Key Points
Question
What is the estimated vaccine effectiveness up to 12 weeks for the first COVID-19 mRNA booster vaccines administered in US nursing homes?
Findings
In this cohort study of 10 949 residents of 202 community nursing homes and 4321 residents of 128 Veterans Health Administration community living centers, booster vaccination was associated with significant reductions in SARS-CoV-2 infections, hospitalizations, and the combined end point of hospitalizations or deaths.
Meaning
These findings suggest that administration of a SARS-CoV-2 mRNA vaccine booster among nursing home residents may have played an important role in preventing COVID-19–associated morbidity and mortality.
Abstract
Importance
A SARS-CoV-2 vaccine booster dose has been recommended for all nursing home residents. However, data on the effectiveness of an mRNA vaccine booster in preventing infection, hospitalization, and death in this vulnerable population are lacking.
Objective
To evaluate the association between receipt of a SARS-CoV-2 mRNA vaccine booster and prevention of infection, hospitalization, or death among nursing home residents.
Design, Setting, and Participants
This cohort study emulated sequentially nested target trials for vaccination using data from 2 large multistate US nursing home systems: Genesis HealthCare, a community nursing home operator (system 1) and Veterans Health Administration community living centers (VHA CLCs; system 2). The cohort included long-term (≥100 days) nursing home residents (10 949 residents from 202 community nursing homes and 4321 residents from 128 VHA CLCs) who completed a 2-dose series of an mRNA vaccine (either BNT162b2 [Pfizer-BioNTech] or mRNA-1273 [Moderna]) and were eligible for a booster dose between September 22 and November 30, 2021. Residents were followed up until March 8, 2022.
Exposures
Receipt of a third mRNA vaccine dose, defined as a booster dose (boosted group), or nonreceipt of a booster dose (unboosted group) on an eligible target trial date. If participants in the unboosted group received a booster dose on a later target trial date, they were included in the booster group for that target trial; thus, participants could be included in both the boosted and unboosted groups.
Main Outcomes and Measures
Test-confirmed SARS-CoV-2 infection, hospitalization, or death was followed up to 12 weeks after booster vaccination. The primary measure of estimated vaccine effectiveness was the ratio of cumulative incidences in the boosted group vs the unboosted group at week 12, adjusted with inverse probability weights for treatment and censoring.
Results
System 1 included 202 community nursing homes; among 8332 boosted residents (5325 [63.9%] female; 6685 [80.2%] White) vs 10 886 unboosted residents (6865 [63.1%] female; 8651 [79.5%] White), the median age was 78 (IQR, 68-87) years vs 78 (IQR, 68-86) years. System 2 included 128 VHA CLCs; among 3289 boosted residents (3157 [96.0%] male; 1950 [59.3%] White) vs 4317 unboosted residents (4151 [96.2%] male; 2434 [56.4%] White), the median age was 74 (IQR, 70-80) vs 74 (IQR, 69-80) years. Booster vaccination was associated with reductions in SARS-CoV-2 infections of 37.7% (95% CI, 25.4%-44.2%) in system 1 and 57.7% (95% CI, 43.5%-67.8%) in system 2. For hospitalization, reductions of 74.4% (95% CI, 44.6%-86.2%) in system 1 and 64.1% (95% CI, 41.3%-76.0%) in system 2 were observed. Estimated vaccine effectiveness for death associated with SARS-CoV-2 was 87.9% (95% CI, 75.9%-93.9%) in system 1; however, although a reduction in death was observed in system 2 (46.6%; 95% CI, −34.6% to 94.8%), this reduction was not statistically significant. A total of 45 SARS-CoV-2–associated deaths occurred in system 1 and 18 deaths occurred in system 2. For the combined end point of SARS-CoV-2–associated hospitalization or death, boosted residents in system 1 had an 80.3% (95% CI, 65.7%-88.5%) reduction, and boosted residents in system 2 had a 63.8% (95% CI, 41.4%-76.1%) reduction.
Conclusions and Relevance
In this study, during a period in which both the Delta and Omicron variants were circulating, SARS-CoV-2 booster vaccination was associated with significant reductions in SARS-CoV-2 infections, hospitalizations, and the combined end point of hospitalization or death among residents of 2 US nursing home systems. These findings suggest that administration of vaccine boosters to nursing home residents may have an important role in preventing COVID-19–associated morbidity and mortality.
This cohort study uses a target trial emulation approach to estimate the vaccine effectiveness of a SARS-CoV-2 mRNA booster dose in preventing infection, hospitalization, and death among nursing home residents in the US.
Introduction
Nursing home residents have experienced substantial SARS-CoV-2–associated morbidity and mortality.1,2 Resurgence with the Delta variant in the late summer and early autumn of 2021 and waning immunity among nursing home residents3 led the Centers for Disease Control and Prevention (CDC) to recommend a booster dose for those vaccinated with a 2-dose mRNA series.4,5 The US Food and Drug Administration (FDA) amended the emergency use authorizations for the BNT162b2 vaccine (Pfizer-BioNTech) in September 2021 and the mRNA-1273 vaccine (Moderna) in October 2021. The FDA recommended a booster dose at least 6 months after receipt of the second dose of an mRNA vaccine for individuals at high risk, including those 65 years or older.6 Both mRNA vaccines were also authorized in August 2021 to be used for additional doses in immunocompromised individuals, which applied to many older adults in long-term care facilities. As of February 1, 2022, approximately 66% of US nursing home residents had received a booster dose.6
Despite the high risk of SARS-CoV-2 morbidity and death among nursing home residents, limited data exist on the effectiveness of boosters in mitigating infection or death in this vulnerable population. Vaccine trials did not include nursing home residents, and timely patient-level observational data are not widely available. Nursing homes are an optimal environment for measurement of vaccine effectiveness (VE) because of residential stability, systematic documentation of immunizations (including boosters), and frequent testing for SARS-CoV-2. Additional clinical evidence of booster effectiveness may support efforts to increase booster distribution for this vulnerable population.
