Abstract
The hepatocyte nuclear factor 1β (HNF1B) gene is involved in the development of specialized epithelia of several organs during the early and late phases of embryogenesis, performing its function mainly by regulating the cell cycle and apoptosis pathways. The first pathogenic variant of HNF1B (namely, R177X) was reported in 1997 and is associated with the maturity-onset diabetes of the young. Since then, more than 230 different HNF1B variants have been reported, revealing a multifaceted syndrome with complex and heterogenous genetic, pathologic, and clinical profiles, mainly affecting the pediatric population. The pancreas and kidneys are the most frequently affected organs, resulting in diabetes, renal cysts, and a decrease in renal function, leading, in 2001, to the definition of HNF1B deficiency syndrome, including renal cysts and diabetes. However, several other organs and systems have since emerged as being affected by HNF1B defect, while diabetes and renal cysts are not always present. Especially, liver involvement has generally been overlooked but recently emerged as particularly relevant (mostly showing chronically elevated liver enzymes) and with a putative relation with tumor development, thus requiring a more granular analysis. Nowadays, HNF1B-associated disease has been recognized as a clinical entity with a broader and more variable multisystem phenotype, but the reasons for the phenotypic heterogeneity are still poorly understood. In this review, we aimed to describe the multifaceted nature of HNF1B deficiency in the pediatric and adult populations: we analyzed the genetic, phenotypic, and clinical features of this complex and misdiagnosed syndrome, covering the most frequent, unusual, and recently identified traits.
Keywords: HNF1B deficiency, non-neoplastic condition, tumor, MODY, kidney cyst, hepatopathy, cholestasis, cognitive impairment
1. HNF1B Deficiency: Genetics and Historical Background
Hepatocyte nuclear factors (HNFs) are transcriptional factors regulating tissue development and function of several organs. Overall, HNFs are classified into four groups based on their functional domains, namely HNF1, HNF3 (or FoxA), HNF4, and HNF6 [(or Onecut (OC)]. Notably, the HNF1 group, that includes Hepatocyte nuclear factor 1α (HNF1A) and Hepatocyte nuclear factor 1β [HNF1B, also known as Transcription Factor 2 (TCF2)], has been increasingly studied due to its association with disease [1]. In particular, heterozygous pathogenic variants in the HNF1B gene are the most commonly identified genetic cause of organ malformations in the pediatric populations, especially affecting kidney development. The gene is located on chromosome 17q12 and encodes hepatocyte nuclear factor 1β, a member of the homeodomain-containing family of transcription factors, which plays a key role in the morphogenesis of several organs, especially the kidney, pancreas, and liver [2].
The first HNF1B pathogenic variant (R177X) was described in a Japanese family with maturity-onset diabetes of the young (MODY), in 1997 [3]. MODY is a monogenic and autosomal dominant form of diabetes mellitus whose onset usually occurs before 25 years of age [4]. This disease is due to a dysfunction of pancreatic β cells characterized by non-ketotic diabetes and absence of pancreatic autoantibodies. Extrapancreatic manifestations are rarely found in the different subtypes of MODY and HNF1B-related disease is an exception in this regard [4]. The involvement of HNF1B pathogenic gene variants in renal disease was observed in pedigrees affected by MODY5: these patients often display renal cysts and renal function decline that preceded pancreatic dysfunction. The disease was then renamed renal cysts and diabetes syndrome in 2001 (RCAD, #137920) [5].
Nowadays, it is known that several other organs and systems can be affected by the perturbation of the HNF1B signaling, and that diabetes and renal cysts are not always present. Therefore, HNF1B-associated disease has been recognized as a clinical entity with a broader and more variable multi-system phenotype, which may be due to the functional promiscuity of the HNF1B transcription factor, as it regulates the development of the urogenital tract, brain, and parathyroid gland, in addition to the kidney, liver, and pancreas, by acting in concert with several other developmental genes [5].
HNF1B-associated disease expresses an autosomal dominant inheritance pattern; however, de novo pathogenic variants account for up to 50% of cases, and, therefore, a family history of the disease may be absent [6]. More than 230 different HNF1B allelic variants have been reported in association with disease, according to the Human Gene Mutation Database, including missense, nonsense, frameshift, and splicing mutations [7]. The most frequently identified variants are clustered in the first 4 exons of the gene, with exons 2 and 4, and the intron 2 splice site being hotspots [6]. There is no available evidence to suggest that patients with whole-gene deletions exhibit a different phenotype from those with pathogenic variants at the coding or splice site, suggesting that the dysfunctions are due to a gene dosage defect (i.e., haploinsufficiency) [8]. The most commonly identified genetic alteration (in approximately 50% of patients) is a complete gene deletion, in the context of 17q12 chromosomal microdeletion, which also includes 14 other genes [2]. Some disease-associated phenotypes, in particular the neurological ones, appear to occur only in the context of this microdeletion and, consequently, might not be directly linked to HNF1B itself [2].
To date, no clear correlation between the type or position of a pathogenic variant within HNF1B and the occurrence of particular clinical features has been clearly demonstrated [9]. The reasons for the phenotypic variation remain poorly understood and may reflect the functional effects of different gene anomalies, stochastic variation in temporal HNF1B gene expression during the earliest developmental stages, or additional genetic and/or environmental modifiers [3].
In particular, the extreme variability of HNF1B deficiency could be due to the deregulation or malfunction of proteins belonging to the signaling cascade of HNF1B. However, although several genes regulated by HFN1B have been identified using candidate gene approaches, e.g., cystic disease and cilia-related proteins, the full set of downstream genes that are responsible for the physiological and pathological functions of HFN1B remains elusive. In particular, the signaling cascade involved in the development of the liver phenotype is completely missing.
Additionally, pediatric and adult populations seem to be differently affected. Although the pediatric population has been more thoroughly studied, adult manifestations of HNF1B-deficiency start being more frequently observed, and kindle the need of a more granular analysis (Table 1).
Table 1.
List of the main studies evaluating the clinical manifestations of HNF1B mutations in the adult population.
| Patients with HNF1B Pathogenic Variants | Mean Age | Main Findings | Reference |
|---|---|---|---|
| 27 | 35 |
|
[10] |
| 28 | 24 |
|
[11] |
| 201 | >18 |
|
[12] |
| 8 | 34.8 |
|
[13] |
| 6 | 23 |
|
[14] |
| 3 | 38.7 |
|
[15] |
| 4 | 43.7 |
|
[16] |
In this Review, we performed an extensive analysis of the published available literature from 1997 (year of publication of the first published pathogenic variant of HNF1B) [3] to date (2022). We queried PubMed, Scopus, Embase, and Web of Science databases using the following keywords and MeSH (Medical Subject Headings) words: (“HNF1B” OR “HNF1β”) AND (“Hepatocyte nuclear factor”) AND (“HNF”) AND (“TCF2” OR “TCF-2”) AND (“Transcription Factor 2”). Pre-clinical (in vitro and in vivo animal models) and clinical studies were both considered, but non-English written papers were excluded. The title and abstract of the studies identified were then evaluated for appropriateness, and corresponding references were revised to grant literature research adequacy. From each study, details about study design and HNF1B-related data (with a particular focus on genetic, histopathologic, and clinical features) were critically analyzed and summarized.
2. Kidney Involvement in HNF1B Deficiency
2.1. Clinical Spectrum
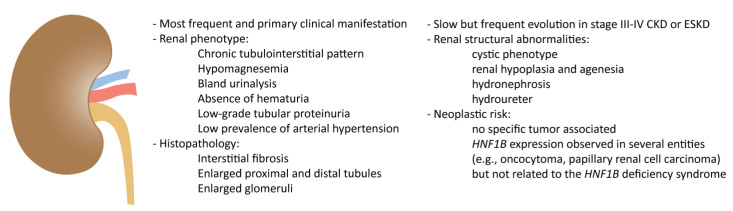
Renal phenotype is predominantly characterized by a chronic tubulointerstitial pattern, with bland urinalysis in most patients, absence of hematuria, low-grade tubular proteinuria, and low prevalence of arterial hypertension. In this setting, a renal biopsy usually shows an interstitial fibrosis with enlarged glomeruli or oligomeganephronia and enlarged proximal and distal tubules [10,17]. These lesions are the consequence of impaired energetic homeostasis of the renal tubule due to HNF1B pathogenic variants [18]. Hypomagnesemia and hypokalemia are both frequent (62% and are 46%, respectively) and are often found even in advanced chronic kidney disease (CKD; stage III–V) [10]. The high prevalence of hypomagnesemia is confirmed also in other studies and is due to magnesium tubular wasting [12]. This defect is ascribed to the altered control of FXYD2 gene expression by HNF1B [19], whereas potassium loss may be secondary to magnesium deficiency [20]. Of note, primary isolated hypomagnesemia can be the first clinical manifestation of HNF1B nephropathy [21] and genetic testing should be considered in this setting [22].
Some data are emerging on the mechanisms and progression trajectory of HNF1B nephropathy towards CKD and end-stage kidney disease (ESKD) [23]. Although the median decrease is reported to be around −2.45 mL/min/year, as expected in tubulointerstitial form, rapid unexplained worsening of renal function has been reported, and CKD is found in more than 90% of adult patients at diagnosis [10]. Dubois-Laforgue et al. reported a high prevalence of stages III and IV of CKD (44%) and even ESKD (21%) [12]. Interestingly, patients with 17q12 deletion appear to have CKD stage III–IV and ESKD less often at diagnosis and a significantly better renal function in the follow-up than patients with different pathogenic variants, suggesting a genotype/phenotype correlation [24]. Arterial hypertension, proteinuria, age at the time of diagnosis of diabetes, and the presence of microvascular complications (retinopathy and neuropathy) are also factors correlated with CKD progression. This group of risk factors may reflect the coexistence of diabetic nephropathy in a subset of patients, who are also at increased cardiovascular risk [12]. This “albuminuric pattern” (as opposed to the more common one with low-grade tubular proteinuria) seems to be characterized by a more rapid functional deterioration in the long-term [25].
Renal structural abnormalities (RSA) are another key feature of HNF1B nephropathy and display significant heterogeneity. HNF1B pathogenic variants putatively account for ∼10% of congenital abnormalities in patients with kidney and urinary tract, both among children and adults [13]. A cystic phenotype is frequently observed, with most adult patients harboring less than 5 cortical cysts. These usually spare the kidney outline, do not determine an increase in kidney size, and do not increase in number over time. No correlation has been found between cyst development and renal function. While this course differentiates HNF1B nephropathy from Autosomal Dominant Polycystic Kidney Disease (ADPKD), a few cases of massive cysts mimicking ADPKD have also been reported [10]. Around 20% of patients showed single-kidney disease that can represent the evolution of a multicystic and dysplastic contralateral kidney. A wide range of other RSAs and renal manifestations have been reported, including hydronephrosis or hydroureter, vesical ureteral reflux, kidney stones, and nephrocalcinosis [10].
The clinical characteristics of HNF1B nephropathy in adults have been investigated mainly in 2 multicentric retrospective studies. In the study by Faguer et al., data of 27 adult patients (mean age: 35 years) were collected and analyzed [10]. Although 4/27 patients were symptom-free (14.8%), in the remaining 23 patients, the most common first manifestation was related to kidney involvement (61%), followed by diabetes (9%), genital tract malformations (18%), and liver test abnormalities (12%) [10]. The more recent and consistent study by Dubois-Laforgue et al. included 201 patients aged 18 or older with a high prevalence of diabetes (82%) and genital tract abnormalities (50% in female and 80% in male) [12]. HNF1B nephropathy is probably underdiagnosed among adult patients with CKD of an unknown cause, even in presence of compatible RSA. The sequencing of HNF1B gene in a cohort of unrelated adult patients with unknown etiology and RSA allowed the identification of HNF1B pathogenic variants in a subset of 9% of patients [14].
