Abstract
Background
Whether a second arterial conduit improves outcomes after multi-vessel coronary artery bypass grafting remains unclear. Consequently, arterial conduits other than the left internal thoracic artery are seldom used in the United States.
Methods
Using a state-maintained clinical registry including all 126 non-federal hospitals in California, we compared all-cause mortality and rates of stroke, myocardial infarction, repeat revascularization, and sternal wound infection between propensity score-matched cohorts who underwent primary, isolated multi-vessel coronary artery bypass grafting with the left internal thoracic artery, and who received a second arterial conduit (right internal thoracic artery or radial artery, N=5,866) or a venous conduit (N=53,566) between 2006 and 2011. Propensity score matching using 34 preoperative characteristics yielded 5,813 matched sets. A sub-group analysis compared outcomes between propensity score-matched recipients of a right internal thoracic artery (N=1,576) or a radial artery (N=4,290).
Results
Second arterial conduit use decreased from 10.7% in 2006 to 9.1% in 2011 (p<0.0001). However, receipt of a second arterial conduit was associated with significantly lower mortality (13.1% vs. 10.6% at 7 years; HR 0.79, 95% CI 0.72–0.87), and lower risks of myocardial infarction (HR 0.78, 95% CI 0.70–0.87) and repeat revascularization (HR 0.82, 95% CI 0.76–0.88). Compared with radial artery grafts, right internal thoracic artery grafts were associated with similar mortality rates (right internal thoracic artery 10.3% vs. radial artery 10.7% at 7 years; HR 1.10, 95% CI 0.89–1.37) and individual risks of cardiovascular events, but the risk of sternal wound infection was increased (risk difference 1.07%, 95% CI 0.15%–2.07%).
Conclusions
Second arterial conduit use in California is low and declining, but arterial grafts were associated with significantly lower mortality and fewer cardiovascular events. A right internal thoracic artery graft offered no benefit over that of a radial artery, but did increase risk of sternal wound infection. These findings suggest surgeons should consider lowering their threshold for using arterial grafts, and the radial artery may be the preferred second conduit.
Keywords: Coronary artery bypass graft surgery
INTRODUCTION
Coronary artery disease is a leading cause of death in the United States1 and Europe.2 When disease is severe, coronary artery bypass grafting (CABG) is more effective than any other therapy.3–6 However, the CABG operation is highly varied; surgeons must choose between biologically-disparate bypass conduits and the manner in which they are implanted. These choices are not well standardized, and each variation may carry different risks and benefits.
The left internal thoracic artery (ITA) is the optimal conduit; its patency rates are unmatched and its anatomic location facilitates anastomosis to the left coronary vessels.7–9 The most common CABG operation performed worldwide bypasses the left anterior descending coronary artery with the left ITA, and the other coronary arteries with the saphenous vein.10 However, higher rates of saphenous vein graft failure relative to the left ITA9 have led surgeons to search for better secondary conduits. The right ITA and radial artery are both promising, but discordant results in single-center observational studies11, 12 and worry about increased risks of sternal wound infection13 or early graft failure14 impede widespread adoption. Guidelines are vague regarding when to use a second arterial conduit,10, 15 and many believe equipoise exists. Most recently, a randomized trial of single versus bilateral ITA conduits for CABG demonstrated no benefit 5 years after surgery.16 However, the trial may have been predisposed to a null result due to crossover between treatments, use of radial artery conduits in the control group, and the near-optimal medical management within the trial. A population-level analysis of “real-world” outcomes is needed to help inform guidelines.
We conducted a statewide retrospective cohort study to compare the effectiveness of second arterial conduits with that of venous conduits for CABG in California.
METHODS
All data are available for purchase from the California Office of Statewide Health Planning and Development (OSHPD).17 The authors are not permitted to share the data directly.
Study Design
We examined patients who underwent primary, multi-vessel CABG in California between January 1, 2006 and July 1, 2011 to evaluate the influence of a second arterial conduit (radial artery or right ITA) on mortality, stroke, myocardial infarction, and repeat revascularization. The California Committee for the Protection of Human Subjects and the institutional review board at Stanford University approved this research. Informed consent was not required. All authors accept responsibility for the accuracy of the analyses.
Study Population and Intervention
Patients were included if they underwent primary, multi-vessel CABG with the left ITA during the study period. Exclusion criteria were: out-of-state residency, single-vessel coronary artery disease, history of prior CABG, receipt of more than two arterial conduits, and any concomitant cardiac or aortic operation. Patients who received either a radial artery or right ITA graft comprised the experimental (arterial conduit) group; patients who received a left ITA graft with venous conduits for remaining grafts comprised the control (venous conduit) group.
All records were obtained from the California CABG Outcomes Reporting Program (CCORP)—a state-maintained and audited, mandatory clinical registry of all CABG patients discharged from the 126 non-federal, California-licensed hospitals—and the California OHSPD Patient Discharge, Emergency Department, and Ambulatory Surgery Center data sets. Operative details and baseline characteristics were collected from the CCORP registry. Additional comorbidities were ascertained from prior hospitalizations, or from diagnoses that were coded as “present on admission” during the index hospitalization (Supplemental Table 1 in the Supplement).
Outcomes
The primary endpoint was all-cause mortality. The OSHPD patient discharge database is linked to the California Department of Public Health Death Statistical Master File (DSMF),18 the annual state death record which is distinct from the Social Security Death Index. Longitudinal clinical follow-up was obtained by matching record linkage number and birth year across all encounters. Secondary endpoints included perioperative mortality (within 30 days of surgery), sternal wound infection, major adverse cardiovascular and cerebrovascular events (MACCE, defined as the composite of stroke, myocardial infarction, and repeat revascularization), and the cumulative incidence of each individual MACCE event. Sternal wound infection was defined as an infected wound with coexisting osteomyelitis, dehiscence, or mediastinitis, or that requiring surgical debridement.19 Stroke was defined as an incident ischemic or hemorrhagic cerebral event. Myocardial infarction included any subsequent visit for treatment of an incident acute myocardial infarction. Repeat revascularization included any reoperative CABG or percutaneous coronary intervention after the index operation (see Supplemental Table 2 in the Supplement for International Classification of Diseases, Ninth Revision, Clinical Modification definitions of nonfatal events). Absent the event of interest, patients were censored on December 31, 2013, the last date of DSMF and clinical follow-up.
