This survey study reports the demographic characteristics and treatment-related organizational aspects of pediatric intensive care units that deliver continuous kidney replacement therapy across Europe.
Key Points
Question
What are the variations in practice during continuous kidney replacement therapy (CKRT) practice among pediatric intensive care units (PICUs) across Europe?
Findings
In this survey study of 161 European PICUs, substantial variation in CKRT practice was found across various organizational aspects, including follow-up, prescription (eg, anticoagulation), liberation from CKRT, and training and education of staff.
Meaning
The survey found that CKRT management in European PICUs varied widely, which calls for concerted educational initiatives and consensus recommendations on the practice of CKRT.
Abstract
Importance
Continuous kidney replacement therapy (CKRT) is the preferred method of kidney support for children with critical illness in pediatric intensive care units (PICUs). However, there are no data on the current CKRT management practices in European PICUs.
Objective
To describe current CKRT practices across European PICUs.
Design, Setting, and Participants
This cross-sectional survey of PICUs in 20 European countries was conducted by the Critical Care Nephrology Section of the European Society of Pediatric and Neonatal Intensive Care from April 1, 2020, to May 31, 2022. Participants included intensivists and nurses working in European PICUs. The survey was developed in English and distributed using SurveyMonkey. One response from each PICU that provided CKRT was included in the analysis. Data were analyzed from June 1 to June 30, 2022.
Main Outcome and Measures
Demographic characteristics of European PICUs along with organizational and delivery aspects of CKRT (including prescription, liberation from CKRT, and training and education) were assessed.
Results
Of 283 survey responses received, 161 were included in the analysis (response rate, 76%). The attending PICU consultant (70%) and the PICU team (77%) were mainly responsible for CKRT prescription, whereas the PICU nurses were responsible for circuit setup (49%) and bedside machine running (67%). Sixty-one percent of permanent nurses received training to use CKRT, with no need for certification or recertification in 36% of PICUs. Continuous venovenous hemodiafiltration was the preferred dialytic modality (51%). Circuit priming was performed with normal saline (67%) and blood priming in children weighing less than 10 kg (56%). Median (IQR) CKRT dose was 35 (30-50) mL/kg/h in neonates and 30 (30-40) mL/kg/h in children aged 1 month to 18 years. Forty-one percent of PICUs used regional unfractionated heparin infusion, whereas 35% used citrate-based regional anticoagulation. Filters were changed for filter clotting (53%) and increased transmembrane pressure (47%). For routine circuit changes, 72 hours was the cutoff in 62% of PICUs. Some PICUs (34%) monitored fluid removal goals every 4 hours, with variation from 12 hours (17%) to 24 hours (13%). Fluid removal goals ranged from 1 to 3 mL/kg/h. Liberation from CKRT was performed with a diuretic bolus followed by an infusion (32%) or a diuretic bolus alone (19%).
Conclusions and Relevance
This survey study found a wide variation in current CKRT practice, including organizational aspects, education and training, prescription, and liberation from CKRT, in European PICUs. This finding calls for concerted efforts on the part of the pediatric critical care and nephrology communities to streamline CKRT education and training, research, and guidelines to reduce variation in practice.
Introduction
Continuous kidney replacement therapy (CKRT) is the preferred method of kidney support in the pediatric intensive care unit (PICU) because it allows for slow fluid removal and solute normalization in children with hemodynamic instability.1,2,3,4 Continuous kidney replacement therapy is increasingly being used in the PICU both for kidney and nonkidney indications (acute kidney injury [AKI], fluid overload [FO], electrolyte instability, acid-base imbalances, sepsis [for removal of inflammatory mediators], acute liver failure, inborn errors of metabolism, or drug/toxin removal).5,6,7,8,9,10 However, due to the lack of evidence and standardized, practice-based recommendations, wide variations in practice exist in all aspects of delivery of CKRT in children with critical illness.11,12,13,14,15,16 Most protocols for CKRT are therefore based on institutional, personal, or historical experience. Although a limited number of pediatric studies2,3,5,6,7,8,9 have described the demographic characteristics and outcome data of children with critical illness requiring CKRT, detailed information on current practices for the management of pediatric CKRT in Europe is lacking.
To address this gap, the Critical Care Nephrology Section of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) conducted a survey with the aim of describing the current CKRT practices across European PICUs. The results of this study may serve as a catalyst for providing training and education in this field to safely deliver CKRT to children with critical illness.
Methods
Study Design and Ethics Approval
The Critical Care Nephrology Section of ESPNIC was launched in 2013 and consists of nursing, medical, and allied health professional members. From April 1, 2020, to May 31, 2022, we conducted a cross-sectional anonymous online survey focused on different aspects of CKRT practices in European PICUs. We followed the American Association for Public Opinion Research (AAPOR) reporting guideline.17 Since no demographic, observational, or interventional data were collected on patients in this survey study, ethical approval was not deemed necessary in accordance with the Common Rule; however, the project was registered as a quality improvement project with King’s College Hospital in London, UK. Informed consent from participants was implied from their completion of the survey.
Survey Development and Testing
The survey was developed in English using the online SurveyMonkey (Momentive Inc) instrument for distribution. The survey was designed to address different aspects of CKRT delivery, which we developed based on an extensive review of the literature and expert consensus. This literature review explored important research on the most controversial aspects of CKRT, such as timing of initiation, choice of vascular access, dose of CKRT, anticoagulation, and liberation from CKRT, among others. We searched all available pediatric literature, including single-center studies (mostly retrospective) as well as studies from the multicenter Prospective Pediatric Continuous Renal Replacement Therapy Registry. However, we explored the literature from adult studies when suitable given that most randomized clinical trials have been conducted in adult patients.
Subsequently, the survey was initially pilot-tested among the authors of this study for clarity and face validity. The survey consisted of 78 questions, was divided into different sections, and required about 25 minutes on average to be completed. Detailed information on the data collected is available in the eMethods in Supplement 1, and the survey is provided in the eAppendix in Supplement 1.
