Abstract
The corticocortical vestibular network (CVN) plays an important role in maintaining balance and stability. In order to clarify the specific relationship between the CVN and the balance ability of patients with mild cognitive impairment (MCI), we recruited 30 MCI patients in the community. According to age and sex, they were 1:1 matched to 30 older adults with normal cognitive function. We evaluated balance ability and performed MRI scanning in the two groups of participants. We analyzed functional connectivity within the CVN based on the region of interest. Then, we performed a Pearson correlation analysis between the functional connection and the Berg Balance Scale scores. The research results show that compared with the control group, there were three pairs of functional connections (hMST_R–Premotor_R, PFcm_R–SMA_L, and hMST_L–VIP_R) that were significantly decreased in the CVNs of the MCI group (p < 0.05). Further correlation analysis showed that there was a significant positive correlation between hMST_R–Premotor_R functional connectivity and BBS score (r = 0.364, p = 0.004). The decline in balance ability and increase in fall risk in patients with MCI may be closely related to the change in the internal connection mode of the corticocortical vestibular network.
Keywords: mild cognitive impairment, fall, corticocortical vestibular network, balance, functional connection
1. Introduction
Falls and fall-related injuries are common and serious problems for elderly populations. Adults over the age of 65 have the highest risk of falling. In total, 30% of adults over 65 years of age and 50% of adults over 80 years of age fall at least once per year [1,2,3]. Falls are caused by the disparity between a person’s physical strength and their environment or the direct needs of ongoing activities. Every fall has the potential to change one’s life and may lead to chronic disability, assisted living, or even death. Even if the fall does not cause serious injury, it can also cause a fear of falling, which will lead to limited activity and increase the risk of future falls [4,5].
Mild cognitive impairment (MCI) is an early stage of Alzheimer’s disease; existing studies show that MCI is an important risk factor for instability and falls in the elderly [6]. Compared with elderly populations with normal cognitive function, the incidence of falls in MCI elderly individuals is higher [7]. Approximately 60% of elderly individuals with MCI fall at least once a year, which is twice the number of falls as those with normal cognitive function [1,8]. Patients with MCI are also more likely to experience traumatic and nonaccidental falls [9,10]. The incidence of hip fracture in elderly people with MCI after falling is three times higher than that in the general elderly population [11]. Therefore, it is important to prevent MCI patients from falling and to reduce the adverse consequences caused by falls.
Decline in balance ability is an important factor in the increased fall risk of MCI patients. A cross-sectional study involving 295 subjects showed that at as early as the stage of subjective cognitive decline, the balance ability of patients decreased significantly [12]. The fall risk index of patients with MCI was significantly increased, while Berg Balance Scale and gait index scores were significantly lower than those of healthy elderly people [13]. The maintenance of balance ability depends on the integration of vestibular inputs into the brain, which then adjust the efferent motor response [14]. The corticocortical vestibular network (CVN) is the core system that integrates vision, hearing and proprioception to maintain balance and stability [15]. Vestibule and other sensorimotor signals are primarily integrated in the dorsal striatum to control the movement of the body and limbs [16]. The central vestibular system receives inputs from two parallel information pathways. Regular signals transmit head rotation information while irregular signals only transmit information on high-frequency characteristics [17,18]. When the human body interacts with its environment, a multi-modal signal inflow is generated. The brain codes and estimates the stability of spontaneous motion and the motion of adjacent parts of the body to ensure the accurate control of behavior and posture in daily life. When the body is out of balance, the vestibular network sends out alarm signals, triggering defensive movement responses. At the level of neurophysiology, a decrease in gray matter in the vestibular network is closely related to a decrease in cognitive control ability and flexibility of posture, which reduces control over body posture balance [19,20]. Nerve conduction in the elderly is slower, and their vestibular network integration ability is weakened, their reaction time is prolonged, and they do not identify and avoid risks in a timely and effective manner [21]. The decline in control ability leads to an imbalance in balance ability, which significantly increases the risk of falls [22].
The change in functional connectivity of the CVN leads to a decline in balance ability and increases fall risk for MCI patients. However, previous studies have not studied the relationship between CVN connectivity and balance ability in patients with MCI in-depth, which has led to a lack of accurate targets in noninvasive brain stimulation techniques and other interventions. This study will use functional connectivity analysis based on the region of interest to observe the relationship between the functional connectivity of the CVN and balance ability in patients with MCI, with the goal of providing therapeutic targets for further intervention to reduce fall risk in MCI patients.
