Abstract
Synthetic peptide vaccines which are derived from functional domains of Streptococcus mutans glucosyltransferases (GTF) have been shown to induce protective immunity in Sprague-Dawley rats after subcutaneous injection in the salivary gland region. Since mucosal induction of salivary immunity would be preferable in humans, we explored methods to induce mucosal antibody in the rat to the GTF peptide vaccines HDS and HDS-GLU after intranasal administration. Several methods of facilitation of the immune response were studied: the incorporation of peptides in bioadhesive poly(d,l-lactide-coglycolide) (PLGA) microparticles, the use of monoepitopic (HDS) or diepitopic (HDS-GLU) peptide constructs, or the use of mucosal adjuvants. Salivary immunoglobulin A (IgA) responses were not detected after intranasal administration of diepitopic HDS-GLU peptide constructs in alum or after incorporation into PLGA microparticles. However, significant primary and secondary salivary IgA and serum IgG antibody responses to HDS were induced in all rats when cholera holotoxin (CT) or a detoxified mutant Escherichia coli heat-labile enterotoxin (R192G LT) were intranasally administered with HDS peptide constructs in PLGA. Coadministration of LT with HDS resulted in predominantly IgG2a responses in the serum, while coadministration with CT resulted in significant IgG1 and IgG2a responses to HDS. Serum IgG antibody, which was induced to the HDS peptide construct by coadministration with these adjuvants, also bound intact mutans streptococcal GTF in an enzyme-linked immunosorbent assay and inhibited its enzymatic activity. Thus, immune responses which are potentially protective for dental caries can be induced to peptide-based GTF vaccines after mucosal administration if combined with the CT or LT R192G mucosal adjuvant.
Mucosal immunization with mutans streptococcal glucosyltransferases (GTF) has been shown to induce immune responses which can protect rats from experimental dental caries (38) and which can reduce mutans streptococcal recolonization in humans (42, 43). These multifunctional enzymes catalyze the formation of glucans from sucrose and also include domains for the binding of glucan (1, 12). Theoretically, GTF subunit vaccines could be constructed in order to increase the enzyme inhibitory capacity of the immune response and to eliminate responses to irrelevant epitopes. Several domains have been associated with the catalytic functions of GTF by using a variety of techniques, including labeling intermediates (14, 32), site-directed mutagenesis (10, 29, 30, 54), and sequence alignment with catalytically similar proteins (10, 24, 28). These studies have suggested that GTF and alpha amylase share several invariant residues important to their catalytic activity which are associated with (β,α)8 barrel structures in their respective catalytic domains (24, 31, 45, 53). Catalytically important residues have been identified in the β4, β5, and β7 strands of a putative GTF (β,α)8 barrel segment. Peptides from within these and other suspected catalytically important regions have been synthesized and shown to induce GTF-inhibitory immune responses after systemic injection (6, 9, 22, 39–41). Several of these peptide constructs have also been shown to induce immune responses which were protective in a rat experimental dental caries model (40, 51, 52).
We have explored the immunological characteristics of a 19-mer peptide (HDS) in the β7 strand, within which several residues are found which are potentially involved in the activity of the enzyme (40). His-561 and Asp-562 in Streptococcus mutans GTF-B are invariant in mutans streptococcal GTF. The analogous histidine in alpha amylases helps to stabilize transition states (45), while the aspartate stabilizes the reaction-intermediate carbonium cation (25). Site-directed mutagenesis of the equivalent histidine and aspartic acid residues in mutans streptococcal GTF catalytically inactivated the enzyme (10, 54). Also contained within the HDS peptide sequence is an aspartate, equivalent to Asp-567 in GTF-B, which has been shown to influence characteristics of glucan synthesized by GTF (30, 36). Aspartic acid is invariant at this position in all mutans streptococcal GTF, although it is not conserved in alpha amylases, presumably because its function is irrelevant to amylolytic activity. The HDS peptide subtends the sequence within which these residues are found. When subcutaneously injected as a four-chain construct, this peptide induces high levels of salivary immunoglobulin A (IgA) and serum IgG antibody to HDS which reacts with and inhibits GTF catalytic activity (41).
Mucosal administration of antigen is a promising route of delivery for dental caries vaccines in humans for several reasons. These routes (oral, topical, or intranasal [i.n.] administration) are considered to be relatively safer than systemic immunizations and may be better tolerated by the young child targeted for immunization. Furthermore, several mucosal routes have already been shown to induce protective responses after GTF administration in experimental systems. Although several peptide constructs based on functional GTF epitopes are markedly immunogenic after local systemic injection, their immunogenicity upon mucosal application has not been explored. Induction of immune responses with peptide-based vaccines generally requires immune facilitation. Since these constructs are promising as components of a human dental caries vaccine, the present study was designed to explore several methods to induce anti-peptide immune responses after i.n. immunization. These methods included administration of peptide constructs in bioadhesive poly(d,l-lactide-coglycolide) (PLGA) microparticles, an approach previously shown to facilitate primary and secondary mucosal antibody formation to i.n. administered intact GTF (44). In addition, we explored the ability of a mutant E. coli heat-labile enterotoxin (LT) (11) to function as a mucosal adjuvant for i.n. administration of HDS peptide constructs, comparing its immune-enhancing properties to that of cholera toxin (CT), an adjuvant often used to increase immune responses to mucosally applied antigens. The toxic properties of this LT had been modified by substitution of arginine for glycine at position 192 (R192G LT). This detoxified LT had been shown to enhance immune responses to mucosally applied antigens (4, 16).
MATERIALS AND METHODS
Peptide constructs.
Monoepitopic and diepitopic peptide constructs were prepared for this study. The monoepitopic peptide sequence, HDS, was based on a putative catalytic region within the predicted (β,α)8 barrel structure of GTF (10, 24). Subcutaneous injection of this peptide construct had previously been shown to induce high levels of serum antibody and moderate levels of salivary antibody to HDS. The 19-mer HDS peptide sequence contained catalytically implicated His-561, Asp-562, and Asp-567 (10, 29, 54). The HDS sequence, VPSYSFIRAHDSEVQDLIA, is highly conserved among all mutans streptococcal GTF and was identical to the respective S. mutans GTF-B sequence (37). The HDS peptide was synthesized (AnaSpec, Inc., San Jose, Calif.) using the stepwise solid-phase method of Merrifield (27) on a core matrix of lysines to yield macromolecules with four identical peptides per molecule, as described by Tam (48). Purity (>90%) was assessed using high-pressure liquid chromatography (HPLC), amino acid analysis, and molecular weight determination was done by mass spectrometry.