Statistical Analysis
The study was designed to achieve a power of at least 99% with an alpha-level of 0.05 to detect a reduction in the hazard of death by 20% over 8 years in second arterial conduit recipients. We hypothesized that the type of arterial conduit (radial artery versus right ITA) would not affect survival, but had at least 80% power with an alpha-level of 0.025 to detect a survival difference of 20% in this planned subgroup comparison.
We used propensity score matching to limit confounding by indication. Non-parsimonious logistic regression was used to estimate each patient’s probability of receiving a second arterial conduit (Supplemental Figure 1).20 Patients were optimally-matched with up to four controls per treated subject (Supplemental Table 3). Controls were weighted to estimate the average treatment effect on the treated (Supplemental Figure 2).21 Balance between treatment groups was assessed with standardized mean differences, with ≤10% deemed ideal balance, and ≤20% deemed acceptable balance.22 As a sensitivity analysis, we also tested our primary hypothesis with a matching-based, instrumental variable design23 (see Supplemental Methods in the Supplement) to try to mitigate unmeasured confounding due to unmeasured characteristics (e.g. target vessel anatomy and frailty). Briefly, we matched similar patients between surgeons who frequently used second arterial conduits with those who did not. Pre-specified sub-group analyses included stratification by number of diseased vessels and a direct comparison of radial artery and right ITA grafts. For the latter sub-group comparison, propensity score matching was performed only among recipients of second arterial conduits to estimate the average treatment effect of receiving a right ITA graft.
We estimated the risk difference (RD) in 30-day mortality and sternal wound infection by calculating the difference in the marginal probability of each outcome; 95% confidence intervals (CI) were obtained with 100,000 bootstrap replicates of the matched sets. Weighted Cox proportional hazards regression with a robust variance estimator was used to compare survival and freedom from MACCE. Additional estimates were obtained after multivariable adjustment for all baseline characteristics, or inclusion of surgeon as a random effect. The restricted mean survival time was calculated as an alternative means of describing the effect of treatment during the study period.24 As an exploratory analysis, the cumulative incidence of stroke, myocardial infarction, and repeat revascularization after index CABG was compared between treatment groups with death as a competing risk. Subdistribution hazards in the matched population were estimated with the Fine and Gray method.25 To explore age-dependent effects of a second arterial conduit on survival, a Cox proportional hazards model was fit to the matched study population with the use of an interaction term for age (modeled as a natural spine) and receipt of a second arterial conduit. Standard errors were computed from 1,000 bootstrap replicates.
A gamma sensitivity analysis was performed to determine the sensitivity of our results to unmeasured confounding.26 All tests of treatment effect were two-tailed with an alpha threshold of 0.05. Statistical analyses were performed in R version 3.2.3 (R Foundation, Vienna, Austria); data management was performed with SAS version 9.2 (SAS Institute, Cary, NC). Further statistical details are in the Supplement.
RESULTS
Patients and Trends in Arterial Conduit Use
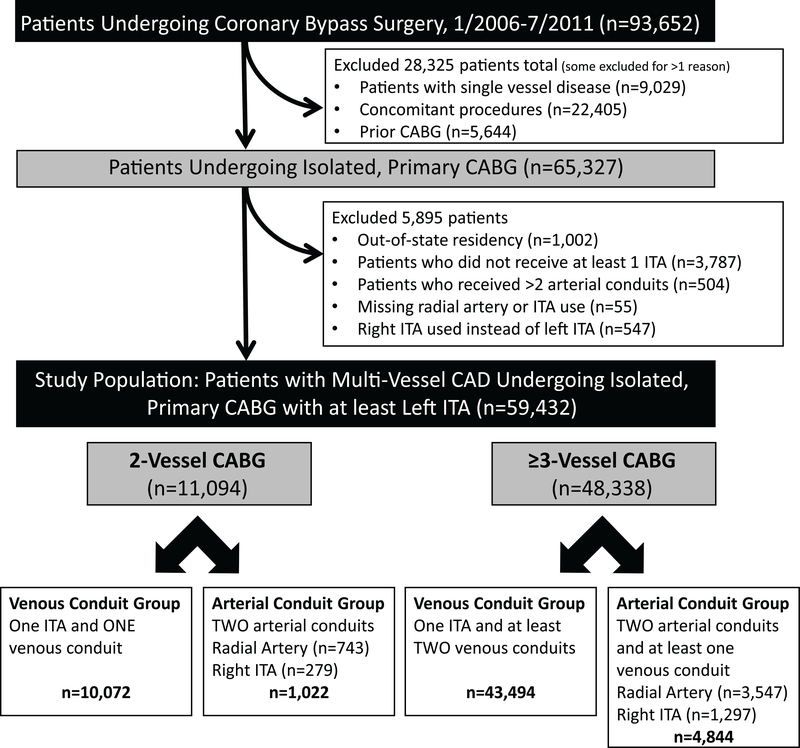
Of 93,652 patients who underwent CABG during the study period, 59,432 were eligible for the investigation (Figure 1). At baseline, patients who received a second arterial conduit were younger and had fewer comorbidities than recipients of venous conduits (Table 1). Propensity score matching successfully balanced the baseline characteristics (Table 1 & Supplemental Table 4). Median follow-up time was 5.3 years (interquartile range (IQR) 3.8 – 6.7 years) for recipients of second arterial conduits and 5.2 years (IQR 3.7 – 6.6 years) for recipients of venous conduits.
Figure 1.
Patient Selection Flow Diagram. CABG, coronary artery bypass grafting; CAD, coronary artery disease; ITA, internal thoracic artery
Table 1.
