION
Aortic dissection is the most common emergent disease of the thoracic aorta, and occurs with an age-dependent incidence of 2.5-10/100,000 person-years.1, 2 Surgery is the standard treatment when the ascending aorta is involved (type A);3, 4 left untreated, the condition is almost uniformly fatal. The timing of intervention is critical because without surgery, mortality historically approached 50% at 48 hours.5, 6 Consequently, immediate operative therapy is the standard of care.7, 8 However, advances in imaging and blood pressure management may reduce this early risk and permit delaying surgery in order to transfer patients to expert aortic surgery centers.
The previously demonstrated relationship between provider experience and outcomes of surgery for aortic dissection9 ignited calls for nationwide regionalization of care.10 However, an inability to distinguish between types A and B dissection, and a failure to account for differences in clinical status or patient selection prior to interfacility transfer undermined conclusions from previous observational studies.9, 11 Nevertheless, delaying emergent surgery to reroute care to experienced providers – even within an institution12, 13 – may represent an opportunity to improve outcomes.8, 10 Although >60% of patients treated for acute type A aortic dissection at specialized centers originate at other hospitals,12, 14 little data exist on the epidemiology and safety of interfacility transfer,15, 16 or on regionalization of care – critical questions for policymakers.
We conducted a retrospective cohort study to evaluate the risks and benefits of interfacility transfer, and to explore the feasibility and efficacy of regionalizing care for patients with acute type A aortic dissection.
METHODS
All data are available for purchase from the Centers for Medicare and Medicaid Services. The authors are not permitted to share the data directly.
Study Design
We examined the hospitalizations of all Medicare beneficiaries diagnosed with an acute type A aortic dissection between January 1, 1999 and December 30, 2014 to identify: 1) the risks and benefits of interfacility transfer; 2) the effect of hospital volume on mortality; and 3) the impact of regionalizing care to high-volume hospitals. We used disease-specific, hospital-level transfer patterns observed over the 15-year study period as a preference-based instrumental variable to isolate the effects of interfacility transfer, hospital volume, and regionalization of care on operative and long-term mortality. The institutional review board at Stanford University approved this study. All authors accept responsibility for the accuracy of the analyses and interpretations of the data.
Study Population
We included patients with an incident diagnosis of aortic dissection (International Classification of Diseases (ICD9-CM) code 441.0) and Current Procedural Terminology code(s) for surgery of the aortic root and/or ascending aorta within 14 days of diagnosis, or within the same hospitalization. We performed an internal validation study to evaluate our definition of type A aortic dissection (see Supplemental Methods). We excluded patients who underwent surgery limited to the descending aorta to minimize misclassification of type B aortic dissection, a disease that is initially treated surgically in <10% of patients.14 We also excluded patients who underwent >1 interfacility transfer due to the complex decision making for such patients, and potential to introduce survivor bias. Comorbidities were obtained from the Chronic Conditions Summary file (a complete list of the procedural codes used in these analyses is provided in the Supplement (Supplemental Tables 1-2)).
Patient disposition within the MEDPAR file is inaccurate and incomplete;17 therefore, we classified patients as having undergone transfer if they were discharged from a short-term, specialty, or critical access hospital and admitted – on the same date – to a different facility where the proximal aortic operation was performed.
To identify high-volume aortic surgery programs, we examined the total number of aortic root, ascending aorta, and transverse arch repairs performed among Medicare beneficiaries – for any indication – at each hospital over the 15-year study period. High-volume was defined a priori as the top decile of hospitals with respect to total number of proximal aortic and arch repairs among Medicare beneficiaries. Hospitals were then divided into three categories: 1) no cardiac/aortic surgery – hospitals that did not perform an open aortic operation; 2) low-volume – hospitals that performed fewer than 105 proximal aortic operations total; and 3) high-volume – hospitals that performed at least 105 proximal aortic operations total.
Outcomes
The primary study endpoint was operative mortality, defined as death during the index hospitalization or within 30 days of surgery. The secondary outcome was all-cause mortality during follow-up. Death data were obtained from the Beneficiary Summary file.
Instrumental Variable Method
Instrumental variables are observable factors that affect treatment assignment, but only affect the outcome through their influence on treatment. A valid instrumental variable is similar to treatment randomization and permits unbiased estimation of treatment effects despite the presence of unobserved confounders.18, 19 In patients with acute type A aortic dissection, selection for interfacility transfer is primarily influenced by hospital capabilities and acute illness severity – factors that are not captured in administrative data. Consequently, our primary analysis does not to rely upon propensity score methods (or similar analyses) which can only adjust for observed factors. In the Supplement, we explore the problematic conclusions a propensity score analysis produces in this setting (Supplemental Methods & Supplemental Tables 3-6).
Aortic dissection is much less common than an acute coronary syndrome, and typically requires imaging for diagnosis. Consequently, aortic dissection is diagnosed at the presenting hospital, not in the field. Emergency medical services bring patients from the field to the closest hospital they believe can provide adequate care. This may be the closest hospital, or if there is a strong belief that the issue is cardiovascular, then it may be the closest hospital capable of cardiac catheterization, but not expert aortic surgery (see Supplemental Methods and Supplemental Table 7 for insight into decision-making underlying transport from the field, and the null effect on outcome of selective transport of patients with aortic dissection to hospitals capable of cardiac catheterization). With respect to thoracic aortic surgery experience, the population is therefore pseudo-randomized to present at high-volume, low-volume, or no-volume (obligate transfer) hospitals. Among aortic surgery-performing hospitals, we restricted our analysis to hospitals that observed >1 aortic dissection during the study period in order to establish the presence of a treatment pattern. Upon examining the disease-specific, hospital-level transfer patterns during the study period, it became clear that the majority of hospitals either never transferred or always transferred patients with an acute type A aortic dissection, and transfers followed a bifurcated pattern (i.e. always to high-volume institutions or always to low-volume institutions) (Supplemental Figure 1). These patterns likely arise from ingrained referral practices between hospitals. Following the guidance in Hernán & Robins20 the idealized “target randomized trial” that this observational study models itself upon is an unbalanced, randomized 2×2 factorial design with the first factor being interfacility transfer or no transfer, and the second factor being assignment to treatment at a high-volume or low-volume hospital. For our primary analysis, patients who presented to no- or low-volume hospitals that did not always employ the same treatment strategy were excluded given the potential for selection bias (Supplemental Table 8 compares patients included versus excluded by the instrumental variable design); this is because hospitals with variable treatment strategies likely used patient-specific information to decide when and where to transfer patients, whereas hospitals without variation in their treatment strategy are less likely to have used patient-specific information in deciding whether, and where, to transfer.