The diepitopic peptide construct, HDS-GLU, contained two copies of the HDS peptide and two copies of a 22-mer peptide, GLU, from the glucan-binding domain of GTF. This peptide, TGAQTIKGQKLYFKANGQQVKG, had complete homology with the derived sequence within the “A” repeat region of S. downei GTF-I (12) and was very similar to sequences in the proposed glucan-binding regions of the S. sobrinus and S. mutans GTF which synthesize insoluble glucan product (1, 34). The GLU peptide construct was immunogenic and protective in the experimental rat caries model as a monoepitopic construct (51) and enhanced immune responses to other epitopes when included in diepitopic constructs (M. A. Taubman, C. J. Holmberg, and D. J. Smith, J. Dent. Res. 76:347, abstr. 2666, 1997). The HDS-GLU construct was also synthesized by AnaSpec on a lysine backbone.
Peptide constructs were incorporated into the PLGA-based biodegradable carrier using 1% gelatin as a bioadhesive agent. The PLGA copolymer (Boehringer Ingelheim Chemicals, Inc.) was used at a lactide/glycolide ratio of 75:25. A low-density polymer foam was prepared by lyophilization of a polymer solution in glacial acetic acid. The polymer foam was cryogenically ground in a Tekmar model A-10 analytical mill (20,000 rpm) equipped with a cryogenic well, cooled with liquid nitrogen, enabling low-temperature particle size reduction.
GTF.
GTF from S. mutans SJ were prepared as previously described (49). After bacterial growth in glucose-containing defined medium, enzymes were isolated from culture medium by affinity chromatography on Sephadex G-150 (Pharmacia Fine Chemicals, Piscataway, N.J.) with 3 M guanidine HCl as the eluting solvent. These GTF-rich pools were then subjected to fast-protein liquid chromatography on Superose 6 (Pharmacia) with 6 M guanidine HCl for elution. These GTF preparations synthesized both water-insoluble and water-soluble glucans in both tube and filter assays and were used for inhibition assays and enzyme-linked immunosorbent assay (ELISA) measurements of antibody activity.
Experimental protocols. (i) Experiment 1.
The first experiment explored the mucosal immunogenicity of the HDS peptide construct when delivered i.n. in PLGA microparticles. Sprague-Dawley CD strain 45-day-old male rats (Charles River Laboratories, Wilmington, Mass.) were used for immunization. Two groups of rats were i.n. immunized on days 0, 7, 14, and 21 with 0.03 ml distributed equally between both nostrils using an Eppendorf pipette. This dose was well tolerated by the IN-immunized animals; thus, no anesthesia was required for antigen administration. The first group (n = 2) was sham immunized with unloaded PLGA microparticles. The second group (n = 6) was immunized with PLGA microparticles loaded with HDS (PLGA-HDS). Each dose contained 60 μg of peptide contained in 800 μg of microparticles. Prior to immunization, all rats were bled from the tail vein, and saliva was collected for 10 min by gravity collection (10 mg of pilocarpine nitrate/kg [rat weight]) under ether anesthesia. All rats were subsequently bled, and saliva was collected on day 28. Sera and salivas were stored at −70°C prior to measurement of antibody.
(ii) Experiment 2.
The second experiment explored the mucosal immunogenicity of HDS when presented in a diepitopic construct with GLU and delivered i.n. in PLGA microparticles. Three groups of seven animals (45-day-old Sprague-Dawley rats) per group were i.n. immunized on days 0, 7, 14, and 21 with 0.03 ml. The first group was sham immunized with unloaded PLGA microparticles. The second group was immunized with PLGA microparticles loaded with HDS-GLU (PLGA HDS-GLU). Each dose contained 60 μg of peptide contained in 800 μg of microparticles. The third group was immunized with 60 μg of HDS-GLU in 10 μl of aluminum hydroxide. Prior to immunization, all rats were bled and saliva was collected using pilocarpine stimulation under ether anesthesia. All rats were subsequently bled, and saliva was collected on days 28, 42, and 55. Animals were reimmunized i.n. on days 70 and 75 with doses identical to those used for primary immunization. Blood and saliva were then collected on days 96, 102, and 109. Serum and saliva samples were stored at −70°C prior to measurement of the antibody.
(iii) Experiment 3.
The third experiment explored the ability of CT to facilitate mucosal responses to PLGA HDS after i.n. administration. Immunization was initiated in Sprague-Dawley CD strain 38-day-old female rats. Three groups of eight animals per group were i.n. immunized on days 0, 7, and 15. The first group was sham immunized with unloaded PLGA microparticles. Rats in the second group were i.n. immunized with PLGA microparticles and 5 μg of CT (List Biological Laboratories, Inc., Campbell, Calif.). Rats in the third group were i.n. immunized with 800 μg of PLGA microparticles containing 60 μg of HDS (PLGA-HDS), together with 5 μg of CT. Prior to immunization, all rats were bled and saliva was collected. In this experiment, rats were first momentarily anesthetized with a gas mixture of 50% carbon dioxide and 50% oxygen and then anesthetized by intraperitoneal injection of a mixture (0.65 ml/kg) of three parts ketamine (Ketaset;100 mg/ml; Fort Dodge Lab, Ft. Dodge, Iowa) and seven parts xylazine (Rompun; 20 mg/ml; Bayer Corp., Shawnee Mission, Kans.). Saliva secretion was stimulated by subcutaneous injection of 0.6 ml of carbachol (containing 0.1 mg/ml in saline; Sigma Chemical Co., St. Louis, Mo.) per kg of rat weight. After fluid collection, rats were injected subcutaneously with yohimbine (Yobine; 2.0 mg/ml; Lloyd Laboratories, Shenandoah, Iowa) at a volume equal to 1.4 times that used for anesthesia. All rats were subsequently bled, and saliva samples were obtained on days 24, 30, 52, and 70. Animals were reimmunized i.n. on day 93 with doses identical to those used for primary immunization. Blood and saliva were then collected on days 100, 114, 129, and 171, followed by a third immunization on days 183 and 197. Final bleeding and saliva collection took place on day 211. Serum and saliva samples were stored at −70°C prior to measurement of the antibody.
