Key Points
Question
Does mindfulness training, exercise, or the combination of these interventions improve cognitive function in older adults with subjective cognitive concerns?
Findings
In this randomized clinical trial that included 585 participants, mindfulness training, exercise, or both did not result in significant differences in improvement in episodic memory or executive function composite scores at 6 months.
Meaning
The findings do not support the use of mindfulness training, exercise, or a combination of both for significantly improving cognitive function in older adults with subjective cognitive concerns.
Abstract
Importance
Episodic memory and executive function are essential aspects of cognitive functioning that decline with aging. This decline may be ameliorable with lifestyle interventions.
Objective
To determine whether mindfulness-based stress reduction (MBSR), exercise, or a combination of both improve cognitive function in older adults.
Design, Setting, and Participants
This 2 × 2 factorial randomized clinical trial was conducted at 2 US sites (Washington University in St Louis and University of California, San Diego). A total of 585 older adults (aged 65-84 y) with subjective cognitive concerns, but not dementia, were randomized (enrollment from November 19, 2015, to January 23, 2019; final follow-up on March 16, 2020).
Interventions
Participants were randomized to undergo the following interventions: MBSR with a target of 60 minutes daily of meditation (n = 150); exercise with aerobic, strength, and functional components with a target of at least 300 minutes weekly (n = 138); combined MBSR and exercise (n = 144); or a health education control group (n = 153). Interventions lasted 18 months and consisted of group-based classes and home practice.
Main Outcomes and Measures
The 2 primary outcomes were composites of episodic memory and executive function (standardized to a mean [SD] of 0 [1]; higher composite scores indicate better cognitive performance) from neuropsychological testing; the primary end point was 6 months and the secondary end point was 18 months. There were 5 reported secondary outcomes: hippocampal volume and dorsolateral prefrontal cortex thickness and surface area from structural magnetic resonance imaging and functional cognitive capacity and self-reported cognitive concerns.
Results
Among 585 randomized participants (mean age, 71.5 years; 424 [72.5%] women), 568 (97.1%) completed 6 months in the trial and 475 (81.2%) completed 18 months. At 6 months, there was no significant effect of mindfulness training or exercise on episodic memory (MBSR vs no MBSR: 0.44 vs 0.48; mean difference, –0.04 points [95% CI, –0.15 to 0.07]; P = .50; exercise vs no exercise: 0.49 vs 0.42; difference, 0.07 [95% CI, –0.04 to 0.17]; P = .23) or executive function (MBSR vs no MBSR: 0.39 vs 0.31; mean difference, 0.08 points [95% CI, –0.02 to 0.19]; P = .12; exercise vs no exercise: 0.39 vs 0.32; difference, 0.07 [95% CI, –0.03 to 0.18]; P = .17) and there were no intervention effects at the secondary end point of 18 months. There was no significant interaction between mindfulness training and exercise (P = .93 for memory and P = .29 for executive function) at 6 months. Of the 5 prespecified secondary outcomes, none showed a significant improvement with either intervention compared with those not receiving the intervention.
Conclusions and Relevance
Among older adults with subjective cognitive concerns, mindfulness training, exercise, or both did not result in significant differences in improvement in episodic memory or executive function at 6 months. The findings do not support the use of these interventions for improving cognition in older adults with subjective cognitive concerns.
Trial Registration
ClinicalTrials.gov Identifier: NCT02665481
This randomized clinical trial examines whether mindfulness-based stress reduction, exercise, or a combination of both interventions improves cognitive function in older adults with subjective cognitive concerns.
Introduction
Most older adults experience deteriorating cognitive function. Declines in episodic memory and executive function parallel volume losses in brain structures, such as the hippocampus and dorsolateral prefrontal cortex (DLPFC).1,2 With the increasing age of the population, lifestyle interventions could provide a scalable means to target modifiable mechanisms of these cognitive and brain changes, thereby helping improve and maintain cognitive functioning.3
Two promising interventions are mindfulness training and exercise. Mindfulness-based stress reduction (MBSR) is a group-based intervention based on mindfulness meditation training.4 From a mechanistic standpoint, practicing mindfulness may enhance cognitive processes such as working memory5; further, mindfulness techniques may reduce stress, thereby affecting physiological parameters such as cortisol levels and sleep.6,7 Aerobic and strength training are both theorized to be associated with cognitive function8; some studies have found exercise-related cognitive changes together with structural brain changes.9,10 Previous studies have suggested changes in insulin sensitivity, aerobic capacity, and body fat as some of the proposed mechanisms.11 MBSR and exercise could have additive benefits because their putative mechanisms may be complementary. Accordingly, a randomized clinical trial was conducted to determine whether MBSR and exercise improve cognitive function and whether the combination of MBSR and exercise has greater benefits than either intervention alone.
Methods
Study Design
The MEDEX (Mindfulness, Education, and Exercise) study was a randomized clinical trial comparing MBSR and exercise, alone or in combination, with a robust control intervention (health education) designed to control for expectancy in older adults with subjective cognitive concerns and without dementia. Outcome assessments evaluated cognitive function and brain structure over 18 months of intervention. For full details of the trial design, protocol, and statistical analysis plan, see Wetherell et al12 and Supplement 1. The study was conducted from 2015 to 2020 in St Louis, Missouri, and San Diego, California, with enrollment from November 19, 2015, through January 23, 2019, and final follow-up on March 16, 2020. Ethics approval was provided by the universities’ institutional review boards. All participants provided written informed consent. Recruitment methods included use of press (eg, television, newspapers), online sources (eg, via social media, websites), printed flyers, presentations at community outreach events, and direct mailings.
Participants
From November 2015 to January 2019, the study enrolled community-dwelling older adults. Inclusion criteria were age 65 to 84 years; self-reported age-related changes in cognitive function, defined by a positive response to questions of whether they or others had noticed trouble with their memory or concentration; and being cognitively intact, defined as scoring less than 10 on the Short Blessed Test, for which scores greater than or equal to 10 suggest impairment consistent with dementia.13 The study allowed mild cognitive impairment, and no clinical rating of dementia status was done. Exclusion criteria were neurodegenerative illness (eg, dementia, Parkinson disease, cerebrovascular disease); not sedentary (current moderate- to high-intensity exercise ≥1 h/wk or light activity ≥1 h/d; see eMethods 1 in Supplement 2 for details); current meditation practice or cognitive training; medical conditions that suggest shortened lifespan, or would prohibit safe participation, would prohibit safe participation in the interventions (eg, metastatic cancer, unstable cardiovascular disease), or would interfere with study assessments (eg, diabetes medication, systemic glucocorticoids, magnetic resonance imaging [MRI] contraindications, severe hearing/visual impairment); and nonfluent English-language speaker.
Randomization
After baseline assessment, participants were randomized to the following groups in a 1:1:1:1 ratio: MBSR alone, exercise alone, combined MBSR and exercise, and health education (control group). Using R software, the study statistician (M.D.Y.) generated the randomization sequence. The study primary investigator and coordinators were kept blinded to the randomization until the study coordinator was ready for the next group to be randomized. Participants learned their randomization assignment at the first intervention group meeting. Randomization was done in groups of approximately 15 individuals (range, 12-17) and was stratified by site.
