Abstract
To better understand the lung and systemic responses of helper T cells mediating memory immunity to Mycobacterium tuberculosis, we used three- and four-color flow cytometry to study the surface phenotype of CD4+ lymphocytes. Bronchoalveolar lavage (BAL) fluid and peripheral blood (PB) samples were obtained from a total of 25 subjects, including 10 tuberculosis (TB)-infected subjects, 8 purified-protein-derivative-negative subjects, and 7 purified-protein-derivative-positive subjects. In marked contrast to CD4+ lymphocytes from PB (9% ± 5% expressing CD45RA and CD29), the majority (55% ± 16%) of CD4+ lymphocytes in BAL (ALs) simultaneously expressed CD45RA, a naïve T-cell marker, and CD29, members of the very late activation family. Further evaluation revealed that CD4+ ALs expressed both CD45RA and CD45RO, a memory T-cell marker. In addition, the proportion of CD4+ lymphocytes expressing CD69, an early activation marker, was drastically increased in BAL fluid (83% ± 9%) compared to PB (1% ± 1%), whereas no significant difference was seen in the expression of CD25, the low-affinity interleukin 2 receptor (34% ± 15% versus 40% ± 16%). More importantly, we identified a minor population of CD69bright CD25bright CD4+ lymphocytes in BAL (10% ± 6%) that were consistently absent from PB (1% ± 1%). Thus, CD4+ lymphocytes in the lung paradoxically coexpress surface molecules characteristic of naïve and memory helper T cells as well as surface molecules commonly associated with early and late stages of activation. No difference was observed for ALs obtained from TB-infected and uninfected lung segments in this regard. It remains to be determined if these surface molecules are induced by the alveolar environment or if CD4+ lymphocytes coexpressing this unusual combination of surface molecules are selectively recruited from the circulation. Our data suggest that ex vivo experiments on helper T-cell subsets that display distinctive phenotypes may be pivotal to studies on the human immune response to potential TB vaccines.
Much of our understanding of the role of pulmonary lymphocytes in host defense comes from the study of cells recovered from bronchoalveolar lavage (BAL) fluid. Lymphocytes account for approximately 10% of the cell types in BAL fluid obtained from healthy individuals (16). The distribution of T lymphocytes in BAL fluid is similar to that in peripheral blood (PB): 65 to 75% are CD3+, 40 to 45% are CD4+, and 20 to 25% are CD8+ (16). Persistent exposure to airborne antigens in the lower respiratory tract does not appear to result in preferential selection of T-cell clones (5, 10, 17, 50).
We previously characterized the BAL fluid from patients with less clinically and radiographically advanced tuberculosis (TB) as lymphocyte predominant (11). The major effector in cell-mediated immunity in TB is the CD4+ T cell (8). Pulmonary TB is characterized by CD4+ T-cell infiltration (2, 19, 23, 48). A preponderance of CD4+ T cells at the site of infection is associated with recovery following anti-TB therapy (2) and more rapid disease regression (49). Furthermore, CD4+ T cells activate and recruit other effector cells to the site of infection, resulting in an inflammatory response characterized by the influx of lymphocytes. This amplification process is diminished in human immunodeficiency virus (HIV)-coinfected patients (42).
Memory immunity is manifested by enhanced reactions that produce a more effective protection against pathogens to which an organism has been previously exposed (13). Following the initial encounter, the frequency of antigen-specific T cells increases, and this increase can persist in the absence of antigen for long periods of time. The ability of memory T cells to infiltrate tissue is fundamental to immune surveillance. Induction of memory CD4+ T cells is central to vaccination against Mycobacterium tuberculosis, and their ability to circulate from the blood to the lung is pivotal to mounting an effective defense against pulmonary TB.
It is known that T lymphocytes from the BAL fluid of purified-protein-derivative-positive (PPD+) healthy donors proliferate in response to PPD and Candida in vitro as well as T lymphocytes from their PB (24). Earlier fluorescence-activated cell sorter (FACS) studies performed on cells recovered from the BAL fluid of healthy controls have determined that alveolar T lymphocytes are CD45RO+ or CD45RA− and express other surface markers characteristic of an activated phenotype, including CD29, CD49a, CD69, CD98, and HLA-DR (3, 4, 12, 27, 31, 43). Thus, the alveolar compartment appear to be dominated by T lymphocytes, most of which are sensitized memory cells capable of responding to T-cell receptor (TCR)-mediated stimulation. With the availability of high-speed multicolor flow cytometry, we sought to reassess the surface phenotype of the helper T-cell population in the lung in order to gain further insight into the immunological potential of these cells in protecting the human host against M. tuberculosis.
MATERIALS AND METHODS
Study population.
Three groups of HIV-negative study subjects were evaluated: healthy volunteers who were PPD−, individuals who were PPD+, and patients with active TB (Table 1). Healthy PPD− and PPD+ subjects had normal chest radiographs. Active pulmonary TB was diagnosed by abnormal chest radiographs with respiratory symptoms and by sputum or BAL fluid specimens being smear and culture positive for M. tuberculosis. All patients and volunteers gave informed consent, and the protocol was approved by the Institutional Board of Research Associates at New York University School of Medicine.
TABLE 1.
Study subject demographics
| Parameter | No. of patients
|
|||
|---|---|---|---|---|
| PPD− | PPD+ | TBc | Total (%) | |
| Total no. | 8 | 7 | 10 | 25 (100) |
| Agea | 35 ± 3 | 33 ± 5 | 39 ± 3 | 36 ± 2 |
| Sex | ||||
| Male | 6 | 7 | 8 | 21 (84) |
| Female | 2 | 0 | 2 | 4 (16) |
| Race | ||||
| Caucasian | 1 | 0 | 1 | 2 (8) |
| African | 7 | 4 | 2 | 13 (52) |
| Hispanic | 0 | 2 | 4 | 6 (24) |
| Asian | 0 | 1 | 3 | 4 (16) |
| Nonsmokersb | 4 | 2 | 5 | 11 (44) |
Mean ± standard error of the mean.
Those who had never smoked and those who had quit smoking >1 year previously.
All were nonanergic with the exception of a single patient with miliary TB.
BAL.