(iv) Experiment 4.
The fourth experiment explored the ability of a detoxified mutant Escherichia coli heat-labile enterotoxin (LT R192G) to facilitate the mucosal response to PLGA HDS after i.n. administration. This mucosal adjuvant was prepared and kindly provided by John D. Clements, Tulane University Medical Center, New Orleans, La. Immunization was initiated in 38-day-old Sprague-Dawley CD strain female rats. Two groups of rats were i.n. immunized on days 0, 8, and 14. The first group (n = 4) was sham immunized with unloaded PLGA microparticles, together with 5 μg of CT. Rats in the second group (n = 7) were i.n. immunized with PLGA microparticles containing 60 μg of HDS (HDS-PLGA), together with 5 μg of R192G LT. Rats in both groups were bled, and saliva samples were obtained on days 0, 26, and 54. Animals were reimmunized i.n. on day 89 with doses identical to those used for primary immunization. Blood and saliva were then collected on days 96, 110, and 169, followed by a third immunization on days 160 and 174. Final bleeding and salivation took place on day 188. Anesthesia and secretion stimulation was performed as in experiment 3. Sera and saliva samples were stored at −70°C prior to measurement of the antibody.
ELISA.
Serum IgG and salivary IgA antibodies were tested by ELISA. Polystyrene microtiter plates (Flow Laboratories) were coated with 2.5 μg of HDS or 0.5 μg of S. sobrinus or S. mutans GTF per ml. Antibody activity was then measured by incubation with 1:400 and 1:4,000 dilutions of sera or 1:4 and 1:8 dilutions of saliva. Plates were then developed for IgG antibody with rabbit anti-rat IgG, followed in sequence by alkaline phosphatase-labeled goat anti-rabbit IgG (Biosource, Inc.) and p-nitrophenylphosphate (Sigma). A mouse monoclonal reagent to rat α chain (Zymed, South San Francisco, Calif.) was used with biotinylated goat anti-mouse IgG (Zymed), followed by avidin-alkaline phosphatase (ICN Biomedicals, Inc., Auroa, Ohio), followed by p-nitrophenylphosphate to reveal levels of salivary IgA antibody to peptides. Reactivity was recorded as the A405 in a microplate reader (Biotek Instruments, Winooski, Vt.). Data are reported as ELISA units (EU), which were calculated relative to the levels of appropriate reference sera or salivas from Sprague-Dawley rats twice immunized with the respective peptide construct. Dilutions of sera producing an A405 of approximately 1.0 were considered 100 EU for serum IgG antibody measurements. Dilutions of saliva producing an A405 of approximately 0.8 were considered 100 EU for salivary IgA antibody.
To measure the relative level of IgA in saliva samples, plates were coated with mouse monoclonal reagent to rat α chain (1:500) (Zymed). After 2 h of incubation with saliva samples at 1:200, the plates were developed with biotinylated mouse monoclonal reagent to rat α chain, followed by avidin-alkaline phosphatase (Cappel) and p-nitrophenylphosphate to reveal relative levels of salivary IgA. Reactivity was recorded as the A405 value. Data are reported as IgA units, a relative indication of IgA concentration, by comparison with a precalibrated rat salivary IgA reagent.
ELISA was also used to detect IgG1 and IgG2a antibody to HDS in rat serum (20). HDS (2.5 μg/ml; sodium bicarbonate buffer, pH 9.7) was coated onto 96-well plates. Rat serum (at a 100 to 1,000 times dilution) was applied, followed by horseradish peroxidase-conjugated sheep anti-rat IgG1 or IgG2a (Binding Site, Birmingham, United Kingdom). Colorimetric reactions were developed with o-phenylenediamine (Sigma) in the presence of 0.02% H2O2. After a 10-min incubation, reactions were stopped with 2 N H2SO4 and measured at 490 nm. Hyperimmune serum to HDS was prepared in Sprague-Dawley rats by subcutaneous immunization with HDS (10 μg/dose) in complete Freund adjuvant (first dose) and incomplete Freund adjuvant (second dose) at intervals of 3 weeks. The absorbancy levels of the immune sera were compared to the absorbancy levels of a standard curve comprised of dilutions of a calibrated rat serum containing 663 mg of IgG1 and 2,063 mg of Ig2a (Binding Site) per liter, captured with affinity-purified goat anti-rat IgG Fc (Chemicon International, Inc., Temecula, Calif.), and developed as described above.
Antibody inhibition of glucan synthesis.
Selected rat sera were evaluated for their ability to inhibit water-insoluble glucan synthesis catalyzed by S. mutans GTF by using a filter assay. We preincubated 10-μl volumes of diluted sera (1:10 dilutions in 0.02 M sodium phosphate-buffered saline and 0.2% sodium azide [PBSA], pH 6.5) with the GTF for 2 h at 37°C in a total volume of 0.04 ml of PBSA. Then, 1.7 mg of sucrose and 24 nCi of 14C-glucose–sucrose (ca. 50,000 cpm) were added in 0.2 ml PBSA in the absence of primer dextran. Incubation proceeded overnight at 37°C, after which water-insoluble glucan was collected on Whatman GF/F glass fiber filters. Water-insoluble glucan collected on filters was washed, and retained radioactivity was determined as previously reported (49). Under the conditions of this assay, approximately 1,200 cpm were incorporated into water-insoluble glucan in the presence of the sham-immune sera. The percent inhibition of enzyme activity was calculated by using these mean sham incorporation counts-per-minute values as the 100% incorporation levels.
Statistical analysis.
The differences in the median values among the treatment groups were analyzed by Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks, followed by Dunnet's or Dunn's multiple comparison procedures for nonparametric analyses.
