Key Points
Question
What is the prevalence of obesity in pediatric patients with type 2 diabetes (T2D)?
Findings
This systematic review and meta-analysis of 53 studies including 8942 participants found that 75.27% of children with T2D had obesity, and 77.24% had obesity at diagnosis. Male participants had significantly higher odds of obesity than female participants, and Asian participants had the lowest prevalence of obesity compared with other racial groups.
Meaning
In this study, not all pediatric patients with T2D had obesity; further studies are needed to elucidate the mechanisms beyond obesity driving this condition in children.
This systematic review and meta-analysis evaluates the global prevalence of obesity in pediatric type 2 diabetes, examines the association of sex and race with obesity risk, and assesses the association of obesity with glycemic control and dyslipidemia.
Abstract
Importance
The childhood obesity epidemic is presumed to drive pediatric type 2 diabetes (T2D); however, the global scale of obesity in children with T2D is unknown.
Objectives
To evaluate the global prevalence of obesity in pediatric T2D, examine the association of sex and race with obesity risk, and assess the association of obesity with glycemic control and dyslipidemia.
Data Sources
MEDLINE, Embase, CINAHL, Cochrane Library, and Web of Science were searched from database inception to June 16, 2022.
Study Selection
Observational studies with at least 10 participants reporting the prevalence of obesity in patients with pediatric T2D were included.
Data Extraction and Synthesis
Following the Meta-analysis of Observational Studies in Epidemiology reporting guideline, 2 independent reviewers in teams performed data extraction and risk of bias and level of evidence analyses. The meta-analysis was conducted using a random-effects model.
Main Outcomes and Measures
The primary outcomes included the pooled prevalence rates of obesity in children with T2D. The secondary outcomes assessed pooled prevalence rates by sex and race and associations between obesity and glycemic control and dyslipidemia.
Results
Of 57 articles included in the systematic review, 53 articles, with 8942 participants, were included in the meta-analysis. The overall prevalence of obesity among pediatric patients with T2D was 75.27% (95% CI, 70.47%-79.78%), and the prevalence of obesity at diabetes diagnosis among 4688 participants was 77.24% (95% CI, 70.55%-83.34%). While male participants had higher odds of obesity than female participants (odds ratio, 2.10; 95% CI, 1.33-3.31), Asian participants had the lowest prevalence of obesity (64.50%; 95% CI, 53.28%-74.99%), and White participants had the highest prevalence of obesity (89.86%; 95% CI, 71.50%-99.74%) compared with other racial groups. High heterogeneity across studies and varying degrees of glycemic control and dyslipidemia were noted.
Conclusions and Relevance
The findings of this systematic review and meta-analysis suggest that obesity is not a universal phenotype in children with T2D. Further studies are needed to consider the role of obesity and other mechanisms in diabetes genesis in this population.
Introduction
In the past few decades, type 2 diabetes (T2D) in children and adolescents has emerged in conjunction with increasing pediatric obesity rates globally.1,2,3,4,5,6,7,8 Children and youth living with obesity also have a higher risk of developing T2D as adults when compared with children with reference range weight, which may contribute to increased cardiovascular risk.9
T2D is an aggressive disease in children with high treatment failure rates. It has early comorbidities and complications, including nonalcoholic fatty liver disease, dyslipidemia, polycystic ovary syndrome, and nephropathy.9,10,11,12,13,14,15,16
While the complex weave of factors driving the pathogenesis of pediatric T2D are not yet fully defined,17,18,19 the biopsychosocial determinants of health with health inequities and social and economic vulnerabilities in this population play an important role in disease risk and outcomes.20 Obesity is a major trigger for screening for T2D in clinical practice,21,22,23 yet the prevalence of obesity in the pediatric T2D population is unknown. It is important to recognize whether T2D is diagnosed through ascertainment bias, whereby only children with obesity are screened and subsequently diagnosed with T2D. If obesity is not a universal phenotype in T2D, there may be children with reference range body mass measures in whom T2D is driven by factors other than obesity, which impacts their treatment and outcomes. Estimating the prevalence of obesity in the pediatric T2D population may have a significant impact on the recommendations of screening guidelines for the disease.
This systematic review and meta-analysis aimed to evaluate the global prevalence of obesity in children and adolescents living with T2D and assess the association of sex and race with obesity prevalence in this population. Furthermore, we explored the association of obesity with T2D-related metabolic profiles, including glycemic control and lipid homeostasis.
Methods
Protocol and Registration
This systematic review was registered with the International Prospective Register of Systematic Reviews (CRD42018091127).24 This review is reported as per the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist.25
Eligibility Criteria
Primary observational studies (cross-sectional, retrospective cohort, or prospective cohort) with a sample size of at least 10 participants reporting the prevalence of obesity in children 18 years or younger with T2D were included. T2D diagnostic criteria were (1) random plasma glucose of at least 200.0 mg/dL and presence of classical symptoms (to convert glucose to millimoles per liter, multiply by 0.0555), (2) fasting plasma glucose of at least 127.9 mg/dL, or (3) 2-hour plasma glucose of at least 200.0 mg/dL in response to oral glucose tolerance test and the absence of pancreatic autoantibodies.21,22,23 We incorporated studies that used age- and sex- adjusted body mass index (BMI)–based measures to define overweight and obesity, with BMIs in the 85th percentile or greater to less than the 95th percentile defining overweight and BMIs in the 95th percentile or greater defining obesity.26 Studies with different definitions of obesity were included but removed in the sensitivity analysis to assess their impact on the results.
We excluded studies reporting on participants with gestational diabetes. If study reports involved serial data publication, we included the report with the largest sample size.
Literature Searches
The literature searches encompassed journal articles, conference abstracts, and gray literature. No language- or time-based restrictions were applied, but the searches were restricted to human studies.
Search strategies were developed by a senior health sciences librarian and conducted in MEDLINE, Embase, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews from the date of inception (eTables 1-5 in Supplement 1). The gray literature searches were conducted in clinicaltrials.gov and Web of Science: Conference Proceedings Citation Index—Science. The initial search was performed on December 14, 2017; updated searches were conducted on February 1, 2019, and June 16, 2022.
In addition, we searched the references of the articles screened for full-text eligibility to retrieve studies for inclusion. We searched for full-text publications where conference abstracts were eligible; if not located, we contacted the principal investigators to determine publication status and obtain relevant data for the analyses.
Study Selection and Data Collection
Two independent reviewers in pairs (M.C., J.D., A.N., M.H., Y.Q., S.S.J.C., A.R., P.P.T., and F.Z.) screened titles, abstracts, and full-text articles and completed data abstraction. Reviewers resolved any differences at all data assessment stages through discussions, and a third reviewer (M.C.S.) resolved persistent disagreements. A standardized data abstraction form was developed and piloted specifically for this study. The data collected included the authors’ names; title; year of publication; country; study design; age at T2D diagnosis; age at study enrollment; diabetes duration; sample size; prevalence of reference-range weight, overweight, and obesity; and hemoglobin A1c (HbA1c) values to assess glycemic control. We also collected data on participants’ lipid profiles to assess for dyslipidemia, including triglycerides, total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol. In addition, we extracted the sex, race and ethnicity, and specific obesity prevalence data when available. If longitudinal studies reported obesity prevalence at multiple time points, we abstracted the data closest to the date of T2D diagnosis. We contacted the principal investigators to retrieve missing data.
Risk of Bias and Level of Evidence Assessment
Two independent reviewers (M.C. and J.D.) assessed the risk of bias using a validated tool developed by Hoy et al.27 A third reviewer (M.C.S.) arbitrated persistent disagreements. The overall level of evidence was assessed according to the Oxford Centre for Evidence-Based Medicine criteria.28
Statistical Analysis
A random-effects meta-analysis was performed when 2 or more eligible studies of similar design, methods, populations, and outcomes were identified.29,30 The primary outcome for this systematic review was assessing the overall pooled global prevalence of obesity in T2D. Because studies in which prevalence trended toward 0% or 100% may affect the meta-analysis, each study’s prevalence values were transformed using the Freeman-Tukey double arcsine method, and the results were then converted back to prevalence estimates for interpretation.30
Both inconsistency index (I2) and χ2 test P values were used to quantify heterogeneity. An I2 value of greater than 75% and 1-sided P < .10 were considered significant.31
Subgroup analyses were performed by sex and race, if data were available.5,13,32 When 2 or more studies reported the prevalence of obesity, we evaluated a pooled prevalence for each sex and pooled odds ratio (OR). We used the National Institutes of Health definitions of racial and ethnic groups to categorize the included studies and used the term Indigenous to refer to Indigenous populations in North America.33
Metaregression analyses were performed to examine the associations between the prevalence of obesity and mean HbA1c as a measure of glycemic control and dyslipidemia. In addition, we conducted sensitivity analyses by removing conference abstracts with no associated full-text publications, sample sizes smaller than 50, studies with mixed ages when pediatric-only data could not be obtained, and those that used different obesity definitions. Subgroup and sensitivity analyses were to be conducted if at least 10 studies were included in the meta-analysis for the specific outcomes.31 We also performed post hoc sensitivity analyses by excluding studies with inclusion criteria of overweight, those with unspecified or unclear diabetes diagnostic criteria, those with patients who had weight loss at presentation or positive pancreatic autoantibodies, those that did not explicitly assess for and exclude maturity-onset diabetes of the young (MODY), and those that were not population based.