2.2. Histopathology of Non-Neoplastic Conditions
The kidney is by far one of the most commonly affected organs in HNF1B syndrome. Although diabetes (in the form of MODY-5) is also frequently observed in HNF1B-deficient patients, the kidney involvement is not related to diabetic dysfunction (i.e., diabetic nephropathy), but rather to an abnormal embryonic development [26].
In line with the key role played by HNF1B in kidney development, the most common structural alteration causing renal dysfunction is related to nephron dysgenesis [26,27,28]. In a rodent model of kidney embryonal development, the HNF1B transcript increased, starting from the early stages of polarized epithelium differentiation, and was maintained until complete maturation of the nephron [28]. Similar findings were also observed in a mouse model, further confirming the involvement of HNF1B in early nephrogenesis [29]. Furthermore, its expression was mostly restricted to the tubular compartment (proximal and distal tubules, and collecting ducts), while the glomeruli and the urothelial system appeared only partially affected [27,28,30,31,32]. This broad involvement in nephron development being specifically expressed by the tubular compartment, explains cystic disease as the main histopathological findings associated to HNF1B syndrome [26].
Additional functions of HNF1B in kidney physiology (such as tubular transport and organ metabolism) can justify the entire spectrum of renal pathological conditions observed. In particular, patients with HNF1B deficiency typically presented renal hypoplasia and agenesia, familial juvenile hyperuricemic nephropathy, glomerulocystic kidney disease, and renal interstitial fibrosis, eventually leading to chronic kidney disease [23,31,33,34,35,36,37,38,39].
2.3. Histopathology of Neoplastic Conditions
In addition to the regulation of organ development during embryogenesis, HNF1B also controls the expression of genes involved in cell cycle regulation and apoptosis, thereby making its assessment also relevant for neoplastic conditions [40,41,42]. Notably, HNF1B seems to be more related to the development of tumors with “clear cell” features, but its specific pathogenetic mechanism remains largely unexplored and thus, ambiguous [43,44,45,46,47].
Focusing on kidney neoplastic disease, the development of kidney tumors in the context of HNF1B deficiency syndrome (with or without underlying kidney disease) was rarely observed and reported [48,49,50]. In contrast, the expression of HNF1B per se in kidney tumors has been widely explored and its immunohistochemical evaluation was proposed to differentiate renal oncocytoma (diffusely and strongly positive, nuclear stain) from chromophobe renal cell carcinoma (negative) [51,52,53,54,55]. In clear cell renal cell carcinoma, down-regulation of HNF1B was mainly observed, and its loss was associated with high-grade tumor and metastatic disease, suggesting both tumor-suppressive functions and prognostic potential [43,49], as also shown in Wilms tumor [56,57]. Conversely, papillary renal cell carcinoma, mucinous tubular spindle cell carcinoma, and metanephric adenoma were characterized by HNF1B overexpression, supporting its role as a proto-oncogene [58,59]. Overall, these apparently contradictory findings confirm the multifaceted role of HNF1B in kidney tumor development and support the need for broader analysis and validation studies.
The main features of the kidney involvement in HNF1B deficiency are summarized in Figure 1.
Figure 1.
The HNF1B deficiency and the kidney.
3. Liver Involvement in HNF1B Deficiency
3.1. Clinical Spectrum
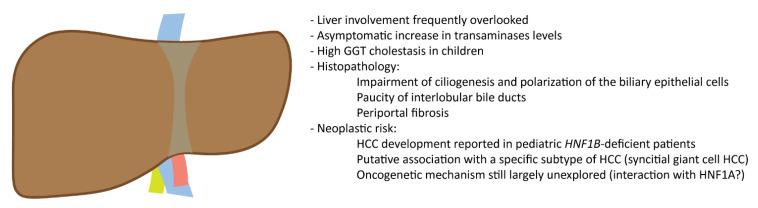
Although HNF1B defect is probably one of the most frequently recognized monogenic causes of developmental renal disease, abnormalities in liver function tests are frequently reported (around 40% in adults), especially in association with MODY5, where they reach 85% of cases [34]. Nevertheless, due to the initial description of HNF1B-deficiency as a syndrome characterized by kidney disease and diabetes, the liver involvement in HNF1B deficiency has been frequently overlooked, and only a few studies reported a comprehensive evaluation of the hepatic disease phenotypes [60]. Therefore, liver involvement has been poorly investigated, and reported so far as asymptomatic increase in transaminase levels or, less frequently, as a cholestatic liver disease.
A cholestatic condition featuring high gamma-glutamyl transferase (GGT) levels was reported in 5 newborns, who were all small for their gestational age (SGA), with a history of intrauterine growth restriction (IUGR), and affected by multiple bilateral renal cysts and moderate renal function impairment. Diabetes requiring insulin therapy occurred at an average age of 10 years in 3/5 cases, while 2/5 showed pancreatic hypoplasia with impaired pancreatic exocrine function [60,61,62,63,64]. Moreover, our group has recently described an additional case of a child with high GGT-cholestasis due to a de novo missense pathogenic variant of HNF1B, thus implicating rare mutations in HNF1B in the pathogenetic role in cholestatic liver diseases with increased GGT (Table 2) [65,66].
Table 2.
Clinical and histopathological features of patients’ with HNF1B variants presenting with neonatal cholestasis.
| Gender/Origin GW/BW g (DS) | Liver Involvement | Liver Histology |
Renal Function and Ultrasound Findings | Pancreatic Involvement |
Growth | Urogenital Malformations/ Cognitive Impairment |
HNF1B Variant | Reference |
|---|---|---|---|---|---|---|---|---|
| ♂/Japan 39/2390 (−2.26) <1 month |
|
PILBD, marked cholestasis |
|
Diabetes requiring insulin therapy at 13 years of age (polyuria and polydipsia, mild metabolic acidosis) | NA | Absent/mild | c.457C>A, p.H135N (missense mutation in exon 2, de novo or paternal: history of liver dysfunction and renal insufficiency in his paternal family) | [63] |
| ♂/Belgium (Sardinian origin) 37/1520 (−3.46) <1 month |
|
PILBD, severe biliary stasis, slight periportal fibrosis |
|
|
Final height of 162.1 cm (−1.86 SD), BMI 19.0 kg/m2 (−0.62 SD) |
Absent/NA | 499–504 delGCTCTG insCCCCT, A167FS (combination of a deletion and insertion in exon 2, de novo) |
[62] |
| ♂/Germany 35/1780 (−1.69) <1 month |
|
PILBD |
|
|
Final height of 133.9 cm (−6.7 SD), BMI 17.3 kg/m2 (−2.1 SD) | Inguinal hernia, abdominal testis/delayed psychomotor development | HNF1B deletion exons 1–9, de novo | [61] |
| ♀/Czech Republic 38/2360 (−1.60) <1 month |
|
PILBD, cholestasis without signs of bile duct proliferation |
|
|
Growth along the 3rd centile | Absent/absent | 1698 kb deletion including HNF1B, de novo | [60] |
| ♂/France 35/NA <1 month |
|
|
|
NA | NA | NA/NA | 1.5 Mb deletion including HNF1B | [64] |
| ♂/Italy 38/2600 (−1.27) <1 month |
|
PILBD, biliary stasis |
|
Initial pancreatic exocrine dysfunction without pancreatic hypoplasia at US |
Growth along the 10th centile | Absent/absent | c.827G>A, p.R276Q (missense mutation in exon 4, de novo) |
[65] |
NA: information not available, GW: gestation weeks, BW: birth weight, PILBD: paucity of intralobular bile ducts, BMI: body mass index, US: ultrasound.
Genes causing low or high GGT cholestatic liver disease have either hepatocyte expression or cholangiocyte expression, respectively. Based on the current evidence, HNF1B has been included among the genetic causes of high GGT cholestasis derived from a cholangiocyte dysfunction, in both children and adults [63].
Interestingly, a similar clinical/histological liver phenotype was observed in 13 children from 9 unrelated consanguineous families with high GGT cholestatic liver disease, all presenting homozygous damaging variants in kinesin family member 12 (KIF12; Table 3) [64,65], a target gene known to be regulated by the HNF1B transcription factor [62].
Table 3.
Clinical and histopathological features of patients with KIF12 variants presenting with neonatal cholestasis.
| Gender/Origin GW/BW g (DS) Age of Presentation |
Liver Involvement | Liver Histology | Renal Function and Ultrasound Findings |
Pancreatic Involvement |
Growth | Urogenital Malformations/ Cognitive Impairment |
KIF12 Homozygous Variant (NM_138424.1) | Reference |
|---|---|---|---|---|---|---|---|---|
| (A) ♀/Syrian (consanguineous) full term 1 month |
|
PILBD, bridging fibrosis with early nodule formation, mixed portal inflammatory infiltrate, multinucleated giant hepatocytes, extensive hepatocanalicular cholestasis, ductular reaction |
Left hydronephrosis and mild increase in left renal pelvic anterior–posterior diameter to 6 mm |
NA | NA | NA | c.655C>T: p. (Arg219 *) | [67] |
| (B) ♂/Turkish (consanguineous) full term 2 months |
|
PILBD, biliary pattern of cirrhosis with nodule formation, mixed portal inflammatory infiltrate, mild ductular reaction, pseudo-acini formation, and nodule formation |
Right renal pelvic anterior–posterior diameter | NA | NA | NA | c.610G>A: p. (Val204Met) |
[67] |
| (C) ♂/Turkish (consanguineous) 9 years; ibling of patient B |
|
Not performed | Caliectasis of the upper pole of the left kidney | NA | NA | NA | c.610G>A: p. (Val204Met) |
[67] |
| (D) ♂/4 months |
|
NA | Normal | Absent | Normal | Absent | c.463C>T: p. (Arg155*) |
[68] |
| (E) ♂/Unknown (consanguineous)/5 years |
|
NA | Normal | Absent | Normal | Absent | c.656G>A: p. (Arg219Gln) |
[68] |
| (F) ♂/Unknown (consanguineous)/14 months |
|
Suggestive of biliary cirrhosis | Normal | Absent | Normal | Absent | c.610G>A: p. (Val204Met) |
[68] |
| (G) ♂/Unknown (consanguineous)/6 months |
|
Suggestive of biliary atresia | Normal | Absent | Normal | Absent | c.610G>A: p. (Val204Met) |
[68] |
| (E) ♀/Kurdish (consanguineous)/ 13 years |
|
Extensive liver fibrosis, only minimal inflammation and proliferated bile ducts | Normal | Absent | Normal | Absent | c.655C>T: p. (Arg219 *) | [69] |
| (F) ♂/Kurdish (consanguineous)/ 13 years |
|
Advanced fibrosis and bile duct proliferation | Normal | Pancreatic lipomatosis | Normal | Absent | c.655C>T: p. (Arg219 *) | [69] |
| (G) ♀/Iraqi (consanguineous)/ 7 years |
|
NA | Normal | Absent | Normal | Absent | c.655C>T: p. (Arg219 *) | [69] |
| (H) ♀/Iraqi (consanguineous)/ 5 years |
|
Canalicular cholestasis, moderate fibrosis, mild inflammation without steatosis | Normal | Absent | Failure to thrive | Absent | c.655C>T: p. (Arg219 *) | [69] |
| (I) ♂/Syrian (consanguineous)/12 years |
|
Cirrhosis, septal hepatitis and ductular proliferation | Normal | Absent | Normal | Absent | c.655C>T: p. (Arg219 *) | [69] |
| (J) ♀/Afghan (consanguineous)/10 years |
|
NA | Normal | Absent | Normal | Absent | c.482-4_500del p. ? | [69] |
NA: information not available, GW: gestation weeks, BW: birth weight, PILBD: paucity of intralobular bile ducts, BMI: body mass index, US: ultrasound. *: stop codon
KIF12 belongs to the large kinesin superfamily of microtubule-associated molecular motors, which are crucial in the intracellular transport of organelles, microtubule cytoskeleton organization, and mitotic spindle function for cell division.
