Key Points
Question
What is the risk of mortality and liver and nonliver complications for patients with chronic hepatitis C (CHC) who are being treated with direct-acting antivirals (DAAs)?
Findings
This cohort study of 245 596 adults with CHC found that DAA treatment (vs no treatment) was independently associated with a lower risk of mortality and liver (ie, hepatocellular carcinoma and decompensation) and nonliver (ie, diabetes, chronic kidney disease, cardiovascular disease, and nonliver cancer) outcomes.
Meaning
These findings support the need for continued efforts to promote hepatitis C screening for diagnosis and treatment of CHC before onset of complications to prevent liver and nonliver complications and to lower all-cause mortality.
Abstract
Importance
Chronic hepatitis C (CHC) and its complications are associated with high rates of morbidity and mortality. However, large-scale data analysis of the long-term liver and nonliver effects of direct-acting antiviral (DAA) treatment has been limited.
Objective
To assess the association of hepatitis C virus elimination through DAA treatment with the risk of liver and nonliver morbidity and mortality during long-term follow-up among a large nationwide cohort of insured patients with CHC in the US.
Design, Setting, and Participants
This was a retrospective cohort study of 245 596 adult patients with CHC using data from the Optum Clinformatics Data Mart database, 2010 to 2021. Of the total cohort, 40 654 patients had received 1 or more prescriptions for DAA medication (without interferon), and 204 942 patients were untreated.
Exposure
Treatment with a DAA.
Main Outcomes and Measures
Incidence of hepatocellular carcinoma (HCC), liver decompensation, relevant nonliver events (nonliver cancer, diabetes, chronic kidney disease, cardiovascular disease), and overall mortality.
Results
The DAA-treated cohort (vs untreated) were older (mean [SD] age, 59.9 [10.8] vs 58.5 [13.0] years; P < .001); more likely to be male (25 060 [62%] vs 119 727 [58%] men; P < .001) and White (23 937 [59%] vs 115 973 [57%]; P < .001) individuals; and more likely to have diabetes (10 680 [26%] vs 52 091 [25%]; P < .001) or cirrhosis (17 971 [44%] vs 60 094 [29%]; P < .001). Comparing DAA-treated with untreated patients, the incidence (per 1000 person-years) of liver outcomes (eg, decompensation, 28.2 [95% CI, 27.0-29.4] vs 40.8 [95% CI, 40.1-41.5]; P < .001, and HCC in compensated cirrhosis, 20.1 [95% CI, 18.4-21.9] vs 41.8 [95% CI, 40.3-43.3]; P < .001) and nonliver outcomes (eg, diabetes, 30.2 [95% CI, 35.4-37.7] vs 37.2 [95% CI, 36.6-37.9]; P < .001; and chronic kidney disease, 31.1 [95% CI, 29.9-32.2] vs 34.1 [95% CI, 33.5-34.7]; P < .001) were significantly lower in treated patients. The all-cause mortality rates per 1000 person-years were also significantly lower in DAA-treated compared with untreated patients (mortality, 36.5 [95% CI, 35.4-37.7] vs 64.7 [95% CI, 63.9-65.4]; P < .001). In multivariable regression analysis, DAA treatment was independently associated with a significant decrease in the risk of liver (adjusted hazard ratio [aHR] for HCC, 0.73; decompensation, 0.36), nonliver (aHR for diabetes, 0.74; chronic kidney disease, 0.81; cardiovascular disease, 0.90; nonliver cancer, 0.89), and mortality outcomes (aHR, 0.43).
Conclusions and Relevance
The findings of this retrospective cohort study indicate that DAA treatment for insured patients with CHC was associated with improved liver- and nonliver outcomes, and ultimately, with long-term overall survival.
This retrospective cohort study of 245 596 adults with CHC examines the association of hepatitis C virus elimination with the risk of liver and nonliver morbidity and overall mortality from 2010 to 2021.
Introduction
Untreated chronic hepatitis C (CHC) commonly becomes progressive liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). In addition to liver-related complications, CHC may cause multiple extrahepatic manifestations that increase morbidity and mortality in addition to the total financial burden. Among patients with CHC who achieve virologic cure, interferon-based treatment has been shown to associate with reduced overall mortality and liver-related complications such as HCC and liver decompensations, as well as nonliver-related complications such as nonliver cancers, diabetes, chronic kidney disease (CKD), and cardiovascular disease (CVD) events. However, interferon-based treatment lacks efficacy and is poorly tolerated, especially for older adult patients and those with comorbidities or advanced fibrosis.
Since 2014, major improvements have been made in therapies for hepatitis C virus (HCV), most notably direct-acting antiviral (DAA) agents. Short-course all-oral DAA treatment can achieve a virologic cure for almost all treated patients, regardless of age, cirrhosis status, prior treatment failure history, and presence of comorbidities such as CKD, making CHC treatment tolerable and applicable to all populations with CHC, including historically difficult-to-treat populations. Some studies have highlighted the association of HCV elimination by DAAs with the risk of HCC and other liver-related complications. In addition, HCV elimination by DAAs has been associated with significant reductions in the risk of certain nonliver diseases such as diabetes. However, the effects of DAAs on most nonliver comorbidities have not been well documented. In addition, data for long-term outcomes after DAA treatment are limited. Large-scale data obtained from clinical practices without the selection bias of tertiary care center data are more generalizable and can better inform future public health and policy planning.
Therefore, using a large nationwide database of insured patients with CHC monitored in routine clinical practice across the US, we investigated and compared the risk of liver-related and nonliver complications as well as overall mortality between DAA-treated and untreated patients with CHC.
