This qualitative study compares wide-field optical coherence tomography imaging with standard hematoxylin and eosin slides for margin analysis in squamous cell carcinoma of the oral cavity and oropharynx.
Key Points
Question
Does wide-field optical coherence tomography (WF-OCT) imaging have potential as an adjunct to standard pathology for margin analysis of squamous cell carcinoma of the oral cavity and oropharynx?
Findings
In this qualitative study including 53 patients undergoing oral cavity or oropharynx surgery for squamous cell carcinoma, nondestructive, real-time visualization of tissue microarchitecture, as well as differentiation among mucosa, submucosa, muscle, dysplastic, and benign tissue, was possible using WF-OCT imaging.
Meaning
These findings suggest that WF-OCT may be a promising imaging modality for intraoperative analysis in head and neck surgery, especially at deep margins.
Abstract
Importance
Involvement of deep margins represents a significant challenge in the treatment of oropharyngeal cancer, and given practical limitations of frozen-section analysis, a need exists for real-time, nondestructive intraoperative margin analysis. Wide-field optical coherence tomography (WF-OCT) has been evaluated as a tool for high-resolution adjunct specimen imaging in breast surgery, but its clinical application in head and neck surgery has not been explored.
Objective
To evaluate the utility of WF-OCT for visualizing microstructures at margins of excised oral and oropharyngeal tissue.
Design, Setting, and Participants
This nonrandomized, investigator-initiated qualitative study evaluated the feasibility of the Perimeter Medical Imaging AI Otis WF-OCT device at a single academic center. Included participants were adults undergoing primary ablative surgery of the oral cavity or oropharynx for squamous cell carcinoma in 2018 and 2019. Data were analyzed in October 2019.
Exposures
Patients were treated according to standard surgical care. Freshly resected specimens were imaged with high-resolution WF-OCT prior to routine pathology. Interdisciplinary interpretation was performed to interpret WF-OCT images and compare them with corresponding digitized pathology slides. No clinical decisions were made based on WF-OCT image data.
Main Outcomes and Measures
Visual comparisons were performed between WF-OCT images and hematoxylin and eosin slides.
Results
A total of 69 specimens were collected and scanned from 53 patients (mean [SD] age, 59.4 [15.2] years; 35 [72.9%] men among 48 patients with demographic data) undergoing oral cavity or oropharynx surgery for squamous cell carcinoma, including 42 tonsillar tissue, 17 base of the tongue, 4 buccal tissue, 3 mandibular, and 3 other specimens. There were 41 malignant specimens (59.4%) and 28 benign specimens (40.6%). In visual comparisons of WF-OCT images and hematoxylin and eosin slides, visual differentiation among mucosa, submucosa, muscle, dysplastic, and benign tissue was possible in real time using WF-OCT images. Microarchitectural features observed in WF-OCT images could be matched with corresponding features within the permanent histology with fidelity.
Conclusions and Relevance
This qualitative study found that WF-OCT imaging was feasible for visualizing tissue microarchitecture at the surface of resected tissues and was not associated with changes in specimen integrity or surgical and pathology workflow. These findings suggest that formal clinical studies investigating use of WF-OCT for intraoperative analysis of deep margins in head and neck surgery may be warranted.
Introduction
The achievement of negative resection margins has important prognostic implications in the treatment of head and neck squamous cell carcinoma (SCC). Patients found to have involved margins after surgery are known to have increased risk of local recurrence,1,2,3,4,5 poorer rates of progression-free survival,5,6,7,8,9,10 and a need for adjuvant treatments, such as radiotherapy and chemotherapy, and additional surgery. These interventions themselves were associated with increased risk of morbidity and mortality.11,12,13
Anatomical constraints inherent to the head and neck play a role in the difficulty of achieving local control, especially at the posterior, deep margin of the tonsils, where resectable tissue is limited and critical nervous and vascular structures are at risk of surgical injury. With so little room for error, confidence in margin status is critical and is further complicated by limitations of current methods for intraoperative pathological assessment of tissue samples.14
An emerging approach is the use of optical coherence tomography (OCT). This nondestructive imaging modality, first described in the 1990s,15 uses the principle of near-infrared interferometry to produce high-resolution, cross-sectional, and volumetric images of tissues of interest in a manner analogous to the use of sound waves for ultrasonography imaging.16,17,18,19 It was previously shown that trained readers can use OCT images to identify heterogenous and disorganized patterns in tissue microarchitecture, including discrepancies in epithelial layer thickness, from a variety of tissue types.19 This capability suggests that OCT may be useful as a tool to distinguish suspicious regions of interest at the surfaces of surgically resected tissue.