Interventions
All interventions were conducted for 18 months, which consisted of a 6-month acute and 12-month maintenance phase.
The MBSR intervention matched the format of the consensus MBSR protocol14; after a brief introductory meeting, the intervention was conducted in 8 weekly 2.5-hour classes plus a half-day retreat. For the remainder of the 6-month acute phase and the subsequent 12 months of maintenance, MBSR classes met monthly. Content included instruction in mindfulness meditation practices and exercises to enhance mindfulness in everyday life. Participants also used A Mindfulness-Based Stress Reduction Workbook.15 Participants received daily at-home assignments with a goal of 60 minutes of daily at-home meditative practice. Additional details are provided in Supplement 2.
The exercise intervention was designed to improve aerobic fitness, strength, balance, mobility, and flexibility. It consisted of facility-based, instructor-supervised 1.5-hour classes twice weekly for 6 months. Sessions included aerobic exercise, resistance training, and functional exercises. Participants were prescribed home exercise with a goal of completing at least 300 minutes per week of combined class plus home exercise. Classes continued once per week during the 12-month maintenance phase with the same exercise goal of at least 300 minutes per week. Additional details are provided in Supplement 2.
Participants in the combined MBSR and exercise intervention underwent both MBSR and exercise, with the above-listed frequency of classes and goals for each intervention.
The health education intervention was an attention placebo to control for nonspecific factors (eg, time spent in groups) and expectancy.16 It matched the MBSR intervention for group setting, class time, frequency of sessions, and attention with weekly assignments, but no goals, related to the amount of time engaged in them. It was based on the Stanford chronic disease self-management book Living a Healthy Life with Chronic Conditions,17 omitting information on mindfulness and exercise.
To monitor fidelity, both sites utilized instructors trained in the respective interventions. Instructor fidelity was maintained by regular supervision calls, measuring session time and confirming adherence to the study protocol, and, in the case of MBSR, video recording sessions with review by MBSR experts according to published fidelity criteria for mindfulness-based interventions18 (all sessions were rated as competent; Supplement 2).
To evaluate participant adherence, class attendance was monitored. Additionally, for MBSR and exercise interventions, home practice during the 6-month acute period was measured and reinforced using daily surveys sent to tablets or smartphones. During the maintenance phase, participants in the MBSR and exercise groups were asked if they had any breaks in their home practice.
Outcomes
All outcomes were measured by blinded assessors. The 2 primary outcomes were episodic memory and executive function (cognitive control) composites (standardized to a mean [SD] of 0 [1]; higher composite scores indicate better cognitive performance) at the 6-month end point. These composite scores were calculated from a neuropsychological test battery conducted at 0, 3, 6, and 18 months. The secondary end point was 18 months. These domains were selected based on previous research on the effects of mindfulness and exercise on cognitive function. Memory tests were immediate and delayed recall using a 16-item word list and 2 paragraphs developed for repeated administrations during longitudinal studies (ie, different lists and paragraphs at each time point)19 and the Picture Sequence Memory Test from the National Institutes of Health (NIH) Toolbox.20 Executive function tests were the Dimensional Change Card Sort test, Flanker Inhibitory Control and Attention Test, and List Sorting Working Memory Test from NIH Toolbox and the following 3 additional computer-based tests: the Consonant-Vowel Odd-Even Switching test,21 the Sustained Attention to Response Test,22 and the Stroop Test.23 For each memory or executive function variable, a Z score was computed for each participant using the mean and SD of that variable computed on all randomized participants at baseline ([participant score − mean]/SD). Composite scores were then created by taking the mean of the Z scores of all available memory or cognitive control variables (additional details are provided in the statistical analysis plan [Supplement 1]). Composite scores, compared with individual test scores, improve both test-retest reliability and the ability to detect subtle changes in scores, as exemplified by the Preclinical Alzheimer Cognitive Composite (a clinical trial outcome that similarly combines multiple cognitive tests).24 For interpretation purposes, if the intervention was effective in improving each individual measure that comprised the composite by 1 SD, the overall composite score would improve by 1 point (compared with the control). The correlations between the baseline (month 0) and 6-month composite scores were 0.81 for the memory composite and 0.80 for the executive function composite, suggesting high reliability.
Secondary outcomes (left and right hippocampal volume and left and right DLPFC surface area and cortical thickness) consisted of high-resolution T1-weighted MRI (MP-RAGE; 1 × 1 × 1 mm; TR = 2300 ms; TI = 900 ms; TE = 2.95 ms; flip angle = 9°), which were acquired at 0, 6, and 18 months. Longitudinal FreeSurfer25 processing generated the measurements. The correlations between the baseline and 6-month MRI measures were 0.99 for hippocampal volume, 0.98 for DLPFC surface area, and 0.92 for DLPFC thickness. At the same time points, resting-state MRI data were collected; these data are presented in another report.26
Additional secondary cognitive outcomes included the Revised Observed Tasks of Daily Living27 score, a performance-based measure of functional cognitive capacity (range, 0-28; higher values indicate greater ability to complete everyday activities) and the Quality of Life in Neurological Disorders Cognitive Function28 score, a self-report measure of cognitive concerns (range, 18-90; higher values indicate better outcomes).
To assess mechanisms of exercise- and mindfulness-induced cognitive benefits, several physiological and performance measures at 0, 6, and 18 months were tested (details of measurement are provided in Supplement 2): aerobic fitness, insulin sensitivity and resistance, body fat and fat-free masses, physical performance, plasma cortisol levels, physical activity, time to fall asleep and total sleep time, mindfulness state, and upper- and lower-body strength.
Race and ethnicity were self-reported by participants based on fixed categories to understand the diversity of enrolled participants and for potential future subgroup analyses examining differences in results based on these characteristics.
Sample Size Calculation
A target sample size of 580 participants was determined based on 80% power to detect either main effects or an interaction of at least a small effect size, of 0.2 (Cohen d). The study was not designed to detect a specific minimal clinically important difference. All power analyses were conducted with G*Power, version 3.1, and assumed 15% attrition for power calculations.
Statistical Analyses
See Supplement 1 for the complete statistical analysis plan. A marginal model was fit for the repeated measures analyses. The model included the between-participant main effects of MBSR and exercise, their interaction, and the 2- and 3-way interactions between time and the between-participant effects. Time (0, 3 [cognitive measures only], 6, and 18 months) was a within-participants effect with an unspecified covariance matrix due to uneven time intervals between visits. Site, age, and sex were included as covariates in the models. Clustering by site was accounted for because site was a factor in all primary and secondary outcome models.
The primary test of effectiveness of each intervention was the change in the composite scores from baseline to 6 months in the participants randomized to undergo the intervention compared with those not receiving the intervention, as computed with the appropriate contrast (eg, MBSR vs no MBSR). The 2 × 2 factorial design was analyzed with the 2 main effects of exercise (underwent exercise intervention vs did not undergo exercise) and MBSR (underwent MBSR intervention vs did not undergo MBSR).