Methods
The Institutional Review Board of Stanford University approved this study. Informed consent was waived because the study used only deidentified publicly available data. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Design and Participants
This was a retrospective cohort study of adults (≥18 years) with HCV infection, using data from the Optum Clinformatics Data Mart (CDM) (Optum Inc) database provided by the Center for Population Health Sciences of Stanford University. The CDM database is a large deidentified administrative health claims database derived from a large, adjudicated claims data warehouse of commercial and Medicare Advantage health plans. We performed a search for patients with CHC from January 1, 2010, to March 31, 2021, using codes from the International Classification of Diseases, Ninth Revision and Tenth Revision, Clinical Modification (ICD-9-CM and ICD-10-CM). Eligible patients were confirmed to have had at least 1 inpatient or 2 outpatient ICD-9-CM or ICD-10-CM diagnosis codes for HCV infection. We excluded patients who were younger than 18 years of age, had a history of interferon prescription at any time, and/or had a history of liver transplantation. The included patients were followed up for the duration of the study.
Study Variables and Outcomes
Baseline demographic information, clinical characteristics, and laboratory test results were obtained for the 6 months prior to the index diagnosis of HCV infection. The demographic information collected included age, sex, race and ethnicity, and geographic region. The clinical characteristics included the presence of cirrhosis, liver decompensation, HCC, and comorbidities, such as hypertension, diabetes, and hyperlipidemia, per ICD-9-CM or ICD-10-CM codes. Cirrhosis was determined by the presence of cirrhosis or any complications of cirrhosis, such as portal hypertension, ascites, varices, or encephalopathy. The definitions of liver decompensation were presence or history of (1) ascites and associated complications, (2) variceal bleeding, and/or (3) liver encephalopathy. The definitions of alcohol use included the presence of alcohol use disorder and complications of alcohol disuse, such as alcohol withdrawal, alcohol intoxication, alcohol-associated gastritis, and/or neuropathy. Nonliver cancers referred to non-HCC. Initiation of DAA treatment was defined as having 1 or more prescriptions for a DAA medication, identified by the National Drug Codes for DAAs in the pharmaceutical claims records of the CDM database.
In the subgroup of patients with available laboratory test results data, we examined baseline laboratory parameters: HCV RNA, HCV genotype, alanine aminotransferase, aspartate aminotransferase, international normalized ratio, total bilirubin, albumin, platelet, hematocrit, creatinine, and fibrosis-4 index. An HCV cure or sustained virologic response (SVR) in this subgroup was defined as a negative result to an HCV RNA test at any time during the 12 weeks after the last DAA prescription was refilled.
Primary End Points
We categorized the patients with HCV into DAA-treated and untreated cohorts. We defined the index date or start point for mortality or incidences of liver and nonliver complications of the DAA-treated patients as the first DAA treatment prescription date and that of nontreated patients as the date of the first HCV diagnosis. The primary study end points were (1) the incidence of liver-related outcomes, including HCC and liver decompensation, and (2) mortality. The secondary study end points were (1) the incidence of nonliver-related outcomes, including nonliver cancer, CKD, CVD, diabetes, and (2) DAA treatment as factors associated with liver, nonliver, and mortality outcomes in patients with HCV infection. Incidence analyses of each outcome excluded patients who had the specific outcome at baseline (eFigure 1 in the Supplement). Mortality data per the CDM database were based on external data sources (eg, the Death Master File from the US Social Security Administration and records from the Centers for Medicare & Medicaid Services) as well as from internal sources, such as the claims records in the CDM database indicating that a patient had died. In addition, the CDM database performs internal checks to validate the date of death.
Statistical Analysis
Categorical variables were reported as counts and percentages; continuous variables were reported as either mean (SD) or median (IQR). In the comparison of subgroups, Pearson χ2 test was used for categorical variables and t test of variance or Wilcoxon rank-sum test was used for continuous variables. Cumulative HCC incidence curves and survival curves were plotted using the Kaplan-Meier method. The log-rank test was used to compare the differences between the DAA-treated and untreated groups. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% CIs for the risk of events. Variables included in the multivariable models either had a univariate of P < .10 or had potential association with the outcomes of interest from prior reports. To confirm that the robustness of findings regarding DAA treatment affecting clinic outcomes among the total cohort, we performed a sensitivity analysis of the subgroup of patients with available laboratory test results to compare patients with confirmed SVR vs untreated patients.
Statistical significance was defined as having a 2-tailed P < .05. All statistical analyses were conducted using R, version 3.5.1 (The R Foundation) from January 2021 to September 2022.
Results
Study Patients
A total of 245 596 patients with HCV (mean [SD] age, 58.7 [12.6] years; 100 809 [41.0%] women; 8652 [3.5%] Asian, 42 854 [17.4%] Black, 29 502 [12.0%] Hispanic, and 139 910 [57.0%] White individuals) met the study inclusion criteria and were included in the analyses (eFigure 1 in the Supplement). Of these, 40 654 (16.6%) patients had received at least 1 prescription for DAA medication, and 204 942 (83.4%) patients had not. All patients were analyzed for liver outcomes and overall mortality (eFigure 1A in the Supplement) as well as for nonliver disease outcomes (eFigure 1B in the Supplement).
Patient baseline characteristics are shown in Table 1. The DAA-treated cohort of patients had a mean (SD) age of 59.9 (10.8) years and the untreated, 58.5 (13.0) years (P < .001). Compared with the untreated patients, DAA-treated patients were more likely to be male (61.6% vs 58.4%; P < .001) and to have compensated cirrhosis (44.2% vs 29.3%; P < .001), HCC (3.1% vs 2.4%; P < .001), and/or diabetes (26.3% vs 25.4%; P < .001). The mean Charlson comorbidity index (CCI) of the DAA-treated patients was significantly higher than that of untreated patients (4.0 vs 3.3; P < .001).