Recruitment of European PICUs and Data Collection
This survey focused on intensivists and nurses working in European PICUs. By using dedicated organizational newsletters, country leads, and personal networks and contacting the ESPNIC Critical Care Nephrology Section members, the questionnaire was spread across 20 countries in Europe. Demographic characteristics of European PICUs along with organizational and delivery aspects of CKRT (including prescription, liberation from CKRT, and training and education) were assessed. No identifiable data regarding staff or patients were collected. We targeted CKRT lead professionals from all units to avoid misdiagnosis of variation in practice based on the level of experience of health care professionals. Responses to the survey were excluded if any of the following conditions were met: the PICU did not perform CKRT, the unit was a neonatal intensive care unit only, and the PICU was outside Europe. We also excluded incomplete responses from the final number of included questionnaires, and, in the case of multiple responses per center, we contacted the CKRT lead of the unit to confirm the entry to be included.
Statistical Analysis
Raw data that were downloaded from SurveyMonkey were checked for data completeness and potential responses meeting the exclusion criteria. Data were analyzed using Stata, version 17.0 (StataCorp LLC) from June 1 to June 30, 2022. Descriptive data were reported as number and frequency (proportion) for categorical variables and as median (IQR) for continuous variables given their nonparametric distribution.
Results
Survey Respondents and PICU Characteristics
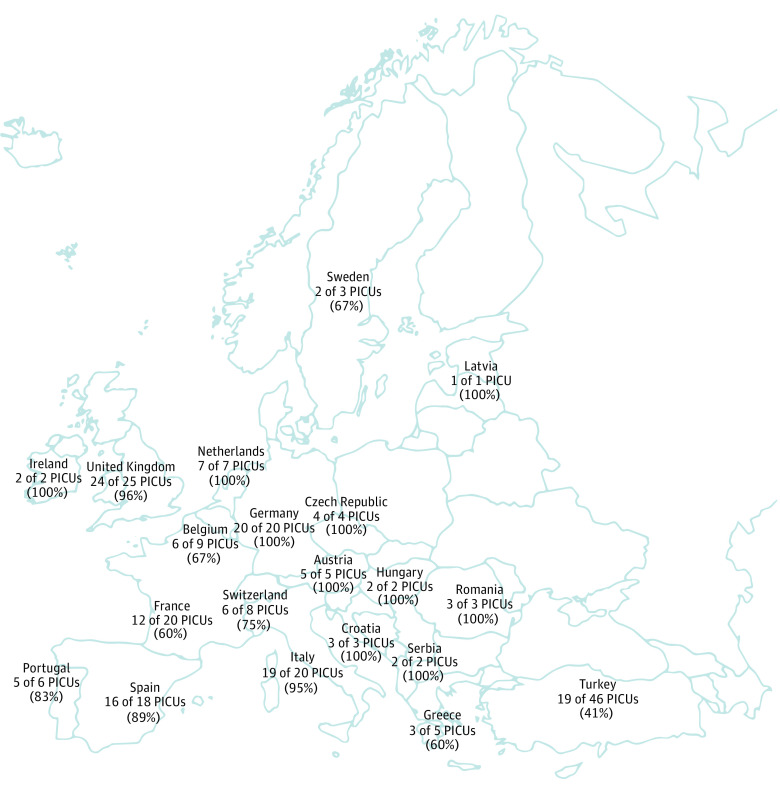
The questionnaire received 283 responses. After exclusion criteria were applied (7 PICUs did not perform CKRT, 5 units were neonatal intensive care units, 12 PICUs were outside Europe, and 98 responses were incomplete or duplicates from the same units), 161 responses were included for a response rate of 76% (n = 213) among European PICUs that performed CKRT (Figure). Table 1 summarizes the characteristics of PICUs and respondents as well as organizational aspects of the PICUs involved in the survey. Respondents were mainly physicians (90%) from PICUs with a median (IQR) annual admission rate of 500 (350-800) children.
Figure. Map Reporting the Distribution of Survey Respondents Across European Countries .
PICU indicates pediatric intensive care unit.
Table 1. Demographic Characteristics and Organizational Aspects of European PICUs Performing CKRT.
| Characteristic | Responses, No. (%) |
|---|---|
| Responder profession | |
| No. | 161 |
| Physician | 145 (90) |
| Nurse | 16 (10) |
| PICU dimensions, median (IQR) | |
| Maximum bed capacity | 12 (8-16) |
| No. of admissions per y | 500 (350-800) |
| Presence of written policy for CKRT | 123 (77) |
| No. | 159 |
| Need for and type of consent for CKRT initiation | |
| No. | 141 |
| Verbal consent | 61 (43) |
| Written consent | 35 (25) |
| No consent needed | 45 (32) |
| Minimum weight for starting KRT, median (IQR) | |
| Peritoneal dialysis | 2 (1-3) |
| CKRT | 3 (2-3.5) |
| Intermittent hemodialysis | 6 (5-10) |
| Person with primary responsibility for prescribing CKRT type or modality to be useda | |
| Attending PICU consultant | 113 (70) |
| Kidney team advice | 53 (33) |
| Unit policy or guidelines | 35 (22) |
| Only 1 KRT modality available | 3 (2) |
| Person with primary responsibility for CKRT management | |
| No. | 141 |
| PICU team | 109 (77) |
| Kidney team | 17 (12) |
| Varies depending on CKRT modality | 9 (6) |
| Combined ICU and kidney team | 5 (4) |
| Technicians | 1 (1) |
| Person with primary responsibility for CKRT setup (lining and priming) | |
| No. | 157 |
| PICU team | |
| Nurse | 77 (49) |
| Physician | 43 (27) |
| Kidney team | |
| Nurse | 28 (18) |
| Physician | 5 (3) |
| Perfusionist | 4 (3) |
| Person with primary responsibility for CKRT bedside machine runninga | |
| PICU team | |
| Nurse | 107 (67) |
| Physician | 58 (36) |
| Kidney team | |
| Nurse | 17 (11) |
| Physician | 10 (6) |
| Perfusionist | 2 (1) |
| Staffing ratio when the patient is both on CKRT and MV | |
| No. | 131 |
| 1 Staff member to 1 patient | 74 (56) |
| 1 Staff member to 2 patients | 32 (24) |
| 2 Staff members to 1 patient | 23 (18) |
| Other ratio | 2 (2) |
| Patients received CKRT follow-up as outpatients by nephrologists | 77 (63) |
| No. | 122 |
| Concern regarding the use of adult CKRT machines in children weighing <8 kg | 81 (60) |
| No. | 136 |
| Availability of CKRT equipment designed for neonates or infants (ie, CARPEDIEM or NIDUS) | 20 (15) |
| No. | 135 |
Abbreviations: CARPEDIEM, Cardio-Renal Pediatric Dialysis Emergency Machine; CKRT, continuous kidney replacement therapy; ICU, intensive care unit; KRT, kidney replacement therapy; MV, mechanical ventilation; NIDUS, Newcastle Infant Dialysis and Ultrafiltration System; PICU, pediatric intensive care unit.