Statistical Analysis
We designed the study to achieve at least 90% power to detect a small risk difference in operative mortality (Supplemental Methods). For each comparison (transferred vs. stayed, high-volume vs. low-volume, and regionalized vs. not regionalized), logistic regression was used to estimate each patient’s predicted probability of the dichotomous instrument given baseline covariates (Supplemental Figure 2). Patients were then matched in a 1:k or k:1 fashion, using different matching algorithms tailored to fit the individual question. For the isolated effect of transfer, patients transferred to high-volume hospitals were only matched to those who presented to and stayed at a high-volume hospital. Patients transferred to low-volume hospitals were only matched to those who presented to and remained at a low-volume hospital. To estimate the effect of hospital volume, transferred patients were only matched to patients that originated at a hospital of the same volume category; and patients that remained at high-volume centers were only matched to those that remained at low-volume centers. To estimate the effect of regionalization, patients transferred to high-volume hospitals were matched to patients who remained at low-volume hospitals or were transferred to low-volume hospitals (because the latter two groups would be rerouted to high-volume hospitals under a policy of regionalization). Balance between groups was assessed with standardized differences.21 A standardized difference ≤10% was deemed ideal balance, and ≤20% was acceptable balance.22
Weighted logistic regression with a robust variance estimator was used to determine the marginal effects of transfer, hospital volume, and regionalization on operative mortality; weighted Cox proportional hazards regression with a robust variance estimator was used to compare survival.23 We estimated the risk difference in operative mortality by calculating the difference in the marginal probability of the outcome, and 95% confidence intervals (CI) were obtained with 100,000 bootstrap replicates. To address non-proportional hazards, the restricted mean survival time was calculated to describe the effect of treatment on long-term survival.24
To evaluate the effect of hospital volume as a continuous variable on operative mortality, a Cox proportional hazards model was fit to the study population with a natural spline fit for volume (3 knots). All tests were two-tailed with an alpha threshold of 0.05. Statistical analyses were performed in R version 3.3.1 (R Foundation, Vienna, Austria).
Sensitivity Analyses
We only included patients who underwent surgery in the analysis. This approach may underestimate the risk of transfer; patients who were transferred and died en route or were declined an operation were not captured. Therefore, we calculated the number of unobserved transfer mortalities needed to discover a statistically significant increase in mortality directly attributable to transfer. We assumed non-transferred patients would incur the same weighted risk of death as those who remained at low- or high-volume hospitals. A similar sensitivity analysis was performed to determine the number of unobserved regionalized patients that would need to die to nullify or reverse a beneficial effect of regionalization. Additionally, gamma parameters were calculated to determine the sensitivity of each outcome comparison to residual unmeasured bias.25
RESULTS
Patients, Hospitals and Temporal Changes
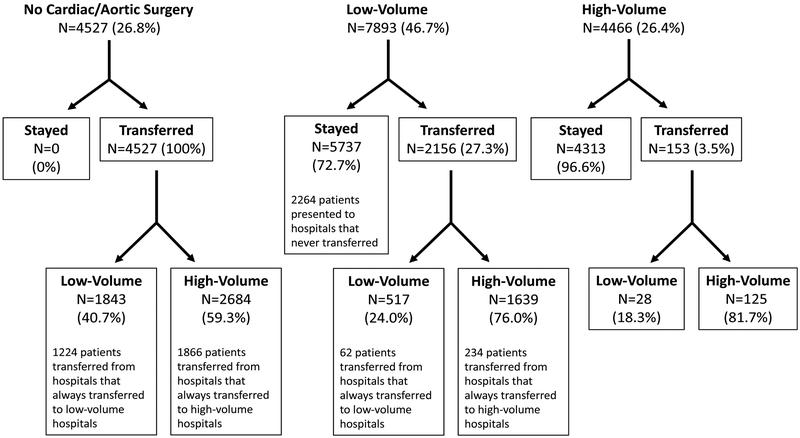
Of 31,320 patients with a diagnosis of thoracic aortic dissection of either type, 16,886 were eligible for inclusion and 8,956 were selected in the primary instrumental variable study (Table 1, Figure 1 & Supplemental Figure 3). Among 3,153 unique hospitals, 453 never transferred and 2,034 always transferred patients with acute type A aortic dissection (Supplemental Figure 1). Hospitals that always transferred patients with type A aortic dissection almost always transferred to either a high-volume or a low- volume hospital (83%); few hospitals (17%) transferred patients to both hospital types (Supplemental Figure 1).
Table 1.
