Abstract
Simple Summary
To date, several systematic reviews and meta-analyses have explored associations between commonly used drugs and gastric cancer (GC) risk, with inconsistent conclusions on relationships and methodological quality. No attempts have been made to quantify the credibility of these findings. Hence, it is necessary to compare the results of individual reviews by looking into existing systematic reviews and meta-analyses, providing an overview of the findings of a particular association. This is the first umbrella review to evaluate the validity and credibility of evidence from previously published systematic reviews and meta-analyses on observational studies and to identify associations between commonly used drugs and GC risk and its subtypes.
Abstract
Recently, attention has been paid to some medications and gastric cancer (GC) risk. This review aimed to evaluate associations between commonly used drugs and GC risk and to grade evidence from published systematic reviews and meta-analyses. This umbrella review was registered in PROSPERO (CRD42022320276). The systematic reviews and meta-analyses of observational studies were retrieved by searching Embase, PubMed, and Web of Science. The evidence strength of commonly used drugs and GC risk was categorized into four grades: weak, suggestive, highly suggestive, and strong. Of 19 associations between commonly used drugs and GC risk and its subtypes, none was supported by convincing or highly suggestive evidence. The risk of GC related to non-steroidal anti-inflammatory drugs (NSAIDs), non-aspirin NSAIDs, and acid-suppressive drugs, as well as the risk of non-cardia GC related to NSAIDs and aspirin, was supported by suggestive evidence. The results showed that a reduced GC risk was associated with two drug types (NSAIDs and non-aspirin NSAIDs), and an increased GC risk was associated with acid-suppressing drugs at the suggestive evidence level. Moreover, NSAIDs and aspirin reduced non-cardia GC risk as supported by suggestive evidence. However, the evidence supporting statins or metformin in reducing GC risk was weak, and thus future studies are required to clarify these associations.
Keywords: drug, gastric cancer, risk, umbrella review
1. Introduction
Globally, it was estimated that over one million new incidences of gastric cancer (GC) and approximately 769,000 deaths were reported in 2020, and GC ranked as the fourth-highest mortality rate and the fifth-highest incidence [1]. According to the International Agency for Research on Cancer Incidence, Eastern Europe and Eastern Asia (China and Japan) have the highest rates. According to 2030 predictions, the GC incidence rate will decrease in most countries [2], however, GC is still a major global cancer burden at present [3]. With improved screening programs and advances in both surgical and endoscopic techniques, the 5-year survival rate for early GC tends to reach >90% [4]. Current treatment strategies include surgery, chemoradiotherapy, molecular-targeted therapy, and immunotherapy [5,6]. Unfortunately, GC is usually diagnosed at advanced stages, often with a poor prognosis. Similarly, the prognosis for patients with recurrent or metastatic disease is poor, with a median survival of eight months [7]. Thus, preventative measures are crucial for controlling disease incidence.
The eradication of Helicobacter pylori (H. pylori) is a pivotal therapeutic step against GC [8]. The established risk factors for GC, apart from H. pylori infection, include family history, salted food, alcohol, and smoking [9,10,11,12]. Recently, several studies reported that some commonly used drugs had potential links with GC risk, including cardiovascular medications, antidiabetics, and acid suppressants [13,14,15]. However, the preventative effects of medications such as statins, aspirin, metformin, and proton pump inhibitors (PPIs) against GC remain controversial. Additionally, several systematic reviews and meta-analyses investigated medication use and GC risk [16,17], but in fact, no attempts have been made to quantify the credibility of these findings. Given the uncertainty of observational research, this quantification of evidence is both timely and critical [18,19]. An umbrella review can be used to summarize evidence from numerous, same-topic meta-analyses, and rank the evidence of each association [20,21]; therefore, such an umbrella review was conducted to generate a better understanding of the evidence’s strength per association.
Overall, this review evaluated the validity and credibility of evidence from previously published systematic reviews and meta-analyses on observational studies and identified associations between commonly used drugs and GC risk.
2. Materials and Methods
This umbrella review is registered at PROSPERO (CRD42022320276). It was conducted following guidelines from the Preferred Reporting Items of Systematic Reviews and Meta-Analyses [22].
2.1. Search Strategy and Selection Criteria
Web of Science, Embase, and PubMed (last updated on 14 January 2022) were searched to retrieve all systematic reviews and meta-analyses of observational studies focusing on associations between commonly used drugs and GC risk (Table S1). All references in identified studies were manually searched to identify eligible articles.
Two investigators (X.B. and S.-Q.D.) independently screened titles and abstracts, and checked full texts. All disagreements were figured out by three authors (X.B., S.-Q.D. and D.-Q.D.) through discussion. Eligibility criteria included: (1) systematic reviews and meta-analyses of observational studies measuring associations between commonly used drugs and GC incidence in any population; (2) results from subgroup analyses stratified by drug type; (3) studies focusing on GC subtypes.
Exclusion criteria included: (1) articles that did not examine the use of any commonly drugs; (2) articles that did not assess outcomes of interest (including but not limited to progression-free survival, disease-free survival, overall survival, and mortality of GC); (3) articles that described some unconventional drugs (such as chemotherapeutic drug, targeted drug, and traditional Chinese medicine); (4) meta-analyses including less than three original studies. In addition, non-English articles, animal studies, and genetic studies were excluded as well. If the same association was examined by two or more meta-analyses, associations in different meta-analyses were considered to be duplicate associations; therefore, the meta-analysis with the largest sample size was selected to avoid overlap, as previously indicated [23,24,25].
2.2. Data Extraction
Two authors (X.B. and S.-Q.D.) carried out extraction independently, and three authors (X.B., S.-Q.D. and D.-Q.D.) resolved discrepancies by consensus. The first author’s name, year of publication, country, drug type, outcome (GC or its subtypes), comparison, total participants, number of GC cases, and number of included studies were extracted from each eligible meta-analysis. The first author’s name, year of publication, number of subjects and cases, as well as maximally adjusted effect size, including corresponding 95% confidence interval (CI), hazard ratio, odds ratio, and relative risk, were recorded from each primary study included in the meta-analysis for further analysis.
2.3. Quality Assessment
A Measurement Tool to Assess Systematic Reviews (AMSTAR) version 2.0, which contained 16 items, was used by two researchers (X.B. and S.-Q.D.) to evaluate the study quality [26]. Disagreements were resolved by discussion. All 16 items used to conduct systematic reviews and meta-analyses were important, but seven were critical for reviewing validity and conclusions—also known as critical domains. On this basis, AMSTAR-2 was used to define the quality of a systematic review, being critically low (more than one critical defect with or without non-critical weaknesses), low (one critical defect with or without non-critical weaknesses), moderate (more than one non-critical weakness), or high (no or one non-critical weakness).
2.4. Statistical Analysis
First, the effect size of each study included in each meta-analysis was extracted, and a random-effect model was used to assess the effect size with 95% CI for each association. The corresponding p-values for summary effects were calculated at the same time [27]. Heterogeneity was evaluated by I2 statistics; an I2 > 50% value indicated significant heterogeneity and an I2 > 75% indicated high heterogeneity [28,29]. To be more conservative, a 95% prediction interval (PI) was calculated to analyze inter-study heterogeneity and to assess the uncertainty of summary effect sizes in the random-effect model. The expected effect size range of future studies was also predicted by 95% PI [30].
Then, Egger’s regression asymmetry tests were used to identify small-study effects, reflecting heterogeneity, genuine opportunity, or other reasons for differences between small and large studies [31,32,33]. A small-study effect bias was indicated by p-value for Egger’s test < 0.10.
Excessive significance bias was applied after accounting for publication bias, selective reporting bias and other potential biases. Whether the expected number (E) with significant results was less than the observed number (O) with significant results was compared [31]. A non-central t distribution was used to calculate an E value from the sum of statistical power estimates for each component study. It was also assumed that the power estimate of each component study depended on the effect size of the largest study (i.e., the smallest standard error) for each association [31,34]. When O > E and the p-value for the excess significance test < 0.10, excess significance was considered positive. The STATA version 16.0 (Stata Corporation, College Station, TX, USA) was used for all analyses.
2.5. Assessment of Evidence Credibility
Evidence credibility was assessed using the established criteria from previous umbrella reviews [35,36,37,38,39]. Associations with significant summary effect sizes (p < 0.05) were rated as four classifications: class I: convincing; class II: highly suggestive; class III: suggestive; class IV: weak.
For class I: p < 10−6, number of cases > 1000, p < 0.05 of the largest study, 95% PI excluding the null value, I2 < 50%, p-value for Egger’s test > 0.10, p-value for excess significance test > 0.10.
For class II: p < 10−6, number of cases > 1000, p < 0.05 of the largest study.
For class III: p < 10−3 and number of cases > 1000.
For class IV: the summary results of p < 0.05.
The summary results of p > 0.05 were ranked as class V with no significance.