All randomized individuals were included in the primary analysis (Figure 1). Participants were analyzed according to their randomization group. A Bonferroni-adjusted 2-tailed significance level of .025 was used for each of the 2 primary outcomes. Effect sizes with 95% CIs for 6- and 18-month effects for all primary and secondary outcomes were computed. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary time points and secondary outcomes should be interpreted as exploratory. The mixed-model analytic approach used is robust in accounting for missing data. Participants were included in the analytic model if they had data for at least 1 time point.
Figure 1. Participant Flow in a Study of the Effect of Mindfulness-Based Stress Reduction (MBSR) and Exercise on Cognitive Function .
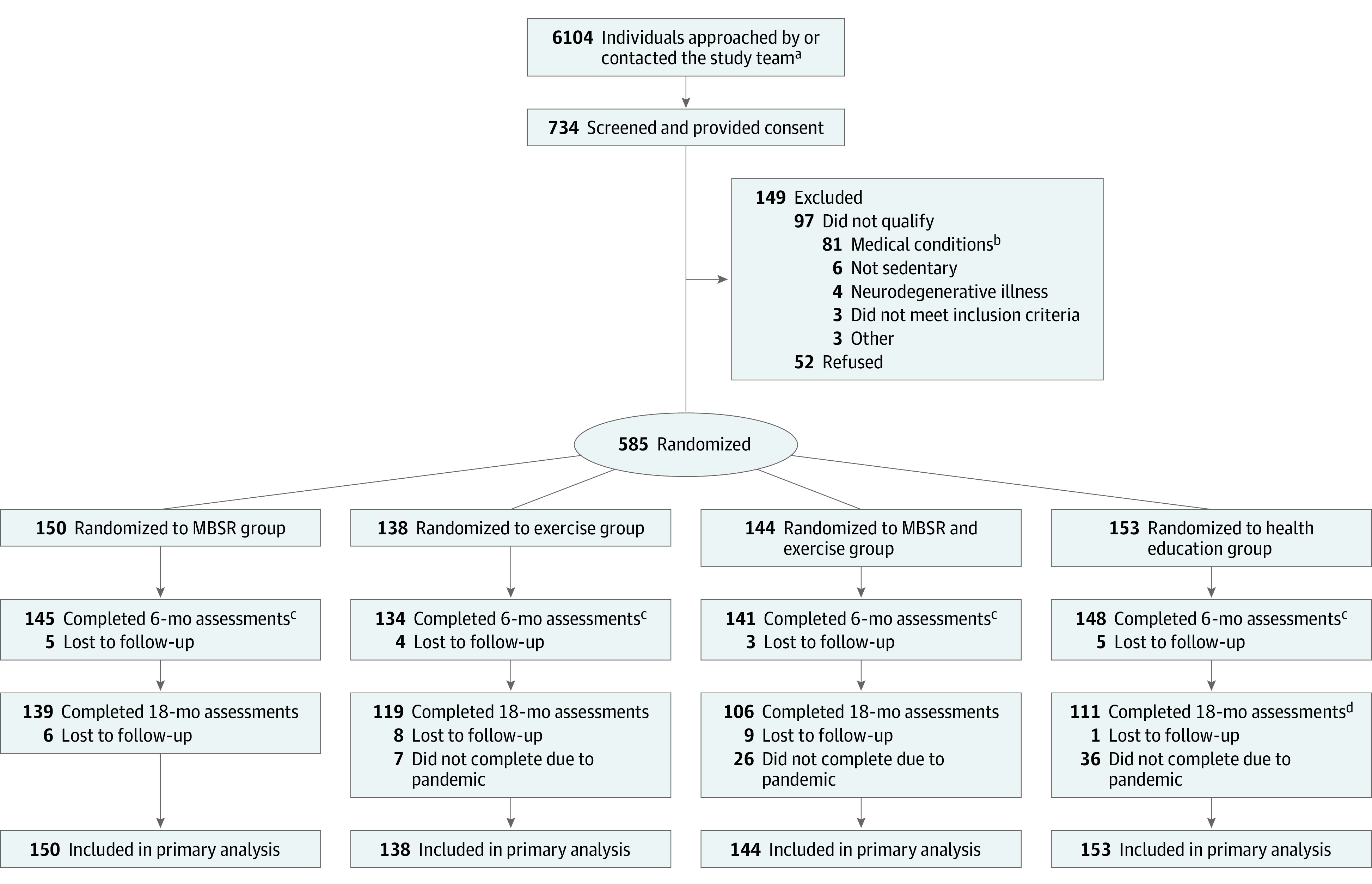
aUnless individuals were screened, they were not fully assessed for eligibility; as such, the study team does not have the results (eg, why they were excluded or declined) for all of these individuals.
bConditions that would suggest shortened lifespan or would prohibit safe participation in the interventions (eg, metastatic cancer, unstable cardiovascular disease) or would interfere with study assessments (eg, diabetes medication, systemic glucocorticoids, magnetic resonance imaging contraindications, severe hearing/visual impairment).
cUnless they officially withdrew, participants who missed the 6-month assessment were not out of the study; they could rejoin for the 18-month assessment.
dA higher number of participants (n = 36) in the health education intervention group were unable to complete the 18-month assessments due to the COVID-19 pandemic because of the randomization schedule (eg, these intervention groups were the last groups to be randomized in the trial). For example, 3 of the last 4 groups randomized in the trial were health education.
Given neutral findings for the primary outcomes, the importance of post hoc analyses became clear. Subsequent per-protocol analyses were conducted, as were subgroup tests examining changes in cognitive outcomes among those who showed the most vs the least change in the physiological and performance markers described above. The 2 per-protocol groups were defined post hoc based on examination of attendance data and home practice data: participants reporting home practice of their randomized intervention (MBSR or exercise) on at least 70% of days and participants attending at least 70% of classes. Both groups also excluded individuals who participated in interventions to which they were not randomized (Supplement 2). Additionally, given that the primary outcomes showed no intervention effect, the original plan to examine MRI structural changes as part of a mediator analysis was modified: rather than examining MRI structural changes as mediators, they were analyzed as secondary outcomes. All analyses were conducted in SAS, version 9.4 (SAS Institute).
Results
Enrollment and Participant Characteristics
A total of 6104 individuals were approached by or directly contacted the study team and 734 completed baseline screening and provided written informed consent; of these individuals, 149 did not qualify or wish to participate further. Thus, 585 individuals met all study criteria and were randomized and included in the analysis. A total of 97.1% of participants completed 6-month assessments and 81.2% completed 18-month assessments (Figure 1).
The full sample had a mean (SD) age of 71.5 (4.8) years and education level of 16.2 (2.2) years and 424 (72.5%) were women, 2 (0.3%) were American Indian, 27 (4.6%) were Asian, 69 (11.8%) were Black, 477 (81.5%) were White (the remaining individuals were unknown or >1 race), and 39 (6.7%) were Hispanic/Latino. Demographic information and other baseline characteristics were well-balanced across intervention groups (Table).