Baseline and Operative Characteristics of the Study Population for the Analysis of Regionalization*
| Characteristic | Overall | Before Instrumental Variable Design | After Instrumental Variable Design & Matching |
||||
|---|---|---|---|---|---|---|---|
| All Acute Type A Aortic Dissection |
Not Rerouted to High- Volume |
Rerouted to High- Volume |
Not Rerouted to High- Volume |
Rerouted to High-Volume |
|||
| (N=16,886) | (N=8,125)† | (N=4,448)† | SMD | (N=3,400) | (N=3,400) | SMD | |
| Age - yr | 72.4 ± 9.5 | 72.3 ± 9.4 | 72.4 ± 9.4 | 0.009 | 72.3 ± 9.3 | 72.4 ± 9.5 | 0.01 |
| Age <65 yrs - no. (%) | 1935 (11.5) | 959 (11.8) | 507 (11.4) | 0.01 | 382 (11.2) | 382 (11.2) | <0.001 |
| Year of surgery - yr | 2006.8 (4.6) | 2006.7 (4.6) | 2006.9 (4.6) | 0.03 | 2006.6 (4.6) | 2006.8 (4.7) | 0.05 |
| Male sex - no. (%) | 9397 (55.6) | 4530 (55.8) | 2474 (55.6) | 0.003 | 1918 (56.4) | 1890 (55.6) | 0.02 |
| Race - no. (%) | |||||||
| White | 14335 (84.9) | 6910 (85.0) | 3766 (84.7) | 0.01 | 2920 (85.9) | 2942 (86.5) | 0.02 |
| Black | 1787 (10.6) | 758 (9.3) | 532 (12.0) | 0.09 | 280 (8.2) | 305 (9) | 0.03 |
| Asian | 268 (1.6) | 182 (2.2) | 47 (1.1) | 0.09 | 75 (2.2) | 40 (1.2) | 0.08 |
| Hispanic | 191 (1.1) | 102 (1.3) | 31 (0.7) | 0.06 | 39 (1.1) | 40 (1.2) | 0.003 |
| Other | 305 (1.8) | 173 (2.1) | 72 (1.6) | 0.04 | 86 (2.5) | 73 (2.1) | 0.03 |
| Prior myocardial infarction - no. (%) | 574 (3.4) | 286 (3.5) | 148 (3.3) | 0.01 | 99 (2.9) | 96 (2.8) | 0.005 |
| Alzheimer's dementia - no. (%) | 806 (4.8) | 386 (4.8) | 224 (5.0) | 0.01 | 154 (4.5) | 161 (4.7) | 0.01 |
| Atrial fibrillation - no. (%) | 2735 (16.2) | 1246 (15.3) | 664 (14.9) | 0.01 | 499 (14.7) | 464 (13.6) | 0.03 |
| Chronic kidney disease - no. (%) | 2555 (15.1) | 1203 (14.8) | 689 (15.5) | 0.02 | 459 (13.5) | 517 (15.2) | 0.05 |
| COPD - no. (%) | 3848 (22.8) | 1797 (22.1) | 1100 (24.7) | 0.06 | 714 (21.0) | 702 (20.6) | 0.009 |
| Congestive heart failure - no. (%) | 4230 (25.1) | 1920 (23.6) | 1097 (24.7) | 0.02 | 789 (23.2) | 761 (22.4) | 0.02 |
| Diabetes mellitus - no. (%) | 3049 (18.1) | 1414 (17.4) | 821 (18.5) | 0.03 | 542 (15.9) | 563 (16.6) | 0.02 |
| Hip fracture - no. (%) | 250 (1.5) | 111 (1.4) | 81 (1.8) | 0.04 | 38 (1.1) | 57 (1.7) | 0.05 |
| Ischemic heart disease - no. (%) | 7842 (46.4) | 3619 (44.5) | 2011 (45.2) | 0.01 | 1442 (42.4) | 1460 (42.9) | 0.01 |
| Arthritis - no. (%) | 2222 (13.2) | 1055 (13.0) | 558 (12.5) | 0.01 | 412 (12.1) | 393 (11.6) | 0.02 |
| Stroke - no. (%) | 1825 (10.8) | 859 (10.6) | 493 (11.1) | 0.02 | 347 (10.2) | 397 (11.7) | 0.05 |
| Cancer - no. (%) | 2039 (12.1) | 965 (11.9) | 546 (12.3) | 0.01 | 392 (11.5) | 419 (12.3) | 0.03 |
| Anemia - no. (%) | 6524 (38.6) | 3008 (37.0) | 1722 (38.7) | 0.04 | 1165 (34.3) | 1211 (35.6) | 0.03 |
| Hyperlipidemia - no. (%) | 9249 (54.8) | 4377 (53.9) | 2444 (54.9) | 0.02 | 1742 (51.2) | 1788 (52.6) | 0.03 |
| Hypertension - no. (%) | 12225 (72.4) | 5780 (71.1) | 3272 (73.6) | 0.05 | 2363 (69.5) | 2350 (69.1) | 0.008 |
| Hypothyroidism - no. (%) | 2329 (13.8) | 1146 (14.1) | 583 (13.1) | 0.03 | 447 (13.1) | 491 (14.4) | 0.04 |
| Region – no. (%) | |||||||
| New England | 947 (5.6) | 274 (3.4) | 363 (8.2) | 0.21 | 83 (2.4) | 190 (5.6) | 0.16 |
| Mideast | 3035 (18.0) | 923 (11.4) | 1084 (24.4) | 0.35 | 210 (6.2) | 478 (14.1) | 0.26 |
| Great Lakes | 3061 (18.1) | 1353 (16.7) | 901 (20.3) | 0.09 | 459 (13.5) | 389 (11.4) | 0.06 |
| Plains | 1267 (7.5) | 689 (8.5) | 263 (5.9) | 0.10 | 354 (10.4) | 470 (13.8) | 0.11 |
| Southeast | 4669 (27.7) | 2259 (27.8) | 1207 (27.1) | 0.02 | 925 (27.2) | 772 (22.7) | 0.10 |
| Southwest | 1504 (8.9) | 875 (10.8) | 300 (6.7) | 0.14 | 437 (12.9) | 432 (12.7) | 0.004 |
| Rocky Mountain | 460 (2.7) | 365 (4.5) | 41 (0.9) | 0.22 | 225 (6.6) | 141 (4.1) | 0.11 |
| Prior Procedures – no. (%) | |||||||
| Aortic valve surgery | 375 (2.2) | 168 (2.1) | 105 (2.4) | 0.02 | 70 (2.1) | 71 (2.1) | 0.002 |
| Thoracic aortic replacement | 36 (0.2) | 11 (0.1) | 15 (0.3) | 0.04 | 3 (0.1) | 6 (0.2) | 0.02 |
| Thoracoabdominal aortic Replacement | 19 (0.1) | 5 (0.1) | 8 (0.2) | 0.03 | 2 (0.1) | 3 (0.1) | 0.01 |
| TEVAR | 22 (0.1) | 9 (0.1) | 7 (0.2) | 0.01 | 2 (0.1) | 1 (0.0) | 0.01 |
| EVAR | 53 (0.3) | 26 (0.3) | 11 (0.2) | 0.01 | 8 (0.2) | 15 (0.4) | 0.04 |
| Abdominal aortic Replacement | 199 (1.2) | 85 (1.0) | 61 (1.4) | 0.03 | 33 (1.0) | 38 (1.1) | 0.01 |