A contour-enhanced funnel plot was used to investigate publication bias. The Egger test and visual inspection were used to assess plot asymmetry.31
The meta-analysis and forest plots were generated using the metafor package in RStudio, version 1.1.383, using the R language version 3.4.3 (R Project for Statistical Computing).34,35,36 The forest plots for the OR by sex meta-analysis were generated using Review Manager version 5.3 software.37
Results
Study Selection
The study screening and selection process is illustrated in the flow diagram (eFigure 1 in Supplement 1). We screened 13 449 nonduplicated records, and 57 studies from unique populations were included in the review. Fifty-three studies, with 8942 participants, were included in our meta-analysis.
Study Characteristics
Overall, 26 studies (45.60%) had a cross-sectional design,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63 23 (40.40%) were retrospective cohort studies,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86 and 8 (14.00%) were prospective cohort studies.3,11,87,88,89,90,91,92 eTable 6 in Supplement 1 reports the characteristics of the included studies.
Of the 57 studies, 12 did not report specific diabetes diagnostic criteria,43,49,59,64,69,70,72,75,76,78,84,91 and 18 did not report measuring autoantibodies.44,49,58,59,62,65,69,70,73,74,75,76,77,78,79,83,84,85 Of the studies reporting autoantibody testing results, 55 patients had positive tests.
The most common clinical presentations included acanthosis nigricans, polyuria, and polydipsia. The most commonly reported risk factors included family history of T2D and maternal gestational diabetes. Most patients were treated with oral hypoglycemic agents, and some were treated with insulin, diet alone, or combination therapies (eTable 7 in Supplement 1).
Several studies did not separate the diagnosis by age and included participants older than 18 years, highlighting the different definitions of pediatric age groups globally. We included some of these studies as long as most study participants were younger than 18 years.
Pooled Prevalence of Obesity
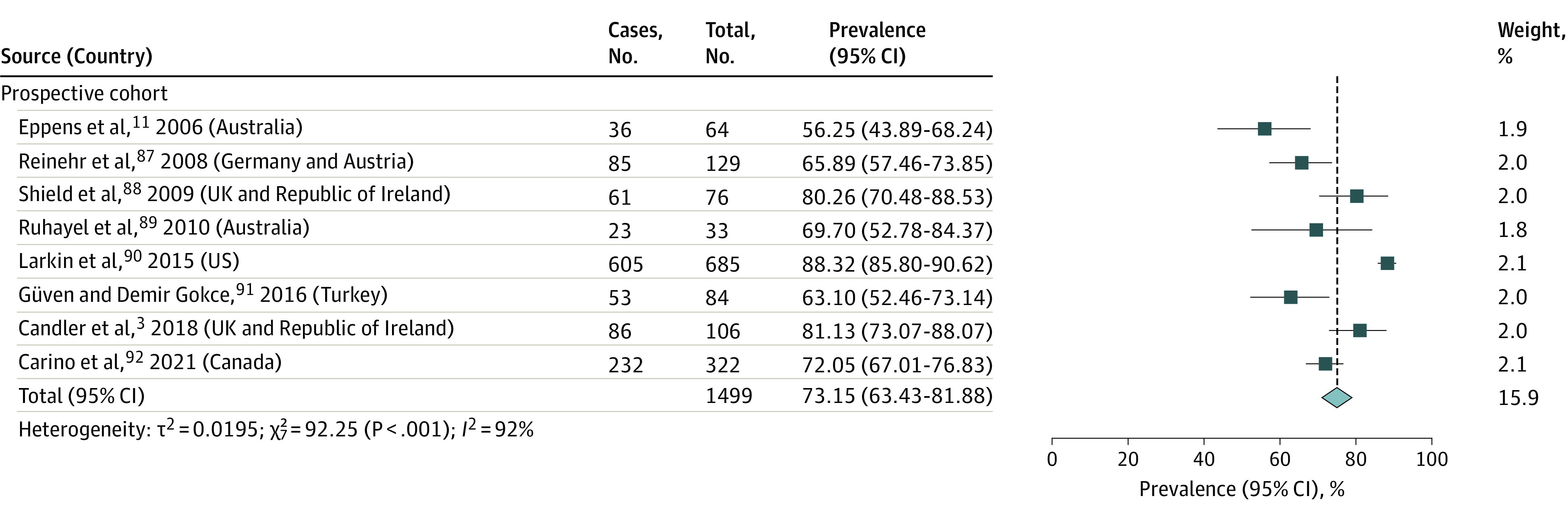
Data from 53 studies with 8942 participants estimated the overall pooled prevalence of obesity in pediatric patients with T2D to be 75.27% (95% CI, 70.47%-79.78%; I2 = 96%; P < .001) (Figure 1 and Figure 2).3,11,38,39,40,41,42,43,44,45,46,47,48,49,50,51,55,56,57,58,59,60,61,62,63,64,65,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92 Four of the 57 studies were not included in this meta-analysis, with 1 study not providing an exact prevalence estimate,66 and 3 containing race-based subgroup data already reported in another article.51,52,53,54 Obesity prevalence was similar across study designs (cross-sectional studies38,39,40,41,42,43,44,45,46,47,48,49,50,51,55,56,57,58,59,60,61,62,63: 76.27%; 95% CI, 67.04%-84.46%; I2 = 96%; P < .001; n = 2444; retrospective cohort studies64,65,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86: 75.18%; 95% CI, 67.92%-81.81%; I2 = 96%; P < .001; n = 4999; prospective cohort studies3,11,87,88,89,90,91,92: 73.15%; 95% CI, 63.43%-81.88%; I2 = 92%; P < .001; n = 1499) (Figure 1 and Figure 2).
Figure 1. Pooled Obesity Prevalence in Cross-sectional and Retrospective Cohort Studies of Pediatric Type 2 Diabetes, by Study Design.
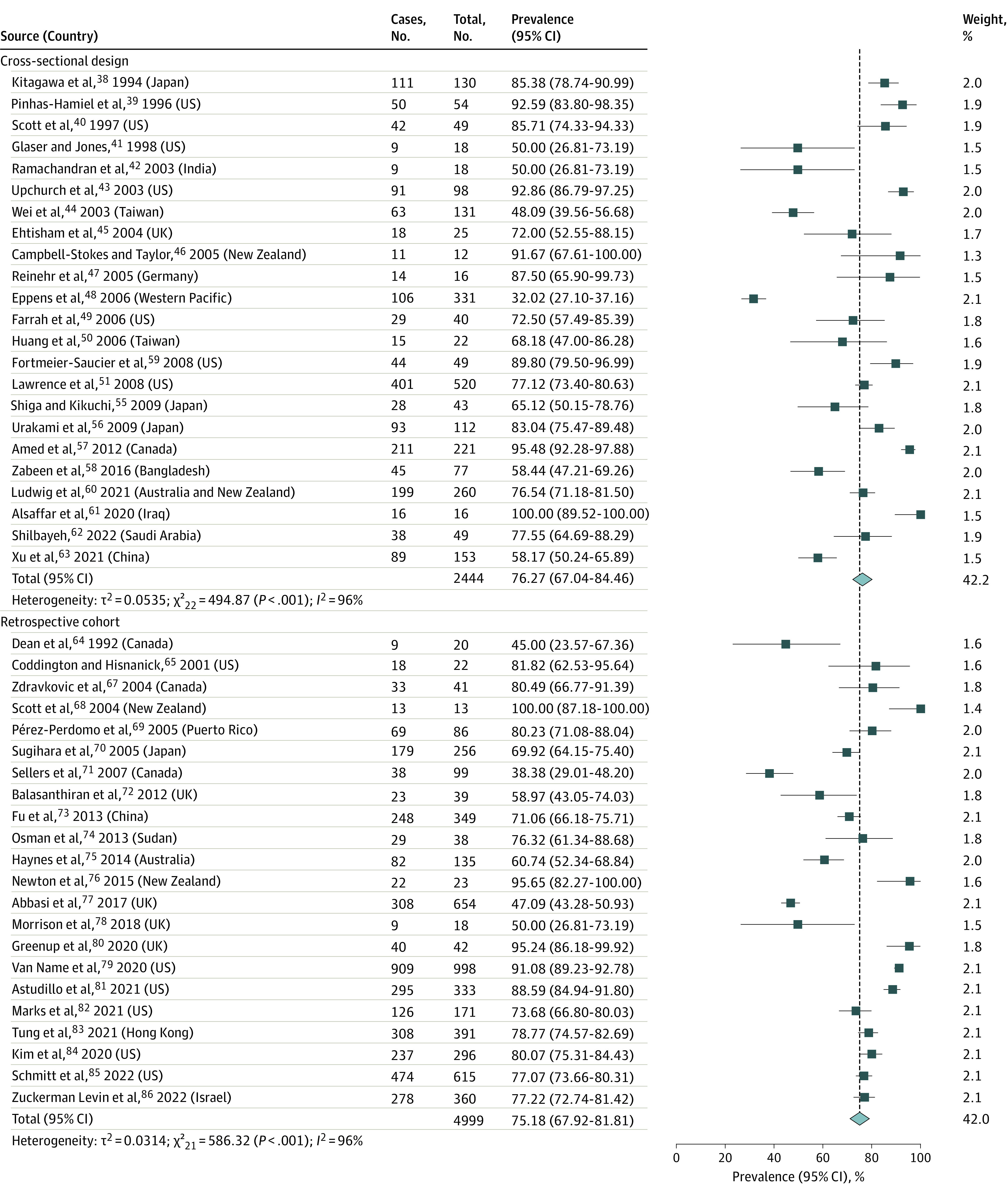
Figure 2. Pooled Obesity Prevalence in Prospective Cohort Studies of Pediatric Type 2 Diabetes.