To address this gap, we used electronic health record (EHR) data updated daily from 2 large multistate long-term care systems. Using a nested target trial emulation method,7 we estimated the VE of an mRNA booster dose (vs no booster dose) among residents who completed the primary series of an mRNA vaccine. Our outcomes included any laboratory-confirmed SARS-CoV-2 infection, hospitalization, or death. We hypothesized that receipt of an mRNA vaccine booster would be associated with lower risk of these outcomes.
Methods
Study Design and Population
This cohort study was designed to emulate a sequence of nested target trials comparing the estimated additional effectiveness of a SARS-CoV-2 mRNA vaccine booster vs the primary vaccine series only among nursing home residents. We planned a priori to analyze 2 cohorts identified in separate health systems and to report results separately. The primary reason for separate cohorts was that the data use agreements did not allow exporting and pooling of person-level data outside of the secure server environment. The first system included 202 nursing homes in 19 states, with a heavy concentration in the northeastern region, operated by Genesis HealthCare (system 1). The second included veterans residing in 128 community living centers (CLCs; analogous to nursing homes) spread nationwide and managed by the Veterans Health Administration (VHA; system 2). The study emulated daily (Monday through Friday) target trials from September 22 through November 30, 2021. September 22 was the date on which the FDA authorized the BNT162b2 vaccine booster for use in nursing home residents. November 30, 2021, was selected to allow at least 12 weeks of follow-up observation time to March 8, 2022. Data were collected from administrative and clinical records in both systems. This study was deemed exempt from informed consent by the institutional review board of Brown University, and the VHA project was approved by the institutional review board of the Providence VA Medical Center. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
The target trial method involves defining a target trial design and emulating the trial in the observational data.7,8 Specific dates are selected as trial dates on which those who meet eligibility criteria and are assigned or not assigned to receive treatment are included. Details about our target trial for both nursing home systems are available in Table 1; this table shows how the trial was emulated in the observational data. On each day, residents were considered eligible for inclusion if they (1) had been present in the nursing home for at least 100 days, with a gap of no more than 10 days (ie, residents could leave and come back, but not for >10 days), and (2) had received a 2-dose mRNA vaccine series at least 120 days earlier. Residents who received the 1-dose Ad26.COV2.S vaccine (Janssen/Johnson & Johnson) were excluded due to small numbers. Residents were also excluded if they had a SARS-CoV-2 infection in the previous 90 days, planned discharge according to their most recent assessment, received treatment with monoclonal antibodies in the previous 90 days, were receiving hospice care, or had already received a third vaccine dose before the index date. After exclusions, the total sample included long-term (≥100 days) nursing home residents (10 949 residents from 202 community nursing homes and 4321 residents from 128 VHA CLCs). Residents were defined as boosted if they received a booster vaccine dose on the index date (boosted group); residents who met all eligibility criteria but did not receive a booster vaccine dose on that day were defined as unboosted (unboosted group).
Table 1. Protocol of the Target Trial to Study the Estimated Effectiveness of Booster Vaccination in Community Nursing Homes and Veterans Health Administration Community Living Centersa.
| Component | Target trial protocol | Consistency with target trial protocol | |
|---|---|---|---|
| System 1: community nursing homes | System 2: VHA community living centers | ||
| Eligibility |
|
|
|
| Treatment | Booster dose (third dose >6 mo after completion of primary series) vs placebo |
|
|
| Assignment | Random assignment by individual |
|
Same as protocol |
| Follow-up |
|
|
|
| Outcome | Positive PCR or antigen test result for SARS-CoV-2 infection and SARS-CoV-2–associated events (infection, hospitalization, or death) | Same as protocol | Same as protocol |
| Statistical analysis |
|
Same as protocol | Same as protocol |
Abbreviations: ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; PCR, polymerase chain reaction; VHA, Veterans Health Administration.
The aim was to evaluate the relative risk of SARS-CoV-2 infection among those who received a booster dose vs a primary series of a vaccine only. Column 2 shows the hypothetical design of a randomized clinical trial involving nursing home residents. Columns 3 and 4 show concordance, deviation, and additions to the target trial protocol for each system.
Study Outcomes and Covariates
We examined 4 postbooster outcomes defined a priori: (1) any incident SARS-CoV-2 infection, (2) hospitalization, (3) death, and (4) combined end point of hospitalization or death. All study outcomes were obtained via the EHR from the 2 systems.
SARS-CoV-2 infection was defined by a positive result on a SARS-CoV-2 rapid antigen or polymerase chain reaction test occurring after the index trial date and with no test confirming infection in the previous 90 days. Testing results were extracted from laboratory results in both systems.9
SARS-CoV-2–associated hospitalization was defined as transfer to an acute care hospital occurring within 21 days of a positive SARS-CoV-2 test. Transfers were identified through census records in system 1 and bed section codes identifying acute care episodes in system 2, which had a single EHR system for both nursing homes and hospitals.