BAL was performed by flexible bronchoscopy with local lidocaine (Xylocaine) anesthesia. Five 20-ml aliquots of sterile saline solution were instilled and subsequently recovered by gentle suction from the right middle lobe and lingula of the PPD− and PPD+ subjects. BAL was performed on TB patients during the first week of conventional antituberculosis treatment. Samples were obtained from the radiographically involved and uninvolved segments of the lung. The BAL fluid was filtered through two layers of sterile cotton gauze to remove mucus. Sodium citrate was added to the BAL fluid to a final concentration of 12.5 mM prior to processing. A total cell count was done in a hemocytometer for each BAL fluid sample. Cell viability was determined by trypan blue exclusion, and all samples had >90% viability. Cytocentrifuge smears were prepared by spinning 5 × 104 to 10 × 104 BAL fluid cells onto microscope slides in 0.5-ml cytofunnels at 500 × g for 5 min in a Cytospin 2 cytocentrifuge (Shandon). Diff-Quik (Dade Behring) staining was performed for each BAL fluid smear. Five hundred cells were counted to obtain the cell differential. A PB sample was obtained from each study subject immediately prior to BAL for simultaneous evaluation by FACS analysis.
Immunofluorescent labeling.
BAL fluid cells were centrifuged in the cold at 750 × g for 10 min. Ten milliliters of prechilled cell dissociation buffer (Sigma) containing 5% fetal calf serum (CDB-FCS) was added to the pellets, and the resuspended BAL fluid cells were passed through a 70-μm-pore-size cell strainer (Falcon). Following centrifugation in the cold at 750 × g for 10 min, pellets were resuspended with CDB-FCS to 1 × 106 to 2 × 106 lymphocytes per ml.
Premixed cocktails containing fluorescent monoclonal antibodies (MAbs) to multiple surface markers were reacted with 100 μl of BAL fluid cell suspension or 100 μl of PB on ice for 30 min. T cells (CD3+), B cells (CD19+), and Fc receptor-expressing (CD16+) NK cells (CD56+) were enumerated. T cells were subtyped for helper (CD4+) and suppressor or cytotoxic (CD8+) lineages, as well as γδ-TCR expression (clone 11F2). Reagents were those commonly used for in vitro diagnosis. In addition, CD4+ lymphocytes were analyzed for CD25 (clone 2A3), CD29 (clone 4B4), CD45RA (clone L48), CD45RO (clone UCHL-1), and CD69 (clone L78). For three-color labeling, MAbs conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), and Cy-Chrome (CC), an energy transfer fluorophore consisting of indodicarbocyanine (Cy5) coupled to PE, were used. For four-color labeling, MAbs conjugated to FITC, PE, ECD (an energy transfer fluorophore consisting of Texas red coupled to PE), and allophycocyanin (APC) were used. Irrelevant MAbs conjugated to the same fluorophores were used to determine nonspecific cell surface binding. Fluorescent reagents were obtained from Becton Dickinson Immunocytometry Systems (BDIS), Pharmingen, or Coulter/Immunotech.
Following surface labeling, cells were treated with FACS lysing solution (BDIS) according to supplier instructions to simultaneously remove erythrocytes and permeabilize lymphocytes, after which cells were washed with Dulbecco's phosphate-buffered saline (DPBS) and fixed with 1% formaldehyde in DPBS. Some cells were washed with DPBS containing 5% FCS following treatment with FACS lysing solution and then reacted with a MAb to the nuclear antigen Ki67 (clone MIB-1) or an irrelevant MAb conjugated to the same fluorophore prior to fixation. When necessary, preserved cells were stored at 4°C in the dark for less than 24 h prior to analysis.
Flow cytometry.
Fluorophores used were discriminated by the specificity of their excitation or emission wavelengths. Cells were analyzed with a FACS Vantage (BDIS) with flow rates of ≤5,000 events/s. An argon ion laser was used for 488-nm excitation (FITC, PE, ECD, or CC) and a mixed-gas Spectrum laser provided 647-nm excitation (APC). Emitted light was detected by logarithmic amplification through barrier filters specific for the emission range of the different fluorophores: 530/22 nm for FITC, 585/42 nm for PE, 630/22 nm for ECD, and 675/20 for CC or APC. Fluorescence spillover detected by inappropriate channels was corrected by electronic compensation. All data were acquired in list mode and subsequently processed using CellQuest software on a Macintosh computer system. Forward-scattered light (size) and 90° angle-scattered light (granularity) intensities at 488 nm were used to exclude debris, blood monocytes, and alveolar macrophages (AMs) and select for lymphocytes. The purity of the lymphocyte gate for CD14− CD45bright cells was 89% ± 11% in BAL fluid and 96% ± 4% in PB. Cells within each subset were reported as percent lymphocytes or percent CD4+ lymphocytes. When needed, absolute counts were obtained by multiplication by the lymphocyte count determined as described above.
Statistics.
Descriptive statistics, including means, standard deviations, and percentages, were used to summarize the demographic variables of the study subjects; medians and first and third quartiles were used to summarize BAL fluid and PB data.
The BAL fluid and PB of the PPD−, PPD+, and TB groups were compared using the Kruskal-Wallis test. If no significant difference was found between the three groups, the groups were combined and one-sample tests were performed using the Wilcoxon signed-rank test to analyze differences between the BAL fluid and PB cells. Comparisons of BAL fluid cells from the involved lobe of TB patients with BAL fluid cells from the lobes of PPD− and PPD+ subjects (combined group) were done using the Wilcoxon rank-sum test. Analyses of differences between the BAL fluid and PB of the TB patients were performed using the Wilcoxon signed-rank test.
To adjust for possible effects of age (35 years or older versus less than 35 years) and smoking (ever versus never), multiple linear regression was also performed on the rank-transformed data. However, adjusted results are not presented, as they were consistent with the unadjusted results. All analyses of data were conducted with two-sided tests of hypotheses at the ≤0.05 significance level. The analyses presented are exploratory, since adjustments were not made for multiple comparisons, and P values are provided for the purpose of data interpretation.
RESULTS
Patient demographics and BAL.
Twenty-five HIV-negative individuals were recruited for this study: 8 PPD− volunteers, 7 PPD+ individuals, and 10 patients with active pulmonary TB (Table 1). The three groups were similar in terms of age, race, and smoking habit. The TB patients all had evidence of active pulmonary disease by chest radiograph, consisting primarily of upper lobe nodular infiltrates (n = 3), cavities (n = 6), or miliary TB (n = 1).
BAL fluid samples were obtained from the lobes of all PPD− and PPD+ subjects and the involved and uninvolved lobes of all but three TB patients, from whom only BAL fluid samples from involved lobes were collected. As we had seen in previous studies, BAL fluid (Table 2) from the involved lobes of TB patients had significantly fewer AMs (P = 0.01), more lymphocytes (P = 0.04), and more neutrophils (P = 0.02) than BAL fluid from the combined group of PPD− and PPD+ patients. TB patients also had significantly fewer AMs in the involved lobe than in the uninvolved lobe (P = 0.05). The TB patients fall into two categories: those whose BAL fluid cell differential was lymphocyte predominant and those with neutrophil predominance. Five TB patients with localized pulmonary disease had BAL fluid lymphocyte counts greater than 20%, as previously described (11). One of these patients had a miliary pattern in the chest radiograph.