3. Results
3.1. Literature Searches
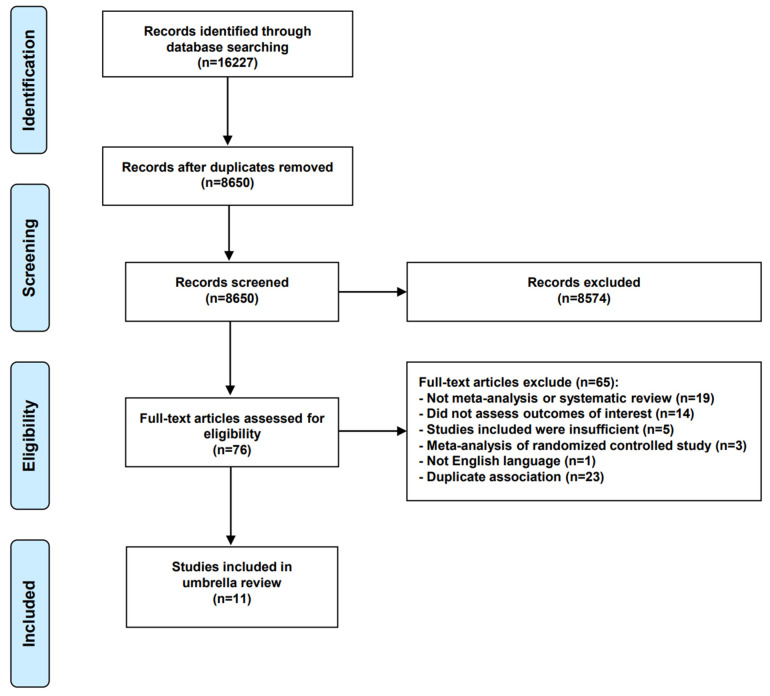
A total of 16,227 studies were screened, and 76 full-text articles were evaluated for eligibility after removing duplicates and screening titles and abstracts. Ultimately, 11 meta-analyses were included in this umbrella review [40,41,42,43,44,45,46,47,48,49,50]. The study selection flow diagram is shown (Figure 1) and the reasons for excluding 65 articles (85.5%) are provided (Table S2).
Figure 1.
Literature search flow diagram.
3.2. Characteristics of Included Articles
19 associations were described in 11 eligible meta-analyses, including 147 individual study estimates of GC risk related to exposure to commonly used drugs. The characteristics of these 11 studies and their distributions related to drug type are shown (Table 1 and Figure 2). The included meta-analyses that were focused on associations of GC risk and its subtypes with any statins, atorvastatin, simvastatin, pravastatin, metformin, aspirin, NSAIDs, non-aspirin NSAIDs, acid suppressing drugs, histamine 2-receptor antagonists (H2RAs), PPIs, as well as any bisphosphonates and alendronates. In addition, three meta-analyses [40,44,46] included six associations between aspirin, NSAIDs, and non-aspirin NSAIDs with the risk of GC subtypes. All articles were published between 2009 and 2021, of which seven (63.6%) were published in the last five years. There were 3 to 32 study estimates combined with each meta-analysis, with a median of seven studies. The number of GC cases and total participants in meta-analyses was 67,866 and 19,505,291, respectively. The minimum number of cases in the meta-analysis was 387, but all except two (pravastatin and alendronate) had more than 1000 cases.
Table 1.
Characteristics of the associations in the included systematic reviews and meta-analyses.
| First Author [Ref], Year | Country | Drug Type | Outcome | Comparison | No. of Included Studies | No. of Cases/Population |
|---|---|---|---|---|---|---|
| You [48], 2018 | China | Any statins | GC | Any users vs. never users | 8 | 3365/7,394,525 |
| Ma [45], 2014 | China | Atorvastatin | GC | Any users vs. never users | 3 | 1580/22,476 |
| China | Simvastatin | GC | Any users vs. never users | 3 | 1757/22,476 | |
| China | Pravastatin | GC | Any users vs. never users | 3 | 463/22,476 | |
| Wang [47], 2021 | China | Aspirin | GC | Regular users vs. never users | 10 | 14,933/2,378,794 |
| Huang [44], 2017 | China | NSAIDs | GC | Any users vs. never users | 32 | 9568/2,633,756 |
| China | NSAIDs | Non-cardia GC | Any users vs. never users | 8 | 2772/488,590 | |
| China | Aspirin | Non-cardia GC | Any users vs. never users | 7 | 2332/487,375 | |
| China | Non-aspirin NSAIDs | Non-cardia GC | Any users vs. never users | 5 | 1507/484,760 | |
| Abnet [40], 2009 | United States | Aspirin | Cardia GC | Any users vs. never users | 7 | 1467/318,431 |
| United States | Non-aspirin NSAIDs | Cardia GC | Any users vs. never users | 5 | 1303/316,734 | |
| Tian [46], 2010 | China | Non-aspirin NSAIDs | GC | Ever users vs. never users | 5 | 3145/328,258 |
| China | NSAIDs | Cardia GC | Ever users vs. never users | 5 | 1036/315,596 | |
| Zhang [50], 2021 | China | Metformin | GC | Ever users vs. never users | 7 | 2253/1,136,484 |
| Ahn [41], 2013 | South Korea | Acid suppressive drugs | GC | Any users vs. never users | 10 | 4628/49,363 |
| South Korea | H2RAs | GC | Any users vs. never users | 9 | 3409/41,432 | |
| Zeng [49], 2021 | China | PPIs | GC | Any users vs. never users | 9 | 7071/2,344,365 |
| Cai [42], 2017 | China | Any bisphosphonates | GC | Any users vs. never users | 8 | 4890/516,849 |
| Deng [43], 2018 | China | Alendronate | GC | Any users vs. never users | 3 | 387/202,551 |
Abbreviations: GC, gastric cancer; NSAIDs, non-steroidal anti-inflammatory drugs; H2RAs, Histamine 2-receptor antagonist; PPIs, proton pump inhibitors.
Figure 2.
Distribution of included articles related to drug type. Abbreviations: NSAIDs, non-steroidal anti-inflammatory drugs.
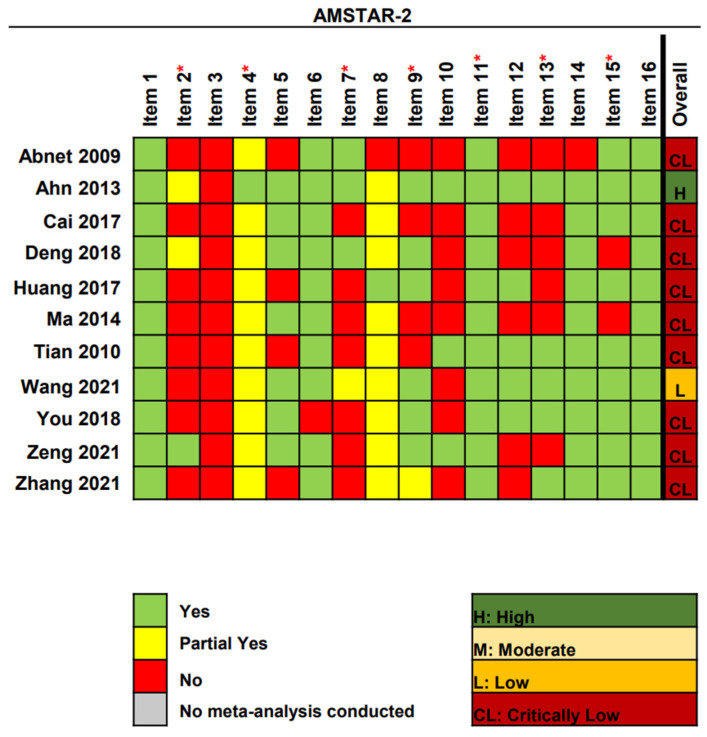
3.3. Methodological Quality Assessment
Using AMSTAR-2, only one (9.1%) meta-analysis was graded as high quality, one (9.1%) was low, and nine (81.8%) were critically low (Figure 3). All studies had more than one critical defect (typically in items 2 (72.7%), 7 (63.6%), and 13 (54.5%)) and several non-critical defects (typically in items 3 (100%), 10 (72.7%), and 12 (54.5%)). The rating criteria for methodological quality assessment are detailed (Table S3).
Figure 3.
Methodological quality of the included systematic reviews [40,41,42,43,44,45,46,47,48,49,50]. Abbreviations: AMSTAR-2, A Measurement Tool to Assess Systematic Reviews-2; * Critical domain.