Baseline Characteristics of the Study Population Before and After Propensity Score Matching*
| Characteristic | Overall | Before Matching | After Matching† | ||||
|---|---|---|---|---|---|---|---|
| Study Population | Venous Second Conduit | Arterial Second Conduit | Venous Second Conduit | Arterial Second Conduit | |||
| (N=59,432) | (N=53,566) | (N=5,866) | SMD | (N=5,813) | (N=5,813) | SMD | |
| Age – yr | 66.0 ± 10.5 | 66.5 ± 10.4 | 62.0 ± 10.5 | 0.43 | 62.5 ±10.4 | 62.0 ± 10.4 | 0.05 |
| Year of surgery – yr | 2008.2 ± 1.6 | 2008.2 ± 1.6 | 2008.1 ± 1.61 | 0.06 | 2008.1 ± 1.6 | 2008.1 ± 1.6 | 0.007 |
| Male sex - no. (%) | 44933 (75.6) | 39930 (74.5) | 5003 (85.3) | 0.27 | 4897.1 (84.2) | 4960.0 (85.3) | 0.03 |
| Race - no. (%) | 0.26 | 0.09 | |||||
| Unknown | 925 (1.6) | 859 (1.6) | 66 (1.1) | 76.1 (1.3) | 66.0 (1.1) | ||
| White | 36518 (61.4) | 32328 (60.4) | 4190 (71.4) | 4161.0 (71.6) | 4161.0 (71.6) | ||
| Black | 2271 (3.8) | 2125 (4.0) | 146 (2.5) | 170.5 (2.9) | 142.0 (2.4) | ||
| Hispanic | 10461 (17.6) | 9796 (18.3) | 665 (11.3) | 655.0 (11.3) | 655.0 (11.3) | ||
| Asian | 6641 (11.2) | 6023 (11.2) | 618 (10.5) | 513.0 (8.8) | 611.0 (10.5) | ||
| Native American | 126 (0.2) | 114 (0.2) | 12 (0.2) | 11.2 (0.2) | 11.0 (0.2) | ||
| Other | 2490 (4.2) | 2321 (4.3) | 169 (2.9) | 226.2 (3.9) | 167.0 (2.9) | ||
| Height – cm | 170.5 ± 10.5 | 170.2 ± 10.5 | 173.3 ± 9.5 | 0.31 | 173.2 ± 9.7 | 173.4 ± 9.5 | 0.02 |
| Weight – kg | 84.2 ± 18.9 | 83.8 ± 18.9 | 88.3 ± 18.6 | 0.24 | 88.0 ± 18.8 | 88.3 ± 18.7 | 0.01 |
| Ejection fraction - % | 52.7 ± 13.5 | 52.4 ± 13.6 | 55.6 ± 12.1 | 0.25 | 55.6 ± 12.0 | 55.6 ± 12.0 | <0.001 |
| Creatinine - mg/dL | 1.26 ± 1.09 | 1.28 ± 1.13 | 1.08 ± 0.54 | 0.23 | 1.09 ± 0.57 | 1.08 ± 0.54 | 0.02 |
| Dialysis - no. (%) | 2244 (3.8) | 2196 (4.1) | 48 (0.8) | 0.21 | 56.2 (1.0) | 47.0 (0.8) | 0.02 |
| Diabetes mellitus - no. (%) | 26558 (44.7) | 24481 (45.7) | 2077 (35.4) | 0.21 | 2065.8 (35.5) | 2051.0 (35.3) | 0.005 |
| Hypertension - no. (%) | 51158 (86.1) | 46414 (86.6) | 4744 (80.9) | 0.16 | 4748.3 (81.7) | 4702.0 (80.9) | 0.02 |
| PVD - no. (%) | 7942 (13.4) | 7384 (13.8) | 558 (9.5) | 0.13 | 573.2 (9.9) | 554.0 (9.5) | 0.01 |
| Cerebrovascular disease - no. (%) | 7979 (13.4) | 7448 (13.9) | 531 (9.1) | 0.15 | 558.7 (9.6) | 527.0 (9.1) | 0.02 |
| Chronic lung disease - no. (%) | 0.20 | <0.001 | |||||
| None | 47094 (79.2) | 42111 (78.6) | 4983 (84.9) | 4957.0 (85.3) | 4957.0 (85.3) | ||
| Mild | 6938 (11.7) | 6346 (11.8) | 592 (10.1) | 579.0 (10.0) | 579.0 (10.0) | ||
| Moderate | 3121 (5.3) | 2936 (5.5) | 185 (3.2) | 178.0 (3.1) | 178.0 (3.1) | ||
| Severe | 2251 (3.8) | 2147 (4.0) | 104 (1.8) | 99.0 (1.7) | 99.0 (1.7) | ||
| Congestive heart failure - no. (%) | 10572 (17.8) | 9989 (18.6) | 583 (9.9) | 0.25 | 610.2 (10.5) | 575.0 (9.9) | 0.02 |
| Prior MI - no. (%) | 0.20 | <0.001 | |||||
| None | 30556 (51.4) | 27224 (50.8) | 3332 (56.8) | 3326.0 (57.2) | 3326.0 (57.2) | ||
| >21 days | 10260 (17.3) | 9250 (17.3) | 1010 (17.2) | 1001.0 (17.2) | 1001.0 (17.2) | ||
| 8 to 21 days | 2673 (4.5) | 2530 (4.7) | 143 (2.4) | 140.0 (2.4) | 140.0 (2.4) | ||
| 1 to 7 days | 13695 (23.0) | 12438 (23.2) | 1257 (21.4) | 1250.0 (21.5) | 1250.0 (21.5) | ||
| >6 hrs but <24 hrs | 1467 (2.4) | 1397 (2.6) | 70 (1.2) | 64.0 (1.1) | 64.0 (1.1) | ||
| ≤6 hrs | 690 (1.2) | 654 (1.2) | 36 (0.6) | 24.0 (0.4) | 24.0 (0.4) | ||
| Prior PCI - no. (%) | 0.06 | 0.01 | |||||
| None | 46692 (78.6) | 42001 (78.4) | 4691 (80.0) | 4663.7 (80.2) | 4653.0 (80.0) | ||
| >6 hrs | 12308 (20.7) | 11154 (20.8) | 1154 (19.7) | 1129.8 (19.4) | 1144.0 (19.7) | ||
| ≤6 hrs | 429 (0.7) | 408 (0.8) | 21 (0.4) | 19.5 (0.3) | 16.0 (0.3) | ||