SARS-CoV-2–associated death was defined as death occurring within 30 days of a positive SARS-CoV-2 test. Deaths were identified through transfers to funeral homes or death records in the daily census in system 1 and through a combination of census and vital status records in system 2.
Immunization records in each EHR were used to identify primary vaccination and booster events.10 We indexed additional resident covariates separately for each eligible trial date (ie, a person’s baseline covariates may have differed for each trial because each trial represented a different index date). Data were obtained from the EHR in both systems. In system 1, additional information from the nursing home Minimum Data Set (MDS) assessments were included.11 The MDS includes demographic characteristics (age, sex, race and ethnicity, and language), clinical diagnoses, MDS measures of cognitive and physical function, an MDS indicator of limited life expectancy, previous SARS-CoV-2 infections, and influenza vaccination for the current season up to the index date. The data in system 2 did not include MDS assessments and relied on demographic characteristics and International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis codes in the EHR (recorded in the 12 months before the index date) for chronic condition information. These data were classified using the Elixhauser comorbidity classification system.12 In both systems, information on resident race and ethnicity was documented by clinicians and included in our analysis due to the potential of race and ethnicity to be confounders of vaccination and outcomes. We characterized residents as immunocompromised if they had received an immunosuppressive medication or had a diagnosis for a qualifying condition (details are available in eTable 1 in Supplement 1). We also measured overall SARS-CoV-2 testing rates at the facility, SARS-CoV-2 positivity rates in the period before each index date (as a measure of local disease risk), and facility-level factors, such as a quality of care and infection control.
Statistical Analysis
A brief discussion of the target trial emulation method is available in the eAppendix in Supplement 1. The target trial emulation approach has been increasingly used to address challenges of immortal time and selection bias in observational data. It is well suited to studying VE in situations in which the unvaccinated group lacks an obvious starting date for follow-up. For each index date, residents meeting eligibility criteria (Table 1) were included. If residents received a booster vaccine dose on a target trial date, they were assigned to the booster arm. After residents received a booster dose, they were ineligible for future trial dates; therefore, data from the boosted arm did not contain repeated observations. Eligible residents who did not receive a booster dose were included in the control arm. If residents were eligible on multiple dates (eg, if they never received a booster), we randomly selected 1 eligible target trial date for analysis.7 The random selection of dates for the control group only determined which target trial date was used and the total amount of follow-up time from that starting date; all individuals in the control group were included.
From each trial date, survival time was computed as the minimum of either time to outcome or time to a censoring event (ie, death or last available date of follow-up [March 8, 2022]). In community nursing homes, residents were censored at transfer because only the nursing home EHR was available. However, VHA facilities captured clinical care outside of CLCs, so transfer outside the CLC was not a censoring event. Residents in the control group were censored if they received a booster dose after the index date but contributed follow-up time until the date of booster administration. If residents in the control group received a booster dose on a later target trial date, they were included in the boosted group for that target trial (thus, residents could be included in both arms).
The same analytical approach was used for both cohorts, but analysis was completed separately by separate analysts (F.D. and C.W.H.). Each event was modeled using pooled logistic regression analysis, with cumulative incidence (ie, risk) measured as 1 minus the probability of event-free survival at each time point (weeks from time 0). The relative ratio of the cumulative incidence curves between groups (boosted vs unboosted) at each time point was computed, with 1 minus relative risk reported as the conventional VE statistic. The cumulative incidence at week 12 was selected as the primary follow-up time of interest for reporting. Final statistical models were adjusted for confounding and informative censoring with inverse probability weighting. Final model covariates and coefficients for each health care system are shown in eTable 4 and eTable 5 in Supplement 1.
Model selection for weights was based on a priori experience with confounders (ie, local COVID-19 rates) and observed baseline imbalances in covariates, which were plausibly common factors associated with treatment and outcome. Absolute standardized mean differences of less than 0.1, categorized by booster status, were used to guide acceptable balance between groups after adjustment. Adjusted models were evaluated for observations with nonmissing data and no imputation. The final weight was a product of treatment and censoring weights. Weights were truncated at their 99% upper quantile. Sampling with replacement by resident (ie, bootstrapping) with 1000 replications was used to generate 95% CIs accounting for the estimated probability weights and crossover of treatment groups by resident. Initial data preparation was conducted using SAS software, version 9.4 (SAS Institute Inc), and Stata software, version 16 (StataCorp LLC). All analyses were performed using R statistical software, version 4.0.1 (R Foundation for Statistical Computing).
Results
Study Population
System 1 included 202 community nursing homes; among 8332 boosted residents (5325 [63.9%] female; 6685 [80.2%] White) vs 10 886 unboosted residents (6865 [63.1%] female; 8651 [79.5%] White), the median age was 78 (IQR, 68-87) years vs 78 (IQR, 68-86) years. System 2 included 128 VHA CLCs; among 3289 boosted residents (3157 [96.0%] male; 1950 [59.3%] White) vs 4317 unboosted residents (4151 [96.2%] male; 2434 [56.4%] White), the median age was 74 (IQR, 70-80) vs 74 (IQR, 69-80) years. Resident counts by inclusion and exclusion criteria for each day are shown in eTable 2 and eTable 3 in Supplement 1.
