Key Points
Question
What is the estimated vaccine effectiveness (VE) and durability of BNT162b2 against infection with the Delta and Omicron variants of SARS-CoV-2 among 5- to 11-year-old children?
Findings
In this test-negative case-control study of 160 002 children who underwent polymerase chain reaction testing for SARS-CoV-2 at locations of a pharmacy chain during Delta- and Omicron-predominant periods, the overall VE of 2 doses of BNT162b2 was 85% against Delta and 20% against Omicron; protection from the 2-dose vaccine against Omicron waned after approximately 3 months and was similar across sublineages (BA.1, BA.2/BA.2.12.1, and BA.4/BA.5). Booster effectiveness was 55% against Omicron and 40% or more against BA.4/BA.5 at 3 or more months after receipt of the booster.
Meaning
This study suggests that 2 BNT162b2 doses provided limited protection against Omicron infection and that a booster dose restored and maintained protection for at least 3 months.
Abstract
Importance
Data describing the vaccine effectiveness (VE) and durability of BNT162b2 among children 5 to 11 years of age are needed.
Objective
To estimate BNT162b2 VE against SARS-CoV-2 infection among children aged 5 to 11 years during Delta and Omicron variant–predominant periods and to further assess VE according to prior SARS-CoV-2 infection status and by sublineage during the Omicron variant–predominant period.
Design, Setting, and Participants
This test-negative case-control study was conducted from November 2 to December 9, 2021 (Delta variant), and from January 16 to September 30, 2022 (Omicron variant), among 160 002 children tested at a large national US retail pharmacy chain, for SARS-CoV-2 via polymerase chain reaction (PCR); 62 719 children were tested during the Delta period, and 97 283 were tested during the Omicron period.
Exposure
Vaccination with BNT162b2 before SARS-CoV-2 testing vs no vaccination.
Main Outcomes and Measures
The primary outcome was SARS-CoV-2 infection confirmed by PCR (regardless of the presence of symptoms), and the secondary outcome was confirmed symptomatic infection. Adjusted estimated VE was calculated from multilevel logistic regression models.
Results
A total of 39 117 children tested positive and 131 686 tested negative for SARS-CoV-2 (total, 170 803; 84 487 [49%] were boys; mean [SD] age was 9 [2] years; 74 236 [43%] were White non-Hispanic or non-Latino; and 37 318 [22%] were Hispanic or Latino). Final VE analyses included 160 002 children without SARS-CoV-2 infection less than 90 days prior. The VE of 2 doses of BNT162b2 against Delta was 85% (95% CI, 80%-89%; median follow-up, 1 month) compared with the Omicron period (20% [95% CI, 17%-23%]; median follow-up, 4 months). The adjusted VE of 2 doses against Omicron at less than 3 months was 39% (95% CI, 36%-42%), and at 3 months or more, it was −1% (95% CI, −6% to 3%). Protection against Omicron was higher among children with vs without infection 90 days or more prior but decreased in all children approximately 3 months after the second dose (58% [95% CI, 49%-66%] with infection vs 37% [95% CI, 34%-41%] without infection at <3 months; 27% [95% CI, 17%-35%] with infection vs −7% [95% CI, −12% to −1%] at ≥3 months without infection). The VE of 2 doses of BNT162b2 at less than 3 months by Omicron sublineage was 40% (95% CI, 36%-43%) for BA.1, 32% (95% CI, 21%-41%) for BA.2/BA.2.12.1, and 50% (95% CI, 37%-60%) for BA.4/BA.5. After 3 months or more, VE was nonsignificant for BA.2/BA.2.12.1 and BA.4/BA.5. The VE of a booster dose was 55% (95% CI, 50%-60%) against Omicron, with no evidence of waning at 3 months or more.
Conclusions and Relevance
This study suggests that, among children aged 5 to 11 years, 2 doses of BNT162b2 provided modest short-term protection against Omicron infection that was higher for those with prior infection; however, VE waned after approximately 3 months in all children. A booster dose restored protection against Omicron and was maintained for at least 3 months. These findings highlight the continued importance of booster vaccination regardless of history of prior COVID-19.
This cross-sectional study estimates BNT162b2 vaccine effectiveness against SARS-CoV-2 infection among children aged 5 to 11 years during Delta and Omicron variant–predominant periods, by prior SARS-CoV-2 infection status and Omicron sublineage.
Introduction
Shortly after being designated a variant of concern on November 26, 2021, the SARS-CoV-2 Omicron (B.1.1.529) variant rapidly replaced the Delta (B.1.617.2) variant globally1 and became the predominant variant in the United States by late December 2021.2 From December 2021 through February 2022, COVID-19 case counts surged to the highest levels ever recorded in the United States.3 By February 2022, an estimated 58% of the US population had serologic evidence of previous SARS-CoV-2 infection, with children aged 5 to 11 years having the highest seropositivity (77%) among all age groups.4
On November 2, 2021, the Pfizer-BioNTech COVID-19 vaccine BNT162b2 was recommended for use in the United States as a 2-dose schedule for children aged 5 to 11 years for the prevention of COVID-19.5 On May 19, 2022, the Centers for Disease Control and Prevention (CDC) recommended a BNT162b2 booster dose administered 5 months or more after completion of the primary series to children aged 5 to 11 years.6,7 Until June 24, 2022, BNT162b2 was the only vaccine recommended for this age group in the United States.8,9 Finally, on October 12, 2022, the CDC recommended the use of updated (bivalent) COVID-19 vaccines as a booster dose administered 2 months or more after completion of primary or booster vaccination for children aged 5 to 11 years.10
The available evidence describing vaccine effectiveness (VE) among children aged 5 to 11 years suggests that 2 doses of BNT162b2 provided modest protection (approximately 30%-60% VE) against Omicron-related infection,11,12,13,14 symptomatic COVID-19,11,14,15 and COVID-19–related urgent or emergency care visits16 through approximately 2 to 3 months after receipt of the second dose.13,15,16 The VE of a booster dose has not yet been reported for this age group, to our knowledge. In addition, previously published VE estimates have not been stratified by history of prior infection—which is becoming increasingly important for understanding vaccine performance given high levels of seroprevalence among this age group at this point in the pandemic.4
We estimated the VE of 1 and 2 doses of BNT162b2 against Delta and Omicron infection and of a booster dose against Omicron infection among children aged 5 to 11 years. For VE against Omicron, we also stratified estimates by reported history of infection, time since last dose, sublineage (BA.1, BA.2/BA.2.12.1, and BA.4/BA.5), and symptom status.
Methods
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The Advarra institutional review board approved the study and granted a waiver of documentation of informed consent under US Department of Health and Human Services regulation 45 CFR 46.104(d)(4) for research using deidentified data and a complete waiver of Health Insurance Portability and Accountability Act authorization.