Pooled Prevalence of Obesity at T2D Diagnosis
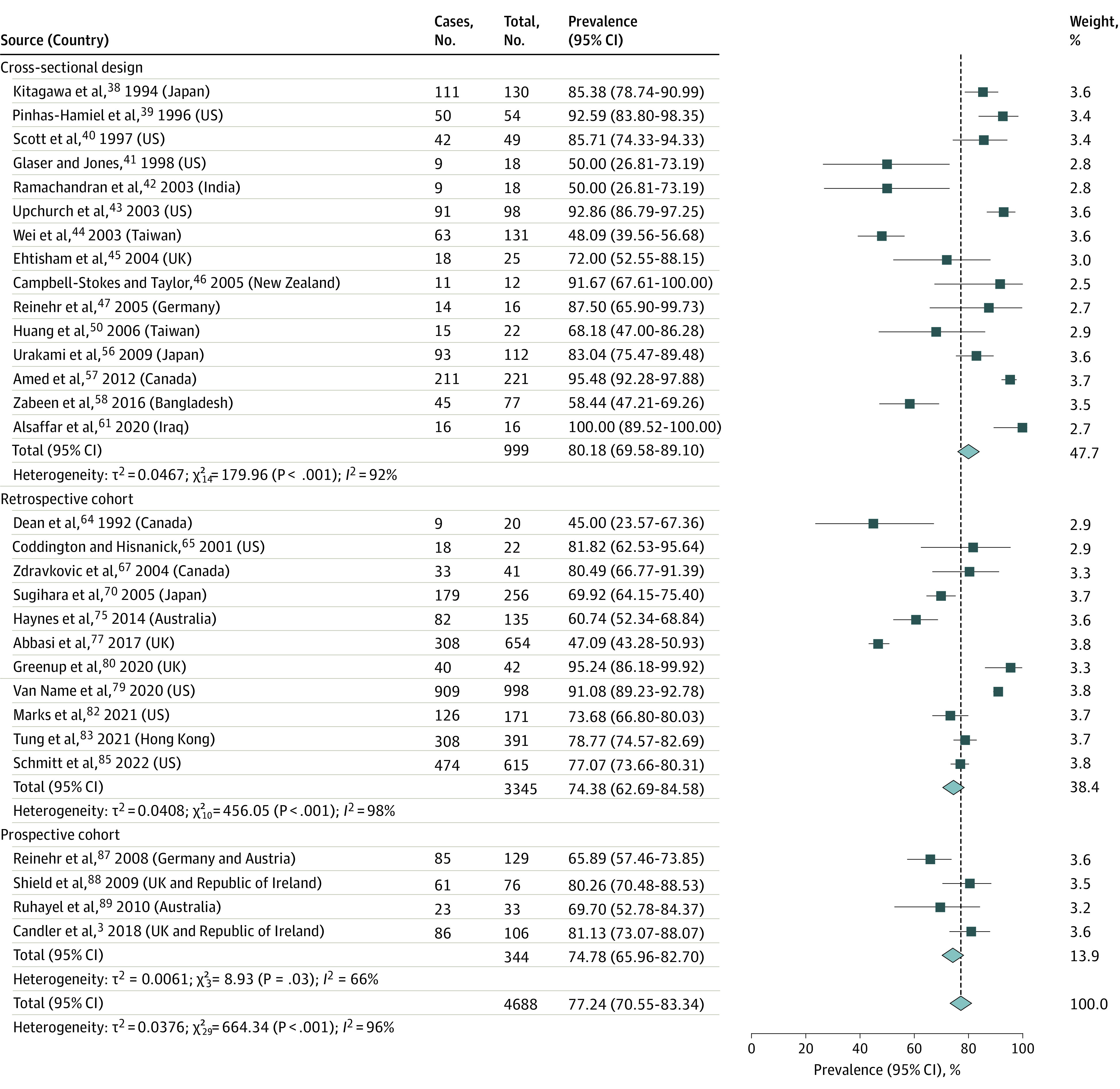
Data from 30 studies with 4688 participants reported an obesity prevalence in T2D at diagnosis of 77.24% (95% CI, 70.55%-83.34%; I2 = 96%; P < .001) (Figure 3).3,38,39,40,41,42,43,44,45,46,47,50,56,57,58,61,64,65,67,70,75,77,79,80,82,83,85,87,88,89 The pooled prevalence in cross-sectional studies was 80.18% (95% CI, 69.58%-89.10%; I2 = 92%; P < .001; n = 999)38,39,40,41,42,43,44,45,46,47,50,56,57,58,61 and 74.38% (95% CI, 62.69%-84.58%; I2 = 98%; P < .001; n = 3345) for retrospective cohort studies.3,64,65,67,70,75,77,79,80,82,83,85,87,88,89 The meta-analysis of prospective cohort studies found a prevalence of 74.78% (95% CI, 65.96%-82.70%; I2 = 66%; P = .03; n = 344).3,87,88,89
Figure 3. Pooled Obesity Prevalence at Pediatric Type 2 Diabetes Diagnosis Across All Included Studies, by Study Design.
As these data indicated that some patients with T2D did not have obesity, we further characterized this population. There were wide variations in the prevalence of overweight and reference-range weight in the included studies (eTable 8 in Supplement 1). The prevalence of overweight ranged from 0.0% to 43.40% and normal weight from 0.0% to 43.60%.3,11,40,42,43,45,48,49,50,51,52,53,54,58,60,63,69,71,72,74,75,77,78,79,81,83,84,87,88,89,92
When assessing glycemic control and lipid homeostasis, studies reported a broad range of HbA1c levels (4.5%-12.6% [to convert to proportion of total hemoglobin, multiply by 0.01]). Metaregression analysis revealed no significant correlations between obesity prevalence and mean HbA1c levels (eTable 8 in Supplement 1).
The prevalence of dyslipidemia was 4.0% to 87.5% across 31 studies, with a mixed dyslipidemia profile including hypertriglyceridemia, high LDL cholesterol levels, and low HDL cholesterol levels. The metaregression analysis indicated significant associations between obesity and low HDL cholesterol levels (P = .04), but not hypercholesterolemia, hypertriglyceridemia, or elevated LDL cholesterol levels.
Subgroup Analyses by Sex and Race
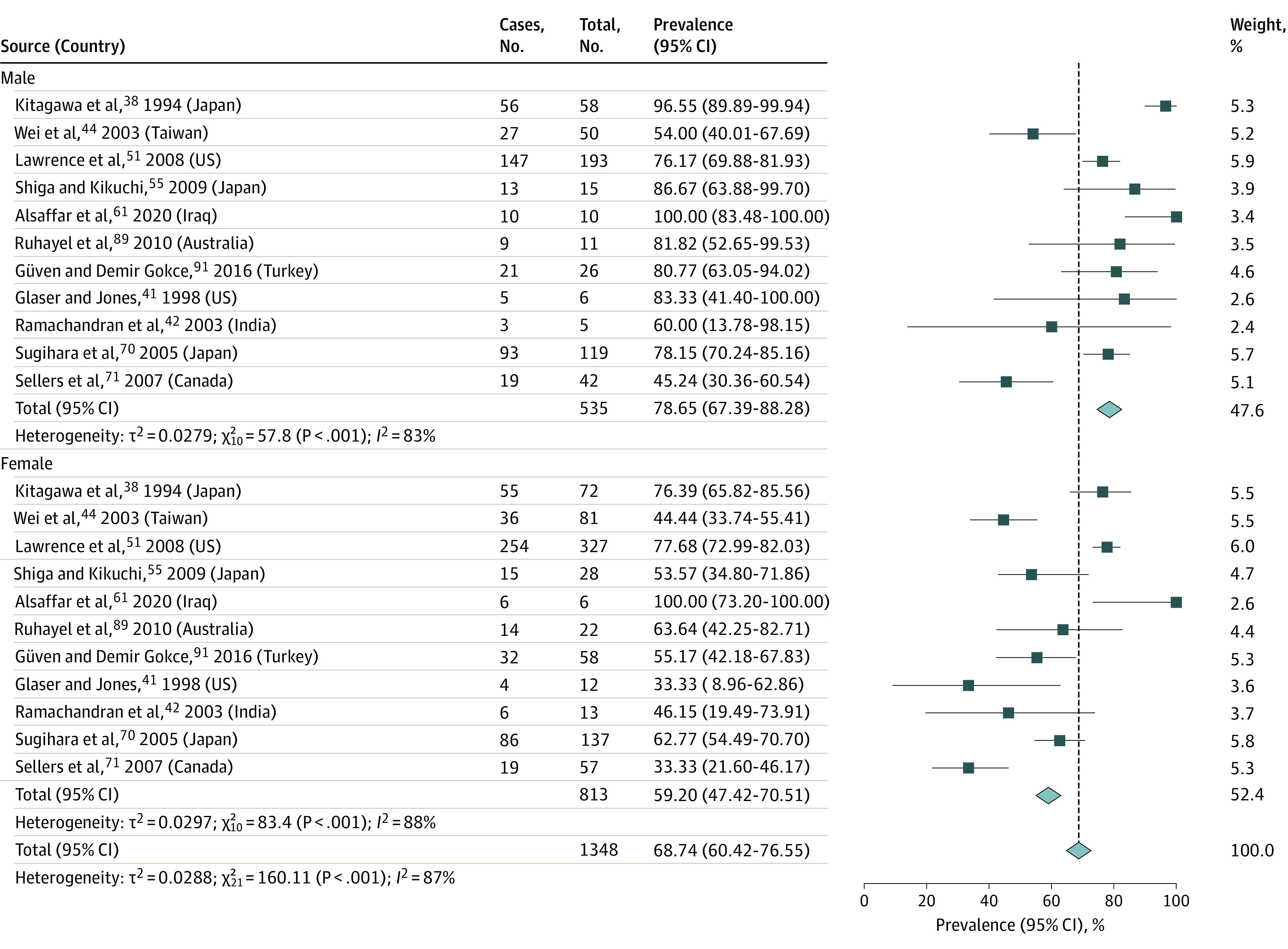
The pooled prevalence of obesity in male participants with T2D was 78.65% (95% CI, 67.39%-88.28%; I2 = 83%; P < .001, n = 535), and the estimate was lower in female participants, at 59.20% (95% CI, 47.42%-70.51%; I2 = 88%; P < .001; n = 813) (Figure 4). The pooled OR of obesity prevalence for male vs female participants was 2.10 (95% CI, 1.33-3.31; I2 = 52%; P = .03) (eFigure 2 in Supplement 1).38,41,42,44,51,55,61,70,71,89,91
Figure 4. Prevalence of Obesity in Pediatric Type 2 Diabetes by Sex.