The sum of percentages is more than 100% because respondents could indicate more than 1 option.
Organizational Aspects and CKRT Education
Most PICUs (77%) had a written policy for CKRT management. Initiation of CKRT required written consent in 25% of PICUs. Seventy percent of attending PICU consultants were responsible for prescribing CKRT type or modality. The PICU team (77%) was mainly responsible for CKRT management, whereas in a minority of the cases a nephrologist (12%) managed the treatments. The PICU nurses were mainly responsible for circuit setup (49%) and bedside machine running (67%). Staffing ratios during CKRT varied widely, with 56% of PICUs reporting a ratio of 1 member of staff to 1 patient.
Sixty percent of PICUs expressed concerns regarding the use of adult machines in children weighing less than 8 kg, but only 15% of the PICUs had available dedicated CKRT equipment designed for these children (ie, CARPEDIEM [Cardio-Renal Pediatric Dialysis Emergency Machine; Medtronic] and NIDUS [Newcastle Infant Dialysis and Ultrafiltration System; Allmed Medical Care Holdings Limited]). Sixty-three percent of respondents provided follow-up for patients as outpatients after they were discharged. Sixty-one percent of permanent PICU nurses were formally trained to use CKRT, with no need for certification or recertification in approximately one-third (36%) of PICUs and with need for yearly certification in another one-third (32%) of PICUs. No regular training on CKRT was offered in 59% of PICUs (eTable in Supplement 1).
Vascular Access Characteristics
eFigure 1 in Supplement 1 describes the current practice regarding location and size for vascular access according to patient age and weight. Ninety-three PICUs used an ultrasonography-guided technique to obtain vascular access to perform CKRT, with the internal jugular vein being the preferred vessel in all age groups (<1 month, 1 month-1 year, 1 year-5 years, and >5 years; preferred by 76%-88%). The size of vascular access increased proportionately with patient weight.
CKRT Initiation and Termination
Table 2 as well as eFigures 2 and 3 in Supplement 1 show various components of the CKRT prescription. Continuous venovenous hemodiafiltration (CVVHDF) was the most commonly used modality (66%), whereas intermittent hemodialysis (IHD) was the least commonly used (16%) (eFigure 2 in Supplement 1), with 47% of PICUs reporting never using IHD in the past 12 months. Eighty percent of the PICUs offered the possibility to perform tandem therapies, mainly CKRT connected to extracorporeal membrane oxygenation (56%) and CKRT with therapeutic plasma exchange (52%) (eFigure 2 in Supplement 1).
Table 2. CKRT Prescription Characteristics.
| Characteristic | Total responses, No. (%) |
|---|---|
| Clinical scenario in which the physician would commence CKRTa | |
| No. | 91 |
| Severe hyperkalemia | 82 (90) |
| Treating fluid overload in a child with critical illness | 77 (85) |
| Hyperammonemia | 77 (85) |
| Persistent refractory metabolic acidosis | 54 (59) |
| Removing soluble mediators of septic shock | 18 (20) |
| Preventing fluid overload in a child with critical illness | 16 (18) |
| Creating space for nutrition by allowing liberalization of intake | 10 (11) |
| Preferred KRT modality for hyperammonemia | |
| No. | 123 |
| CVVHDF | 63 (51) |
| CVVHD | 38 (31) |
| CVVH | 14 (11) |
| IHD | 7 (6) |
| PD | 1 (1) |
| Dialysate or effluent dose, median (IQR), mL/kg/h | |
| In neonates | 35 (30-50) |
| In children | 30 (30-40) |
| Blood flow rate, median (IQR), mL/min | |
| Minimum | 20 (10-20) |
| Maximum | 200 (150-200) |
| Solutions used for circuit priming, excluding blooda | |
| No. | 126 |
| Normal saline | 84 (67) |
| Human albumin solution | 59 (47) |
| Plasmalyte | 17 (14) |
| Hartmann solution | 8 (6) |
| Fresh frozen plasma | 7 (6) |
| Indications for blood priming of the circuita | |
| No. | 121 |
| Weight <10 kg | 68 (56) |
| Hemodynamic instability or inotropic requirement | 57 (47) |
| Extracorporeal volume >10% of circulating blood volume | 48 (40) |
| Anemia | 45 (37) |
| Never used | 19 (16) |
| Blood priming performed in all patients receiving CKRT | 2 (2) |
| Modality of blood priming | |
| No. | 128 |
| Priming the whole CKRT circuit with blood | 50 (39) |
| Combining blood and crystalloids for a normal hematocrit | 38 (30) |
| Administering a blood transfusion before starting CKRT | 21 (16) |
| Priming the whole CKRT circuit with blood, or administering a blood transfusion | 4 (3) |
| Combining blood and colloids for a normal hematocrit | 2 (2) |
| Otherb | 1 (1) |
| Never performed | 12 (10) |
| Preferred dialysate solution | |
| No. | 147 |
| Bicarbonate base solution | 107 (73) |
| Lactate base solution | 40 (27) |
| Customization of the dialysate bag (eg, adding glucose or sodium) | |
| No. | 137 |
| With customization | 69 (50) |
| Position of the replacement fluid | |
| No. | 130 |
| Combination of predilution and postdilution | 46 (35) |
| Postdilution | 29 (22) |
| Predilution | 29 (22) |
| Variable practice depending on patient scenario | 26 (20) |
| Filtration-to-dialysis ratio during CVVHDF | |
| No. | 111 |
| 50/50 | 45 (41) |
| 30/70 | 35 (32) |
| Dependent on molecular weight of the target solute | 31 (28) |
| Diuretics (eg, furosemide) during CKRT | |
| No. | 126 |
| Used diuretics | 49 (39) |
| Nutrition support changes during CKRTa | |
| No. | 130 |
| Change in protein | 62 (48) |
| Change in calories | 49 (38) |
| Change in trace elements | 40 (31) |
| No changes | 52 (40) |
Abbreviation: CKRT, continuous kidney replacement therapy; CVVH, continuous venovenous hemofiltration; CVVHD, continuous venovenous hemodialysis; CVVHDF, continuous venovenous hemodiafiltration; IHD, intermittent hemodialysis; PD, peritoneal dialysis.