Study Design and Participants
This test-negative case-control study used electronic health records from Walgreens, a national pharmacy chain with more than 7200 retail pharmacy locations throughout the United States and Puerto Rico. In fall 2020, Walgreens formed a partnership with Aegis Sciences Corp, a national health care laboratory,17 to improve the access, efficiency, and reliability of COVID-19 testing at more than 5200 Walgreens pharmacies. As of September 30, 2022, Walgreens and Aegis have completed nearly 10 million SARS-CoV-2 polymerase chain reaction (PCR) tests, with 626 000 tests conducted among children aged 5 to 11 years.
The study population included children aged 5 to 11 years who were tested for SARS-CoV-2 via PCR at a Walgreens pharmacy between November 2, 2021, and September 30, 2022. Parents or guardians completed an online appointment scheduler to select a Walgreens location and SARS-CoV-2 test type (PCR, rapid nucleic acid amplification test [NAAT], or rapid antigen).18 At the time of scheduling, information on demographic characteristics, current symptoms, and clinical history was collected via a self-reported questionnaire (available in English or Spanish). Race and ethnicity have been associated with COVID-19 morbidity and vaccine uptake.19,20 Race and ethnicity were self-reported by the parent or guardian from among categories defined by the CDC; the categories American Indian or Alaska Native and Native Hawaiian or Other Pacific Islander were combined into 1 group (Native) due to limited sample size.21 Patients without an appointment scheduled in advance were not included in this study. Patients experiencing severe symptoms were directed to contact emergency services. At the time of appointment scheduling, the parent or guardian acknowledged that the patient’s anonymized data may be used for research purposes as described in the Notice of Privacy Practices.22 At the appointment, individuals self-collected swab specimens of the anterior nares under the supervision of trained Walgreens staff. Specimens were placed into a collection tube and transferred to Aegis for processing using the TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific).
Exposures
The number of BNT162b2 doses and the month and year of administration of each dose were self-reported in the questionnaire by the child’s parent or guardian. Children who had not received any COVID-19 vaccine at the time of questionnaire completion were defined as unvaccinated. Children were excluded if they had received a COVID-19 vaccine other than BNT162b2, were vaccinated before the month of recommendation by the Advisory Committee on Immunization Practices (November 2021), received a third dose less than 5 months after the second dose, or self-reported having an immunocompromising condition.
Outcomes
Vaccine effectiveness was evaluated by comparing the odds of vaccination among children testing positive for SARS-CoV-2 (cases) and those testing negative (controls) via PCR. The primary outcome was confirmed SARS-CoV-2 infection (regardless of the presence of symptoms), and the secondary outcome was confirmed symptomatic infection. Variant lineage was assigned based on testing date; children who tested positive from November 2 to December 9, 2021, were considered to have Delta, and those who tested positive from January 16 to September 30, 2022, were considered to have Omicron based on national laboratory surveillance estimates for when Delta and Omicron, respectively, comprised 95% or more of circulating strains.23 Children were excluded from the study if they were tested between December 10, 2021, and January 15, 2022. Omicron sublineage periods were defined as 75% or more predominance: January 16 to March 5, 2022, for BA.1; March 27 to June 4, 2022, for BA.2/BA.2.12.1; and July 3 to September 30, 2022, for BA.4/BA.5.
On December 10, 2021, the appointment scheduling questionnaire was updated to add questions about the history and timing of prior SARS-CoV-2 infection and the reason for testing, as well as to revise the list of symptoms. This updated information was used to exclude children from analyses evaluating Omicron infection who were infected less than 90 days prior because a recent prior positive test result may be due to prolonged NAAT positivity.24
Statistical Analysis
Patient characteristics were summarized using descriptive statistics stratified by variant period, SARS-CoV-2 test result, and vaccination status. Differences between groups according to case status and vaccination status were evaluated using 2-sided χ2 tests with P < .05. Estimated VE was calculated as 1 minus the odds ratio from logistic regression models multiplied by 100% using SAS, version 9 (SAS Institute Inc). In adjusted multilevel models, we controlled for (1) demographic characteristics and clinical history modeled as fixed effects (ie, age [on the testing date; continuous], gender, race and ethnicity, and reported history of any chronic medical condition [vs none; including a heart condition, chronic lung disease, diabetes, high blood pressure, overweight or obesity, or kidney failure]), reported recent close contact with someone who had confirmed or presumed COVID-19 (vs no recent contact), testing related to travel (vs not; Omicron period only), and previous SARS-CoV-2 infection (Omicron period only); (2) calendar week using a categorical term in 2-week increments; and (3) pharmacy-level characteristics modeled as random effects (ie, rural, suburban, or urban Walgreens trade area designation and SARS-CoV-2 testing volume [number of tests conducted relative to the estimated catchment population of the pharmacy; as tertile ranks]).25 Other covariates (US Department of Health and Human Services region and county-level Social Vulnerability Index score) were considered but excluded from final models because they did not meet criteria for model inclusion (≥1% association with odds ratio estimates). The Wald method was used to calculate 95% CIs for significance testing.26
Results
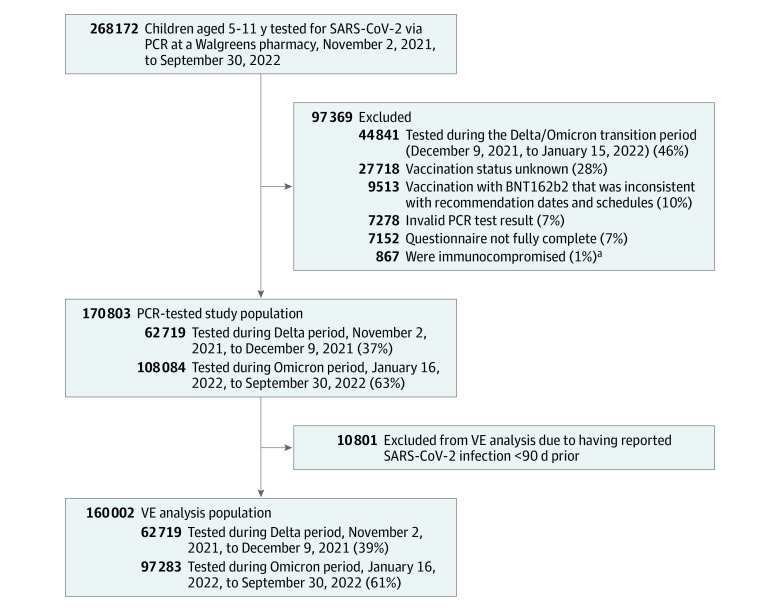
A total of 268 172 children aged 5 to 11 years underwent PCR testing for SARS-CoV-2 infection at a Walgreens location between November 2, 2021, and September 30, 2022. A total of 97 369 children (36%) were excluded from the study: 44 841 (46%) were tested during the Delta or Omicron transition period, 27 718 (28%) had unknown vaccination status, 9513 (10%) were not vaccinated with BNT162b2 consistent with recommendation dates and schedules, 7278 (7%) had an invalid PCR test result, 7152 (7%) did not have a fully complete questionnaire, and 867 (1%) were immunocompromised. The PCR-tested study population included 170 803 children, of whom 62 719 (37%) were tested during the Delta period and 108 084 (63%) were tested during the Omicron period (Figure 1).