3.4. Summary Effect Size
The random-effect model was used to re-perform meta-analyses of 19 associations (Table 2). Twelve associations (63.2%) were statistically significant at p ≤ 0.05, and only one association (5.3%) between NSAIDs and GC risk reached p < 10−6. Moreover, four associations (21.1%) showed moderate statistical significance (p < 10−3), including non-aspirin NSAIDs and GC risk, NSAIDs and non-cardia GC risk, aspirin and non-cardia GC risk, as well as acid-suppressing drugs and GC risk. The majority of associations that reached statistical significance suggested potential preventative effects on GC risk, including statins, metformin, NSAIDs, aspirin, and non-aspirin NSAIDs. However, acid-suppressing drugs, including H2RAs and PPIs, increased the risk of GC. The effect of the largest study per association indicated that 7 of 19 were significant at p < 0.05.
Table 2.
Evidence-rating results on associations between commonly used drugs and the risk of gastric cancer.
| First Author [Ref], Year |
Drug Type | Outcome | Random-Effects Summary Effect Size (95% CI) |
Random p Value |
I2 | 95% PI | Egger p Value |
LS | p Value * | Influential Factors | CE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| You [48], 2018 | Any statins | GC | 0.82 (0.69–0.97) | 0.019 | 69.5% | (0.52–1.29) | 0.041 | No | 0.476 | Protective factor |
IV |
| Ma [45], 2014 | Atorvastatin | GC | 0.60 (0.31–1.17) | 0.136 | 96.2% | (0.00–3080.40) | 0.198 | No | NP | NA | V |
| Simvastatin | GC | 0.70 (0.49–1.00) | 0.052 | 85.6% | (0.01–49.50) | 0.077 | No | 1.000 | NA | V | |
| Pravastatin | GC | 0.64 (0.32–1.31) | 0.221 | 88.0% | (0.00–3939.07) | 0.180 | No | NP | NA | V | |
| Wang [47], 2021 | Aspirin | GC | 0.67 (0.52–0.87) | 0.003 | 96.2% | (0.26–1.76) | 0.531 | No | 0.764 | Protective factor |
IV |
| Huang [44], 2017 | NSAIDs | GC | 0.76 (0.70–0.84) | 5.72 × 10−9 | 64.8% | (0.51–1.14) | 0.034 | No | 0.143 | Protective factor |
III |
| NSAIDs | Non-cardia GC | 0.70 (0.59–0.84) | 1.33 × 10−4 | 69.4% | (0.40–1.24) | 0.054 | Yes | 0.139 | Protective factor |
III | |
| Aspirin | Non-cardia GC | 0.64 (0.53–0.78) | 9.60 × 10−6 | 69.2% | (0.35–1.18) | 0.105 | Yes | 0.111 | Protective factor |
III | |
| Non-aspirin NSAIDs | Non-cardia GC | 0.74 (0.60–0.93) | 8.58 × 10−3 | 58.8% | (0.37–1.50) | 0.023 | No | 0.687 | Protective factor |
IV | |
| Abnet [40], 2009 | Aspirin | Cardia GC | 0.82 (0.64–1.04) | 0.099 | 68.1% | (0.39–1.70) | 0.653 | No | NP | NA | V |
| Non-aspirin NSAIDs | Cardia GC | 0.80 (0.67–0.95) | 0.013 | 35.6% | (0.49–1.29) | 0.195 | Yes | NP | Protective factor |
IV | |
| Tian [46], 2010 | Non-aspirin NSAIDs | GC | 0.83 (0.76–0.92) | 1.53 × 10−4 | 0.0% | (0.72–0.97) | 0.118 | No | 0.181 | Protective factor |
III |
| NSAIDs | Cardia GC | 0.89 (0.75–1.05) | 0.167 | 28.3% | (0.58–1.36) | 0.313 | No | 1.000 | NA | V | |
| Zhang [50], 2021 | Metformin | GC | 0.82 (0.68–0.99) | 0.037 | 73.2% | (0.48–1.41) | 0.914 | Yes | NP | Protective factor |
IV |
| Ahn [41], 2013 | Acid suppressive drugs | GC | 1.45 (1.17–1.80) | 7.07 × 10−4 | 53.8% | (0.82–2.56) | 0.819 | Yes | 0.289 | Risk factor |
III |
| H2RAs | GC | 1.46 (1.14–1.86) | 2.51 × 10−3 | 60.3% | (0.74–2.85) | 0.699 | Yes | 0.289 | Risk factor |
IV | |
| Zeng [49], 2021 | PPIs | GC | 1.74 (1.25–2.43) | 1.14 × 10−3 | 94.0% | (0.53–5.71) | 0.186 | Yes | NP | Risk factor |
IV |
| Cai [42], 2017 | Any bisphosphonates | GC | 1.11 (0.92–1.35) | 0.266 | 59.1% | (0.64–1.93) | 0.407 | No | 0.489 | NA | V |
| Deng [43], 2018 | Alendronate | GC | 0.81 (0.57–1.14) | 0.229 | 39.0% | (0.03–22.61) | 0.631 | No | 1.000 | NA | V |
Abbreviations: GC, gastric cancer; NSAIDs, non-steroidal anti-inflammatory drugs; H2RAs, Histamine 2-receptor antagonist; PPIs, proton pump inhibitors; CI, confidence interval; PI, prediction interval; LS, largest study with significant effect; CE, class of evidence; NP, not pertinent because of fewer-than-expected number of observed studies; NA, not applicable. * p value of excess significance test. All statistical tests two sided.
3.5. Study Heterogeneity
Out of 19 associations, 15 (78.9%) showed significant heterogeneity. Besides, high heterogeneity (I2 > 75%) was observed in five associations (26.3%) with GC risk: atorvastatin, simvastatin, pravastatin, aspirin, and PPIs. The inter-study heterogeneity was analyzed by calculating the 95% PI value, and only one association (5.3%) between non-aspirin NSAIDs and GC risk excluded the null value (Table 2).
3.6. Small-Study Effects
The small-study effect was found in five associations (26.3%): any statins and GC risk, simvastatin and GC risk, NSAIDs and GC risk, NSAIDs and non-cardia GC risk, as well as non-aspirin NSAIDs and non-cardia GC risk (Table 2). However, only three associations (15.8%) included 10 or more studies, providing sufficient statistical power for Egger’s test to fully determine the small-study effect of NSAIDs, aspirin, and acid-suppressing drugs associated with GC risk.
3.7. Excess Significance
No evidence of excess significance bias was observed when the plausible effect size was supposed to be equal to the largest study estimate (Table 2).
3.8. Evidence Grading
Of 19 associations between commonly used drugs and GC risk and its subtypes, no association was supported by convincing or highly suggestive evidence. Five associations (26.3%) were supported by suggestive evidence: the association between NSAIDs use, non-aspirin NSAIDs use and decreased GC risk, the association between NSAIDs use, aspirin use and decreased non-cardia GC risk, and the association between acid-suppressing drug use and increased GC risk (Table 2 and Figure 4). Notably, the meta-analysis indicated an association between acid-suppressing drug use and GC risk, attaining a high-quality level by AMSTAR-2 [41]. For the remaining associations, either weak evidence (36.8%) or non-significant evidence (36.8%) was found.
Figure 4.
Summary estimates of commonly used drugs and GC risk by class of evidence. Abbreviations: GC, gastric cancer; NSAIDs, non-steroidal anti-inflammatory drugs; H2RAs, Histamine 2-receptor antagonist; PPIs, proton pump inhibitors; CI, confidence interval.
4. Discussion
4.1. Main Findings
Commonly used drugs may have potential links with various cancer types during the treatment of chronic diseases. To date, several systematic reviews or meta-analyses have explored associations between commonly used drugs and GC risk [51,52,53,54,55]. However, conclusions on relationships and methodological quality were inconsistent. Hence, the next step is to review and grade evidence from the existing systematic reviews and meta-analyses, thus providing an overview of the findings of a specific association. This umbrella review was therefore conducted.
In this study, two pieces of suggestive evidence showed that the intake of NSAIDs and non-aspirin NSAIDs could reduce the incidence of GC, while another one showed that the intake of acid-suppressing drugs was positively related to a higher risk of GC. For subtypes of GC, two pieces of suggestive evidence suggested that the increased intake of NSAIDs and aspirin was related to a lower risk of non-cardia GC. Five pieces of weak evidence suggested that the increased intake of aspirin, metformin and any statins was negatively associated with the incidence of GC, while the intake of H2RAs and PPIs was positively associated with a higher risk of GC. Two pieces of weak evidence showed that the increased intake of non-aspirin NSAIDs intake could have a negative correlation with the incidence of both cardia GC and non-cardia GC. The remaining drugs had no association with GC risk. All of these were previously summarized in Figure 4.