Table 1. Baseline Characteristics of DAA-Treated and Untreated Patients With Chronic Hepatitis C.
| Characteristics | Patients, No. (%) | P value | |
|---|---|---|---|
| DAA-treated (n = 40 654) | Untreated (n = 204 942) | ||
| Age, mean (SD), y | 59.9 (10.8) | 58.5 (13.0) | <.001 |
| Female sex | 15 594 (38.4) | 85 215 (41.6) | <.001 |
| Male sex | 25 060 (61.6) | 119 727 (58.4) | |
| Race and ethnicity | |||
| Asian | 988 (2.4) | 7664 (3.7) | <.001 |
| Black | 8070 (19.9) | 34 784 (17.0) | |
| Hispanic | 4839 (11.9) | 24 663 (12.0) | |
| White | 23 937 (58.9) | 115 973 (56.6) | |
| Other/unknowna | 2820 (6.9) | 21 858 (10.7) | |
| Comorbidity status | |||
| CCI, mean (SD) | 4.0 (3.5) | 3.3 (3.5) | <.001 |
| Cirrhosis | 17 971 (44.2) | 60 094 (29.3) | <.001 |
| Compensated cirrhosis | 10 208 (25.1) | 35 813 (17.5) | <.001 |
| Hepatocellular carcinoma | 1249 (3.1) | 4968 (2.4) | <.001 |
| Diabetes | 10 680 (26.3) | 52 091 (25.4) | <.001 |
| Chronic kidney disease | 4708 (11.6) | 26 884 (13.1) | <.001 |
| Cardiovascular disease | 28 385 (69.8) | 136 399 (66.6) | <.001 |
| Nonliver cancer | 4335 (10.7) | 21 032 (10.3) | .02 |
Abbreviations: CCI, Charlson comorbidity index; DAA, direct-acting antiviral.
If response was “unknown” or blank.
Liver Outcomes
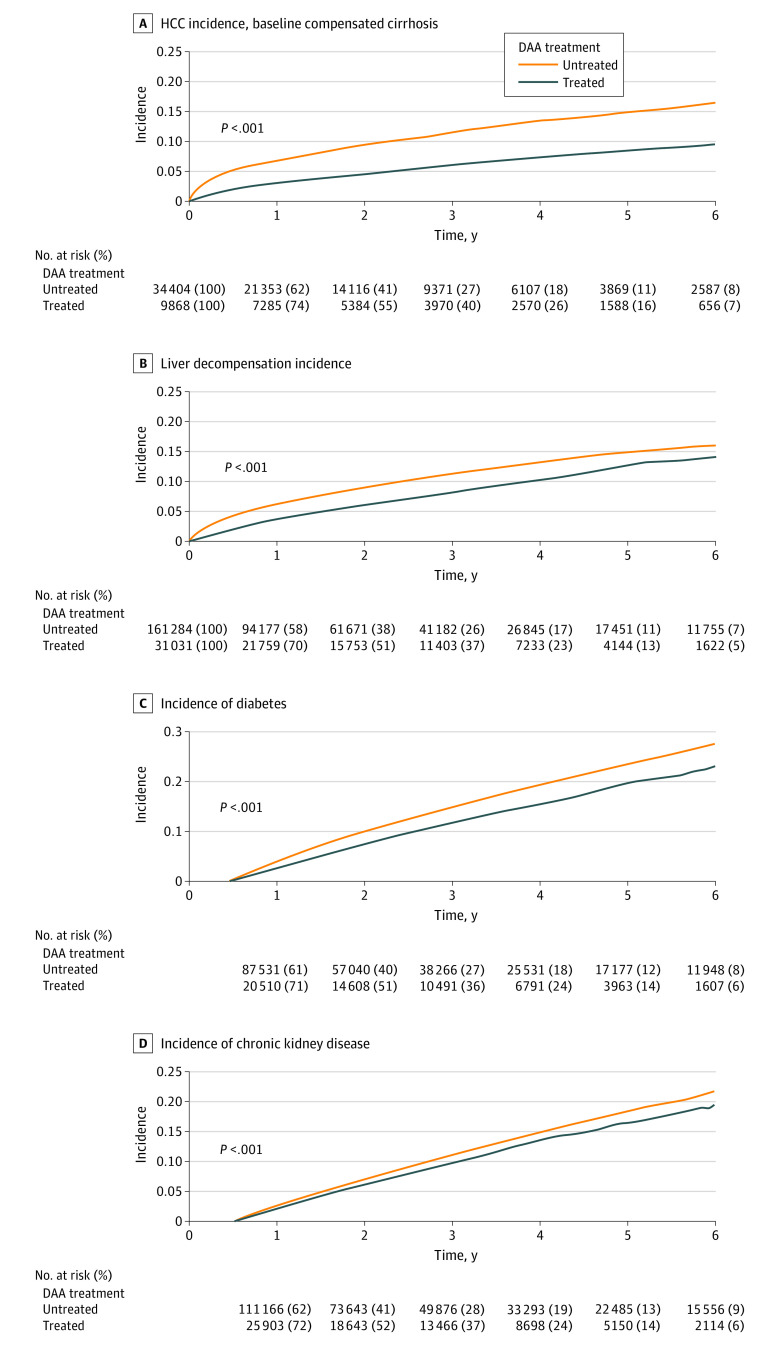
Although the HCC incidence rates (per 1000 patient-years) for patients without cirrhosis at baseline were low and not significantly different in DAA-treated compared with untreated patients (3.8 [95% CI, 3.3-4.3] vs 4.0 [95% CI, 3.8-4.2]; P = .42; Table 2), HCC incidence for patients with cirrhosis at baseline was significantly lower (20.1 [95% CI, 18.4-21.9] vs 41.8 [95% CI, 40.3-43.3]; P < .001; Figure 1A and Table 2). Similarly, the incidence of liver decompensation was significantly lower among DAA-treated compared with untreated patients (28.2 [95% CI, 27.0-29.4] vs 40.8 [95% CI, 40.1-41.5]; P < .001).