Table. Baseline Characteristics by Intervention Group.
| Characteristic | MBSR (n = 150) | Exercise (n = 138) | MBSR and exercise (n = 144) | Health education (n = 153) |
|---|---|---|---|---|
| Age, mean (SD), y | 71.2 (4.2) | 71.1 (4.9) | 72.4 (5.3) | 71.1 (4.6) |
| Sex, No. (%) | ||||
| Women | 108 (72.0) | 108 (78.3) | 102 (70.8) | 106 (69.3) |
| Men | 42 (28.0) | 30 (21.7) | 42 (29.2) | 47 (30.7) |
| Race, No. (%) | ||||
| American Indian or Alaska Native | 0 | 1 (0.7) | 1 (0.7) | 0 |
| Asian | 3 (2.0) | 9 (6.5) | 10 (6.9) | 5 (3.3) |
| Black or African American | 18 (12.0) | 14 (10.1) | 18 (12.5) | 19 (12.4) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 0 |
| White | 127 (84.7) | 109 (79.0) | 112 (77.8) | 129 (84.3) |
| More than 1 race | 1 (0.7) | 0 | 3 (2.1) | 0 |
| Unknown/not reported | 1 (0.7) | 5 (3.6) | 0 | 0 |
| Hispanic, No. (%) | 7 (4.7) | 9 (6.5) | 14 (9.7) | 9 (5.9) |
| Current smoker, No. (%) | 4 (2.7) | 1 (0.7) | 0 | 5 (3.3) |
| Education level, mean (SD), y | 16.0 (2.2) | 16.6 (2.2) | 16.0 (2.3) | 16.1 (2.1) |
| APOE*E4–positive, No. (%) | 49/149 (32.9) | 44 (31.9) | 37/143 (25.9) | 44 (28.8) |
| CIRS-G Score, mean (SD)a | 6.7 (2.9) | 6.7 (2.8) | 6.9 (2.8) | 6.7 (3.0) |
| Comorbidities, No. (%) | ||||
| Arthritis | 69 (46.0) | 73 (52.9) | 67 (46.5) | 63 (41.2) |
| Hypertension | 53 (35.3) | 58 (42.0) | 60 (41.7) | 73 (47.7) |
| High blood cholesterol | 46 (30.7) | 56 (40.6) | 62 (43.1) | 71 (46.4) |
| Credibility and expectations for improvement, mean (SD)b | ||||
| Credibility | 30.1 (6.8) [n = 143] | 33.5 (5.8) [n = 123] | 32.9 (6.4) [n = 127] | 26.6 (7.4) [n = 143] |
| Improvement | 59.4 (24.2) [n = 143] | 61.9 (23.5) [n = 123] | 68.4 (23.0) [n = 127] | 56.1 (22.5) [n = 139] |
| BMI classification, No. (%)c | ||||
| Normal (16-24.9) | 57 (38.0) | 34 (24.6) | 35 (24.3) | 47 (30.7) |
| Overweight (25-29.9) | 51 (34.0) | 51 (37.0) | 63 (43.8) | 54 (35.3) |
| Obese (≥30.0) | 42 (28.0) | 53 (38.4) | 46 (31.9) | 52 (34.0) |
| WTAR standard score, mean (SD)d | 113.6 (10.6) | 114.1 (10.2) | 113.4 (10.4) | 112.2 (10.3) |
| SPPB modified score, mean (SD)e | 8.8 (1.8) | 8.7 (1.9) [n = 137] | 9.0 (2.0) | 8.8 (2.0) |
| Paragraph recall score, mean (SD) | ||||
| Immediatef | 43.0 (10.7) [n = 149] | 43.4 (10.2) [n = 137] | 41.9 (10.8) | 42.6 (9.8) |
| Delayedf | 36.4 (12.0) | 37.5 (10.8) | 35.8 (11.3) | 36.7 (10.1) |
| Word List score, mean (SD) | ||||
| Learningg | 32.4 (7.4) | 31.3 (7.3) | 31.2 (7.6) | 31.1 (7.4) |
| Recallh | 6.9 (3.1) | 7.1 (3.2) | 6.6 (3.0) | 6.5 (3.2) |
| Neuro-QoL Cognitive Function score, mean (SD)i | 62.4 (12.6) [n = 149] | 63.2 (12.1) | 65.5 (11.4) | 63.3 (11.4) |
| OTDL score, mean (SD)j | 20.7 (3.2) | 20.3 (3.5) | 20.3 (3.5) | 20.2 (3.7) |
| CAMS-R score, mean (SD)k | 38.1 (6.2) | 37.0 (5.6) | 37.8 (5.9) | 36.7 (5.6) |
| NIH Toolbox Fluid Composite score, mean (SD)l | 92.0 (8.7) | 92.0 (10.0) | 91.4 (8.4) | 91.5 (9.1) |
| Cortisol area under curve, mean (SD)m | 5580 (2471) [n = 134] | 5703 (2606) [n = 116] | 6565 (2950) [n = 123] | 5749 (2500) [n = 133] |
| Insulin sensitivity, mean (SD) | ||||
| HOMA-IRn | 2.5 (1.7) [n = 149] | 3.0 (2.2) [n = 135] | 3.0 (2.1) [n = 143] | 3.1 (2.3) |
| OGIS, mL/min−1/m−2o | 352 (61) [n = 139] | 344 (63) [n = 128] | 348 (67) [n = 136] | 346 (67) [n = 145] |
Abbreviations: MBSR, mindfulness-based stress reduction; NIH, National Institutes of Health; OTDL, Observed Tasks of Daily Living.
The Cumulative Illness Rating Scale-Geriatric (CIRS-G) is a 14-item instrument that measures the number and severity of physical health problems (13 organ systems; 0-4 score for each system; overall range, 0-56). Higher scores are indicative of more comorbidities and severe medical conditions. The mean score was between 6.7 and 6.9 (dependent on the intervention group), which suggests the sample was generally healthy, with approximately 3 moderate-severity medical conditions per participant.
The Credibility and Expectations Questionnaire35 was administered after the first intervention class to evaluate participants’ perception of the credibility of the intervention to which they were assigned (4 questions; score range, 4-40; higher scores indicate greater credibility), and their expectations for improvement (1 question; range, 0%-100%; higher percentages indicate expectations for greater improvement). Participants generally rated the credibility of the interventions as high, with mean ranges above 30 for all intervention groups except for health education (mean [SD] of 26.6 [7.4]). Expectations for improvement were above 50% for all groups.
The percentage of people with body mass index (BMI) ≥30.0 in this study is slightly lower than the value reported for older adults (≥60 y) of 41.5% in the National Health and Nutrition Examination Survey 2017-2020.36
Wechsler Test of Adult Reading (WTAR) measures intelligence and has 50 items. The standard score ranges from 52 to 128, with higher scores indicating higher estimated IQ; a standard score is equivalent to an IQ score. The normative score is 100. Based on the WTAR, this sample was above-normal in terms of IQ; this aligns with the advanced educational levels.
