Abstract
We previously identified the Legionella pneumophila ptsP (phosphoenolpyruvate phosphotransferase) ortholog gene as a putative virulence factor in a study of signature-tagged mutagenesis using a guinea pig pneumonia model. In this study, we further defined the phenotypic properties of L. pneumophila ptsP and its complete sequence. The L. pneumophila ptsP was 2,295 bases in length. Its deduced amino acid sequence had high similarity with ptsP orthologs of Pseudomonas aeruginosa, Azotobacter vinelandii, and Escherichia coli, with nearly identical lengths. Here we show that while the mutant grew well in laboratory media, it was defective in both lung and spleen multiplication in guinea pigs. It grew slowly in guinea pig alveolar macrophages despite good uptake into the cells. Furthermore, there was minimal growth in a human alveolar epithelial cell line (A549). Transcomplementation of the L. pneumophila ptsP mutant almost completely rescued its growth in alveolar macrophages, in A549 cells, and in guinea pig lung and spleen. The L. pneumophila ptsP mutant was capable of evasion of phagosome-lysosome fusion and resided in ribosome-studded phagosomes. Pore formation activity of the mutant was normal. The L. pneumophila ptsP mutant expressed DotA and IcmX in apparently normal amounts, suggesting that the ptsP mutation did not affect dotA and icmX regulation. In addition, the mutant was resistant to serum and neutrophil killing. Taken together, these findings show that L. pneumophila ptsP is required for full in vivo virulence of L. pneumophila, most probably by affecting intracellular growth.
Legionella pneumophila is the most common etiologic agent of Legionnaires' disease, a type of pneumonia affecting immunocompromised and immunocompetent humans (13). This gram-negative bacterium is a facultative intracellular parasite of mononuclear cells in vivo and in vitro (19) and evades phagosome-lysosome fusion within these cells (16). The phagosomes harboring L. pneumophila are studded by ribosomes during certain periods (37). Several L. pneumophila virulence factors facilitating intracellular growth have been identified in screens using macrophages or macrophage-like cell lines (24, 25). One important set of virulence factors is the dot/icm system, which is required for evasion of phagosome-lysosome fusion (3, 34, 35) and establishment of the phagosomes permissive for growth of L. pneumophila within them (6).
In a previous study, we described a broad range of potential L. pneumophila virulence genes in a guinea pig pneumonia model by using a signature-tagged mutagenesis method (12). In that study, three different classes of macrophage virulence phenotypes were discovered. One group of mutants had a markedly reduced ability to multiply within macrophages and included mutants of the already known dot/icm complex (3, 35). Another group of mutants was able to multiply efficiently within macrophages. A third group of mutants had an initial defect in intracellular multiplication but were able to multiply in macrophages as well as the wild-type strain after prolonged incubation. Partial sequencing of the transposon-interrupted genes of two prototrophic mutants of this third group showed homology to the Escherichia coli phosphoenolpyruvate phosphotransferase (ptsP) (33).
The E. coli ptsP ortholog facilitates nitrogen utilization via a complex two-component sensing and regulatory phosphate transfer system. The E. coli ptsP gene encodes enzyme INtr (EINtr), consisting of two domains: an N-terminal domain of 127 amino acids homologous to the N-terminal sensory domain of the NifA protein of Azoto bacter vinelandii, and a C-terminal domain of 578 amino acids homologous to all other currently sequenced EI proteins (33). Sequence analysis suggests that EINtr serves a sensory function linking carbon and nitrogen metabolism (33). The C-terminal domain of EINtr transfers a phosphate from phosphoenolpyruvate to a histidine residue of the phosphocarrier protein NPr (32). NPr in turn transfers a phosphate to the cell membrane EIIANtr, which has a role in the regulation of ς54 -dependent transcriptional initiation of genes concerned with organic nitrogen utilization (31). NPr and EIIANtr are encoded in the rpoN operon, suggesting that ptsP is involved in the transcriptional regulation of rpoN-dependent operons (31).
In this study, the L. pneumophila ptsP ortholog gene was completely sequenced and its deduced amino acid sequence was analyzed. The ptsP mutant was characterized phenotypically, and complementation studies were performed to confirm that the interrupted gene itself was required for in vivo and in vitro virulence.
MATERIALS AND METHODS
Bacteria and plasmids.
L. pneumophila serogroup1 strain AA100jm (12) is a spontaneous streptomycin-resistant mutant of strain 130b (28) which is virulent in guinea pigs, macrophages, and amoebae (12, 29). Clones 47:3h and 47:4a are ptsP mutants, and clone 47:2f is a dotO mutant of AA100jm. dotO is within the icm/dot gene cluster and involved in intracellular growth and evasion of the endocytic pathway (1). They were made by transposon mutagenesis of AA100jm using Tn903HT (Tn903 harboring a signature tag) (12). L. pneumophila strains were grown at 35°C in a humidified incubator either on MOPS [3-(N-morpholino)propanesulfonic acid]-buffered charcoal yeast extract agar medium supplemented with α-ketoglutarate (BCYE-α) (11) or in ACES [N-(2-acetamido)-2-aminoethanesulfonic acid]-buffered yeast extract broth supplemented with α-ketoglutarate (BYE-α) (11). E. coli K-12 and K29 were serum sensitive and serum resistant, respectively (17), and were gifts from Marcus Horwitz. E. coli strain XL-1 Blue (Stratagene) was grown at 37°C either on Luria-Bertani agar or in Luria-Bertani broth. Selective antimicrobial agents were added to the growth media when appropriate and included kanamycin (30 μg/ml), streptomycin (200 μg/ml), and chloramphenicol (10 [L. pneumophila] or 30 [E. coli] μg/ml). Plasmid pSU2719 (26) was a gift from Nicolas Cianciotto. Plasmid pSU2719 carries a P15A replicon, chloramphenicol acetyltransferase, and LacZα and can multiply within L. pneumophila. Plasmid pUC18 was purchased from Life Technologies, Gaithersburg, Md.
Nucleic acid manipulation.
All nucleic acid manipulations were accomplished according to standard molecular biology techniques (2).
Complete sequencing of the ptsP gene.