TABLE 2.
Comparison of BAL cell differentials for subject groups
| Subject group (BAL fluid source) | No. of cells/ml (104) | % Leukocytes ina:
|
|||
|---|---|---|---|---|---|
| AM | L | N | E | ||
| PPD− | 34 (18–54) | 95 (93–97) | 4 (2–6) | 0.6 (0–1) | ≤1 |
| PPD+ | 54 (38–57) | 94 (92–95) | 4 (3–4) | 2 (1–3) | ≤1 |
| TB (involved lobe) | 39 (30–50) | 35b (27–67) | 13c (4–24) | 25c (1–54) | ≤1 |
| TB (uninvolved lobe) | 22 (21–29) | 92 (73–95) | 7 (3–17) | 3 (1–3) | ≤1 |
Values are medians; values in parentheses are first to third quartiles. AM, alveolar macrophages; L, lymphocytes; N, neutrophils; E, eosinophils.
P ≤ 0.05 compared to other values in column.
P ≤ 0.05 compared to values for PPD+ and PPD− groups.
Lymphocyte subsets.
Table 3 illustrates the distribution of lymphocyte subsets in the PB and BAL fluid of our study subjects (1 PB and 11 BAL fluid specimens were excluded because of technical difficulties encountered in the collection or processing of these specimens).
TABLE 3.
Comparison of lymphocyte subsets in PB and BAL fluid
| Subset | Phenotype | % Lymphocytesa
|
||||||
|---|---|---|---|---|---|---|---|---|
| PPD− subjects
|
PPD+ subjects
|
TB patients
|
||||||
| PB | BAL fluid | PB | BAL fluid | PB | BAL fluid (involved lobe) | BAL fluid (uninvolved lobe) | ||
| B cells | CD19+ | 13 (12–7) | 2b (1–4) | 9 (9–14) | 2b (2–4) | 6c (3–8) | 1b (1–3) | 2b (2–3) |
| NK cells | CD56+ CD16− | 9 (8–9) | 12 (7–14) | 9 (8–12) | 9 (8–9) | 11 (7–19) | 7 (5–8) | 5 (5–7) |
| CD56+ CD16+ | 6 (4–8) | 2b (1–3) | 9 (4–11) | 2b (2–2) | 13c (10–19) | 2b (2–4) | 2b (2–4) | |
| T cells | CD3+ | 69 (66–74) | 87b (79–93) | 73 (70–79) | 89b (81–91) | 70 (64–75) | 93b (80–95) | 89b (89–90) |
| γδ TCR+ | 2 (1–2) | 1 (1–2) | 3 (3–4) | 2 (1–4) | 3d (2–6) | 1 (1–2) | 3 (2–4) | |
| CD3+ CD4+ | 45 (42–47) | 57 (50–61) | 45 (37–57) | 45 (37–54) | 35e (31–43) | 52 (50–76) | 60 (52–64) | |
| Abs CD4+f | 83 (57–106) | 40 (34–100) | 540g (129–1,924) | 117 (74–360) | ||||
| CD3+ CD8+ | 25 (22–26) | 29 (20–36) | 27 (21–30) | 33 (24–34) | 24 (20–43) | 21 (16–35) | 34 (26–38) | |
| CD4/CD8 | 2 (1–2) | 2 (1–3) | 1 (1–3) | 1 (1–2) | 1 (1–2) | 3h (2–4) | 2 (1–3) | |
Values are medians; values in parentheses are first to third quartiles.
P ≤ 0.05 compared to values for PB of all groups.
P ≤ 0.05 compared to values for PB of PPD− and PPD+ subjects.
P ≤ 0.05 compared to values for BAL fluid (involved lobe) of TB patients.
P ≤ 0.05 compared to values for all BAL fluid of TB patients.
Absolute CD3+ CD4+ cells; data are 106 cells per milliliter of BAL fluid.
P ≤ 0.05 compared to values for BAL fluid of PPD− and PPD+ groups.
P ≤ 0.05 compared to values for PB of all groups.
We found that PB lymphocytes (BLs) of TB patients contained fewer B cells (P = 0.005) and more immunoglobulin G Fc receptor III-positive NK cells (P = 0.03) than BLs of PPD− or PPD+ subjects. In contrast, there were no significant differences in the distribution of B cells or NK cells in BAL lymphocytes (ALs) among the three groups. However, combined data from the BAL fluid and PB of all study subjects indicated that lymphocytes in BAL fluid contained fewer B cells and NK cells than those in PB (P = 0.001 and P = 0.006, respectively).
There were no significant differences in the distribution of T cells in PB or BAL fluid lymphocytes among the three subject groups. However, we found that ALs contained more T cells than BLs for the combined groups (P = 0.01).
For PPD− or PPD+ subjects, there were no significant differences in the distribution of CD4+ T cells in BAL fluid compared to PB. For TB patients, the percent CD4+ T cells in BAL fluid lymphocytes was marginally higher than that in PB (P = 0.06). However, analysis by CD4+ count indicated that the absolute number of helper T cells in the involved lobe was considerably greater than that in the uninvolved lobe, as well as the lobes of PPD− and PPD+ subjects (P = 0.01).
There were no significant differences in the distribution of CD8+ T cells in PB or BAL fluid lymphocytes among the three subject groups. Furthermore, there were no significant differences in the CD4/CD8 ratio except for the involved lobe of TB patients, which had a higher CD4/CD8 ratio than PB (P = 0.05).
We did not observe any significant differences in the distribution of γδ T cells in PB or BAL fluid lymphocytes among the three subject groups.
Naïve and memory helper T cells.
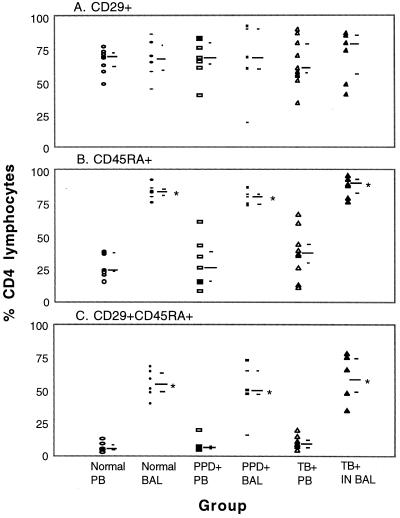
To better define helper T cells resident in the lung and compare them to helper T cells in the circulation, we evaluated the coordinate expression of CD45RA and CD29 by three-color FACS analysis. Similar percentages of CD4+ lymphocytes from PB and BAL fluid expressed CD29 (Fig. 1A). However, a much higher percentage (P = 0.001) of CD4+ lymphocytes from BAL fluid expressed CD45RA than from PB (Fig. 1B). More strikingly, the majority of CD4+ ALs simultaneously expressed CD29 and CD45RA (Fig. 1C). CD29+ and CD45RA+ cells are known to constitute discrete subsets within the helper T-lymphocyte population in PB. Thus, in marked contrast to BAL fluid, only a small percentage of CD4+ BLs were CD29+ CD45RA+ (P = 0.001). These findings were consistent for all three subject groups. It was a surprise to see that molecules characteristic of naïve (CD45RA) and previously activated (CD29) phenotypes were coexpressed on alveolar helper T cells.