Figure 1. Study Participant Flowchart.
PCR indicates polymerase chain reaction; VE, vaccine effectiveness.
aReported having a previous diagnosis of immunocompromised state, such as from immunocompromising medications, solid organ or blood stem cell transplant, human immunodeficiency virus, or other immunocompromising condition.
Patient Characteristics
During the study period, 39 117 of 170 803 (23%) children tested positive for SARS-CoV-2, while 131 686 of 170 803 (77%) tested negative (Table 1). The mean (SD) age of children in the study was 9 (2) years; 84 487 (49%) were male, 74 236 (43%) were White non-Hispanic or non-Latino, and 37 318 (22%) were Hispanic or Latino. A total of 3302 of 170 803 children (2%) were vaccinated with 3 doses, 44 372 (26%) with 2 doses, 7395 (4%) with 1 dose, and 115 689 (68%) were unvaccinated. The proportion of children who received 2 or more vaccine doses increased from 2% (1369 of 62 719) during the Delta period to 43% (46 305 of 108 084) during the Omicron period. Vaccination with 2 doses was more common in the least vulnerable counties, and vaccination with 3 doses was more common among children who were older and White non-Hispanic and lived in the least vulnerable counties (eTable 1 in Supplement 1).
Table 1. Demographic, Clinical, and Testing Characteristics of Children 5 to 11 Years of Age by Variant Period and SARS-CoV-2 PCR Test Result.
| Characteristic | Delta period (November 2 to December 9, 2021) | Omicron period (January 16 to September 30, 2022) | ||||
|---|---|---|---|---|---|---|
| SARS-CoV-2 | P value | SARS-CoV-2 | P value | |||
| Positive (cases) (n = 10 609) | Negative (controls) (n = 52 110) | Positive (cases) (n = 28 508) | Negative (controls) (n = 79 576) | |||
| Age, y | ||||||
| 5-8 | 5465 (52) | 29 244 (56) | <.001 | 15 533 (55) | 44 103 (55) | <.001 |
| 9-11 | 5144 (49) | 22 866 (44) | 12 975 (46) | 35 473 (45) | ||
| Mean (SD) | 8.8 (2.0) | 8.5 (2.0) | 8.6 (2.0) | 8.6 (2.0) | ||
| Gender | ||||||
| Female | 5221 (49) | 26 612 (51) | <.001 | 14 000 (49) | 40 372 (51) | <.001 |
| Male | 5385 (51) | 25 457 (49) | 14 488 (51) | 39 157 (49) | ||
| Othera | 3 (0.03) | 41 (0.1) | 20 (0.1) | 47 (0.1) | ||
| Race and ethnicity | ||||||
| Hispanic or Latino (any race) | 1979 (19) | 11 232 (22) | <.001 | 7175 (25) | 16 932 (21) | <.001 |
| Non-Hispanic or Latino | ||||||
| Asian | 775 (7) | 6676 (13) | 4084 (14) | 11 470 (14) | ||
| Black or African American | 1180 (11) | 5685 (11) | 3824 (13) | 10 697 (13) | ||
| Nativeb | 119 (1) | 671 (1) | 289 (1) | 742 (1) | ||
| White | 5884 (56) | 24 065 (46) | 11 034 (39) | 33 253 (42) | ||
| Declined to answer | 672 (6) | 3781 (7) | 2102 (7) | 6482 (8) | ||
| US Department of Health and Human Services region | ||||||
| Boston, Massachusetts | 1112 (11) | 5666 (11) | <.001 | 1817 (6) | 5286 (7) | <.001 |
| New York, New York | 841 (8) | 5261 (10) | 2633 (9) | 7782 (10) | ||
| Philadelphia, Pennsylvania | 905 (9) | 3985 (8) | 1707 (6) | 4843 (6) | ||
| Atlanta, Georgia | 747 (7) | 5596 (11) | 6877 (24) | 19 659 (25) | ||
| Chicago, Illinois | 4063 (38) | 13 788 (27) | 5847 (21) | 16 569 (21) | ||
| Dallas, Texas | 766 (7) | 3267 (6) | 3128 (11) | 7868 (10) | ||
| Kansas City, Missouri | 537 (5) | 1692 (3) | 751 (3) | 2113 (3) | ||
| Denver, Colorado | 365 (3) | 1544 (3) | 526 (2) | 1983 (3) | ||
| San Francisco, California | 1213 (11) | 10 630 (20) | 4923 (17) | 12 485 (16) | ||
| Seattle, Washington | 60 (1) | 681 (1) | 299 (1) | 988 (1) | ||
| Rural or urban area | ||||||
| Rural | 3189 (30) | 11 014 (21) | <.001 | 6283 (22) | 16 695 (21) | <.001 |
| Suburban | 6895 (65) | 35 585 (68) | 19 209 (67) | 54 147 (68) | ||
| Urban | 525 (5) | 5511 (11) | 3016 (11) | 8734 (11) | ||
| Social vulnerability index, quartile | ||||||
| First (least vulnerable) | 2681 (25) | 13 753 (26) | <.001 | 7412 (26) | 21 959 (28) | <.001 |
| Second | 2910 (27) | 13 039 (25) | 6652 (23) | 19 909 (25) | ||
| Third | 2683 (25) | 13 064 (25) | 7219 (25) | 19 264 (24) | ||
| Fourth (most vulnerable) | 2335 (22) | 12 254 (24) | 7225 (25) | 18 444 (23) | ||
| Chronic conditions | ||||||
| Any | 439 (4) | 1839 (4) | <.001 | 1032 (4) | 2374 (3) | <.001 |
| Diabetes | 25 (0.2) | 96 (0.2) | 38 (0.1) | 178 (0.2) | ||
| Heart condition | 15 (0.1) | 70 (0.1) | 171 (1) | 452 (1) | ||
| Respiratory condition | 350 (3) | 1474 (3) | 587 (2) | 1242 (2) | ||
| Otherc | 61 (1) | 212 (0.4) | 291 (1) | 608 (1) | ||
| None | 10 170 (96) | 50 271 (97) | 27 476 (96) | 77 202 (97) | ||
| Prior SARS-CoV-2 infectiond | ||||||
| No | NA | NA | NA | 20 506 (72) | 59 827 (75) | <.001 |
| Yes | ||||||
| <90 d Ago | NA | NA | 5505 (19) | 5251 (7) | ||
| ≥90 d Ago | NA | NA | 2497 (9) | 14 498 (18) | ||
| Recent close contact with someone diagnosed with or presumed to have COVID-19e | ||||||
| No | 2885 (27) | 33 813 (65) | <.001 | 9467 (33) | 54 558 (69) | <.001 |
| Yes | 7724 (73) | 18 297 (35) | 19 041 (67) | 25 018 (31) | ||