RESULTS
The ability of monoepitopic (HDS) or diepitopic (HDS-GLU) peptide constructs to induce a salivary IgA response to the HDS peptide after i.n. administration was studied in experiments 1 and 2, respectively. After four weekly doses of peptide constructs in the monoepitopic HDS (0.01 ± 0.01 EU; mean ± the standard error) or diepitopic HDS-GLU format in PLGA (0.05 ± 0.02 EU) or with aluminum hydroxide (0.01 ± 0.01 EU), salivary IgA antibody levels were not different from those of sham-immunized controls given unloaded PLGA microparticles i.n. (0.04 ± 0.03 EU [experiment 1] or 0.03 ± 0.01 EU [experiment 2], respectively). These experiments indicated, therefore, that i.n. administration of GTF peptides in either monoepitopic or diepitopic MAP constructs in PLGA microparticles alone was not sufficient to induce the formation of detectable salivary IgA antibody.
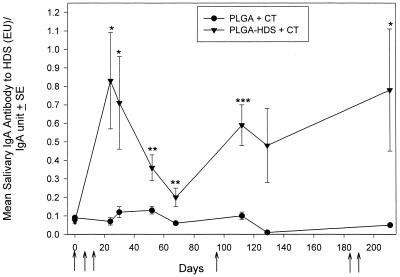
CT has been shown to be an effective mucosal adjuvant with other peptides. Therefore, we explored the ability of using CT to induce a mucosal immune response to PLGA HDS in saliva after i.n. administration. Figure 1 illustrates the salivary IgA antibody to HDS per relative IgA level in the sham-immune unloaded PLGA+CT group and in the immune PLGA-HDS+CT group. Combination of the peptide construct with CT induced a significant primary IgA response to HDS in the salivas of all rats given the peptide antigen microparticles plus CT. The primary response was highest 10 days after the last i.n. dose was administered (day 24, P < 0.001). Salivary IgA antibody to HDS levels then fell incrementally but remained significantly higher than the sham-immune control levels on days 52 (P < 0.003) and 68 (P < 0.03).
FIG. 1.
Salivary IgA responses to HDS peptide after i.n. administration of PLGA-HDS with 5 μg of CT. Closed triangles indicate the mean salivary IgA EU/relative salivary IgA unit ratios in rats immunized with PLGA-HDS microparticles and CT. Closed circles indicate the antibody levels of rats with unloaded PLGA microparticles and CT. The brackets enclose two standard errors. Arrows indicate the days of administration. Significance (∗, P < 0.001; ∗∗, P < 0.04; ∗∗, = P < 0.01) was determined with respect to the sham group response on the respective day. Salivas were tested at a 1:8 dilution.
Figure 1 also shows the salivary IgA responses in sham- and HDS+CT-immunized rats after boosting. A single i.n. dose of PLGA-HDS+CT was given on day 93 which induced a salivary IgA response that was significantly higher (P < 0.03) than the salivary antibody level measured on day 68 but remained less than the peak primary antibody levels. A second course of two i.n. doses approximately 100 days later modestly increased salivary IgA antibody levels. (P < 0.004). Thus, CT was effective in the primary response and in a second and third induction of salivary IgA immune responses to the HDS peptide construct. A vigorous salivary IgA response to CT was also observed in all animals receiving this immunoadjuvant (not shown).
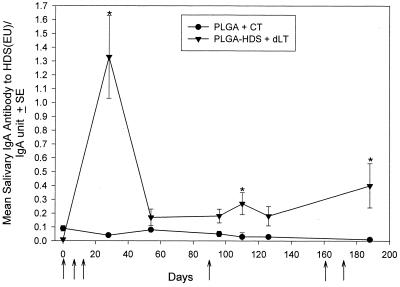
Since it is likely that the toxic effects of CT would preclude i.n. administration to humans, we also explored the ability of detoxified LT (R192G LT) to enhance salivary anti-peptide antibody induction after i.n. application. Figure 2 illustrates the salivary IgA antibody to HDS/relative IgA unit ratio in the sham and PLGA-HDS+R192G LT (dLT) groups. A significant (P < 0.001) salivary IgA response to HDS occurred in all rats coadministered with dLT by day 26. The primary response was of similar kinetics and magnitude to those observed using CT. A second i.n. exposure to peptide and dLT on day 89 and on days 160 and 172 increased the salivary IgA antibody to significant levels. However, the magnitude of these subsequent salivary IgA responses to HDS using dLT (Fig. 2) was not as great as with CT (Fig. 1).
FIG. 2.
Salivary IgA responses to HDS peptide after i.n. administration of PLGA-HDS with 5 μg of R192G LT (dLT). Closed triangles indicate the mean salivary IgA EU/relative salivary IgA unit ratios in rats immunized with PLGA-HDS microparticles and dLT. Closed circles indicate the antibody levels of rats with unloaded PLGA microparticles and CT. Brackets enclose two standard errors. Arrows indicate the days of i.n. administration. Significance (∗, P < 0.001) was determined with respect to the sham group response on the respective day. Salivas were tested at a 1:8 dilution.
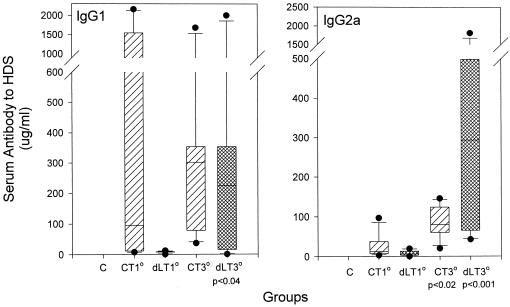
The IgG antibody levels to HDS in serum were also measured after primary and secondary i.n. coadministration with CT or dLT (Table 1). Similar to the observed salivary responses in Fig. 1 and 2, the sera of all rats in the PLGA-HDS groups given either adjuvant contained significant levels (P < 0.001) of IgG antibody within 10 days of completion of the initial immunization regime. At this time a prominent IgG1 antibody response to HDS was observed in association with CT administration, whereas the most prominent primary serum response after the use of dLT was in the IgG2a subclass (Fig. 3). Subsequent doses of the peptide construct with CT or dLT gave efficient boosting of serum IgG responses. For example, the day-211 serum IgG antibody levels in PLGA-HDS +CT or +dLT groups were significantly greater than those observed in the respective group after primary (P < 0.001) or secondary (P < 0.004) immunization. The median responses to HDS increased in both IgG1 and IgG2a subclasses after being boosted with either adjuvant (Fig. 3). The CT-assisted secondary response paralleled the primary response, i.e., by being predominantly IgG1. Boosting with the dLT adjuvant resulted in significantly increased anti-HDS responses in both the IgG1 and IgG2a subclasses.