Since the knowledge of genetic cholestasis is continuously evolving, several transporters, cell–cell junctional proteins, adaptive processes, and intracellular signaling pathways involved in normal hepatobiliary homeostasis and bile acid metabolism may be regarded as new potential candidate genes for cholestasis.
Among them, within the new classification of progressive familial intrahepatic cholestasis (PFIC) [68], KIF12 pathogenic variants have been included as driving mutations responsible for PFIC-8. PFIC-8 pediatric patients are characterized by increased serum levels of GGT and transaminases, hyperbilirubinemia, hypercholesterolemia, pruritus, and by a rapid development of bridging fibrosis associated with ductopenia. Of note, recent data demonstrated that KIF12 is under the control and downstream effector of HNF1B. This transcription factor regulates KIF12 expression in both cultured cells, and knockout mice, by altering co-factor recruitment and histone modification, which supports a potential role of KIF12 in the function of cholangiocytes [70,71]. It is currently unclear which are the mechanisms linking these two types of chronic cholestasis and whether they can depend on common molecular bases. Considering the mutual interactions between KIF12 and HNF1B and the overlapping clinical profile, we speculate that HNF1B pathogenic variants should also be considered as part of the PFIC spectrum. A recent work by Stalke et al. has shown that, in 6 pediatric patients harboring KIF12 variants, cholestasis may be due to a perturbed polarization of hepatocytes, which leads to an incorrect positioning of the hepatocanalicular membrane of channel proteins, such as ATP binding cassette subfamily B member (ABCB) 4 and ABCB11, regulating the transport and extrusion of bile salts into the bile canaliculus [69]. Similarly, patients with HNF1B pathogenic variants, whose malfunction can negatively regulate KIF12 expression, may present a similar derangement of the correct exposure of canalicular transporters. Future research directions will help understanding the pathogenesis of PFIC-8 and its relationship with HNF1β-related cholestasis, hopefully unveiling novel targets for therapeutic intervention. In keeping with this observation, ileal bile acid transporter (IBAT) inhibitors, have been proposed for the treatment of patients with PFIC and Alagille syndrome, and approved in mid-2021 to treat intractable pruritus related to cholestasis [72,73,74]. Moreover, these new drugs already applied to other types of PFIC and cholestatic liver disease [69], could be considered in children and adults with KIF12 and HNF1B defective function. Indeed, although genetic cholestatic liver diseases are more frequently observed in children, novel genetic alterations are continuously identified thanks to high-throughput genetic techniques, thereby providing candidate gene variants that may apply to some causes of unknown cholestasis in adults. In addition, further research may provide new insights on the complex mechanisms that combine cholestasis, hepatic repair/regenerative response, and biliary fibrosis, which could also be relevant for acquired cholestasis, according to the hypothesis that genetic diseases can serve as a ‘pathophysiological roadmap’ to improve the understanding of other pathogenic factors [75].
3.2. Histopathology of Non-Neoplastic Conditions
As HNF1B is involved in the development of specialized epithelia, its dysfunction in the liver is primarily related to the bile duct system. In fact, mouse models harboring liver-specific HNF1B inactivation are characterized by abnormal development of gallbladder and intrahepatic bile ducts, coupled with their respective interlobular arteries, clinically resulting in jaundice and failure to thrive [76,77,78]. The liver of these mice showed histopathological alterations (i.e., senescence alterations and epithelial metaplasia) in the gallbladder and intrahepatic bile ducts. Additionally, hepatic ductal plate remnants were still observed in adult mice [76,77,78]. This evidence is particularly relevant, as it supports the notion that HNF1B is key in the development of the bile duct epithelia, in both extra- and intrahepatic systems. Furthermore, ciliogenesis and cell polarization were found to be primarily and severely impaired [79,80,81], defining the HNF1B-related dysmorphogenic biliary phenotype. These defects may have an underlying developmental origin coming from anomalies in ductal plate remodeling, resulting in ductal plate malformations (DPMs), with the persistence of post-natal embryonic biliary structures, biliary cell clusters or duct-like structures [82]. According to this hypothesis, HNF1B may behave as a pivotal regulator of primitive ductal structures (PDS). Indeed, in a new pathogenic classification, DPMs are not the result of a lack of PDS remodeling, but rather the common endpoint of different defects of differentiation, maturation, expansion, polarity and/or ciliogenesis of PDS, affecting distinct stages of bile duct morphogenesis. For example, mice with HNF1B deficiency showed a normal hepatic differentiation, but an abnormal PDS maturation (73). The cilium hosts many proteins that can sense physical and chemical properties of the biliary milieu, and its dysfunction is associated with increased cholangiocyte proliferation and profound changes in cholangiocyte intracellular signaling. The role of HNF1B in ciliogenesis emerged with ultrastructural analysis of 3 adults with late-onset cholestasis, demonstrating a significant loss of primary cilia in cholangiocytes, but no structural intra- or extrahepatic bile duct defects [15]. Based on this evidence, HNF1B deficiency is included in the wide spectrum of syndromic ciliopathies with liver involvement [83].
The involvement of the extrahepatic bile ducts secondary to HNF1B deficiency has been rarely documented, and mainly reported as the complete absence [60] or (choledochal) cystic degeneration, with atypical morphology on imaging studies [84]. Kettunen et al. described biliary abnormalities, identified by magnetic resonance (MR) cholangiography, in 6 patients with HNF1B mutations. Most of them had varying types of bile duct cysts (BDCs) in the extrahepatic bile ducts, with an atypical morphology for any given Todani classification.
On this basis, it is not surprising that the liver phenotype of patients with HNF1B deficiency shows a cholestatic profile [15,60,61,62,63,85,86], especially in the neonatal age. From a histopathological perspective, all the cholestatic patients reported above [60,61,62,63] had comparable histological features, showing a pattern of paucity of interlobular bile ducts (PILBD), associated with marked cholestasis and variable degrees of periportal fibrosis. Clinical PILBD is not such a rare finding at histology, especially in infants with cholestasis and accounts for the 11% of pediatric liver biopsies [87]. PILBD has been categorized as syndromic (S-PILBD) and non-syndromic (NS-PILBD) entities. S-PILBD is associated with Alagille Syndrome (AGS), whereas a source of diagnostic dilemma to clinicians is the presence of ductopenia in patients who otherwise do not fit the clinical AGS description or are non-syndromic. The paucity of bile ducts is in fact not pathognomonic for AGS and can be found in several disorders of different etiologies, as shown in Table 4.
Table 4.
Paucity of bile ducts and associated disorders.
| Genetic Disorders | Congenital Infections | Immune Disorders |
Drug Related |
|
|---|---|---|---|---|
| AGS (OMIM # 118450, OMIM # 610205) | HNF1B deficiency syndrome (OMIM # 137920) | Cytomegalovirus | Sclerosing cholangitis | Vanishing bile duct syndrome |
| Cystic fibrosis (OMIM # 219700) | KIF12-associated cholestasis (OMIM # 619662) | Rubella | Hemophagocytic lymphohistiocytosis |
|
| α1-antitrypsin deficiency (OMIM # 613490) | ABBC12-associated cholestasis [88] | Syphilis | ||
| Niemann Pick type C (OMIM # 257220) | ||||
| Williams-Beuren syndrome (OMIM # 194050) | ||||
| Trisomy 21 (OMIM # 190685) | ||||
When these additional clinical traits are absent, PILBDs require a broad diagnostic work-up, needing supplementary testing and characterization. Therefore, the identification of HNF1B deficiency as a possible cause of PILBD partially addresses the question regarding bile duct paucity in patients who do not meet the criteria for AGS, the most frequent cause of PILBD. In accordance with these findings, it has recently been stated that a ciliopathy like HNF1B deficiency must be regarded as a new condition to be included in the diagnostic work-up of neonatal/infantile cholestasis, when PILBD is observed at liver biopsy and other features of AGS are absent [66].
A defective HNF1B function may also play a role in the pathogenesis of neonatal sclerosing cholangitis (NSC) similarly to patients with DCDC2 (doublecortin domain containing protein 2) pathogenic variants. To date, 13 patients with NSC have been reported [89,90,91]. They show high GGT cholestasis, acholic stool, and progression to portal hypertension with radiological findings revealing typical structuring with dilatations in the intrahepatic and/or extrahepatic biliary tree. Renal and neurological abnormalities were also frequently present. Liver histology showed peripheral ductopenia, DPM, fibrosis, and eventually, cirrhosis. Interestingly, DCDC2 has been shown to be closely related to the ciliary kinesin-2 subunit KIF3a. However, cases of HNF1B deficiency with a less prominent involvement of the bile duct system were also reported, showing no significant characteristics or steatotic/steatohepatitic changes, mainly in adolescent/adult patients [15,16,34,61,85].
Altogether, these observations pinpoint HNF1B-deficiency as a significant cause of liver involvement, especially in the neonatal/pediatric settings, ultimately suggesting HNF1B to be included in the diagnostic algorithm of patients presenting with a cholestatic profile [66].
3.3. Histopathology of Neoplastic Conditions
The association of HNF1B deficiency with the development of primary liver cancer is largely unexplored. Single nucleotide polymorphisms (SNPs) of HNF1B have been identified and associated through genotyping arrays with cancer development. HNF1B somatic mutations were observed in several human cancers, among which hepatocellular carcinoma (HCC), confirming further, as previously discussed, its role as an oncogene/tumor suppressor gene [50]. Nevertheless, the association of HCC with germline HNF1B deficiency is largely uncharted [6,65]. However, although at a very low risk, HNF1B deficiency may be associated with HCC, as in some PFIC disorders. Interesting enough, although HNF1B deficiency mainly affects the biliary epithelium, only HCC but not cholangiocarcinoma, has been observed so far. HCC is the second most common malignant liver tumor in children after hepatoblastoma. Both hepatoblastoma and HCC account together for the 0.5–1.5% of all childhood malignancies and for the 4% of all pediatric liver transplantations [92]. It differs from adult HCC with respect to the etiological predisposition, biological behavior, and lower frequency of cirrhosis. HNF1A mutations occur in 1–2% of HCC.
HCC was firstly reported in HNF1B deficiency in a 16-month-old newborn with a germline pathogenic variant in HNF1B, presenting renal hyperechogenicity, transient renal neonatal failure, and progressive neonatal cholestasis, ultimately evolving to micronodular cirrhosis and HCC. According to this report, the little patient underwent liver transplantation with no relapse (1 year of follow-up) [64]. Additionally, a report described a 9-month-old girl with ductopenic cholestasis, hyperparathyroidism, growth retardation, and delayed motor development who developed HCC featuring a syncytial giant cell subtype [93]. Notably, the perilesional liver showed injured bile ducts or complete bile duct loss, similar to AGS. Although HNF1B deficiency was not considered in the differential diagnosis, we hint at the possibility that this HCC could be a misdiagnosed case of HNF1B deficiency. Similarly, we described a case of HNF1B deficiency affected by chronic renal disease due to multicystic kidney involvement, bilateral cryptorchidism, and autism spectrum disorder [94]. Noteworthy, our patient also developed a syncytial giant cell subtype of HCC, which led us to hypothesize that the syncytial giant cell variant of pediatric HCC could be strictly related to the HNF1B faulty-driven oncogenetic mechanism, as it has never been reported elsewhere [95,96,97,98].