2. Materials and Methods
2.1. Participants
In total, 30 patients with MCI and 30 older adults with normal cognition were recruited; they were matched for age, sex, and education level. We recruited participants in communities near Shenzhen Bao’an Clinical Medical School, Guangdong Medical University, through posters, WeChat, and phone calls. The participants were assessed by doctors with more than 5 years of experience, according to the inclusion and exclusion criteria. The inclusion criteria of the MCI group included: (1) met the MCI diagnostic criteria (Petersen, 2004) [23]; (2) over 60 years old; and (3) right handedness. The inclusion criteria of the normal cognition (NC) group included: (1) did not meet the MCI diagnostic criteria (Petersen, 2004) [23]; (2) over 60 years old; and (3) right handedness. The exclusion criteria included: (1) cerebral infarction, cerebral hemorrhage, brain tumor, or other brain diseases that may affect the results of magnetic resonance imaging; (2) metal implants in the body, such as dentures, pacemakers, or intramedullary nails; (3) drug use that affects cognitive function; (4) accompanying neuropsychiatric disease, preventing patients from completing the cognitive assessment and balance ability test; (5) patients with existing vestibular or other neurological issues; and (6) accompanying claustrophobia, preventing patients from completing the MRI examination. The Shenzhen Bao’an Clinical Medical School of the Guangdong Medical University Ethics Committee for Clinical Research approved this study. All participants provided written informed consent after receiving information about the study.
2.2. Balance Ability Assessment
All participants were asked to fill in the Chinese version of the Berg Balance Scale (BBS). The scale has 38 items, and each item is scored from 0 to 4 points. The higher the score, the better the balance ability. The intragroup reliability of the scale in evaluating healthy elderly people was 0.97, showing good reliability [24].
2.3. Functional Magnetic Resonance Imaging Acquisition
A 3.0 T Siemens Prisma MRI scanner (Siemens, Erlangen, Germany) was used to obtain the T1 and resting state functional data of all participants. Resting state scans were acquired with the participants’ eyes closed and an echo planar sequence with the following parameters: TE/TR = 30 ms/2000 ms, flip angle = 90°, FOV = 230 mm, slices = 37, slice thickness = 3.6 mm, and time points = 300. A high-resolution T1-weighted MPRAGE was obtained (TR = 2300 ms, TE = 2.27 ms, flip angle = 8°, 160 slices, and voxel size = 0.98 × 0.98 × 1.0 mm3).
2.4. Functional Network Construction
Preprocessing of the fMRI data was performed using Functional Connectivity Toolbox (CONN) pipeline version 21a (www.nitrc.org/projects/conn, accessed on 5 September 2022). The preprocessing included slice-timing correction, segmentation, normalization, smoothing (Gaussian kernel = 8 mm), and band-pass filtering (0.008 Hz to 0.08 Hz). The specific preprocessing details were the same as those in Fang’s research [25].
We performed a functional connectivity analysis of the corticocortical vestibular network based on the region of interest (ROI). The ROIs of this study came from 20 activated brain regions induced by Raiser through tasks, including bilateral Area 2v, Area 3av, the premotor area, the cingulate sulcus visual (CSv), and the ventral intraparietal area (VIP), among others [26]. The ROIs were defined as a sphere with a radius of 5 mm in the Montreal Neurological Institute (MNI) space; we used the CONN toolbox to calculate the measured value of the ROI-to-ROI functional connection. To compare the functional connectivity of the vestibular network between patients with MCI and the NC group, white matter, cerebrospinal fluid signal, age, sex, education level, and cognitive function were controlled as covariates. Significant connections were identified by a two-sample t-test calculated as voxel threshold (uncorrected p-value of <0.05 and a cluster threshold of p-FDR < 0.05).
2.5. Statistical Analysis
The data were analyzed using SPSS 27.0 (IBM Corp., Armonk, NY, USA) for comparison of the clinical characteristics of the participants, and the independent sample t-test or the Kruskal–Wallis test as a nonparametric test was used for continuous variables and the chi-square test was used for binary variables. A p-value of <0.05 indicated that there was a statistical difference between groups. To analyze the correlation between the FC and the balance ability, a Pearson correlation coefficient test was performed.
3. Results
3.1. Subject Characteristics
Sex, age, and education level were not significantly different between the MCI and the control groups (all p > 0.05). The MoCA score and BBS score in the MCI group were significantly lower than those in the normal cognitive control group (p < 0.001) (Table 1).
Table 1.