For race-reported data, the pooled obesity prevalence was 89.86% (95% CI, 71.50%-99.74%; I2 = 87%; P < .001; n = 178) in White patients,47,52,57 84.47% (95% CI, 77.64%-90.37%; I2 = 0%; P = .75; n = 144) in African American and African Canadian patients,53,67 82.19% (95% CI, 58.89%-97.46%; I2 = 88%; P < .001; n = 149) in Middle Eastern patients,61,62,91 81.30% (95% CI, 65.32%-93.46%; I2 = 75%; P = .02; n = 194) in Hispanic and Latino patients,41,53,59 76.73% (95% CI, 57.47%-91.73%; I2 = 87%; P < .001; n = 220) in Indigenous patients,53,57,64,65 and 64.50% (95% CI, 53.28%-74.99%; I2 = 95%; P < .001; n = 1661) in Asian patients (Figure 5).38,42,44,48,50,54,55,56,58,63,67,70,73 One study reported the prevalence of obesity at 72.20% in a mixed population of Asian and Pacific Islander patients (11 total patients),54 and another reported a prevalence of 64.70% in 51 Australian Indigenous patients and 93.50% in 49 Māori youths.60
Figure 5. Prevalence of Obesity in Pediatric Type 2 Diabetes by Race.
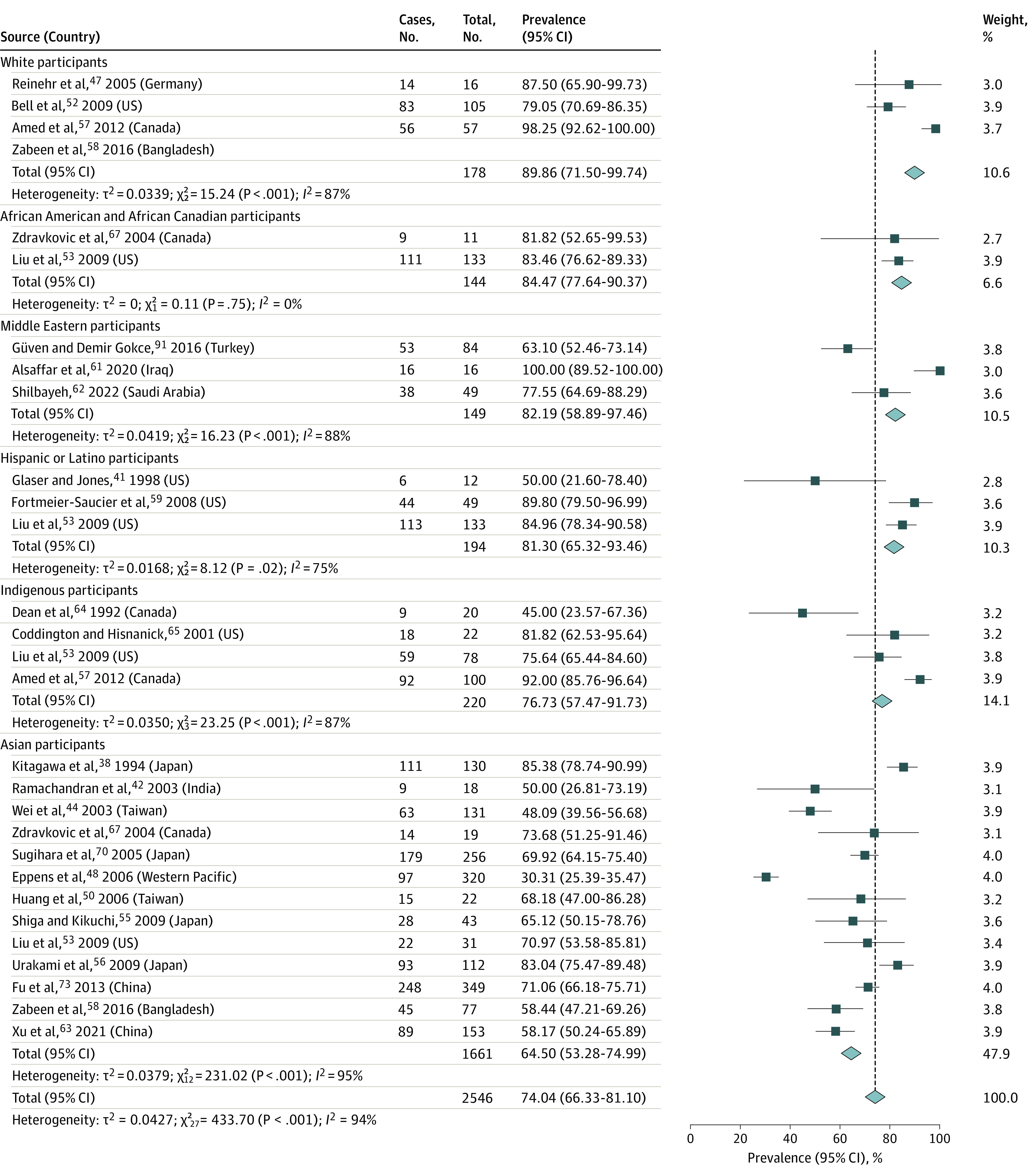
Region-based analysis revealed that North America had the highest prevalence of obesity in patients with T2D at 81.14% (95% CI, 75.99%-85.83%; I2 = 94%; P < .001; n = 4779).39,40,41,43,49,51,57,59,64,65,67,69,71,79,80,81,82,84,85,90,92 The Middle East had the second highest, at 78.41% (95% CI, 68.29%-87.12%; I2 = 77%; P < .001; n = 547),61,62,74,86,91 followed by Oceania at 74.03% (95% CI, 55.52%-89.15%; I2 = 96%; P < .001; n = 871),11,46,48,60,68,75,76,89 Asia at 68.54% (95% CI, 61.16%-75.51%; I2 = 89%; P < .001; n = 1682),4,38,42,50,55,56,58,63,70,73,83 and Europe at 68.30% (95% CI, 54.84%-80.43%; I2 = 92%, P < .001, n = 1063) (eFigure 3 in Supplement 1).3,45,47,72,77,78,87,88
We also analyzed the prevalence of reference-range BMI measures in patients with T2D by region. The highest prevalence was in studies from Oceania (16.43%; 95% CI, 5.37%-31.51%; I2 = 95%; P < .001; n = 836)11,48,60,68,75,89 and Asia (13.95%; 95% CI, 4.52%-26.93%; I2 = 91%; P < .001; n = 661),42,50,58,63,83 whereas Europe (9.52%; 95% CI, 0.46%-25.83%; I2 = 97%; P < .001; n = 1063),3,45,47,72,77,78,87,88 the Americas (4.21%; 95% CI, 1.55%-7.93%; I2 = 95%; P < .001; n = 3568)40,43,49,51,69,71,79,80,81,84,90,92 and the Middle East (1.26%; 95% CI, 0.00%-7.32%; I2 = 0%; P < .001; n = 54)61,74 had lower prevalence (eFigure 4 in Supplement 1).
Sensitivity Analyses
The sensitivity analyses assessed whether the diagnostic criteria for obesity and diabetes, autoimmunity, or the potential for the initial weight loss at diabetes presentation would affect obesity prevalence. There were no studies with a high risk of bias.
Most studies used the 95th percentile of BMI for age and sex to define obesity.26 However, some studies used the adult obesity cutoff (BMI [calculated as weight in kilograms divided by height in meters squared] ≥30), and some did not report the obesity definition used. These studies were removed in the sensitivity analysis (eTable 9 in Supplement 1).38,39,41,42,47,49,55,56,57,61,70,72,75,78,83,85,91 Three studies enrolled only patients with overweight or obesity.47,79,90 We conducted another sensitivity analysis for prevalence estimates excluding these studies. The overall pooled prevalence differed very slightly, with substantial heterogeneity noted.