Although not yet unraveled, HNF1B deficiency-dependent mechanisms promoting HCC could be secondary to its repressive effect on HNF1A transcriptional activity, since HNF1B forms a heterodimer with HNF1A and binds to the same target elements as investigated by Kitanaka et al. [63]. We can therefore speculate that the phenotypic variability of patients with HNF1B pathogenic variants might be caused by different HNF1B activity in conjunction with repression of HNF1A activity, in selected promoters and tissues. Further functional studies on these effects are sorely needed to clarify this issue. From this perspective, we are generating both an animal model of HNF1B-KO mouse and mouse organoids to understand the pathophysiology of liver disease associated with perturbations in the HNF1B signaling pathway (EASL Daniel Alagille Award 2021).
The main features of the liver involvement in HNF1B deficiency are summarized in Figure 2.
Figure 2.
The HNF1B deficiency and the liver.
4. Pancreas Involvement in HNF1B Deficiency
4.1. Clinical Spectrum
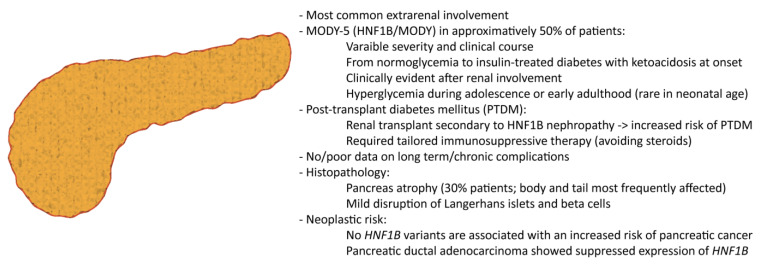
Pancreatic atrophy is reported in approximately 30% of patients and diabetes mellitus in about 50%. Diabetes mellitus is the most common extrarenal feature identified in patients with HNF1B-associated disease, mostly developing as a typical MODY, which is a form of diabetes mellitus secondary to pancreatic beta cell dysfunction, with a typical onset before 25 years of age, the absence of specific anti-beta cell antibodies, and autosomal dominant inheritance. This subtype of MODY is usually known as MODY5 or HNF1B/MODY, according to the most recent nomenclature.
HNF1B/MODY represents up to 6% of all MODY cases and is one of the most frequent causes of syndromic diabetes [99,100], showing a decrease in insulin secretion with progressive worsening of blood glucose control. The severity and course of diabetes are variable and glucose homeostasis ranges from normoglycemia to insulin-treated diabetes with ketoacidosis at onset [101]. Hyperglycemia usually occurs during adolescence or early adulthood [6], when there is a physiological increase in insulin resistance. Hyperglycemia can rarely occur early during the neonatal age [35,102]. The presence of decreased insulin secretion and increased insulin resistance lead to irreversible chronic hyperglycemia, which appears similar to other forms of HNFs/MODY. In the study of Faguer et al., almost 70% of patients required insulin in the follow-up, but no case of retinopathy and neuropathy was reported [34], whereas in the experience of Dubois-Laforgue et al., microvascular complications were detected in 40% of patients after a 15 year-observation period [12].
Extrapancreatic features are evident before the onset of diabetes, and the presence of CKD at the onset of diabetes is a typical feature [101]. Indeed, patients with HNF1B-deficient syndrome rarely show only diabetes and this diagnosis should be suspected at diabetes onset in all the patients with renal cysts and/or other suggestive extra-pancreatic features, even when a family history is absent.
Of note, patients with HNF1B nephropathy who undergo renal transplant are at increased risk of developing post-transplant diabetes mellitus (PTDM) [10]. Therefore, HNF1B defects should be considered when PTDM develops after kidney transplantation [103,104], and should also be ruled out also in the absence of any apparent risk factor, particularly in a young kidney transplant recipient with compatible features and an unknown causal nephropathy [105]. Calcineurin inhibitor treatment after transplant seems to reduce the expression of the wild-type allele of HNF1B gene, leading to insufficient transcriptional activity. The evidence that carriers of the HNF1B deficiency are at a greater risk for diabetes mellitus raised the question of whether transplant management must be modified to avoid drugs that can cause hyperglycemia. In this light, immunosuppressive agents other than corticosteroids should be preferred. Therefore, a tailored immunosuppressive regimen could be considered in HNF1B patients [106]. Finally, diabetic patients with HNF1B nephropathy reaching end-stage renal disease (ESRD) are a potential candidate for simultaneous pancreatic and kidney transplantation.
Regarding the treatment of HNF1B/MODY, patients present hepatic insulin resistance to some extent [107], and thus the treatment with oral hypoglycemic agents such as sulfonylureas may not be satisfactory, and early insulin therapy may be requested [108]. In the large study cohort described by Dubois-Laforgue et al. [12], 49% of the patients were treated with insulin since diabetes onset, but the rate increased to 79% during the follow-up because of the worsening glucose control. In a small subgroup of patients, the treatment with oral hypoglycemic agents (sulphonylurea or meglitinide repaglinide) was started after a mean time of 8 months from diagnosis and it was successful in 57% of patients. In these patients, HbA1c dropped from 7.1% to 6.1% and the beta-cell function result improved after 5 years of treatments. A case report described the switch from insulin to glimepiride after reaching excellent metabolic control. Sitagliptin was added during follow-up, allowing excellent glucose control for 6 years. After this period, the blood glucose control worsened and insulin treatment was necessary [109].
Chronic complications are poorly described in the literature, even if it is likely that they may occur as frequently as in other types of diabetes and are related to glycemic control [12]. However, long-term data are missing. Similarly, data on care during pregnancy are not available in the literature, but insulin treatment is usually necessary to keep blood glucose within normal limits. Birth weight is normal, as long as maternal hyperglycemia is properly treated, but if the fetus carriers HNF1B-deficiency, the birth weight is typically low [35].
4.2. Histopathology of Non-Neoplastic Condition
HNF1B plays a key role in the early development and differentiation of the pancreas by regulating the expression of proteins related to the organ embryogenesis, such as HNF4A and SLC2A2, and variations in HNF1B gene activity can impact both the exocrine and endocrine compartments. Indeed, beta-cells are another cell type where HNF1B drives embryological development and functionality, as demonstrated by Hnf1B-knock out mice models showing hypoplastic and atrophic pancreas, as well as beta-cell impaired terminal differentiation [110,111]. Similar findings were also observed in HNF1B-deficient fetuses and patients [112,113], leading to the well-described MODY-5 clinical phenotype [3,34,112,113,114]. However, most significant changes in pancreas morphology described so far are based on imaging studies [CT/MRI of the pancreas and magnetic resonance cholangiopancreatography (MRCP)], while only a few studies report histopathological changes [34,113,115,116]. Based on imaging description, the pancreas showed no pancreatic body and tail, and the accessory and dorsal ducts were lacking as well, whereas the beta-cell impairment was mainly based on clinical, functional, and serological evaluations. In contrast to the extrahepatic bile duct involvement reported above, no cystic dilatations were reported. Overall, these findings suggested a predominant involvement/agenesis of the dorsal pancreatic component, but the ventral bud (and the head of the pancreas), although mostly spared, also appeared to be partially involved [3,34,113,115,116]. It is noteworthy that parenchymal calcifications were also reported, but not constantly and without a correlation with patient condition or pancreatic morphology, although an association with late-phase dysfunction following diabetes development has been suggested [113].
Histopathological analysis of HNF1B-mutated fetal pancreatic tissue confirmed imaging studies, showing a severe hypoplasia of the pancreatic body and tail, further suggesting its cause as severe acinar component underdevelopment. Indeed, most patients with HNF1B-related pancreatic hypoplasia show a subclinical pancreatic exocrine dysfunction, assessed through fecal elastase deficiency [6]. Furthermore, Langerhans islets were morphologically disorganized and beta cells slightly reduced [112].
4.3. Histopathology of Neoplastic Condition
The role of HNF1B in pancreatic cancer development was mainly related to tumor suppressor functions [50,117]. Indeed, several studies performed in different models of pancreatic ductal adenocarcinoma (including organoids, cell cultures, mice models, and human tissue samples) demonstrated an overall suppressed expression of HNF1B, as genomic transcript or immunohistochemical nuclear stain, compared to the pancreatic acinar parenchyma and ducts [117,118,119,120]. Similarly, down-regulation of HNF1B was observed in mouse models and organoids of intraductal papillary mucinous neoplasms [120,121].
The mechanism of tumor development related to reduced HNF1B levels appeared to be related to a hypermethylation of the promoter region, eventually leading to: (i) loss of the adhesion molecule E-cadherin in neoplastic cells, (ii) expanded epithelial-to-mesenchymal transition, and (iii) increased tumor cell migration [50,117]. However, no variants of HNF1B have been hitherto associated with an increased risk of pancreatic cancer development [122]. The main features of pancreatic involvement in HNF1B deficiency are summarized in Figure 3.
Figure 3.
The HNF1B deficiency and the pancreas.
5. Additional Extrarenal Involvement
Genital tract abnormalities are found in around 40% of female patients, usually presenting major and severe malformations (e.g., bicornuate or rudimentary uterus, vaginal aplasia (Müllerian aplasia)) that were also observed in the context of the Mayer-Rokitansky-Küster-Hauser syndrome [10,123,124]. However, the exact prevalence is most likely largely underestimated due to the presence of minor abnormalities with no/minor clinical significance that could potentially be overlooked [10,123,124]. Notably, HNF1B pathogenic variants were found in 18% of patients with structural alterations in both the kidney and the uterus, but were not found in isolated uterine malformations [125]. Male genital defects, such as cryptorchidism, abnormal descent of testes, hypospadias, and prostatic hypoplasia, were also reported [126].
Mild cognitive impairment, seizures, autism, schizophrenia, and structural alterations of the brain have only been reported in patients with deletions of chromosome 17q12 [127,128,129,130]. However, mild neurological involvement is frequently observed and deserves a more thorough analysis, especially in the adult population [10].
Finally, anecdotal abnormalities and malformations of other districts were also reported, including facial dysmorphic features [131], duodenal atresia [131], prune belly syndrome (also known as Eagle-Barrett syndrome) [126,132], congenital diaphragmatic hernia [133], and congenital joint laxity [134].
Considering this complex landscape, two types of scores have been proposed to select patients for genetic screening based on clinical criteria [13,135]. In the latter, the coexistence of cysts of unknown origin and hypomagnesemia determines a high screening priority, as it is associated with a 6-fold increased probability of finding HNF1B-related pathogenic variants.
6. Conclusions
HNF1B-associated disease has been recognized as a clinical entity with a broader and more variable multisystem phenotype than previously reported. Despite the frequent and peculiar involvement of the kidneys and pancreas, HNF1B deficiency has increasingly emerged as a multifaceted syndromic ciliopathy affecting several organs, among which the liver seems to be quite relevant. The consequent complex and heterogeneous clinical phenotype, most likely related to the functional interaction of HNF1B with other developmental genes, could impinge on patient management. We believe that this comprehensive review, the first providing a broad overview of the genetic, histopathologic, and clinical phenotypes of HNF1B-deficiency syndrome, will kindle a more detailed analysis of the effect of HNF1B pathogenic variants and the related clinical landscape. Joint efforts from multidisciplinary research groups are then required to foster the understanding of the pathophysiology and molecular mechanisms of this highly complex syndrome, with the aim to identify clinically relevant implications, that may translate the evolving pediatric knowledge to adult clinicians.