Clinical characteristics of the participants.
| Variable | MCI (N = 30) | NC (N = 30) | χ2/t Value | p-Value |
|---|---|---|---|---|
| Sex (M/F) | 14/16 | 16/14 | 0.267 | 0.606 |
| Age (years): mean ± SD | 66.73 ± 5.20 | 67.50 ± 5.67 | −0.546 | 0.587 |
| Education level (years): Mean ± SD | 9.53 ± 2.80 | 9.40 ± 2.58 | 0.192 | 0.849 |
| MoCA (scores): mean ± SD | 21.13 ± 2.46 | 27.60 ± 1.28 | −12.782 | <0.001 |
| BBS (scores): mean ± SD | 41.70 ± 5.93 | 48.67 ± 4.16 | −5.269 | <0.001 |
MCI, mild cognitive impairment; NC, normal cognitive control; MoCA, Montreal Cognitive Assessment; BBS, Berg Balance Scale; M, male; F, female; SD, standard deviation.
3.2. Function Connection Analysis Results
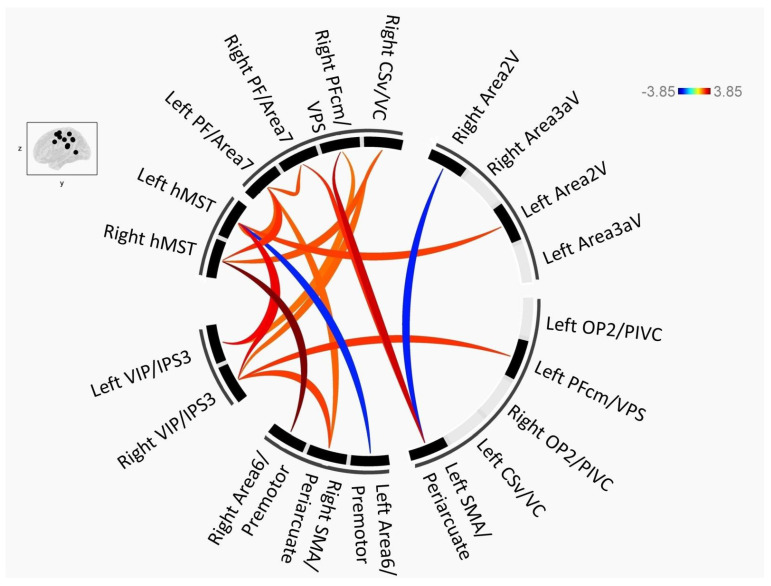
The functional connection between the ROIs of the cortical vestibular network in patients with MCI mainly decreased, and only the connection of two pairwise ROIs increased (Figure 1, where red indicates that the NC group was larger than the MCI and blue indicates that the NC group was smaller than the MCI). At the peak level, the MCI group had 2 pairwise functional connections that were enhanced and 15 pairwise function connections that were decreased compared with the NC group (p < 0.05). At the cluster level, after multiple comparison and correction (false discovery rate (FDR) corrected), three pairwise (hMST_R–premotor_R, PFcm_R–SMA_L, and hMST_L–VIP_R) functional connections in the MCI group were significantly lower than those in the NC group (p < 0.05) (Table 2).
Figure 1.
Connectogram depicting the differences in brain connectivity between the MCI group and the NC group. Voxel threshold: uncorrected p < 0.05 and cluster threshold: p-FDR < 0.05.
Table 2.
Resting state pairwise connections in the MCI group compared to those of the NC group.
| Compare | Analysis Unit | T | p-unc | p-FDR | |
|---|---|---|---|---|---|
| Seed | Target | ||||
| NC < MCI | Left periarcuate/SMA | Right Area 2V | −2.68 | 0.009524 | 0.079966 |
| Left hMST | Left Area 6/premotor | −2.61 | 0.011444 | 0.072479 | |
| NC > MCI | Right hMST | Right Area 6/premotor | 3.85 | 0.000300 | 0.005707 * |
| Right hMST | Left PF/area 7 | 2.57 | 0.012911 | 0.122658 | |
| Right hMST | Right CSv | 2.28 | 0.026072 | 0.165124 | |
| Right PFcm/VPS | Left periarcuate/SMA | 3.26 | 0.001883 | 0.035785 * | |
| Right PFcm/VPS | Left VIP | 2.05 | 0.044867 | 0.275219 | |
| Left periarcuate/SMA | Right PF/area 7 | 2.57 | 0.012626 | 0.079823 | |
| Left PF/area 7 | Right PF/area 7 | 2.36 | 0.021922 | 0.164857 | |
| Left PF/area 7 | Left hMST | 2.26 | 0.027363 | 0.103979 | |
| Left PF/area 7 | Right periarcuate/SMA | 2.16 | 0.034707 | 0.164876 | |
| Left hMST | Right VIP | 3.00 | 0.003958 | 0.048749 * | |
| Left hMST | Left VIP | 2.91 | 0.005131 | 0.097498 | |
| Left hMST | Left area 2V | 2.43 | 0.018107 | 0.086009 | |
| Right VIP | Left PFcm/VPS | 2.52 | 0.014564 | 0.113398 | |
| Right VIP | Right periarcuate/SMA | 2.44 | 0.017905 | 0.125867 | |
| Right VIP | Right CSv | 2.16 | 0.034913 | 0.165835 | |
MCI, mild cognitive impairment; NC, normal cognitive control; p-unc, p-value at peak level; p-FDR, p-value at cluster level; FDR, corrected false discovery rate; * p < 0.05; SMA, supplementary motor area; hMST, human medial superior temporal area; PF, prefrontal cortex; CSv, cingulate sulcus visual; PFcm, medial prefrontal cortex; VPS, visual posterior sylvian area; VIP, ventral intraparietal area; R, right; L, left.