TABLE 1.
Serum IgG antibody reactive with HDS or S. mutans GTF after i.n. administration of PLGA-HDS in CT or detoxified E. coli enterotoxin (dLT)
| Antigen (expt no.) | Adjuvant | Mean serum IgG EU ± SE (P)b reactive with:
|
||||
|---|---|---|---|---|---|---|
| HDS
|
S. mutans GTF
|
|||||
| 1° responsea | 2° response | 3° response | 1° response | 3° response | ||
| PLGA (3) | CT | <1 | <1 | <1 | 9 ± 3 | 7 ± 1 |
| PLGA-HDS (3) | CT | 70 ± 17 (<0.001) | 109 ± 12 (<0.001) | 165 ± 9 (<0.001; <0.001) | 25 ± 10 (<0.03) | 66 ± 43 (<0.04) |
| PLGA-HDS (4) | dLT | 46 ± 16 (<0.001) | 153 ± 7 (<0.001; <0.001) | 175 ± 3 (<0.001; <0.001) | 19 ± 6 (<0.05) | 123 ± 48 (<0.001) |
Samples were collected on days 24, 112, and 211 for the CT groups and on days 26, 110, and 188 for the dLT group.
P indicates the level of significance (one-way ANOVA) compared to the control group serum response on the same day. Where two P values are given, the second value indicates the level of significance (one-way ANOVA) compared to the respective immune group serum response of the previous collection period.
FIG. 3.
Box plots of serum IgG1 and IgG2a antibody levels to HDS after i.n. administration of PLGA-HDS with CT or R192G LT (dLT). Groups immunized i.n. are designated as control (C; unloaded PLGA microparticles and CT), CT (PLGA-HDS microparticles plus CT), or dLT (PLGA-HDS microparticles plus R192GLT). Primary (1°) serum measurements were made on day 24 for CT rats and on day 26 for dLT rats. Tertiary (3°) serum measurements were made on day 211 for CT rats and on day 188 for dLT rats. Boxes enclose the 25th to 75th percentiles, and lines within the boxes indicate the median values. The levels of significance beneath the x axes were calculated with respect to the primary response levels.
The serum IgG antibody levels to S. mutans GTF in either adjuvant group also were significant after the first course of immunization and continued to increase with subsequent immunization (Table 1). Again, the responses using dLT were at least as prominent as those using CT. Many of these sera (day 188 and 211 collections) were able to inhibit the ability of S. mutans GTF to synthesize water-insoluble glucan from sucrose (Table 2). The group immunized i.n. with PLGA-HDS plus CT demonstrated a significant level of enzyme inhibition (13.0% ± 3.6%; P < 0.02) compared to sera from the sham+CT control group (1.4% ± 0.9%). The group immunized i.n. with PLGA-HDS plus LT R192G demonstrated a low level of GTF inhibition (4.4% ± 0.8%), which nearly achieved statistical significance at the P < 0.05 level (P = 0.052) compared to the control group inhibition.
TABLE 2.
Percent inhibition of water-insoluble glucan synthesized by S. mutans GTF in sera from rats immunized i.n. with PLGA-HDS in CT (day 211) or in detoxified E. coli enterotoxin (dLT) (day 188)
| Antigen | n | Adjuvant | Mean % inhibition of S. mutans GTF activity ± SE | P |
|---|---|---|---|---|
| PLGA | 7 | CT | 1.4 ± 0.9 | |
| PLGA-HDS | 6 | CT | 13.0 ± 3.5 | < 0.02 |
| PLGA-HDS | 6 | dLT | 4.4 ± 0.8 |
DISCUSSION
Systemic immunization with peptide constructs which are derived from functional domains of mutans streptococcal GTF have been shown to induce protective immunity in rats to infection and disease with cariogenic mutans streptococci (40, 51). The induction of salivary IgA antibody is considered to be the principal mediator of these protective effects. The present study examined several mucosal immunization strategies using these GTF peptide constructs in order to induce potentially protective levels of salivary IgA antibody by the i.n. route. This route was selected because the nasal region has been shown to contain organized nasal-associated lymphoid tissue (NALT) with inductive properties (57) which, after antigen exposure, results in the formation of significant levels of salivary IgA antibody (41, 56). This route also has been used for human immunization with attenuated influenza (26). The first strategy (experiment 1) involved the incorporation of peptide constructs into polylactide-coglycolide microparticles since mucosal sampling is considered to favor particulate antigen (21) and since this method significantly increased salivary IgA antibody formation to intact GTF (41). However, microparticle incorporation of peptide constructs, either in mono- or diepitopic formats, was not by itself sufficient to induce a detectable salivary IgA response to peptide epitopes after i.n. administration (Table 1).
CT is a powerful immunoadjuvant which is frequently used to enhance the induction of mucosal and systemic immunity to a variety of bacterial and viral pathogens (13). Immunization i.n. with chimeric synthetic peptide containing two copies of a T-cell epitope and one copy of a B-cell epitope (TTB) from measles virus fusion protein, administered with the B subunit of CT, induced antibody that neutralized measles virus and TTB-specific IgA antibodies in saliva and nasal washes (17). Immunization i.n. of BALB/c mice with a known cytotoxic-T-lymphocyte (CTL) epitope in human immunodeficiency virus type 1 glycoprotein 120, administrated with CT, induced peptide-specific CTLs in spleen cells (33). Also, i.n. immunization with an alanine-rich peptide derived from an S. mutans adhesin, coupled to the CT B subunit, suppressed colonization of murine teeth by S. mutans (47). We incorporated CT into the immunization regime with the GTF-peptide PLGA microparticles. The addition of CT induced a significant (P < 0.001) primary salivary IgA antibody response to the HDS peptide in all animals 10 days after the third of three weekly doses of CT+HDS-PLGA (day 24; Fig. 1). The pattern of the salivary IgA antibody response to the peptide antigen was very similar to the CT-enhanced response to i.n. immunization with intact streptococcal antigens (55). The adjuvant effects of CT are broad based and can include increased mucosal epithelial cell and macrophage production of proinflammatory cytokines (2, 3), upregulation of B7–2 costimulatory factors on antigen-presenting cells (APCs) (8), and increased antigen transfer from the mucosal to the systemic compartment (23). Of special interest are studies of i.n. CT-induced anti-peptide responses on isolated NALT cells that have suggested that the CT adjuvant effect is, at least in part, locally manifest and that NALT dendritic cells are the predominating APC population involved (33). Our results support the ability of CT to serve as an effective mucosal adjuvant for peptide antigens.