Abbreviations
| AGS | Alagille syndrome |
| BDCs | bile duct cysts |
| BMI | body mass index |
| BW | birth weight |
| CKD | chronic kidney disease |
| DCDC2 | doublecortin domain containing protein 2 |
| DPMs | ductal plate malformations |
| EASL | European Association for the Study of the Liver |
| ESKD | end-stage kidney disease |
| HCC | hepatocellular carcinoma |
| HNF | hepatocyte nuclear factor |
| HNF1B | hepatocyte nuclear factor 1β |
| IBATis | ileal bile acid transporter inhibitors |
| IUGR | intrauterine growth restriction |
| KIF12 | kinesin family member 12 |
| MeSH | Medical Subject Headings |
| NA | information not available |
| NSC | neonatal sclerosing cholangitis |
| PDS | primitive ductal structures |
| PFIC | progressive familial intrahepatic cholestasis |
| PILBD | paucity of intralobular bile ducts |
| RSA | renal structural abnormalities |
| SGA | small for gestational age |
| SNPs | single nucleotide polymorphisms |
| TCF2 | transcription factor 2 |
| US | ultrasound |
Author Contributions
A.G., M.D., L.F. and M.P.: conceptualization. A.G.: writing—original draft preparation. A.G., S.K., M.C., M.Q., M.D., L.F. and M.P.: writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lau H.H., Ng N.H.J., Loo L.S.W., Jasmen J.B., Teo A.K.K. The molecular functions of hepatocyte nuclear factors—In and beyond the liver. J. Hepatol. 2018;68:1033–1048. doi: 10.1016/j.jhep.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Bockenhauer D., Jaureguiberry G. HNF1B-associated clinical phenotypes: The kidney and beyond. Pediatr. Nephrol. 2016;31:707–714. doi: 10.1007/s00467-015-3142-2. [DOI] [PubMed] [Google Scholar]
- 3.El-Khairi R., Vallier L. The role of hepatocyte nuclear factor 1beta in disease and development. Diabetes Obes. Metab. 2016;18((Suppl. 1)):23–32. doi: 10.1111/dom.12715. [DOI] [PubMed] [Google Scholar]
- 4.Nyunt O., Wu J.Y., McGown I.N., Harris M., Huynh T., Leong G.M., Cowley D.M., Cotterill A.M. Investigating maturity onset diabetes of the young. Clin. Biochem. Rev. 2009;30:67–74. [PMC free article] [PubMed] [Google Scholar]
- 5.Verhave J.C., Bech A.P., Wetzels J.F., Nijenhuis T. Hepatocyte Nuclear Factor 1beta-Associated Kidney Disease: More than Renal Cysts and Diabetes. J. Am. Soc. Nephrol. 2016;27:345–353. doi: 10.1681/ASN.2015050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clissold R.L., Hamilton A.J., Hattersley A.T., Ellard S., Bingham C. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat. Rev. Nephrol. 2015;11:102–112. doi: 10.1038/nrneph.2014.232. [DOI] [PubMed] [Google Scholar]
- 7.Stenson P.D., Mort M., Ball E.V., Chapman M., Evans K., Azevedo L., Hayden M., Heywood S., Millar D.S., Phillips A.D., et al. The Human Gene Mutation Database (HGMD((R))): Optimizing its use in a clinical diagnostic or research setting. Hum. Genet. 2020;139:1197–1207. doi: 10.1007/s00439-020-02199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pal A., Reidy K.J. Genetic Syndromes Affecting Kidney Development. Results Probl. Cell Differ. 2017;60:257–279. doi: 10.1007/978-3-319-51436-9_10. [DOI] [PubMed] [Google Scholar]
- 9.Mitchel M.W., Moreno-De-Luca D., Myers S.M., Levy R.V., Turner S., Ledbetter D.H., Martin C.L. 17q12 Recurrent Deletion Syndrome. [(accessed on 15 October 2020)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK401562/2020.
- 10.Faguer S., Decramer S., Chassaing N., Bellanné-Chantelot C., Calvas P., Beaufils S., Bessenay L., Lengelé J.P., Dahan K., Ronco P., et al. Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int. 2011;80:768–776. doi: 10.1038/ki.2011.225. [DOI] [PubMed] [Google Scholar]
- 11.Bellanné-Chantelot C., Clauin S., Chauveau D., Collin P., Daumont M., Douillard C., Dubois-Laforgue D., Dusselier L., Gautier J.F., Jadoul M., et al. Large genomic rearrangements in the hepatocyte nuclear factor-1beta (TCF2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes. 2005;54:3126–3132. doi: 10.2337/diabetes.54.11.3126. [DOI] [PubMed] [Google Scholar]
- 12.Dubois-Laforgue D., Cornu E., Saint-Martin C., Coste J., Bellanné-Chantelot C., Timsit J., Monogenic Diabetes Study Group of the Société Francophone du Diabète Diabetes, Associated Clinical Spectrum, Long-term Prognosis, and Genotype/Phenotype Correlations in 201 Adult Patients with Hepatocyte Nuclear Factor 1B (HNF1B) Molecular Defects. Diabetes Care. 2017;40:1436–1443. doi: 10.2337/dc16-2462. [DOI] [PubMed] [Google Scholar]
- 13.Raaijmakers A., Corveleyn A., Devriendt K., van Tienoven T.P., Allegaert K., Van Dyck M., van den Heuvel L., Kuypers D., Claes K., Mekahli D., et al. Criteria for HNF1B analysis in patients with congenital abnormalities of kidney and urinary tract. Nephrol. Dial. Transplant. 2015;30:835–842. doi: 10.1093/ndt/gfu370. [DOI] [PubMed] [Google Scholar]
- 14.Musetti C., Quaglia M., Mellone S., Pagani A., Fusco I., Monzani A., Giordano M., Stratta P. Chronic renal failure of unknown origin is caused by HNF1B mutations in 9% of adult patients: A single centre cohort analysis. Nephrology. 2014;19:202–209. doi: 10.1111/nep.12199. [DOI] [PubMed] [Google Scholar]
- 15.Roelandt P., Antoniou A., Libbrecht L., Van Steenbergen W., Laleman W., Verslype C., Van der Merwe S., Nevens F., De Vos R., Fischer E., et al. HNF1B deficiency causes ciliary defects in human cholangiocytes. Hepatology. 2012;56:1178–1181. doi: 10.1002/hep.25876. [DOI] [PubMed] [Google Scholar]
- 16.Montoli A., Colussi G., Massa O., Caccia R., Rizzoni G., Civati G., Barbetti F. Renal cysts and diabetes syndrome linked to mutations of the hepatocyte nuclear factor-1 beta gene: Description of a new family with associated liver involvement. Am. J. Kidney Dis. 2002;40:397–402. doi: 10.1053/ajkd.2002.34538. [DOI] [PubMed] [Google Scholar]
- 17.Sagen J.V., Bostad L., Njolstad P.R., Sovik O. Enlarged nephrons and severe nondiabetic nephropathy in hepatocyte nuclear factor-1beta (HNF-1beta) mutation carriers. Kidney Int. 2003;64:793–800. doi: 10.1046/j.1523-1755.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 18.Piedrafita A., Balayssac S., Casemayou A., Saulnier-Blache J.S., Lucas A., Iacovoni J.S., Breuil B., Chauveau D., Decramer S., Malet-Martino M., et al. Hepatocyte nuclear factor-1beta shapes the energetic homeostasis of kidney tubule cells. FASEB J. 2021;35:e21931. doi: 10.1096/fj.202100782RR. [DOI] [PubMed] [Google Scholar]
- 19.Adalat S., Woolf A.S., Johnstone K.A., Wirsing A., Harries L.W., Long D.A., Hennekam R.C., Ledermann S.E., Rees L., Van′t Hoff W., et al. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J. Am. Soc. Nephrol. 2009;20:1123–1131. doi: 10.1681/ASN.2008060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L., Frindt G., Palmer L.G. Magnesium modulates ROMK channel-mediated potassium secretion. J. Am. Soc. Nephrol. 2010;21:2109–2116. doi: 10.1681/ASN.2010060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Der Made C.I., Hoorn E.J., De La Faille R., Karaaslan H., Knoers N.V., Hoenderop J.G., Poussou R.V., De Baaij J.H. Hypomagnesemia as First Clinical Manifestation of ADTKD-HNF1B: A Case Series and Literature Review. Am. J. Nephrol. 2015;42:85–90. doi: 10.1159/000439286. [DOI] [PubMed] [Google Scholar]
- 22.Musetti C., Quaglia M., Stratta P., Giordano M. Hypomagnesemia and progressive chronic kidney disease: Thinking of HNF1B and other genetic nephropathies. Kidney Int. 2015;88:641. doi: 10.1038/ki.2015.187. [DOI] [PubMed] [Google Scholar]
- 23.Chan S.C., Zhang Y., Shao A., Avdulov S., Herrera J., Aboudehen K., Pontoglio M., Igarashi P. Mechanism of Fibrosis in HNF1B-Related Autosomal Dominant Tubulointerstitial Kidney Disease. J. Am. Soc. Nephrol. 2018;29:2493–2509. doi: 10.1681/ASN.2018040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge S., Yang M., Cui Y., Wu J., Xu L., Dong J., Liao L. The Clinical Characteristics and Gene Mutations of Maturity-Onset Diabetes of the Young Type 5 in Sixty-One Patients. Front. Endocrinol. 2022;13:911526. doi: 10.3389/fendo.2022.911526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua Tan C.S., Ang S.F., Yeoh E., Goh B.X., Loh W.J., Shum C.F., May Ping Eng M., Yan Lun Liu A., Wan Ting Chan L., Goh L.X., et al. MODY5 Hepatocyte Nuclear Factor 1ss (HNF1ss)-Associated Nephropathy: Experience from a regional monogenic diabetes referral centre in Singapore. J. Investig. Med. High Impact Case Rep. 2022;10:23247096211065626. doi: 10.1177/23247096211065626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bingham C., Ellard S., Allen L., Bulman M., Shepherd M., Frayling T., Berry P.J., Clark P.M., Lindner T., Bell G.I., et al. Abnormal nephron development associated with a frameshift mutation in the transcription factor hepatocyte nuclear factor-1 beta. Kidney Int. 2000;57:898–907. doi: 10.1046/j.1523-1755.2000.057003898.x. [DOI] [PubMed] [Google Scholar]
- 27.Horster M.F., Braun G.S., Huber S.M. Embryonic renal epithelia: Induction, nephrogenesis, and cell differentiation. Physiol. Rev. 1999;79:1157–1191. doi: 10.1152/physrev.1999.79.4.1157. [DOI] [PubMed] [Google Scholar]
- 28.Lazzaro D., De Simone V., De Magistris L., Lehtonen E., Cortese R. LFB1 and LFB3 homeoproteins are sequentially expressed during kidney development. Development. 1992;114:469–479. doi: 10.1242/dev.114.2.469. [DOI] [PubMed] [Google Scholar]
- 29.Lokmane L., Heliot C., Garcia-Villalba P., Fabre M., Cereghini S. vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development. 2010;137:347–357. doi: 10.1242/dev.042226. [DOI] [PubMed] [Google Scholar]