3.3. Correlation Analysis
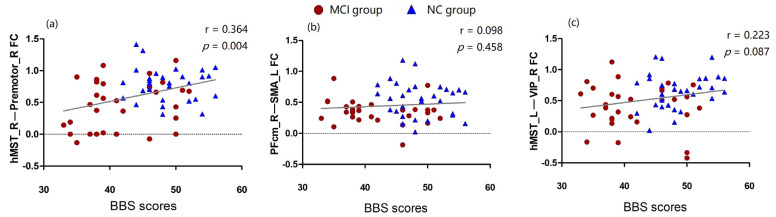
We extracted the z-values of three pairwise functional connections that retained statistical differences after the correction, and we conducted a correlation analysis with the participants’ BBS scores. There was a significant correlation between the hMST_R–premotor_R functional connectivity and the BBS score (r = 0.364 and p = 0.004) (Figure 2a). There was no significant correlation between the PFcm_R–SMA_L and hMST_L–VIP_R functional connectivity and the BBS score (p > 0.05) (Figure 2b,c).
Figure 2.
The linear correlation between the functional connection and the BBS scores. (a) correlation analysis of hMST_R–premotor_R functional connectivity and the BBS score(r = 0.364, p = 0.004); (b) correlation analysis of PFcm_R–SMA_L functional connectivity and the BBS score(r = 0.098, p = 0.458); (c) correlation analysis of hMST_L–VIP_R functional connectivity and the BBS score(r = 0.223, p = 0.087).
4. Discussion
In this study, we compared the functional connections within the cortical vestibular network between patients with MCI and normal elderly people and found that there were significant differences among the three pairs of functional connections (hMST_R–premotor_R, PFcm_R–SMA_L, and hMST_L–VIP_R). Further correlation analysis showed that there was a significant positive correlation between the hMST_R–premotor_R functional connectivity and the BBS scores.
The CVN is composed of several distinct and separate areas which receive bilateral vestibular inputs from the vestibular nucleus, and they project directly down to the vestibular nucleus. Among them, parieto-insular vestibular cortex (PIVC) is regarded as the core of multisensory information processing [27,28]. However, in this study, the functional connection between bilateral PIVC and other components of CVN in MCI patients did not change significantly. This shows that the vestibular network damage of MCI patients has not yet affected PIVC. Nevertheless, proprioception, vision, hearing, and other signal input channels are not immune. The MST is thought to play an important role in heading perception, where the neurons in the MST are tuned to both head movement and vestibular signals [29,30,31,32]. The MST regulates the planning and execution of smooth pursuit eye movements, and its activities are affected by cognitive factors such as attention and working memory [33]. In addition, the MST has a causal effect on human self-motion perception and plays a key role in visually guided navigation [34]. The dorsal premotor cortex plays an essential role in visually guided goal-directed motor behavior [35]. In this study, we observed that the hMST_R–premotor_R functional connectivity in patients with MCI was significantly reduced. This may be the main manifestation of the impairment of patients’ vestibular network function, which has a negative impact on self-perception and exercise plans, thus leading to a decline in balance and an increase in the risk of falls for patients.
The VPS area shows vestibular-dominant heading tuning [36]. In the MST, the more extensive directional tuning of the putative interneurons is used for visual stimulation, while in VPS, the putative interneurons are more widely tuned to the vestibular state. It is assumed that the regional diversity of the tuning width difference between interneurons and pyramidal neurons is likely due to their dominant inputs [37]. The SMA is critical to many aspects of motor behavior, including motion preparation, motion initiation, and the selection, motion control, and monitoring of motion outcomes; at the same time, it plays an important role in auditory processing and participates in the sensorimotor process to guide auditory perception [38,39]. The decreased functional connectivity of the VPS–SMA may be due to the impaired auditory perception of patients with MCI, which leads to the weakening of multisensory integration and a decline in CVN function. The selectivity of the vestibular and visual flow signals lies between the MST and VPS, and as the VIP is an important brain region combining vestibular and visual flow signals [40,41,42], it may be proximal to the MST in terms of vestibular processing, but it is hierarchically similar to the MST in terms of optic flow processing [37]. It has also been found that the deactivation of the VIP area does not lead to behavioral defects and may not participate in visual heading discrimination [43]. Therefore, the weakening of the functional connection of the MST–VIP may be caused by the decreased ability of patients with MCI to process visual/vestibular signals.