| Mitral valve surgery | 155 (0.9) | 70 (0.9) | 40 (0.9) | 0.004 | 24 (0.7) | 35 (1) | 0.04 |
| Triscuspid valve surgery | 15 (0.1) | 5 (0.1) | 6 (0.1) | 0.02 | 4 (0.1) | 3 (0.1) | 0.009 |
| CABG | 988 (5.9) | 450 (5.5) | 272 (6.1) | 0.03 | 183 (5.4) | 180 (5.3) | 0.004 |
| VAD/ECMO | 14 (0.1) | 5 (0.1) | 6 (0.1) | 0.02 | 3 (0.1) | 2 (0.1) | 0.01 |
| Other cardiac surgery | 82 (0.5) | 33 (0.4) | 28 (0.6) | 0.03 | 15 (0.4) | 21 (0.6) | 0.02 |
| Index Surgical Procedures – no. (%) | |||||||
| Aortic valve surgery | 2428 (14.4) | 1098 (13.5) | 575 (12.9) | 0.02 | 413 (12.1) | 404 (11.9) | 0.008 |
| Aortic root replacement | 4816 (28.5) | 2319 (28.5) | 1184 (26.6) | 0.04 | 1002 (29.5) | 975 (28.7) | 0.02 |
| Ascending aortic replacement | 12228 (72.4) | 5878 (72.3) | 3297 (74.1) | 0.04 | 2427 (71.4) | 2446 (71.9) | 0.01 |
| Aortic arch replacement | 3044 (18.0) | 851 (10.5) | 1199 (27.0) | 0.43 | 345 (10.1) | 681 (20) | 0.28 |
| Descending thoracic aortic replacement | 195 (1.2) | 76 (0.9) | 59 (1.3) | 0.04 | 30 (0.9) | 28 (0.8) | 0.006 |
| Thoracoabdominal aortic replacement | 25 (0.1) | 13 (0.2) | 5 (0.1) | 0.01 | 4 (0.1) | 9 (0.3) | 0.03 |
| TEVAR | 154 (0.9) | 41 (0.5) | 60 (1.3) | 0.09 | 12 (0.4) | 33 (1.0) | 0.08 |
| EVAR | 19 (0.1) | 8 (0.1) | 6 (0.1) | 0.01 | 2 (0.1) | 3 (0.1) | 0.01 |
| Abdominal aortic replacement | 39 (0.2) | 24 (0.3) | 7 (0.2) | 0.03 | 10 (0.3) | 4 (0.1) | 0.04 |
| CABG | 4181 (24.8) | 2089 (25.7) | 849 (19.1) | 0.16 | 851 (25) | 683 (20.1) | 0.12 |
| Other valve surgery | 8 (0.0) | 5 (0.1) | 0 (0.0) | 0.04 | 2 (0.1) | 0 (0.0) | 0.03 |
| Other cardiac surgery | 340 (2.0) | 135 (1.7) | 77 (1.7) | 0.005 | 52 (1.5) | 72 (2.1) | 0.04 |
| Transferred - no. (%) | 6836 (40.5) | 2388 (29.4) | 4448 (100.0) | 2.19 | 1205 (35.4) | 3400 (100.0) | 1.91 |
| Surgery at high-volume center - no. (%) | 8761 (51.9) | 0 (0.0) | 4448 (100.0) | - | 0 (0.0) | 3400 (100.0) | - |
| Rerouted to high-volume center- no. (%) | 4448 (35.4) | 0 (0.0) | 4448 (100.0) | - | 0 (0.0) | 3400 (100.0) | - |
Plus-minus values are means +/− standard deviation. Variables excluded from table though well-balanced (SMD <0.1): cataracts, glaucoma, osteoporosis, asthma, hyperparathyroidism, and depression. CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; EVAR, endovascular aortic repair; SMD, standardized mean difference; TEVAR, thoracic endovascular aortic repair; VAD, ventricular assist device
The 4,313 patients who initially presented to high-level hospitals were excluded from the analysis of the effect of regionalization
Figure 1.
Landscape of study population. The top row represents the presenting hospital type for each patient. Percentages are row percentages within initial group. Patients transferred from high-volume hospitals were excluded from analyses.
During the study, 40.5% of patients were transferred, and 51.9% of patients received surgery at high-volume centers. The rate of transfer and the proportion of patients both presenting to and having surgery at high-volume centers remained stable during the study period (Supplemental Figures 4-6). Operative mortality from acute type A aortic dissection significantly decreased over time at both low- and high-volume hospitals (Supplemental Figure 7). Median follow-up time was 2.4 years (interquartile range (IQR) 0.1 to 6.5 years) and maximum follow-up time was 16 years.
Interfacility Transfer
Before and after matching, patients who were transferred were similar to those who remained at their presenting hospital (Supplemental Table 9). Interfacility transfer was associated with a reduction in the proportion of patients having surgery on the day of presentation (60.9% vs 45.4%, P<0.001); the median distance travelled in transfer was 28.5 miles (IQR 11.3 to 50.4 miles).
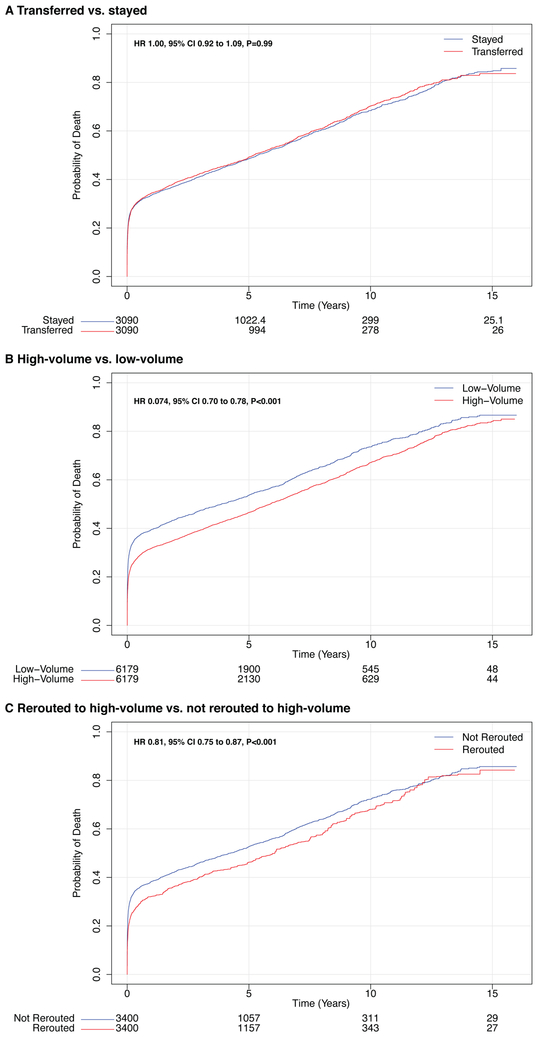
Despite a delay in surgery, interfacility transfer did not appear to affect the operative mortality rate (Table 2). A sensitivity analysis determined that 3.6% of all presenting patients would need to die as a result of transfer (i.e. death during transfer or clinical decompensation to a degree that precludes surgery) in order for the risk attributable to transfer to be statistically significant. Similarly, long-term mortality did not differ between patients who were transferred compared with those who remained at their presenting hospital (Figure 2A, Table 2).