4.2. Comparison with Other Studies
NSAIDs, including aspirin and non-aspirin NASIDs, are commonly used to treat fever, pain, and inflammation. Recently, the anti-cancer effects of NSAIDs have also been reported in several cancer prevention studies [56,57,58]. However, a number of studies evaluating associations between NSAIDs and GC risk have been controversial [59,60]. This study found that the use of NSAIDs was related to a reduced risk of GC and non-cardia GC, which is consistent with a previous meta-analysis [44]. Moreover, aspirin is one of the world’s most prescribed NSAIDs. A cohort study in Korea showed that the long-term use of aspirin was negatively related to GC risk [59]. A large population-based case–control study concluded that the use of aspirin was inversely associated with the risk of GC and non-cardia GC [61]. Similarly, in this study, aspirin use lowered the risk of GC and non-cardia GC. In addition, it was found that the use of non-aspirin NSAIDs was negatively related to GC risk, which was also found in both cardia GC and non-cardia GC. Interestingly, one meta-analysis, including five clinical studies, explored the risk of non-aspirin NSAIDs and cardia GC [40]. All but one study reported no evidence that non-aspirin NSAIDs reduced the risk of cardia GC [62]. As for the risk of cardia GC associated with NSAIDs and aspirin, previous studies showed that aspirin or NSAIDs was not related to the risk of cardia GC [63,64,65], which is consistent with our findings. The anti-tumor mechanisms underpinning NSAIDs have not yet been fully elucidated. As inflammation is a critical component of tumor progression, a plausible explanation could be the anti-inflammatory effects of NASIDs [66]. Furthermore, the cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) pathway is associated with GC development, and PGE2 also plays a pro-inflammatory mediator role in GC development [67]. Thus, NSAIDs could reduce GC risk by inhibiting COX-2 expression [68].
Acid-suppressing drugs, including H2RAs and PPIs, are primary treatments for peptic ulcer disease, gastroesophageal reflux disease, and other digestive diseases [69,70]. This study observed that acid-suppressing drugs, either H2RAs or PPIs, could increase the risk of GC. One possible mechanism could be that acid-suppressing drugs increase gastrin secretion to reduce gastric acidity, thus leading to hypergastrinemia [71]. As a result, elevated serum gastrin may facilitate GC [72]. Recent studies also showed that these drugs were positively related to the risk of GC [73,74], whereas other studies provided evidence to the contrary. Lee et al. found that PPIs use was not related to an increased risk of GC [75]. Additionally, the results of a large prospective cohort suggested no association between H2RAs use and GC risk [15]. The inclusion of people from different regions may contribute to the inconsistency, and differences in the length of follow-up may be one of the reasons for the inconsistency.
In addition to PPIs, the role of statins and metformin in GC development has also been widely studied [76,77,78]. Statins reduce cholesterol and the incidence of cardiovascular disease [79]. Metformin is a first-line oral antidiabetic agent for the treatment of Type 2 diabetes [80]. Previous studies reported that statins and metformin exerted protective effects toward multiple cancers, including GC [81,82,83]. In this study, the increased intake of statins and metformin was found to be negatively correlated with GC risk. However, there was no negative relationship between any of three statin types (atorvastatin, simvastatin, and pravastatin) and GC risk, which is consistent with the findings of Vinogradova et al. [84]. Currently, there is also limited mechanistic evidence on how statins reduce the incidence of GC. One possible mechanism could be that statins evoke apoptosis and anti-angiogenesis [85]. The underlying anti-tumor mechanism of metformin is to activate the 5′ adenosine monophosphate-activated protein kinase pathway [86].
4.3. Limitations
This is the first umbrella review to systematically explore evaluate associations between commonly used drugs and the risk of GC and its subtypes through published systematic reviews and meta-analyses of observational studies. However, there are several limitations to this study. First, the evidence from meta-analyses of observational studies was graded only. Observational studies often have more potential for bias and confounding issues when compared with randomized controlled studies. Thus, the associations identified in observational studies did not necessarily imply causality. Second, the credibility of the umbrella review depended directly on meta-analyses and indirectly on individual studies; therefore, some biases were inevitable. Third, the methodological quality of most systematic reviews and meta-analyses was critically low from the perspective of AMSTAR-2 analyses. Fourth, drug use and the risk of GC subtypes was not comprehensively reviewed due to a lack of data. For example, associations between acid-suppressing drugs and the risk of GC subtypes were not reviewed because the number of GC subtypes from an individual study was not reported [87]. Furthermore, drug dose, frequency, and duration of drug use and GC risk were not evaluated due to data limitations.
5. Conclusions
To conclude, three associations between GC risk and commonly used drugs (NSAIDs, non-aspirin NSAIDs, and acid-suppressing drugs) were supported by suggestive evidence. It was found that the intake of NSAIDs or non-aspirin NSAIDs could reduce GC risk, whereas the intake of acid-suppressing drugs could increase GC risk. Moreover, the decreased risk of non-cardia GC associated with NSAIDs or aspirin was also supported by suggestive evidence. However, caution should be exercised when interpreting these relationships, and high-quality studies are required in the future to determine whether these associations have absolute causality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15020372/s1, Table S1: Search strategy; Table S2: The list of excluded studies; Table S3: Rating criteria for methodological quality assessment. References [40,41,42,43,44,45,46,47,48,49,50,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, X.B. and D.-Q.D.; methodology, X.B. and S.-Q.D.; software, X.B.; validation, X.B., S.-Q.D. and D.-Q.D.; formal analysis, X.-P.Z. and M.-H.H.; resources, X.B., S.-Q.D. and D.-Q.D.; data curation, X.B. and D.-Q.D.; writing—original draft preparation, X.B.; writing—review and editing, S.-Q.D., X.-P.Z., M.-H.H. and D.-Q.D.; visualization, D.-Q.D.; supervision, D.-Q.D.; project administration, D.-Q.D.; funding acquisition, D.-Q.D. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that they have no competing interest.
Funding Statement
Dong-Qiu Dai was partly funded by the National Natural Science Foundation of China (No. 81972322).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y., Zheng Y., Wang H.L., Wu J. Global Patterns and Trends in Gastric Cancer Incidence Rates (1988–2012) and Predictions to 2030. Gastroenterology. 2021;161:116–127.e8. doi: 10.1053/j.gastro.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Thrift A.P., El-Serag H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020;18:534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki H., Oda I., Abe S., Sekiguchi M., Mori G., Nonaka S., Yoshinaga S., Saito Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2016;19:198–205. doi: 10.1007/s10120-015-0469-0. [DOI] [PubMed] [Google Scholar]
- 5.Takei S., Kawazoe A., Shitara K. The New Era of Immunotherapy in Gastric Cancer. Cancers. 2022;14:1054. doi: 10.3390/cancers14041054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 7.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 8.Yan L., Chen Y., Chen F., Tao T., Hu Z., Wang J., You J., Wong B.C.Y., Chen J., Ye W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report from a Randomized Controlled Trial with 26.5 Years of Follow-up. Gastroenterology. 2022;163:154–162.e3. doi: 10.1053/j.gastro.2022.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Choi I.J., Kim C.G., Lee J.Y., Kim Y.I., Kook M.C., Park B., Joo J. Family History of Gastric Cancer and Helicobacter pylori Treatment. N. Engl. J. Med. 2020;382:427–436. doi: 10.1056/NEJMoa1909666. [DOI] [PubMed] [Google Scholar]