Genomic DNA from mutant clone 47:3h (AA100jm ptsP838::Tn903HT) or mutant clone 47:4a (AA100jm ptsP1240::Tn903HT) was digested with restriction enzymes known not to cut the transposon insertion upstream of the kanamycin resistance gene (Kmr) cassette. Digested DNA was ligated into pUC18, which was appropriately digested. E. coli strain XL-1 Blue was transformed with the ligated product by electroporation. Plasmid DNA of Kmr transformants was restriction digestion mapped to confirm proper insertion of the desired DNA fragment into the plasmid. Plasmid DNA was purified by using a Quiagen spin filter (Quiagen), and the insert DNA was sequenced by a primer walking technique. An ABI Big Dye Taq FS terminator sequencing kit (Applied Biosystems) was used to synthesize the dye-terminated DNA, which then was sequenced by using an ABI 377 automated sequencer (University of Pennsylvania Sequencing Facility). Whole sequence data were analyzed and aligned using SeqMan II software, version 4.03 (DNASTAR Inc., Madison, Wis.). GenBank sequence database searching was performed with the BLASTX and BLASTN search algorithms. Deduced amino acid sequences were analyzed with Motif (http://www.motif.genome.ad.jp) and SOSUI (http://azusa.proteome.bio.tuat.ac.jp/sosui/). Multiple sequence alignments were performed using Megalign, version 4.03, with the Jotun Hein method (DNASTAR).
Macrophages and alveolar epithelial cells.
Guinea pig alveolar macrophages were prepared as previously described (12) and cultured in medium 199 (M199; Life Technologies) supplemented with 10% fetal bovine serum (Bio Whittaker, Walkersville, Md.). A549, a human alveolar epithelial cell line received as a gift from Michael Beers, was maintained in M199–10% fetal bovine serum. A549 cells were harvested at logarithmic growth phase, using 10 mM EDTA-phosphate-buffered saline (PBS) solution and 5% trypsin–PBS. The alveolar epithelial cells (1.25 × 105/well) were cultured overnight in 24-well culture tray in 5% CO2 air at 37°C and then used for experiments. Murine bone marrow-derived macrophages were prepared from A/J mice as reported previously (34).
Serum killing assay.
Normal serum was collected from healthy guinea pigs. The antibody titer of the serum against L. pneumophila SG1 was 1:32, as measured by indirect immunofluorescence, as described previously, but modified to detect guinea pig antibodies by use of fluorescein-labeled goat anti-guinea pig immunoglobulin G antibody (ICN Biomedicals, Aurora, Ohio) (8). Immune guinea pig serum was obtained from animals infected with sublethal doses of L. pneumophila and had an indirect immunofluorescence assay titer of 1:256. Serum was heat inactivated at 56°C for 30 min when needed. Bacteria (108 CFU/ml) were incubated in phosphate buffer supplemented with Ca2+ and Mg2+ (pH 7.35) with or without serum at 37°C for 1 h. The suspensions were then diluted in decimal dilutions with Mueller-Hinton broth (MHB) and plated onto BCYE-α agar plates, which were incubated for 3 days. Surviving bacteria were enumerated by counting CFU on the plates.
Neutrophil killing assay.
Human peripheral polymorphonuclear leukocytes were purified by density gradient centrifugation and dextran sedimentation as described previously (4). The killing assay was performed using 20% human serum, as previously described (18), with one exception. M199 was used to suspend the bacteria and neutrophils rather than Hanks' balanced salt solution, as the Legionella bacteria were killed by the salt solution.
Determination of flagellation.
Plate-grown Legionella bacteria were suspended in sterile distilled water and stained for the presence of flagellae, using the Ryu stain (Remel Laboratories, Lenexa, Kans.), as described previously (9, 22).
Invasion assay.
Guinea pig alveolar macrophages were infected with bacterial (multiplicity of infection [MOI] at 50) in 24-well microplates, after which the plates were centrifuged at 100 × g for 8 min at room temperature. The infected macrophages were then incubated at 37°C in 5% CO2 air for 2 h. The infected macrophages were washed with warm M199 three times and incubated with or without gentamicin (50 μg/ml) for 1 h. The macrophages were then washed with warm M199 three times. The infected macrophages were harvested at indicated points in sterile distilled water and then lysed by vortex mixing for 1 min. The lysate was plated quantitatively on BCYE-α medium. A separate experiment showed that there were no significant increases in intracellular bacterial concentrations during the 1-h gentamicin incubation period (data not shown).
Intracellular growth assay.
Macrophages or alveolar epithelial cells were prepared as described above, infected with L. pneumophila (MOI of 0.1), and then incubated in 5% CO2 air at 37°C. Culture supernatants were harvested at indicated times, diluted appropriately with MHB, and then plated onto BCYE-α agar plates. In some experiments the cultured cells were lysed in the tissue culture wells either by low-energy sonication or by hypotonic lysis with distilled water; neither method affects the viability of L. pneumophila.
Pore formation assay.
L. pneumophila contact-induced pore formation in the macrophage membrane was assayed as described previously (40). In these assays, L. pneumophila was added at the given MOIs to 1.5 × 105 mouse bone marrow-derived macrophages plated on coverslips in 24-well microplates. Rabbit anti-L. pneumophila polyclonal antibody was added to each well. The tissue culture plates were centrifuged at 150 × g for 5 min at room temperature and incubated for 1 h at 37°C. The coverslips were then stained with ethidium bromide (25 μg/ml) and acridine orange (5 μg/ml). All cells will stain with acridine orange, whereas an intact macrophage membrane will exclude ethidium bromide. Pore-forming activity was measured as the percentage of macrophages that stain positive with ethidium bromide. Coverslips were examined with a Zeiss Axioplan II microscope. A rhodamine bandpass filter set was used to detect ethidium bromide, and a fluorescein isothiocyanate bandpass filter set was used to detect acridine orange staining.
Phagosome trafficking assay.
Trafficking of phagosomes harboring L. pneumophila within murine bone marrow-derived macrophages was assayed as described previously (34). Briefly, the macrophages (8 × 104) on glass coverslips in a 24-well microplate were infected with L. pneumophila at an MOI of 50. The plates were centrifuged at 150 × g for 5 min at room temperature to optimize bacterial uptake. Infected macrophages were incubated for 30 min at 37°C in 5% CO2 air; then the cells were washed and fixed in paraformaldehyde. The coverslips were immersed in ice-cold methanol for 10 s to permeabilize the cells and then blocked in PBS containing 2% goat serum for 1 h. Lysosomes were stained with anti-murine Lamp-1 rat antibody (1D4B; 1:250), followed by fluorescein isothiocyanate-labeled anti-rat immunoglobulin secondary antibody (1:250). Bacteria were stained with 4′,6-diamidino-2-phenylindole. All antibody washes were in PBS. Coverslips were inverted onto 2 μl of mounting media before viewing. Bacterial phagosomes were scored for Lamp-1 staining by standard epifluorescence microscopy.