FIG. 1.
Expression of CD29 and CD45RA and simultaneous expression of CD29 and CD45RA on helper T cells from BAL fluid and PB. Viable cells were labeled with FITC–anti-CD45RA, PE–anti-CD29, and CC–anti-CD4, fixed, and evaluated by FACS analysis. A minimum of 2,500 CD4+ lymphocyte-sized events were analyzed. The long bar indicates the median and the short bars indicate the first and third quartiles for each subject group. IN, involved lobe. BAL fluid values significantly different from PB values (P ≤ 0.05) are marked with an asterisk. All TB patients were nonanergic.
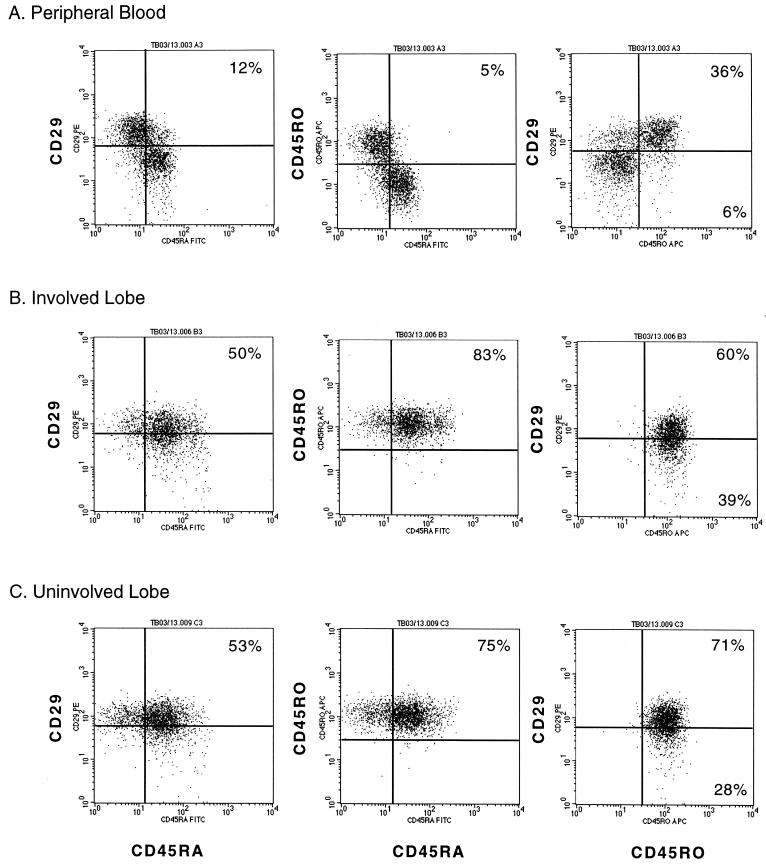
In order to resolve this dichotomy, we studied the expression of CD29, CD45RA, and CD45RO on CD4+ lymphocytes from the PB and BAL fluid of additional subjects by four-color FACS analysis. The histograms obtained from a representative TB patient are shown in Fig. 2. CD4+ BLs displayed either CD29 or CD45RA, and only a few cells expressed both (Fig. 2A, left histogram). Similarly, CD4+ BLs displayed either CD45RA or CD45RO but rarely both (Fig. 2A). However, the majority of CD4+ BLs that expressed CD45RO also expressed CD29 (Fig. 2A). In marked contrast, most CD4+ ALs, independent of whether they were collected from the involved or uninvolved lobe, coexpressed CD29 and CD45RA (Fig. 2B and C). More remarkably, these CD45RA+ cells also expressed CD45RO (Fig. 2B and C). Identical findings were obtained for the PB and BAL fluid from a healthy PPD− subject and two other TB patients (data not shown).
FIG. 2.
Simultaneous expression of CD29, CD45RA, and CD45RO on CD4+ lymphocytes from the PB and BAL fluid obtained from the involved and uninvolved lobes of a TB patient. Viable cells were labeled with FITC–anti-CD45RA, PE–anti-CD29, APC–anti-CD45RO, and ECD–anti-CD4, fixed, and evaluated by FACS analysis. A minimum of 2,500 CD4+ lymphocyte-sized events were analyzed. The distribution of events in each quadrant is given as percent CD4+ lymphocytes. Quadrants located in the same position are used to analyze histograms within the same panel.
Variability in reagents or instrumentation cannot account for our discrepant findings, since CD4+ BLs displayed the largely reciprocal pattern of CD45RA and CD29 or CD45RO expression. Differences in the ex vivo handling of BAL fluid specimens could be a major contributing factor. We did not isolate ALs by density gradient fractionation or adherence depletion. Instead, all the cells in the BAL fluid were collected by centrifugation and then resuspended in a nonenzymatic cell dissociation buffer to promote detachment of ALs from AMs. Identical volumes of BAL fluid cell suspension and whole blood were labeled side by side, treated with a red blood cell-lysing solution, and fixed with formaldehyde. Unfractionated leukocyte populations were discriminated by cell size and granularity during FACS analysis. It is conceivable that CD45RA+ T cells tightly adhering to AMs were removed by others during density gradient fractionation or adherence depletion (4, 12, 27, 41, 48). It is also conceivable that incubation with AMs at 37°C during adherence depletion modulated CD45RA from the T-cell surface. The possibility remained that we induced the expression of CD45RA ex vivo. This is, however, unlikely, since our BAL fluid specimens were kept at 4°C immediately after bronchoscopy, in the presence of a divalent cation chelator.
Activation phenotype of helper T cells.
Besides CD29, a very late activation (VLA) molecule, we also compared the expression of other markers of immune activation on helper T cells from the PB and BAL fluid of our study subjects by three-color FACS analysis.