A resident flowchart and information about assignment to each group are provided in eFigure 1 in Supplement 1. More residents in system 1 received a BNT162b2 (Pfizer-BioNTech) booster dose (5616 of 8332 individuals [67.4%]) than residents in system 2 (1433 of 3289 individuals [43.6%]). The follow-up time contributed was 1 163 155 days (median [IQR], 37 [13-117] days per resident) in system 1 and 356 049 days (median [IQR], 41 [11-84] days per resident) in system 2.
Other baseline characteristics of both cohorts, including the boosted and unboosted groups, are shown in Table 2. Boosted vs unboosted residents differed greatest by length of stay (system 1: median [IQR], 764 [428-1279] days vs 698 [355-1210] days; system 2: median [IQR], 761 [378-1558] days vs 694 [273-1413] days) and time since second vaccination (system 1: median [IQR], 265 [252-277] days vs 248 [232-260] days; system 2: median [IQR], 270 [252-285] days vs 260 [252-280] days).The balance across covariates after inverse probability weights were applied is shown in eFigure 2 in Supplement 1. Compared with boosted residents in system 2, those in system 1 were older (median [IQR], 78 [68-87] years vs 74 [70-80] years), less likely to be of Black race (857 of 8332 individuals [10.3%] vs 731 of 3289 individuals [22.3%]) and more likely to be female (5325 of 8332 individuals [63.9%] vs 132 of 3289 individuals [4.0%]).
Table 2. Baseline Characteristics of Nursing Home Residents by Booster Status and Nursing Home Systema.
| Characteristic | Participants, No. (%) | |||
|---|---|---|---|---|
| System 1: community nursing homesb | System 2: VHA community living centersc | |||
| Unboosted group (n = 10 886) | Boosted group (n = 8332) | Unboosted group (n = 4317) | Boosted group (n = 3289) | |
| Age, median (IQR), y | 78 (68-86) | 78 (68-87) | 74 (69-80) | 74 (70-80) |
| Sex | ||||
| Male | 4021 (36.9) | 3007 (36.1) | 4151 (96.2) | 3157 (96.0) |
| Female | 6865 (63.1) | 5325 (63.9) | 166 (3.8) | 132 (4.0) |
| Preindex length of stay, median (IQR), d | 698 (355-1210) | 764 (428-1279) | 694 (273-1413) | 761 (378-1558) |
| Race and ethnicity | ||||
| Black | 1141 (10.5) | 857 (10.3) | 962 (22.3) | 731 (22.2) |
| Hispanic | 574 (5.3) | 423 (5.1) | 184 (4.3) | 134 (4.1) |
| White | 8651 (79.5) | 6685 (80.2) | 2434 (56.4) | 1950 (59.3) |
| Otherd | 522 (4.8) | 369 (4.4) | 737 (17.1) | 474 (14.4) |
| Comorbidities | ||||
| Current smoker | 371 (3.4) | 275 (3.3) | 548 (12.7) | 368 (11.2) |
| Alzheimer disease and related dementias | 5319 (48.9) | 4242 (50.9) | 2871 (66.5) | 2217 (67.4) |
| Immunocompromised | 1258 (11.6) | 1006 (12.1) | 1254 (29.0) | 916 (27.9) |
| Pulmonary disease | 2514 (23.1) | 1880 (22.6) | 1380 (32.0) | 1043 (31.7) |
| Diabetes (any type) | 4205 (38.6) | 3205 (38.5) | 2124 (49.2) | 1613 (49.0) |
| Paralysis | 1614 (14.8) | 1241 (14.9) | 1058 (24.5) | 756 (23.0) |
| Time from second dose to booster dose, median (IQR), d | 248 (232-260) | 265 (252-277) | 260 (252-280) | 270 (252-285) |
| No. of SARS-CoV-2 tests, mean (SD)e | ||||
| Past 14 d | 0.5 (1.7) | 0.5 (1.7) | 1.9 (1.7) | 1.9 (1.7) |
| Past 90 d | 2.9 (7.7) | 3.4 (8.7) | 11.8 (8.6) | 11.9 (8.7) |
Abbreviation: VHA, Veterans Health Administration.
Among nursing home residents eligible to receive an mRNA vaccine booster dose between September 22 and November 30, 2021. All diagnoses were as defined by the Charlson Comorbidity Index and identified via codes from the International Classification of Diseases, Tenth Revision, Clinical Modification.
Includes 202 community nursing homes.
Includes 128 VHA community living centers.
Includes American Indian, Asian, Pacific Islander, and unreported race and/or ethnicity.
Includes all tests received, regardless of positive or negative results.
Estimated Vaccine Effectiveness of Booster Dose
Cumulative incidence curves for the 4 COVID-19 outcomes up to 12 weeks are shown in Figure 1. Overall, compared with boosted residents in system 2, those in system 1 had higher rates of SARS-CoV-2 infection over 12 weeks (100.1 [95% CI, 92.8-107.3] infections/1000 residents vs 72.5 [95% CI, 62.4-81.9] infections/1000 residents) and SARS-CoV-2–associated death (1.4 [95% CI, 0.8-2.2] deaths/1000 residents vs 1.3 [95% CI, 0.2-2.6] deaths/1000 residents) but lower rates of SARS-CoV-2–associated hospitalization (3.9 [95% CI, 2.7-5.2] hospitalizations/1000 residents vs 31.2 [95% CI, 25.0-38.0] hospitalizations/1000 residents) associated with SARS-CoV-2. A total of 45 SARS-CoV-2–associated deaths were observed in system 1 vs 18 SARS-CoV-2–associated deaths in system 2.