While OCT was originally developed for ophthalmologic applications,15,20 a summary of research from 201516 demonstrated the potential application of the technology in numerous other tissue types. There have also been preliminary studies17,21,22,23,24,25,26 exploring OCT’s utility for imaging soft tissues of the head, neck, and oral cavity. To date, the form factor of investigational OCT tissue-scanning devices has been handheld probes of various designs.25,27,28,29,30,31,32,33 While handheld scanners can allow for in situ imaging of a tumor or cavity, this design has thus far been unable to provide images at sufficient power, resolution, and detail to be clinically useful.
Since 2017, a novel OCT imaging system based on a different approach to tissue scanning has been in use by our institution’s breast surgical service.34,35 This system differs from those based on handheld probes in that it uses a flatbed scanner designed for rapid acquisition of wide-field OCT (WF-OCT) images of whole, excised (ex vivo) surgical specimens prior to processing for standard permanent pathology. The goal of this study was to evaluate the feasibility of using this WF-OCT system to visualize microstructures at the margins of tissue excised from the oral cavity and oropharynx with the aim of investigating whether benign and suspicious features could be observed in WF-OCT images in a way that corresponded to use of permanent hematoxylin and eosin (H&E) pathology slides.
Methods
The protocol for this nonrandomized, investigator-initiated qualitative study evaluating feasibility was approved by the institutional review board of the Icahn School of Medicine at Mount Sinai, and all patients gave signed, informed consent before participating. This study was reported using an adaptation of the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized feasibility and pilot trials.36 Specimens were deidentified, and surgeons (M.Y., M.S.T., E.M.G., and B.A.M.) were blinded to results of the imaging process. Only the principal investigator (B.A.M.) was able to link each patient’s WF-OCT image data to their final pathology report for image comparisons. This was a prospective, single-center feasibility study.
Wide-Field Optical Coherence Tomography
Principles of OCT have been described previously.17,37 Briefly, low-coherence light from the near-infrared spectrum (1250-1350 nm) is split into sample and reference beams; these are then focused at the target tissue or at a reference interferometer, respectively. Backscattered and reflected light recombine at the beam splitter, and the resulting interference patterns allow digital reconstruction of 2-dimensional optical slices at an axial resolution of 6 to 15 μm and a penetration depth of up to 2 mm. Slices can be further stacked to create a volumetric representation of the specimen.
The WF-OCT platform used in this study (Otis version 2.0; Perimeter Medical Imaging AI) is a stand-alone, cart-mounted system. User-operable parts are a flatbed scanning window and a console with a touch screen user interface system for setting parameters, observing image acquisition in real time, and performing postacquisition image review, analysis, and annotation. After excision and prior to imaging, specimens are placed within a single-use, lidded tissue-handling tray that connects to the console to enable application of gentle vacuum pressure to hold the specimen in place against the scanning window during imaging.
At the time of this study, the system was an investigational device. It was subsequently approved by the US Food and Drug Administration for general use as an imaging tool in the evaluation of excised human tissue by providing 2-dimensional, cross-sectional, real-time visualization of human tissues with image review manipulation software for identifying and annotating regions of interest. The system does not have a specific clinical indication for use in oropharyngeal tissue.
Patient Selection
Consecutive adult patients (aged ≥18 years) referred for primary ablative surgery of the oral cavity or oropharynx for biopsy-proven squamous cell carcinoma from 2018 to 2019 were prospectively enrolled in the study. Patients undergoing revision surgery and those previously treated with oral cavity radiation or chemotherapy were excluded. Target enrollment was up to 100 patients.