The anamnestic effect of CT on anti-peptide responses was more pronounced in the systemic compartment than in the mucosal compartment. Salivary IgA responses to HDS remained elevated above those of controls after subsequent immunizations but, as previously reported by Wu and Russell (55), antibody levels were not increased above peak primary responses (Fig. 1). In contrast, the serum IgG responses to peptide were boosted to high levels during the 7-month course of the study (Table 1). The small size of the peptide antigen, coupled with the potential for CT-induced enhanced mucosal permeability (23), may have increased the amount of peptide available in the systemic compartment for subsequent IgG immune response, presumably in the cervical lymph nodes.
Although similar to CT in structure and function, the heat-labile E. coli LT enterotoxin has been reported to demonstrate activation potential for both Th1 and Th2 CD4+ cells and to be somewhat less inherently toxic (45). Interest in this immunoadjuvant has increased since the toxicity of LT can be reduced by substituting a glycine for an arginine at position 192 in the A subunit (11). This substitution interferes with trypsin-mediated cleavage of the 187CGNSSRTITGDTC199 loop of LT, which is a necessary antecedent for the expression of its complete toxic activity (7). The resulting mutant LT (R192G) has been reported to be much less toxic than the LT holotoxin in several in vitro assays (13), while the mutant retains many of its adjuvant properties, even when expressed in Vibrio cholerae (35).
The current studies demonstrate that mutant LT enterotoxin (R192G) and CT holotoxin similarly enhance the induction of salivary IgA and serum IgG antibody responses to either the HDS peptide (Fig. 2 and 3 and Table 1) or the intact GTF protein (Table 1) after coadministration i.n. with HDS in PLGA microparticles. These results reinforce the observation that mucosal adjuvanticity is preserved in R192G with respect to the formation of humoral responses. The consequences of the administration of CT holotoxin and the mutant LT did, however, differ with respect to the serum IgG isotype pattern of antibody expression. Antibody in the IgG1 subclass was most prominent after primary and secondary i.n. coadministration of peptide with CT. In contrast, IgG2a antibody to HDS was favored after use of the R192G mucosal adjuvant. This shift in response to the IgG2a isotype has been previously observed in CBA/J mice who were given R192G and heat-inactivated Candida albicans i.n., followed by challenge with viable C. albicans (5). Significant levels of antibody of both isotypes were observed late in the response using either adjuvant.
The ability of R192G LT to induce and sustain salivary immune responses to HDS peptide at a level similar to that for the CT holotoxin suggests that mutant LT has value as a mucosal adjuvant for subunit-based dental caries vaccines which require additional components to improve their immunogenicity. The application of R192G LT at mucosal sites that have limited proteolytic activity would forestall the reported possibility that toxic activity could appear via non-trypsin proteolytic activation of the mutant LT (15). Ryan et al. (35) have shown that R192G LT can be expressed in attenuated vaccine strains of V. cholerae which, subsequent to expression, demonstrated an adjuvant effect for immune response to the infecting Vibrio strain. Recombinant polypeptides subtending the portion of the GTF catalytic domain, which includes HDS, have been cloned into and expressed by Salmonella enterica serovar Typhimurium and shown to induce mucosal IgA responses after i.n. immunization in mice (19). Serovar Typhimurium also has been used to coexpress the salivary binding region of the S. mutans antigen I and II adhesins and the A2/B subunit of CT, resulting in protective immunity against S. mutans colonization (18). Thus, the possibility exists that effective and safe dental caries vaccines could be constructed of attenuated intestinal pathogens which express functional domains of GTF or other virulence components of mutans streptococci, together with detoxified LT or CT immunoadjuvant components.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants DE-06153 and DE-04733 from the National Institute of Dental and Craniofacial Research.
We thank John D. Clements, Tulane University Medical Center, New Orleans, La., for his generous gift of the mutant E. coli heat-labile enterotoxin R192G.