The Short Physical Performance Battery (SPPB) assesses walking speed, lower extremity strength, and balance. Modified scoring was used (range, 1-12; higher scores indicate better physical functioning). The sample had mean scores between 8.7 and 9.0, suggestive of relatively high physical functioning.
The Paragraph Recall task is used to quantify memory performance. This measure involved the participant listening to 2 stories and being asked to recall and report as many of the paragraph elements as possible, with each story having 44 elements (range, 0-88 for immediate and delayed recall tests; higher scores are better). The immediate positive total mean score was slightly higher across all intervention groups (mean, 41.9-43.4) than the delayed positive total mean score (mean, 35.8-37.5).
This task involved the participant recalling as many words as possible from a list of 16 words (4 learning trials were presented) (range, 0-64; higher scores are better). Across all intervention groups, the mean score was between 31.1 and 32.4, suggesting that the sample was able to recall slightly less than half of the words over the course of 4 trials.
This task occurs 20 minutes from the learning task. The participant was asked to recall as many words as possible from the 16-word list (range, 0-16; higher scores are better). The mean score was between 6.5 and 7.1 across all intervention groups, suggesting that the sample was able to recall less than half of the words after the delayed period.
The Quality of Life in Neurological Disorders Cognitive Function (Neuro-QoL) is an 18-item self-report measure that assess health-related quality of life (range, 18-90; higher scores are better). The mean score across all interventions was between 62.4 and 65.5, suggesting that in general participants had only mild decrements in self-reported everyday cognitive function.
The Revised Observed Tasks of Daily Living measures functional capacity and has a range of 0 to 28. Higher scores suggest better functional capacity. Across all intervention groups, the mean score was between 20.2 and 20.7. This suggests high functional capacity at baseline.
The self-report Cognitive and Affective Mindfulness Scale-Revised (CAMS-R) measures state mindfulness (range, 12-48; higher scores indicate greater state of mindfulness). The mean score across intervention groups ranged from 36.7 to 38.1, indicating this sample reported a high level of state mindfulness at baseline.
This score was derived from the mean standard scores of the Flanker, Dimensional Change Card Sort, Picture Sequence Memory, List Sorting, and Pattern Comparison tasks and then deriving standard scores based on this new distribution. An uncorrected standard score at or near 100 indicates ability that is average compared with others nationally. A standard score of approximately 85 suggests significantly below-average fluid cognitive ability. The mean score of this sample was 91.4 and 92.0.
Cortisol area under the curve is based on salivary measurements collected at waking, 30 minutes after waking, and bedtime on 3 consecutive days. There is no normative range for cortisol AUC for this specific assay.
Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) calculated using fasting glucose and insulin. A HOMA-IR less than 1.0 indicates insulin sensitivity. The mean HOMA-IR was above 1.0 across all intervention groups.
Oral Glucose Insulin Sensitivity (OGIS) calculated using a 75 g 2-hour oral glucose tolerance test obtained at the 0, 90, and 120 time points. The higher the OGIS index, the more insulin sensitive an individual is. An OGIS score of 302 mL/min−1/m−2 (+/− 17) suggests impaired glucose tolerance. The mean OGIS score was greater than 302 mL/min−1/m−2 across all intervention groups.
Primary Outcomes
Figure 2 shows changes over 18 months in the 2 primary outcome measures: composite variables of memory and executive function. At 6 months, there were no significant differences in these measures when comparing participants with and without MBSR (memory composite score, 0.44 vs 0.48; mean difference, –0.04 points [95% CI, –0.15 to 0.07]; P = .50; executive function score, 0.39 vs 0.31; mean composite difference, 0.08 [95% CI, –0.02 to 0.19]; P = .12) and with vs without exercise (memory composite, 0.49 vs 0.42; mean difference, 0.07 points [95% CI, –0.04 to 0.17]; P = .23; executive function composite, 0.39 vs 0.32; mean difference, 0.07 points [95% CI, –0.03 to 0.18]; P = .17).
Figure 2. Memory and Executive Function Composite Changes Over 18 Months.

The composite scores were the standardized mean of several neuropsychological test scores for the domain of interest. A Z score was computed for each participant ([participant score − mean]/SD), using the mean and SD of that variable computed on all randomized participants at baseline. For example, the memory composite variable was created by the mean Z scores of all available memory variables. For composite interpretation purposes, if the intervention was effective in improving each individual measure that comprised the composite by 1 SD, the overall composite score would improve by 1 point (compared with the control). The ranges for memory and executive function are −3.3 to 3.7 and −5.0 to 3.0, respectively. See eTable 2 in Supplement 2 for numerical/model data of intervention effects. The boxplot inner horizontal lines represent the median values, the boxes represent the IQR (25% and 75%), the vertical whiskers extend to the upper and lower adjacent values (the furthest points within 1.5 IQRs of the 25th and 75th percentiles), and the dots indicate outlier values.
Secondary Outcomes
There were also no significant differences at 18 months (secondary end point) for the composite variables of memory (MBSR vs no MBSR: 0.61 vs 0.53; mean difference, 0.08 [95% CI, –0.04 to 0.19]; P = .18; exercise vs no exercise: 0.55 vs 0.59; mean difference, –0.04 [95% CI, –0.15 to 0.07]; P = .47) and executive function (MBSR vs no MBSR: 0.27 vs 0.31; mean difference, –0.04 [95% CI, –0.15 to 0.07]; P = .44; exercise vs no exercise: 0.28 vs 0.29; mean difference, –0.01 [95% CI, –0.12 to 0.11]; P = .93)
Secondary outcomes included structural MRI measures (Figure 3) and additional cognitive outcomes (Supplement 2). At 6 months, there were no significant intervention effects on hippocampal volume (MBSR vs no MBSR: difference, –3.46 mm3 [95% CI, –14.27 to 7.34]; P = .53; exercise vs no exercise: difference, 3.04 mm3 [95% CI, –7.76 to 13.85]; P = .58), DLPFC surface area (MBSR vs no MBSR: difference, 22.71 mm2 [95% CI, –22.95 to 68.36]; P = .33; exercise vs no exercise: difference, –17.18 mm2 [95% CI, –62.83 to 28.48]; P = .46), or cortical thickness (MBSR vs no MBSR: difference, –0.01 mm [95% CI, –0.02 to 0.01]; P = .37; exercise vs no exercise: difference, 0.01 mm [95% CI, 0.00-0.02]; P = .21). At the secondary time point of 18 months, there was also no significant intervention effects on DLPFC surface area (MBSR vs no MBSR: difference, 25.35 mm2 [95% CI, –23.18 to 73.88]; P = .31; exercise vs no exercise: difference, 21.11 mm2 [95% CI, –27.41 to 69.64]; P = .39) or cortical thickness (MBSR vs no MBSR: difference = –0.01 mm, [95% CI, –0.02 to 0.00], P = .10; exercise vs no exercise: difference, –0.01 mm [95% CI, –0.02 to 0.00]; P = .09). One exception was that hippocampal volume showed a significantly greater reduction over 18 months with MBSR compared with no MBSR (difference, –20.16 mm3 [95% CI, –33.88 to –6.44]; P = .004), contrary to the hypothesized direction of change; however, there was no significant intervention effect with exercise compared with no exercise (difference, –6.26 mm3 [95% CI, –19.98 to 7.46]; P = .37). There was also a main effect of time for hippocampal volume (P < .001) and DLPFC cortical thickness (P < .001) (but not DLPFC surface area [P = .68]), which declined in all groups over 18 months. There were no significant intervention effects on the secondary cognitive outcomes (Observed Tasks of Daily Living or Neurological Disorders Cognitive Function score; eFigure 2 in Supplement 2).