| Mitral regurgitation - no. (%) | 0.15 | 0.02 | |||||
| None | 39354 (66.2) | 35367 (66.0) | 3987 (68.0) | 3940.2 (67.8) | 3952.0 (68.0) | ||
| Trivial | 6834 (11.5) | 6006 (11.2) | 828 (14.1) | 799.8 (13.8) | 820.0 (14.1) | ||
| Mild | 8042 (13.5) | 7382 (13.8) | 660 (11.3) | 681.2 (11.7) | 654.0 (11.3) | ||
| Moderate | 2573 (4.3) | 2414 (4.5) | 159 (2.7) | 169.7 (2.9) | 157.0 (2.7) | ||
| Severe | 246 (0.4) | 235 (0.4) | 11 (0.2) | 9.8 (0.2) | 11.0 (0.2) | ||
| Cardiogenic shock - no. (%) | 634 (1.1) | 616 (1.1) | 18 (0.3) | 0.10 | 22.2 (0.4) | 15.0 (0.3) | 0.02 |
| CPR - no. (%) | 259 (0.4) | 247 (0.5) | 12 (0.2) | 0.05 | 13.0 (0.2) | 10.0 (0.2) | 0.01 |
| Atrial fibrillation - no. (%) | 16803 (28.3) | 15278 (28.5) | 1525 (26.0) | 0.06 | 1533.4 (26.4) | 1513.0 (26.0) | 0.008 |
| Liver disease - no. (%) | 2934 (4.9) | 2737 (5.1) | 197 (3.4) | 0.09 | 198.8 (3.4) | 195.0 (3.4) | 0.004 |
| Cancer - no. (%) | 2116 (3.6) | 1971 (3.7) | 145 (2.5) | 0.07 | 148.2 (2.5) | 144.0 (2.5) | 0.005 |
| Osteoporosis - no. (%) | 1492 (2.5) | 1416 (2.6) | 76 (1.3) | 0.10 | 86.3 (1.5) | 76.0 (1.3) | 0.02 |
| Hip fracture - no. (%) | 229 (0.4) | 219 (0.4) | 10 (0.2) | 0.04 | 12.8 (0.2) | 10.0 (0.2) | 0.01 |
| Malnutrition - no. (%) | 1852 (3.1) | 1762 (3.3) | 90 (1.5) | 0.12 | 100.2 (1.7) | 90.0 (1.5) | 0.01 |
| Anemia - no. (%) | 27908 (47.0) | 25096 (46.9) | 2812 (47.9) | 0.02 | 2762.0 (47.5) | 2787.0 (47.9) | 0.009 |
| Hypothyroidism - no. (%) | 5574 (9.4) | 5142 (9.6) | 432 (7.4) | 0.08 | 449.9 (7.7) | 431.0 (7.4) | 0.01 |
| Asthma - no. (%) | 4079 (6.9) | 3722 (6.9) | 357 (6.1) | 0.04 | 339.5 (5.8) | 349.0 (6.0) | 0.007 |
| Dementia - no. (%) | 340 (0.6) | 328 (0.6) | 12 (0.2) | 0.06 | 17.4 (0.3) | 12.0 (0.2) | 0.02 |
| Immunosuppressed - no. (%) | 1318 (2.2) | 1228 (2.3) | 90 (1.5) | 0.06 | 95.5 (1.6) | 89.0 (1.5) | 0.009 |
| Surgical status - no. (%) | 0.19 | <0.001 | |||||
| Elective | 21641 (36.4) | 19102 (35.7) | 2539 (43.3) | 2521.0 (43.4) | 2521.0 (43.4) | ||
| Urgent | 35563 (59.8) | 32350 (60.4) | 3213 (54.8) | 3193.0 (54.9) | 3193.0 (54.9) | ||
| Emergent | 2209 (3.7) | 2096 (3.9) | 113 (1.9) | 99.0 (1.7) | 99.0 (1.7) | ||
| Emergent salvage | 17 (0.0) | 16 (0.0) | 1 (0.0) | 0 (0) | 0 (0) | ||
| Redo sternotomy - no. (%) | 89 (0.1) | 78 (0.1) | 11 (0.2) | 0.01 | 11.4 (0.2) | 11.0 (0.2) | 0.002 |
| ≥3 vessel disease - no. (%) | 48338 (81.3) | 43494 (81.2) | 4844 (82.6) | 0.04 | 4810.0 (82.7) | 4810.0 (82.7) | <0.001 |
| Surgeon volume - isolated CABG | 332 ± 178 | 334 ± 180 | 321 ± 164 | 0.07 | 325 ± 174 | 322 ± 164 | 0.02 |
Plus-minus valves are means +/− standard deviation. 4.1% of patients missing data for mitral regurgitation, otherwise no variable with >0.2% missingness. If any missing data present, groups were balanced on missingness for each variable. CABG, coronary artery bypass grafting; CPR, cardiopulmonary resuscitation; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SMD, standardized mean difference
The number of patients and proportions presented are weighted due to variable 1:k matching and for estimation of the average treatment effect on the treated. Total number matched: arterial conduit = 5,813, venous conduit = 17,930.
339 surgeons performed at least one CABG operation across 126 hospitals, and 239 of these surgeons (70.5%) used a second arterial conduit at least once (Supplemental Figure 3). Between 2006 and 2011, the annual number of isolated, multi-vessel CABGs declined (Supplemental Figure 4). Use of radial artery and bilateral ITA conduits also monotonically decreased over the study period (radial artery: 7.8%, 2006 vs. 6.6%, 2011; P<0.001, right ITA: 3.0%, 2006 vs. 2.4%, 2011; P=0.03, either arterial conduit: 10.7%, 2006 vs. 9.1%, 2011; P<0.001).