- 30.Lindström N.O., McMahon J.A., Guo J., Tran T., Guo Q., Rutledge E., Parvez R.K., Saribekyan G., Schuler R.E., Liao C., et al. Conserved and Divergent Features of Human and Mouse Kidney Organogenesis. J. Am. Soc. Nephrol. 2018;29:785–805. doi: 10.1681/ASN.2017080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferre S., Igarashi P. New insights into the role of HNF-1beta in kidney (patho)physiology. Pediatr. Nephrol. 2019;34:1325–1335. doi: 10.1007/s00467-018-3990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naylor R.W., Davidson A.J. Hnf1beta and nephron segmentation. Pediatr. Nephrol. 2014;29:659–664. doi: 10.1007/s00467-013-2662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidet L., Decramer S., Pawtowski A., Morinière V., Bandin F., Knebelmann B., Lebre A.S., Faguer S., Guigonis V., Antignac C., et al. Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin. J. Am. Soc. Nephrol. 2010;5:1079–1090. doi: 10.2215/CJN.06810909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellanné-Chantelot C., Chauveau D., Gautier J.F., Dubois-Laforgue D., Clauin S., Beaufils S., Wilhelm J.M., Boitard C., Noël L.H., Velho G., et al. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann. Intern. Med. 2004;140:510–517. doi: 10.7326/0003-4819-140-7-200404060-00009. [DOI] [PubMed] [Google Scholar]
- 35.Edghill E.L., Bingham C., Ellard S., Hattersley A.T. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J. Med. Genet. 2006;43:84–90. doi: 10.1136/jmg.2005.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bingham C., Bulman M.P., Ellard S., Allen L.I., Lipkin G.W., van′t Hoff W.G., Woolf A.S., Rizzoni G., Novelli G., Nicholls A.J., et al. Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am. J. Hum. Genet. 2001;68:219–224. doi: 10.1086/316945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzoni G., Loirat C., Levy M., Milanesi C., Zachello G., Mathieu H. Familial hypoplastic glomerulocystic kidney. A new entity? Clin. Nephrol. 1982;18:263–268. [PubMed] [Google Scholar]
- 38.Kaplan B.S., Gordon I., Pincott J., Barratt T.M. Familial hypoplastic glomerulocystic kidney disease: A definite entity with dominant inheritance. Am. J. Med. Genet. 1989;34:569–573. doi: 10.1002/ajmg.1320340423. [DOI] [PubMed] [Google Scholar]
- 39.Mache C.J., Preisegger K.H., Kopp S., Ratschek M., Ring E. De novo HNF-1 beta gene mutation in familial hypoplastic glomerulocystic kidney disease. Pediatr. Nephrol. 2002;17:1021–1026. doi: 10.1007/s00467-002-0975-2. [DOI] [PubMed] [Google Scholar]
- 40.Hojny J., Michalkova R., Krkavcova E., Bui Q.H., Bartu M., Nemejcova K., Kalousova M., Kleiblova P., Dundr P., Struzinska I. Comprehensive quantitative analysis of alternative splicing variants reveals the HNF1B mRNA splicing pattern in various tumour and non-tumour tissues. Sci. Rep. 2022;12:199. doi: 10.1038/s41598-021-03989-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki E., Kajita S., Takahashi H., Matsumoto T., Tsuruta T., Saegusa M. Transcriptional upregulation of HNF-1beta by NF-kappaB in ovarian clear cell carcinoma modulates susceptibility to apoptosis through alteration in bcl-2 expression. Lab. Invest. 2015;95:962–972. doi: 10.1038/labinvest.2015.73. [DOI] [PubMed] [Google Scholar]
- 42.Tsuchiya A., Sakamoto M., Yasuda J., Chuma M., Ohta T., Ohki M., Yasugi T., Taketani Y., Hirohashi S. Expression profiling in ovarian clear cell carcinoma: Identification of hepatocyte nuclear factor-1 beta as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. Am. J. Pathol. 2003;163:2503–2512. doi: 10.1016/S0002-9440(10)63605-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchner A., Castro M., Hennig A., Popp T., Assmann G., Stief C.G., Zimmermann W. Downregulation of HNF-1B in renal cell carcinoma is associated with tumor progression and poor prognosis. Urology. 2010;76:507 e6-11. doi: 10.1016/j.urology.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 44.Köbel M., Kalloger S.E., Carrick J., Huntsman D., Asad H., Oliva E., Ewanowich C.A., Soslow R.A., Gilks C.B. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am. J. Surg. Pathol. 2009;33:14–21. doi: 10.1097/PAS.0b013e3181788546. [DOI] [PubMed] [Google Scholar]
- 45.Amano Y., Mandai M., Yamaguchi K., Matsumura N., Kharma B., Baba T., Abiko K., Hamanishi J., Yoshioka Y., Konishi I. Metabolic alterations caused by HNF1beta expression in ovarian clear cell carcinoma contribute to cell survival. Oncotarget. 2015;6:26002–26017. doi: 10.18632/oncotarget.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuff J., Salari K., Clarke N., Esheba G.E., Forster A.D., Huang S., West R.B., Higgins J.P., Longacre T.A., Pollack J.R. Integrative bioinformatics links HNF1B with clear cell carcinoma and tumor-associated thrombosis. PLoS ONE. 2013;8:e74562. doi: 10.1371/journal.pone.0074562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Němejcová K., Tichá I., Kleiblová P., Bártů M., Cibula D., Jirsová K., Dundr P. Expression, Epigenetic and Genetic Changes of HNF1B in Endometrial Lesions. Pathol. Oncol. Res. 2016;22:523–530. doi: 10.1007/s12253-015-0037-2. [DOI] [PubMed] [Google Scholar]
- 48.Lebrun G., Vasiliu V., Bellanné-Chantelot C., Bensman A., Ulinski T., Chrétien Y., Grünfeld J.P. Cystic kidney disease, chromophobe renal cell carcinoma and TCF2 (HNF1 beta) mutations. Nat. Clin. Pract. Nephrol. 2005;1:115–119. doi: 10.1038/ncpneph0054. [DOI] [PubMed] [Google Scholar]
- 49.Bártů M., Hojný J., Hájková N., Michálková R., Krkavcová E., Hadravský L., Kleissnerová L., Bui Q.H., Stružinská I., Němejcová K., et al. Analysis of expression, epigenetic, and genetic changes of HNF1B in 130 kidney tumours. Sci. Rep. 2020;10:17151. doi: 10.1038/s41598-020-74059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandra S., Srinivasan S., Batra J. Hepatocyte nuclear factor 1 beta: A perspective in cancer. Cancer Med. 2021;10:1791–1804. doi: 10.1002/cam4.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.An J., Park C.K., Kim M., Joo J.W., Cho N.H. HNF-1beta as an immunohistochemical marker for distinguishing chromophobe renal cell carcinoma and hybrid oncocytic tumors from renal oncocytoma. Virchows Arch. 2021;478:459–470. doi: 10.1007/s00428-020-02912-7. [DOI] [PubMed] [Google Scholar]
- 52.Kato N., Motoyama T. Hepatocyte nuclear factor-1beta(HNF-1beta) in human urogenital organs: Its expression and role in embryogenesis and tumorigenesis. Histol. Histopathol. 2009;24:1479–1486. doi: 10.14670/HH-24.1479. [DOI] [PubMed] [Google Scholar]
- 53.Wang C.C., Mao T.L., Yang W.C., Jeng Y.M. Underexpression of hepatocyte nuclear factor-1beta in chromophobe renal cell carcinoma. Histopathology. 2013;62:589–594. doi: 10.1111/his.12026. [DOI] [PubMed] [Google Scholar]
- 54.Conner J.R., Hirsch M.S., Jo V.Y. HNF1beta and S100A1 are useful biomarkers for distinguishing renal oncocytoma and chromophobe renal cell carcinoma in FNA and core needle biopsies. Cancer Cytopathol. 2015;123:298–305. doi: 10.1002/cncy.21530. [DOI] [PubMed] [Google Scholar]
- 55.Sun M., Tong P., Kong W., Dong B., Huang Y., Park I.Y., Zhou L., Liu X.D., Ding Z., Zhang X., et al. HNF1B Loss Exacerbates the Development of Chromophobe Renal Cell Carcinomas. Cancer Res. 2017;77:5313–5326. doi: 10.1158/0008-5472.CAN-17-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y., Kanyomse Q., Xie Y. Tumor-suppressive activity of Hnf1beta in Wilms’ tumor. Biosci. Biotechnol. Biochem. 2019;83:2008–2015. doi: 10.1080/09168451.2019.1611409. [DOI] [PubMed] [Google Scholar]
- 57.Bártů M., Dundr P., Němejcová K., Tichá I., Hojný H., Hájková N. The Role of HNF1B in Tumorigenesis of Solid Tumours: A Review of Current Knowledge. Folia Biol. 2018;64:71–83. doi: 10.14712/fb2018064030071. [DOI] [PubMed] [Google Scholar]
- 58.Szponar A., Yusenko M.V., Kuiper R., van Kessel A.G., Kovacs G. Genomic profiling of papillary renal cell tumours identifies small regions of DNA alterations: A possible role of HNF1B in tumour development. Histopathology. 2011;58:934–943. doi: 10.1111/j.1365-2559.2011.03795.x. [DOI] [PubMed] [Google Scholar]
- 59.Banyai D., Sarlos D.P., Nagy A., Kovacs G. Recalling Cohnheim’s Theory: Papillary Renal Cell Tumor as a Model of Tumorigenesis from Impaired Embryonal Differentiation to Malignant Tumors in Adults. Int. J. Biol. Sci. 2018;14:784–790. doi: 10.7150/ijbs.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kotalova R., Dusatkova P., Cinek O., Dusatkova L., Dedic T., Seeman T., Lebl J., Pruhova S. Hepatic phenotypes of HNF1B gene mutations: A case of neonatal cholestasis requiring portoenterostomy and literature review. World J. Gastroenterol. 2015;21:2550–2557. doi: 10.3748/wjg.v21.i8.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raile K., Klopocki E., Holder M., Wessel T., Galler A., Deiss D., Muller D., Riebel T., Horn D., Maringa M., et al. Expanded clinical spectrum in hepatocyte nuclear factor 1b-maturity-onset diabetes of the young. J. Clin. Endocrinol. Metab. 2009;94:2658–2664. doi: 10.1210/jc.2008-2189. [DOI] [PubMed] [Google Scholar]
- 62.Beckers D., Bellanne-Chantelot C., Maes M. Neonatal cholestatic jaundice as the first symptom of a mutation in the hepatocyte nuclear factor-1beta gene (HNF-1beta) J. Pediatr. 2007;150:313–314. doi: 10.1016/j.jpeds.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Kitanaka S., Miki Y., Hayashi Y., Igarashi T. Promoter-specific repression of hepatocyte nuclear factor (HNF)-1 beta and HNF-1 alpha transcriptional activity by an HNF-1 beta missense mutant associated with Type 5 maturity-onset diabetes of the young with hepatic and biliary manifestations. J. Clin. Endocrinol. Metab. 2004;89:1369–1378. doi: 10.1210/jc.2003-031308. [DOI] [PubMed] [Google Scholar]
- 64.de Leusse C., De Paula A.M., Aschero A., Parache C., Hery G., Cailliez M., Missirian C., Fabre A. Hepatocarcinoma and Cholestasis Associated to Germline Hemizygous Deletion of Gene HNF1B. J. Pediatr. Gastroenterol. Nutr. 2019;68:e85. doi: 10.1097/MPG.0000000000002015. [DOI] [PubMed] [Google Scholar]