The sum of percentages is more than 100% because respondents could indicate more than 1 option.
Priming the whole circuit first with crystalloid and then blood prime.
eFigure 3 in Supplement 1 shows the modality of choice according to the underlying disease. Continuous venovenous hemodiafiltration was the preferred CKRT modality for all indications except chronic kidney failure, in which IHD and peritoneal dialysis were preferred (eFigure 3A in Supplement 1). The clinical scenarios that prompted initiation of CKRT included severe hyperkalemia (90%), FO (85%), and hyperammonemia (85%). There was wide variation in preference for priming solutions, with normal saline (67%) being most preferred and blood priming being mainly used in children weighing less than 10 kg (56%) and children with hemodynamic instability receiving vasoactive agents (47%) (Table 2). Blood priming was performed by different techniques, including priming the whole CKRT circuit with blood (39%) or combining blood and crystalloids aiming for a normal hematocrit (30%). A bicarbonate base solution (73%) was the preferred solution, and 50% of the PICUs claimed to customize the dialysate bag. The replacement fluid was mainly (35%) administered as a combination of predilution and postdilution, with a filtration to dialysis ratio of 50/50 (41%). More than one-third (39%) of the PICUs used diuretics while the patient was receiving CKRT, and more than half (60%) of the PICUs altered the prescription of nutritional support mainly by changing the amount of protein (48%) and calories (38%) (Table 2).
In case of insufficient desired toxin clearance, respondents preferred to change the CKRT modality, followed by increasing the effluent rate (eFigure 3B in Supplement 1). The CKRT dose was calculated mainly (72%) using a weight-based formula (mL/kg/h), and the median (IQR) CKRT dose among the PICUs was 35 (30-50) mL/kg/h in neonates and 30 (30-40) mL/kg/h in children aged 1 month to 18 years. Only 30% of PICUs reported ever using a CKRT dose over 40 mL/kg/h, with a median (IQR) dose of 80 (45-120) mL/kg/h. The reported median (IQR) minimum blood flow rate was 20 (10-20) mL/min, with a median (IQR) maximum blood flow rate of 200 (150-200) mL/min.
Anticoagulation Management
Detailed description of anticoagulation strategies is reported in Table 3. First-line anticoagulants of choice were unfractionated heparin (41%) and regional citrate anticoagulation (RCA) (35%). In case of heparin use, activated clotting time (53%) and activated partial thromboplastin time (51%) were the 2 tests used for monitoring. Filters were routinely changed every 72 hours (62%), and 48 to 72 hours were considered an adequate filter life for the majority of PICUs (62%). Filters were changed due to filter clotting (53%) and an increase of transmembrane pressure (47%).
Table 3. Anticoagulation Management During CKRT.
| Characteristic | Responses, No. (%) |
|---|---|
| First-line anticoagulation of choice | |
| No. | 133 |
| Regional unfractionated heparin infusion | 54 (41) |
| Citrate-based regional anticoagulation | 47 (35) |
| Regional heparin and protamine anticoagulation | 15 (11) |
| Systemic unfractionated heparin infusion | 10 (8) |
| Systemic LMWH | 5 (4) |
| Regional prostacyclin | 1 (1) |
| Otherb | 1 (1) |
| Second-line anticoagulation of choice | |
| No. | 131 |
| Citrate-based regional anticoagulation | 45 (34) |
| Regional unfractionated heparin | 28 (21) |
| Systemic unfractionated heparin infusion | 17 (13) |
| No anticoagulation | 10 (8) |
| Systemic LMWH | 10 (8) |
| Regional heparin and protamine anticoagulation | 9 (7) |
| Regional prostacyclin | 9 (7) |
| Systemic prostacyclin | 1 (1) |
| Otherc | 1 (1) |
| Preferred test to measure heparin anticoagulationa | |
| ACT | 84 (53) |
| aPTT | 82 (51) |
| Anti–Xa levels | 38 (24) |
| No monitoring | 2 (1) |
| What is considered an adequate filter life in patients undergoing CKRT? | |
| No. | 132 |
| 72 h | 19 (14) |
| 48-72 h | 82 (62) |
| 24-48 h | 25 (19) |
| ≤24 h | 6 (5) |
| When is the filter routinely changed (according to manufacturer recommendations) if there are no issues? | |
| No. | 130 |
| Every 72 h | 80 (62) |
| Every 48 h | 13 (10) |
| Every 24 h | 8 (6) |
| We do not follow manufacturer recommendations | 29 (22) |
| What situation triggers the change of the filter? | |
| No. | 132 |
| Filter clotting | 70 (53) |
| Increase of transmembrane pressure | 62 (47) |
| Most common complications using anticoagulation during CKRT | |
| No. | 91 |
| Catheter and/or circuit thrombosis | 27 (30) |
| Bleeding | 22 (24) |
| Catheter site and/or mucosal bleeding | 15 (17) |
| Citrate overload | 12 (13) |
| Thrombocytopenia | 9 (10) |
| Difficulty achieving adequate anticoagulation | 6 (7) |
Abbreviations: ACT, activated clotting time; aPTT, activated partial thromboplastin time; CKRT, continuous kidney replacement therapy; KRT, kidney replacement therapy; LMWH, low-molecular-weight heparin.