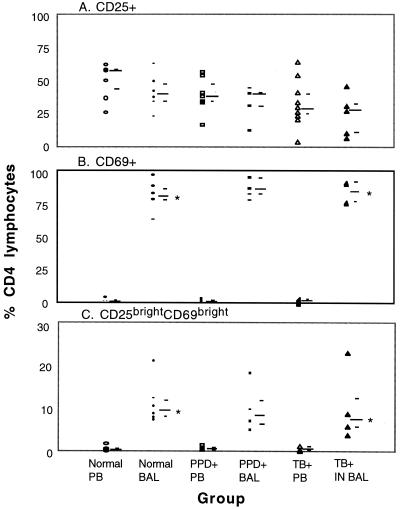
We used CD25, the low-affinity interleukin 2 receptor (IL-2Rα), as a marker of intermediate activation (Fig. 3A). There were no significant differences in the percent CD25-expressing CD4+ lymphocytes in BAL fluid compared to PB for all three subject groups. However, the percent CD25-expressing CD4+ BLs of TB patients was lower than that in the combined group of PPD− and PPD+ subjects (P = 0.02).
FIG. 3.
Expression of CD25 and CD69 and enhanced expression of both markers on helper T cells from BAL fluid and PB. Viable cells were labeled with FITC–anti-CD69, PE–anti-CD25, and CC–anti-CD4, fixed, and analyzed with a FACS. A minimum of 2,500 CD4+ lymphocyte-sized events were analyzed. The long bar indicates the median and the short bars indicate the first and third quartiles for each subject group. IN, involved lobe. BAL fluid values significantly different from PB values (P ≤ 0.05) are marked with an asterisk. All TB patients were nonanergic.
We also used CD69 as a marker of early activation (Fig. 3B). In marked contrast to CD25, the percent CD69-expressing CD4+ lymphocytes in BAL fluid was drastically elevated compared to that in PB for all three subject groups (P = 0.01). The high percentages of CD69+ helper T cells in BAL fluid were reminiscent of that observed for PB following in vitro stimulation with phorbol myristic acid and ionomycin (data not shown).
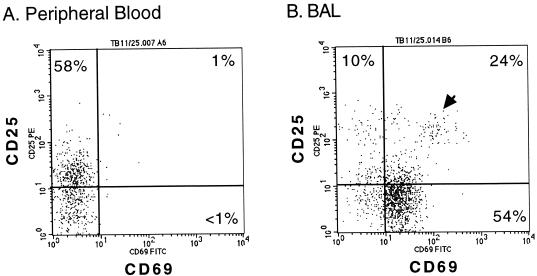
Most interestingly, we identified a small population of CD4+ ALs (Fig. 4B) that expressed high levels of both early and intermediate activation markers (CD25bright CD69bright). CD4+ lymphocytes that displayed this phenotype were essentially absent from the PB of the same individual (Fig. 4A). As shown in Fig. 3C, this finding was similar for all three subject groups. When data from these groups were combined, it was obvious that, unlike PB (median = 0.4%), CD25bright CD69bright helper T cells were consistently detectable in the BAL fluid (median = 8.5%) as a significant, albeit minor, subset (P = 0001). Moreover, no significant difference was found between the percent CD25bright CD69bright CD4+ ALs of TB patients and those of PPD− and PPD+ subjects.
FIG. 4.
Expression of CD25 and CD69 on CD4+ lymphocytes from the PB and BAL fluid of a healthy PPD− subject. Viable cells were labeled and analyzed as described for Fig. 3. The distribution of events in each quadrant is given as percent CD4+ lymphocytes. Quadrants located in the same position are used to analyze both histograms. A cluster of CD25bright CD69bright events in the right histogram is indicated by an arrowhead. This subset constituted 8.4% of the CD4+ lymphocytes in this BAL fluid specimen.
CD69 is constitutively expressed by monocytes (28), and we recently reported the in vivo and in vitro induction of IL-2Rα expression in mononuclear phagocytes by M. tuberculosis (45). In our FACS analyses, ALs and AMs were discriminated on the basis of cell size and granularity. The purity of the AL population (CD45bright CD14−) was often <90%. Nonetheless, it was unlikely that the CD25bright CD69bright CD4+ cells that we detected were technical artifacts caused by AMs inadvertently distributing within the “gate” that we used to select for ALs. There was no correlation (r = −0.08) between the percent CD25bright CD69bright CD4+ cells and the percent CD45bright CD14− cells recovered within the lymphocyte gate. One can argue that AMs do not always express CD14 (16), a monocyte-specific marker. However, <1% of the CD4+ lymphocytes in our BAL fluid specimens were found to display high levels of CD54 and HLA-DR as CD4+ AMs.
To see if the “recently activated” helper T cells in the lung were induced to proliferate in situ, we also determined the expression of Ki67, a nuclear antigen present in all phases of the cell cycle except G0 (14). Combined data from the BAL fluid of the three subject groups showed that very few CD4+ ALs coexpressed Ki67 and CD25 (median = 1.4%), and there was no significant difference in the percent CD25+ Ki67+ helper T cells in the involved lobe of TB patients compared to that in the BAL fluid of PPD− and PPD+ subjects.
DISCUSSION
Some, but not all, of our observations from this relatively small study of 10 TB patients and 15 PPD− or PPD+ healthy controls are in agreement with previous findings from our group, as well as other laboratories (19, 23, 25, 31, 43). We found a fourfold increase in the number of lymphocytes from the radiographically involved lobes of TB patients compared to the lobes of healthy controls. Both site-specific and generalized inflammation was seen, with a twofold increase in the number of lymphocytes from the radiographically uninvolved lobes of TB patients. These observations confirm earlier reports that pulmonary TB was characterized by the influx of lymphocytes (2, 19, 23, 43). No significant differences in the percentage of B cells, NK cells, γδ T cells, or CD4+ or CD8+ T cells were found when we compared BAL fluid lymphocytes from TB-infected lung segments with those from uninfected lung segments. The predominant lymphocyte is the CD4+ T cell. Our group has noted that lymphocyte subpopulations recovered in BAL fluid are similar to those at the site of granuloma formation (23).
In agreement with previous reports (3, 12), we found that most alveolar CD4+ lymphocytes expressed CD29. However, at variance with previous reports (4, 12, 27, 41, 43, 48), we also found that most CD4+ lymphocytes expressed CD45RA. Most surprisingly, CD4+ lymphocytes that expressed CD45RA also expressed CD45RO. Human helper T cells were first divided using MAbs to CD45RA and CD29 into two major subsets with divergent in vitro functions associated with their surface phenotypes. CD45RA+ CD29− helper T cells respond poorly to recall antigens, while CD45RA− CD29+ helper T cells respond vigorously (33, 34). Subsequently, T cells were found to express CD45RA or CD45RO, isoforms within the CD45 family of transmembrane tyrosine phosphatases generated by alternative splicing (47). CD45RA+ CD45RO− and CD45RA− CD45RO+ subpopulations are commonly referred to as “naïve” and “memory” T cells, respectively. Nonetheless, CD45RA and CD45RO phenotypes are known to undergo bidirectional conversion (6). Human CD4+ CD45RA+ T-cell lines generated from the PB of healthy donors coexpressed CD45RO and displayed a cyclic pattern of CD45RA but not CD45RO expression following mitogen stimulation (40). Indeed, T cells coexpressing CD45RA and CD45RO were considered intermediates in the naïve-to-memory transition and 2 to 10% of T cells in secondary lymphoid tissue were found to express this phenotype (37). Another group reported that a minor subset (<1%) of CD4+ lymphocytes in the PB of healthy donors coexpressed high levels of CD45RA and CD45RO (15). More recently, investigators discovered that a subset of CD4+ CD45RA+ cells in PB was able to produce gamma interferon, a characteristic of antigen-experienced Th1 cells (46), while studies on the development of protective T-cell responses showed that during primary cytomegalovirus infection, the first virus-specific CD4+ T cells to appear in PB displayed a CD45RA+ CD45RO+ phenotype (39).