Figure 1. SARS-CoV-2 Cumulative Incidence in 2 US Nursing Home Systems by Booster Status.
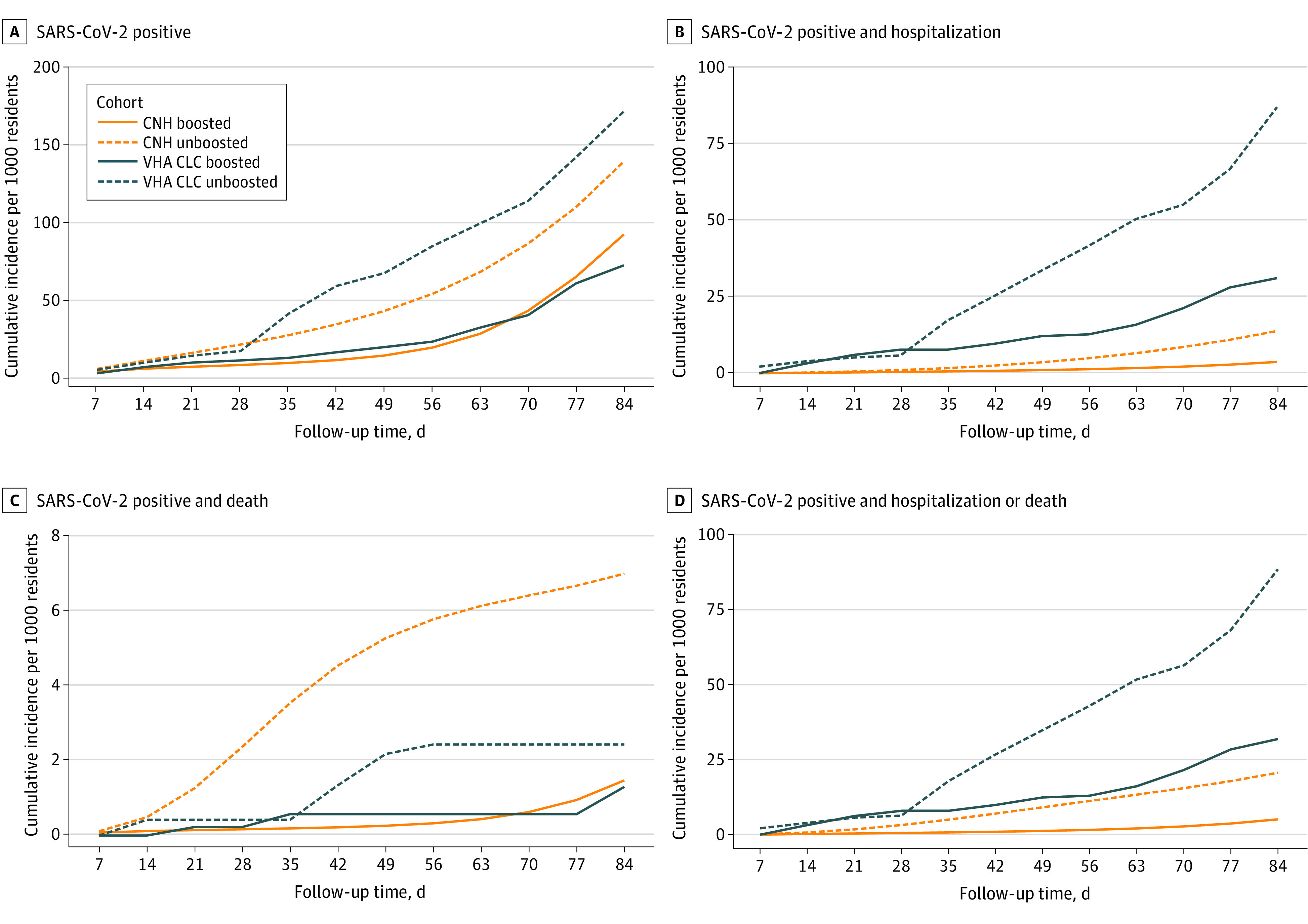
Among nursing home residents eligible to receive an mRNA vaccine booster dose between September 22 to November 30, 2021. Days of follow-up were measured from the index date of the target trial. A total of 202 community nursing homes (CNHs) and 128 Veterans Health Administration community living centers (VHA CLCs) were included.
At week 12, booster vaccination was associated with reductions in the risk of SARS-CoV-2 infection of 37.7% (95% CI, 25.4%-44.2%) in system 1 and 57.7% (95% CI, 43.5%-67.8%) in system 2 (Table 3). The estimated VE for SARS-CoV-2–associated hospitalization was 74.4% (95% CI, 44.6%-86.2%) in system 1 and 64.1% (95% CI, 41.3%-76.0%) in system 2. In system 1, the VE for SARS-CoV-2–associated death was 87.9% (95% CI, 75.9%-93.9%); in system 2, a reduction in death was observed (46.6%; 95% CI, −34.6% to 94.8%), but this reduction was compatible with chance. The VE for the combined end point of hospitalization or death was 80.3% (95% CI, 65.7%-88.5%) in system 1 vs 63.8% (95% CI, 41.4%-76.1%) in system 2.