- 65.Pinon M., Carboni M., Colavito D., Cisarò F., Peruzzi L., Pizzol A., Calosso G., David E., Calvo P.L. Not only Alagille syndrome. Syndromic paucity of interlobular bile ducts secondary to HNF1beta deficiency: A case report and literature review. Ital. J. Pediatr. 2019;45:27. doi: 10.1186/s13052-019-0617-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandato C., Zollo G., Vajro P. Cholestatic jaundice in infancy: Struggling with many old and new phenotypes. Ital. J. Pediatr. 2019;45:83. doi: 10.1186/s13052-019-0679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ünlüsoy Aksu A., Das S.K., Nelson-Williams C., Jain D., Özbay Hoşnut F., Evirgen Şahin G., Lifton R.P., Vilarinho S. Recessive Mutations in KIF12 Cause High Gamma-Glutamyltransferase Cholestasis. Hepatol. Commun. 2019;3:471–477. doi: 10.1002/hep4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maddirevula S., Alhebbi H., Alqahtani A., Algoufi T., Alsaif H.S., Ibrahim N., Abdulwahab F., Barr M., Alzaidan H., Almehaideb A., et al. Identification of novel loci for pediatric cholestatic liver disease defined by KIF12, PPM1F, USP53, LSR, and WDR83OS pathogenic variants. Genet. Med. 2019;21:1164–1172. doi: 10.1038/s41436-018-0288-x. [DOI] [PubMed] [Google Scholar]
- 69.Stalke A., Sgodda M., Cantz T., Skawran B., Lainka E., Hartleben B., Baumann U., Pfister E.D. KIF12 Variants and Disturbed Hepatocyte Polarity in Children with a Phenotypic Spectrum of Cholestatic Liver Disease. J. Pediatr. 2022;240:284–291 e9. doi: 10.1016/j.jpeds.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 70.Gong Y., Ma Z., Patel V., Fischer E., Hiesberger T., Pontoglio M., Igarashi P. HNF-1beta regulates transcription of the PKD modifier gene Kif12. J. Am. Soc. Nephrol. 2009;20:41–47. doi: 10.1681/ASN.2008020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mrug M., Li R., Cui X., Schoeb T.R., Churchill G.A., Guay-Woodford L.M. Kinesin family member 12 is a candidate polycystic kidney disease modifier in the cpk mouse. J. Am. Soc. Nephrol. 2005;16:905–916. doi: 10.1681/ASN.2004121083. [DOI] [PubMed] [Google Scholar]
- 72.Kamath B.M., Stein P., Houwen R.H.J., Verkade H.J. Potential of ileal bile acid transporter inhibition as a therapeutic target in Alagille syndrome and progressive familial intrahepatic cholestasis. Liver Int. 2020;40:1812–1822. doi: 10.1111/liv.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karpen S.J., Kelly D., Mack C., Stein P. Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders. Hepatol. Int. 2020;14:677–689. doi: 10.1007/s12072-020-10070-w. [DOI] [PubMed] [Google Scholar]
- 74.Gonzales E., Hardikar W., Stormon M., Baker A., Hierro L., Gliwicz D., Lacaille F., Lachaux A., Sturm E., Setchell K.D., et al. Efficacy and safety of maralixibat treatment in patients with Alagille syndrome and cholestatic pruritus (ICONIC): A randomised phase 2 study. Lancet. 2021;398:1581–1592. doi: 10.1016/S0140-6736(21)01256-3. [DOI] [PubMed] [Google Scholar]
- 75.Fabris L., Fiorotto R., Spirli C., Cadamuro M., Mariotti V., Perugorria M.J., Banales J.M., Strazzabosco M. Pathobiology of inherited biliary diseases: A roadmap to understand acquired liver diseases. Nat. Rev. Gastroenterol. Hepatol. 2019;16:497–511. doi: 10.1038/s41575-019-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coffinier C., Gresh L., Fiette L., Tronche F., Schutz G., Babinet C., Pontoglio M., Yaniv M., Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829–1838. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- 77.Yamasaki H., Sada A., Iwata T., Niwa T., Tomizawa M., Xanthopoulos K.G., Koike T., Shiojiri N. Suppression of C/EBPalpha expression in periportal hepatoblasts may stimulate biliary cell differentiation through increased Hnf6 and Hnf1b expression. Development. 2006;133:4233–4243. doi: 10.1242/dev.02591. [DOI] [PubMed] [Google Scholar]
- 78.Clotman F., Libbrecht L., Gresh L., Yaniv M., Roskams T., Rousseau G.G., Lemaigre F.P. Hepatic artery malformations associated with a primary defect in intrahepatic bile duct development. J. Hepatol. 2003;39:686–692. doi: 10.1016/S0168-8278(03)00409-4. [DOI] [PubMed] [Google Scholar]
- 79.Raynaud P., Tate J., Callens C., Cordi S., Vandersmissen P., Carpentier R., Sempoux C., Devuyst O., Pierreux C.E., Courtoy P., et al. A classification of ductal plate malformations based on distinct pathogenic mechanisms of biliary dysmorphogenesis. Hepatology. 2011;53:1959–1966. doi: 10.1002/hep.24292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanimizu N., Miyajima A., Mostov K.E. Liver progenitor cells fold up a cell monolayer into a double-layered structure during tubular morphogenesis. Mol. Biol. Cell. 2009;20:2486–2494. doi: 10.1091/mbc.e08-02-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanimizu N., Miyajima A., Mostov K.E. Liver progenitor cells develop cholangiocyte-type epithelial polarity in three-dimensional culture. Mol. Biol. Cell. 2007;18:1472–1479. doi: 10.1091/mbc.e06-09-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raynaud P., Carpentier R., Antoniou A., Lemaigre F.P. Biliary differentiation and bile duct morphogenesis in development and disease. Int. J. Biochem. Cell Biol. 2011;43:245–256. doi: 10.1016/j.biocel.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 83.Gunay-Aygun M. Liver and kidney disease in ciliopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2009;151:296–306. doi: 10.1002/ajmg.c.30225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kettunen J.L., Parviainen H., Miettinen P.J., Färkkilä M., Tamminen M., Salonen P., Lantto E., Tuomi T. Biliary Anomalies in Patients With HNF1B Diabetes. J. Clin. Endocrinol. Metab. 2017;102:2075–2082. doi: 10.1210/jc.2017-00061. [DOI] [PubMed] [Google Scholar]
- 85.Gonc E.N., Ozturk B.B., Haldorsen I.S., Molnes J., Immervoll H., Ræder H., Molven A., Søvik O., Njølstad P.R. HNF1B mutation in a Turkish child with renal and exocrine pancreas insufficiency, diabetes and liver disease. Pediatr. Diabetes. 2012;13:e1–e5. doi: 10.1111/j.1399-5448.2011.00773.x. [DOI] [PubMed] [Google Scholar]
- 86.Francis Y., Tiercelin C., Alexandre-Heyman L., Larger E., Dubois-Laforgue D. HNF1B-MODY Masquerading as Type 1 Diabetes: A Pitfall in the Etiological Diagnosis of Diabetes. J. Endocr. Soc. 2022;6:bvac087. doi: 10.1210/jendso/bvac087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ayoub M.D., Kamath B.M. Alagille Syndrome: Diagnostic Challenges and Advances in Management. Diagnostics. 2020;10:907. doi: 10.3390/diagnostics10110907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ibrahim S.H., Kamath B.M., Loomes K.M., Karpen S.J. Cholestatic liver diseases of genetic etiology: Advances and controversies. Hepatology. 2022;75:1627–1646. doi: 10.1002/hep.32437. [DOI] [PubMed] [Google Scholar]
- 89.Li J.Q., Lu Y., Qiu Y.L., Wang J.S. Neonatal sclerosing cholangitis caused by DCDC2 variations in two siblings and literature review. Zhonghua Er Ke Za Zhi. 2018;56:623–627. doi: 10.3760/cma.j.issn.0578-1310.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 90.Grammatikopoulos T., Sambrotta M., Strautnieks S., Foskett P., Knisely A.S., Wagner B., Deheragoda M., Starling C., Mieli-Vergani G., Smith J., et al. Mutations in DCDC2 (doublecortin domain containing protein 2) in neonatal sclerosing cholangitis. J. Hepatol. 2016;65:1179–1187. doi: 10.1016/j.jhep.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Girard M., Bizet A.A., Lachaux A., Gonzales E., Filhol E., Collardeau-Frachon S., Jeanpierre C., Henry C., Fabre M., Viremouneix L., et al. DCDC2 Mutations Cause Neonatal Sclerosing Cholangitis. Hum. Mutat. 2016;37:1025–1029. doi: 10.1002/humu.23031. [DOI] [PubMed] [Google Scholar]
- 92.Khanna R., Verma S.K. Pediatric hepatocellular carcinoma. World J. Gastroenterol. 2018;24:3980–3999. doi: 10.3748/wjg.v24.i35.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atra A., Al-Asiri R., Wali S., Al-Husseini H., Al-Bassas A., Zimmermann A. Hepatocellular carcinoma, syncytial giant cell: A novel variant in children: A case report. Ann. Diagn. Pathol. 2007;11:61–63. doi: 10.1016/j.anndiagpath.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 94.Pinon M., Gambella A., Giugliano L., Chiadò C., Kalantari S., Bracciamà V., Deaglio S., Tinti D., Peruzzi L., Cotti R., et al. New case of syncytial giant-cell variant of hepatocellular carcinoma in a pediatric patient with HNF1B deficiency: Does it fit with the syndrome? BMJ Open Gastroenterol. 2022;9:e001013. doi: 10.1136/bmjgast-2022-001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gambella A., Mastracci L., Caporalini C., Francalanci P., Mescoli C., Ferro J., Alaggio R., Grillo F. Not only a small liver—The pathologist’s perspective in the pediatric liver transplant setting. Pathologica. 2022;114:89–103. doi: 10.32074/1591-951X-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kakos C.D., Ziogas I.A., Demiri C.D., Esagian S.M., Economopoulos K.P., Moris D., Tsoulfas G., Alexopoulos S.P. Liver Transplantation for Pediatric Hepatocellular Carcinoma: A Systematic Review. Cancers. 2022;14:1294. doi: 10.3390/cancers14051294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Varol F.I. Pediatric Hepatocellular Carcinoma. J. Gastrointest. Cancer. 2020;51:1169–1175. doi: 10.1007/s12029-020-00494-w. [DOI] [PubMed] [Google Scholar]
- 98.Torbenson M., Zen Y., Yeh M.M. Tumors of the Liver: American Registry of Pathology. American Registry of Pathology; Rockville, MD, USA: 2018. [Google Scholar]
- 99.Colclough K., Ellard S., Hattersley A., Patel K. Syndromic Monogenic Diabetes Genes Should Be Tested in Patients with a Clinical Suspicion of Maturity-Onset Diabetes of the Young. Diabetes. 2022;71:530–537. doi: 10.2337/db21-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saint-Martin C., Bouvet D., Bastide M., Bellanne-Chantelot C. Gene Panel Sequencing of Patients with Monogenic Diabetes Brings to Light Genes Typically Associated with Syndromic Presentations. Diabetes. 2022;71:578–584. doi: 10.2337/db21-0520. [DOI] [PubMed] [Google Scholar]
- 101.Bingham C., Hattersley A.T. Renal cysts and diabetes syndrome resulting from mutations in hepatocyte nuclear factor-1beta. Nephrol. Dial. Transplant. 2004;19:2703–2708. doi: 10.1093/ndt/gfh348. [DOI] [PubMed] [Google Scholar]