In addition, we found that the connectivity of MCI patients’ periarcuate_L–area 2V_R increased. The periarcuate area plays an important role in coordinating eye and hand movements [44], and area 2V, located in the postcentral sulcus, can integrate optical flow clues in the processing of self-movement [45,46]. Enhancement of the periarcuate_L–area 2V_R functional connection may compensate for the motor coordination dysfunction caused by a reduction in visual signal input. Another interesting finding is that hMST_L–premotor_L functional connectivity is enhanced in patients with MCI, which is completely opposite to the result on the right side. Existing research cannot explain this result, and we speculate that it may be a partial compensation for the decline in contralateral functional connectivity.
The results of the correlation analysis showed that there was a significant positive correlation between the strength of hMST_R–premotor_R functional connection and BBS scores, but it has no significant correlation with MoCA scores. Previous evidence has suggested that the decline in balance function is not related to the severity of dementia or cognitive decline [47]. This study found similar results, indicating that cognitive function is a separate risk factor. The vestibular network is involved in two pathways that are critical to maintaining balance: the vestibulo-ocular reflex and the vestibulospinal reflex [48,49], which play key roles in controlling posture, gait, and fall risk [50,51]. On the basis of previous studies’ methods, this study used a more refined CVN template [26] to find the neuroimaging markers of impaired balance in patients with MCI. The results showed that the decreased functional connectivity of the hMST_R–premotor_R may be an early neuroimaging marker of impaired balance function in patients with MCI.
To summarize, the results of this study showed that functional connectivity within the CVN in patients with MCI is weakened, especially in the key brain regions of visual, auditory, and vestibular signal integration, and this is synchronous with a decrease in balance ability in patients with MCI. A decline in the functional connectivity of hMST_R and premotor_R may be an early sensitive marker for the decline in balance ability of MCI patients, which can be considered as an important target for future intervention.
This study had several limitations. Firstly, this was a preliminary study of a small sample, and it aimed to provide a more accurate examination for future interventions. Therefore, we did not perform more detailed assessments of vision, hearing, gait, dynamic balance ability, and other factors that affect falls. This may affect the stability of our results due to the interference of confounding factors; thus, we should collect information on other control variables in more detail in future research. Secondly, the corticocortical vestibular network seed points that we used were task-activated. However, this study used resting-state MRI data, which may have omitted some changes in the functional connectivity of the inactive brain regions in the resting state. Thirdly, this study was a case–control study, and the results of the correlation analysis cannot be used as the basis for causal inference. In future research, various factors that affect vestibular network function should be considered and evaluated in more detail. At the same time, tasks can be designed to activate the vestibular network or the sample size can be increased to improve the validity of the results. In addition, we can use noninvasive brain function regulation technology to stimulate the significantly different cortical vestibular network components found in this study in order to verify the value of these seed points in treatment.
5. Conclusions
The decline in balance ability and the increase in fall risk among patients with MCI may be closely related to a change in the internal connection mode of the corticocortical vestibular network. In particular, the functional connectivity of hMST_R–premotor_R in patients with MCI is closely related to balance function score, which may be a sensitive marker for predicting falls.