Table 3. Estimated Vaccine Effectiveness at 12 Weeks Among Nursing Home Residents Who Did vs Did Not Receive a Booster Dosea.
| Outcome | Cumulative incidence per 1000 residents (95% CI) | Relative vaccine effectiveness, % (95% CI)b | Risk difference in cumulative incidence (95% CI) | |
|---|---|---|---|---|
| Boosted group | Unboosted group | |||
| Infection | ||||
| System 1: CNHs | 100.1 (92.8 to 107.3) | 160.5 (133.2 to 179.9) | 37.7 (25.4 to 44.2) | –60.4 (–72.5 to –40.4) |
| System 2: VHA CLCs | 72.5 (62.4 to 81.9) | 171.2 (127.9 to 216.2) | 57.7 (43.5 to 67.8) | –98.8 (–146.0 to –57.0) |
| Hospitalization | ||||
| System 1: CNHs | 3.9 (2.7 to 5.2) | 15.1 (7.7 to 24.4) | 74.4 (44.6 to 86.2) | –11.2 (–19.3 to –4.9) |
| System 2: VHA CLCs | 31.2 (25.0 to 38.0) | 86.9 (54.8 to 121.8) | 64.1 (41.3 to 76.0) | –55.8 (–93.0 to –23.0) |
| Death | ||||
| System 1: CNHs | 1.4 (0.8 to 2.2) | 11.3 (6.5 to 16.9) | 87.9 (75.9 to 93.9) | –9.9 (–14.7 to –5.7) |
| System 2: VHA CLCs | 1.3 (0.2 to 2.6) | 2.4 (0.3 to 5.5) | 46.6 (–34.6 to 94.8) | –1.1 (–4.5 to 1.3) |
| Composite outcome (hospitalization or death) | ||||
| System 1: CNHs | 5.2 (3.8 to 6.6) | 26.3 (15.9 to 38.3) | 80.3 (65.7 to 88.5) | –21.1 (–31.6 to –12.2) |
| System 2: VHA CLCs | 31.8 (25.4 to 38.9) | 87.8 (56.2 to 123.8) | 63.8 (41.4 to 76.1) | –56.1 (–93.2 to –24.8) |
Abbreviations: VHA CLC, Veterans Health Administration community living center; CNH, community nursing home.
Among nursing home residents eligible to receive an mRNA vaccine booster dose between September 22 and November 30, 2021. Includes residents from 202 CNHs and 128 VHA CLCs.
Relative vaccine effectiveness was calculated as 1 minus the cumulative incidence ratio multiplied by 100.
The estimated VE in absolute differences is shown in Figure 2. The estimated VE in each system differed mostly in hospitalization rates. We estimated that 47 residents in system 1 and 18 residents in system 2 needed to receive a booster dose to prevent 1 SARS-CoV-2–associated hospitalization or death. Relative VE is provided in eFigure 2 in Supplement 1.
Figure 2. Estimated Vaccine Effectiveness for Absolute Risk of Outcomes Associated With SARS-CoV-2 Among Residents Receiving Booster Doses in 2 US Nursing Home Systems.
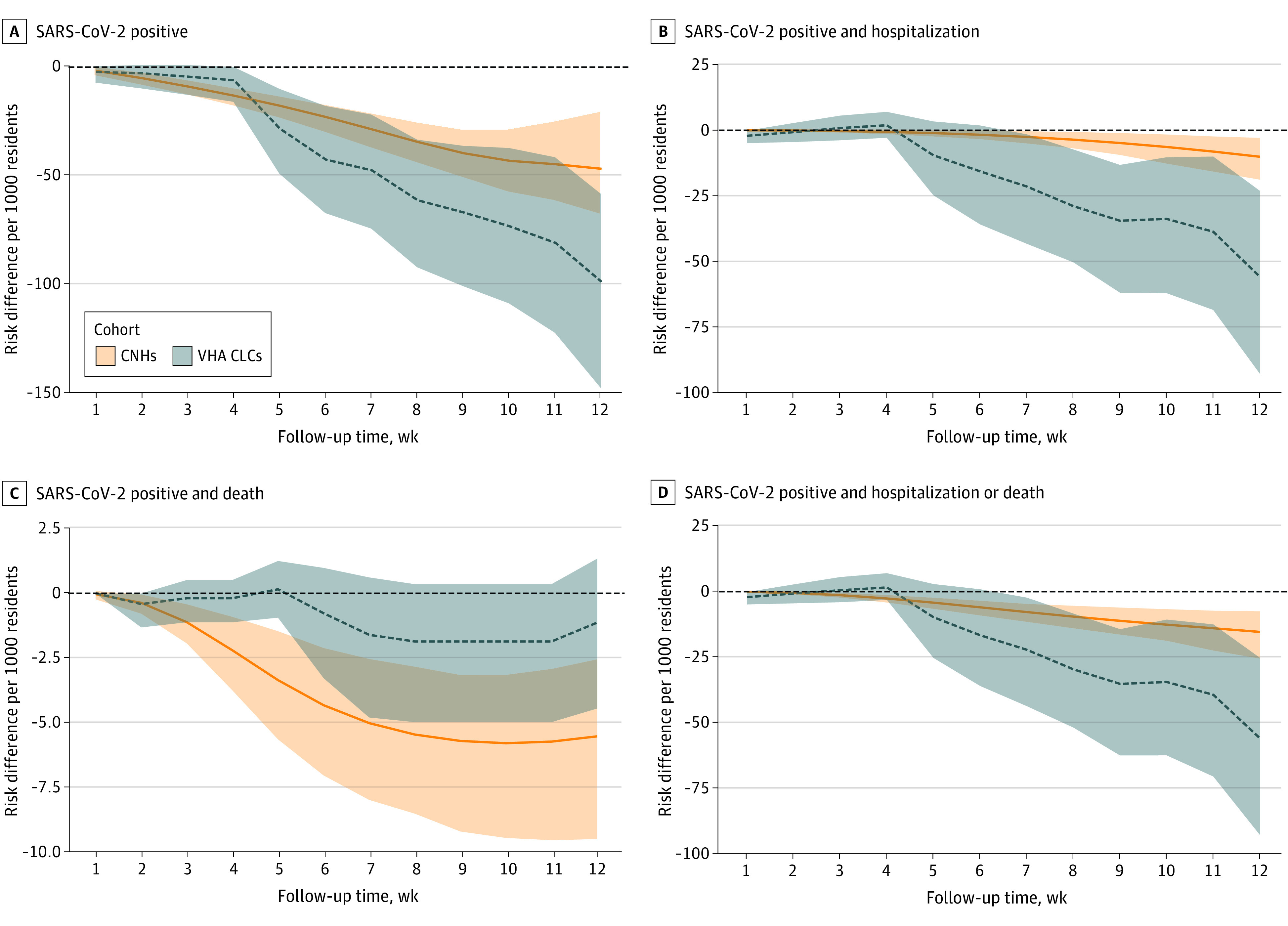
Among residents eligible to receive an mRNA vaccine booster dose between September 22 to November 30, 2021. A total of 202 community nursing homes (CNHs) and 128 Veterans Health Administration community living centers (VHA CLCs) were included. Shading represents 95% CIs.