The sum of percentages is more than 100% because respondents could indicate more than one option.
Regional heparin without protamine.
Direct thrombin inhibitors or no answer.
Fluid Removal Strategies and Liberation From CKRT
Regarding fluid removal during CKRT, 7% of PICUs combined both fluid balance and hemodynamic status in their decision to initiate fluid removal during CKRT. Thirty-two percent of PICUs set a maximum net ultrafiltration rate, ranging from 1 to 3 mL/kg/h. The achievement of the desired fluid removal goals was assessed every 4 hours (34%), with some PICUs assessing this factor on an ad hoc basis, varying from 12 hours (17%) to 24 hours (13%).
Regarding liberation from CKRT, native urine output and the resolution of FO were the 2 factors most often associated with the decision to perform a trial of liberation from CKRT (eFigure 4A in Supplement 1). Liberation from CKRT was performed mainly with a diuretic bolus followed by an infusion (32%) or by a diuretic bolus alone (19%), and 34% of PICUs used a variable strategy depending on the patient’s clinical characteristics (eFigure 4B in Supplement 1). Nine percent of PICUs did not use any agent (ie, fluid and/or diuretic) to trial a patient off of CKRT.
Discussion
Although a few studies have explored practices and attitudes toward the management of CKRT in adult patients in the ICU, to our knowledge, this cross-sectional survey was the first to explore the current status of CKRT management in European PICUs.18,19 The response rate among PICUs performing CKRT was high (76%), and therefore the results can be considered representative of current CKRT practice across European PICUs. We found wide variation in current CKRT management, reinforcing the need for consensus on best practice guidelines.
Continuous kidney replacement therapy is a complex extracorporeal therapy requiring a high level of expertise, training, and resources.1,20 Given that children with critical illness receive CKRT in the PICU, it is not surprising that, in this study, the multidisciplinary PICU team was primarily in charge of CKRT in most units and, in a few units, CKRT was primarily managed by nephrologists. Each strategy has advantages and disadvantages, with a collaborative approach being the best strategy.
We found low rates of education and training being offered to health care professionals who perform CKRT. It has been shown that troubleshooting skills and adverse incidents are directly associated with regular training of staff involved in providing CKRT.21,22 This outcome is an important finding on which the ESPNIC Critical Care Nephrology Section will base the design of ongoing education and training in this field.
Most PICUs offer a range of CKRT modalities (continuous venovenous hemofiltration, continuous venovenous hemodialysis, and CVVHDF), perhaps reflecting the versatility of modern CKRT machines. The survey results showed CVVHDF to be the most used modality in all proposed clinical scenarios except for chronic kidney failure, in which peritoneal dialysis and IHD were most often used. Theoretically, CVVHDF has the advantage of combining small-molecule elimination by diffusion with medium-sized solutes by convection. Moreover, adding a replacement solution in predilution reduces the risk of clotting and, when combined with RCA, facilitates citrate elimination by diffusion.23,24 However, to our knowledge, no study has examined the superiority of 1 modality over the other, and typically each PICU applies the modality they are most comfortable using.
Regarding the indications for initiation of CKRT, respondents indicated that the most common clinical scenarios triggering prompt initiation of CKRT were severe hyperkalemia, FO, and hyperammonemia. Fluid overload with or without AKI is common in children with critical illness,25,26 and it has been shown to be associated with increased mortality; therefore, expert consensus recommends the initiation of CKRT when FO reaches the threshold of 10% and response to diuretics is insufficient.5,6,7,27 More than one-third of PICUs used diuretics during CKRT treatment, although the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines do not recommend their use to increase urine output or reduce the duration of CKRT.16
Sixty percent of respondents expressed concerns regarding the use of adult CKRT machines in children weighing less than 8 kg. A dedicated neonatal or infant dialysis machine, CARPEDIEM, was introduced in clinical practice, while another machine, NIDUS, is currently under investigation.28,29,30,31 However, only 15% of PICUs described availability of one of these dedicated neonatal or infant machines. Nonetheless, it should be highlighted that third- and fourth-generation machines have repeatedly been shown to have a high level of accuracy in children, and dedicated technology should be strongly recommended in children weighing less than 8 to 10 kg, especially because of smaller priming volumes and accurate ultrafiltration rates.32,33
There appeared to be a consensus that blood priming of the circuit is needed in cases of body weight less than 10 kg, hemodynamic instability, or extracorporeal blood volume exceeding 10% of the patient´s blood volume. However, wide variations existed in the technique to perform blood priming. Since blood priming can be technically challenging, having a common ground for PICUs that practice this technique might be helpful.