Mortality
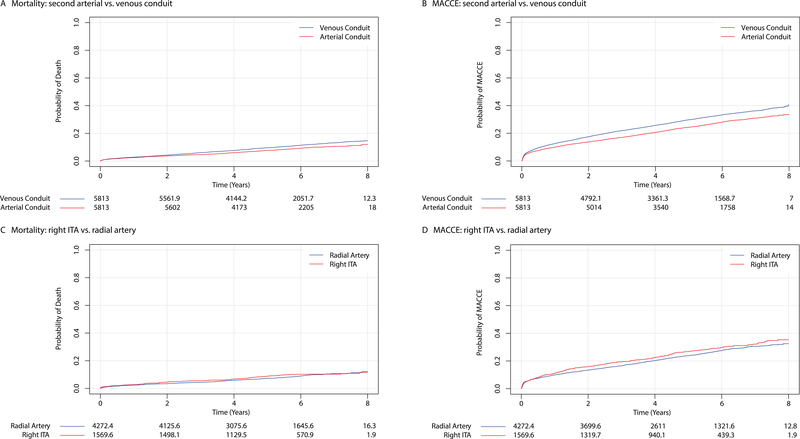
Thirty-day mortality did not differ between second arterial conduit recipients versus venous conduit recipients (arterial 0.81% vs. venous 0.86%; RD −0.05%, 95% CI −0.31% – 0.22%). However, a second arterial conduit, compared with a venous conduit, was associated with a significantly lower risk of death during follow-up (13.1% vs. 10.6% at 7 years; hazard ratio (HR) 0.79, 95% CI 0.72 – 0.87, P<0.001) (Figure 2). An exploratory analysis of restricted mean survival times demonstrated that survival significantly diverged 4 years after the index surgery (Supplemental Figure 5). The benefit associated with a second arterial conduit persisted even after adjusting for baseline covariates and allowing surgeon-specific effects (Table 2), but individual surgeons had a near-negligible effect on the baseline hazard of death after CABG. Our instrumental variable analysis corroborated the overall findings: a second arterial conduit did not affect 30-day mortality but was associated with lower mid-term mortality (HR 0.70, 95% CI 0.62 – 0.80) and MACCE after CABG (Table 2, Supplemental Table 5 & Supplemental Figure 6).
Figure 2.
Mortality and Major Adverse Cardiovascular and Cerebrovascular Events after Coronary Artery Bypass Surgery. All-cause mortality (Panels A and C) and the incidence of major adverse cardiovascular and cerebrovascular events (Panels B and D) are plotted against time after surgery and stratified according to conduit type. Numbers of patients at risk are included below each figure. Note that some numbers are not necessarily integers due to matched pairs with variable controls. ITA, internal thoracic artery; MACCE, major adverse cardiovascular and cerebrovascular events
Table 2.
Between-Group Differences in All-Cause Mortality and Major Adverse Cardiovascular and Cerebrovascular Events
| All-Cause Mortality | Second Arterial vs. Venous Conduit (reference) | Right Internal Thoracic Artery vs. Radial Artery Conduit (reference) | ||||
| Group Contrast Measure | Hazard Ratio | 95 % CI | P Value | Hazard Ratio | 95% CI | P Value |
| Propensity Score-Matched Population (PH model)* | 0.79 | 0.72 – 0.87 | <0.001 | 1.10 | 0.89 – 1.37 | 0.38 |
| Propensity Score-Matched Population (PH model, with multivariable adjustment)† | 0.80 | 0.72 – 0.89 | <0.001 | 1.16 | 0.93 – 1.46 | 0.19 |
| Propensity Score-Matched Population (PH model, with surgeon as random effect)‡ | 0.79 | 0.70 – 0.89 | <0.001 | 1.06 | 0.85 – 1.32 | 0.62 |
| Instrumental Variable Analysis with Near-Far Matched-Population (PH model) | 0.70 | 0.62 – 0.80 | <0.001 | - | - | - |
| Major Adverse Cardiovascular & Cerebrovascular Events | Second Arterial vs. Venous Conduit (reference) | Right Internal Thoracic Artery vs. Radial Artery Conduit (reference) | ||||
| Group Contrast Measure | Hazard Ratio | 95 % CI | P Value | Hazard Ratio | 95% CI | P Value |
| Propensity Score-Matched Population (PH model)* | 0.80 | 0.76 – 0.85 | <0.001 | 1.12 | 0.99 – 1.27 | 0.06 |
| Propensity Score-Matched Population (PH model, with multivariable adjustment)† | 0.80 | 0.76 – 0.85 | <0.001 | 1.17 | 1.03 – 1.33 | 0.02 |
| Propensity Score-Matched Population (PH model, with surgeon as random effect) | 0.80 | 0.75 – 0.87 | <0.001 | 1.17 | 1.01 – 1.34 | 0.03 |
| Instrumental Variable Analysis with Near-Far Matched-Population (PH model) | 0.77 | 0.69 – 0.81 | <0.001 | - | - | - |
Gamma = 1.15 for all-cause mortality and 1.23 for major adverse cardiovascular and cerebrovascular events between recipients of second arterial and venous conduits. The gamma parameter estimates the amount of unmeasured bias necessary to render the finding null. For interpretation, an unobserved covariate would need to increase the odds of treatment 2-fold, and increase the odds of all-cause mortality 1.5-fold, to render the presented finding null. Similarly, an unobserved covariate would need to increase the odds of treatment 2-fold, and increase the odds of major adverse cardiovascular and cerebrovascular events 1.9-fold, to render the presented finding null.
Adjusted for baseline variables in Table 1
The standard deviation of the random effect for surgeon was 0.009. Therefore, the 15% of surgeons expected to be one standard deviation above the mean increased the relative risk of death by 0.9%.