Discussion
This cohort study estimated the clinical effectiveness of an mRNA SARS-CoV-2 vaccine booster among 2 large multistate populations of nursing home residents. We sought to estimate VE among those eligible for our hypothetical target trial. We found that residents in system 1 who received a booster dose had a substantially lower risk of SARS-CoV-2 infection, hospitalization, and death. Residents in system 2 had a similarly lower risk of infection and hospitalization; however, risk of death was similar in boosted vs unboosted residents in this system, possibly because only 18 COVID-19–associated deaths occurred in the observed time period. With the exception of the outcome of SARS-CoV-2–associated deaths in system 2, all measured outcomes were associated with significantly lower risk in boosted vs unboosted residents. Although the estimated VE varied somewhat by nursing home system (community nursing homes vs VHA CLCs), the findings between cohorts were consistent overall; residents who received a booster dose had a significantly lower risk of SARS-CoV-2 infection and morbidity (as measured by SARS-CoV-2–associated hospitalization).
To evaluate the robustness of VE, we examined 2 substantially different nursing home populations: a large private US operator providing nursing home care and a national health care system caring specifically for older and/or disabled veterans. These 2 health systems were demographically different (eg, few women were present in VHA CLCs). Notably, the rate of hospitalization was substantially higher in VHA CLCs vs community nursing homes (31.2 hospitalizations/1000 residents vs 3.9 hospitalizations/1000 residents over 12 weeks). The rates and reasons for acute hospitalization may differ for non–COVID-19 related differences in the resident comorbidity burden and differences in the availability of hospice care, advance planning, and clinician practice regarding hospitalization. It should be noted that the sample size of system 2 (VHA CLCs) was 39.5% the size of the sample in system 1 (community nursing homes), with shorter follow-up time and fewer facilities. However, consistent VE findings in these 2 disparate cohorts suggest the importance of boosters for the overall US nursing home population.
There are few other studies of booster effectiveness in nursing home residents to date. A recent study published in the Morbidity and Mortality Weekly Report13 evaluated publicly reported data for more than 15 000 US nursing homes and estimated a 46% VE for preventing SARS-CoV-2 infection, which was similar to our VE estimate; however, the researchers did not report results for death or hospitalization. Bar-On et al14 reported observational data from Israeli community-dwelling adults 60 years and older who received a booster dose 5 months after completing the primary vaccine series. Comparing confirmed infections and adjusting for person-days at risk, older adults had approximately 88% fewer confirmed infections in the 12 to 42 days after receipt of the booster dose,14 during a period in which the Delta variant was the predominant virus in circulation. During the same period, Muhsen at al15 reported on more than 41 000 Israeli nursing home residents who received a booster dose approximately 5 months after completing the primary series compared with community-dwelling adults. The boosted nursing home residents had a 71% lower rate of infection and an 80% lower rate of hospitalization than unboosted community dwellers.15 A substantial limitation of this previous work is that it lacked extrapolation to the Omicron variant (BA.4 and BA.5 strains), which was not yet circulating; however, our analysis included the early period of Omicron circulation through February 2022. We found a sustained protective benefit up to at least 3 months in persons receiving a booster dose vs persons not receiving a booster dose. This finding was consistent with other work16,17 suggesting a third dose of an mRNA vaccine offered protection against Omicron variants vs 2 doses alone.
The present work evaluated VE by using an emulated trial design, which allowed us to include unboosted participants with characteristics comparable with those of boosted participants who differed primarily by the date on which they received a SARS-CoV-2 vaccine rather than by indication, baseline covariates, or exposure risk to circulating strains. The study included residents in facilities across broad geographic and socioeconomic distributions. Differences in our estimates vs others could arise from differences in methods, population health, virus characteristics, or other reasons.