Assessment of periodontal tissue showed that nearly half of the CD4+ lymphocytes extracted from diseased lesions coexpressed CD29 and CD45RA (44). CD29 is the β1 subunit of a number of integrins from the VLA family. The presence of VLA-4 (α4β1) on T cells is associated with their adhesion to and migration through endothelial cell layers (38) by interaction with VCAM-1, receptors upregulated at proinflammatory sites (26). This can easily explain why only CD4+ lymphocytes expressing moderate to high levels of CD29 can enter the alveolar space. A chemokine produced by cells of monocytic lineage, PARC (also known as DC-CK1), preferentially attracts CD45RA+ T cells (1, 18). PARC mRNA is expressed constitutively in human lung. Perhaps CD29+ CD45RA+ helper T cells, which constitute approximately 10% of the CD4+ lymphocytes in the PB of healthy subjects (35, 44), are selectively recruited.
CD69 is one of the earliest surface markers expressed following TCR ligation. While its ligand has not been identified, CD69 is known to serve as a costimulatory molecule for T-cell activation, proliferation, and differentiation (28). In an in vitro model of transendothelial migration, CD4+ T cells expressing high levels of VLA-4 were found to migrate (9). However, only a minority of the migrating cells coexpressed CD69. It is quite plausible, therefore, that CD69 expression on alveolar helper T cells was induced following their arrival in the lung. CD69 expression on γδ as well as αβ human T cells can be driven by polyclonal stimulators such as bacterial lipopolysaccharide or tumor necrosis factor alpha (21). Our group previously reported that tumor necrosis factor alpha is spontaneously released by BAL fluid cells and that the amount released by cells recovered from infected lung segments is 10-fold higher than that released by cells from uninfected lung segments (22). If CD69 expression is a relevant indication, then BAL fluid CD4+ lymphocytes from all three subject groups appear to be uniformly sensitized.
Polyclonal helper T-cell activation in the lung did not, however, progress beyond the early stage. In agreement with reports from other laboratories (3, 31), we found that only half as many CD4+ lymphocytes in BAL fluid expressed CD25 as expressed CD69. Using coordinate detection, we were able to identify CD4+ lymphocytes expressing high levels of both CD69 and CD25. This subset constituted approximately 10% of the helper T-cell population in the BAL fluid of all three subject groups. Unlike CD45RA+ CD45RO+ T cells in secondary lymphoid tissues (37), the ones in BAL fluid, including the CD25bright CD69bright subset, were mostly in the G0 phase of the cell cycle. Our finding is confirmed by transbronchial biopsies from clinically well lung transplant recipients, which showed few (0 to 3%) proliferating T lymphocytes (32). While TCR ligation is sufficient to induce IL-2Rα expression, IL-2 production is dependent on costimulatory signals (36). IL-2, secreted mainly by CD4+ T cells, is required for progression through G1.
Our observations strongly implicate the alveolar space as an environment in which the majority of CD4+ T cells are sensitized, or appropriately poised, to respond to exogenous pathogens. A minor population of CD4+ T cells, presumably those that have encountered a specific antigen, are driven further along the activation pathway. They are not, however, sufficiently triggered to initiate replication in the lung. It remains to be determined if these stimulated helper T cells subsequently acquire homing receptors that endow them with the capacity to migrate to peripheral lymphoid organs, where costimulatory signals from accessory cells are available to potentiate IL-2 production.
The major difference between TB-infected and uninfected lung segments in our study appears to be the number of CD4+ lymphocytes. Seymour et al. (44) reported that a higher proportion of CD4+ lymphocytes from diseased periodontal tissue were CD29+ CD45RA+ than in nondiseased tissue. Therefore, it is perplexing that no discernible differences in the distribution of helper T-cell subsets or variations in the level of activation molecules that they expressed were found in the lung segments of our TB patients. There were numerous reports that the proliferative response of human PB lymphocytes to the whole bacilli and recombinant or secreted proteins of M. tuberculosis was diminished in TB patients relative to that in tuberculin-positive healthy controls (7, 29, 30), and while patients with minimal disease gave a response comparable to that of M. tuberculosis BCG-vaccinated donors, patients with active advanced disease did not (7). Thus, it will be intriguing to determine if there are discernible differences in CD4+ lymphocytes of anergic and nonanergic TB patients. Likewise, it will be interesting to determine if CD4+ CD45RA+ CD45RO+ cells from the alveolar space display discordant levels of CD49d-CD29. It was proposed that heterogeneity in integrin expression could represent distinctive functional and homing capacities (20). Studying additional phenotypic markers on helper T cells in the alveolar space will allow us to pursue relevant populations in PB, obviating invasive bronchoscopy procedures, in order to evaluate potential TB vaccines. Assessing the cytokine profile of freshly explanted T cells in the BAL fluid of TB patients and healthy controls, as well as the role of alveolar CD8+ T cells in regulating CD4+ T-cell activity, will better our understanding of normal immunophysiology in the lung as well as elucidating the immunopathology underlying pulmonary TB.
ACKNOWLEDGMENTS
We thank M. Bodkin, D. Chan, and J. Law for technical assistance and E. Ching for manuscript preparation.
This work was supported by a Heiser Foundation Postdoctoral Fellowship Award (B. Raju), NIH grants MO1 00096 and HL62055 (W. Rom), NIH grants AI41949 and AI44729 (D. B. Tse), and the GCRC and CFAR at New York University School of Medicine.