- 10.Lam B.Q., Srivastava R., Morvant J., Shankar S., Srivastava R.K. Association of Diabetes Mellitus and Alcohol Abuse with Cancer: Molecular Mechanisms and Clinical Significance. Cells. 2021;10:3077. doi: 10.3390/cells10113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butt J., Varga M.G., Wang T., Tsugane S., Shimazu T., Zheng W., Abnet C.C., Yoo K.Y., Park S.K., Kim J., et al. Smoking, Helicobacter Pylori Serology, and Gastric Cancer Risk in Prospective Studies from China, Japan, and Korea. Cancer Prev. Res. 2019;12:667–674. doi: 10.1158/1940-6207.CAPR-19-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu B., Yang D., Yang S., Zhang G. Dietary Salt Intake and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Front. Nutr. 2021;8:801228. doi: 10.3389/fnut.2021.801228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho M.H., Yoo T.G., Jeong S.M., Shin D.W. Association of Aspirin, Metformin, and Statin Use with Gastric Cancer Incidence and Mortality: A Nationwide Cohort Study. Cancer Prev. Res. 2021;14:95–104. doi: 10.1158/1940-6207.CAPR-20-0123. [DOI] [PubMed] [Google Scholar]
- 14.Zheng J., Xie S.H., Santoni G., Lagergren J. Metformin use and risk of gastric adenocarcinoma in a Swedish population-based cohort study. Br. J. Cancer. 2019;121:877–882. doi: 10.1038/s41416-019-0598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu P., McMenamin Ú.C., Johnston B.T., Murchie P., Iversen L., Lee A.J., Vissers P.A.J., Cardwell C.R. Use of proton pump inhibitors and histamine-2 receptor antagonists and risk of gastric cancer in two population-based studies. Br. J. Cancer. 2020;123:307–315. doi: 10.1038/s41416-020-0860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su C.H., Islam M.M., Jia G., Wu C.C. Statins and the Risk of Gastric Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022;11:7180. doi: 10.3390/jcm11237180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang P., Zhou Y., Chen B., Wan H.W., Jia G.Q., Bai H.L., Wu X.T. Aspirin use and the risk of gastric cancer: A meta-analysis. Dig. Dis. Sci. 2010;55:1533–1539. doi: 10.1007/s10620-009-0915-0. [DOI] [PubMed] [Google Scholar]
- 18.Benson K., Hartz A.J. A comparison of observational studies and randomized, controlled trials. N. Engl. J. Med. 2000;342:1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- 19.Kyriacou D.N., Lewis R.J. Confounding by Indication in Clinical Research. JAMA. 2016;316:1818–1819. doi: 10.1001/jama.2016.16435. [DOI] [PubMed] [Google Scholar]
- 20.Ioannidis J. Next-generation systematic reviews: Prospective meta-analysis, individual-level data, networks and umbrella reviews. Br. J. Sport. Med. 2017;51:1456–1458. doi: 10.1136/bjsports-2017-097621. [DOI] [PubMed] [Google Scholar]
- 21.Ioannidis J.P. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ Can. Med. Assoc. J. J. De L’association Med. Can. 2009;181:488–493. doi: 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radua J., Ramella-Cravaro V., Ioannidis J.P.A., Reichenberg A., Phiphopthatsanee N., Amir T., Yenn Thoo H., Oliver D., Davies C., Morgan C., et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry Off. J. World Psychiatr. Assoc. (WPA) 2018;17:49–66. doi: 10.1002/wps.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J.H., Kim J.Y., Lee J., Jeong G.H., Lee E., Lee S., Lee K.H., Kronbichler A., Stubbs B., Solmi M., et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: An umbrella review. Lancet Psychiatry. 2020;7:955–970. doi: 10.1016/S2215-0366(20)30312-6. [DOI] [PubMed] [Google Scholar]
- 25.Raglan O., Kalliala I., Markozannes G., Cividini S., Gunter M.J., Nautiyal J., Gabra H., Paraskevaidis E., Martin-Hirsch P., Tsilidis K.K., et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer. 2019;145:1719–1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 26.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Ioannidis J.P., Patsopoulos N.A., Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patsopoulos N.A., Evangelou E., Ioannidis J.P. Heterogeneous views on heterogeneity. Int. J. Epidemiol. 2009;38:1740–1742. doi: 10.1093/ije/dyn235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley R.D., Higgins J.P., Deeks J.J. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 31.Ioannidis J.P., Trikalinos T.A. An exploratory test for an excess of significant findings. Clin. Trials. 2007;4:245–253. doi: 10.1177/1740774507079441. [DOI] [PubMed] [Google Scholar]
- 32.Sterne J.A., Sutton A.J., Ioannidis J.P., Terrin N., Jones D.R., Lau J., Carpenter J., Rücker G., Harbord R.M., Schmid C.H., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 33.Sun H., Gong T.T., Xia Y., Wen Z.Y., Zhao L.G., Zhao Y.H., Wu Q.J. Diet and ovarian cancer risk: An umbrella review of systematic reviews and meta-analyses of cohort studies. Clin. Nutr. 2021;40:1682–1690. doi: 10.1016/j.clnu.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Lubin J.H., Gail M.H. On power and sample size for studying features of the relative odds of disease. Am. J. Epidemiol. 1990;131:552–566. doi: 10.1093/oxfordjournals.aje.a115530. [DOI] [PubMed] [Google Scholar]
- 35.Bortolato B., Köhler C.A., Evangelou E., León-Caballero J., Solmi M., Stubbs B., Belbasis L., Pacchiarotti I., Kessing L.V., Berk M., et al. Systematic assessment of environmental risk factors for bipolar disorder: An umbrella review of systematic reviews and meta-analyses. Bipolar Disord. 2017;19:84–96. doi: 10.1111/bdi.12490. [DOI] [PubMed] [Google Scholar]
- 36.Dragioti E., Evangelou E., Larsson B., Gerdle B. Effectiveness of multidisciplinary programmes for clinical pain conditions: An umbrella review. J. Rehabil. Med. 2018;50:779–791. doi: 10.2340/16501977-2377. [DOI] [PubMed] [Google Scholar]
- 37.He Y., Li X., Gasevic D., Brunt E., McLachlan F., Millenson M., Timofeeva M., Ioannidis J.P.A., Campbell H., Theodoratou E. Statins and Multiple Noncardiovascular Outcomes: Umbrella Review of Meta-analyses of Observational Studies and Randomized Controlled Trials. Ann. Intern. Med. 2018;169:543–553. doi: 10.7326/M18-0808. [DOI] [PubMed] [Google Scholar]
- 38.Kalliala I., Markozannes G., Gunter M.J., Paraskevaidis E., Gabra H., Mitra A., Terzidou V., Bennett P., Martin-Hirsch P., Tsilidis K.K., et al. Obesity and gynaecological and obstetric conditions: Umbrella review of the literature. BMJ. 2017;359:j4511. doi: 10.1136/bmj.j4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veronese N., Solmi M., Caruso M.G., Giannelli G., Osella A.R., Evangelou E., Maggi S., Fontana L., Stubbs B., Tzoulaki I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018;107:436–444. doi: 10.1093/ajcn/nqx082. [DOI] [PubMed] [Google Scholar]
- 40.Abnet C.C., Freedman N.D., Kamangar F., Leitzmann M.F., Hollenbeck A.R., Schatzkin A. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: Results from a cohort study and a meta-analysis. Br. J. Cancer. 2009;100:551–557. doi: 10.1038/sj.bjc.6604880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn J.S., Eom C.S., Jeon C.Y., Park S.M. Acid suppressive drugs and gastric cancer: A meta-analysis of observational studies. World J. Gastroenterol. 2013;19:2560–2568. doi: 10.3748/wjg.v19.i16.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai D., Qin J., Chen G., Feng W., Liu J. Bisphosphonates use and risk of gastric cancer: An updated meta-analysis of cohort and case-control studies. Minerva Med. 2017;108:464–472. doi: 10.23736/S0026-4806.17.05055-8. [DOI] [PubMed] [Google Scholar]
- 43.Deng Y., Zhang Z., Jia X., Cheng W., Zhou X., Liu Y., Wang M. Oral bisphosphonates and incidence of cancers in patients with osteoporosis: A systematic review and meta-analysis. Arch. Osteoporos. 2018;14:1. doi: 10.1007/s11657-018-0552-3. [DOI] [PubMed] [Google Scholar]
- 44.Huang X.Z., Chen Y., Wu J., Zhang X., Wu C.C., Zhang C.Y., Sun S.S., Chen W.J. Aspirin and non-steroidal anti-inflammatory drugs use reduce gastric cancer risk: A dose-response meta-analysis. Oncotarget. 2017;8:4781–4795. doi: 10.18632/oncotarget.13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma Z., Wang W., Jin G., Chu P., Li H. Effect of statins on gastric cancer incidence: A meta-analysis of case control studies. J. Cancer Res. Ther. 2014;10:859–865. doi: 10.4103/0973-1482.138218. [DOI] [PubMed] [Google Scholar]
- 46.Tian W., Zhao Y., Liu S., Li X. Meta-analysis on the relationship between nonsteroidal anti-inflammatory drug use and gastric cancer. Eur. J. Cancer Prev.: Off. J. Eur. Cancer Prev. Organ. (ECP) 2010;19:288–298. doi: 10.1097/CEJ.0b013e328339648c. [DOI] [PubMed] [Google Scholar]