| Testing related to past or future traveld | ||||||
| No | NA | NA | NA | 24 928 (87) | 46 178 (58) | <.001 |
| Yes | NA | NA | 3580 (13) | 33 398 (42) | ||
| Testing volume of pharmacy | ||||||
| Low | 4898 (46) | 23 547 (45) | .14 | 21 244 (75) | 63 279 (80) | <.001 |
| Medium | 3775 (36) | 18 738 (36) | 5095 (18) | 11 227 (14) | ||
| High | 1936 (18) | 9825 (19) | 2169 (8) | 5070 (6) | ||
| Symptoms | ||||||
| Any symptoms | 5266 (50) | 18 397 (35) | <.001 | 20 762 (73) | 30 048 (38) | <.001 |
| Chills | 736 (7) | 1929 (4) | 2985 (10) | 2600 (3) | ||
| Congestion or runny nosed | NA | NA | 9408 (33) | 14 780 (19) | ||
| Fatigued | NA | NA | 5623 (20) | 6149 (8) | ||
| Headache | 2397 (23) | 5846 (11) | 8036 (28) | 8836 (11) | ||
| Low-grade fever (temperature, <38.9 °C) | 1965 (19) | 5415 (10) | 9784 (34) | 8980 (11) | ||
| Muscle pain | 664 (6) | 1521 (3) | 2570 (9) | 2246 (3) | ||
| New loss of taste or smell | 467 (4) | 500 (1) | 435 (2) | 570 (1) | ||
| New or worsening cough | 2476 (23) | 8677 (17) | 12 986 (46) | 17 166 (22) | ||
| Shortness of breath or difficulty breathing (not severe) | 241 (2) | 893 (2) | 827 (3) | 1079 (1) | ||
| Sore throat | 2385 (22) | 9814 (19) | 8995 (32) | 12 442 (16) | ||
| Vomiting or diarrhea | 629 (6) | 3092 (6) | 2497 (9) | 4087 (5) | ||
| Asymptomatic | 5343 (50) | 33 713 (65) | 7746 (27) | 49 528 (62) | ||
| Vaccination status and time since last dosef | ||||||
| Unvaccinated | 10 218 (96) | 48 199 (93) | <.001 | 15 804 (55) | 41 468 (52) | <.001 |
| 1 Dose only (any time since dose) | 336 (3) | 2597 (5) | 1361 (5) | 3101 (4) | ||
| 2 Doses only | ||||||
| Any time since last dose | 55 (1) | 1314 (3) | 10 784 (38) | 32 219 (41) | ||
| <3 mo | 55 (1) | 1314 (3) | 4017 (14) | 12 800 (16) | ||
| ≥3 mo | NA | NA | 6767 (24) | 19 419 (24) | ||
| 3-5 mo | NA | NA | 4148 (15) | 13 307 (17) | ||
| 6-8 mo | NA | NA | 2478 (9) | 5838 (7) | ||
| ≥9 mo | NA | NA | 141 (1) | 274 (0.3) | ||
| 3 Doses (any time since last dose) | NA | NA | 553 (2) | 2749 (4) | ||
| <3 mo | NA | NA | 135 (1) | 844 (1) | ||
| ≥3 mo | NA | NA | 418 (2) | 1905 (2) | ||
Abbreviations: NA, not applicable; PCR, polymerase chain reaction.
For gender, the response options were male, female, or other.
Includes American Indian, Alaska Native, Native Hawaiian, or Other Pacific Islander.
Includes cirrhosis of the liver, kidney disease, and obesity.
On December 10, 2021, the appointment scheduling questionnaire was updated to add questions asking about the history and timing of prior SARS-CoV-2 infection, whether the reason for testing was travel related, and to revise the list of symptoms.
Defined as reported contact for more than 15 minutes and within 6 feet, in the last 14 days, with someone suspected or confirmed to have COVID-19.
Follow-up at 2 months or more since last dose was not available for the Delta period.
The SARS-CoV-2 positivity rate was higher during the Omicron period (26% [28 508 of 108 084]) than during the Delta period (17% [10 609 of 62 719]) (Table 1). During both the Delta and Omicron periods, compared with children who tested negative, those who tested positive were more likely to have had recent close contact with someone diagnosed or presumed to have COVID-19 (Delta, 7724 of 10 609 [73%] vs 18 297 of 52 110 [35%]; Omicron, 19 041 of 28 508 [67%] vs 25 018 of 79 576 [31%]) or to have symptomatic illness (Delta, 5266 of 10 609 [50%] vs 18 397 of 52 110 [35%]; Omicron, 20 762 of 28 508 [73%] vs 30 048 of 79 576 [38%]), particularly the presence of low-grade fever (<38.9 °C: Delta, 1965 of 10 609 [19%] vs 5415 of 52 110 [10%]; Omicron, 9784 of 28 508 [34%] vs 8980 of 79 576 [11%]), new or worsening cough (Delta, 2476 of 10 609 [23%] vs 8677 of 52 110 [17%]; Omicron, 12 986 of 28 508 [46%] vs 17 166 of 79 576 [22%]), muscle pain (Delta, 664 of 10 609 [6%] vs 1521 of 52 110 [3%]; Omicron, 2570 of 28 508 [9%] vs 2246 of 79 576 [3%]), chills (Delta, 736 of 10 609 [7%] vs 1929 of 52 110 [4%]; Omicron, 2985 of 28 508 [10%] vs 2600 of 79 576 [3%]), headache (Delta, 2397 of 10 609 [22%] vs 5846 of 52 110 [11%]; Omicron, 8036 of 28 508 [28%] vs 8836 of 79 576 [11%]), or sore throat (Delta, 2385 of 10 609 [23%] vs 9814 of 52 110 [19%]; Omicron, 8995 of 28 508 [32%] vs 12 442 of 79 576 [16%]). During the Omicron period, fatigue (5623 of 28 508 [20%] vs 6149 of 79 576 [8%]) and congestion or runny nose (9408 of 28 508 [33%] vs 14 780 of 79 576 [19%]) were also more common among cases than controls. Children were less likely to report any symptoms if they had been vaccinated during the Delta period or had received a booster vaccination during the Omicron period (eTable 1 in Supplement 1). Reported infection 90 or more days prior (2497 of 28 508 [9%] vs 14 498 of 79 576 [18%]) and vaccination with 2 or more doses (11 337 of 28 508 [40%] vs 34 968 of 79 576 [44%]) were less common among cases than controls during the Omicron period (Table 1).