Table 2. Incidence Rates for Liver, Nonliver, and Overall Mortality Outcomes in DAA-Treated and Untreated Patients With Chronic Hepatitis C.
| Outcome | Patient, No. | Event | Incidence rate per 1000 patient-years (95% CI) | P value |
|---|---|---|---|---|
| Hepatocellular carcinoma | ||||
| No baseline cirrhosis | .42 | |||
| DAA-treated | 22 954 | 218 | 3.8 (3.3-4.3) | |
| Untreated | 144 425 | 1266 | 4.0 (3.8-4.2) | |
| Baseline compensated cirrhosis | <.001 | |||
| DAA-treated | 9868 | 523 | 20.1 (18.4-21.9) | |
| Untreated | 34 404 | 3020 | 41.8 (40.3-43.3) | |
| Liver decompensation | ||||
| DAA-treated | 31 031 | 2146 | 28.2 (27.0-29.4) | <.001 |
| Untreated | 161 284 | 13 078 | 40.8 (40.1-41.5) | |
| Diabetes | ||||
| DAA-treated | 28 775 | 2151 | 30.2 (28.9-31.5) | <.001 |
| Untreated | 143 149 | 11 114 | 37.2 (36.6-37.9) | |
| Chronic kidney disease | ||||
| DAA-treated | 35 946 | 2812 | 31.1 (29.9-32.2) | <.001 |
| Untreated | 178 058 | 13 007 | 34.1 (33.5-34.7) | |
| Cardiovascular disease | ||||
| DAA-treated | 12 269 | 3075 | 128.0 (123.5-132.6) | .007 |
| Untreated | 68 543 | 14 327 | 118.7 (116.8-120.7) | |
| Nonliver cancer | ||||
| DAA-treated | 36 319 | 2103 | 22.5 (21.6-23.5) | .07 |
| Untreated | 183 910 | 9311 | 23.0 (22.6-23.5) | |
| Mortality | ||||
| Total | ||||
| DAA-treated | 40 654 | 4002 | 36.5 (35.4-37.7) | <.001 |
| Untreated | 204 942 | 30 887 | 64.7 (63.9-65.4) | |
| No baseline cirrhosis | ||||
| DAA-treated | 22 683 | 1164 | 19.8 (18.6-20.9) | <.001 |
| Untreated | 144 848 | 11 933 | 36.1 (35.5-36.8) | |
| Baseline compensated cirrhosis | ||||
| DAA-treated | 10 208 | 1196 | 42.7 (40.4-45.2) | <.001 |
| Untreated | 35 813 | 9891 | 121.2 (118.8-123.6) | |
| Baseline decompensated cirrhosis | ||||
| DAA-treated | 7763 | 1642 | 72.7 (69.2-76.3) | <.001 |
| Untreated | 24 281 | 9063 | 137.8 (135.0-140.7) | |
Abbreviation: DAA, direct-acting antiviral.
Figure 1. Liver and Nonliver Outcomes in DAA-Treated and Untreated Patients With Chronic Hepatitis C.
DAA indicates direct-acting antiviral medication, and HCC, hepatocellular carcinoma.
Nonliver Outcomes
We evaluated 4 major nonliver outcomes: diabetes, CKD, CVD, and nonliver cancers. Compared with untreated patients, DAA-treated patients had significantly lower incidence rates for diabetes and CKD (Figures 1C and D and Table 2). The crude incidence of nonliver cancers in the DAA-treated and untreated patients appeared to be similar (22.5 [95% CI, 21.6-23.5] vs 23.0 [95% CI, 22.6-23.5]; P = .07; eFigure 2B in the Supplement; Table 2). In contrast, the crude incidence of CVD was significantly higher in DAA-treated than the untreated patients (128.0 [95% CI, 123.5-132.6] vs 118.7 [95% CI, 116.8-120.7]; P = .007; eFigure 2A in the Supplement; Table 2).
Mortality
For the total study cohort, the mortality rate per 1000 person-years was twice higher among untreated compared with the DAA-treated patients (64.7 vs 36.5; P < .001; Figure 2A and Table 2). Similar findings were observed in the subgroup of patients without cirrhosis at baseline and the subgroup of patients with compensated cirrhosis as well as those with decompensated cirrhosis (Figures 2B-D and Table 2). Notably, although the mortality rates were approximately twice higher among the untreated compared with the DAA-treated patients in the cohort without baseline cirrhosis (36.1 vs 19.8; P < .001) and the cohort with baseline liver decompensation (137.8 vs 72.7; P < .001), the mortality rate was 3 times lower in the DAA-treated compared with the untreated patients (42.7 vs 121.2; P < .001) in the cohort of patients with baseline compensated cirrhosis (Table 2).
Figure 2. Mortality in DAA-Treated and Untreated Patients With Chronic Hepatitis C.