Limitations
This study has several limitations. Vaccination status was not randomly assigned, so our observational results are potentially limited by unmeasured confounding. The relatively small differences between groups are encouraging and may be explained by the eligibility requirement of primary series vaccination, resulting in greater cohort homogeneity. In addition, the cumulative incidence rates by group in the first week reveal similar disease risk. This similarity suggests a relatively low risk of residual confounding if the 2 groups had similar baseline event risk in the period after vaccination but before developing postbooster immunity. We did not account for whether the overall facility vaccination rate impacts individual VE (ie, interference). A 12-week follow-up period was selected because recommendations for a fourth dose began in March 2022, which complicated follow-up of residents (ie, some would receive another vaccine). In addition, deaths in the community nursing homes (system 1) are clearly recorded; however, for residents who die after transfer, records are more sporadic. System 2 (VHA CLCs) links deaths to vital statistics files and does not have this limitation. This discrepancy is the reason a composite end point, in which the outcome is hospitalization or death, is reported. We could not evaluate effectiveness against specific variants, including currently circulating strains like BA.4 and BA.5, because genotyping was not routinely collected in this clinical practice setting.
Conclusions
This cohort study found lower risk of SARS-CoV-2 infection and hospitalization after booster adoption by 2 large US nursing home systems during a period in which Delta and Omicron variants were circulating. These findings suggest the importance of SARS-CoV-2 booster vaccination for the nursing home population.
eAppendix. Details on Analytical Approach and Statistical Analysis
eFigure 1. Flowchart of Eligibility for Target Trials of mRNA Boosters in 2 US Nursing Home Systems
eFigure 2. Covariate Balance Preprobability and Postprobability Weights
eTable 1. Definition of Immunocompromised Status
eTable 2. System 1 Trial Eligibility by Date
eTable 3. System 2 Trial Eligibility by Date
eFigure 3. Relative Booster Effectiveness in First 12 Weeks Among Nursing Home Residents
eTable 4. Coefficients From Model Estimating Inverse Probability of Treatment Weights
eTable 5. Coefficients From Model Estimating Inverse Probability of Censoring Weights
Data Sharing Statement
References
- 1.McGarry BE, Barnett ML, Grabowski DC, Gandhi AD. Nursing home staff vaccination and Covid-19 outcomes. N Engl J Med. 2022;386(4):397-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 nursing home data. Centers for Medicare & Medicaid Services. Updated October 16, 2022. Accessed December 23, 2021. https://data.cms.gov/covid-19/covid-19-nursing-home-data
- 3.Canaday DH, Oyebanji OA, Keresztesy D, et al. Significant reduction in vaccine-induced antibody levels and neutralization activity among healthcare workers and nursing home residents 6 months following coronavirus disease 2019 BNT162b2 mRNA vaccination. Clin Infect Dis. 2022;75(1):e884-e887. doi: 10.1093/cid/ciab963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1545-1552. doi: 10.15585/mmwr.mm7044e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC statement on ACIP booster recommendations. News release. Centers for Disease Control and Prevention; September 24, 2021. Accessed December 28, 2021. https://www.cdc.gov/media/releases/2021/p0924-booster-recommendations-.html
- 6.COVID data tracker: nursing home COVID-19 vaccination data dashboard. Centers for Disease Control and Prevention. Accessed December 28, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations-nursing-homes
- 7.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Albéniz X, Hsu J, Hernán MA. The value of explicitly emulating a target trial when using real world evidence: an application to colorectal cancer screening. Eur J Epidemiol. 2017;32(6):495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santostefano CM, White EM, Feifer RA, Mor V. Accuracy of ICD-10 codes for identifying skilled nursing facility residents with lab-confirmed COVID-19. J Am Geriatr Soc. 2021;69(12):3397-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White EM, Yang X, Blackman C, Feifer RA, Gravenstein S, Mor V. Incident SARS-CoV-2 infection among mRNA-vaccinated and unvaccinated nursing home residents. N Engl J Med. 2021;385(5):474-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 cognitive function scale. Med Care. 2017;55(9):e68-e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698-705. [DOI] [PubMed] [Google Scholar]
- 13.Prasad N, Derado G, Nanduri SA, et al. Effectiveness of a COVID-19 additional primary or booster vaccine dose in preventing SARS-CoV-2 infection among nursing home residents during widespread circulation of the Omicron variant—United States, February 14–March 27, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(18):633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385(15):1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhsen K, Maimon N, Mizrahi A, et al. Effects of BNT162b2 COVID-19 vaccine booster in long-term care facilities in Israel. N Engl J Med. 2022;386(4):399-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zedan HT, Nasrallah GK. Effectiveness of mRNA booster doses against the Omicron variant. Lancet Infect Dis. 2022;22(9):1257-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monge S, Rojas-Benedicto A, Olmedo C, et al. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 Omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect Dis. 2022;22(9):1313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Details on Analytical Approach and Statistical Analysis
eFigure 1. Flowchart of Eligibility for Target Trials of mRNA Boosters in 2 US Nursing Home Systems
eFigure 2. Covariate Balance Preprobability and Postprobability Weights
eTable 1. Definition of Immunocompromised Status
eTable 2. System 1 Trial Eligibility by Date
eTable 3. System 2 Trial Eligibility by Date
eFigure 3. Relative Booster Effectiveness in First 12 Weeks Among Nursing Home Residents
eTable 4. Coefficients From Model Estimating Inverse Probability of Treatment Weights
eTable 5. Coefficients From Model Estimating Inverse Probability of Censoring Weights
Data Sharing Statement