Another sensitivity analysis excluded studies with uncertain or unspecified T2D diagnostic criteria.43,49,51,56,59,64,69,70,72,75,76,78,84,91 We also performed sensitivity analyses excluding patients with positive tests for islet cell, glutamic acid decarboxylase, and islet tyrosine phosphatase 2 pancreatic autoantibodies (n = 55).43,45,46,64,80,91 The results of these analyses led to a pooled prevalence of obesity in the pediatric T2D population of 74.81% (95% CI, 69.72%-79.59%; I2 = 96%; P < .001) (eTable 9 in Supplement 1). We also excluded studies of patients who presented with weight loss.3,39,40,43,49,65,67,72,74,80,82,83,84,86 The pooled obesity prevalence was 72.87% (95% CI, 66.58%-78.75%, I2 = 97%, P < .001). We also performed a sensitivity analysis removing studies that specifically excluded patients with MODY based on genetic testing results,3,39,45,51,60,72,80,82,86,87,88 and the pooled prevalence was 78.87%; (95% CI, 74.70%-82.77%; I2 = 85%; P < .001). In conclusion, our results did not differ significantly with any of these sensitivity analyses.
Publication Bias
No publication bias was identified for the prevalence of obesity at study visit or diagnosis from the funnel plots or Egger tests. eFigures 5 and 6 in Supplement 1 present these analyses.
Risk of Bias Within Studies
Studies had either a low (n = 32)3,38,39,44,45,46,48,50,51,52,53,58,60,61,62,63,64,65,67,70,71,73,74,77,81,83,84,85,86,88,90,92 or moderate risk of bias (n = 25)11,40,41,42,43,47,49,54,55,56,57,59,66,68,69,72,75,76,78,79,80,82,87,89,91 (eTable 10 in Supplement 1). Risk of bias was present in studies with sampling frames that were not a close representation of the target population, likely driven by the rarity of the diagnosis of T2D in children38,40,42,43,48,49,55,56,57,59,66,69,72,78,80,91 or used convenience sampling instead of a census or random sample selection.40,43,49,55,56,57,59,66,78,79,80,90,91
Some studies had 25% or higher rates of missing data, potentially indicating a nonresponse bias.11,43,47,54,60,62,63,66,69,70,75,76,82,87,89 In some studies, it was unclear that all individuals were examined using the same methods, as participants were tested in different clinics with no reported standardized protocols.3,41,46,57,68,69,77,79,80,87,88 Most studies only assessed obesity in patients in a particular city or clinic.11,39,40,41,42,43,47,49,50,52,54,55,56,58,59,61,64,65,66,67,68,71,72,74,75,76,78,80,81,82,84,85,89,91
Level of Evidence
Based on Oxford Centre for Evidence-Based Medicine criteria, 28 studies (49.1%) had a level of evidence of 1,3,11,38,39,44,48,51,52,53,54,58,60,63,69,70,71,73,75,77,81,82,83,84,85,86,87,88,92 16 (28.1%) had a level of evidence of 2,41,42,45,46,47,50,61,62,64,65,67,68,72,74,76,89 and 13 (22.8%) had a level of evidence of 3.40,43,49,55,56,57,59,66,78,79,80,90,91 A significant portion of studies did not use a random sample or census to estimate prevalence, which may limit the assessment of the level of evidence of the prevalence estimate.
Discussion
Childhood obesity is a global health crisis affecting approximately 340 million children and is a major driver of T2D risk.93,94,95 Understanding the contribution of body mass to the evolution of insulin resistance, glucose intolerance, and T2D and its comorbidities and complications is crucial for creating personalized interventions to improve outcomes.
While acknowledging the low to moderate risk of bias, variable levels of evidence, and high heterogeneity, up to 1 in 4 children with T2D do not have obesity, and some have reference-range body mass measures. While the obvious conclusions of the analysis are that there are limitations of BMI-based measures to predict diabetes and that mechanisms beyond obesity are involved in T2D evolution in children, the selection for screening of at-risk children to establish the diagnosis becomes more complex. Guidelines generally look for elevated body mass measures as a main screening indication. While factors such as ethnicity and in utero exposure to diabetes are already combined with BMI-based measures to justify screening, more sophisticated prediabetes and diabetes prediction models are needed to justify a broader screening approach. These models may need to incorporate family history, in utero exposure to diabesity, lifestyle factors, hormones, puberty, growth, sex, race and ethnicity, markers of insulin resistance, insulin production capacity, and others to refine the identification of those in need of screening.21,22,23
The 2 main mechanisms driving T2D include insulin resistance and insulin deficiency. In children with T2D, beta cell dysfunction manifests with substantial impairments in first- and second-phase insulin secretion,96,97,98 and children with T2D and normal weight have lower insulin secretory capacity than patients with T2D and obesity.99 The decline in beta cell function in children with T2D is 20% or greater per annum, which is almost double the rate seen in adult T2D.97,100,101,102,103,104 Patients with positive autoimmunity have more severe insulin deficiency compared with patients with autoantibody-negative T2D, who are more likely to have severe insulin resistance.105 The pathogenic mechanisms driving diabetes in these 2 subgroups may be different and need further study.
Recent evidence from adult studies suggests that adult diabetes subtypes can be classified based on age, BMI, diabetes-related autoantibodies, HbA1c, islet function, and insulin resistance.106 This classification system defined several patient subpopulations, including those with normal BMI with insulin deficiency with or without islet autoimmunity, those with high BMI with or without severe insulin resistance, and a mild form of diabetes of old age.106 Similarly, there may be subtypes of pediatric T2D in which children may or may not have obesity and autoimmunity, with varying degrees of metabolic end-organ insulin resistance or defects in beta cell insulin secretion. These phenotypes may be driven by glucolipotoxicity, genetic defects of beta cells, epigenetics, autoimmunity, and inflammation as drivers of diabetes risk. Further studies are needed to define the different potential subgroups of children with T2D.
While it is already known that more girls develop T2D than boys,1,2,3,4,5 our data suggest that boys with T2D were more likely to have obesity than girls. The mechanisms driving sex differences in T2D risk in children are not fully understood.107 Increased adiposity and insulin resistance are physiological changes during puberty, and increased weight during puberty may be driven by and contribute to hyperinsulinemia.107 However, obesity is likely one factor that augments peripubertal insulin resistance and may contribute to diabetes risk.107
Although patients of other racial and ethnic groups are at a higher risk of T2D than White patients,2,108 there were only a few studies that reported on the prevalence of obesity in different subpopulations, with some overlap of confidence intervals. Within this limitation, Asian children with T2D tended to have a lower prevalence of obesity than the other racial groups; there is evidence that these children develop T2D at lower BMI levels than other groups.18 There are subgroups of children in Japan with a nonobese, nonautoimmune phenotype with T2D and reduced insulin secretion with insulin resistance, and female patients with a history of low birth weight are at particular risk.109 Having a higher total and visceral adiposity than other groups are postulated mechanisms driving T2D in this population.19
While African American and Hispanic and Latino children have higher rates of T2D than White children, these populations have similar rates of obesity with T2D. Further analysis is needed to understand the mechanisms driving racial and ethnic variations in T2D risk.
The identification of patients with T2D and normal body mass defines a path to T2D genesis in which obesity is not a factor. It is likely that obesity-independent insulin secretory defects and insulin resistance and other factors play important roles in the development of diabetes in this group, and further analyses of this group are essential.
A trend that emerged from the analysis was that most studies reported a mean or median HbA1c level greater than 7.0%, which is higher than targeted glycemic control.21,22 These results confirm the challenges in achieving adequate glycemic control in this population, the attainment of which can reduce diabetes-related complications such as retinopathy and albuminuria.110,111
We did not identify significant associations between the prevalence of obesity and dyslipidemia or HbA1c levels in patients with pediatric T2D. As not all studies reported data on HbA1c levels and dyslipidemia, it is possible that there was insufficient power to detect a significant association or that obesity-driven insulin resistance has an indirect association with dyslipidemia. Insulin resistance disrupts hepatic fatty acids flux, reduces muscle fatty acid uptake, and upregulates adipose tissue lipolysis due to resistance to the antilipolytic effects of insulin that can propagate dyslipidemia.112
Limitations
This study has limitations. One limitation of the study is the high heterogeneity and that not all studies reported on the exclusion of MODY or other forms of diabetes. The high heterogeneity encountered affects the certainty of our estimate. While subgroup analysis by racial and ethnic groups did identify different prevalence values for different races, this analysis did not fully explain the heterogeneity, and thus, factors beyond race and ethnicity likely affect the association of obesity and T2D. Patients with MODY tend to have lower BMI than those with T2D,113 so if patients had MODY, that could lower the obesity prevalence estimate. However, it is unlikely that a large enough proportion of patients had MODY to affect our results, given that MODY is rare, its diagnosis requires the fulfillment of certain diagnostic criteria, and there are practical and cost considerations that limit having large screening programs for MODY in the T2D population.21 The sensitivity analyses demonstrated similar prevalence of obesity in T2D when studies with no genetic testing for MODY were removed (eTable 9 in Supplement 1), so it is unlikely that this issue has significant implications on the results.
Importantly, as clinical guidelines generally use overweight and obesity as one of the main criteria to screen for T2D in children,21,22,23 it is possible that the prevalence value is underestimated due to the likelihood that children with normal body mass are not necessarily screened for T2D. However, population-based screening is not cost-effective in most parts of the world, and clarification of the screening criteria is warranted to include those with normal body mass. In addition, the expansion of the visceral adipose compartment is a crucial risk factor for developing T2D independent of BMI and total adiposity.114,115,116 There were no data on visceral adiposity in the included studies, and this possibility requires further study.
Conclusions
In this study, while obesity was an important risk factor for the development of T2D in children, not all patients with T2D had obesity. Screening for and diagnosing T2D may consider obesity as a risk factor for T2D but not a prerequisite to screening when other risk factors are present.