- 47.Wang L., Zhang R., Yu L., Xiao J., Zhou X., Li X., Song P., Li X. Aspirin Use and Common Cancer Risk: A Meta-Analysis of Cohort Studies and Randomized Controlled Trials. Front. Oncol. 2021;11:690219. doi: 10.3389/fonc.2021.690219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You S., Sun G., Yao Q., Wan Z., Huang X. Statin use and risk of gastrointestinal cancer: A meta-analysis of cohort studies. Int. J. Clin. Exp. Med. 2018;11:1437–1447. [Google Scholar]
- 49.Zeng R., Cheng Y., Luo D., Wang J., Yang J., Jiang L., Zhuo Z., Guo K., Wu H., Leung F.W., et al. Comprehensive analysis of proton pump inhibitors and risk of digestive tract cancers. Eur. J. Cancer. 2021;156:190–201. doi: 10.1016/j.ejca.2021.07.030. [DOI] [PubMed] [Google Scholar]
- 50.Zhang K., Bai P., Dai H., Deng Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim. Care Diabetes. 2021;15:52–58. doi: 10.1016/j.pcd.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Kong P., Wu R., Liu X., Liu J., Chen S., Ye M., Yang C., Song Z., He W., Yin C., et al. The Effects of Anti-inflammatory Drug Treatment in Gastric Cancer Prevention: An Update of a Meta-analysis. J. Cancer. 2016;7:2247–2257. doi: 10.7150/jca.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh P.P., Singh S. Statins are associated with reduced risk of gastric cancer: A systematic review and meta-analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013;24:1721–1730. doi: 10.1093/annonc/mdt150. [DOI] [PubMed] [Google Scholar]
- 53.Song H.J., Jeon N., Squires P. The association between acid-suppressive agent use and the risk of cancer: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2020;76:1437–1456. doi: 10.1007/s00228-020-02927-8. [DOI] [PubMed] [Google Scholar]
- 54.Wright E., Schofield P.T., Molokhia M. Bisphosphonates and evidence for association with esophageal and gastric cancer: A systematic review and meta-analysis. BMJ Open. 2015;5:e007133. doi: 10.1136/bmjopen-2014-007133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou X.L., Xue W.H., Ding X.F., Li L.F., Dou M.M., Zhang W.J., Lv Z., Fan Z.R., Zhao J., Wang L.X. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: A meta-analysis of cohort studies. Oncotarget. 2017;8:55622–55631. doi: 10.18632/oncotarget.16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cairat M., Al Rahmoun M., Gunter M.J., Severi G., Dossus L., Fournier A. Use of nonsteroidal anti-inflammatory drugs and breast cancer risk in a prospective cohort of postmenopausal women. Breast Cancer Res. BCR. 2020;22:118. doi: 10.1186/s13058-020-01343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doat S., Cénée S., Trétarre B., Rebillard X., Lamy P.J., Bringer J.P., Iborra F., Murez T., Sanchez M., Menegaux F. Nonsteroidal anti-inflammatory drugs (NSAIDs) and prostate cancer risk: Results from the EPICAP study. Cancer Med. 2017;6:2461–2470. doi: 10.1002/cam4.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuo C.N., Pan J.J., Huang Y.W., Tsai H.J., Chang W.C. Association between Nonsteroidal Anti-Inflammatory Drugs and Colorectal Cancer: A Population-Based Case-Control Study. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2018;27:737–745. doi: 10.1158/1055-9965.EPI-17-0876. [DOI] [PubMed] [Google Scholar]
- 59.Kim Y.I., Kim S.Y., Kim J.H., Lee J.H., Kim Y.W., Ryu K.W., Park J.H., Choi I.J. Long-Term Low-Dose Aspirin Use Reduces Gastric Cancer Incidence: A Nationwide Cohort Study. Cancer Res. Treat. 2016;48:798–805. doi: 10.4143/crt.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sørensen H.T., Friis S., Nørgård B., Mellemkjaer L., Blot W.J., McLaughlin J.K., Ekbom A., Baron J.A. Risk of cancer in a large cohort of nonaspirin NSAID users: A population-based study. Br. J. Cancer. 2003;88:1687–1692. doi: 10.1038/sj.bjc.6600945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akre K., Ekström A.M., Signorello L.B., Hansson L.E., Nyrén O. Aspirin and risk for gastric cancer: A population-based case-control study in Sweden. Br. J. Cancer. 2001;84:965–968. doi: 10.1054/bjoc.2001.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sadeghi S., Bain C.J., Pandeya N., Webb P.M., Green A.C., Whiteman D.C. Aspirin, nonsteroidal anti-inflammatory drugs, and the risks of cancers of the esophagus. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008;17:1169–1178. doi: 10.1158/1055-9965.EPI-07-2852. [DOI] [PubMed] [Google Scholar]
- 63.Cheung K.S., Chan E.W., Wong A.Y.S., Chen L., Seto W.K., Wong I.C.K., Leung W.K. Aspirin and Risk of Gastric Cancer After Helicobacter pylori Eradication: A Territory-Wide Study. J. Natl. Cancer Inst. 2018;110:743–749. doi: 10.1093/jnci/djx267. [DOI] [PubMed] [Google Scholar]
- 64.Duan L., Wu A.H., Sullivan-Halley J., Bernstein L. Nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric adenocarcinomas in Los Angeles County. Cancer Epidemiol. Biomark. Prev.: A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008;17:126–134. doi: 10.1158/1055-9965.EPI-07-0664. [DOI] [PubMed] [Google Scholar]
- 65.Figueroa J.D., Terry M.B., Gammon M.D., Vaughan T.L., Risch H.A., Zhang F.F., Kleiner D.E., Bennett W.P., Howe C.L., Dubrow R., et al. Cigarette smoking, body mass index, gastro-esophageal reflux disease, and non-steroidal anti-inflammatory drug use and risk of subtypes of esophageal and gastric cancers by P53 overexpression. Cancer Causes Control CCC. 2009;20:361–368. doi: 10.1007/s10552-008-9250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang D., Cabalag C.S., Clemons N.J., DuBois R.N. Cyclooxygenases and Prostaglandins in Tumor Immunology and Microenvironment of Gastrointestinal Cancer. Gastroenterology. 2021;161:1813–1829. doi: 10.1053/j.gastro.2021.09.059. [DOI] [PubMed] [Google Scholar]
- 68.Jiang X.H., Wong B.C. Cyclooxygenase-2 inhibition and gastric cancer. Curr. Pharm. Des. 2003;9:2281–2288. doi: 10.2174/1381612033453983. [DOI] [PubMed] [Google Scholar]
- 69.Mori H., Suzuki H. Role of Acid Suppression in Acid-related Diseases: Proton Pump Inhibitor and Potassium-competitive Acid Blocker. J. Neurogastroenterol. Motil. 2019;25:6–14. doi: 10.5056/jnm18139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scarpignato C., Hongo M., Wu J.C.Y., Lottrup C., Lazarescu A., Stein E., Hunt R.H. Pharmacologic treatment of GERD: Where we are now, and where are we going? Ann. N. Y. Acad. Sci. 2020;1482:193–212. doi: 10.1111/nyas.14473. [DOI] [PubMed] [Google Scholar]
- 71.Singh P., Indaram A., Greenberg R., Visvalingam V., Bank S. Long term omeprazole therapy for reflux esophagitis:follow-up in serum gastrin levels, EC cell hyperplasia and neoplasia. World J. Gastroenterol. 2000;6:789–792. doi: 10.3748/wjg.v6.i6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Solomon T.E. Trophic effects of pentagastrin on gastrointestinal tract in fed and fasted rats. Gastroenterology. 1986;91:108–116. doi: 10.1016/0016-5085(86)90446-4. [DOI] [PubMed] [Google Scholar]
- 73.Møller H., Nissen A., Mosbech J. Use of cimetidine and other peptic ulcer drugs in Denmark 1977–1990 with analysis of the risk of gastric cancer among cimetidine users. Gut. 1992;33:1166–1169. doi: 10.1136/gut.33.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poulsen A.H., Christensen S., McLaughlin J.K., Thomsen R.W., Sørensen H.T., Olsen J.H., Friis S. Proton pump inhibitors and risk of gastric cancer: A population-based cohort study. Br. J. Cancer. 2009;100:1503–1507. doi: 10.1038/sj.bjc.6605024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J.K., Merchant S.A., Schneider J.L., Jensen C.D., Fireman B.H., Quesenberry C.P., Corley D.A. Proton Pump Inhibitor Use and Risk of Gastric, Colorectal, Liver, and Pancreatic Cancers in a Community-Based Population. Am. J. Gastroenterol. 2020;115:706–715. doi: 10.14309/ajg.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 76.Kato K., Gong J., Iwama H., Kitanaka A., Tani J., Miyoshi H., Nomura K., Mimura S., Kobayashi M., Aritomo Y., et al. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol. Cancer Ther. 2012;11:549–560. doi: 10.1158/1535-7163.MCT-11-0594. [DOI] [PubMed] [Google Scholar]
- 77.Kim D.S., Kim H.J., Ahn H.S. Statins and the risk of gastric, colorectal, and esophageal cancer incidence and mortality: A cohort study based on data from the Korean national health insurance claims database. J. Cancer Res. Clin. Oncol. 2022;148:2855–2865. doi: 10.1007/s00432-022-04075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang P.R., Tsai Y.Y., Chen K.J., Yang Y.H., Shih W.T. Statin Use Improves Overall Survival of Patients with Gastric Cancer after Surgery and Adjuvant Chemotherapy in Taiwan: A Nationwide Matched Cohort Study. Cancers. 2020;12:2055. doi: 10.3390/cancers12082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bellosta S., Corsini A. Statin drug interactions and related adverse reactions: An update. Expert Opin. Drug Saf. 2018;17:25–37. doi: 10.1080/14740338.2018.1394455. [DOI] [PubMed] [Google Scholar]