Study Procedures
Patients were treated according to institutional standards of care (SOC) for surgical management and specimen pathology (Figure 1). Surgical excision of tissue was performed under general anesthesia using electrocautery or an ultrasonic surgical dissection device (Harmonic Scalpel, Ethicon).
Figure 1. Study Workflow.
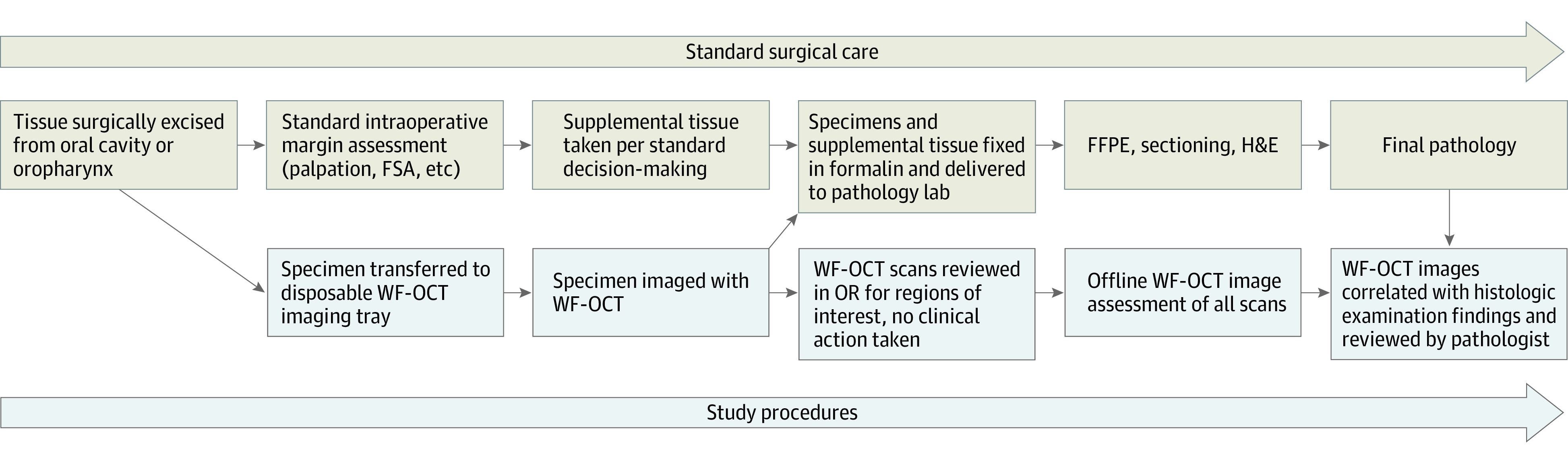
FFPE indicates formalin fixation and paraffin embedding; FSA, frozen section analysis; H&E, hematoxylin and eosin; OR, operating room; WF-OCT, wide-field optical coherence tomography.
Immediately after excision, fresh tissue specimens were passed out of the sterile field to the circulating nurse in the operating room (Figure 1). Additional or supplemental tissue was taken based on the surgeon’s detailed gross visual and physical inspection of the resected specimen and the resection cavity per SOC. Then, prior to frozen section analysis (FSA) or fixation in neutral buffered formalin, primary specimens were marked with ink or sutures for orientation, then placed in a disposable specimen-handling tray and then the scanning window of the WF-OCT imaging system (Video 1).
Video 1. Intraoperative Wide-Field Optical Coherence Tomography Scanning Process.
Real-time intraoperative use of the imaging device on a head and neck tissue specimen. This demonstrates the nondeforming nature of the device, which allows for rapid and accurate images and is not associated with changes in standard-of-care frozen-section analysis. The scan time is 3 minutes for a 10 × 10 cm specimen.
Vacuum was applied to gently hold the specimen in place during scanning. Predetermined system settings for low or high vacuum were selected for each tissue depending on the fragility or density of the specimen. The superior, inferior, medial, lateral, and deep specimen margins, corresponding to those marked with ink and examined during routine pathology sectioning, were scanned for each oropharyngeal specimen (Video 2). Volumes were captured at approximately 15 μm resolution, up to 8.5 × 8.5 cm of tissue surface, and to a penetration depth of up to 2 mm. The WF-OCT scanning system also captured optical photographs of each specimen for orientation and identification.