CI, confidence interval; PH, proportional hazards
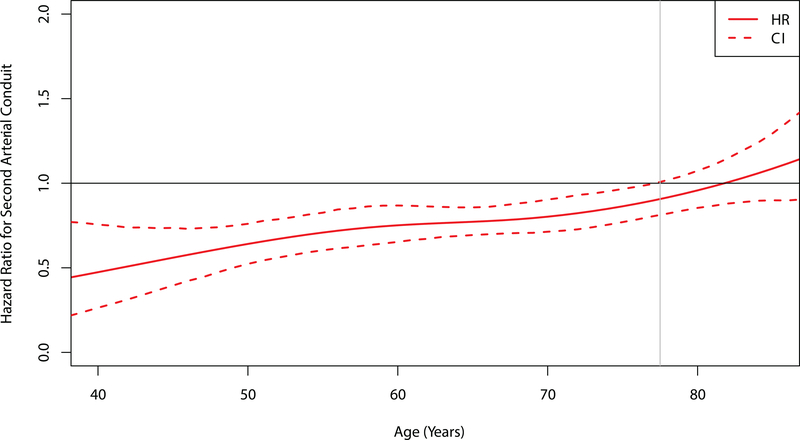
A second arterial conduit exhibited similar stratum-specific influences on mortality in patients with two-vessel or three-or-more-vessel disease (Supplemental Figure 7). When age was examined as a continuous variable, visualization of the interaction between conduit type and age suggested that second arterial conduits were associated with significantly lower mortality in patients up to 78 years old at the time of surgery (Figure 3).
Figure 3.
Age-Dependent Hazard of Death for Second Arterial versus Venous Conduits. The hazard ratio of death for recipients of second arterial versus venous conduits is plotted against age as a continuous variable. The dashed lines represent 95% confidence intervals obtained from bootstrap resampling. The horizontal black line at 1 denotes no difference between conduit types. The vertical grey line at 78 years denotes the age when the upper 95% confidence interval crosses the null.
Major Adverse Cardiovascular and Cerebrovascular Events
Risk of MACCE was significantly lower among recipients of second arterial conduits compared with recipients of venous conduits (31.0% vs. 36.2% at 7 years; HR 0.80, 95% CI 0.76 – 0.84, P<0.001) (Figure 2), and individual risks of myocardial infarction (HR 0.78, 95% CI 0.70 – 0.87) and repeat revascularization (HR 0.82, 95% CI 0.76 – 0.88) were also lower (Supplemental Figure 8). There was no difference in the cumulative incidence of stroke after CABG between groups (HR 0.88, 95% CI 0.77 – 1.01), or in the incidence of sternal wound infection within 1 year of surgery (arterial 1.38% vs. venous 1.44%; RD −0.06%, 95% CI −0.41% – 0.31%).
Radial Artery versus Right Internal Thoracic Artery Grafts
In a planned sub-group analysis, we compared similar propensity score-matched populations who received a radial artery with those who received a right ITA as a second conduit (Supplemental Table 6). There was no difference in 30-day mortality (right ITA 1.20% vs. radial artery 0.62%; RD 0.58%, 95% CI −0.04% – 1.27%) or longer-term mortality (right ITA 10.3% vs. radial artery 10.7% at 7 years; HR 1.10, 95% CI 0.89 – 1.37, P=0.38) between recipients of radial artery grafts or right ITA grafts (Table 2 & Figure 2). The cumulative incidence of stroke, myocardial infarction, and repeat revascularization also did not differ significantly between groups (Supplemental Figure 9). There was no difference in the composite endpoint of MACCE in the primary analysis (HR 1.12, 95% CI 1.00 – 1.26), but a significant increase in the risk of MACCE was noted among right ITA recipients after multivariable adjustment and allowing for surgeon-specific effects (Table 2 & Figure 2). The risk of a sternal wound infection within 1 year of surgery was also significantly higher in the right ITA group (right ITA 2.29% vs. radial artery 1.22%; RD 1.07%, 95% CI 0.15% – 2.07%).
DISCUSSION
The patients with coronary artery disease who benefit most from CABG are well described, but the optimal operation—and in particular, the conduits surgeons should use—remain unclear. While some observational evidence supports the use of second arterial conduits,11, 12 the benefits are uncertain,16, 27 and evidence supporting preferential use of the right ITA over the radial artery is even weaker.10, 15, 28 The low and declining rate of second arterial conduit use in California suggests many physicians are concerned that the risks of sternal wound infection13 and early graft failure14 outweigh potential benefits. In a population-based examination of CABG operations in California, we found that second arterial conduits were associated with lower mortality compared with venous conduits, and among second arterial conduits, the radial artery and right ITA affected mortality similarly.
Although our results concur with findings from some single-center observational studies and meta-analyses,11, 12 they contradict that of the Arterial Revascularization Trial (ART)16 and a recent post-hoc analysis of the Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) trial.27 The ART investigators randomized 3,102 patients at 28 centers to receive CABG with either single or bilateral ITA grafts, and found no difference in survival or cardiovascular events 5 years after surgery. But cross-over was high: over 15% of patients randomized to bilateral ITA grafts received a single ITA graft instead, and 22% of patients randomized to receive a single ITA graft also received a radial artery graft—which may perform as well as a second ITA graft, as our study and others suggest.29 Also, each patient in the ART received near-perfect medical management, which may have reduced the difference in survival between study arms. Together, these factors may have biased the ART towards the null. The post-hoc analysis of the SYNTAX trial defined the treatment arm as receipt of either a radial artery or second ITA graft.27 However, investigators found no difference in survival or cardiovascular events 5 years after surgery.27 But, this analysis was underpowered and only followed patients for 5 years. In our study, significant differences in survival only started to appear 4 years after surgery. That mortality differences appear earlier in our study than in the ART may suggest that residual confounding influenced our results, but differences in study design may also play a role. In fact, a recent examination of CABG outcomes in British Columbia demonstrated results similar to ours.30
The vague recommendations that arterial conduits be “considered in appropriate patients”10 or in those “with reasonable life expectancy”15 offer little guidance to surgeons. Coupled with contradictory evidence between studies, it is not surprising that arterial conduits are used in less than 10% of CABG operations in California. The guidelines are based on single-center observational studies and meta-analyses of these studies.10, 15 However, few studies try to account for confounding by indication, and the single-center design of each study limits statistical power and raises further concern for selection bias as well as generalizability. Examining outcomes across the state of California, we found that second arterial conduits, compared with venous conduits, were associated with lower mortality in patients as old as 78 years. We also observed that mortality differences between groups appeared as early as 4 years after surgery, but 85% to 90% of patients will survive beyond 5 years.4, 16 Collectively, our data suggest that many patients may be clinically “appropriate” candidates and with “reasonable life expectancy”; in other words, second arterial conduits may be grossly underutilized.