Electron microscopic observation.
Guinea pig alveolar macrophages were cultured on sterile plastic coverslips and then infected with L. pneumophila (MOI of 0.1). The infected cells were incubated for 2 or 3 days. Then the infected macrophages were fixed with 2% glutaraldehyde in PBS and washed with ice-cold PBS. Fixed materials were processed using standard technique and examined in an electron microscope (Biomedical Image Core Facility, University of Pennsylvania).
Trans-complementation of L. pneumophila ptsP mutation.
A DNA fragment containing the L. pneumophila ptsP gene was amplified by the PCR from L. pneumophila AA100jm genomic DNA, using the upstream sense primer (mu-3h10; 5′-AATACTGCAGTGGGTGGATTTTCAT-3′) and the downstream antisense primer (mu-3h11; 5′-TTAGGATCCCGCCATTATTCCTG-3′). BamHI and PstI sites respectively (underlined), were incorporated into these primers. Amplification was performed using Vent polymerase (New England Biolabs, Beverly, Mass.). The amplified products of the ptsP gene were digested with BamHI and PstI and then ligated with pSU2719, which had been digested with appropriate enzymes and dephosphorylated. E. coli XL-1 Blue was transformed with the ligated product by electroporation. Plasmid DNA of chloramphenicol-resistant (Cmr) transformants was restriction digested-mapped to confirm proper insertion of the desired DNA fragment into the plasmid. The cloned ptsP gene was sequenced as stated above to verify no erroneous incorporation of nucleotides during amplification. A single clone containing the whole ptsP gene was picked for further study, and the plasmid was designated pHT28a. Plasmid DNA was purified by using a Quiagen spin filter. The ptsP mutant of AA100jm, clone 3h, was transformed with pHT28a by electroporation. Plasmid DNAs from several Cmr transformants were restriction digestion mapped to confirm the presence of the desired plasmid. One of the transformants containing the desired plasmid was picked for further studies and designated HT31a. PCR testing of HT31a using ptsP-specific primers showed that it contained the full-length ptsP gene, in contrast to the noncomplemented mutant. Empty pSU2719 was electroporated into the mutant (clone 47:3h) as well the parent (AA100jm) to serve as negative controls; these were designated HT32a and HT34a, respectively.
Animal model.
The guinea pig model of L. pneumophila pneumonia was used as described previously (10). L. pneumophila was grown in BYE-α broth under the appropriate selective conditions and was diluted in sterile water at a concentration of 3.3 × 106 CFU/ml; 106 CFU was injected into the surgically exposed tracheas of Hartley strain male guinea pigs weighing ∼250 g. The animals were killed 2 days later. The right lower lung lobe and spleen were removed aseptically, weighed and ground in MHB, and then diluted in the same broth type in decimal dilutions. Diluted tissue homogenates were plated onto BCYE-α with or without kanamycin. Another experiment extended the postinfection observation time to 7 days.
Immunoblot analysis.
To identify expression of DotA protein and IcmX protein in the ptsP mutant and its parent, Western blotting was used (27, 34). Plate-grown colonies of bacteria were sonicated (for DotA) or boiled (for IcmX), then lysed in Laemmli sample buffer, and applied to a sodium dodecyl sulfate-polyacrylamide gel. Separated proteins were transferred electrophoretically to Immobilon-P membranes (Millipore). The membranes were probed with a polyclonal rabbit antibody against DotA (gift from Craig R. Roy; 1:1,000) or a polyclonal rabbit antibody against IcmX (gift from Craig R. Roy; 1:500) and alkaline-phosphatase conjugated anti-rabbit secondary antibody (Boehringer Mannheim). The proteins were visualized using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate. All blocking and antibody dilutions were performed in 1 × PBS containing 5% nonfat dry milk and 0.1% Tween 20.
Nucleotide sequence accession number.
The ptsP sequence has been deposited in the GenBank database at the National Center for Biotechnology Information under accession number AF181870.
RESULTS
Complete sequence of the ptsP gene.
Sequencing of the region surrounding the transposon insertion sites of mutant clones 47:3h and 47:4a was completed and verified for a region 3,624 bp in length. The largest open reading frame (ORF1) consisted of 2,295 bp. The two transposon insertion sites were within this ORF, 827 and 1241 bp downstream of the start site, for clones 47:3h and 47:4a, respectively (Fig. 1). No significant homology was discovered with sequences deposited in the nonredundant GenBank database, using the BLASTN search algorithm. However, use of the BLASTX search algorithm revealed that ORF1 had high homology with the EINtrptsP gene of Pseudomonas aeruginosa (score, 825; identities, 413/759 [54%]; positives, 551/759 [72%]; expected, 0.0), the phosphotransferase EI of A. vinelandii (822; 413/759 [54%]; 558/759 [73%]; 0.0), and the ptsP EINtr of E. coli (610; 337/767 [43%]; 471/767 [56%]; e−173). ORF1 was designated L. pneumophila ptsP. All four of these homologous proteins shared many conserved regions, especially the region around the histidine residue that is the putative phosphorylation site. Also highly conserved was the region that represents the motif of the phosphoenolpyruvate-utilizing enzyme signature 2 (Prosite PS00472; positions 628 to 646 in amino acid sequence of L. pneumophila ptsP). Both the N-terminal and C-terminal domains of the E. coli EINtr were highly conserved in the L. pneumophila ptsP. SOSUI analysis (15) predicted that L. pneumophila ptsP is a soluble protein and located in the inner membrane.
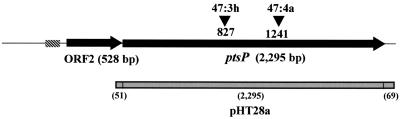
FIG. 1.
Scheme of the ptsP operon. Each arrow represents an ORF. The transposon insertion sites of mutants 47:3h and 47:4a are shown as arrowheads. The gene region used for complementation is shown as a halftone box. The nucleotide numbering refers to that of L. pneumophila ptsP as published in GenBank (AF181870).