Estimated VE
After excluding 10 801 children with reported SARS-CoV-2 infection less than 90 days prior, the population for VE analyses included 160 002 children, of whom 97 283 (61%) were tested during the Omicron period (Figure 1). The overall adjusted VE of 2 doses of BNT162b2 (vs no vaccination) against infection was higher during the Delta period (85% [95% CI, 80%-89%]; median follow-up, 1 month) compared with the Omicron period (20% [95% CI, 17%-23%]; median follow-up, 4 months) (Table 2). The VE of only 1 dose was 56% (95% CI, 50%-61%) during the Delta period and 14% (95% CI, 6%-21%) during the Omicron period (median follow-up, 1 month for both estimates). During the Omicron period, prior SARS-CoV-2 infection 90 or more days prior was associated with higher VE against infection with 2 doses (36% [95% CI, 28%-44%] vs 19% [95% CI, 16%-22%] for children with vs without prior infection, respectively; P < .001 for interaction). Vaccine effectiveness by age, gender, chronic conditions, and symptom status was similar for 2 doses (Table 2).
Table 2. BNT162b2 Adjusted Vaccine Effectiveness for Children 5 to 11 Years of Age by Variant Period, Number of Doses, and Patient Characteristicsa.
| Characteristic | % (95% CI) | ||||
|---|---|---|---|---|---|
| Delta period (November 2 to December 9, 2021)b | Omicron period (January 16 to September 30, 2022)b | ||||
| 1 Dose | 2 Doses | 1 Dose | 2 Doses | 3 Dosesc | |
| Overall | 56 (50 to 61) | 85 (80 to 89) | 14 (6 to 21) | 20 (17 to 23) | 55 (50 to 60) |
| Age, y | |||||
| 5-8 | 50 (41 to 58) | 88 (81 to 92) | 15 (5 to 23) | 22 (18 to 26) | 54 (45 to 61) |
| 9-11 | 60 (52 to 67) | 82 (75 to 88) | 13 (1 to 23) | 18 (14 to 22) | 54 (47 to 61) |
| Gender | |||||
| Female | 56 (47 to 63) | 84 (76 to 89) | 15 (4 to 24) | 20 (16 to 24) | 55 (47 to 62) |
| Male | 55 (47 to 62) | 86 (79 to 90) | 13 (2 to 22) | 20 (16 to 24) | 55 (47 to 62) |
| Chronic condition | |||||
| No | 55 (49 to 61) | 85 (80 to 89) | 14 (6 to 21) | 21 (18 to 24) | 55 (49 to 60) |
| Yes | 63 (22 to 82) | 84 (48 to 95) | 11 (−33 to 41) | 15 (−2 to 30) | 63 (32 to 80) |
| Prior SARS-CoV-2 infection ≥90 d agod | |||||
| No | NA | NA | 12 (4 to 19) | 19 (16 to 22) | 51 (44 to 57) |
| Yes | NA | NA | 32 (12 to 48) | 36 (28 to 44) | 70 (60 to 78) |
| Symptom | |||||
| Asymptomatic | 52 (44 to 59) | 82 (75 to 87) | 23 (12 to 33) | 23 (18 to 28) | 36 (23 to 47) |
| Symptomatic, ≥1 symptom(s) | 49 (37 to 59) | 84 (75 to 91) | 7 (−4 to 16) | 24 (20 to 27) | 61 (55 to 67) |
Abbreviation: NA, not applicable.
Adjusted for age; gender; race and ethnicity; presence of a chronic medical condition; prior SARS-CoV-2 infection (Omicron period only); recent close contact with someone suspected or confirmed to have COVID-19; testing related to travel (Omicron period only); testing volume of pharmacy; rural, suburban, or urban trade area; and calendar week (in 2-week increments).
Median time since last dose was 4 months for children who received 2 doses during the Omicron period and 1 month for all other vaccination categories. For 3 doses, the maximum time since last dose was 5 months.
The most common Omicron sublineages circulating after the booster dose was recommended (on May 19, 2022) were BA.2, BA.2.12.1, BA.4, and BA.5.
On December 10, 2021, the appointment scheduling questionnaire was updated to add questions asking about history and timing of prior SARS-CoV-2 infection.
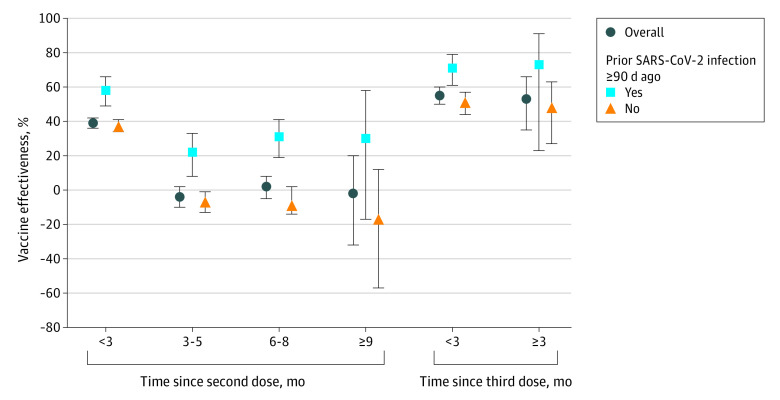
Owing to limited follow-up time, waning of VE during the Delta period could not be assessed. Vaccine effectiveness against infection of 2 doses during the Omicron period appeared to wane after approximately 3 months (Figure 2; eTable 2 and eTable 3 in Supplement 1). Against Omicron infection, the adjusted VE was 39% (95% CI, 36%-42%) at less than 3 months after the second dose and was not significant thereafter (−1% [95% CI, −6% to 3%] at ≥3 months after the second dose). Vaccine effectiveness was higher among children with (vs without) reported prior SARS-CoV-2 infection 90 or more days prior through 6 to 8 months after the second dose. Among children with SARS-CoV-2 infection 90 or more days prior, VE against Omicron infection was 58% (95% CI, 49%-66%) at less than 3 months and decreased to 27% (95% CI, 17%-35%) by 3 months or more. Among children without prior SARS-CoV-2 infection 90 or more days or more prior, VE against Omicron infection was 37% (95% CI, 34%-41%) at less than 3 months and decreased to −7% (95% CI, −12% to −1%) by 3 months or more. Monthly estimates were similar to 3-month interval results (eTable 2 and eFigure in Supplement 1).
Figure 2. Adjusted Vaccine Effectiveness of 2 and 3 Doses of BNT162b2 at 3-Month Intervals.
Adjusted vaccine effectiveness of 2 and 3 doses of BNT162b2 against Omicron infection among children aged 5 to 11 years by reported history of prior SARS-CoV-2 infection and time since receipt of the last dose at 3-month intervals. Vertical lines indicate 95% CIs.
Two-dose VE at less than 3 months against Omicron infection by sublineage was 40% (95% CI, 36%-43%) for BA.1, 32% (95% CI, 21%-41%) for BA.2/BA.2.12.1, and 50% (95% CI, 37%-60%) for BA.4/BA.5 (Table 3). Monthly estimates were similar across sublineage periods for about 2 months but then appeared to decrease faster at month 3 for BA.2/BA.2.12.1 and BA.4/BA.5 compared with BA.1 (eTable 4 in Supplement 1). Vaccine effectiveness against BA.2/BA.2.12.1 and BA.4/BA.5 infection was nonsignificant by 3 months or more after dose 2. Sublineage-specific VE estimates for infection and symptomatic infection were similar (Table 3).