Abbreviations
| Abbreviations | Full Name |
| MCI | Mild Cognitive Impairment |
| NC | Normal Cognitive Control |
| CVN | Corticocortical Vestibular Network |
| BBS | Berg Balance Scale |
| MoCA | Montreal Cognitive Assessment |
| MRI | Magnetic Resonance Imaging |
| ROI | Region Of Interest |
| CSv | Cingulate Sulcus Visual |
| VIP | Ventral Intraparietal Area |
| MNI | Montreal Neurological Institute |
| FDR | False Discovery Rate Corrected |
| SMA | Supplementary Motor Area |
| hMST | Human Medial Superior Temporal Area |
| PF | Prefrontal Cortex |
| PFcm | Medial Prefrontal Cortex |
| VPS | Visual Posterior Sylvian Area |
| ECG | Electrocardiogram |
| SD | Standard Deviation |
Author Contributions
Conceptualization, Z.W. and S.C.; methodology, R.X. and J.R.; investigation, X.L. and J.L.; data curation, Y.D.; writing—original draft preparation, R.X.; writing—review and editing, R.X. and J.R.; visualization, Z.W.; supervision, Y.K.; project administration, S.C.; funding acquisition, S.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Shenzhen Bao’an Clinical Medical School, Guangdong Medical University (protocol code: BYL20210905; date of approval: 3 September 2021).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study. Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the Guangdong Basic and Applied Basic Research Foundation (2021A1515110764) and the Science and Technology Program of Shenzhen (JCYJ20220530142806014 and JCYJ20210324110809025).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tinetti M.E., Speechley M., Ginter S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 2.Bergen G., Stevens M.R., Burns E.R. Falls and Fall Injuries Among Adults Aged ≥65 Years—United States, 2014. Morb. Mortal. Wkly. Rep. 2016;65:993–998. doi: 10.15585/mmwr.mm6537a2. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence Falls in Older People: Assessing Risk and Prevention (NICE Clinical Guideline 161) 2013. [(accessed on 1 September 2022)]. Available online: www.nice.org.uk/guidance/cg161. [PubMed]
- 4.Yardley L., Smith H. A prospective study of the relationship between feared consequences of falling and avoidance of activity in community-living older people. Gerontologist. 2002;42:17–23. doi: 10.1093/geront/42.1.17. [DOI] [PubMed] [Google Scholar]
- 5.Delbaere K., Crombez G., Vanderstraeten G., Willems T., Cambier D. Fear-related avoidance of activities, falls and physical frailty. A prospective community-based cohort study. Age Ageing. 2004;33:368–373. doi: 10.1093/ageing/afh106. [DOI] [PubMed] [Google Scholar]
- 6.Lach H.W., Harrison B.E., Phongphanngam S. Falls and Fall Prevention in Older Adults with Early-Stage Dementia: An Integrative Review. Res. Gerontol. Nurs. 2017;10:139–148. doi: 10.3928/19404921-20160908-01. [DOI] [PubMed] [Google Scholar]
- 7.Ansai J.H., Andrade L.P., Masse F., Gonçalves J., Takahashi A., Vale F., Rebelatto J.R. Risk Factors for Falls in Older Adults with Mild Cognitive Impairment and Mild Alzheimer Disease. J. Geriatr. Phys. Ther. 2019;42:E116–E121. doi: 10.1519/JPT.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 8.Van Dijk P.T., Meulenberg O.G., van de Sande H.J., Habbema J.D. Falls in dementia patients. Gerontologist. 1993;33:200–204. doi: 10.1093/geront/33.2.200. [DOI] [PubMed] [Google Scholar]
- 9.Delbaere K., Kochan N.A., Close J.C., Menant J.C., Sturnieks D.L., Brodaty H., Sachdev P.S., Lord S.R. Mild cognitive impairment as a predictor of falls in community-dwelling older people. Am. J. Geriatr. Psychiatry. 2012;20:845–853. doi: 10.1097/JGP.0b013e31824afbc4. [DOI] [PubMed] [Google Scholar]
- 10.Tyrovolas S., Koyanagi A., Lara E., Santini Z.I., Haro J.M. Mild cognitive impairment is associated with falls among older adults: Findings from the Irish Longitudinal Study on Ageing (TILDA) Exp. Gerontol. 2016;75:42–47. doi: 10.1016/j.exger.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell R., Draper B., Brodaty H., Close J., Ting H.P., Lystad R., Harris I., Harvey L., Sherrington C., Cameron I.D., et al. An 11-year review of hip fracture hospitalisations, health outcomes, and predictors of access to in-hospital rehabilitation for adults ≥ 65 years living with and without dementia: A population-based cohort study. Osteoporos. Int. 2020;31:465–474. doi: 10.1007/s00198-019-05260-8. [DOI] [PubMed] [Google Scholar]
- 12.Yoon B., Choi S.H., Jeong J.H., Park K.W., Kim E.J., Hwang J., Jang J., Kim J.H., Hong J.Y., Lee J., et al. Balance and Mobility Performance Along the Alzheimer’s Disease Spectrum. J. Alzheimers Dis. 2020;73:633–644. doi: 10.3233/JAD-190601. [DOI] [PubMed] [Google Scholar]