- 80.McCreight L.J., Bailey C.J., Pearson E.R. Metformin and the gastrointestinal tract. Diabetologia. 2016;59:426–435. doi: 10.1007/s00125-015-3844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beckwitt C.H., Brufsky A., Oltvai Z.N., Wells A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. BCR. 2018;20:144. doi: 10.1186/s13058-018-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee M.S., Hsu C.C., Wahlqvist M.L., Tsai H.N., Chang Y.H., Huang Y.C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leung H.W., Chan A.L., Lo D., Leung J.H., Chen H.L. Common cancer risk and statins: A population-based case-control study in a Chinese population. Expert Opin. Drug Saf. 2013;12:19–27. doi: 10.1517/14740338.2013.744392. [DOI] [PubMed] [Google Scholar]
- 84.Vinogradova Y., Coupland C., Hippisley-Cox J. Exposure to statins and risk of common cancers: A series of nested case-control studies. BMC Cancer. 2011;11:409. doi: 10.1186/1471-2407-11-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheung K.S., Chan E.W., Wong A.Y.S., Chen L., Seto W.K., Wong I.C.K., Leung W.K. Statins Were Associated with a Reduced Gastric Cancer Risk in Patients with Eradicated Helicobacter Pylori Infection: A Territory-Wide Propensity Score Matched Study. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2020;29:493–499. doi: 10.1158/1055-9965.EPI-19-1044. [DOI] [PubMed] [Google Scholar]
- 86.Daugan M., Dufaÿ Wojcicki A., d’Hayer B., Boudy V. Metformin: An anti-diabetic drug to fight cancer. Pharmacol. Res. 2016;113:675–685. doi: 10.1016/j.phrs.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 87.Tamim H., Duranceau A., Chen L.Q., Lelorier J. Association between use of acid-suppressive drugs and risk of gastric cancer. A nested case-control study. Drug Saf. 2008;31:675–684. doi: 10.2165/00002018-200831080-00004. [DOI] [PubMed] [Google Scholar]
- 88.Wang X., Luo Y., Chen T., Zhang K. Low-dose aspirin use and cancer-specific mortality: A meta-analysis of cohort studies. J. Public Health. 2021;43:308–315. doi: 10.1093/pubmed/fdz114. [DOI] [PubMed] [Google Scholar]
- 89.Sun C., Chen Y., Ismail M.R., Tuason J.P.W., Cheng X., Hu L., Bhan C., Kim N.H., Prasad A., Manem N., et al. Is Acid Suppression Therapy Associated With Increased Risk of Cardia Gastric Cancer? A Meta-Analysis. Am. J. Gastroenterol. 2021;116:S655. doi: 10.14309/01.ajg.0000779240.78130.d8. [DOI] [Google Scholar]
- 90.Sun C., Tuason J.P.W., Kim K.Y., Cheng C., Bhan C., Manem R., Sundararajan N., Gerais Y.A., Gandam M.R., Lising J.F., et al. Is Metformin Associated With Decreased Mortality of Gastric Cancer? A Meta-Analysis. Am. J. Gastroenterol. 2021;116:S637–S638. doi: 10.14309/01.ajg.0000779092.11123.c5. [DOI] [Google Scholar]
- 91.Song H.J., Rhew K., Lee Y.J., Ha I.-H. Acid-suppressive agents and survival outcomes in patients with cancer: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2021;26:34–50. doi: 10.1007/s10147-020-01795-7. [DOI] [PubMed] [Google Scholar]
- 92.Seo S.I., Park C.H., Kim T.J., Bang C.S., Kim J.Y., Lee K.J., Kim J., Kim H.H., You S.C., Shin W.G. Aspirin, metformin, and statin use on the risk of gastric cancer: A nationwide population-based cohort study in Korea with systematic review and meta-analysis. Cancer Med. 2021;11:1217–1231. doi: 10.1002/cam4.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Segna D., Brusselaers N., Glaus D., Krupka N., Misselwitz B. Association between proton-pump inhibitors and the risk of gastric cancer: A systematic review with meta-analysis. Ther. Adv. Gastroenterol. 2021;14:17562848211051463. doi: 10.1177/17562848211051463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goyal H., Sachdeva S., Perisetti A., Aloysius M.M., Chandan S., Tharian B., Thosani N. Continued Aspirin Use and Bleeding Risk After Endoscopic Submucosal Dissection of Gastric Neoplasms: A Meta-Analysis. Am. J. Gastroenterol. 2021;116:S473–S474. doi: 10.14309/01.ajg.0000777504.53047.2f. [DOI] [Google Scholar]
- 95.Zhou Q., Chen D.-S., Xin L., Zhou L.-Q., Zhang H.-T., Liu L., Yuan Y.-W., Li S.-H. The renin-angiotensin system blockers and survival in digestive system malignancies: A systematic review and meta-analysis. Medicine. 2020;99:e19075. doi: 10.1097/MD.0000000000019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng J., He J., Wang W., Zhou H., Cai S., Zhu L., Qian X., Wang J., Lu Z., Huang C. The impact of pain and opioids use on survival in cancer patients: Results from a population-based cohort study and a meta-analysis. Medicine. 2020;99:e19306. doi: 10.1097/MD.0000000000019306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Win T.T., Aye S.N., Fern J.L.C., Fei C.O. Aspirin and Reducing Risk of Gastric Cancer: Systematic Review and Meta-Analysis of the Observational Studies. J. Gastrointest. Liver Dis. 2020;29:191–198. doi: 10.15403/jgld-818. [DOI] [PubMed] [Google Scholar]
- 98.Thomas J.P., Loke Y.K., Alexandre L. Efficacy and safety profile of statins in patients with cancer: A systematic review of randomised controlled trials. Eur. J. Clin. Pharmacol. 2020;76:1639–1651. doi: 10.1007/s00228-020-02967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shuai Y., Li C., Zhou X. The effect of metformin on gastric cancer in patients with type 2 diabetes: A systematic review and meta-analysis. Clin. Transl. Oncol. 2020;22:1580–1590. doi: 10.1007/s12094-020-02304-y. [DOI] [PubMed] [Google Scholar]
- 100.Segna D., Brusselaers N., Glaus D., Krupka N., Misselwitz B. Association between long-term use of proton pump inhibitors and the risk of gastric cancer: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2020;8:227. doi: 10.1177/2050640620927345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niikura R., Hirata Y., Hayakawa Y., Kawahara T., Yamada A., Koike K. Effect of aspirin use on gastric cancer incidence and survival: A systematic review and meta-analysis. JGH Open. 2020;4:117–125. doi: 10.1002/jgh3.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin J.-L., Lin J.-X., Zheng C.-H., Li P., Xie J.-W., Wang J.-b., Lu J., Chen Q.-Y., Cao L.-l., Lin M., et al. Relationship between aspirin use of esophageal, gastric and colorectal cancer patient survival: A meta-analysis. BMC Cancer. 2020;20:638. doi: 10.1186/s12885-020-07117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Indini A., Petrelli F., Tomasello G., Rijavec E., Facciorusso A., Grossi F., Ghidini M. Impact of Use of Gastric-Acid Suppressants and Oral Anti-Cancer Agents on Survival Outcomes: A Systematic Review and Meta-Analysis. Cancers. 2020;12:998. doi: 10.3390/cancers12040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bosetti C., Santucci C., Gallus S., Martinetti M., La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: An updated meta-analysis through 2019. Ann. Oncol. 2020;31:558–568. doi: 10.1016/j.annonc.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 105.Bindu S., Mazumder S., Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020;180:114147. doi: 10.1016/j.bcp.2020.114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abdel-Rahman O., Karachiwala H., Easaw J.C. Outcomes of advanced gastrointestinal (GI) cancer patients in relationship to opioid use: An individual patient data pooled analysis from eight clinical trials. J. Clin. Oncol. 2020;38:687. doi: 10.1200/JCO.2020.38.4_suppl.687. [DOI] [PubMed] [Google Scholar]
- 107.Wan Q.-Y., Wu X.-T., Li N., Du L., Zhou Y. Long-term proton pump inhibitors use and risk of gastric cancer: A meta-analysis of 926 386 participants. Gut. 2019;68:762–764. doi: 10.1136/gutjnl-2018-316416. [DOI] [PubMed] [Google Scholar]
- 108.Jiang K., Jiang X., Wen Y., Liao L., Liu F.B. Relationship between long-term use of proton pump inhibitors and risk of gastric cancer: A systematic analysis. J. Gastroenterol. Hepatol. 2019;34:1898–1905. doi: 10.1111/jgh.14759. [DOI] [PubMed] [Google Scholar]
- 109.Huang C., Lin J., Lin J., Zheng C., Li P., Xie J., Wang J., Lu J., Chen Q., Cao L., et al. Long-term use of proton pump inhibitors may increase the incidence of non-cardiac gastric cancer. Surg. Endosc. 2019;33:S754. doi: 10.1007/s00464-019-07109-x. [DOI] [Google Scholar]