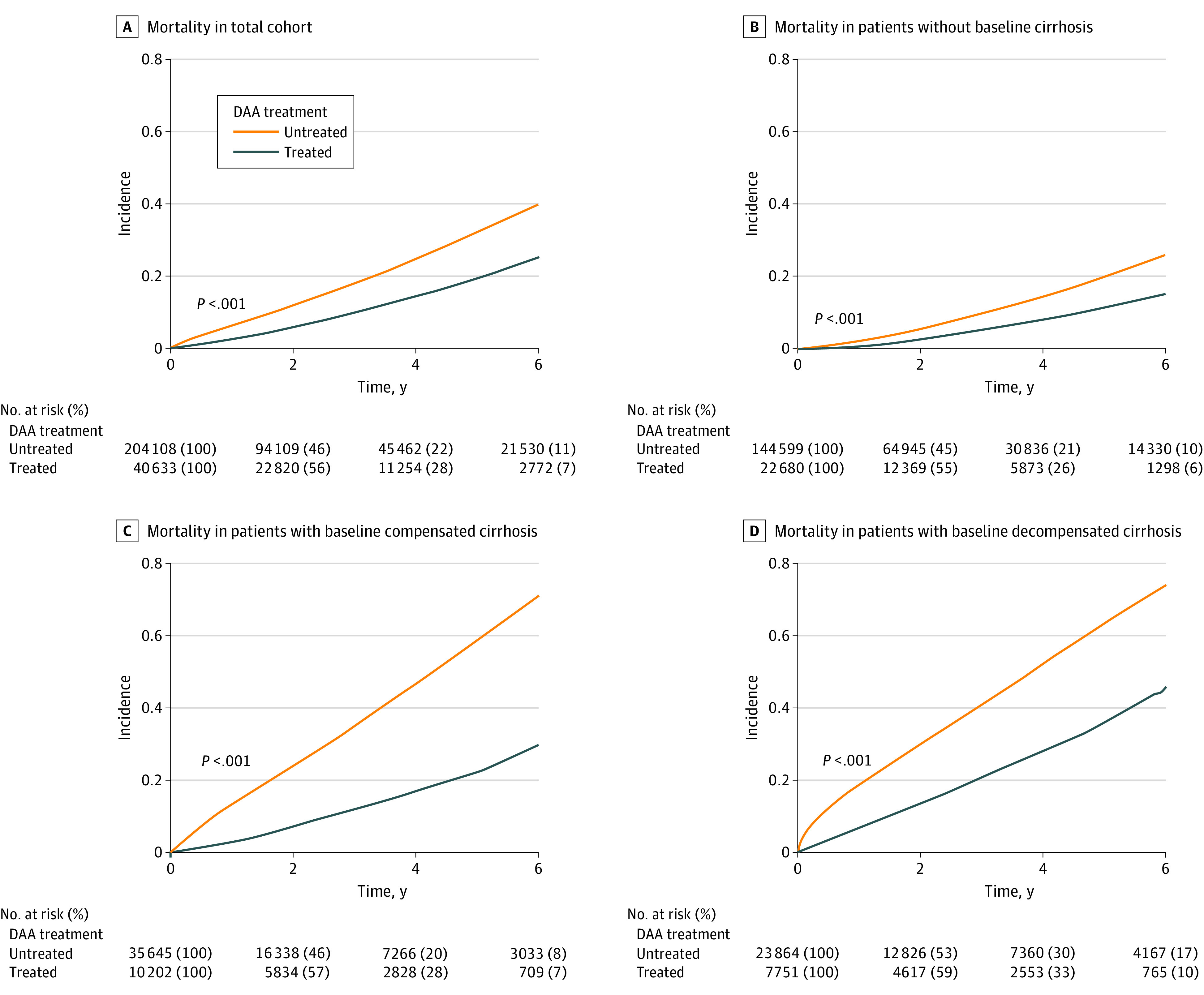
DAA indicates direct-acting antiviral medication.
Association of DAA Treatment With Study Outcomes
In multivariable Cox proportional hazards analysis, DAA treatment was independently associated with decreased risk of liver, nonliver, and mortality outcomes (Table 3). After adjusting for age, sex, race and ethnicity, alcohol use, and cirrhosis, DAA treatment was independently associated with a 27% lower risk of developing HCC (adjusted HRs [aHRs], 0.73; 95% CI, 0.68-0.77; P < .001). After adjusting for age, sex, race and ethnicity, alcohol use, cirrhosis, and HCC, DAA treatment was independently associated with a 64% reduction in the risk of developing liver decompensation (aHR, 0.36; 95% CI, 0.35-0.38; P < .001).
Table 3. DAA Treatment as Factors Associated With Liver, Nonliver, and Mortality Outcomes in Patients With Chronic Hepatitis C.
| Outcome | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Hepatocellular carcinomaa | ||||
| DAA treated | 0.96 (0.90-1.02) | .22 | 0.73 (0.68-0.77) | <.001 |
| Untreated | 1 [Reference] | NA | 1 [Reference] | NA |
| Liver decompensationb | ||||
| DAA treated | 0.73 (0.70-0.77) | <.001 | 0.36 (0.35-0.38) | <.001 |
| Untreated | 1 [Reference] | NA | 1 [Reference] | NA |
| Diabetesc | ||||
| DAA treated | 0.79 (0.76-0.83) | <.001 | 0.74 (0.70-0.77) | <.001 |
| Untreated | 1 [Reference] | NA | 1 [Reference] | NA |
| Chronic kidney diseasec | ||||
| DAA treated | 0.89 (0.86-0.93) | <.001 | 0.81 (0.78-0.85) | <.001 |
| Untreated | 1 [Reference] | NA | 1 [Reference] | NA |
| Cardiovascular diseasec | ||||
| DAA treated | 1.06 (1.02-1.10) | .007 | 0.90 (0.86-0.94) | <.001 |
| Untreated | 1 [Reference] | NA | 1 [Reference] | NA |
| Nonliver cancerc | ||||
| DAA treated | 0.96 (0.91-1.00) | .07 | 0.89 (0.85-0.94) | <.001 |
| Untreated | 1 [Reference] | NA | 1 [Reference] | NA |
| Mortalityd | ||||
| DAA treated | 0.57 (0.55-0.59) | <.001 | 0.43 (0.42-0.45) | <.001 |
| Untreated | 1 [Reference] | NA | 1 [Reference] | NA |
Abbreviations: DAA, direct-acting antiviral; HR, hazard ratio; NA, not applicable.
Adjusted for age, sex, race and ethnicity, alcohol use, cirrhosis, and DAA treatment.
Adjusted for age, sex, race and ethnicity, alcohol use, cirrhosis, hepatocellular carcinoma, and DAA treatment.
Adjusted for age, sex, race and ethnicity, and DAA treatment.
Adjusted for age, sex, race and ethnicity, Charlson comorbidity index, alcohol use, cirrhosis, hepatocellular carcinoma, and DAA treatment.