Table 2.
Between-Group Differences in Operative Mortality and Long-Term Mortality For Comparisons of Transfer, Hospital Volume, and Regionalization
| Group Contrast Measure | Transferred vs. Stayed | High-Volume vs. Low-Volume* | Rerouted to High-Volume vs. Not Rerouted to High-Volume† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Transferred | Stayed | P Value |
High-Volume | Low-Volume | P Value |
Rerouted to High- Volume |
Not Rerouted to High- Volume |
P Value |
|
| Operative Mortality | |||||||||
| Operative mortality - % | 24.8 | 25.5 | - | 22.0 | 29.8 | - | 22.6 | 29.9 | - |
| Absolute risk difference - % (95% CI) | −0.69 (−2.70 to 1.35) | Reference | 0.35 | −8.1 (−10.0 to −6.2) | Reference | <0.001 | −7.2 (−10.3 to −4.1) | Reference | <0.001 |
| Odds ratio (95% CI) | 0.96 (0.87 to 1.07) | Reference | 0.50 | 0.66 (0.60 to 0.73) | Reference | <0.001 | 0.69 (0.58 to 0.81) | Reference | <0.001 |
| Number needed to treat - no. (95% CI) | - | - | - | - | - | - | 14 (10 to 25) | Reference | - |
| Long-Term Mortality | |||||||||
| Mortality at 15 yrs, % | 83.6 | 84.6 | - | 84.1 | 86.6 | - | 84.2 | 85.7 | - |
| Hazard ratio (95% CI) | 1.00 (0.92 to 1.09) | Reference | 0.99 | 0.74 (0.70 to 0.78) | Reference | <0.001 | 0.81 (0.75 to 0.87) | Reference | <0.001 |
| Restricted mean survival time at 15 yrs | |||||||||
| Difference - days (95% CI) | −45.5 (−142.2 to 51.2) | Reference | 0.36 | 288.2 (194.6 to 381.7) | Reference | <0.001 | 213.2 (85.7 to 340.7) | Reference | 0.001 |
| Ratio (95% CI) | 0.98 (0.94 to 1.02) | Reference | 0.36 | 1.14 (1.11 to 1.19) | Reference | <0.001 | 1.11 (1.04 to 1.17) | Reference | 0.001 |
| Ratio of restricted mean time lost (95% CI) | 1.02 (0.98 to 1.05) | Reference | 0.36 | 0.91 (0.88 to 0.94) | Reference | <0.001 | 0.93 (0.89 to 0.97) | Reference | 0.001 |
Gamma = 1.36 for this comparison. The gamma parameter estimates the amount of unmeasured bias necessary to render the finding null. For interpretation, a Gamma = 1.36, implies unobserved covariates would need to increase the odds of presenting at a hospital that would result in care at a low level hospital 2.00-fold, and increase the odds of operative mortality 1.72-fold, to render the presented finding null.
Gamma = 1.28 for this comparison. For interpretation, a Gamma = 1.28, implies unobserved covariates would need to increase the odds of presenting at a non-regionalized hospital 2.00-fold, and increase the odds of operative mortality 1.56-fold, to render the presented finding null.
CI, confidence interval
Figure 2.
Mortality after surgery for acute type A aortic dissection stratified by transfer status, volume status, and reroute status. All-cause mortality is plotted against time after surgery and stratified by whether patients (A) were transferred, (B) had surgery at a high-volume or low-volume hospital, and (C) were rerouted to a high-volume hospital. Numbers of patients at risk are included below each figure. Note that some numbers are not integers due to matched pairs with variable controls. CI, confidence interval; HR, hazard ratio
Hospital Volume
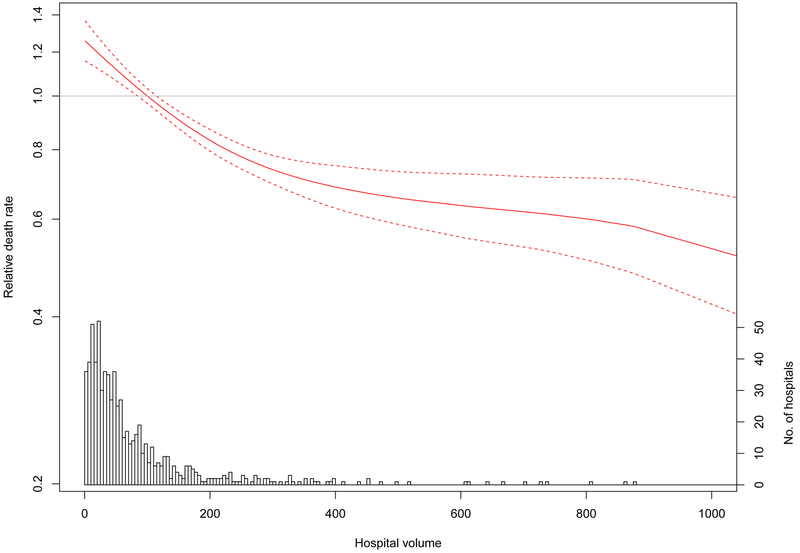
Patients who had surgery at high- versus low-volume hospitals were similar with respect to baseline comorbidities, though differed in the extent of operation received (Supplemental Table 10). Having surgery at a high-volume hospital was associated with a reduction in operative mortality from 29.9% to 21.8% (risk difference −8.1%, 95% CI −10.0 to −6.2, P<0.001) (Table 2). The observed reduction in operative mortality afforded by having the operation at a high-volume hospital also improved long-term survival (Figure 2B, Table 2). When examined as a continuous variable, the beneficial effect of increasing aortic surgery volume on reducing operative mortality persisted beyond the threshold used to define high-volume hospitals in our study (105 cases); the volume-outcome relationship plateaued at approximately 200 cases (Figure 3).