REFERENCES
- 1.Abo H, Masumura T, Kodama T, Ohta H, Fukui K, Kato K, Kagawa H. Peptide sequences for sucrose splitting and glucan binding within Streptococcus sobrinus glucosyltransferase (Water-insoluble glucan synthetase) J Bacteriol. 1991;173:989–996. doi: 10.1128/jb.173.3.989-996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromander A, Holmgren J, Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991;146:2908–294. [PubMed] [Google Scholar]
- 3.Bromander A, Kjerrulf M, Holmgren J, Lycke N. Cholera toxin enhances alloantigen presentation by cultured intestinal epithelial cells. Scand J Immunol. 1993;37:452–458. doi: 10.1111/j.1365-3083.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas-Freytag L, Cheng E, Mayeux P, Domer J E, Clements J D. Effectiveness of a vaccine composed of heat-killed Candida albicans and a novel mucosal adjuvant, LT (R192G), against systemic candidiasis. Infect Immun. 1999;67:826–833. doi: 10.1128/iai.67.2.826-833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardenas-Freytag L, Cheng E, Mirza A. New approaches to mucosal immunization. Adv Exp Med Biol. 1999;473:319–337. doi: 10.1007/978-1-4615-4143-1_34. [DOI] [PubMed] [Google Scholar]
- 6.Chia J-S, Lin R-H, Lin S-W, Chen J-Y, Yang C-S. Inhibition of glucosyltransferase activities of Streptococcus mutans by a monoclonal antibody to a subsequence peptide. Infect Immun. 1993;61:4689–4695. doi: 10.1128/iai.61.11.4689-4695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements J D, Finkelstein R A. Isolation and characterization of homologous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979;21:760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cong Y, Weaver C T, Elson C O. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J Immunol. 1997;159:5301–5308. [PubMed] [Google Scholar]
- 9.Dertzbaugh M T, Macrina F L. Inhibition of Streptococcus mutans glucosyltransferase activity by antiserum to a subsequence peptide. Infect Immun. 1990;58:1509–1513. doi: 10.1128/iai.58.6.1509-1513.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devulapalle K S, Goodman S D, Gao Q, Hemsley A, Mooser G. Knowledge-based model of a glucosyltransferase from the oral bacterial group of mutans streptococci. Protein Sci. 1997;6:2489–2493. doi: 10.1002/pro.5560061201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickenson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferretti J J, Gilpin M L, Russell R R. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987;169:4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freytag L C, Clements J D. Bacterial toxins as mucosal adjuvants. Curr Top Microbiol Immunol. 1999;236:25–36. doi: 10.1007/978-3-642-59951-4_11. [DOI] [PubMed] [Google Scholar]
- 14.Funane K, Shiraiwa M, Hashimoto K, Ichishima E, Kobayashi M. An active-site peptide containing the second essential carboxyl group of dextransucrase from Leuconostoc mesenteroides by chemical modifications. Biochemistry. 1993;32:13696–13702. doi: 10.1021/bi00212a039. [DOI] [PubMed] [Google Scholar]
- 15.Giannelli V, Fontana M R, Giuliani M M, Guangcai D, Rappuoli R, Pizza M. Protease susceptibility and toxicity of heat-labile enterotoxins with a mutation in the active site or in the protease-sensitive loop. Infect Immun. 1997;65:331–334. doi: 10.1128/iai.65.1.331-334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillobel H C R, Carinhanha J I, Cardenas L, Clements J D, de Almeida D F, Ferreira L C S. Adjuvant activity of a nontoxic mutant of Escherichia coli heat-labile enterotoxin on systemic and mucosal immune responses elicited against a heterologous antigen carried by a live Salmonella enterica serovar Typhimurium vaccine strain. Infect Immun. 2000;68:4349–4353. doi: 10.1128/iai.68.7.4349-4353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hathaway L J, Obeid O E, Steward M A. Protection against measles virus-induced encephalitis by antibody from mice immunized intranasally with a synthetic peptide immunogen. Vaccine. 1998;16:135–141. doi: 10.1016/s0264-410x(97)88326-0. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Hajishengallis G, Michalek S M. Induction of protective immunity against Streptococcus mutans colonization after mucosal immunization with attenuated Salmonella enterica serovar Typhimurium expressing an S. mutans adhesin under the control of in vivo-inducible nirB promoter. Infect Immun. 2001;69:2154–2161. doi: 10.1128/IAI.69.4.2154-2161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jespersgaard C, Hajishengallis G, Greenway T E, Smith D J, Russell M W, Michalek S M. Functional and immunogenic characterization of two cloned regions of Streptococcus mutans glucosyltransferase I. Infect Immun. 1999;67:810–816. doi: 10.1128/iai.67.2.810-816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T, Eisen-Lev R, Seki M, Eastcott J W, Wilson M E, Taubman M A. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164:2102–2109. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 21.Kuper C F, Koornstra P J, Hameleers D M, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 22.Laloi P, Munro C L, Jones K R, Macrina F L. Immunologic characteristics of a Streptococcus mutans glucosyltransferase B sucrose-binding site peptide-cholera toxin B-subunit chimeric protein. Infect Immun. 1996;64:28–36. doi: 10.1128/iai.64.1.28-36.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lycke N, Karlsson U, Sjolander A, Mangnusson K E. The adjuvant action of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scand J Immunol. 1991;33:691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 24.MacGregor E A, Jespersen H M, Svensson B. A circularly permuted alpha-amylase-type alpha/beta barrel structure in glucan-synthesizing glucosyltransferases. FEBS Lett. 1996;378:263–266. doi: 10.1016/0014-5793(95)01428-4. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura Y, Kusunoki M, Harada W, Kakudo M. Structure and possible catalytic residues of taka-amylase. S. J Biochem. 1984;95:697–702. doi: 10.1093/oxfordjournals.jbchem.a134659. [DOI] [PubMed] [Google Scholar]
- 26.Mendelman P M, Cordova J, Cho I. Safety, efficacy, and effectiveness of the influenza virus vaccine, trivalent, types A and B, live, cold-adapted (CAIV-T) in healthy children and healthy adults. Vaccine. 2001;19:2221–2226. doi: 10.1016/s0264-410x(00)00449-7. [DOI] [PubMed] [Google Scholar]
- 27.Merrifield R B. Solid-phase peptide synthesis. I. The synthesis of a tetrapeptide. J Am Chem Soc. 1963;85:2149–2154. [Google Scholar]
- 28.Monchois V, Vignon M, Escalier P C, Svensson B, Russell R R. Involvement of Gln937 of Streptococcus downei GTF-I glucansucrase in transition-state stabilization. Eur J Biochem. 2000;267:4127–4136. doi: 10.1046/j.1432-1327.2000.01448.x. [DOI] [PubMed] [Google Scholar]
- 29.Monchois V, Vignon M, Russell R R B. Isolation of key amino acid residues at the N-terminal end of the core region Streptococcus downei glucansucrase, GTF-I. Appl Microbiol Biotechnol. 1999;52:60–665. doi: 10.1007/s002530051575. [DOI] [PubMed] [Google Scholar]