Figure 3. Structural Brain Changes Over 18 Months.
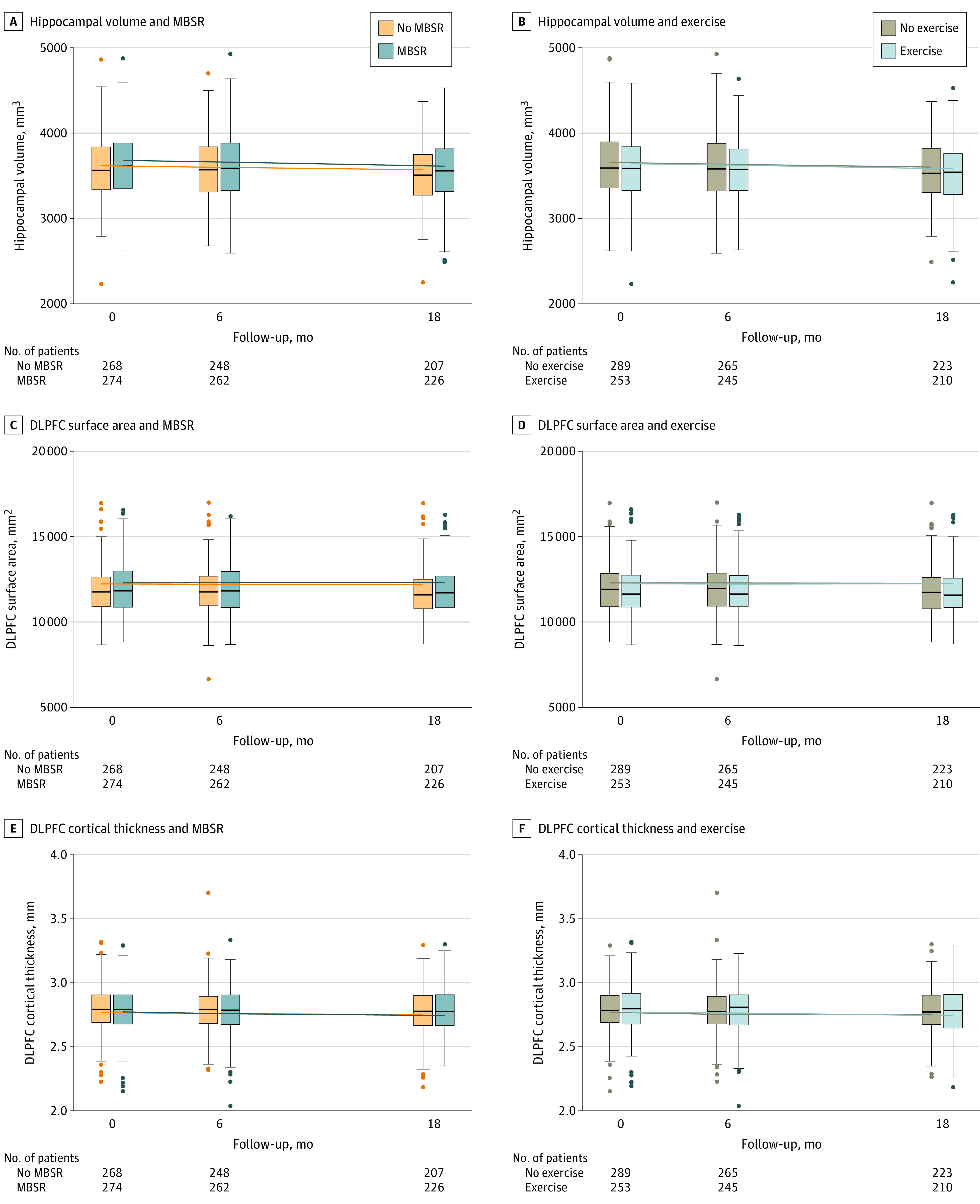
Shown are the mean of the right- and left-sided brain structures. The volumes of the brain regions in this article are somewhat dependent on the measurement technique; existing literature has found that both the volumes and their rate of change are consistent with studies in healthy aging. For example, Fraser et al37 found a rate of hippocampal atrophy of approximately 1% per year and Frangou et al38 reported a frontal cortical thickness change of 0.005 mm per year. These are within the range of changes reported in the current sample. The ranges for hippocampal volume, DLPFC surface area, and DLPFC cortical thickness are 2232 to 4926; 6642 to 16 992; and 2.0 to 3.7, respectively. See eTable 2 in Supplement 2 for numerical/model data of intervention effects. The boxplot inner horizontal lines represent the median values, the boxes represent the IQR (25% and 75%), the vertical whiskers extend to the upper and lower adjacent values (the furthest points within 1.5 IQRs of the 25th and 75th percentiles), and the dots indicate outlier values.
Tests of Combination MBSR and Exercise and Intervention Interactions
Interactions between the 2 factors in the 2 × 2 design (MBSR vs no MBSR and exercise vs no exercise) were tested. Because none of the interaction test results were significant at 6 months (memory composite, P = .93; executive function composite, P = .29; hippocampal volume, P = .76; DLPFC surface area, P = .19; and DLPFC cortical thickness, P = .52), the primary analyses described above were conducted by pooling the factorial groups. eTable 1 in Supplement 2 presents a 4-group analysis (MBSR alone, exercise alone, combined MBSR and exercise, and health education), along with full data on the 3-way interactions tested for the primary outcomes and secondary MRI outcomes. This comparison shows that combined MBSR and exercise showed no significant improvement compared with MBSR alone, exercise alone, or health education (eFigure 1 in Supplement 2).
Adherence to the Interventions and Per-Protocol Analysis
Participants had a median (IQR) attendance of 90% (80.0%-100.0%) at MBSR classes and 83.3% (71.7%-91.7%) at exercise classes in the first 6 months. eFigure 3 in Supplement 2 shows adherence to the interventions based on home practice and class attendance. eTable 2 in Supplement 2 compares intervention effects in the entire sample and the per-protocol subgroups; results are unchanged for all primary and secondary outcomes.