Figure 3.
Hospital volume-dependent hazard of operative mortality after repair of acute type A aortic dissection. The hazard ratio for operative mortality among patients undergoing repair of acute type A aortic dissection at a hospital of a given volume, compared with patients undergoing surgery at a hospital at the upper decile for volume, is plotted against hospital aortic surgery volume as a continuous variable (red solid line). Dashed red lines represent the 95% confidence interval. Hospital volume is based on the number of proximal aortic surgeries performed during the study period. The reference is set to 105 cases (upper decile of surgical volume and definition used for “high volume” in the present study. The horizontal gray line represents a relative hazard of 1. The histogram at the bottom of the figure depicts the number of hospitals with that particular volume.
Regionalizing Care
To estimate the impact of regionalization, patients who were transferred to high-volume centers for surgery (rerouted) were compared with patients who presented to and stayed at low-volume centers or were transferred to low-volume centers (Table 1). Rerouting patients to high-volume hospitals was associated with a significantly lower operative mortality compared with having surgery at a low-volume hospital (30.1% vs. 22.9%, risk difference −7.2%, 95% CI −10.3% to −4.1%, P<0.001) (Table 2). A sensitivity analysis demonstrated that 4.4% of patients rerouted to high-volume hospitals would need to die to nullify the significant survival benefit afforded by regionalization, and 16.6% of patients would need to die to render regionalization harmful. When age was examined as a continuous variable, visualization of the interaction between age and rerouting to high-volume hospitals suggested that the beneficial effect of regionalization on mortality extended below age 65 (Supplemental Figure 8). Early reductions in operative morality due to regionalization also persisted in the long term (Figure 2C, Table 2).
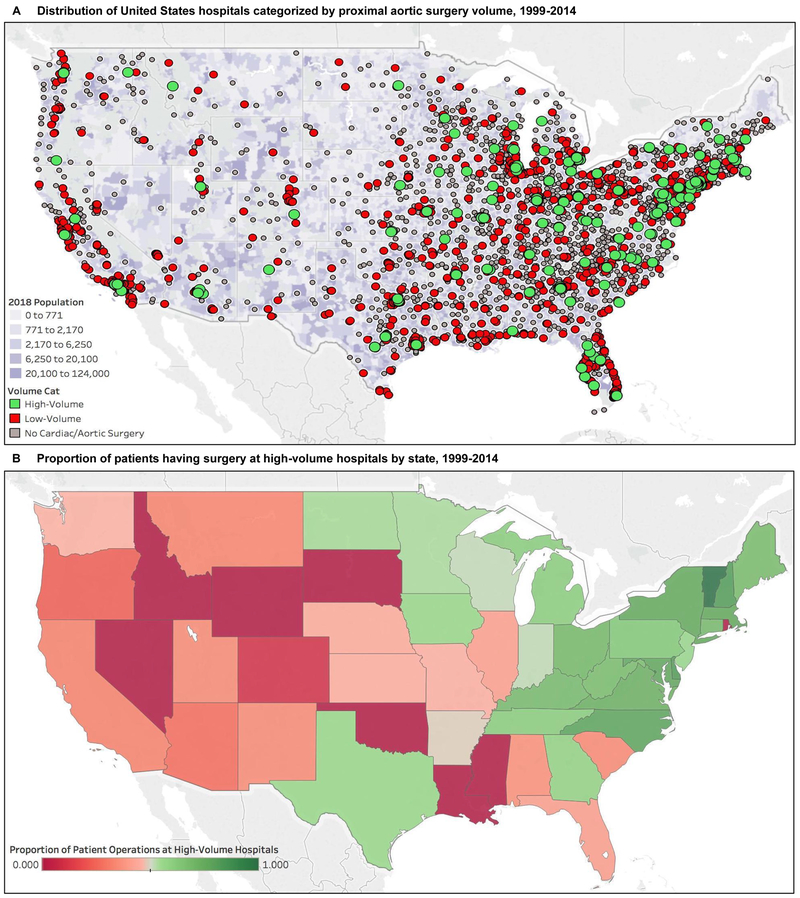
Among patients receiving surgery at low-volume institutions, the median additional travel distance needed to reroute each patient to a high-volume center was 50.1 miles (IQR 12.4 to 105.4 miles). High-volume hospitals were well distributed throughout the eastern United States, but were sparse in the west (Figure 4A). Greater access to high-volume hospitals in the northeast and mid-Atlantic regions was associated with a higher proportion of patients receiving surgical care at high-volume centers (Figure 4B).
Figure 4.
Location of United States hospitals categorized by aortic surgery volume and proportion of patients having surgery at high-volume hospitals by state. (A) The geographic location of each hospital in the study that treated a Medicare beneficiary with a type A aortic dissection stratified by proximal aortic and arch surgery volume. The hospital locations are plotted over a population density map of the counties within the continental United States (not shown: Alaska and Hawaii, 0 high-volume hospitals). (B) The proportion of patients that received surgical treatment for an acute type A aortic dissection at a high-volume hospital within each state of the continental United States (not shown: Alaska, 0%; Hawaii, 0%).
DISCUSSION
Acute type A aortic dissection is a surgical emergency and delayed treatment may be catastrophic.5, 6 Perioperative management and surgery for this disease is complex; in our analysis, outcomes varied significantly with aortic surgery experience. Nearly half of patients did not receive surgery at high-volume hospitals, though many of these patients already incurred the potential risk associated with interfacility transfer. Our study provides strong evidence for the hypothesis that, despite delaying surgical intervention, transferring Medicare beneficiaries with acute type A aortic dissection to higher-volume aortic surgery hospitals would significantly reduce mortality; only 14 patients would need to be rerouted to high-volume hospitals to save an additional life.
Surgeon and hospital experience are associated with improved outcomes in elective26 and emergent thoracic aortic surgery.9, 11 However, prior studies failed to distinguish between types A and B pathology (which are managed very differently), and did not account for selection bias due to disease severity. In the Supplement, we use our data to demonstrate how disregarding bias arising from unobserved factors leads to biased and inaccurate results. Furthermore, previous studies failed to address interfacility transfer: a factor with critical policy implications that can bias results if neglected. By limiting our analysis to patients who were obligate transfers or non-transfers, we minimized important unmeasured confounding and confirmed a robust effect of hospital aortic surgery experience on mortality.