Understanding the causes of T2D in children without obesity is crucial to define the etiology of their diabetes and to create effective management strategies for this cohort. Further research is needed to evaluate the causes of sex- and race and ethnicity–based associations of diabetes with obesity and explore additional factors that may affect the risk of developing T2D apart from obesity in children.
eTable 1. Search Strategy: MEDLINE
eTable 2. Search Strategy: Embase
eTable 3. Search Strategy: CINAHL
eTable 4. Search Strategy: Cochrane Library: Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews
eTable 5. Search Strategy: Web of Science: Conference Proceedings Citation Index-Science
eTable 6. Characteristics of Included Studies
eTable 7. Symptoms at Presentation and Risk Factors of Patients in Included and Type 2 Diabetes Diagnostic Criteria Used Across Studies
eTable 8. Prevalence of Type 2 Diabetes in Patients Without Obesity and Association With Glycemic Control and Dyslipidemia
eTable 9. Results of Sensitivity Analyses
eTable 10. Risk of Bias of Included Studies
eFigure 1. Study Flow Diagram
eFigure 2. Forest Plot Illustrating Odds Ratio of Obesity in Pediatric T2DM by Sex
eFigure 3. Forest Plot Illustrating Prevalence of Obesity in Pediatric T2DM by Region
eFigure 4. Forest Plot Illustrating Prevalence of Normal BMI-Based Measures in Pediatric T2DM by Region
eFigure 5. Funnel Plot for Publication Bias for Pooled Prevalence of Obesity in Pediatric Type 2 Diabetes
eFigure 6. Funnel Plot for Publication Bias for Pooled Prevalence of Obesity at Type 2 Diabetes Diagnosis in Patients With Pediatric Type 2 Diabetes
eReferences.
Data Sharing Statement
References
- 1.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146(5):693-700. doi: 10.1016/j.jpeds.2004.12.042 [DOI] [PubMed] [Google Scholar]
- 2.Amed S, Dean HJ, Panagiotopoulos C, et al. Type 2 diabetes, medication-induced diabetes, and monogenic diabetes in Canadian children: a prospective national surveillance study. Diabetes Care. 2010;33(4):786-791. doi: 10.2337/dc09-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH. Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabet Med. 2018;35(6):737-744. doi: 10.1111/dme.13609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tajima N, Morimoto A. Epidemiology of childhood diabetes mellitus in Japan. Pediatr Endocrinol Rev. 2012;10(suppl 1):44-50. [PubMed] [Google Scholar]
- 5.Dabelea D, Mayer-Davis EJ, Saydah S, et al. ; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778-1786. doi: 10.1001/jama.2014.3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116(2):473-480. doi: 10.1542/peds.2004-2536 [DOI] [PubMed] [Google Scholar]
- 7.Fryar CD, Carroll MD, Ogden CL; Division of Health and Nutrition Examination Surveys . Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2015–2016. September 2018. Accessed November 9, 2022. https://www.cdc.gov/nchs/data/hestat/obesity_child_15_16/obesity_child_15_16.pdf
- 8.Fang X, Zuo J, Zhou J, et al. Childhood obesity leads to adult type 2 diabetes and coronary artery diseases: a 2-sample mendelian randomization study. Medicine (Baltimore). 2019;98(32):e16825-e16825. doi: 10.1097/MD.0000000000016825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003;26(11):2999-3005. doi: 10.2337/diacare.26.11.2999 [DOI] [PubMed] [Google Scholar]
- 10.Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369(9575):1823-1831. doi: 10.1016/S0140-6736(07)60821-6 [DOI] [PubMed] [Google Scholar]
- 11.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29(6):1300-1306. doi: 10.2337/dc05-2470 [DOI] [PubMed] [Google Scholar]
- 12.Song SH, Hardisty CA. Early onset type 2 diabetes mellitus: a harbinger for complications in later years—clinical observation from a secondary care cohort. QJM. 2009;102(11):799-806. doi: 10.1093/qjmed/hcp121 [DOI] [PubMed] [Google Scholar]
- 13.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. ; SEARCH for Diabetes in Youth Research Group . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825-835. doi: 10.1001/jama.2017.0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cioana M, Deng J, Hou M, et al. Prevalence of hypertension and albuminuria in pediatric type 2 diabetes: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(4):e216069. doi: 10.1001/jamanetworkopen.2021.6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeitler P, Hirst K, Pyle L, et al. ; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247-2256. doi: 10.1056/NEJMoa1109333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cioana M, Deng J, Nadarajah A, et al. Prevalence of polycystic ovary syndrome in patients with pediatric type 2 diabetes: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(2):e2147454-e2147454. doi: 10.1001/jamanetworkopen.2021.47454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabelea D, Dolan LM, D’Agostino R Jr, et al. Association testing of TCF7L2 polymorphisms with type 2 diabetes in multi-ethnic youth. Diabetologia. 2011;54(3):535-539. doi: 10.1007/s00125-010-1982-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2011;8(4):228-236. doi: 10.1038/nrendo.2011.183 [DOI] [PubMed] [Google Scholar]
- 19.Yoon K-H, Lee J-H, Kim J-W, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368(9548):1681-1688. doi: 10.1016/S0140-6736(06)69703-1 [DOI] [PubMed] [Google Scholar]
- 20.Carino M, Elia Y, Sellers E, et al. Comparison of clinical and social characteristics of Canadian youth living with type 1 and type 2 diabetes. Can J Diabetes. 2021;45(5):428-435. doi: 10.1016/j.jcjd.2021.01.008 [DOI] [PubMed] [Google Scholar]
- 21.Zeitler P, Arslanian S, Fu J, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19(S27)(suppl 27):28-46. doi: 10.1111/pedi.12719 [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association . 13. Children and adolescents: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S148-S164. doi: 10.2337/dc19-S013 [DOI] [PubMed] [Google Scholar]
- 23.Panagiotopoulos C, Hadjiyannakis S, Henderson M; Diabetes Canada Clinical Practice Guidelines Expert Committee . Type 2 diabetes in children and adolescents. Can J Diabetes. 2018;42(suppl 1):S247-S254. doi: 10.1016/j.jcjd.2017.10.037 [DOI] [PubMed] [Google Scholar]
- 24.Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1(1):2. doi: 10.1186/2046-4053-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of Observational Studies in Epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 26.US Centers for Disease Control and Prevention . Defining childhood obesity. Updated 2018. Accessed May 26, 2020. https://www.cdc.gov/obesity/childhood/defining.html
- 27.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934-939. doi: 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 28.Centre for Evidence-Based Medicine. EBM Levels of Evidence Working Group . OCEBM levels of evidence. Accessed November 9, 2022. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
- 29.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97-111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 30.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974-978. doi: 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 31.Deeks J, Higgins J, Altman DG, eds. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. Accessed November 9, 2022. http://www.training.cochrane.org/handbook
- 32.Arslanian SA. Type 2 diabetes mellitus in children: pathophysiology and risk factors. J Pediatr Endocrinol Metab. 2000;13(suppl 6):1385-1394. doi: 10.1515/jpem-2000-s612 [DOI] [PubMed] [Google Scholar]
- 33.National Institutes of Health . Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. Accessed November 9, 2022. https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html
- 34.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 35.Posit. RStudio. Accessed November 14, 2022. https://www.rstudio.com/
- 36.The R Project for Statistical Computing. Accessed Nomvember 14, 2022. https://www.R-project.org/
- 37.Cochrane Training. RevMan 5. Accessed November 14, 2022. https://www.rstudio.com/
- 38.Kitagawa T, Owada M, Urakami T, Tajima N. Epidemiology of type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in Japanese children. Diabetes Res Clin Pract. 1994;24(suppl):S7-S13. doi: 10.1016/0168-8227(94)90221-6 [DOI] [PubMed] [Google Scholar]
- 39.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128(5 Pt 1):608-615. doi: 10.1016/S0022-3476(96)80124-7 [DOI] [PubMed] [Google Scholar]
- 40.Scott CR, Smith JM, Cradock MM, Pihoker C. Characteristics of youth-onset noninsulin-dependent diabetes mellitus and insulin-dependent diabetes mellitus at diagnosis. Pediatrics. 1997;100(1):84-91. doi: 10.1542/peds.100.1.84 [DOI] [PubMed] [Google Scholar]
- 41.Glaser NS, Jones KL. Non-insulin dependent diabetes mellitus in Mexican-American children. West J Med. 1998;168(1):11-16. [PMC free article] [PubMed] [Google Scholar]
- 42.Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Type 2 diabetes in Asian-Indian urban children. Diabetes Care. 2003;26(4):1022-1025. doi: 10.2337/diacare.26.4.1022 [DOI] [PubMed] [Google Scholar]