- 110.Cheung K.S., Leung W.K. Long-term use of proton-pump inhibitors and risk of gastric cancer: A review of the current evidence. Ther. Adv. Gastroenterol. 2019;12:1756284819834511. doi: 10.1177/1756284819834511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bao C., Wang K., Ding Y., Kong J. Association between Anti-bacterial Drug Use and Digestive System Neoplasms: A Systematic Review and Meta-analysis. Front. Oncol. 2019;9:1298. doi: 10.3389/fonc.2019.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qiao Y., Yang T., Gan Y., Li W., Wang C., Gong Y., Lu Z. Associations between aspirin use and the risk of cancers: A meta-analysis of observational studies. BMC Cancer. 2018;18:288. doi: 10.1186/s12885-018-4156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jang H.J., Kim H.S., Kim J.H., Lee J. The effect of statin added to systemic anticancer therapy: A meta-analysis of randomized, controlled trials. J. Clin. Med. 2018;7:325. doi: 10.3390/jcm7100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Farooqi M.A.M., Malhotra N., Mukherjee S.D., Sanger S., Dhesy-Thind S.K., Ellis P., Leong D.P. Statin therapy in the treatment of active cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2018;13:e0209486. doi: 10.1371/journal.pone.0209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang T., Yang X., Zhou J., Liu P., Wang H., Li A., Zhou Y. Benzodiazepine drug use and cancer risk: A dose-response meta analysis of prospective cohort studies. Oncotarget. 2017;8:102381–102391. doi: 10.18632/oncotarget.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tran-Duy A., Spaetgens B., Hoes A.W., De Wit N.J., Stehouwer C.D.A. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis Reply. Clin. Gastroenterol. Hepatol. 2017;15:790. doi: 10.1016/j.cgh.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 117.Palmer R.H. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017;15:790. doi: 10.1016/j.cgh.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 118.Mei Z., Liang M., Li L., Zhang Y., Wang Q., Yang W. Effects of statins on cancer mortality and progression: A systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int. J. Cancer. 2017;140:1068–1081. doi: 10.1002/ijc.30526. [DOI] [PubMed] [Google Scholar]
- 119.Long L., Cao G., Li Y., Tang S. Association of ACEIs/ARBs therapy with digestive system neoplasms: A meta-analysis. Chin. J. Evid.-Based Med. 2017;17:1051–1059. doi: 10.7507/1672-2531.201703036. [DOI] [Google Scholar]
- 120.Kim H.B., Myung S.K., Park Y.C., Park B. Use of benzodiazepine and risk of cancer: A meta-analysis of observational studies. Int. J. Cancer. 2017;140:513–525. doi: 10.1002/ijc.30443. [DOI] [PubMed] [Google Scholar]
- 121.Kamal F., Khan M.A., Akbar H., Haq K.F., Cholankeril G., Hammad T.A., Ali B., Ismail M.K., Satapathy S.K., Howden C.W. Metformin does not reduce the risk of gastric cancer in type 2 diabetics: Systematic review and meta-analysis. Gastroenterology. 2017;152:S336–S337. doi: 10.1016/S0016-5085(17)31389-6. [DOI] [Google Scholar]
- 122.Joo M.K., Park J.-J., Chun H.J. Additional Benefits of Routine Drugs on Gastrointestinal Cancer: Statins, Metformin, and Proton Pump Inhibitors. Dig. Dis. 2017;36:1–14. doi: 10.1159/000480149. [DOI] [PubMed] [Google Scholar]
- 123.Wang A., Wakelee H.A., Aragaki A.K., Tang J.Y., Kurian A.W., Manson J.E., Stefanick M.L. Protective Effects of Statins in Cancer: Should They Be Prescribed for High-Risk Patients? Curr. Atheroscler. Rep. 2016;18:72. doi: 10.1007/s11883-016-0625-y. [DOI] [PubMed] [Google Scholar]
- 124.Tran-Duy A., Spaetgens B., Hoes A.W., de Wit N.J., Stehouwer C.D.A. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016;14:1706–1719. doi: 10.1016/j.cgh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 125.Stryjkowska-Gora A., Karczmarek-Borowska B., Gora T., Krawczak K. Statins and cancers. Contemp. Oncol. 2015;19:167–175. doi: 10.5114/wo.2014.44294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stegeman I., Bossuyt P.M., Yu T., Boyd C., Puhan M.A. Aspirin for primary prevention of cardiovascular disease and cancer. A benefit and harm analysis. PLoS ONE. 2015;10:e0127194. doi: 10.1371/journal.pone.0127194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang W.-K., Tu H.-T., See L.-C. Aspirin Use on Incidence and Mortality of Gastrointestinal Cancers: Current State of Epidemiological Evidence. Curr. Pharm. Des. 2015;21:5108–5115. doi: 10.2174/1381612821666150915110450. [DOI] [PubMed] [Google Scholar]
- 128.Vallianou N.G., Kostantinou A., Kougias M., Kazazis C. Statins and Cancer. Anti-Cancer Agents Med. Chem. 2014;14:706–712. doi: 10.2174/1871520613666131129105035. [DOI] [PubMed] [Google Scholar]
- 129.Song H., Zhu J., Lu D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst. Rev. 2014:CD010623. doi: 10.1002/14651858.CD010623.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ye X., Fu J., Yang Y., Gao Y., Liu L., Chen S. Frequency-Risk and Duration-Risk Relationships between Aspirin Use and Gastric Cancer: A Systematic Review and Meta-Analysis. PLoS ONE. 2013;8:e71522. doi: 10.1371/journal.pone.0071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu X.-D., Zeng K., Xue F.-Q., Chen J.-H., Chen Y.-Q. Statins are associated with reduced risk of gastric cancer: A meta-analysis. Eur. J. Clin. Pharmacol. 2013;69:1855–1860. doi: 10.1007/s00228-013-1547-z. [DOI] [PubMed] [Google Scholar]
- 132.Malek M., Aghili R., Emami Z., Khamseh M.E. Risk of cancer in diabetes: The effect of metformin. ISRN Endocrinol. 2013;1:636927. doi: 10.1155/2013/636927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Franciosi M., Lucisano G., Lapice E., Strippoli G.F.M., Pellegrini F., Nicolucci A. Metformin Therapy and Risk of Cancer in Patients with Type 2 Diabetes: Systematic Review. PLoS ONE. 2013;8:e0071583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eslami L., Nasseri-Moghaddam S. Meta-analyses: Does Long-term PPI use Increase the Risk of Gastric Premalignant Lesions? Arch. Iran. Med. 2013;16:449–458. [PubMed] [Google Scholar]
- 135.Oh Y.H., Yoon C., Park S.M. Bisphosphonate use and gastrointestinal tract cancer risk: Meta-analysis of observational studies. World J. Gastroenterol. 2012;18:5779–5788. doi: 10.3748/wjg.v18.i40.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Colmers I.N., Bowker S.L., Johnson J.A. Thiazolidinedione use and cancer incidence in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2012;38:475–484. doi: 10.1016/j.diabet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Bosetti C., Rosato V., Gallus S., Cuzick J., La Vecchia C. Aspirin and cancer risk: A quantitative review to 2011. Ann. Oncol. 2012;23:1403–1415. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 138.Algra A.M., Rothwell P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 139.Rothwell P.M., Fowkes F.G., Belch J.F., Ogawa H., Warlow C.P., Meade T.W. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 140.Matsushita Y., Sugihara M., Kaburagi J., Ozawa M., Iwashita M., Yoshida S., Saito H., Hattori Y. Pravastatin use and cancer risk: A meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol. Drug Saf. 2010;19:196–202. doi: 10.1002/pds.1870. [DOI] [PubMed] [Google Scholar]
- 141.Kuoppala J., Lamminpaa A., Pukkala E. Statins and cancer: A systematic review and meta-analysis. Eur. J. Cancer. 2008;44:2122–2132. doi: 10.1016/j.ejca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 142.Browning D.R., Martin R.M. Statins and risk of cancer: A systematic review and metaanalysis. Int. J Cancer. 2007;120:833–843. doi: 10.1002/ijc.22366. [DOI] [PubMed] [Google Scholar]
- 143.Wang W.H., Huang J.Q., Zheng G.F., Lam S.K., Karlberg J., Wong B.C.Y. Non-steroidal anti-inflammatory drug use and the risk of gastric cancer: A systematic review and meta-analysis. JNCI-J. Natl. Cancer Inst. 2003;95:1784–1791. doi: 10.1093/jnci/djg106. [DOI] [PubMed] [Google Scholar]
- 144.Gonzalez-Perez A., Rodriguez L.A.G., Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: A meta-analysis. BMC Cancer. 2003;3:28. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jolly K., Cheng K.K., Langman M.J.S. NSAIDs and gastrointestinal cancer prevention. Drugs. 2002;62:945–956. doi: 10.2165/00003495-200262060-00006. [DOI] [PubMed] [Google Scholar]
- 146.Bosetti C., Gallus S., La Vecchia C. Aspirin and cancer risk: An update to 2001. Eur. J. Cancer Prev. 2002;11:535–542. doi: 10.1097/00008469-200212000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.