REFERENCES
- 1.Adema G J, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T, Bacon K B, Figdor C G. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387:713–717. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie G M, Solomon J A, Bateman E D. Lymphocyte and lymphocyte subset numbers in blood and in bronchoalveolar lavage and pleural fluid in various forms of human pulmonary tuberculosis at presentation and during recovery. Thorax. 1992;47:513–518. doi: 10.1136/thx.47.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancochea J, Gonzalez A, Sanchez M J, Aspa J, Lopez-Botet M. Expression of lymphocyte activation surface antigens in bronchoalveolar lavage and peripheral blood cells from young healthy subjects. Chest. 1993;104:32–37. doi: 10.1378/chest.104.1.32. [DOI] [PubMed] [Google Scholar]
- 4.Becker S, Harris D T, Koren H S. Characterization of normal human lung lymphocytes and interleukin-2-induced lung T cell lines. Am J Respir Cell Mol Biol. 1990;3:441–448. doi: 10.1165/ajrcmb/3.5.441. [DOI] [PubMed] [Google Scholar]
- 5.Bellocq A, Lecossier D, Pierre-Audigier C, Tazi A, Valeyre D, Hance A J. T cell receptor repertoire of T lymphocytes recovered from the lung and blood of patients with sarcoidosis. Am J Respir Crit Care Med. 1994;149:646–654. doi: 10.1164/ajrccm.149.3.7906994. [DOI] [PubMed] [Google Scholar]
- 6.Beverley P C. Functional analysis of human T cell subsets defined by CD45 isoform expression. Semin Immunol. 1992;4:35–41. [PubMed] [Google Scholar]
- 7.Boesen H, Jensen B N, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boom W H. The role of T-cell subsets in Mycobacterium tuberculosis infection. Infect Agents Dis. 1996;5:73–81. [PubMed] [Google Scholar]
- 9.Brezinschek R I, Lipsky P E, Galea P, Vita R, Oppenheimer-Marks N. Phenotypic characterization of CD4+ T cells that exhibit a transendothelial migratory capacity. J Immunol. 1995;154:3062–3077. [PubMed] [Google Scholar]
- 10.Burastero S E, Borgonovo B, Gaffi D, Frittoli E, Wack A, Rossi G A, Crimi E. The repertoire of T-lymphocytes recovered by bronchoalveolar lavage from healthy nonsmokers. Eur Respir J. 1996;9:319–327. doi: 10.1183/09031936.96.09020319. [DOI] [PubMed] [Google Scholar]
- 11.Condos R, Rom W N, Liu Y M, Schluger N W. Local immune responses correlate with presentation and outcome in tuberculosis. Am J Respir Crit Care Med. 1998;157:729–735. doi: 10.1164/ajrccm.157.3.9705044. [DOI] [PubMed] [Google Scholar]
- 12.Dominique S, Bouchonnet F, Smiejan J M, Hance A J. Expression of surface antigens distinguishing “naive” and previously activated lymphocytes in bronchoalveolar lavage fluid. Thorax. 1990;45:391–396. doi: 10.1136/thx.45.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutton R W, Bradley L M, Swain S L. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 14.Gerdes J, Lemke H, Baisch H, Wacker H H, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 15.Hamann D, Baars P A, Hooibrink B, van Lier R W. Heterogeneity of the human CD4+ T-cell population: two distinct CD4+ T-cell subsets characterized by coexpression of CD45RA and CD45RO isoforms. Blood. 1996;88:3513–3521. [PubMed] [Google Scholar]
- 16.Harbeck R J. Immunophenotyping of bronchoalveolar lavage lymphocytes. Clin Diagn Lab Immunol. 1998;5:271–277. doi: 10.1128/cdli.5.3.271-277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauk P J, Wenzel S E, Trumble A E, Szefler S J, Leung D Y. Increased T-cell receptor vbeta8+ T cells in bronchoalveolar lavage fluid of subjects with poorly controlled asthma: a potential role for microbial superantigens. J Allergy Clin Immunol. 1999;104:37–45. doi: 10.1016/s0091-6749(99)70111-9. [DOI] [PubMed] [Google Scholar]
- 18.Hieshima K, Imai T, Baba M, Shoudai K, Ishizuka K, Nakagawa T, Tsuruta J, Takeya M, Sakaki Y, Takatsuki K, Miura R, Opdenakker G, Van Damme J, Yoshie O, Nomiyama H. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 alpha/LD78 alpha and chemotactic for T lymphocytes, but not for monocytes. J Immunol. 1997;159:1140–1149. [PubMed] [Google Scholar]
- 19.Hoheisel G B, Tabak L, Teschler H, Erkan F, Kroegel C, Costabel U. Bronchoalveolar lavage cytology and immunocytology in pulmonary tuberculosis. Am J Respir Crit Care Med. 1994;149:460–463. doi: 10.1164/ajrccm.149.2.8306046. [DOI] [PubMed] [Google Scholar]
- 20.Horgan K J, Luce G E, Tanaka Y, Schweighoffer T, Shimizu Y, Sharrow S O, Shaw S. Differential expression of VLA-alpha 4 and VLA-beta 1 discriminates multiple subsets of CD4+CD45R0+ “memory” T cells. J Immunol. 1992;149:4082–4087. [PubMed] [Google Scholar]
- 21.Lahn M, Kalataradi H, Mittelstadt P, Pflum E, Vollmer M, Cady C, Mukasa A, Vella A T, Ikle D, Harbeck R, O'Brien R, Born W. Early preferential stimulation of gamma delta T cells by TNF-alpha. J Immunol. 1998;160:5221–5230. [PubMed] [Google Scholar]
- 22.Law K, Weiden M, Harkin T, Tchou-Wong K, Chi C, Rom W N. Increased release of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am J Respir Crit Care Med. 1996;153:799–804. doi: 10.1164/ajrccm.153.2.8564135. [DOI] [PubMed] [Google Scholar]
- 23.Law K F, Jagirdar J, Weiden M D, Bodkin M, Rom W N. Tuberculosis in HIV-positive patients: cellular response and immune activation in the lung. Am J Respir Crit Care Med. 1996;153:1377–1384. doi: 10.1164/ajrccm.153.4.8616569. [DOI] [PubMed] [Google Scholar]
- 24.Lecossier D, Valeyre D, Loiseau A, Cadranel J, Tazi A, Battesti J P, Hance A J. Antigen-induced proliferative response of lavage and blood T lymphocytes. Comparison of cells from normal subjects and patients with sarcoidosis. Am Rev Respir Dis. 1991;144:861–868. doi: 10.1164/ajrccm/144.4.861. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Rossman M D, Imir T, Oner-Eyuboglu A F, Lee C W, Biancaniello R, Carding S R. Disease-specific changes in gammadelta T cell repertoire and function in patients with pulmonary tuberculosis. J Immunol. 1996;157:4222–4229. [PubMed] [Google Scholar]