- 43.Upchurch SL, Brosnan CA, Meininger JC, et al. Characteristics of 98 children and adolescents diagnosed with type 2 diabetes by their health care provider at initial presentation. Diabetes Care. 2003;26(7):2209-2209. doi: 10.2337/diacare.26.7.2209 [DOI] [PubMed] [Google Scholar]
- 44.Wei J-N, Sung F-C, Lin C-C, Lin R-S, Chiang C-C, Chuang L-M. National surveillance for type 2 diabetes mellitus in Taiwanese children. JAMA. 2003;290(10):1345-1350. doi: 10.1001/jama.290.10.1345 [DOI] [PubMed] [Google Scholar]
- 45.Ehtisham S, Hattersley AT, Dunger DB, Barrett TG; British Society for Paediatric Endocrinology and Diabetes Clinical Trials Group . First UK survey of paediatric type 2 diabetes and MODY. Arch Dis Child. 2004;89(6):526-529. doi: 10.1136/adc.2003.027821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell-Stokes PL, Taylor BJ; New Zealand Children’s Diabetes Working Group . Prospective incidence study of diabetes mellitus in New Zealand children aged 0 to 14 years. Diabetologia. 2005;48(4):643-648. doi: 10.1007/s00125-005-1697-3 [DOI] [PubMed] [Google Scholar]
- 47.Reinehr T, Andler W, Kapellen T, et al. Clinical characteristics of type 2 diabetes mellitus in overweight European caucasian adolescents. Exp Clin Endocrinol Diabetes. 2005;113(3):167-170. doi: 10.1055/s-2005-837522 [DOI] [PubMed] [Google Scholar]
- 48.Eppens MC, Craig ME, Jones TW, Silink M, Ong S, Ping YJ; International Diabetes Federation Western Pacific Region Steering Committee . Type 2 diabetes in youth from the Western Pacific region: glycaemic control, diabetes care and complications. Curr Med Res Opin. 2006;22(5):1013-1020. doi: 10.1185/030079906X104795 [DOI] [PubMed] [Google Scholar]
- 49.Farah SE, Wals KT, Friedman IB, Pisacano MA, DiMartino-Nardi J. Prevalence of retinopathy and microalbuminuria in pediatric type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2006;19(7):937-942. doi: 10.1515/JPEM.2006.19.7.937 [DOI] [PubMed] [Google Scholar]
- 50.Huang C-Y, Li H-J, Lo F-S, et al. Metabolic disorders in children and adolescents with type 2 diabetes mellitus. Acta Paediatr Taiwan. 2006;47(4):187-191. [PubMed] [Google Scholar]
- 51.Lawrence JM, Liese AD, Liu L, et al. Weight-loss practices and weight-related issues among youth with type 1 or type 2 diabetes. Diabetes Care. 2008;31(12):2251-2257. doi: 10.2337/dc08-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bell RA, Mayer-Davis EJ, Beyer JW, et al. ; SEARCH for Diabetes in Youth Study Group . Diabetes in non-Hispanic White youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(suppl 2):S102-S111. doi: 10.2337/dc09-S202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu LL, Lawrence JM, Davis C, et al. ; SEARCH for Diabetes in Youth Study Group . Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11(1):4-11. doi: 10.1111/j.1399-5448.2009.00519.x [DOI] [PubMed] [Google Scholar]
- 54.Liu LL, Yi JP, Beyer J, et al. ; SEARCH for Diabetes in Youth Study Group . Type 1 and Type 2 diabetes in Asian and Pacific Islander U.S. youth: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(suppl 2):S133-S140. doi: 10.2337/dc09-S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shiga K, Kikuchi N. Children with type 2 diabetes mellitus are at greater risk of macrovascular complications. Pediatr Int. 2009;51(4):563-567. doi: 10.1111/j.1442-200X.2009.02836.x [DOI] [PubMed] [Google Scholar]
- 56.Urakami T, Suzuki J, Yoshida A, et al. Prevalence of components of the metabolic syndrome in schoolchildren with newly diagnosed type 2 diabetes mellitus. Pediatr Diabetes. 2009;10(8):508-512. doi: 10.1111/j.1399-5448.2009.00533.x [DOI] [PubMed] [Google Scholar]
- 57.Amed S, Hamilton JK, Sellers EAC, et al. Differing clinical features in Aboriginal vs. non-Aboriginal children presenting with type 2 diabetes. Pediatr Diabetes. 2012;13(6):470-475. doi: 10.1111/j.1399-5448.2012.00859.x [DOI] [PubMed] [Google Scholar]
- 58.Zabeen B, Nahar J, Tayyeb S, Mohsin F, Nahar N, Azad K. Characteristics of children and adolescents at onset of type 2 diabetes in a tertiary hospital in Bangladesh. Indian J Endocrinol Metab. 2016;20(5):638-642. doi: 10.4103/2230-8210.190544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fortmeier-Saucier L, Savrin C, Heinzer M, Hudak C. BMI and lipid levels in Mexican American children diagnosed with type 2 diabetes. Worldviews Evid Based Nurs. 2008;5(3):142-147. doi: 10.1111/j.1741-6787.2008.00122.x [DOI] [PubMed] [Google Scholar]
- 60.Ludwig K, Craig ME, Donaghue KC, Maguire A, Benitez-Aguirre PZ. Type 2 diabetes in children and adolescents across Australia and New Zealand: a 6-year audit from the Australasian Diabetes Data Network (ADDN). Pediatr Diabetes. 2021;22(3):380-387. doi: 10.1111/pedi.13169 [DOI] [PubMed] [Google Scholar]
- 61.Alsaffar Y, Hussain AMA, Selman NA. Prevalence of type 2 diabetes in pediatrics and adolescents newly diagnosed with diabetes in Babylon Governorate, Iraq. Arch Venez Farmacol Ter. 2020;39(7):839-843. Accessed November 9, 2022. https://www.redalyc.org/journal/559/55965388008/55965388008.pdf [Google Scholar]
- 62.Shilbayeh S. Type 2 diabetes mellitus and its effect on quality of life in adolescents: a retrospective cohort study in Saudi Arabia. Pediatr Endocrinol Diabetes Metab. 2022;28(1):54-63. doi: 10.5114/pedm.2022.113988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Z-R, Du H-W, Cui L-W, et al. Association of β-cell function and insulin resistance with pediatric type 2 diabetes among Chinese children. World J Diabetes. 2021;12(8):1292-1303. doi: 10.4239/wjd.v12.i8.1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dean HJ, Mundy RL, Moffatt M. Non-insulin-dependent diabetes mellitus in Indian children in Manitoba. CMAJ. 1992;147(1):52-57. [PMC free article] [PubMed] [Google Scholar]
- 65.Coddington DA, Hisnanick JJ. Clinical characteristics of non-insulin-dependent diabetes mellitus among southwestern American Indian youths. J Health Popul Nutr. 2001;19(1):12-17. [PubMed] [Google Scholar]
- 66.Grinstein G, Muzumdar R, Aponte L, Vuguin P, Saenger P, DiMartino-Nardi J. Presentation and 5-year follow-up of type 2 diabetes mellitus in African-American and Caribbean-Hispanic adolescents. Horm Res. 2003;60(3):121-126. doi: 10.1159/000072523 [DOI] [PubMed] [Google Scholar]
- 67.Zdravkovic V, Daneman D, Hamilton J. Presentation and course of type 2 diabetes in youth in a large multi-ethnic city. Diabet Med. 2004;21(10):1144-1148. doi: 10.1111/j.1464-5491.2004.01297.x [DOI] [PubMed] [Google Scholar]
- 68.Scott A, Whitcombe S, Bouchier D, Dunn P. Diabetes in children and young adults in Waikato Province, New Zealand: outcomes of care. N Z Med J. 2004;117(1207):U1219. [PubMed] [Google Scholar]
- 69.Pérez-Perdomo R, Pérez-Cardona CM, Allende-Vigo M, Rivera-Rodríguez MI, Rodríguez-Lugo LA. Type 2 diabetes mellitus among youth in Puerto Rico, 2003. P R Health Sci J. 2005;24(2):111-117. [PubMed] [Google Scholar]
- 70.Sugihara S, Sasaki N, Kohno H, Amemiya S, Tanaka T, Matsuura N; Committee for the Medical Treatment of Childhood-Onset Type 2 Diabetes Mellitus, The Japanese Society for Pediatric Endocrinology . Survey of current medical treatments for childhood-onset type 2 diabetes mellitus in Japan. Clin Pediatr Endocrinol. 2005;14(2):65-75. doi: 10.1297/cpe.14.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sellers EAC, Yung G, Dean HJ. Dyslipidemia and other cardiovascular risk factors in a Canadian First Nation pediatric population with type 2 diabetes mellitus. Pediatr Diabetes. 2007;8(6):384-390. doi: 10.1111/j.1399-5448.2007.00284.x [DOI] [PubMed] [Google Scholar]
- 72.Balasanthiran A, O’Shea T, Moodambail A, et al. Type 2 diabetes in children and young adults in East London: an alarmingly high prevalence. Pract Diabetes. 2012;29(5):193-198a. doi: 10.1002/pdi.1689 [DOI] [Google Scholar]
- 73.Fu J-F, Liang L, Gong C-X, et al. Status and trends of diabetes in Chinese children: analysis of data from 14 medical centers. World J Pediatr. 2013;9(2):127-134. doi: 10.1007/s12519-013-0414-4 [DOI] [PubMed] [Google Scholar]
- 74.Osman HAM, Elsadek N, Abdullah MA. Type 2 diabetes in Sudanese children and adolescents. Sudan J Paediatr. 2013;13(2):17-23. [PMC free article] [PubMed] [Google Scholar]
- 75.Haynes A, Kalic R, Curran J, et al. Type 2 diabetes and associated complications in Western Australian children: a population-based study (1990-2012). Diabetologia. 2014;57(suppl 1):S510-S510. [Google Scholar]
- 76.Newton K, Stanley J, Wiltshire E. Audit of type 2 diabetes in youth in Wellington, New Zealand 2001–2013. Pediatr Diabetes. 2015;16(suppl 21):50-150. [Google Scholar]
- 77.Abbasi A, Juszczyk D, van Jaarsveld CHM, Gulliford MC. Body mass index and incident type 1 and type 2 diabetes in children and young adults: a retrospective cohort study. J Endocr Soc. 2017;1(5):524-537. doi: 10.1210/js.2017-00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morrison A, Chatterjee S, Greening J, et al. Phenotype and burden of comorbidities in adolescents with type 2 diabetes in a multiethnic population. Diabet Med. 2018;35(suppl 1):107. 29078006 [Google Scholar]