- 13.Kuan Y.C., Huang L.K., Wang Y.H., Hu C.J., Tseng I.J., Chen H.C., Lin L.F. Balance and gait performance in older adults with early-stage cognitive impairment. Eur. J. Phys. Rehabil. Med. 2021;57:560–567. doi: 10.23736/S1973-9087.20.06550-8. [DOI] [PubMed] [Google Scholar]
- 14.Cronin T., Arshad Q., Seemungal B.M. Vestibular Deficits in Neurodegenerative Disorders: Balance, Dizziness, and Spatial Disorientation. Front. Neurol. 2017;8:538. doi: 10.3389/fneur.2017.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noohi F., Kinnaird C., De Dios Y., Kofman I.S., Wood S.J., Bloomberg J., Mulavara A., Sienko K.H., Polk T.A., Seidler R.D. Age Differences in Vestibular Brain Connectivity Are Associated With Balance Performance. Front. Aging Neurosci. 2020;12:566331. doi: 10.3389/fnagi.2020.566331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiles L., Smith P.F. The vestibular-basal ganglia connection: Balancing motor control. Brain Res. 2015;1597:180–188. doi: 10.1016/j.brainres.2014.11.063. [DOI] [PubMed] [Google Scholar]
- 17.Atomi T., Noriuchi M., Oba K., Atomi Y., Kikuchi Y. Self-recognition of one’s own fall recruits the genuine bodily crisis-related brain activity. PLoS ONE. 2014;9:e115303. doi: 10.1371/journal.pone.0115303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cullen K.E. The vestibular system: Multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35:185–196. doi: 10.1016/j.tins.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malcolm B.R., Foxe J.J., Butler J.S., De Sanctis P. The aging brain shows less flexible reallocation of cognitive resources during dual-task walking: A mobile brain/body imaging (MoBI) study. NeuroImage. 2015;117:230–242. doi: 10.1016/j.neuroimage.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makizako H., Shimada H., Doi T., Park H., Yoshida D., Uemura K., Tsutsumimoto K., Liu-Ambrose T., Suzuki T. Poor balance and lower gray matter volume predict falls in older adults with mild cognitive impairment. BMC Neurol. 2013;13:102. doi: 10.1186/1471-2377-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ide R., Ota M., Hada Y., Watanabe S., Takahashi T., Tamura M., Nemoto K., Arai T. Dynamic balance deficit and the neural network in Alzheimer’s disease and mild cognitive impairment. Gait. Postur. 2022;93:252–258. doi: 10.1016/j.gaitpost.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Seidler R.D., Bernard J.A., Burutolu T.B., Fling B.W., Gordon M.T., Gwin J.T., Kwak Y., Lipps D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 24.Berg K.O., Maki B.E., Williams J.I., Holliday P.J., Wood-Dauphinee S.L. Clinical and laboratory measures of postural balance in an elderly population. Arch. Phys. Med. Rehabil. 1992;73:1073–1080. [PubMed] [Google Scholar]
- 25.Fang T.C., Chen C.M., Chang M.H., Wu C.H., Guo Y.J. Altered Functional Connectivity and Sensory Processing in Blepharospasm and Hemifacial Spasm: Coexistence and Difference. Front. Neurol. 2021;12:759869. doi: 10.3389/fneur.2021.759869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raiser T.M., Flanagin V.L., Duering M., van Ombergen A., Ruehl R.M., Zu Eulenburg P. The human corticocortical vestibular network. NeuroImage. 2020;223:117362. doi: 10.1016/j.neuroimage.2020.117362. [DOI] [PubMed] [Google Scholar]
- 27.Grüsser O.J., Pause M., Schreiter U. Localization and responses of neurons in the parieto-insular vestibular cortex of awake monkeys (Macaca fascicularis) J. Physiol. 1990;430:537–557. doi: 10.1113/jphysiol.1990.sp018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grüsser O.J., Pause M., Schreiter U. Vestibular neurons in the Parieto-insular cortex of monkeys (Macaca fascicularis): Visual and neck receptor responses. J. Physiol. 1990;430:559–583. doi: 10.1113/jphysiol.1990.sp018307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaafsma S.J., Duysens J. Neurons in the ventral intraparietal area of awake macaque monkey closely resemble neurons in the dorsal part of the medial superior temporal area in responses to optic flow patterns. J. Neurophysiol. 1996;76:4056–4068. doi: 10.1152/jn.1996.76.6.4056. [DOI] [PubMed] [Google Scholar]