- 26.Lobb R R, Hemler M E. The pathophysiologic role of alpha 4 integrins in vivo. J Clin Investig. 1994;94:1722–1728. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marathias K P, Preffer F I, Pinto C, Kradin R L. Most human pulmonary infiltrating lymphocytes display the surface immune phenotype and functional responses of sensitized T cells. Am J Respir Cell Mol Biol. 1991;5:470–476. doi: 10.1165/ajrcmb/5.5.470. [DOI] [PubMed] [Google Scholar]
- 28.Marzio R, Mauel J, Betz-Corradin S. CD69 and regulation of the immune function. Immunopharmacol Immunotoxicol. 1999;21:565–582. doi: 10.3109/08923979909007126. [DOI] [PubMed] [Google Scholar]
- 29.Mehra V, Gong J H, Iyer D, Lin Y, Boylen C T, Bloom B R, Barnes P F. Immune response to recombinant mycobacterial proteins in patients with tuberculosis infection and disease. J Infect Dis. 1996;174:431–434. doi: 10.1093/infdis/174.2.431. [DOI] [PubMed] [Google Scholar]
- 30.Mendez-Samperio P, Gonzalez-Garcia L, Pineda-Fragoso P R, Ramos-Sanchez E. Specificity of T cells in human resistance to Mycobacterium tuberculosis infection. Cell Immunol. 1995;162:194–201. doi: 10.1006/cimm.1995.1069. [DOI] [PubMed] [Google Scholar]
- 31.Meyer K C, Soergel P. Variation of bronchoalveolar lymphocyte phenotypes with age in the physiologically normal human lung. Thorax. 1999;54:697–700. doi: 10.1136/thx.54.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milne D S, Moy J V, Corris P A, Robertson H, De Soyza A, Kirby J A, Cunningham A C. Intragraft proliferating T lymphocytes are associated with moderate acute pulmonary rejection. Transplantation. 2000;69:1981–1984. doi: 10.1097/00007890-200005150-00045. [DOI] [PubMed] [Google Scholar]
- 33.Morimoto C, Letvin N L, Boyd A W, Hagan M, Brown H M, Kornacki M M, Schlossman S F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985;134:3762–3769. [PubMed] [Google Scholar]
- 34.Morimoto C, Letvin N L, Distaso J A, Aldrich W R, Schlossman S F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985;134:1508–1515. [PubMed] [Google Scholar]
- 35.Neidhart M, Pataki F, Schonbachler J, Bruhlmann P. Flow cytometric characterisation of the “false naive” (CD45RA+, CD45RO−, CD29 bright+) peripheral blood T-lymphocytes in health and in rheumatoid arthritis. Rheumatol Int. 1996;16:77–87. doi: 10.1007/BF01816439. [DOI] [PubMed] [Google Scholar]
- 36.Nelson B H, Willerford D M. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 37.Picker L J, Treer J R, Ferguson-Darnell B, Collins P A, Buck D, Terstappen L W. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selection on T cells during the virgin to memory cell transition. J Immunol. 1993;150:1105–1121. [PubMed] [Google Scholar]
- 38.Pietschmann P, Cush J J, Lipsky P E, Oppenheimer-Marks N. Identification of subsets of human T cells capable of enhanced transendothelial migration. J Immunol. 1992;149:1170–1178. [PubMed] [Google Scholar]
- 39.Rentenaar R J, Gamadia L E, van DerHoek N, van Diepen F N, Boom R, Weel. Wertheim-van Dillen J F P M, van Lier R A, ten Berge I J. Development of virus-specific CD4(+) T cells during primary cytomegalovirus infection. J Clin Investig. 2000;105:541–548. doi: 10.1172/JCI8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothstein D M, da Silva A, Sugita K, Yamamoto M, Prasad K V, Morimoto C, Schlossman S F, Rudd C E. Human CD4/CD45RA+ and CD4/CD45RA− T cell subsets express CD4–p56lck complexes, CD4-associated lipid kinases, TCR/CD3–p59fyn complexes, and share similar tyrosine kinase substrates. Int Immunol. 1993;5:409–418. doi: 10.1093/intimm/5.4.409. [DOI] [PubMed] [Google Scholar]
- 41.Saltini C, Kirby M, Trapnell B C, Tamura N, Crystal R G. Biased accumulation of T lymphocytes with “memory”-type CD45 leukocyte common antigen gene expression on the epithelial surface of the human lung. J Exp Med. 1990;171:1123–1140. doi: 10.1084/jem.171.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schluger N W, Rom W N. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–691. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 43.Schwander S K, Sada E, Torres M, Escobedo D, Sierra J G, Alt S, Rich E A. T lymphocytic and immature macrophage alveolitis in active pulmonary tuberculosis. J Infect Dis. 1996;173:1267–1272. doi: 10.1093/infdis/173.5.1267. [DOI] [PubMed] [Google Scholar]
- 44.Seymour G J, Taubman M A, Eastcott J W, Gemmell E, Smith D J. CD29 expression on CD4+ gingival lymphocytes supports migration of activated memory T lymphocytes to diseased periodontal tissue. Oral Microbiol Immunol. 1997;12:129–134. doi: 10.1111/j.1399-302x.1997.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 45.Tchou-Wong K M, Tanabe O, Chi C, Yie T A, Rom W N. Activation of NF-kappaB in Mycobacterium tuberculosis-induced interleukin-2 receptor expression in mononuclear phagocytes. Am J Respir Crit Care Med. 1999;159:1323–1329. doi: 10.1164/ajrccm.159.4.9710105. [DOI] [PubMed] [Google Scholar]
- 46.Thiel A, Schmitz J, Miltenyi S, Radbruch A. CD45RA-expressing memory/effector Th cells committed to production of interferon-gamma lack expression of CD31. Immunol Lett. 1997;57:189–192. doi: 10.1016/s0165-2478(97)00056-4. [DOI] [PubMed] [Google Scholar]
- 47.Trowbridge I S, Thomas M L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 48.Urdaneta E, Feo-Figarella E, Montalvo C, Talamo C, Castillo Y, Carrasco D, Rivera H, Blanca I, Machado I, Echeverria de Perez G, De Sanctis J B, Bianco N E. Characterization of local memory cells in stage-classified pulmonary tuberculosis: preliminary observations. Scand J Immunol. 1998;47:496–501. doi: 10.1046/j.1365-3083.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- 49.Yu C T, Wang C H, Huang T J, Lin H C, Kuo H P. Relation of bronchoalveolar lavage T lymphocyte subpopulations to rate of regression of active pulmonary tuberculosis. Thorax. 1995;50:869–874. doi: 10.1136/thx.50.8.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yurovsky V V, Bleecker E R, White B. Restricted T-cell antigen receptor repertoire in bronchoalveolar T cells from normal humans. Hum Immunol. 1996;50:22–37. doi: 10.1016/0198-8859(96)00126-7. [DOI] [PubMed] [Google Scholar]