A second ORF, designated ORF2, was immediately upstream of the ptsP ortholog and in the same reading frame. This 528-bp ORF shared significant homology with the P. aeruginosa invasion protein homolog gene (invA; score, 184; identity, 83/147 [56%]; positives, 111/147 [75%] expected, 4e−46; AF116285). These ptsP and invA orthologs are in contiguous regions and in the same orientation in both bacteria (39). ORF2 was also homologous to MutT-like proteins in a variety of other bacteria and to invA of Rickettsia prowazekii and other bacteria. No putative promoter regions were identified immediately upstream of the ptsP ortholog, but a possible promoter was found starting 39 bp upstream of ORF2 (http://www.fruitfly.org/seq_tools/promoter.html).
Growth of the ptsP mutants and complemented mutants in guinea pigs.
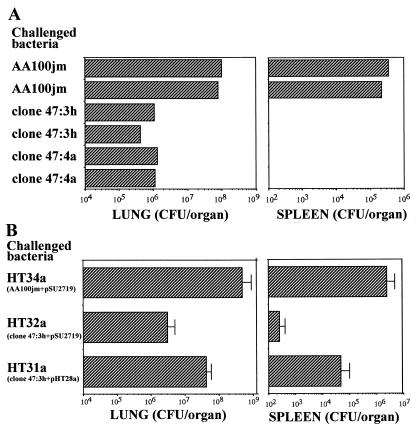
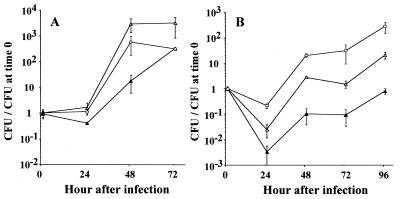
Intrapulmonary growth of two different ptsP mutants was assessed using a guinea pig pneumonia model. Two days after intratracheal inoculation of guinea pigs, the parent strain multiplied by at least 100-fold in the lungs, whereas neither of the mutants multiplied in the lungs (Fig. 2). Parent, but not mutant, strain bacteria were recovered in high concentrations from the spleens. Three of four animals inoculated with the mutant bacteria had no detectable bacteria recovered from their spleens, and the fourth animal's spleen contained bacteria in a concentration just above the detection limit of 100 CFU/spleen. Concentrations of the mutant in the lung and spleen were about 1 and <0.02%, respectively, of the parent concentrations for the same organs in different animals.
FIG. 2.
(A) Growth of ptsP mutants generated by transposon insertion (clones 47:3h and 47:4a) and their parent (AA100jm) in guinea pigs. Bacteria (106 CFU) were injected into the surgically exposed tracheas of guinea pigs. The animals were killed 2 days later, and their lungs and spleens were recovered aseptically. Bacterial burdens in the lungs and the spleens of infected animals were determined as stated in the text. (B) Recovery of L. pneumophila from guinea pig lung and spleen for the transcomplemented ptsP mutant HT31a (ptsP mutant carrying pHT28a), for the mutant with empty plasmid HT32a (ptsP mutant carrying pSU2719), or for the parent with empty plasmid, HT34a (parent carrying pSU2719). Plasmid pSU2719 is the plasmid vector; pHT27a consists of pSU2719 and the ptsP ORF. These strains were inoculated into guinea pig tracheas, and bacterial burdens in the lungs and spleens were determined 2 days later.
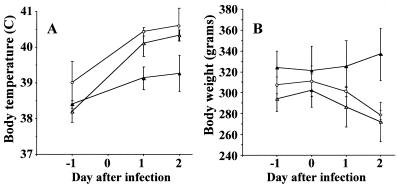
The transcomplemented mutant, HT31a, had partial restoration of the parenteral phenotype (HT34a) in the guinea pig pneumonia model and was recovered from both lung and spleen in 10- and 100-fold-higher concentrations, respectively, than the mutant with empty vector, HT32a (Fig. 2). Animals infected with HT31a experienced significant weight loss and fever, in contrast to animals infected with HT32a, which had neither significant weight loss nor fever (Fig. 3). There was considerable in vivo plasmid loss from all three strains; about 27.5, 1.9 and 5.8% of bacteria recovered from the lungs of animals challenged with HT31a, HT32a, and HT34a, respectively, were Cmr, whereas 100% of the starting inocula for all three strains were Cmr. All eight randomly chosen Cmr colonies of HT31a isolated from guinea pig lungs contained the expected plasmid and complete ptsP gene, by plasmid restriction mapping and PCR testing, respectively. This confirmed that the HT31a animal group had indeed received HT31a. The overall results showed that a large portion of virulence defect of the ptsP mutants could be attributed to a mutation in ptsP, although additional downstream virulence genes could not be completely excluded.
FIG. 3.
Signs of L. pneumophila pulmonary infection. Rectal temperature (A) and body weight (B) of guinea pigs infected with HT31a (ptsP mutant carrying pHT28a; open triangles), HT32a (ptsP mutant carrying pSU2719; closed triangles), or HT34a (parent carrying pSU2719; open circles) were monitored between days 0 and 2 after inoculation of the bacteria. Each point represents the mean ± SD from three animals.
To determine if the ptsP mutant would cause delayed disease in the guinea pig, mirroring the delayed growth of the mutant in guinea pig alveolar macrophages, four guinea pigs each were infected with either the parent (1.4 × 106 CFU/animal) or ptsP mutant (1.9 × 106 CFU/animal) and observed for 7 days postinfection. All four guinea pigs infected with the parent strain appeared clinically ill, developed fever (≥ + 1.5°C change from baseline), and exhibited weight loss (mean, −21% from baseline); three of four animals died of pneumonia by day 5 postinfection, and one survived to day 7. In contrast, all four animals infected with the mutant bacterium appeared clinically well, survived to 7 days postinfection, and gained weight (mean, +18% from baseline). The only evidence of disease in the animals infected with the mutant bacterium was a slight (mean, +0.4°C), though statistically significant (P = 0.01, paired t test), increase in body temperature on postinfection day 2 only. Of note, this slight fever peak occurred 1 day later than did the maximum fever observed for the parent-infected animals. Postmortem findings in the two animal groups showed that the animals infected with the ptsP mutant had significantly lower lung weights (mean, 6.2 versus 3.0 g), bacterial lung counts (mean, 2.4 × 109 versus 6.7 × 103 CFU/lung), and bacterial spleen counts (9.6 × 103 versus 4.5 × 101 CFU/spleen) than did animals infected with the parent strain (P < 0.03 for all comparisons). These findings show that ptsP is required for full virulence of L. pneumophila in guinea pigs and that delayed animal virulence is not observed for infection with a ptsP mutant.