European guidelines recommend the radial artery as a reasonable, though less desirable, alternative to the right ITA as a second conduit.10, 15 Prior investigations demonstrate its superiority over saphenous vein grafts,31 but inferiority to the right ITA.28 Although radial artery patency is related to target vessel size and stenosis,14 the only randomized trial comparing radial artery grafts with free right ITA grafts demonstrated no difference in patency, but improved survival among recipients of radial artery grafts.29 We found a lower incidence of sternal wound infection in recipients of radial artery grafts but no difference in overall survival or individual cardiovascular events. Theoretically, a right ITA graft should perform better when it is pedicled rather than free, but pedicled grafts are shorter and restricted to bypassing proximal lesions. That the effect of a radial artery graft on mortality was no different from a combination of pedicled and free right ITA grafts suggests that the increased versatility of the radial artery may be a side benefit. However, concern that radial artery conduits may be more susceptible to competitive flow may have led to preferential use of radial arteries for grafting lateral wall targets with high-grade stenoses, and this may have biased our results. Nevertheless, it is biologically plausible that either artery is better equipped than the saphenous vein to withstand systemic pressures, and coupled with the excess risk of sternal wound infection noted in our study and others,16 perhaps future guidelines should de-emphasize the distinction between radial artery and right ITA grafts.
Coronary bypass with multiple arterial grafts is a more challenging operation, and variability in surgeon experience and hospital resources may produce heterogeneous treatment effects. Professional societies must consider this procedural variability when creating recommendations for specific components of the operation. Although surgeon and hospital volume may influence immediate results,32 individual surgeons contributed near-negligible effects to the longer-term hazard of death over the study period. This suggests that most surgeons can perform the operation effectively. Across California, 70% of surgeons performed at least one CABG operation with a second arterial conduit, and the 20% improvement in the hazard of death afforded by a second arterial conduit that we observed in our study may be more representative of widespread community adoption of such a technique.
This study has several limitations. Propensity score matching cannot account for residual confounding owing to unmeasured variables; and information about medication use, conduit harvest technique (e.g. skeletonized vs. non-skeletonized), target vessel and conduit size, target vessel stenosis, and graft configuration was not available in CCORP. Therefore, we cannot rule out a systematic bias introduced by more frail patients with less-optimal targets receiving venous conduits. OSHPD does not track patients who leave California, and this can bias our results if emigration rates differed between treatment groups. Finally, complications of radial artery harvest were not tracked in the CCORP registry and are difficult to identify with diagnosis codes. To our knowledge, this is the largest study to date that compares outcomes between secondary conduits for CABG, and the “real-world” examination of conduit types implanted and managed by a broad mix of providers is unique.
In this population-level comparison of secondary conduits, arterial grafts were associated with significantly lower mortality in patients undergoing multi-vessel CABG compared with venous grafts, but a right ITA graft offered no benefit over that of a radial artery. Second arterial conduits were also associated with lower risks of myocardial infarction and repeat revascularization. That a survival benefit arose within 5 years of surgery and even extended to the elderly suggests that surgeons should lower their threshold for using arterial grafts. Although the type of arterial conduit did not influence all-cause mortality, the association with a reduction in the risk of sternal wound infection suggests a radial artery may be the preferred second conduit.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
In this population-level, retrospective cohort study of 59,432 California residents who underwent primary, isolated multi-vessel coronary artery bypass grafting with the left internal thoracic artery, receipt of a second arterial conduit was associated with lower mortality and adverse cardiovascular events compared with receipt of a venous conduit.
The survival benefit associated with use of a second arterial conduit extended to patients up to 78 years old.
As a second arterial conduit, the right internal thoracic artery offered no benefit compared with the radial artery, but it was associated with an increased risk of sternal wound infection.
What are the clinical implications?
Current practice recommendations are vague regarding when to use a second arterial conduit, and in California, surgeons use a second arterial conduit in less than 10% of isolated coronary artery bypass grafting operations.
Based on our results, surgeons should lower their threshold for using additional arterial grafts.
The radial artery may be the preferred second conduit over the right internal thoracic artery and saphenous vein.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the National Institutes of Health, National Center for Advancing Translational Science, Clinical and Translational Science Awards (NIH TL1 TR001084, to Dr. Goldstone; NIH KL2 TR001083, to Dr. Chiu; and NIH UL1 TR001085, to the Stanford Center for Clinical Translational Education and Research), and by a grant (KHS022192A, to Dr. Baiocchi) from the Agency for Healthcare Research and Quality.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Heart Network. European Cardiovascular Disease Statistics 2017. http://www.ehnheart.org/cvd-statistics.html. Accessed October 21, 2017.