- 30.Gu Y., Watkins P.V., Angelaki D.E., DeAngelis G.C. Visual and nonvisual contributions to three-dimensional heading selectivity in the medial superior temporal area. J. Neurosci. 2006;26:73–85. doi: 10.1523/JNEUROSCI.2356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu Y., Angelaki D.E., Deangelis G.C. Neural correlates of multisensory cue integration in macaque MSTd. Nat. Neurosci. 2008;11:1201–1210. doi: 10.1038/nn.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Y., Deangelis G.C., Angelaki D.E. Causal links between dorsal medial superior temporal area neurons and multisensory heading perception. J. Neurosci. 2012;32:2299–2313. doi: 10.1523/JNEUROSCI.5154-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wild B., Treue S. Primate extrastriate cortical area MST: A gateway between sensation and cognition. J. Neurophysiol. 2021;125:1851–1882. doi: 10.1152/jn.00384.2020. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt C., Baltaretu B.R., Crawford J.D., Bremmer F. A Causal Role of Area hMST for Self-Motion Perception in Humans. Cereb. Cortex Commun. 2020;1:tgaa042. doi: 10.1093/texcom/tgaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama Y., Sugawara S.K., Fukunaga M., Hamano Y.H., Sadato N., Nishimura Y. The dorsal premotor cortex encodes the step-by-step planning processes for goal-directed motor behavior in humans. NeuroImage. 2022;256:119221. doi: 10.1016/j.neuroimage.2022.119221. [DOI] [PubMed] [Google Scholar]
- 36.Chen A., DeAngelis G.C., Angelaki D.E. Representation of vestibular and visual cues to self-motion in ventral intraparietal cortex. J. Neurosci. 2011;31:12036–12052. doi: 10.1523/JNEUROSCI.0395-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Li S., Jiang D., Chen A. Response Properties of Interneurons and Pyramidal Neurons in Macaque MSTd and VPS Areas During Self-Motion. Front. Neural Circuits. 2018;12:105. doi: 10.3389/fncir.2018.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welniarz Q., Gallea C., Lamy J.C., Méneret A., Popa T., Valabregue R., Béranger B., Brochard V., Flamand-Roze C., Trouillard O., et al. The supplementary motor area modulates interhemispheric interactions during movement preparation. Hum. Brain Mapp. 2019;40:2125–2142. doi: 10.1002/hbm.24512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima C.F., Krishnan S., Scott S.K. Roles of Supplementary Motor Areas in Auditory Processing and Auditory Imagery. Trends Neurosci. 2016;39:527–542. doi: 10.1016/j.tins.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bremmer F., Klam F., Duhamel J.R., Ben Hamed S., Graf W. Visual-vestibular interactive responses in primate ventral intraparietal area (VIP) Eur. J. Neurosci. 2002;16:1569–1586. doi: 10.1046/j.1460-9568.2002.02206.x. [DOI] [PubMed] [Google Scholar]
- 41.Bremmer F., Duhamel J.R., Ben Hamed S., Graf W. Heading encoding in the macaque ventral intraparietal area (VIP) Eur. J. Neurosci. 2002;16:1554–1568. doi: 10.1046/j.1460-9568.2002.02207.x. [DOI] [PubMed] [Google Scholar]
- 42.Schlack A., Hoffmann K.P., Bremmer F. Interaction of linear vestibular and visual stimulation in the macaque ventral intraparietal area (VIP) Eur. J. Neurosci. 2022;16:1877–1886. doi: 10.1046/j.1460-9568.2002.02251.x. [DOI] [PubMed] [Google Scholar]
- 43.Chen A., Gu Y., Liu S., DeAngelis G.C., Angelaki D.E. Evidence for a Causal Contribution of Macaque Vestibular, But Not Intraparietal, Cortex to Heading Perception. J. Neurosci. 2016;36:3789–3798. doi: 10.1523/JNEUROSCI.2485-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurata K. Movement-related activity in the periarcuate cortex of monkeys during coordinated eye and hand movements. J. Neurophysiol. 2017;118:3293–3310. doi: 10.1152/jn.00279.2017. [DOI] [PubMed] [Google Scholar]
- 45.Cardin V., Smith A.T. Sensitivity of human visual and vestibular cortical regions to egomotion-compatible visual stimulation. Cereb. Cortex. 2010;20:1964–1973. doi: 10.1093/cercor/bhp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardin V., Smith A.T. Sensitivity of human visual cortical area V6 to stereoscopic depth gradients associated with self-motion. J. Neurophysiol. 2011;106:1240–1249. doi: 10.1152/jn.01120.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kosse N.M., De Groot M.H., Vuillerme N., Hortobágyi T., Lamoth C.J.C. Factors related to the high fall rate in long-term care residents with dementia. Int. Psychogeriatr. 2015;27:803–814. doi: 10.1017/S104161021400249X. [DOI] [PubMed] [Google Scholar]
- 48.Allum J.H.J. Recovery of vestibular ocular reflex function and balance control after a unilateral peripheral vestibular deficit. Front. Neurol. 2012;3:83. doi: 10.3389/fneur.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterka R.J. Sensorimotor integration in human postural control. J. Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 50.Agrawal Y., Carey J.P., Della Santina C.C., Schubert M.C., Minor L.B. Disorders of balance and vestibular function in US adults: Data from the National Health and Nutrition Examination Survey, 2001–2004. Arch. Intern. Med. 2009;169:938–944. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 51.Whitney S.L., Marchetti G.F., Pritcher M., Furman J.M. Gaze stabilization and gait performance in vestibular dysfunction. Gait. Postur. 2009;29:194–198. doi: 10.1016/j.gaitpost.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.