Intracellular growth and uptake characteristics.
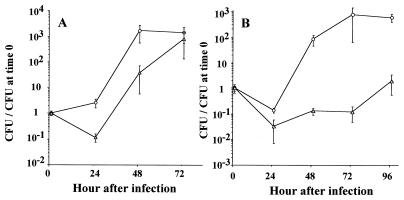
To determine if the reduced guinea pig virulence of the ptsP mutant was due to an intracellular growth defect, we examined its growth within explanted guinea pig alveolar macrophages and a human alveolar epithelial cell line. The parent strain grew well within both cell types, contrast to the deficient growth of the mutant (Fig. 4). In alveolar macrophages, the ptsP mutant supernatant concentration decreased on day 1 in comparison to its starting concentration and that of the parent strain, but there after the mutant grew as fast as the parent (Fig. 4A).
FIG. 4.
Growth of L. pneumophila within guinea pig alveolar macrophages and human alveolar epithelial cells. (A) Guinea pig alveolar macrophage Triangles, ptsP mutant (clone 47:3h); circles, parent (AA100jm). (B) A549, human alveolar epithelial cell line. Symbols have the same meaning as in panel A. CFU in each well was determined at indicated times. Each time point represents mean ± SD of triplicate wells. Bacterial growth is expressed as the log10 of the ratio of the bacterial concentration at the indicated time to the bacterial concentration at the start of the experiment.
To exclude the possibility that growth of the mutant was normal in alveolar macrophages but blocked from cellular release, several additional studies were performed with alveolar macrophages. First, microscopic examination of ptsP mutant-infected alveolar macrophages showed that the infected macrophages appeared morphologically identical to those observed for the parent strain-infected macrophages. Specifically, there was not an abundance of nonlysed heavily infected macrophages for the ptsP mutant-infected macrophages. Second, a study that examined the parent and mutant bacterial concentrations from cell culture lysates and supernatants showed that the ptsP mutant had the same growth defect versus the parent strain, for both the tissue culture lysates and supernatants (data not shown).
In contrast to findings in alveolar macrophages, the ptsP mutant apparently did not multiply at all within the A549 human alveolar epithelial cell line, whereas the parent strain grew well (Fig. 4B). Subsequent experiments that examined A549 cell lysates showed that there was very slow intracellular growth of the ptsP mutant in A549 cells, which was masked by the higher extracellular bacterial concentration in the supernatant alone. By day 4 of the experiment, the concentration of the ptsP mutant in the lysate was equivalent to that of the extracellular concentration (data not shown). This was in contrast to the roughly equivalent concentrations of the parent strain in the supernatant and cell lysate from day 1 on.
Because the initial slow growth of the ptsP mutant in the guinea pig alveolar macrophages could be due to reduced uptake of the mutant by the macrophages, invasion of macrophages by the bacterium was assessed using a gentamicin protection assay. This showed that the fractions of internalized bacteria were about the same for the both the parent and mutant strains. The total number of bacteria for macrophages infected with the parent strain was (48 ± 14 [standard deviation {SD}]) × 104 CFU and the number of intracellular bacteria remaining after gentamicin incubation was (6.2 ± 1.2) × 104 CFU, or an intracellular-to-total bacteria ratio of 13%. For the 47:3h ptsP mutant, the respective numbers were (32 ± 9) × 104 CFU, (6.8 ± 2) × 104 CFU, and 21%.
Effect of ptsP transcomplementation on intracellular growth.
To determine if transcomplementation of the ptsP mutant restored its ability to grow within cells, it was used to infect guinea pig alveolar macrophages and A549 cells. The transcomplemented mutant (HT31a) grew within macrophages almost as well as its parent containing empty vector, HT34a (Fig. 5A). Complementation of ptsP also restored growth of the mutant within A549 cells to levels intermediate between the mutant containing empty vector and the parent with empty vector (Fig. 5B).
FIG. 5.
Complementation of ptsP in trans rescues the intracellular growth of L. pneumophila within guinea pig alveolar macrophages and human alveolar epithelial cells. (A) Guinea pig alveolar macrophage. (B) A549, human alveolar epithelial cell line. Cells were infected with HT31a (transcomplemented ptsP mutant harboring pHT28a; open triangles), HT32a (ptsP mutant harboring empty pSU2719; closed triangles), or HT34a (parent harboring empty pSU2719; open circles) at an MOI of 0.1. pSU2719 is the plasmid vector used; pHT28a consists of pSU2719 and ptsP ORF. Each time point in panel A represents mean ± SD of six (at days 0 and 1) or seven (at days 2 and 3) wells. Each time point in (panel B) represents mean ± SD of quadruplicate wells. Bacterial growth is expressed as the log10 of the ratio of the bacterial concentration at the indicated time to the bacterial concentration at the start of the experiment.
Extracellular phenotypic characterization of the mutant.
Extracellular growth of the ptsP mutant (clone 47:3h) in BYE-α broth was the same as was observed for the parent type strain (AA100jm) (data not shown). We previously showed that both clones 47:3h and 47:4a are prototrophic (34). Microscopically, the parent strain appeared as short thin rods, while the ptsP mutant was longer, but as thin as its parent, at log-growth phase. At stationary phase (optical density at 660 nm of >1.0), more filamentous forms were seen with the ptsP mutant than wild type. The mutant showed the same Sudan black B-positive deposits as the parent strain, both at log phase and at stationary phase. Both the parent and mutant produced monopolar and dipolar flagella. Colonial morphology of the mutants and the parent was indistinguishable.
Both the mutant and its parent were resistant to serum complement-mediated killing. No reductions in bacterial concentrations were observed in the presence of 20% fresh serum, whereas the same concentration of serum combined with immune serum resulted in 4.4 and 4.7 log10 killing, respectively, of both parent and mutant. In contrast, a serum-sensitive strain of E. coli was killed by 4.8 log10 in the presence of both 10 and 20% fresh serum. Neither the parent nor the mutant were killed by human neutrophils; there was less than a 0.5 log10 decrease in bacterial numbers after incubation with neutrophils for 1 h, which was indistinguishable from the decrease in bacterial counts observed in the presence of tissue culture medium and serum, in the absence of neutrophils. This was in contrast to a 2 log10 decrease of the control E. coli strain in the presence, but not absence, of neutrophils.