- 102.Adalat S., Bockenhauer D., Ledermann S.E., Hennekam R.C., Woolf A.S. Renal malformations associated with mutations of developmental genes: Messages from the clinic. Pediatr. Nephrol. 2010;25:2247–2255. doi: 10.1007/s00467-010-1578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tudorache E., Sellier-Leclerc A.L., Lenoir M., Toubiana N., Bensman A., Bellanne-Chantelot C., Ulinski T. Childhood onset diabetes posttransplant in a girl with TCF2 mutation. Pediatr. Diabetes. 2012;13:e35–e39. doi: 10.1111/j.1399-5448.2011.00842.x. [DOI] [PubMed] [Google Scholar]
- 104.Zuber J., Bellanné-Chantelot C., Carette C., Canaud G., Gobrecht S., Gaha K., Mallet V., Martinez F., Thervet E., Timsit J., et al. HNF1B-related diabetes triggered by renal transplantation. Nat Rev Nephrol. 2009;5:480–484. doi: 10.1038/nrneph.2009.98. [DOI] [PubMed] [Google Scholar]
- 105.Lopes A.M., Teixeira S. New-onset diabetes after kidney transplantation revealing HNF1B-associated disease. Endocrinol. Diabetes Metab. Case Rep. 2021;2021 doi: 10.1530/EDM-20-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Faguer S., Esposito L., Casemayou A., Pirson Y., Decramer S., Cartery C., Hazzan M., Garrigue V., Roussey G., Cointault O., et al. Calcineurin Inhibitors Downregulate HNF-1beta and May Affect the Outcome of HNF1B Patients After Renal Transplantation. Transplantation. 2016;100:1970–1978. doi: 10.1097/TP.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 107.Pearson E.R., Badman M.K., Lockwood C.R., Clark P.M., Ellard S., Bingham C., Hattersley A.T. Contrasting diabetes phenotypes associated with hepatocyte nuclear factor-1alpha and -1beta mutations. Diabetes Care. 2004;27:1102–1107. doi: 10.2337/diacare.27.5.1102. [DOI] [PubMed] [Google Scholar]
- 108.Hattersley A.T., Greeley S.A., Polak M., Rubio-Cabezas O., Njølstad P.R., Mlynarski W., Castano L., Carlsson A., Raile K., Chi D.V., et al. ISPAD Clinical Practice Consensus Guidelines 2018: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr. Diabetes. 2018;19((Suppl. 27)):47–63. doi: 10.1111/pedi.12772. [DOI] [PubMed] [Google Scholar]
- 109.Carrillo E., Lomas A., Pines P.J., Lamas C. Long-lasting response to oral therapy in a young male with monogenic diabetes as part of HNF1B-related disease. Endocrinol. Diabetes Metab. Case Rep. 2017;2017 doi: 10.1530/EDM-17-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haumaitre C., Barbacci E., Jenny M., Ott M.O., Gradwohl G., Cereghini S. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc. Natl. Acad. Sci. USA. 2005;102:1490–1495. doi: 10.1073/pnas.0405776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maestro M.A., Boj S.F., Luco R.F., Pierreux C.E., Cabedo J., Servitja J.M., German M.S., Rousseau G.G., Lemaigre F.P., Ferrer J. Hnf6 and Tcf2 (MODY5) are linked in a gene network operating in a precursor cell domain of the embryonic pancreas. Hum. Mol. Genet. 2003;12:3307–3314. doi: 10.1093/hmg/ddg355. [DOI] [PubMed] [Google Scholar]
- 112.Haumaitre C., Fabre M., Cormier S., Baumann C., Delezoide A.L., Cereghini S. Severe pancreas hypoplasia and multicystic renal dysplasia in two human fetuses carrying novel HNF1beta/MODY5 mutations. Hum. Mol. Genet. 2006;15:2363–2375. doi: 10.1093/hmg/ddl161. [DOI] [PubMed] [Google Scholar]
- 113.Haldorsen I.S., Vesterhus M., Raeder H., Jensen D.K., Søvik O., Molven A., Njølstad P.R. Lack of pancreatic body and tail in HNF1B mutation carriers. Diabet. Med. 2008;25:782–787. doi: 10.1111/j.1464-5491.2008.02460.x. [DOI] [PubMed] [Google Scholar]
- 114.Vesterhus M., Raeder H., Johansson S., Molven A., Njolstad P.R. Pancreatic exocrine dysfunction in maturity-onset diabetes of the young type 3. Diabetes Care. 2008;31:306–310. doi: 10.2337/dc07-1002. [DOI] [PubMed] [Google Scholar]
- 115.Edghill E.L., Oram R.A., Owens M., Stals K.L., Harries L.W., Hattersley A.T., Ellard S., Bingham C. Hepatocyte nuclear factor-1beta gene deletions--a common cause of renal disease. Nephrol. Dial. Transplant. 2008;23:627–635. doi: 10.1093/ndt/gfm603. [DOI] [PubMed] [Google Scholar]
- 116.Edghill E.L., Bingham C., Slingerland A.S., Minton J.A.L., Noordam C., Ellard S., Hattersley A.T. Hepatocyte nuclear factor-1 beta mutations cause neonatal diabetes and intrauterine growth retardation: Support for a critical role of HNF-1beta in human pancreatic development. Diabet. Med. 2006;23:1301–1306. doi: 10.1111/j.1464-5491.2006.01999.x. [DOI] [PubMed] [Google Scholar]
- 117.Janky R.S., Binda M.M., Allemeersch J., Govaere O., Swinnen J.V., Roskams T., Aerts S., Topal B. Prognostic relevance of molecular subtypes and master regulators in pancreatic ductal adenocarcinoma. BMC Cancer. 2016;16:632. doi: 10.1186/s12885-016-2540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kondratyeva L.G., Chernov I.P., Zinovyeva M.V., Kopantzev E.P., Sverdlov E.D. Expression of master regulatory genes of embryonic development in pancreatic tumors. Dokl. Biochem. Biophys. 2017;475:250–252. doi: 10.1134/S1607672917040020. [DOI] [PubMed] [Google Scholar]
- 120.Kato H., Tateishi K., Fujiwara H., Nakatsuka T., Yamamoto K., Kudo Y., Hayakawa Y., Nakagawa H., Tanaka Y., Ijichi H., et al. MNX1-HNF1B Axis Is Indispensable for Intraductal Papillary Mucinous Neoplasm Lineages. Gastroenterology. 2022;162:1272–1287 e16. doi: 10.1053/j.gastro.2021.12.254. [DOI] [PubMed] [Google Scholar]
- 121.Roy N., Malik S., Villanueva K.E., Urano A., Lu X., Von Figura G., Seeley E.S., Dawson D.W., Collisson E.A., Hebrok M. Brg1 promotes both tumor-suppressive and oncogenic activities at distinct stages of pancreatic cancer formation. Genes Dev. 2015;29:658–671. doi: 10.1101/gad.256628.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Elliott K.S., Zeggini E., McCarthy M.I., Gudmundsson J., Sulem P., Stacey S.N., Thorlacius S., Amundadottir L., Grönberg H., Xu J., et al. Evaluation of association of HNF1B variants with diverse cancers: Collaborative analysis of data from 19 genome-wide association studies. PLoS ONE. 2010;5:e10858. doi: 10.1371/journal.pone.0010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomson E., Tran M., Robevska G., Ayers K., van der Bergen J., Gopalakrishnan Bhaskaran P., Haan E., Cereghini S., Vash-Margita A., Margetts M., et al. Functional genomics analysis identifies loss of HNF1B function as a cause of Mayer-Rokitansky-Kuster-Hauser syndrome. Hum. Mol. Genet. 2022 doi: 10.1093/hmg/ddac262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lindner T.H., Njolstad P.R., Horikawa Y., Bostad L., Bell G.I., Sovik O. A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum. Mol. Genet. 1999;8:2001–2008. doi: 10.1093/hmg/8.11.2001. [DOI] [PubMed] [Google Scholar]
- 125.Oram R.A., Edghill E.L., Blackman J., Taylor M.J., Kay T., Flanagan S.E., Ismail-Pratt I., Creighton S.M., Ellard S., Hattersley A.T., et al. Mutations in the hepatocyte nuclear factor-1beta (HNF1B) gene are common with combined uterine and renal malformations but are not found with isolated uterine malformations. Am. J. Obstet. Gynecol. 2010;203:364 e1-5. doi: 10.1016/j.ajog.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 126.Haeri S., Devers P.L., Kaiser-Rogers K.A., Moylan V.J., Torchia B.S., Horton A.L., Wolfe H.M., Aylsworth A.S. Deletion of hepatocyte nuclear factor-1-beta in an infant with prune belly syndrome. Am. J. Perinatol. 2010;27:559–563. doi: 10.1055/s-0030-1248943. [DOI] [PubMed] [Google Scholar]
- 127.Moreno-De-Luca D., Mulle J.G., Kaminsky E.B., Sanders S.J., Myers S.M., Adam M.P., Pakula A.T., Eisenhauer N.J., Uhas K., Weik L., et al. Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am. J. Hum. Genet. 2010;87:618–630. doi: 10.1016/j.ajhg.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Loirat C., Bellanne-Chantelot C., Husson I., Deschenes G., Guigonis V., Chabane N. Autism in three patients with cystic or hyperechogenic kidneys and chromosome 17q12 deletion. Nephrol. Dial. Transplant. 2010;25:3430–3433. doi: 10.1093/ndt/gfq380. [DOI] [PubMed] [Google Scholar]
- 129.Nagamani S.C.S., Erez A., Shen J., Li C., Roeder E., Cox S., Karaviti L., Pearson M., Kang S.H.L., Sahoo T., et al. Clinical spectrum associated with recurrent genomic rearrangements in chromosome 17q12. Eur. J. Hum. Genet. 2010;18:278–284. doi: 10.1038/ejhg.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mefford H.C., Clauin S., Sharp A.J., Moller R.S., Ullmann R., Kapur R., Pinkel D., Cooper G.M., Ventura M., Ropers H.H., et al. Recurrent reciprocal genomic rearrangements of 17q12 are associated with renal disease, diabetes, and epilepsy. Am. J. Hum. Genet. 2007;81:1057–1069. doi: 10.1086/522591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Quintero-Rivera F., Woo J.S., Bomberg E.M., Wallace W.D., Peredo J., Dipple K.M. Duodenal atresia in 17q12 microdeletion including HNF1B: A new associated malformation in this syndrome. Am. J. Med. Genet. A. 2014;164:3076–3082. doi: 10.1002/ajmg.a.36767. [DOI] [PubMed] [Google Scholar]
- 132.Murray P.J., Thomas K., Mulgrew C.J., Ellard S., Edghill E.L., Bingham C. Whole gene deletion of the hepatocyte nuclear factor-1beta gene in a patient with the prune-belly syndrome. Nephrol. Dial. Transplant. 2008;23:2412–2415. doi: 10.1093/ndt/gfn169. [DOI] [PubMed] [Google Scholar]
- 133.Hendrix N.W., Clemens M., Canavan T.P., Surti U., Rajkovic A. Prenatally diagnosed 17q12 microdeletion syndrome with a novel association with congenital diaphragmatic hernia. Fetal Diagn. Ther. 2012;31:129–133. doi: 10.1159/000332968. [DOI] [PubMed] [Google Scholar]
- 134.Hinkes B., Hilgers K.F., Bolz H.J., Goppelt-Struebe M., Amann K., Nagl S., Bergmann C., Rascher W., Eckardt K.U., Jacobi J. A complex microdeletion 17q12 phenotype in a patient with recurrent de novo membranous nephropathy. BMC Nephrol. 2012;13:27. doi: 10.1186/1471-2369-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Faguer S., Chassaing N., Bandin F., Prouheze C., Garnier A., Casemayou A., Huart A., Schanstra J.P., Calvas P., Decramer S., et al. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86:1007–1015. doi: 10.1038/ki.2014.202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.