- 3.Mohr FW, Morice MC, Kappetein AP, Feldman TE, Stahle E, Colombo A, Mack MJ, Holmes DR Jr., Morel MA, Van Dyck N, Houle VM, Dawkins KD and Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. [DOI] [PubMed] [Google Scholar]
- 4.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S 3rd, Bertrand M, Fuster V and Investigators FT. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 5.Stone GW, Sabik JF, Serruys PW, Simonton CA, Genereux P, Puskas J, Kandzari DE, Morice MC, Lembo N, Brown WM 3rd, Taggart DP, Banning A, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi I, Bogats G, Mansour S, Noiseux N, Sabate M, Pomar J, Hickey M, Gershlick A, Buszman P, Bochenek A, Schampaert E, Page P, Dressler O, Kosmidou I, Mehran R, Pocock SJ, Kappetein AP and Investigators ET. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med. 2016;375:2223–2235. [DOI] [PubMed] [Google Scholar]
- 6.Makikallio T, Holm NR, Lindsay M, Spence MS, Erglis A, Menown IB, Trovik T, Eskola M, Romppanen H, Kellerth T, Ravkilde J, Jensen LO, Kalinauskas G, Linder RB, Pentikainen M, Hervold A, Banning A, Zaman A, Cotton J, Eriksen E, Margus S, Sorensen HT, Nielsen PH, Niemela M, Kervinen K, Lassen JF, Maeng M, Oldroyd K, Berg G, Walsh SJ, Hanratty CG, Kumsars I, Stradins P, Steigen TK, Frobert O, Graham AN, Endresen PC, Corbascio M, Kajander O, Trivedi U, Hartikainen J, Anttila V, Hildick-Smith D, Thuesen L, Christiansen EH and investigators Ns. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388:2743–2752. [DOI] [PubMed] [Google Scholar]
- 7.Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, Golding LA, Gill CC, Taylor PC, Sheldon WCand et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Cameron A, Davis KB, Green G and Schaff HV. Coronary bypass surgery with internal-thoracic-artery grafts--effects on survival over a 15-year period. N Engl J Med. 1996;334:216–219. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD and Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. [DOI] [PubMed] [Google Scholar]
- 10.Aldea GS, Bakaeen FG, Pal J, Fremes S, Head SJ, Sabik J, Rosengart T, Kappetein AP, Thourani VH, Firestone S, Mitchell JD and Society of Thoracic S. The Society of Thoracic Surgeons Clinical Practice Guidelines on Arterial Conduits for Coronary Artery Bypass Grafting. Ann Thorac Surg. 2016;101:801–809. [DOI] [PubMed] [Google Scholar]
- 11.Taggart DP, D’Amico R and Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358:870–875. [DOI] [PubMed] [Google Scholar]
- 12.Weiss AJ, Zhao S, Tian DH, Taggart DP and Yan TD. A meta-analysis comparing bilateral internal mammary artery with left internal mammary artery for coronary artery bypass grafting. Ann Cardiothorac Surg. 2013;2:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taggart DP, Altman DG, Gray AM, Lees B, Nugara F, Yu LM, Campbell H, Flather M and Investigators ART. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J. 2010;31:2470–2481. [DOI] [PubMed] [Google Scholar]
- 14.Desai ND, Cohen EA, Naylor CD, Fremes SE and Radial Artery Patency Study I. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Engl J Med. 2004;351:2302–2309. [DOI] [PubMed] [Google Scholar]
- 15.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W and Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 16.Taggart DP, Altman DG, Gray AM, Lees B, Gerry S, Benedetto U, Flather M and Investigators ART. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N Engl J Med. 2016;375:2540–2549. [DOI] [PubMed] [Google Scholar]
- 17.California Office of Statewide Health Planning and Development. Types of OSHPD patient-level data. https://www.oshpd.ca.gov/HID/Data_Request_Center/Types_of_Data.html. Accessed October 21, 2017.
- 18.Zingmond DS, Ye Z, Ettner SL and Liu H. Linking hospital discharge and death records--accuracy and sources of bias. J Clin Epidemiol. 2004;57:21–29. [DOI] [PubMed] [Google Scholar]
- 19.Southern DA, Doherty C, De Souza MA, Quan H, Harrop AR, Nickerson D and Rabi D. Charts versus Discharge ICD-10 Coding for Sternal Wound Infection Following Coronary Artery Bypass Grafting. Perspect Health Inf Manag. 2015;12:1e. [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum P and Rubin D. The central role of the propensity score in observational studies. Biometrika. 1983;70:41–55. [Google Scholar]
- 21.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silber JH, Rosenbaum PR, Trudeau ME, Even-Shoshan O, Chen W, Zhang X and Mosher RE. Multivariate matching and bias reduction in the surgical outcomes study. Med Care. 2001;39:1048–1064. [DOI] [PubMed] [Google Scholar]
- 23.Baiocchi M, Small DS, Lorch S and Rosenbaum PR. Building a stronger instrument in an observational study of perinatal care for premature infants. J Am Stat Assoc. 2010;105:1285–1296. [Google Scholar]
- 24.Uno H, Claggett B, Tian L, Inoue E, Gallo P, Miyata T, Schrag D, Takeuchi M, Uyama Y, Zhao L, Skali H, Solomon S, Jacobus S, Hughes M, Packer M and Wei LJ. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32:2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fine JP and Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 26.Rosenbaum PR. Sensitivity analysis for m-estimates, tests and confidence intervals in matched observational studies. Biometrics. 2007;63:456–464. [DOI] [PubMed] [Google Scholar]
- 27.Parasca CA, Head SJ, Mohr FW, Mack MJ, Morice MC, Holmes DR Jr., Feldman TE, Colombo A, Dawkins KD, Serruys PW, Kappetein AP and Investigators S. The impact of a second arterial graft on 5-year outcomes after coronary artery bypass grafting in the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery Trial and Registry. J Thorac Cardiovasc Surg. 2015;150:597–606. [DOI] [PubMed] [Google Scholar]
- 28.Benedetto U, Caputo M, Gaudino M, Marsico R, Rajakaruna C, Bryan A and Angelini GD. Right internal thoracic artery or radial artery? A propensity-matched comparison on the second-best arterial conduit. J Thorac Cardiovasc Surg. 2017;153:79–88. [DOI] [PubMed] [Google Scholar]
- 29.Buxton BF, Hayward PA, Matalanis G, Moten SC, Horrigan M, Rosalion A, Raman J and Hare DL. 10-year endpoint of RAPCO is reached: clinical and angiographic results of a randomised trial of radial artery versus right internal thoracic artery or saphenous vein for the second graft. Paper presented at: 96th Annual Meeting of the American Association for Thoracic Surgery; May 14–18, 2016; Baltimore, MD, USA. [Google Scholar]
- 30.Pu A, Ding L, Shin J, Price J, Skarsgard P, Wong DR, Bozinovski J, Fradet G and Abel JG. Long-term Outcomes of Multiple Arterial Coronary Artery Bypass Grafting: A Population-Based Study of Patients in British Columbia, Canada. JAMA Cardiol. 2017;2:1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tranbaugh RF, Dimitrova KR, Friedmann P, Geller CM, Harris LJ, Stelzer P, Cohen BM, Ko W, DeCastro H, Lucido D and Hoffman DM. Coronary artery bypass grafting using the radial artery: clinical outcomes, patency, and need for reintervention. Circulation. 2012;126:S170–175. [DOI] [PubMed] [Google Scholar]
- 32.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE and Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.