Pore formation, intracellular trafficking, and phagosome ultrastructural morphology.
To determine if the initial slow growth of the ptsP mutant was related to decreased pore formation of macrophages, bacterium-induced pore formation in murine bone marrow-derived macrophages was compared to that of the parent strain, as well as to that of a dotO mutant, using cell permeability to ethidium bromide as a marker of cell pore formation.
DotO mutants are unable to form pores in macrophages (1). Both the parent and ptsP mutant formed pores in the majority of macrophages studied, while macrophages infected with the dotO mutant did not form pores. At an MOI of 1,000, the ptsP mutant formed pores in 60.0% ± 10.8% of cells, versus 79.3% ± 3.0% and 1.6% ± 1.0% for cells infected by the parent and dotO mutants, respectively. At an MOI of 10, the frequencies of cell pore formation were 52.9% ± 7.5%, 60.7% ± 25.3%, and 2.0% ± 1.1% for the ptsP mutant, parent, and dotO mutant, respectively. These results show that the ptsP mutation had no effect on cytotoxicity.
L. pneumophila normally blocks maturation of its phagosome (16), and a defect in this ability could affect intracellular growth. The ability of the ptsP mutant to inhibit the colocalization of the lysosomal membrane marker Lamp-1 within the phagosome was assessed by microscopy using murine bone marrow-derived macrophages. Thirty minutes after infection, only 8.7% (10/114) of phagosomes harboring the ptsP mutant (clone 47:3h) and 5.5% (6/109) of the parent strain were colocalized with Lamp-1, in contrast to the 73.8% (93/126) colocalization frequency for phagosomes containing the dotO mutant. This result indicated that the ptsP mutation did not impair the ability of the bacterium to inhibit phagosome maturation.
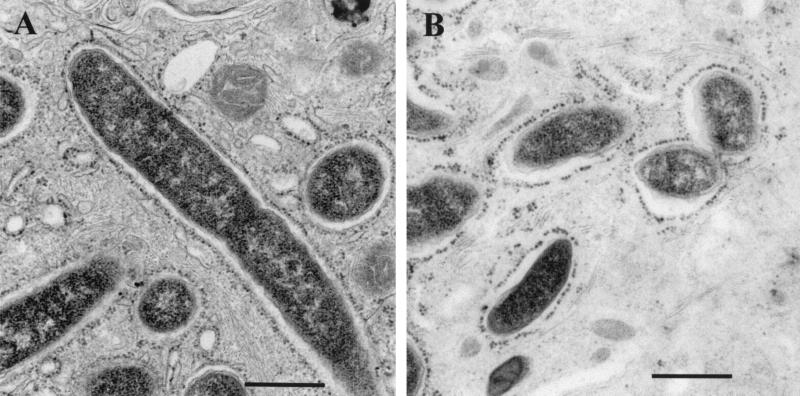
The ultrastructure of phagosomes containing the ptsP mutant or its parent was examined using guinea pig alveolar macrophages to determine if mutant-containing phagosomes were ribosome studded, as is observed for wild-type L. pneumophila. Two days after infection of macrophages with the parent strain (MOI = 0.1), 14 (38.9%) of 36 infected macrophages contained ribosome-studded phagosomes (Fig. 6A). Too few macrophages to score by electron microscopy were infected with the ptsP mutant at the same MOI 2 days after inoculation. Three days after infection of macrophages with the mutant, most macrophages were infected; among 40 macrophages infected, 15 contained ribosome-studded phagosomes (37.5%) (Fig. 6B). The phagosomes containing the ptsP mutant appeared to be slightly more spacious than the phagosomes containing the parent strain, but quantitative morphometric studies were not performed.
FIG. 6.
Electron microscopy of phagosomes containing a ptsP mutant or its parent. Guinea pig alveolar macrophages were infected with bacteria as described in the text. (A) Parent (AA100jm) at day 2 after infection. (B) ptsP mutant (clone 47:3h) at day 3 after infection. Magnification, ×40,000. A 500-nm size marker is shown.
DotA and IcmX production.
To determine if the ptsP mutation had an effect on the dot/icm system, we examined DotA protein and Icm X protein expression of the mutant. Crude lysates of both the parent and ptsP mutant applied in equivalent protein amounts to the polyacrylamide gel showed apparently identical amounts of DotA by immunoblot assay. Similarly, boiled samples of both the parent and ptsP mutant and F2345 applied in equivalent protein amounts to the polyacrylamide gel showed apparently identical amounts of IcmX protein (data not shown). These data indicate that the ptsP mutation does not affect dotA and icmX regulation.
DISCUSSION
We have demonstrated that the L. pneumophila ptsP gene is an important virulence factor for cell and guinea pig infection. Mutations at two different sites in the gene dramatically reduced the ability of the bacterium to multiply in guinea pig lungs and also eliminated the extrapulmonary invasiveness of the bacterium. This reduced virulence is attributable to the reduced ability of the mutant bacterium to multiply within macrophages without affecting its ability to invade cells. The architecture of the L. pneumophila ptsP gene is very similar to that found in P. aeruginosa in that both the ptsP and invA orthologs are in contiguous regions, and in the same orientation (39). There is also considerable homology between the L. pneumophila, P. aeruginosa (39), and A. vinelandii (36) orthologs of the ptsP gene itself, indicating a common genetic origin.
Proof that the ptsP mutation itself is responsible for the reduced-virulence phenotype was provided by the complementation studies. Transcomplementation of ptsP increased the virulence of the noncomplemented mutant about 50-fold in cultured cells and 10-fold and 250-fold in guinea pig lung and spleen, respectively, and fully restored the clinical virulence of the bacterium. In comparison with the parent strain, transcomplementation of ptsP almost completely restored the ability of the mutant to grow within alveolar epithelial cells and reversed its initial growth defect within alveolar macrophages. Also, ptsP transcomplementation almost completely restored the ability of the mutant to multiply in the lung and invade the spleen of guinea pigs. We attribute partial, rather than full, restoration of the mutant to the parenteral phenotype to plasmid loss in the absence of antibiotic selection in the animal and cellular infection models. There is no practical way to maintain chloramphenicol selection in vivo or in cell culture, as chloramphenicol appears to be ineffective against normally Cms L. pneumophila in both systems (7). Nonantimicrobial selective pressure favored the growth of the complemented mutant, in that it was more able to multiply in tissues when it contained the plasmid than when it lacked it; this is demonstrated by the greater than 10-fold difference in plasmid retention between the complemented mutant and the mutant with the empty plasmid.