For nonliver outcomes, after adjusting for age, sex, race and ethnicity, DAA treatment was associated with a 26% lower risk of developing diabetes (aHR, 0.74; 95% CI, 0.70-0.77; P < .001), a 19% lower risk of CKD (aHR, 0.81; 95% CI, 0.78-0.85; P < .001), a 10% lower risk of CVD (aHR, 0.90; 95% CI, 0.86-0.94; P < .001). There was also an 11% lower risk of nonliver cancers (aHR, 0.89; 95% CI, 0.85-0.94; P < .001) after adjusting for potential confounders despite the lack of a significant difference in the crude incidence of nonliver cancers between treated and untreated patients. Treatment with a DAA was independently associated with a 57% lower risk of mortality among treated patients compared with the untreated cohort (aHR, 0.43; 95% CI, 0.42-0.45; P < .001) after adjusting for age, sex, race and ethnicity, CCI, alcohol use, cirrhosis, and HCC.
Sensitivity Analysis of Cohort With Laboratory Data
We performed sensitivity analysis to confirm how DAA medication use affected the subgroup of 114 819 patients with HCV whose records had laboratory testing results data—to compare patients with confirmed SVR (n = 12 305; 10.7%) with untreated patients (n = 102 514; 89.3%) (eFigure 3 in the Supplement). The mean age of this cohort of patients with DAA-SVR was 61.2 years vs 59.0 years for the untreated patients (P < .001; eTable 1 in the Supplement); the distribution of other characteristics between the 2 groups was similar to those of the main cohort.
We found similar differences and findings in the incidence rates of liver complications (eFigures 4A and B in the Supplement), nonliver outcomes (eFigures 4C-F in the Supplement), and overall mortality (eFigures 5A-D in the Supplement) between the DAA-treated with SVR and untreated patients for this cohort as we did with the main cohort (eTable 2 in the Supplement).
Consistent with findings in the analysis of the main cohort, after adjusting for the same potential confounders as we did for the total cohort, we also found SVR with DAA treatment to be associated with an approximately 30% lower risk of HCC, 65% lower risk of liver decompensation, 25% lower risk of diabetes, and a 10% to 20% lower risk of CKD, CVD, and nonliver cancers (aHRs, 0.68, 0.35, 0.73, 0.77, 0.85, and 0.87, respectively; P < .001; eTable 3 in the Supplement). The risk of overall mortality was also significantly lower for the treated patients with SVR as those in the total study cohort.
Discussion
To our knowledge, this is the largest clinical study to date that comprehensively evaluates overall mortality and liver as well as nonliver outcomes associated with DAA treatment for patients with CHC. Of note, a significant reduction in mortality was found, even for patients without cirrhosis, a population previously considered to receive less benefit from an HCV cure than patients with cirrhosis. In these study findings, DAA treatment was associated with a reduced risk of both liver and extrahepatic outcomes, ie, HCC, liver decompensation, diabetes, CKD, CVD, nonliver cancer, and ultimately, overall survival. Because HCV treatment with a DAA regimen is well tolerated for nearly all patients, we believe these findings provide further support for universal HCV treatment coverage for all patients affected by HCV.
The ultimate goal of most medical treatments, including HCV treatment, is to reduce mortality. Long-term follow-up studies of patients who received interferon-based regimens have shown lower liver-related and all-cause mortality for patients who achieved HCV cure compared with those who were not treated. Those studies also found a reduction in HCC risk but not for liver decompensation. In the present study, DAA treatment (vs no treatment) was associated with a large and significant reduction (57%) in all-cause mortality, regardless of age, sex, race and ethnicity, comorbidity, and the presence of alcohol use, cirrhosis, or HCC. However, there can be a selection bias for patients who are less sick, and thus a larger mortality difference between DAA-treated and untreated patients. Indeed, the mean CCI of the DAA-treated patients in our study was significantly higher than that of untreated patients. As a result, we attempted to account for this by adjusting for differences in comorbidities between the 2 study groups using the CCI in our multivariable regression analysis. After adjusting for potential confounders including CCI, we still found that DAA therapy was an independent factor associated with improved survival. Additionally, the 57% lower mortality rate we observed among treated vs untreated patients was aligned with results reported by a prospective study of approximately 10 000 adult patients with chronic HCV infection in 32 expert hepatologic centers in France. In that study, a significant decrease of 52% (aHR, 0.48; 95% CI, 0.33-0.70; P < .001) in all-cause mortality was noted in patients receiving DAAs compared with untreated patients. The subgroup analyses of patients with less vs more advanced disease showed treated patients having had only half the mortality rates compared with untreated patients. However, treated patients with compensated cirrhosis at baseline had even more mortality decline, 3 times lower than the rate of untreated patients. Together with large-scale data inclusive of community insured patients, these findings suggest that it is “never too early” to treat patients without cirrhosis nor too late to treat patients with liver decompensation; however, those with early cirrhosis may benefit the most from treatment. These study results are aligned with results from a recent study using Medicare claims data, in which DAA treatment was associated with a decrease in mortality among Medicare beneficiaries with or without cirrhosis. However, our study expanded on this prior study by providing comparative data for DAA-treated and untreated patients younger than 65 years and for liver and nonliver outcomes as well as mortality outcome data.
Because all-oral DAA treatment became widely available in 2014, studies based on large data sets have included mostly male patients of a single race, as well as data from several clinical registries mostly derived from specialized centers; these studies have consistently shown lower HCC risk after SVR. This study adds to the current knowledge by providing further data to support the evidence of lower risk of HCC in DAA-treated patients of diverse genders, races, and ethnicities from all regions of the US, including community patients. In addition, this study provides data for lower risk of liver decompensation among DAA-treated patients, which is different from results of a study of patients from specialized liver centers; this may be related to selection bias inherent in studies from tertiary care centers and those using smaller patient sample size. Given that our study cohort of 245 596 insured patients with HCV infection was drawn from the CDM database, which includes the 61 million insured individuals from across all regions of the US, the findings may be more generalizable to the overall population with private health insurance.