We found good adherence to the KDIGO guidelines recommendation that vascular access be placed under ultrasonographic guidance in the internal jugular vein.16 The recommendation has traditionally been to insert a large vascular catheter to maximize blood flow, but also to be mindful of vascular thromboembolism if the catheter-to-vessel ratio exceeded 45%.34,35
The majority of PICUs used weight-based CKRT doses as opposed to body surface area–based calculations. It seems important to get a consensus in children because dose calculations based on body surface area tend to be higher in neonates and infants (eg, in an infant, a dose of 2 L/1.73 m2/h corresponds to approximately 60 mL/kg/h).36 We found considerable variation in dose used, with only one-third of PICUs reporting the use of a CKRT dose over 40 mL/kg/h. The role of high-volume CKRT is still uncertain in both pediatric and adult populations.35,37,38,39 However, one-third of respondents increased the effluent dose in case of inadequate clearance of toxins. Although large randomized clinical trials performed in adult patients have not shown any survival benefit when high-intensity CKRT was compared with standard-dose CKRT, there is no concrete evidence in pediatrics.35,37,38 Surviving sepsis guidelines for children do not recommend high-volume hemofiltration for sepsis.40 However, high-volume CKRT might be associated with underdosing of essential drugs (eg, antibiotics and antiepileptics) and an exacerbated catabolic state already present in AKI by removing essential nutrients. As with every other aspect of CKRT, optimal delivery of CKRT should be a dynamic and patient-specific therapy, taking into account the potential discrepancy between the prescribed and delivered dose, which could be as high as 20% to 30%.35
The 2 most common anticoagulants used were regional unfractionated heparin and RCA. Regional citrate anticoagulation has been recommended by KDIGO guidelines as the first choice in patients who do not have a contraindication for citrate because RCA is strictly an extracorporeal anticoagulant and reduces bleeding complications.16 Several prospective randomized clinical trials41,42 in adult patients have demonstrated increased circuit life in CKRT with RCA. Acceptable filter life as well as the safety and efficacy of RCA have also been demonstrated in case series of children aged 1 month to 10 years.43,44,45,46,47 Although the largest study by the Prospective Pediatric Continuous Renal Replacement Therapy Registry45 showed no difference in circuit life between these 2 anticoagulation methods, RCA was associated with fewer bleeding complications. A recent systematic review48 seemed to highlight the improved safety and efficacy profile of RCA compared with systemic heparin anticoagulation in the pediatric population. In rare cases where both RCA and systemic heparin anticoagulation are contraindicated, such as in acute liver failure, prostacyclin or nafamostat mesylate have been shown to be promising alternatives49; however, in the present survey, these agents were rarely used.
Timing, amount of fluid removal, and fluid removal goals are all important practical issues encountered by health care professionals. The aim of fluid removal during CKRT is to balance the desired clinical goals (eg, oxygenation and control of FO) with avoiding the adverse effects of overzealous fluid removal (eg, hypotension, acid-base disturbances, and end-organ ischemia).50,51,52 The best approach for initial and subsequent fluid removal rates in neonates and children is dynamic and in accordance with the physiological and hemodynamic status of the patient. In this survey study, different PICUs set hourly, 4- to 24-hour, or ad hoc fluid assessment goals. Achievement of the net ultrafiltration goal is an important aspect of the CKRT prescription that is often overlooked, leading to suboptimal achievement of fluid balance goals. Based on hemodynamic status, 32% of PICUs set a maximum net ultrafiltration rate: the actual outcome of the interaction between cumulative FO and maximum net ultrafiltration remains to be clarified.
The KDIGO guidelines recommend cessation of CKRT when it is no longer needed because native kidney function has adequately recovered and/or in accordance with the patient´s goals of care. In this survey study, we found that current practice for deciding the timing of liberation from CKRT varied widely. Some clinicians used native urine output during CKRT (variable amounts), whereas others evaluated clinical improvement and/or resolution of FO, with some studies suggesting that biomarkers could be used to estimate successful liberation from CKRT. A recent systematic review and meta-analysis53 in adults showed 16 variables associated with successful CKRT discontinuation. Of these variables, urine output seemed to be the most reliable.53 In cases of unsuccessful liberation from CKRT, timing and choice of transition to other therapeutic modalities remain unclear. Similarly, there remains a wide variation in the practice adopted by clinicians to trial patients off CKRT, as found in this study.
Strengths and Limitations
To our knowledge, this survey study is one of the largest studies to explore CKRT practices in European PICUs. Getting responses from CKRT lead professionals at each PICU meant that we could potentially avoid misdiagnosing variation in practice based on the level of experience of health care professionals. However, the study had some limitations. First, we could not retrieve data from each European country partly due to lack of contacts, knowledge about the respective infrastructure, and language barriers. Nevertheless, we had a response rate of 76%; therefore, we believe the findings provide a fair representation of CKRT practices across Europe. Second, we could not explore all aspects of CKRT due to how extensive the survey would have become. Third, since the survey was limited to European PICUs, we could not extrapolate the findings to PICUs in other regions where CKRT may be provided by varying health care professionals or where resources may differ. Fourth, the possibility of respondent bias could not be avoided, being inherent to every survey study.
Conclusions
We found wide variations in practice even among experienced clinicians offering CKRT in European PICUs. This finding calls for dedicated pediatric critical care nephrology consortia, such as the Acute Disease Quality Initiative and the Critical Care Nephrology Section of ESPNIC, to engage in global initiatives to streamline education and training, research, and guidelines that can be easily accessed by stakeholders in the field to reduce variation in practice.