Table 3. BNT162b2 Adjusted Vaccine Effectiveness for Children 5 to 11 Years of Age by Number of Doses, Time Since Last Dose (in 3-Month Increments), Symptomatic Status, and Omicron Sublineage Periodsa.
| Characteristic | % (95% CI) | ||||
|---|---|---|---|---|---|
| BA.1 (January 16 to March 5, 2022)b | BA.2/BA.2.12.1 (March 27 to June 4, 2022)b | BA.4/BA.5 (July 3 to September 30, 2022)b | |||
| 2 Doses | 2 Doses | 3 Doses | 2 Doses | 3 Doses | |
| Overall, mo | 40 (37 to 43) | 4 (−2 to 11) | 59 (34 to 75) | 10 (2 to 17) | 48 (39 to 55) |
| <3 | 40 (36 to 43) | 32 (21 to 41) | 59 (34 to 75) | 50 (37 to 60) | 48 (39 to 56) |
| 3-5 | 32 (17 to 44) | −1 (−9 to 6) | NA | −3 (−21 to 13) | 40 (16 to 57) |
| 6-8 | NA | 13 (−1 to 25) | NA | 7 (−2 to 16) | NA |
| ≥9 | NA | NA | NA | −6 (−36 to 17) | NA |
| Symptomatic, mo | 38 (33 to 43) | 13 (4 to 20) | 61 (27 to 79) | 7 (−3 to 16) | 56 (47 to 63) |
| <3 | 38 (33 to 43) | 31 (16 to 43) | 61 (27 to 79) | 45 (28 to 59) | 57 (47 to 64) |
| 3-5 | 30 (11 to 45) | 8 (−1 to 16) | NA | 5 (−16 to 22) | 48 (24 to 65) |
| 6-8 | NA | 22 (5 to 35) | NA | 2 (−10 to 12) | NA |
| ≥9 | NA | NA | NA | −4 (−37 to 21) | NA |
Abbreviation: NA, not applicable.
Adjusted for age; gender; race and ethnicity; presence of a chronic medical condition; prior SARS-CoV-2 infection; recent close contact with someone suspected or confirmed to have COVID-19; testing related to travel; testing volume of pharmacy; rural, suburban, or urban trade area; and calendar week (in 2-week increments).
Median time since last dose for children who received 2 doses was 2 months during the BA.1 period, 5 months during the BA.2/BA.2.12.1 period, and 7 months during the BA.4/5 period. For 3 doses, the maximum time since last dose was 1 month during the BA.2/BA.2.12.1 period and 5 months during the BA.4/5 period.
The overall adjusted VE for a booster dose against Omicron infection was 55% (95% CI, 50%-60%) (Table 2). Booster dose VE was higher among children with a history of infection 90 or more days prior compared with those without (70% [95% CI, 60%-78%] vs 51% [95% CI, 44%-57%]; P = .01 for interaction). Booster dose VE was also higher against symptomatic infection compared with asymptomatic infection (61% [95% CI, 55%-67%] vs 36% [95% CI, 23%-47%]; P < .001 for interaction). Booster dose VE against infection at 3 months or more after the dose assessed during the BA.4/BA.5 period was 40% or more regardless of history of infection 90 or more days prior (Figure 2; eTable 3 in Supplement 1) or presence of symptoms (Table 3). Crude VE estimates were similar to adjusted estimates (eTables 2, eTable 5, eTable 6, and eTable 7 in Supplement 1).
Discussion
This study estimated VE of BNT162b2 against infection among children 5 to 11 years of age who underwent testing for SARS-CoV-2 at a large national US pharmacy chain during periods of Delta and Omicron predominance. Overall, 2-dose VE was high against Delta infection (85% [95% CI, 80%-89%]) but lower against Omicron infection (20% [95% CI, 17%-23%]). Protection varied by history of prior infection. Specifically, overall 2-dose VE against Omicron infection was 36% (95% CI, 28%-44%) among children with a reported infection 90 days or more prior and 19% (95% CI, 16%-22%) among children without a reported infection 90 or more days prior. Although VE against Omicron was consistently higher for children with vs without SARS-CoV-2 infection 90 or more days prior, protection decreased in both groups approximately 3 months after the second dose (58% [95% CI, 49%-66%] vs 37% [95% CI, 34%-41%] at <3 months and 27% [95% CI, 17%-35%] vs −7% [95% CI, −12% to −1%] at ≥3 months). Two-dose VE at less than 3 months was relatively similar across Omicron sublineages, ranging from 50% (95% CI, 37%-60%) against BA.4/BA.5 to 32% (95% CI, 21%-41%) against BA.2/BA.2.12.1. A booster dose increased VE against Omicron infection to levels higher than that achieved after 2 doses to 55% (95% CI, 50%-60%) overall and to 70% (95% CI, 60%-78%) among children with a history of infection 90 or more days prior. Booster dose VE was higher against symptomatic infections than asymptomatic infections (61% [95% CI, 55%-67%] vs 36% [95% CI, 23%-47%]). Against BA.4/BA.5 (for which data beyond 3 months after a booster dose were available), there was no evidence of waning VE at 3 months or more after the booster dose.
Our findings for 2-dose VE are consistent with prior studies among children 5 to 11 years of age, which have reported modest protection in the first 3 months after a second dose. Cohen-Stavi et al14 reported VE of 51% (95% CI, 39%-61%) against infection (regardless of symptoms) 7 to 21 days after a second dose among Israeli children. Three other studies have reported lower VE of approximately 30% against Omicron infection after 2 doses of BNT162b2, although follow-up periods were longer. Fowlkes et al13 reported VE of 31% (95% CI, 9%-48%) 14 to 82 days after dose 2 among US children, Sacco et al12 reported VE of 29% (95% CI, 29%-30%) 14 to 98 days after dose 2 among children in Italy, and Tan et al11 reported VE of 26% (95% CI, 19%-32%) 60 days or more after dose 2 among children in Singapore. All 4 studies11,12,13,14 evaluated VE among children without a history of prior SARS-CoV-2 infection. Against symptomatic COVID-19, studies11,14,15,16 have reported approximately 50% to 60% VE in the first 1 to 2 months after receipt of a second dose. Our results are consistent with those reported by Fleming-Dutra et al,15 who showed that VE against symptomatic infection was 60% (95% CI, 55%-65%) at 2 to 4 weeks after a second dose but that this effectiveness waned to 29% (95% CI, 25%-33%) by 2 months after dose 2 among children tested in a pharmacy setting. Seventy-three percent of the Omicron infections captured in our study were symptomatic, which may explain why 2-dose VE was similar among all infections and symptomatic infections and may explain the high degree of consistency between our results for infection (with or without symptoms) and those from the study by Fleming-Dutra et al,15 which examined effectiveness against symptomatic infection.