More than 60% of patients treated for acute type A aortic dissection at experienced centers originate from other hospitals.12, 14, 16, 27 Whereas transfer rates in the International Registry of Acute Aortic Dissection (IRAD) registry are high and increasing (62% to 71% over 17 years),14 Medicare beneficiaries with aortic dissection are transferred at a much lower rate (40%), and this rate has not changed. This is particularly concerning given that most – but not all – patients with acute type A aortic dissection can be stabilized and survive transfer. In separate analyses of acute aortic syndromes, reported transfer mortality rates ranged from 0% to 2.7%.15, 16 Our study corroborates such findings; interfacility transfer did not increase the risk of operative mortality, and >4% of transferred patients would need to die from transfer to render regionalization ineffective.
Our definition of “high-volume” was set a priori and appears to be conservative; the effect of volume likely extends beyond our threshold of 105 proximal aortic and transverse arch operations used in this study. Hospital volumes were calculated using only Medicare beneficiary information, which underestimates the true volume of such surgeries in hospitals. However, this definition permitted 1 high-volume hospital per 3-4 million people in the United States. The resultant geographic distribution of high-volume hospitals may represent a feasible blueprint for regionalization. Directing patients to high-performing hospitals may be preferable to indiscriminately concentrating care via certificate-of-need legislation, a tactic that has thus far yielded lackluster effects on outcomes.28
The additional travel distance needed to reroute a patient to a high-volume center is reasonable. In the Medicare population we examined, instituting a policy of regionalization would require a change of behavior for 66% of hospitals. Approximately two-thirds of these hospitals would need to change from transferring patients to low-volume facilities to transferring them to high-volume facilities, and approximately one-third would need to transfer patients to high-volume facilities instead of operating on them. For 90% of patients, transit time for rerouting to high-volume centers (≤220 miles) would only delay surgery 1 to 2 hours if traveling by rotary wing transport. Yet, transit time represents only a fraction of the total time required for interfacility transfer. Delays often occur while securing transport or awaiting beds at the accepting facility, but a regionalization protocol may abrogate such inefficiencies.
A plan for regionalization of care for acute type A aortic dissection might be feasible across the United States, but nationwide transfer systems – like that which exists in North West London29 – with defined targets for hemodynamic management are needed to facilitate widespread regionalization.30 Although transfer delays surgical intervention, it may be an opportunity to improve early medical care for aortic dissection. Among patients undergoing transfer for suspected acute aortic dissection, pre-transport hemodynamic therapy was frequently absent or inadequate at the originating hospital, and blood pressure and heart rate medications were instead initiated by the transport team in over 30% of cases.31 Therefore, coordinated transfer systems for rerouting patients with suspected acute aortic syndromes to high-volume hospitals – as occurs in Cleveland15 and Minnesota16 – might decrease risk while awaiting surgery.
Our study has several limitations. Because our diagnosis of type A dissection required evidence of surgical intervention, we did not capture patients who died without surgery. This limitation leaves our estimates susceptible to selection bias, although our sensitivity analyses demonstrate that the magnitude of the bias would have to be large to change the overall inference. Medicare data are subject to coding error and lack important clinical details, including hemodynamic stability. The older age of the Medicare population may limit generalizability to younger patients, but it is possible that younger patients may stand to benefit more from regionalization because more extensive operations performed at high-volume hospitals may enhance durability. Our study is the largest investigation of acute type A aortic dissection to date, and the examination of transfer patterns across the entire United States is unique. It is unlikely that a prospective clinical trial of necessary size and duration can be feasibly conducted; and limitations in state or national databases, or in the Society of Thoracic Surgeons or IRAD registries, preclude valid analyses of interfacility transfer and regionalization.
Half of patients with acute type A aortic dissection receive operations at low-volume hospitals, and over one-third of transferred patients are sent to low-volume centers. Regionalizing care by rerouting patients with type A aortic dissection to high-volume hospitals may substantially reduce perioperative mortality and improve long-term survival. We estimate only 14 patients would need to be rerouted to a high-volume hospital to save an additional life. Whenever possible, the emergency surgical treatment of acute type A aortic dissection should be concentrated within specialized aortic surgery centers.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
In this national, retrospective cohort study of 16,886 Medicare beneficiaries with acute type A aortic dissection, operative and long-term mortality were significantly lower in patients with acute type A aortic dissection who received surgery at high-volume hospitals.
Despite delaying surgery, a regionalization policy that transfers patients to high-volume hospitals instead of low-volume aortic surgery hospitals was associated with a substantial reduction in operative mortality; this association persisted in the long term.
What are the clinical implications?
Nearly half of patients with acute type A aortic dissection do not receive surgery at high-volume hospitals, though many of these patients already incur the potential risk associated with interfacility transfer.
Our study provides strong evidence for the hypothesis that, despite delaying surgical intervention, transferring Medicare beneficiaries with acute type A aortic dissection to higher-volume aortic surgery hospitals would significantly reduce mortality.