Post Hoc Analysis of Subgroups That Showed Putatively Beneficial Effects of Interventions
eTable 3 in Supplement 2 shows the effects of the interventions on multiple performance and physiological measures. Physical performance, aerobic fitness, and strength increased and sleep quality significantly improved (sleep latency was reduced and total sleep time was increased) with exercise (eTable 3A in Supplement 2). No variables were influenced by MBSR, including self-reported mindfulness (eTable 3B in Supplement 2).
Subgroups of participants who had the most change (top tertile) vs those who had the least change (bottom tertile) in these performance and physiological variables were then evaluated in terms of changes in their cognitive performance. eTable 4 in Supplement 2 quantifies these tertiles and eFigure 4 in Supplement 2 compares their episodic memory and executive function changes over 18 months. As shown in eFigure 4 in Supplement 2, there were, at most marginal, and, in the majority of cases, no differences in subgroups, which suggests limited to no evidence that MBSR or exercise differentially affected cognitive performance of participants in the top vs bottom tertiles; therefore, no inferential statistics were calculated.
Discussion
In this multicenter trial involving older adults with subjective cognitive concerns, mindfulness training, exercise, or both did not result in significant differences in improvement in episodic memory or executive function composite scores at 6 months. In secondary analyses, there were no significant improvements due to the interventions at 18 months in secondary outcomes, including structural brain measures of hippocampus and DLPFC. The findings do not support the hypothesis that these interventions improve cognitive performance in older adults.
These null findings differ from positive findings in some randomized clinical trials of exercise29 and epidemiological data that have suggested that exercise was associated with improved cognitive and brain health in older adults,30 as well as a smaller body of literature supporting the beneficial role of mindfulness.31 There are several potential causes for these null findings. First, all groups showed increases in cognitive performance over time, so it could be posited that all interventions (including health education) benefited participants equally and these increases reflect those benefits, and thus the study failed due to lack of a proper negative control. Arguing against this idea is that the health education intervention was designed for this study so that it would not specifically target cognition (eg, it did not include a mindfulness or exercise regimen). Further, if cognitive performance increases represented true benefits, one would expect to see a reflection of those benefits in brain structures (ie, increase or attenuated decrease in the size of hippocampus and DLPFC, structures involved in episodic memory, and executive function), yet both structures showed longitudinal declines with all conditions, consistent with age-related atrophy not attenuated by the interventions. In addition, the combination of MBSR and exercise showed no greater change than each intervention alone. Thus, the increases in cognitive performance likely reflect expectancy or practice effects from repeated exposure to the assessments.
Another potential cause of the null findings was failure in target engagement (ie, failure in having the desired effect from the interventions), which could result from poor participant adherence, low intervention fidelity by instructors, low intensity of interventions, or low reliability of outcome measures. However, none of these problems were apparent: participants demonstrated high adherence and retention in the study, instructors were trained and supervised for fidelity, the intensity of interventions was similar to that in prior trials, and outcome reliability was good. Furthermore, per-protocol analyses of participants that were more highly adherent to the interventions showed no significant differences from the overall sample. In the exercise intervention, physiological and performance changes suggest participants benefited from exercise. Thus, the findings are similar to the Lifestyle Interventions and Independence for Elders Study, which showed a beneficial effect of 24 months of exercise on disability prevention, but not cognitive performance.32 In contrast, MBSR was not associated with significant change in any physiological or performance measure, which raises the question of whether the implementation of MBSR was sufficient; however, given adequate instructor fidelity, participant class attendance, and home practice, the lack of a measurable effect of mindfulness training may reflect a lack of clearly-measurable targets in mindfulness-based intervention.
Another possibility accounting for lack of detectable effect of interventions is that participants were generally healthy and potentially insufficiently sedentary at baseline, thereby limiting potential for benefiting from lifestyle interventions. To test this, subgroup analyses of those who showed the greatest changes in physiological or performance variables posited to underlie cognitive health (eg, improved insulin sensitivity) were conducted. These analyses found that, even when the interventions produced beneficial changes in these putative mechanisms, they still did not lead to significant cognitive benefits. Thus, the health of the participants does not appear to explain the null results. As a whole, these results suggest that the underlying hypothesis is unsupported.
Limitations
This study has several limitations. First, the participants were largely White and the majority were college-educated; this limited diversity reduces generalizability of findings. Second, the study focused on structural characteristics of hippocampus and DLPFC as proxy measures of the brain’s health; other regions or assessment techniques might be more sensitive to intervention effects.33 Third, the study tested interventions over 18 months; a longer period of intervention may be needed to show beneficial effects. Fourth, the study focused on healthy older adults who were objectively cognitive intact; some studies have found beneficial effects of exercise on cognitive function in more physically or cognitively ill and frail older adults,34 as well as benefits of MBSR in older adults with depression and anxiety.7 Fifth, individuals with subjective cognitive concerns are a heterogeneous group that could include those with incipient dementia as well as individuals experiencing the influence of medications, medical conditions, or nutrition status. These and other potentially remediable mechanisms beyond cortisol, insulin sensitivity, and aerobic fitness were not examined in this study and should be considered in future research.
Conclusions
Among older adults with subjective cognitive concerns, mindfulness training, exercise, or both did not result in significant differences in improvement in episodic memory or executive function composite scores at 6 months. The findings do not support the use of these interventions for improving cognition in older adults with subjective cognitive concerns.