- 79.Van Name MA, Cheng P, Gal RL, et al. ; Pediatric Diabetes Consortium . Children and adolescents with type 1 and type 2 diabetes mellitus in the Pediatric Diabetes Consortium Registries: comparing clinical characteristics and glycaemic control. Diabet Med. 2020;37(5):863-867. doi: 10.1111/dme.14233 [DOI] [PubMed] [Google Scholar]
- 80.Greenup E, Sunil B, Barr MM, Ashraf AP. Glycaemic control and outcomes in children with type 2 diabetes diagnosed at or before 10 years of age. Endocrinol Diabetes Metab. 2020;4(2):e00192. doi: 10.1002/edm2.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Astudillo M, Tosur M, Castillo B, et al. Type 2 diabetes in prepubertal children. Pediatr Diabetes. 2021;22(7):946-950. doi: 10.1111/pedi.13254 [DOI] [PubMed] [Google Scholar]
- 82.Marks BE, Khilnani A, Meyers A, et al. Increase in the diagnosis and severity of presentation of pediatric type 1 and type 2 diabetes during the COVID-19 pandemic. Horm Res Paediatr. 2021;94(7-8):275-284. doi: 10.1159/000519797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tung JYL, Kwan EYW, But BWM, et al. Incidence and clinical characteristics of pediatric-onset type 2 diabetes in Hong Kong: the Hong Kong childhood diabetes registry 2008 to 2017. Pediatr Diabetes. 2022;23(5):556-561. doi: 10.1111/pedi.13231 [DOI] [PubMed] [Google Scholar]
- 84.Kim G, DeSalvo D, Guffey D, et al. Dyslipidemia in adolescents and young adults with type 1 and type 2 diabetes: a retrospective analysis. Int J Pediatr Endocrinol. 2020;2020(1):11. doi: 10.1186/s13633-020-00081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmitt JA, Ashraf AP, Becker DJ, Sen B. Changes in type 2 diabetes trends in children and adolescents during the COVID-19 pandemic. J Clin Endocrinol Metab. 2022;107(7):e2777-e2782. doi: 10.1210/clinem/dgac209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zuckerman Levin N, Cohen M, Phillip M, et al. Youth-onset type 2 diabetes in Israel: a national cohort. Pediatr Diabetes. 2022;23(6):649-659. doi: 10.1111/pedi.13351 [DOI] [PubMed] [Google Scholar]
- 87.Reinehr T, Schober E, Roth CL, Wiegand S, Holl R; DPV-Wiss Study Group . Type 2 diabetes in children and adolescents in a 2-year follow-up: insufficient adherence to diabetes centers. Horm Res. 2008;69(2):107-113. doi: 10.1159/000111814 [DOI] [PubMed] [Google Scholar]
- 88.Shield JPH, Lynn R, Wan KC, Haines L, Barrett TG. Management and 1 year outcome for UK children with type 2 diabetes. Arch Dis Child. 2009;94(3):206-209. doi: 10.1136/adc.2008.143313 [DOI] [PubMed] [Google Scholar]
- 89.Ruhayel SD, James RA, Ehtisham S, Cameron FJ, Werther GA, Sabin MA. An observational study of type 2 diabetes within a large Australian tertiary hospital pediatric diabetes service. Pediatr Diabetes. 2010;11(8):544-551. doi: 10.1111/j.1399-5448.2010.00647.x [DOI] [PubMed] [Google Scholar]
- 90.Larkin ME, Walders-Abramson N, Hirst K, et al. Effects of comorbid conditions on health-related quality of life in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Manag (Lond). 2015;5(6):431-439. doi: 10.2217/dmt.15.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Güven A, Demir Gokce EC. Cardiovascular risk and long term follow-up of Turkish children with type 2 diabetes: single center experience. Horm Res Paediatr. 2016;86(suppl 1):244. [Google Scholar]
- 92.Carino M, Elia Y, Sellers E, et al. Comparison of clinical and social characteristics of Canadian youth living with type 1 and type 2 diabetes. Can J Diabetes. 2021;45(5):428-435. doi: 10.1016/j.jcjd.2021.01.008 [DOI] [PubMed] [Google Scholar]
- 93.Pinhas-Hamiel O, Zeitler P. “Who is the wise man?—the one who foresees consequences:” childhood obesity, new associated comorbidity and prevention. Prev Med. 2000;31(6):702-705. doi: 10.1006/pmed.2000.0752 [DOI] [PubMed] [Google Scholar]
- 94.Han JC, Lawlor DA, Kimm SYS. Childhood obesity. Lancet. 2010;375(9727):1737-1748. doi: 10.1016/S0140-6736(10)60171-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.World Health Organization (WHO) . Obesity and overweight. Accessed November 9, 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 96.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care. 2009;32(1):100-105. doi: 10.2337/dc08-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care. 2010;33(10):2225-2231. doi: 10.2337/dc10-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taha D, Umpaichitra V, Banerji MA, Castells S; Comparative Study Controlled Clinical Trial . Type 2 diabetes mellitus in African-American adolescents: impaired beta-cell function in the face of severe insulin resistance. J Pediatr Endocrinol Metab. 2006;19(2):135-142. doi: 10.1515/JPEM.2006.19.2.135 [DOI] [PubMed] [Google Scholar]
- 99.Urakami T, Kuwabara R, Habu M, et al. Clinical characteristics of non-obese children with type 2 diabetes mellitus without involvement of β-cell autoimmunity. Diabetes Res Clin Pract. 2013;99(2):105-111. doi: 10.1016/j.diabres.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 100.Narasimhan S, Weinstock RS. Youth-onset type 2 diabetes mellitus: lessons learned from the TODAY study. Mayo Clin Proc. 2014;89(6):806-816. doi: 10.1016/j.mayocp.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kahn SE, Lachin JM, Zinman B, et al. ; ADOPT Study Group . Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60(5):1552-1560. doi: 10.2337/db10-1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC; UK Prospective Diabetes Study (UKPDS) Group . UKPDS 26: sulphonylurea failure in non-insulin-dependent diabetic patients over six years. Diabet Med. 1998;15(4):297-303. doi: [DOI] [PubMed] [Google Scholar]
- 103.RISE Consortium . Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care. 2018;41(8):1717-1725. doi: 10.2337/dc18-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.TODAY Study Group . Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013;36(6):1749-1757. doi: 10.2337/dc12-2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tfayli H, Bacha F, Gungor N, Arslanian S. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus -positive patients. Diabetes. 2009;58(3):738-744. doi: 10.2337/db08-1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361-369. doi: 10.1016/S2213-8587(18)30051-2 [DOI] [PubMed] [Google Scholar]
- 107.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140(3):399-410. doi: 10.1530/REP-10-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. ; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017;376(15):1419-1429. doi: 10.1056/NEJMoa1610187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Urakami T. Clinical characteristics in Japanese children with nonobese type 2 diabetes. Ann Pediatr Endocrinol Metab. 2018;23(3):113-118. doi: 10.6065/apem.2018.23.3.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.TODAY Study Group . Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36(6):1735-1741. doi: 10.2337/dc12-2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.TODAY Study Group . Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36(6):1772-1774. doi: 10.2337/dc12-2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218-1240. doi: 10.3390/nu5041218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schober E, Rami B, Grabert M, et al. ; DPV-Wiss Initiative of the German Working Group for Paediatric Diabetology and . Phenotypical aspects of maturity-onset diabetes of the young (MODY diabetes) in comparison with type 2 diabetes mellitus (T2DM) in children and adolescents: experience from a large multicentre database. Diabet Med. 2009;26(5):466-473. doi: 10.1111/j.1464-5491.2009.02720.x [DOI] [PubMed] [Google Scholar]
- 114.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39-48. doi: 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 115.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419-5426. doi: 10.1210/jc.2010-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23(4):465-471. doi: 10.2337/diacare.23.4.465 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategy: MEDLINE
eTable 2. Search Strategy: Embase
eTable 3. Search Strategy: CINAHL
eTable 4. Search Strategy: Cochrane Library: Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews
eTable 5. Search Strategy: Web of Science: Conference Proceedings Citation Index-Science
eTable 6. Characteristics of Included Studies
eTable 7. Symptoms at Presentation and Risk Factors of Patients in Included and Type 2 Diabetes Diagnostic Criteria Used Across Studies
eTable 8. Prevalence of Type 2 Diabetes in Patients Without Obesity and Association With Glycemic Control and Dyslipidemia
eTable 9. Results of Sensitivity Analyses
eTable 10. Risk of Bias of Included Studies
eFigure 1. Study Flow Diagram
eFigure 2. Forest Plot Illustrating Odds Ratio of Obesity in Pediatric T2DM by Sex
eFigure 3. Forest Plot Illustrating Prevalence of Obesity in Pediatric T2DM by Region
eFigure 4. Forest Plot Illustrating Prevalence of Normal BMI-Based Measures in Pediatric T2DM by Region
eFigure 5. Funnel Plot for Publication Bias for Pooled Prevalence of Obesity in Pediatric Type 2 Diabetes
eFigure 6. Funnel Plot for Publication Bias for Pooled Prevalence of Obesity at Type 2 Diabetes Diagnosis in Patients With Pediatric Type 2 Diabetes
eReferences.
Data Sharing Statement