To our knowledge, this is the first study to report VE for a booster dose of BNT162b2 among children aged 5 to 11 years, which was assessed during periods predominated by the BA.2/BA.2.12.1 and BA.4/BA.5 sublineages. Although children receive a lower vaccine dose (10 μg) than given to older age groups (30 μg), our results were consistent with previous findings for adults reported by Link-Gelles et al27 of limited and short-lived protection against Omicron-related urgent or emergency care, in particular due to BA.2/BA.2.12.1. Link-Gelles et al27 observed modest protection (<70%) with 2 or 3 doses of a messenger RNA vaccine against BA.2/BA.2.12.1–related hospitalization that may decrease over time, and another study12 has reported low 2-dose VE of 41% (95% CI, 22%-55%) against BA.1-related hospital admission or death among children aged 5 to 11 years. With the authorization of bivalent COVID-19 vaccines as a booster dose for children aged 5 to 11 years in the United States, the (monovalent) BNT162b2 vaccine is no longer authorized as a booster dose.28 As new variants and sublineages emerge and outcompete earlier strains, it is critical to continually monitor the level and duration of protection offered by currently available vaccines.
Limitations
Our study findings are subject to the following limitations. Since October 2021, children aged 5 to 11 years have had the highest SARS-CoV-2 seroprevalence of all age groups in the United States. Between December 2021 and February 2022 (when COVID-19 cases surged in the US owing to the emergence of Omicron), seroprevalence among this age group increased markedly from 47% to 77%.4 Thus, during our study period, the prevalence of prior SARS-CoV-2 infection and corresponding population levels of infection-acquired immunity changed rapidly. Bias resulting from these rapid changes could have resulted in underestimation of VE, particularly for BA.2/BA.2.12.1 and BA.4/BA.5 sublineages. If unvaccinated children were more likely than vaccinated children to acquire infection-derived immunity during the first Omicron wave—a phenomenon known as “differential depletion of susceptibles”—this factor could bias VE estimates against subsequent Omicron sublineages downward and, in some cases, yield negative VE estimates even when a vaccine provides protection.29 Although we gathered information on prior SARS-CoV-2 infection, these data were self-reported. Despite this potential for bias, we observed a clear pattern of waning effectiveness against Omicron infection after 2 doses, consistent with previous reports.12,15 This analysis did not include children experiencing moderate or severe symptoms. However, our analysis describes asymptomatic and mild illness, which comprises most pediatric cases (>95%).30 The limited follow-up period available to assess booster VE allowed for the evaluation of only short-term effectiveness. Information on demographic characteristics, clinical history, and current symptoms was reported by a parent or guardian, which may be subject to recall bias or error. Vaccination dates were reported as month and year only, which may affect VE estimates over time owing to misclassification of time since vaccination. Although VE estimates were based on adjusted multilevel models that accounted for patient characteristics, pharmacy-level factors, and time trends, bias from residual confounding remains possible. The test-negative design is less susceptible to bias caused by differences in health care–seeking behavior between cases and controls compared with other designs. Nonetheless, differential testing by vaccine status may occur based on testing policies, perceived risk, or other factors. Finally, SARS-CoV-2 epidemiology changes rapidly; thus, these results may not be generalizable beyond the context captured in this study. Our estimates for the short-term VE of 2 doses of BNT162b2 in this age group, however, are consistent with prior studies conducted in the United States, Israel, Italy, and Singapore.
Conclusions
This test-negative case-control study suggests that, in the current setting of Omicron predominance and high seroprevalence, 2 doses of BNT162b2 provided modest short-term protection against Omicron infection among children aged 5 to 11 years. Estimated effectiveness was higher among children with a history of infection; however, protection decreased in all children about 3 months after the second dose. A (monovalent) booster dose restored and provided moderate protection against Omicron infection for at least 3 months after vaccination. These findings highlight the continued benefits associated with booster doses in maintaining protection against SARS-CoV-2 in individuals with or without a history of COVID-19. Future VE studies are needed to describe the level of protection and longer-term durability provided by monovalent and bivalent COVID-19 vaccines against more severe end points among this age group.
eTable 1. Demographic, Clinical, and Testing Characteristics of Children 5-11 Years of Age by Variant Period and COVID-19 Vaccination Status
eTable 2. BNT162b2 Crude and Adjusted Vaccine Effectiveness for Children 5-11 Years of Age by Number of Doses, Time Since Last Dose, and Prior SARS-CoV-2 Infection Status During the Overall Omicron Period
eTable 3. Sample Counts for Figure 2 (Adjusted Vaccine Effectiveness of 2 and 3 Doses of BNT162b2 Against Omicron Infection Among Children Aged 5-11 Years by Reported History of Prior SARS-CoV-2 Infection and Time Since Receipt of the Last Dose at 3-Month Intervals)
eTable 4. BNT162b2 Adjusted Vaccine Effectiveness for Children 5-11 Years of Age by Number of Doses, Time Since Last Dose, and Omicron Sublineage Periods
eTable 5. BNT162b2 Crude Vaccine Effectiveness (95% Confidence Interval) for Children 5-11 Years of Age by Variant Period, Number of Doses, and Patient Characteristics
eTable 6. BNT162b2 Crude Vaccine Effectiveness for Children 5-11 Years of Age by Number of Doses, Time Since Last Dose, and Omicron Sublineage Periods
eTable 7. BNT162b2 Crude Vaccine Effectiveness for Children 5-11 Years of Age by Number of Doses, Months Since Last Dose (in 3-Month Increments), Symptomatic Status, and Omicron Sublineage Periods
eFigure. Adjusted Vaccine Effectiveness of 2 Doses of BNT162b2 Against Omicron Infection Among Children Aged 5-11 Years by History of Prior SARS-CoV-2 Infection and Number of Months Since Receipt of the Second Dose
Data Sharing Statement
References
- 1.World Health Organization . COVID-19 weekly epidemiological update, edition 74. Updated January 11, 2022. Accessed May 4, 2022. https://apps.who.int/iris/handle/10665/351044
- 2.World Health Organization . COVID-19 weekly epidemiological update, edition 72. Updated December 28, 2022. Accessed July 12, 2022. https://apps.who.int/iris/handle/10665/350973
- 3.Centers for Disease Control and Prevention . COVID data tracker. Accessed July 12, 2022. https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance
- 4.Clarke KEN, Kim Y, Jones J, et al. Pediatric infection-induced SARS-CoV-2 seroprevalence estimation using commercial laboratory specimens: how representative is it of the general U.S. pediatric population? SSRN. Published online May 4, 2022. doi: 10.2139/ssrn.4092074 [DOI] [Google Scholar]