Policy-makers should evaluate the feasibility and benefits of regionalizing the surgical treatment of acute type A aortic dissection to specialized aortic surgery programs.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the National Institutes of Health, National Center for Advancing Translational Science, Clinical and Translational Science Awards (NIH TL1 TR000084, to Dr. Goldstone; NIH KL2 TR000083, to Dr. Chiu; and NIH UL1 TR001085, to the Stanford Center for Clinical Translational Education and Research), and by a grant (KHS022192A, to Dr. Baiocchi) from the Agency for Healthcare Research and Quality.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- CI
confidence interval
- ICD9-CM
International Classification of Diseases, 9th Edition, Clinical Modification
- IRAD
International Registry of Acute Aortic Dissection
- IQR
interquartile range
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Clouse WD, Hallett JW Jr., Schaff HV, Spittell PC, Rowland CM, Ilstrup DM Melton LJ 3rd. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79:176–80. [DOI] [PubMed] [Google Scholar]
- 2.Meszaros I, Morocz J, Szlavi J, Schmidt J, Tornoci L, Nagy L Szep L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–8. [DOI] [PubMed] [Google Scholar]
- 3.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237–47. [DOI] [PubMed] [Google Scholar]
- 4.Nienaber CA Clough RE. Management of acute aortic dissection. Lancet. 2015;385:800–11. [DOI] [PubMed] [Google Scholar]
- 5.Hirst AE Jr., Johns VJ Jr. Kime SW Jr. Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore). 1958;37:217–79. [DOI] [PubMed] [Google Scholar]
- 6.Austen WG Desanctis RW. Surgical Treatment of Dissecting Aneurysm of the Thoracic Aorta. N Engl J Med. 1965;272:1314–7. [DOI] [PubMed] [Google Scholar]
- 7.Bavaria JE, Pochettino A, Brinster DR, Gorman RC, McGarvey ML, Gorman JH, Escherich A Gardner TJ. New paradigms and improved results for the surgical treatment of acute type A dissection. Ann Surg. 2001;234:336–42; discussion 342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu P Miller DC. Evolution of surgical therapy for Stanford acute type A aortic dissection. Ann Cardiothorac Surg. 2016;5:275–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chikwe J, Cavallaro P, Itagaki S, Seigerman M, Diluozzo G Adams DH. National outcomes in acute aortic dissection: influence of surgeon and institutional volume on operative mortality. Ann Thorac Surg. 2013;95:1563–9. [DOI] [PubMed] [Google Scholar]
- 10.Miller DC. Another meiosis in the specialty of cardiovascular and thoracic surgery: birth of the purebred “thoracic aortic surgeon”? J Am Coll Cardiol. 2014;63:1804–6. [DOI] [PubMed] [Google Scholar]
- 11.Knipp BS, Deeb GM, Prager RL, Williams CY, Upchurch GR Jr. Patel HJ. A contemporary analysis of outcomes for operative repair of type A aortic dissection in the United States. Surgery. 2007;142:524–8; discussion 528 e1. [DOI] [PubMed] [Google Scholar]
- 12.Andersen ND, Ganapathi AM, Hanna JM, Williams JB, Gaca JG Hughes GC. Outcomes of acute type a dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol. 2014;63:1796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. [DOI] [PubMed] [Google Scholar]
- 14.Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, Myrmel T, Larsen M, Harris KM, Greason K, Di Eusanio M, Bossone E, Montgomery DG, Eagle KA, Nienaber CA, Isselbacher EM O’Gara P. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66:350–8. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal B, Raymond C, Jacob J, Kralovic D, Kormos K, Holloway D Menon V. Transfer of patients with suspected acute aortic syndrome. Am J Cardiol. 2013;112:430–5. [DOI] [PubMed] [Google Scholar]
- 16.Harris KM, Strauss CE, Duval S, Unger BT, Kroshus TJ, Inampudi S, Cohen JD, Kapsner C, Boland LL, Eales F, Rohman E, Orlandi QG, Flavin TF, Kshettry VR, Graham KJ, Hirsch AT Henry TD. Multidisciplinary standardized care for acute aortic dissection: design and initial outcomes of a regional care model. Circ Cardiovasc Qual Outcomes. 2010;3:424–30. [DOI] [PubMed] [Google Scholar]
- 17.Kahn JM Iwashyna TJ. Accuracy of the discharge destination field in administrative data for identifying transfer to a long-term acute care hospital. BMC Res Notes. 2010;3:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baiocchi M, Cheng J Small DS. Instrumental variable methods for causal inference. Stat Med. 2014;33:2297–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClellan M, McNeil BJ Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA. 1994;272:859–66. [PubMed] [Google Scholar]
- 20.Hernan MA Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol. 2016;183:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum PR. Observational studies. 2nd ed. New York: Springer; 2002. [Google Scholar]
- 22.Silber JH, Rosenbaum PR, Trudeau ME, Even-Shoshan O, Chen W, Zhang X Mosher RE. Multivariate matching and bias reduction in the surgical outcomes study. Med Care. 2001;39:1048–64. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC Stuart EA. Optimal full matching for survival outcomes: a method that merits more widespread use. Stat Med. 2015;34:3949–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uno H, Claggett B, Tian L, Inoue E, Gallo P, Miyata T, Schrag D, Takeuchi M, Uyama Y, Zhao L, Skali H, Solomon S, Jacobus S, Hughes M, Packer M Wei LJ. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32:2380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum PR Rubin DB. Assessing sensitivity to an unobserved binary covariate in an observational study with binary outcome. J R Statist Soc. 1983;45:212–218. [Google Scholar]
- 26.Hughes GC, Zhao Y, Rankin JS, Scarborough JE, O’Brien S, Bavaria JE, Wolfe WG, Gaca JG, Gammie JS, Shahian DM Smith PK. Effects of institutional volumes on operative outcomes for aortic root replacement in North America. J Thorac Cardiovasc Surg. 2013;145:166–70. [DOI] [PubMed] [Google Scholar]
- 27.Raghupathy A, Nienaber CA, Harris KM, Myrmel T, Fattori R, Sechtem U, Oh J, Trimarchi S, Cooper JV, Booher A, Eagle K, Isselbacher E, Bossone E International Registry of Acute Aortic Dissection I. Geographic differences in clinical presentation, treatment, and outcomes in type A acute aortic dissection (from the International Registry of Acute Aortic Dissection). Am J Cardiol. 2008;102:1562–6. [DOI] [PubMed] [Google Scholar]
- 28.DiSesa VJ, O’Brien SM, Welke KF, Beland SM, Haan CK, Vaughan-Sarrazin MS Peterson ED. Contemporary impact of state certificate-of-need regulations for cardiac surgery: an analysis using the Society of Thoracic Surgeons’ National Cardiac Surgery Database. Circulation. 2006;114:2122–9. [DOI] [PubMed] [Google Scholar]
- 29.Vaja R, Talukder S, Norkunas M, Hoffman R, Nienaber C, Pepper J, Rosendahl U, Asimakopoulos G Quarto C. Impact of a streamlined rotational system for the management of acute aortic syndrome: sharing is caring. Eur J Cardiothorac Surg. 2019;55:984–989. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal B, Raymond CE, Randhawa MS, Roselli E, Jacob J, Eagleton M, Kralovic DM, Kormos K, Holloway D Menon V. Transfer metrics in patients with suspected acute aortic syndrome. Circ Cardiovasc Qual Outcomes. 2014;7:780–2. [DOI] [PubMed] [Google Scholar]
- 31.Winsor G, Thomas SH, Biddinger PD Wedel SK. Inadequate hemodynamic management in patients undergoing interfacility transfer for suspected aortic dissection. Am J Emerg Med. 2005;23:24–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.