Data on the association of DAA treatment with extrahepatic diseases are more limited. Prior studies of diabetes, CVD, and other nonliver event risks in DAA-treated compared with untreated patients have been limited by single-center design, small sample size, and/or study sample inclusive mainly of men or White patients. Our analyses elucidated the subsequent risk of a wide range of nonliver diseases (ie, diabetes, CKD, CVD, and nonliver cancer), which were all consistently lower for DAA-treated patients. Although the crude incidence of nonliver cancer was similar between the DAA-treatment and untreated patients and the crude incidence of CVD was higher among DAA-treated compared with untreated patients, the DAA-treated patients were significantly older and more likely to have diabetes than their untreated counterparts, factors that can affect risk of cancer and CVD. Therefore, after adjustment for background risk, including age, DAA treatment was significantly associated with lower risk of nonliver and CVD, findings that were consistent in both the main study cohort analyses as well as in the sensitivity analysis that included only patients with available laboratory test results to confirm SVR among the treated group.
Strengths and Limitations
The strengths of this study are its large study sample of DAA-treated and untreated patients from diverse racial and ethnic groups and from across US regions. The CDM database included more than 360 000 individuals with CHC, including more than 40 000 who were treated with DAA medication; to our knowledge, this is among the largest samples analyzed to date. Also, being an insurance claim database, the CDM includes patients from diverse medical practice settings, not just tertiary centers. Moreover, the proportion of cirrhosis in the CDM database was relatively high, among 44% of the DAA-treated and 29% of the untreated patients. One of the study objectives was to evaluate the long-term outcomes of DAA treatment among patients with CHC in a clinical setting. Also, the duration of follow-up was adequate for making meaningful conclusions regarding long-term outcomes.
The study also had limitations. First, the total study cohort included only patients diagnosed with HCV and covered by private insurance; therefore, the findings may not be generalizable to individuals who are underinsured or not insured, many of whom have fewer resources and/or may be incarcerated or active users of alcohol or illicit drugs. Also, because only 2.4% of the study patients were Asian, the results may not generalize to this racial group.
Second, the study period (January 1, 2014-March 31, 2021) was delineated to ensure the compatibility of both comparators and to minimize the immortal time bias; however, residual bias may remain. Third, large claim databases can include miscoding and misclassification. Although we used ICD-9-CM and ICD-10-CM codes, they may not have exactly matched the actual diagnosis. Fourth, not all patients with a DAA prescription adhered to or completed the course nor attained SVR. Additionally, although DAA treatment was associated with lower nonliver cancer risk, not all nonliver cancers were associated with HCV, and further studies are needed to clarify the association between DAA treatment and specific nonliver cancers. Lastly, we excluded patients with a history of interferon use to avoid confounding data with patients treated with interferon-DAA combination to avoid bias toward a higher SVR because it is generally lower in patients with prior interferon failure. However, SVR was not a primary outcome of this study. More importantly, patients with interferon failure would have comprised less than 5% given that the pre-DAA treatment rate for CHC with interferon was only 10% among insured US patients.
Conclusions
This retrospective cohort study provides nationwide, large-scale, clinical data results consistent with the improved outcomes of DAA therapy for insured patients with CHC—not just liver-related complications of HCC and liver decompensation but also for nonliver diseases, ie, diabetes, CKD, CVD, and nonliver cancers, and ultimately, for long-term overall survival. These results were found in patients without cirrhosis, with compensated cirrhosis, and with existing liver decompensation.
The study findings also highlight a substantial need to provide DAA therapy to all patients with HCV, regardless of disease stage or financial status. Additional national efforts are needed to reach and treat US population groups that are underinsured or not insured, incarcerated, and otherwise marginalized, such as users of illicit drugs, who are also at higher risk of disease complication and reinfection. Our findings advocate for continued efforts to promote hepatitis C screening and early diagnosis and treatment—prior to the onset of CHC complications—to prevent liver and nonliver morbidity and mortality.
eTable 1. Baseline characteristics of DAA-SVR and untreated patients with chronic hepatitis C.
eTable 2. Incidence rates for liver, non-liver, and overall mortality outcomes in DAA-SVR and untreated patients with chronic hepatitis C.
eTable 3. DAA-SVR as factors associated with liver, non-liver, and mortality outcomes in patients with chronic hepatitis C.
eFigure 1. Patient flow chart for liver, non-liver, and mortality outcome analyses in DAA-treated and untreated patients.
eFigure 2. Liver and non-liver outcomes in DAA-treated and untreated patients with chronic hepatitis C. (A) Cardiovascular disease and (B) Non-liver cancer.
eFigure 3. Patient flow chart for liver, non-liver, and mortality outcome analyses in DAA-SVR and untreated patients.
eFigure 4. Liver and non-liver outcomes in DAA-SVR and untreated patients with chronic hepatitis C. (A) HCC incidence in baseline compensated cirrhosis, (B) Hepatic decompensation incidence, (C) Diabetes mellitus, (D) Chronic kidney disease, (E) Cardiovascular disease, and (F) Non-liver cancer.
eFigure 5. Mortality in DAA-SVR and untreated patients with chronic hepatitis C.