eMethods. Data Collected With the Survey
eTable. Nursing Staff Education and Training for CKRT Among the PICUs
eFigure 1. Vascular Access Location and Size Preferences According to Age and Weight
eFigure 2. KRT Modalities Most Commonly Used in the Previous 12 Months in the PICUs (eFigure 2A) and Tandem Therapies Available (eFigure 2B)
eFigure 3. KRT Modality According to the Disease (eFigure 3A) and CKRT Strategies in Case of Lack of Toxins Clearance (eFigure 3B)
eFigure 4. Factors Influencing Decision to Stop CKRT (eFigure 4A) and Strategies for CKRT Discontinuation (eFigure 4B)
eAppendix. Survey
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Ricci Z, Goldstein SL. Pediatric continuous renal replacement therapy. Contrib Nephrol. 2016;187:121-130. doi: 10.1159/000442370 [DOI] [PubMed] [Google Scholar]
- 2.Goldstein SL, Currier H, Graf CD, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309-1312. doi: 10.1542/peds.107.6.1309 [DOI] [PubMed] [Google Scholar]
- 3.Symons JM, Chua AN, Somers MJ, et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol. 2007;2(4):732-738. doi: 10.2215/CJN.03200906 [DOI] [PubMed] [Google Scholar]
- 4.Deep A, Goldstein SL, eds. Critical Care Nephrology and Renal Replacement Therapy in Children. Springer International Publishing AG; 2018. doi: 10.1007/978-3-319-90281-4 [DOI] [Google Scholar]
- 5.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316-325. doi: 10.1053/j.ajkd.2009.10.048 [DOI] [PubMed] [Google Scholar]
- 6.Cortina G, McRae R, Hoq M, et al. Mortality of critically ill children requiring continuous renal replacement therapy: effect of fluid overload, underlying disease, and timing of initiation. Pediatr Crit Care Med. 2019;20(4):314-322. doi: 10.1097/PCC.0000000000001806 [DOI] [PubMed] [Google Scholar]
- 7.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771-1776. doi: 10.1097/01.CCM.0000132897.52737.49 [DOI] [PubMed] [Google Scholar]
- 8.Santiago MJ, López-Herce J, Urbano J, et al. Clinical course and mortality risk factors in critically ill children requiring continuous renal replacement therapy. Intensive Care Med. 2010;36(5):843-849. doi: 10.1007/s00134-010-1858-9 [DOI] [PubMed] [Google Scholar]
- 9.Modem V, Thompson M, Gollhofer D, Dhar AV, Quigley R. Timing of continuous renal replacement therapy and mortality in critically ill children*. Crit Care Med. 2014;42(4):943-953. doi: 10.1097/CCM.0000000000000039 [DOI] [PubMed] [Google Scholar]
- 10.Deep A, Stewart CE, Dhawan A, Douiri A. Effect of continuous renal replacement therapy on outcome in pediatric acute liver failure. Crit Care Med. 2016;44(10):1910-1919. doi: 10.1097/CCM.0000000000001826 [DOI] [PubMed] [Google Scholar]
- 11.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028-1035. doi: 10.1038/sj.ki.5002231 [DOI] [PubMed] [Google Scholar]
- 12.Basu RK, Zappitelli M, Brunner L, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(3):659-667. doi: 10.1038/ki.2013.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators . Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(1):11-20. doi: 10.1056/NEJMoa1611391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jetton JG, Boohaker LJ, Sethi SK, et al. ; Neonatal Kidney Collaborative (NKC) . Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1(3):184-194. doi: 10.1016/S2352-4642(17)30069-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554-561. doi: 10.2215/CJN.01900214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KDIGO AKI Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;17:1-138. [Google Scholar]
- 17.American Association for Public Opinion Research . Standard definitions report. Accessed October 10, 2022. https://www.aapor.org/Standards-Ethics/Standard-Definitions-(1).aspx
- 18.Ronco C, Zanella M, Brendolan A, et al. Management of severe acute renal failure in critically ill patients: an international survey in 345 centres. Nephrol Dial Transplant. 2001;16(2):230-237. doi: 10.1093/ndt/16.2.230 [DOI] [PubMed] [Google Scholar]
- 19.Legrand M, Darmon M, Joannidis M, Payen D. Management of renal replacement therapy in ICU patients: an international survey. Intensive Care Med. 2013;39(1):101-108. doi: 10.1007/s00134-012-2706-x [DOI] [PubMed] [Google Scholar]
- 20.Maclaren G, Butt W. Controversies in paediatric continuous renal replacement therapy. Intensive Care Med. 2009;35(4):596-602. doi: 10.1007/s00134-009-1425-4 [DOI] [PubMed] [Google Scholar]
- 21.Rhee H, Jang GS, Han M, et al. The role of the specialized team in the operation of continuous renal replacement therapy: a single-center experience. BMC Nephrol. 2017;18(1):332. doi: 10.1186/s12882-017-0746-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz EF, Ortiz-Soriano VM, Talbott M, et al. ; University of Kentucky CRRT Quality Assurance Group . Development, implementation and outcomes of a quality assurance system for the provision of continuous renal replacement therapy in the intensive care unit. Sci Rep. 2020;10(1):20616. doi: 10.1038/s41598-020-76785-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joannidis M, Oudemans-van Straaten HM. Clinical review: patency of the circuit in continuous renal replacement therapy. Crit Care. 2007;11(4):218. doi: 10.1186/cc5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raina R, Sethi S, Khooblall A, et al. Non-anticoagulation pediatric continuous renal replacement therapy methods to increase circuit life. Hemodial Int. 2022;26(2):147-159. doi: 10.1111/hdi.13003 [DOI] [PubMed] [Google Scholar]
- 25.Fleming GM, Askenazi DJ, Bridges BC, et al. A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J. 2012;58(4):407-414. doi: 10.1097/MAT.0b013e3182579218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selewski DT, Cornell TT, Blatt NB, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40(9):2694-2699. doi: 10.1097/CCM.0b013e318258ff01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selewski DT, Goldstein SL. The role of fluid overload in the prediction of outcome in acute kidney injury. Pediatr Nephrol. 2018;33(1):13-24. doi: 10.1007/s00467-016-3539-6 [DOI] [PubMed] [Google Scholar]
- 28.Ronco C, Garzotto F, Brendolan A, et al. Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturised machine (CARPEDIEM). Lancet. 2014;383(9931):1807-1813. doi: 10.1016/S0140-6736(14)60799-6 [DOI] [PubMed] [Google Scholar]