- 5.Woodworth KR, Moulia D, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5-11 years—United States, November 2021. MMWR Morb Mortal Wkly Rep. 2021;70(45):1579-1583. doi: 10.15585/mmwr.mm7045e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. CDC strengthens recommendations and expands eligibility for COVID-19 booster shots. May 19, 2022. Accessed October 28, 2022. https://www.cdc.gov/media/releases/2022/s0519-covid-booster-acip.html
- 7.U.S. Food & Drug Administration. Coronavirus (COVID-19) update: FDA expands eligibility for Pfizer-BioNTech COVID-19 vaccine booster dose to children 5 through 11 years. May 17, 2022. Accessed October 28, 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-pfizer-biontech-covid-19-vaccine-booster-dose
- 8.Centers for Disease Control and Prevention. CDC recommends Moderna COVID-19 vaccine for children and adolescents. June 24, 2022. Accessed October 28, 2022. https://www.cdc.gov/media/releases/2022/s0623-moderna-children.html
- 9.U.S. Food & Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Moderna and Pfizer-BioNTech COVID-19 vaccines for children down to 6 months of age. June 17, 2022. Accessed October 28, 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-covid-19-vaccines-children
- 10.Centers for Disease Control and Prevention . CDC expands updated COVID-19 vaccines to include children ages 5 through 11. Accessed October 28, 2022. https://www.cdc.gov/media/releases/2022/s1012-COVID-19-Vaccines.html
- 11.Tan SHX, Cook AR, Heng D, Ong B, Lye DC, Tan KB. Effectiveness of BNT162b2 vaccine against Omicron in children 5 to 11 years of age. N Engl J Med. 2022;387(6):525-532. doi: 10.1056/NEJMoa2203209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacco C, Del Manso M, Mateo-Urdiales A, et al. ; Italian National COVID-19 Integrated Surveillance System and the Italian COVID-19 vaccines registry . Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5-11 years in Italy: a retrospective analysis of January-April, 2022. Lancet. 2022;400(10346):97-103. doi: 10.1016/S0140-6736(22)01185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowlkes AL, Yoon SK, Lutrick K, et al. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5-11 years and adolescents aged 12-15 years—PROTECT Cohort, July 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(11):422-428. doi: 10.15585/mmwr.mm7111e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen-Stavi CJ, Magen O, Barda N, et al. BNT162b2 vaccine effectiveness against Omicron in children 5 to 11 years of age. N Engl J Med. 2022;387(3):227-236. doi: 10.1056/NEJMoa2205011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming-Dutra KE, Britton A, Shang N, et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during Omicron predominance. JAMA. 2022;327(22):2210-2219. doi: 10.1001/jama.2022.7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19–associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5-17 years—VISION Network, 10 states, April 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(9):352-358. doi: 10.15585/mmwr.mm7109e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aegis Sciences Corporation. About Aegis. Accessed February 1, 2022. https://www.aegislabs.com/our-team/labs
- 18.Walgreens. COVID-19 testing for ages 3+. Accessed November 10, 2022. https://www.walgreens.com/findcare/covid19/testing
- 19.Van Dyke ME, Mendoza MC, Li W, et al. Racial and ethnic disparities in COVID-19 incidence by age, sex, and period among persons aged <25 years—16 U.S. jurisdictions, January 1–December 31, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(11):382-388. doi: 10.15585/mmwr.mm7011e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy NC, Zell E, Fast HE, et al. Disparities in first dose COVID-19 vaccination coverage among children 5-11 years of age, United States. Emerg Infect Dis. 2022;28(5):986-989. doi: 10.3201/eid2805.220166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon P, Hall J, Fuld J, et al. Alternative methods for grouping race and ethnicity to monitor COVID-19 outcomes and vaccination coverage. MMWR Morb Mortal Wkly Rep. 2021;70(32):1075-1080. doi: 10.15585/mmwr.mm7032a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walgreens. Notice of privacy practices. Accessed July 26, 2021. https://www.walgreens.com/topic/help/general/noticeprivacypractices.jsp?foot=privacy
- 23.Centers for Disease Control and Prevention . COVID data tracker: variant proportions. Accessed March 22, 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 24.Centers for Disease Control and Prevention . Overview of testing for SARS-CoV-2, the virus that causes COVID-19. Updated June 15, 2022. Accessed July 12, 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html
- 25.Agency for Toxic Substances and Disease Registry . CDC/ATSDR Social Vulnerability Index. Updated March 15, 2022. Accessed July 12, 2022. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- 26.Agresti A. Categorical Data Analysis. 3rd ed. John Wiley & Sons; 2013. [Google Scholar]
- 27.Link-Gelles R, Levy ME, Gaglani M, et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA vaccine doses among immunocompetent adults during periods when SARS-CoV-2 Omicron BA.1 and BA.2/BA.2.12.1 sublineages predominated—VISION Network, 10 states, December 2021–June 2022. MMWR Morb Mortal Wkly Rep. 2022;71(29):931-939. doi: 10.15585/mmwr.mm7129e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Food & Drug Administration . Coronavirus (COVID-19) update: FDA authorizes Moderna and Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose in younger age groups. Updated October 12, 2022. Accessed October 28, 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-bivalent-covid-19-vaccines
- 29.World Health Organization . Evaluation of COVID-19 vaccine effectiveness in a changing landscape of COVID-19 epidemiology and vaccination: interim guidance, 1 October 2022: second addendum to evaluation of COVID-19 vaccine effectiveness: interim guidance. Accessed October 28, 2022. https://apps.who.int/iris/handle/10665/363344
- 30.Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5(10):708-718. doi: 10.1016/S2352-4642(21)00198-X [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Demographic, Clinical, and Testing Characteristics of Children 5-11 Years of Age by Variant Period and COVID-19 Vaccination Status
eTable 2. BNT162b2 Crude and Adjusted Vaccine Effectiveness for Children 5-11 Years of Age by Number of Doses, Time Since Last Dose, and Prior SARS-CoV-2 Infection Status During the Overall Omicron Period
eTable 3. Sample Counts for Figure 2 (Adjusted Vaccine Effectiveness of 2 and 3 Doses of BNT162b2 Against Omicron Infection Among Children Aged 5-11 Years by Reported History of Prior SARS-CoV-2 Infection and Time Since Receipt of the Last Dose at 3-Month Intervals)
eTable 4. BNT162b2 Adjusted Vaccine Effectiveness for Children 5-11 Years of Age by Number of Doses, Time Since Last Dose, and Omicron Sublineage Periods
eTable 5. BNT162b2 Crude Vaccine Effectiveness (95% Confidence Interval) for Children 5-11 Years of Age by Variant Period, Number of Doses, and Patient Characteristics
eTable 6. BNT162b2 Crude Vaccine Effectiveness for Children 5-11 Years of Age by Number of Doses, Time Since Last Dose, and Omicron Sublineage Periods
eTable 7. BNT162b2 Crude Vaccine Effectiveness for Children 5-11 Years of Age by Number of Doses, Months Since Last Dose (in 3-Month Increments), Symptomatic Status, and Omicron Sublineage Periods
eFigure. Adjusted Vaccine Effectiveness of 2 Doses of BNT162b2 Against Omicron Infection Among Children Aged 5-11 Years by History of Prior SARS-CoV-2 Infection and Number of Months Since Receipt of the Second Dose
Data Sharing Statement