References
- 1.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1)(suppl):S58-S68. doi: 10.1016/j.jhep.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599-1608. doi: 10.1053/j.gastro.2016.02.039 [DOI] [PubMed] [Google Scholar]
- 3.Morgan TR, Ghany MG, Kim HY, et al. ; HALT-C Trial Group . Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52(3):833-844. doi: 10.1002/hep.23744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahale P, Engels EA, Li R, et al. The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection. Gut. 2018;67(3):553-561. doi: 10.1136/gutjnl-2017-313983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa E, Furusyo N, Kajiwara E, et al. ; Kyushu University Liver Disease Study (KULDS) Group . Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: a prospective, multicenter study. J Hepatol. 2013;58(3):495-501. doi: 10.1016/j.jhep.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 6.Cacoub P, Desbois AC, Comarmond C, Saadoun D. Impact of sustained virological response on the extrahepatic manifestations of chronic hepatitis C: a meta-analysis. Gut. 2018;67(11):2025-2034. doi: 10.1136/gutjnl-2018-316234 [DOI] [PubMed] [Google Scholar]
- 7.Ogawa E, Furusyo N, Kajiwara E, et al. ; Kyushu University Liver Disease Study [KULDS] Group . Evaluation of the adverse effect of premature discontinuation of pegylated interferon α-2b and ribavirin treatment for chronic hepatitis C virus infection: results from Kyushu University Liver Disease Study. J Gastroenterol Hepatol. 2012;27(7):1233-1240. doi: 10.1111/j.1440-1746.2011.06965.x [DOI] [PubMed] [Google Scholar]
- 8.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi: 10.7326/M16-2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrat F, Fontaine H, Dorival C, et al. ; French ANRS CO22 Hepather cohort . Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393(10179):1453-1464. doi: 10.1016/S0140-6736(18)32111-1 [DOI] [PubMed] [Google Scholar]
- 10.Dang H, Yeo YH, Yasuda S, et al. Cure with interferon-free direct-acting antiviral is associated with increased survival in patients with hepatitis C virus-related hepatocellular carcinoma from both east and west. Hepatology. 2020;71(6):1910-1922. doi: 10.1002/hep.30988 [DOI] [PubMed] [Google Scholar]
- 11.Emery JS, Kuczynski M, La D, et al. Efficacy and safety of direct acting antivirals for the treatment of mixed cryoglobulinemia. Am J Gastroenterol. 2017;112(8):1298-1308. doi: 10.1038/ajg.2017.49 [DOI] [PubMed] [Google Scholar]
- 12.Saadoun D, Pol S, Ferfar Y, et al. Efficacy and safety of sofosbuvir plus daclatasvir for treatment of HCV-associated cryoglobulinemia vasculitis. Gastroenterology. 2017;153(1):49-52.e5. doi: 10.1053/j.gastro.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 13.Hum J, Jou JH, Green PK, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care. 2017;40(9):1173-1180. doi: 10.2337/dc17-0485 [DOI] [PubMed] [Google Scholar]
- 14.Stanford Center for Population Health Sciences . Optum DOD (v 5.0). Redivis Dataset; 2021. Accessed November 7, 2022. https://redivis.com/StanfordPHS
- 15.D’Amico G, Bernardi M, Angeli P. Towards a new definition of decompensated cirrhosis. J Hepatol. 2022;76(1):202-207. doi: 10.1016/j.jhep.2021.06.018 [DOI] [PubMed] [Google Scholar]
- 16.Kalidindi Y, Jung J, Feldman R, Riley T III. Association of direct-acting antiviral treatment with mortality among Medicare beneficiaries with hepatitis C. JAMA Netw Open. 2020;3(7):e2011055. doi: 10.1001/jamanetworkopen.2020.11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi: 10.1053/j.gastro.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 18.Calvaruso V, Cabibbo G, Cacciola I, et al. ; Rete Sicilia Selezione Terapia–HCV (RESIST-HCV) . Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018;155(2):411-421.e4. doi: 10.1053/j.gastro.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 19.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017;68:25-32. doi: 10.1016/j.jhep.2017.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka Y, Ogawa E, Huang CF, et al. ; REAL-C Investigators . HCC risk post-SVR with DAAs in East Asians: findings from the REAL-C cohort. Hepatol Int. 2020;14(6):1023-1033. doi: 10.1007/s12072-020-10105-2 [DOI] [PubMed] [Google Scholar]
- 21.Butt AA, Yan P, Aslam S, Shaikh OS, Abou-Samra AB. Hepatitis C virus (HCV) Treatment with directly acting agents reduces the risk of incident diabetes: results from electronically retrieved cohort of HCV infected veterans (ERCHIVES). Clin Infect Dis. 2020;70(6):1153-1160. doi: 10.1093/cid/ciz304 [DOI] [PubMed] [Google Scholar]
- 22.Butt AA, Yan P, Shuaib A, Abou-Samra AB, Shaikh OS, Freiberg MS. Direct-acting antiviral therapy for HCV infection is associated with a reduced risk of cardiovascular disease events. Gastroenterology. 2019;156(4):987-996.e8. doi: 10.1053/j.gastro.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 23.D’Ambrosio R, Degasperi E, Anolli MP, et al. Incidence of liver- and non-liver-related outcomes in patients with HCV-cirrhosis after SVR. J Hepatol. 2022;76(2):302-310. doi: 10.1016/j.jhep.2021.09.013 [DOI] [PubMed] [Google Scholar]
- 24.Vutien P, Hoang J, Brooks L Jr, Nguyen NH, Nguyen MH. Racial disparities in treatment rates for chronic hepatitis C: analysis of a population-based cohort of 73,665 patients in the United States. Medicine (Baltimore). 2016;95(22):e3719. doi: 10.1097/MD.0000000000003719 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline characteristics of DAA-SVR and untreated patients with chronic hepatitis C.
eTable 2. Incidence rates for liver, non-liver, and overall mortality outcomes in DAA-SVR and untreated patients with chronic hepatitis C.
eTable 3. DAA-SVR as factors associated with liver, non-liver, and mortality outcomes in patients with chronic hepatitis C.
eFigure 1. Patient flow chart for liver, non-liver, and mortality outcome analyses in DAA-treated and untreated patients.
eFigure 2. Liver and non-liver outcomes in DAA-treated and untreated patients with chronic hepatitis C. (A) Cardiovascular disease and (B) Non-liver cancer.
eFigure 3. Patient flow chart for liver, non-liver, and mortality outcome analyses in DAA-SVR and untreated patients.
eFigure 4. Liver and non-liver outcomes in DAA-SVR and untreated patients with chronic hepatitis C. (A) HCC incidence in baseline compensated cirrhosis, (B) Hepatic decompensation incidence, (C) Diabetes mellitus, (D) Chronic kidney disease, (E) Cardiovascular disease, and (F) Non-liver cancer.
eFigure 5. Mortality in DAA-SVR and untreated patients with chronic hepatitis C.