- 29.Goldstein SL, Vidal E, Ricci Z, et al. Survival of infants treated with CKRT: comparing adapted adult platforms with the Carpediem. Pediatr Nephrol. 2022;37(3):667-675. doi: 10.1007/s00467-021-05180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulthard MG, Crosier J, Griffiths C, et al. Haemodialysing babies weighing <8 kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol. 2014;29(10):1873-1881. doi: 10.1007/s00467-014-2923-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert HJ, Sharma S, Matthews JNS. I-KID study protocol: evaluation of efficacy, outcomes and safety of a new infant haemodialysis and ultrafiltration machine in clinical use: a randomised clinical investigation using a cluster stepped-wedge design. BMJ Paediatr Open. 2021;5(1):e001224. doi: 10.1136/bmjpo-2021-001224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rödl S, Marschitz I, Mache CJ, Koestenberger M, Madler G, Zobel G. Continuous renal replacement therapy with Prismaflex HF20 disposable set in children from 4 to 15 kg. ASAIO J. 2011;57(5):451-455. doi: 10.1097/MAT.0b013e31822d2132 [DOI] [PubMed] [Google Scholar]
- 33.Liu ID, Ng KH, Lau PY, Yeo WS, Koh PL, Yap HK. Use of HF20 membrane in critically ill unstable low-body-weight infants on inotropic support. Pediatr Nephrol. 2013;28(5):819-822. doi: 10.1007/s00467-012-2394-3 [DOI] [PubMed] [Google Scholar]
- 34.Szeps I, Östlund Å, Norberg Å, Fläring U, Andersson A. Thromboembolic complications of vascular catheters used for pediatric continuous renal replacement therapy: prevalence in a single-center, retrospective cohort. Pediatr Crit Care Med. 2021;22(8):743-752. doi: 10.1097/PCC.0000000000002754 [DOI] [PubMed] [Google Scholar]
- 35.Vesconi S, Cruz DN, Fumagalli R, et al. ; DOse REsponse Multicentre International Collaborative Initiative (DO-RE-MI Study Group) . Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Crit Care. 2009;13(2):R57. doi: 10.1186/cc7784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci Z, Guzzi F, Tuccinardi G, Romagnoli S. Dialytic dose in pediatric continuous renal replacement therapy patients. Minerva Pediatr. 2016;68(5):366-373. [PubMed] [Google Scholar]
- 37.Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356(9223):26-30. doi: 10.1016/S0140-6736(00)02430-2 [DOI] [PubMed] [Google Scholar]
- 38.Bellomo R, Cass A, Cole L, et al. ; RENAL Replacement Therapy Study Investigators . Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627-1638. doi: 10.1056/NEJMoa0902413 [DOI] [PubMed] [Google Scholar]
- 39.Monti G, Herrera M, Kindgen-Milles D, et al. ; Dose Response Multicentre International Collaborative Initiative Scientific Committee . The DOse REsponse Multicentre International Collaborative Initiative (DO-RE-MI). Contrib Nephrol. 2007;156:434-443. doi: 10.1159/000102137 [DOI] [PubMed] [Google Scholar]
- 40.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-1247. doi: 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oudemans-van Straaten HM, Bosman RJ, Koopmans M, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med. 2009;37(2):545-552. doi: 10.1097/CCM.0b013e3181953c5e [DOI] [PubMed] [Google Scholar]
- 42.Schilder L, Nurmohamed SA, Bosch FH, et al. ; CASH Study Group . Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: a multi-center randomized clinical trial. Crit Care. 2014;18(4):472. doi: 10.1186/s13054-014-0472-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raymakers-Janssen PAMA, Lilien M, van Kessel IA, Veldhoen ES, Wösten-van Asperen RM, van Gestel JPJ. Citrate versus heparin anticoagulation in continuous renal replacement therapy in small children. Pediatr Nephrol. 2017;32(10):1971-1978. doi: 10.1007/s00467-017-3694-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soltysiak J, Warzywoda A, Kociński B, et al. Citrate anticoagulation for continuous renal replacement therapy in small children. Pediatr Nephrol. 2014;29(3):469-475. doi: 10.1007/s00467-013-2690-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brophy PD, Somers MJ, Baum MA, et al. Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant. 2005;20(7):1416-1421. doi: 10.1093/ndt/gfh817 [DOI] [PubMed] [Google Scholar]
- 46.Cortina G, McRae R, Chiletti R, Butt W. The effect of patient- and treatment-related factors on circuit lifespan during continuous renal replacement therapy in critically ill children. Pediatr Crit Care Med. 2020;21(6):578-585. doi: 10.1097/PCC.0000000000002305 [DOI] [PubMed] [Google Scholar]
- 47.Rico MP, Fernández Sarmiento J, Rojas Velasquez AM, et al. Regional citrate anticoagulation for continuous renal replacement therapy in children. Pediatr Nephrol. 2017;32(4):703-711. doi: 10.1007/s00467-016-3544-9 [DOI] [PubMed] [Google Scholar]
- 48.Buccione E, Bambi S, Rasero L, et al. Regional citrate anticoagulation and systemic anticoagulation during pediatric continuous renal replacement therapy: a systematic literature review. J Clin Med. 2022;11(11):3121. doi: 10.3390/jcm11113121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deep A, Zoha M, Dutta Kukreja P. Prostacyclin as an anticoagulant for continuous renal replacement therapy in children. Blood Purif. 2017;43(4):279-289. doi: 10.1159/000452754 [DOI] [PubMed] [Google Scholar]
- 50.Hall A, Crichton S, Dixon A, Skorniakov I, Kellum JA, Ostermann M. Fluid removal associates with better outcomes in critically ill patients receiving continuous renal replacement therapy: a cohort study. Crit Care. 2020;24(1):279. doi: 10.1186/s13054-020-02986-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tehranian S, Shawwa K, Kashani KB. Net ultrafiltration rate and its impact on mortality in patients with acute kidney injury receiving continuous renal replacement therapy. Clin Kidney J. 2019;14(2):564-569. doi: 10.1093/ckj/sfz179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murugan R, Kerti SJ, Chang CH, et al. Association between net ultrafiltration rate and renal recovery among critically ill adults with acute kidney injury receiving continuous renal replacement therapy: an observational cohort study. Blood Purif. 2022;51(5):397-409. doi: 10.1159/000517281 [DOI] [PubMed] [Google Scholar]
- 53.Katulka RJ, Al Saadon A, Sebastianski M, et al. Determining the optimal time for liberation from renal replacement therapy in critically ill patients: a systematic review and meta-analysis (DOnE RRT). Crit Care. 2020;24(1):50. doi: 10.1186/s13054-020-2751-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Data Collected With the Survey
eTable. Nursing Staff Education and Training for CKRT Among the PICUs
eFigure 1. Vascular Access Location and Size Preferences According to Age and Weight
eFigure 2. KRT Modalities Most Commonly Used in the Previous 12 Months in the PICUs (eFigure 2A) and Tandem Therapies Available (eFigure 2B)
eFigure 3. KRT Modality According to the Disease (eFigure 3A) and CKRT Strategies in Case of Lack of Toxins Clearance (eFigure 3B)
eFigure 4. Factors Influencing Decision to Stop CKRT (eFigure 4A) and Strategies for CKRT Discontinuation (eFigure 4B)
eAppendix. Survey
Nonauthor Collaborators
Data Sharing Statement