Statistical analysis plan
eMethods and eResults
Data sharing statement
References
- 1.Bettio LEB, Rajendran L, Gil-Mohapel J. The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev. 2017;79:66-86. doi: 10.1016/j.neubiorev.2017.04.030 [DOI] [PubMed] [Google Scholar]
- 2.Head D, Kennedy KM, Rodrigue KM, Raz N. Age differences in perseveration: cognitive and neuroanatomical mediators of performance on the Wisconsin Card Sorting Test. Neuropsychologia. 2009;47(4):1200-1203. doi: 10.1016/j.neuropsychologia.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653-666. doi: 10.1038/s41582-018-0070-3 [DOI] [PubMed] [Google Scholar]
- 4.Crane RS, Brewer J, Feldman C, et al. What defines mindfulness-based programs? the warp and the weft. Psychol Med. 2017;47(6):990-999. doi: 10.1017/S0033291716003317 [DOI] [PubMed] [Google Scholar]
- 5.Lao SA, Kissane D, Meadows G. Cognitive effects of MBSR/MBCT: a systematic review of neuropsychological outcomes. Conscious Cogn. 2016;45:109-123. doi: 10.1016/j.concog.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 6.Brand S, Holsboer-Trachsler E, Naranjo JR, Schmidt S. Influence of mindfulness practice on cortisol and sleep in long-term and short-term meditators. Neuropsychobiology. 2012;65(3):109-118. doi: 10.1159/000330362 [DOI] [PubMed] [Google Scholar]
- 7.Wetherell JL, Hershey T, Hickman S, et al. Mindfulness-based stress reduction for older adults with stress disorders and neurocognitive difficulties: a randomized controlled trial. J Clin Psychiatry. 2017;78(7):e734-e743. doi: 10.4088/JCP.16m10947 [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Pinilla F, Hillman C. The influence of exercise on cognitive abilities. Compr Physiol. 2013;3(1):403-428. doi: 10.1002/cphy.c110063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166-1170. doi: 10.1093/gerona/61.11.1166 [DOI] [PubMed] [Google Scholar]
- 10.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017-3022. doi: 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voss MW, Jain S. Getting fit to counteract cognitive aging: evidence and future directions. Physiology (Bethesda). 2022;37(4):0. doi: 10.1152/physiol.00038.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wetherell JL, Ripperger HS, Voegtle M, et al. ; MEDEX Research Group . Mindfulness, Education, and Exercise for age-related cognitive decline: study protocol, pilot study results, and description of the baseline sample. Clin Trials. 2020;17(5):581-594. doi: 10.1177/1740774520931864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140(6):734-739. doi: 10.1176/ajp.140.6.734 [DOI] [PubMed] [Google Scholar]
- 14.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4(1):33-47. doi: 10.1016/0163-8343(82)90026-3 [DOI] [PubMed] [Google Scholar]
- 15.Stahl B, Goldstein E. A Mindfulness-Based Stress Reduction Workbook. New Harbinger Pubns Inc; 2010. [Google Scholar]
- 16.Haddad R, Lenze EJ, Nicol G, Miller JP, Yingling M, Wetherell JL. Does patient expectancy account for the cognitive and clinical benefits of mindfulness training in older adults? Int J Geriatr Psychiatry. 2020;35(6):626-632. doi: 10.1002/gps.5279 [DOI] [PubMed] [Google Scholar]
- 17.Lorig K, Holman H, Sobel D. Living a Healthy Life With Chronic Conditions: Self-Management of Heart Disease, Arthritis, Diabetes, Depression, Asthma, Bronchitis, Emphysema and Other Physical And Mental Health Conditions. Bull Publishing; 2012. [Google Scholar]
- 18.Crane RS, Eames C, Kuyken W, et al. Development and validation of the mindfulness-based interventions: teaching assessment criteria (MBI:TAC). Assessment. 2013;20(6):681-688. doi: 10.1177/1073191113490790 [DOI] [PubMed] [Google Scholar]
- 19.Lenze EJ, Hickman S, Hershey T, et al. Mindfulness-based stress reduction for older adults with worry symptoms and co-occurring cognitive dysfunction. Int J Geriatr Psychiatry. 2014;29(10):991-1000. doi: 10.1002/gps.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11)(suppl 3):S54-S64. doi: 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson JD, Balota DA, Duchek JM, Head D. White matter integrity and reaction time intraindividual variability in healthy aging and early-stage Alzheimer disease. Neuropsychologia. 2012;50(3):357-366. doi: 10.1016/j.neuropsychologia.2011.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Schie MK, Thijs RD, Fronczek R, Middelkoop HA, Lammers GJ, Van Dijk JG. Sustained attention to response task (SART) shows impaired vigilance in a spectrum of disorders of excessive daytime sleepiness. J Sleep Res. 2012;21(4):390-395. doi: 10.1111/j.1365-2869.2011.00979.x [DOI] [PubMed] [Google Scholar]
- 23.Balota DA, Tse CS, Hutchison KA, Spieler DH, Duchek JM, Morris JC. Predicting conversion to dementia of the Alzheimer’s type in a healthy control sample: the power of errors in Stroop color naming. Psychol Aging. 2010;25(1):208-218. doi: 10.1037/a0017474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donohue MC, Sperling RA, Salmon DP, et al. ; Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing; Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease Cooperative Study . The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71(8):961-970. doi: 10.1001/jamaneurol.2014.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402-1418. doi: 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder AZ, Nishino T, Shimony JS, et al. Covariance and correlation analysis of resting state functional magnetic resonance imaging data acquired in a clinical trial of mindfulness-based stress reduction and exercise in older individuals. Front Neurosci. 2022;16:825547. doi: 10.3389/fnins.2022.825547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diehl M, Marsiske M, Horgas AL, Rosenberg A, Saczynski JS, Willis SL. The revised observed tasks of daily living: a performance-based assessment of everyday problem solving in older adults. J Appl Gerontol. 2005;24(3):211-230. doi: 10.1177/0733464804273772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cella D, Lai JS, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860-1867. doi: 10.1212/WNL.0b013e318258f744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner DT, Hu MX, Generaal E, et al. Physical exercise interventions targeting cognitive functioning and the cognitive domains in nondementia samples: a systematic review of meta-analyses. J Geriatr Psychiatry Neurol. 2021;34(2):91-101. doi: 10.1177/0891988720915523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregory MA, Gill DP, Petrella RJ. Brain health and exercise in older adults. Curr Sports Med Rep. 2013;12(4):256-271. doi: 10.1249/JSR.0b013e31829a74fd [DOI] [PubMed] [Google Scholar]
- 31.Whitfield T, Barnhofer T, Acabchuk R, et al. The effect of mindfulness-based programs on cognitive function in adults: a systematic review and meta-analysis. Neuropsychol Rev. 2022;32(3):677-702. doi: 10.1007/s11065-021-09519-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sink KM, Espeland MA, Castro CM, et al. ; LIFE Study Investigators . Effect of a 24-Month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE randomized trial. JAMA. 2015;314(8):781-790. doi: 10.1001/jama.2015.9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weng TB, Pierce GL, Darling WG, Falk D, Magnotta VA, Voss MW. The acute effects of aerobic exercise on the functional connectivity of human brain networks. Brain Plast. 2017;2(2):171-190. doi: 10.3233/BPL-160039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi PG, Carnavale BF, Farche ACS, Ansai JH, de Andrade LP, Takahashi ACM. Effects of physical exercise on the cognition of older adults with frailty syndrome: a systematic review and meta-analysis of randomized trials. Arch Gerontol Geriatr. 2021;93:104322. doi: 10.1016/j.archger.2020.104322 [DOI] [PubMed] [Google Scholar]
- 35.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73-86. doi: 10.1016/S0005-7916(00)00012-4 [DOI] [PubMed] [Google Scholar]
- 36.Stierman B, Afful J, Carroll MD, et al. National Health and Nutrition Examination Survey 2017-March 2020 Prepandemic Data Files: Development of Files and Prevalence Estimates for Selected Health Outcomes; National Center for Health Statistics; 2021 Accessed November 16, 2022. https://stacks.cdc.gov/view/cdc/106273
- 37.Fraser MA, Shaw ME, Cherbuin N. A systematic review and meta-analysis of longitudinal hippocampal atrophy in healthy human ageing. NeuroImage. 2015/05/15/ 2015;112:364-374. doi: 10.1016/j.neuroimage.2015.03.035 [DOI] [PubMed] [Google Scholar]
- 38.Frangou S, Modabbernia A, Williams SCR, et al. ; Karolinska Schizophrenia Project (KaSP) . Cortical thickness across the lifespan: data from 17,075 healthy individuals aged 3-90 years. Hum Brain Mapp. 2022;43(1):431-451. doi: 10.1002/hbm.25364 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical analysis plan
eMethods and eResults
Data sharing statement