An equally valid alternative explanation for the incomplete restoration of the parenteral virulence phenotype by transcomplementation is that there was a polar effect on downstream virulence genes in the same operon. Examination of the published sequence of a related L. pneumophila serogroup 1 strain shows that there are two large ORFs downstream of ptsP in what may be the same operon (http://genome3.cpmc.columbia.edu/%7Elegion/). Since the ptsP trancomplementation by itself had a dramatic effect on the virulence phenotype, these putative downstream virulence genes would have to act in concert with ptsP to cause full virulence. Several attempts at making an unmarked nonpolar mutation of ptsP were unsuccessful in our hands, precluding definitive resolution of this point.
Our studies indicate that the ptsP mutant invades macrophages normally and hence that its intracellular multiplication is slowed due to poor growth once the bacterium is intracellular. This poor growth could be due either to growth of only a small fraction of invading bacteria or to a longer than normal lag phase of growth of all the invading bacteria. In alveolar macrophages the bacteria eventually multiply to levels observed for the parent strain, indicating that the intracellular growth rate normalizes after a long lag. Normalization of the growth rate is consistent with the results of the ultrastructural and endosome maturation studies, which showed that the mutant resides in a parenteral-type phagosome with inhibition of endosomal maturation. The significance of the slightly more spacious nature of the phagosomes containing the mutant is unknown. Taken together, these findings suggest that the intracellular multiplication defect is an early event, eventually bypassed by unknown factors. The delayed normalization of growth within macrophages did not have an in vivo correlate, probably because guinea pig host defenses develop quickly, enabling the host defenses to overcome an initially slowly growing bacterium.
Growth of L. pneumophila within alveolar epithelial cells has been suggested to be a virulence determinant, in that bacterial mutants capable of growing within alveolar epithelial cells, but not alveolar macrophages, retained their virulence in a mouse pneumonia model (14). Defective growth of the ptsP mutant in alveolar epithelial cells may partially or wholly explain why the guinea pig virulence of the mutant is so attenuated, despite the ability of the bacterium to eventually multiply normally in alveolar macrophages and to possess the parenteral phagosomal phenotype. Alteration in the regulation of the dot/icm system is not an explanation for the reduced virulence of the ptsP mutant. Unlike dot/icm mutants, the ptsP mutant established ribosome-studded phagosomes, normally inhibited endosomal maturation, and formed host cell membrane pores. In addition, DotA and IcmX appear to be normally produced by the ptsP mutant.
The function and the pathogenic and nonpathogenic roles of the L. pneumophila ptsP gene are unknown. The deduced amino acid sequences of known ptsP homologs and L. pneumophila ptsP were well conserved. Among them, E. coli ptsP has been investigated most intensively. The E. coli ptsP gene encodes EINtr, which is thought to serve a sensory function linking carbon and nitrogen metabolism (33). In addition, EINtr may play a role in the transcriptional regulation of rpoN-dependent operons (31). A variety of bacterial virulence traits are linked to rpoN-dependent operons, including Pseudomonas syringae virulence for tomato plants (23), Vibrio anguillarum fish virulence (30), Agrobacterium tumefaciens plant virulence (5), and V. cholerae virulence for mice (21). We speculate that the L. pneumophila ptsP ortholog is involved in signal transduction for expression of a virulence factor, which may be through regulation of the rpoN operon.
There are few studies on the phenotypic effects of ptsP mutations of other bacteria. Mutation of the P. aeruginosa ptsP ortholog results in reduced virulence for Caenorhabditis elegans and mice, by an unknown mechanism (38, 39). Mutational inactivation of the A. vinelandii ptsP ortholog affects poly-β-hydroxybutyrate accumulation (36). L. pneumophila is known to deposit β-hydroxybutyrate within its cytoplasm as a nutrition source for long-term starvation survival (20). Our results show that the L. pneumophila ptsP gene is apparently not responsible for β-hydroxybutyrate accumulation, as the broth-grown ptsP mutant showed the same amount of Sudan black B-positive deposits as its parent.
In summary, this study demonstrates that the ptsP ortholog is required for full expression of virulence of L. pneumophila in vivo. The ptsP mutation results in an initial defect of growth within macrophages and inability to grow within alveolar epithelial cells. The sequence homology search suggests that the gene may be involved in signal transduction of virulence, which requires further study to elucidate the mechanism. Such studies will give us new insights into the molecular pathogenesis of L. pneumophila.
ACKNOWLEDGMENTS
We thank Martha Edelstein for excellent technical assistance; Craig Roy for helpful discussion, provision of reagents, and instruction in the intracellular trafficking experiments; and Lalita Ramakrishnan for critical review of the manuscript.
Futoshi Higa was supported by a study grant from Japanese Ministry of Education, Sports, and Culture.