- 30.Monchois V, Vignon M, Russell R R B. Mutagenesis of Asp-569 of glucosyltransferase I glucansucrase modulates glucan and oligosaccharide synthesis. Appl Environ Microbiol. 2000;66:1923–1927. doi: 10.1128/aem.66.5.1923-1927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mooser G. Glycosidases and glucosyltransferases. In: Sigman D, editor. The enzymes. Vol. 20. London, England: Academic Press; 1992. pp. 187–221. [Google Scholar]
- 32.Mooser G, Hefta S A, Paxton R J, Shively J E, Lee T. Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus glucosyltransferases. J Biol Chem. 1991;266:8916–8922. [PubMed] [Google Scholar]
- 33.Porgador A, Staats H F, Faiola B, Gilboa E, Palker T J. Intranasal immunization with CTL epitope peptides from HIV-1 or ovalbumin and the mucosal adjuvant cholera toxin induces peptide-specific CTLs and protection against tumor development in vivo. J Immunol. 1997;158:834–841. [PubMed] [Google Scholar]
- 34.Russell R R, Shiroza T, Kuramitsu H K, Ferretti J J. Homology of glucosyltransferase gene and protein sequences from Streptococcus sobrinus and Streptococcus mutans. J Dent Res. 1988;67:543–547. doi: 10.1177/00220345880670030401. [DOI] [PubMed] [Google Scholar]
- 35.Ryan E T, Crean T I, John M, Butterton J R, Clements J D, Calderwood S B. In vivo expression and immunoadjuvanticity of a mutant of heat-labile enterotoxin of Escherichia coli in vaccine and vector strains of Vibrio cholerae. Infect Immun. 1999;67:1694–1701. doi: 10.1128/iai.67.4.1694-1701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimamura A, Nakano Y J, Mukasa H, Kuramitsu H K. Identification of amino acid residues in Streptococcus mutans glucosyltransferases influencing the structure of the glucan product. J Bacteriol. 1994;176:4845–4850. doi: 10.1128/jb.176.16.4845-4850.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiroza T, Ueda S, Kuramitsu H K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987;169:4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith D J, Taubman M A, Ebersole J L. Effect of oral administration of glucosyltransferase antigens on experimental dental caries. Infect Immun. 1979;26:81–89. doi: 10.1128/iai.26.1.82-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith D J, Taubman M A, King W F, Eida S, Powell J R, Eastcott J W. Immunological characteristics of a synthetic peptide associated with a catalytic domain of mutans streptococcal glucosyltransferase. Infect Immun. 1994;62:5470–5476. doi: 10.1128/iai.62.12.5470-5476.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith D J, Shoushtari B, Heschel R L, King W F, Taubman M A. Immunogenicity and protective immunity induced by synthetic peptides associated with a catalytic subdomain of mutans group streptococcal glucosyltransferase. Infect Immun. 1997;65:4424–4430. doi: 10.1128/iai.65.11.4424-4430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith D J, Heschel R, King W F, Taubman M A. Antibody to glucosyltransferase induced by synthetic peptides associated with catalytic regions of alpha-amylases. Infect Immun. 1999;67:2638–2642. doi: 10.1128/iai.67.5.2638-2642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith D J, Taubman M A. Oral immunization of humans with Streptococcus sobrinus GTF. Infect Immun. 1987;55:2562–2569. doi: 10.1128/iai.55.11.2562-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith D J, Taubman M A, King W F. Effect of local deposition of antigen on salivary immune responses and reaccumulation of mutans streptococci. J Clin Immunol. 1990;10:273–281. doi: 10.1007/BF00916703. [DOI] [PubMed] [Google Scholar]
- 44.Smith D J, Trantolo D J, King W F, Gusek E J, Fackler P H, Gresser J D, De Souza V L, Wise D H. Induction of secretory immunity with bioadhesive poly(d,1-lactide-co-glycolide) microparticles containing Streptococcus sobrinus glucosyltransferase. Oral Microbiol Immunol. 2000;15:124–130. doi: 10.1034/j.1399-302x.2000.150209.x. [DOI] [PubMed] [Google Scholar]
- 45.Sogaard M, Kadziola A, Haser R, Svensson B. Site-directed mutagenesis of histidine 93, aspartic acid 180, glutamic acid 205, histidine 290, and aspartic acid 291 at the active site and tryptophan 279 at the raw starch binding site in barley alpha-amylase 1. J Biol Chem. 1993;268:22480–22484. [PubMed] [Google Scholar]
- 46.Takahashi M, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fijihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvanticity of Escherichia coli labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi I, Okahashi N, Matsushita K, Tokuda M, Kanamoto T, Munekata E, Russell M W, Koga T. Immunogenicity and protective effect against oral colonization by Streptococcus mutans of synthetic peptides of a streptococcal surface protein antigen. J Immunol. 1991;146:332–336. [PubMed] [Google Scholar]
- 48.Tam J P. Synthetic peptide vaccine design: synthesis and properties of high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taubman M A, Smith D J, King W F, Eastcott J W, Bergey E J, Levine M J. Immune properties of glucosyltransferases from Streptococcus sobrinus. J Oral Pathol. 1988;17:466–470. doi: 10.1111/j.1600-0714.1988.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 50.Taubman M A, Holmberg C, Smith D J, Eastcott J W. T and B cell epitopes from peptide sequences associated with glucosyltransferase function. Clin Immunol Immunopathol. 1995;76:S95. [Google Scholar]
- 51.Taubman M A, Holmberg C J, Smith D J. Immunization of rats with synthetic peptide constructs from the glucan binding or catalytic regions of mutans streptococcal glucosyltransferase protects against dental caries. Infect Immun. 1995;63:3088–3093. doi: 10.1128/iai.63.8.3088-3093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taubman M A, Smith D J, Holmberg C J, Eastcott J W. Coimmunization with complementary glucosyltransferase peptides results in enhanced immunogenicity and protection against dental caries. Infect Immun. 2000;68:2698–2703. doi: 10.1128/iai.68.5.2698-2703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai Y-W, Chia J-S, Shiau Y-Y, Chou H-C, Liaw Y-C, Lou K-L. Three-dimensional modeling of the catalytic domain of Streptococcus mutans glucosyltransferase GtfB. FEMS Microbiol Lett. 2000;188:75–79. doi: 10.1111/j.1574-6968.2000.tb09171.x. [DOI] [PubMed] [Google Scholar]
- 54.Tsumori H, Minami T, Kuramitsu H K. Identification of essential amino acids in the Streptococcus mutans glucosyltransferases. Infect Immun. 1997;179:3391–3396. doi: 10.1128/jb.179.11.3391-3396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu H Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu H Y, Nahm M H, Guo Y, Russell M W, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 57.Wu H Y, Nguyen H H, Russell M W. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]