REFERENCES
- 1.Andrews H L, Vogel J P, Isberg R R. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley; 1995. [Google Scholar]
- 3.Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 4.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Investig Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 5.Chesnokova O, Coutinho J B, Khan I H, Mikhail M S, Kado C I. Characterization of flagella genes of Agrobacterium tumefaciens, and the effect of a bald strain on virulence. Mol Microbiol. 1997;23:579–590. doi: 10.1046/j.1365-2958.1997.d01-1875.x. [DOI] [PubMed] [Google Scholar]
- 6.Coers J, Monahan C, Roy C R. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat Cell Biol. 1999;1:451–453. doi: 10.1038/15687. [DOI] [PubMed] [Google Scholar]
- 7.Edelstein P H. Antimicrobial chemotherapy for legionnaires' disease: a review. Clin Infect Dis. 1995;21(Suppl 3):5265–5276. doi: 10.1093/clind/21.supplement_3.s265. [DOI] [PubMed] [Google Scholar]
- 8.Edelstein P H. Detection of antibodies to Legionella spp. In: Rose N R, de Macario E C, Folds J D, Lane H C, Nakamura R M, editors. Manual of clinical laboratory immunology. 5th ed. Washington, D.C.: American Society Microbiology; 1997. pp. 502–509. [Google Scholar]
- 9.Edelstein P H. Legionnaires' disease laboratory manual. Chantilly, Va: National Technical Information Service; 1985. [Google Scholar]
- 10.Edelstein P H, Calarco K, Yasui V K. Antimicrobial therapy of experimentally induced Legionnaires' disease in guinea pigs. Am Rev Respir Dis. 1984;130:849–856. doi: 10.1164/arrd.1984.130.5.849. [DOI] [PubMed] [Google Scholar]
- 11.Edelstein P H, Edelstein M A. Comparison of three buffers used in the formulation of buffered charcoal yeast extract medium. J Clin Microbiol. 1993;31:3329–3330. doi: 10.1128/jcm.31.12.3329-3330.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelstein P H, Edelstein M A, Higa F, Falkow S. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc Natl Acad Sci USA. 1999;96:8190–8195. doi: 10.1073/pnas.96.14.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein P H, Meyer R D. Legionnaires' disease. A review. Chest. 1984;85:114–120. doi: 10.1378/chest.85.1.114. [DOI] [PubMed] [Google Scholar]
- 14.Gao L Y, Stone B J, Brieland J K, Abu Kwaik Y. Different fates of Legionella pneumophila pmi and mil mutants within macrophages and alveolar epithelial cells. Microb Pathog. 1998;25:291–306. doi: 10.1006/mpat.1998.0237. [DOI] [PubMed] [Google Scholar]
- 15.Hirokawa T, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz M A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz M A, Silverstein S C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Investig. 1980;65:82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz M A, Silverstein S C. Interaction of the legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. II. Antibody promotes binding of L. pneumophila to monocytes but does not inhibit intracellular multiplication. J Exp Med. 1981;153:398–406. doi: 10.1084/jem.153.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz M A, Silverstein S C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Investig. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James B W, Mauchline W S, Dennis P J, Keevil C W, Wait R. Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low-nutrient environments. Appl Environ Microbiol. 1999;65:822–827. doi: 10.1128/aem.65.2.822-827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klose K E, Mekalanos J J. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol Microbiol. 1998;28:501–520. doi: 10.1046/j.1365-2958.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 22.Kodaka H, Armfield A Y, Lombard G L, Dowell V R., Jr Practical procedure for demonstrating bacterial flagella. J Clin Microbiol. 1982;16:948–952. doi: 10.1128/jcm.16.5.948-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorang J M, Keen N T. Characterization of avrE from Pseudomonas syringae pv. tomato: a hrp-linked avirulence locus consisting of at least two transcriptional units. Mol Plant Microbe Interact. 1995;8:49–57. doi: 10.1094/mpmi-8-0049. [DOI] [PubMed] [Google Scholar]
- 24.Marra A, Blander S J, Horwitz M A, Shuman H A. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA. 1992;89:9607–9611. doi: 10.1073/pnas.89.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marra A, Horwitz M A, Shuman H A. The HL-60 model for the interaction of human macrophages with the Legionnaires' disease bacterium. J Immunol. 1990;144:2738–2744. [PubMed] [Google Scholar]
- 26.Martinez E, Bartolome B, de la Cruz F. pACYC184-derived cloning vectors containing the multiple cloning site and lacZ alpha reporter gene of pUC8/9 and pUC18/19 plasmids. Gene. 1988;68:159–162. doi: 10.1016/0378-1119(88)90608-7. [DOI] [PubMed] [Google Scholar]
- 27.Matthews M, Roy C R. Identification and subcellular localization of the Legionella pneumophila IcmX protein: a factor essential for establishment of a replicative organelle in eukaryotic host cells. Infect Immun. 2000;68:3971–3982. doi: 10.1128/iai.68.7.3971-3982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer R D, Edelstein P H, Kirby B D, Louie M H, Mulligan M E, Morgenstein A A, Finegold S M. Legionnaires' disease: unusual clinical and laboratory features. Ann Intern Med. 1980;93:240–243. doi: 10.7326/0003-4819-93-2-240. [DOI] [PubMed] [Google Scholar]
- 29.Moffat J F, Edelstein P H, Regula D P, Jr, Cirillo J D, Tompkins L S. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol Microbiol. 1994;12:693–705. doi: 10.1111/j.1365-2958.1994.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 30.O'Toole R, Milton D L, Horstedt P, Wolf-Watz H. RpoN of the fish pathogen Vibrio (Listonella) anguillarum is essential for flagellum production and virulence by the water-borne but not intraperitoneal route of inoculation. Microbiology. 1997;143:3849–3859. doi: 10.1099/00221287-143-12-3849. [DOI] [PubMed] [Google Scholar]
- 31.Powell B S, Court D L, Inada T, Nakamura Y, Michotey V, Cui X, Reizer A, Saier M H, Jr, Reizer J. Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli. Enzyme IIANtr affects growth on organic nitrogen and the conditional lethality of an erats mutant. J Biol Chem. 1995;270:4822–4839. doi: 10.1074/jbc.270.9.4822. [DOI] [PubMed] [Google Scholar]
- 32.Rabus R, Reizer J, Paulsen I, Saier M H., Jr Enzyme I(Ntr) from Escherichia coli. A novel enzyme of the phosphoenolpyruvate-dependent phosphotransferase system exhibiting strict specificity for its phosphoryl acceptor. NPr. J Biol Chem. 1999;274:26185–26191. doi: 10.1074/jbc.274.37.26185. [DOI] [PubMed] [Google Scholar]
- 33.Reizer J, Reizer A, Merrick M J, Plunkett G, III, Rose D J, Saier M H., Jr Novel phosphotransferase-encoding genes revealed by analysis of the Escherichia coli genome: a chimeric gene encoding an enzyme I homologue that possesses a putative sensory transduction domain. Gene. 1996;181:103–108. doi: 10.1016/s0378-1119(96)00481-7. [DOI] [PubMed] [Google Scholar]
- 34.Roy C R, Berger K H, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 35.Sadosky A B, Wiater L A, Shuman H A. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segura D, Espin G. Mutational inactivation of a gene homologous to Escherichia coli ptsP affects poly-β-hydroxybutyrate accumulation and nitrogen fixation in Azotobacter vinelandii. J Bacteriol. 1998;180:4790–4798. doi: 10.1128/jb.180.18.4790-4798.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan M W, Mahajan-Miklos S, Ausubel F M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan M W, Rahme L G, Sternberg J A, Tompkins R G, Ausubel F M. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci USA. 1999;96:2408–2413. doi: 10.1073/pnas.96.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuckman D M, Hung J B, Roy C R. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol Microbiol. 1999;32:990–1001. doi: 10.1046/j.1365-2958.1999.01410.x. [DOI] [PubMed] [Google Scholar]