Video 2. Real-Time Volumetric Review and Analysis of Intraoperative Wide-Field Optical Coherence Tomography Imaging Data.
Wide-field optical coherence tomography and the imaging device allowed real-time volumetric review and analysis of images taken from a tissue specimen. This allows the surgeon to review margins of interest at high resolution in a scrollable format. This is placed side by side with the still image of the specimen on the device console.
Research staff immediately reviewed WF-OCT imaging data in the operating room to ensure that images were of sufficient quality for subsequent detailed analysis. No actions or changes to SOC were taken based on WF-OCT image assessment. Scanning procedures were completed within the cold ischemic window. In some patients undergoing tonsillectomy in which the healthy, contralateral tonsil was also resected as SOC, that tissue was also imaged for comparative purposes. After scanning was completed, specimens were placed in neutral buffered formalin for transport to pathology and processed for sectioning, H&E staining, slide digitization, and histopathological analysis according to institutional SOC.
Data Analysis
An author (A.K.B.) and a trained image reader assessed WF-OCT images of each scanned margin postoperatively. Observed tissue architecture and features were annotated, and regions of interest identified in the WF-OCT images were compared with corresponding regions in digitized histology slide images for each specimen. These were located based on orientations recorded on the device during scanning. A head and neck pathologist (B.A.V. or W.H.W.) then reviewed annotated comparisons and confirmed that images could be used to visibly discern muscle, lymphatic vessels, and other regions from each other on WF-OCT and H&E images. Data were analyzed in October 2019. Analyses of visual comparisons were qualitative, and no formal measures of performance are presented.
Results
Between June 2018 and May 2019, 69 specimens from 53 patients (mean [SD] age, 59.4 [15.2] years; 35 [72.9%] men among 48 patients with demographic data) were collected from a variety of tissue types within the oral cavity and oropharynx and successfully scanned using WF-OCT before being analyzed per SOC (Table; eTable in the Supplement). Analyzed specimens included 42 tonsillar tissue, 17 base of the tongue, 4 buccal tissue, 3 mandibular, and 3 other specimens. There were 41 malignant specimens (59.4%) and 28 benign specimens (40.6%). These were successfully analyzed by SOC histology after WF-OCT scanning. It took approximately 1 to 2 minutes per margin to scan excised specimens, which was not associated with changes in the integrity of specimens for downstream pathology.
Table. Patient Baseline Characteristics.
| Characteristic | Value, No. (%) (N = 48)a |
|---|---|
| Age, mean (SD), y | 59.4 (15.2) |
| Sex | |
| Men | 35 (72.9) |
| Women | 13 (27.1) |
| p16 Test status | |
| Positive | 21 (43.8) |
| Negative | 2 (4.2) |
| Not tested | 26 (54.2) |
| HPV test status | |
| Type 16 | 20 (41.7) |
| Type 35 | 2 (4.2) |
| Type 69 | 1 (2.1) |
| Negative | 2 (4.2) |
| Not tested | 24 (50.0) |
| Diagnosis | |
| SCC | |
| HPV related | 23 (47.9) |
| Not HPV related | 12 (25.0) |
| Otherb | 10 (20.8) |
| No tumor or residual invasive disease found | 3 (6.3) |
Abbreviations: HPV, human papillomavirus; SCC, squamous cell carcinoma.
Data were available for 48 patients.
Other diagnoses included reactive lymphoid hyperplasia (3 patients), cellular pleomorphic adenoma (1 patient), diffuse large B cell lymphoma (1 patient), epithelial hyperplasia (1 patient), invasive malignant melanoma (1 patient), lymphangioma (1 patient), squamous dysplasia (1 patient), and squamous papilloma (1 patient).
Representative comparisons of WF-OCT and H&E images from patients with invasive, human papillomavirus (HPV)–negative and –positive, and p16-negative and -positive SCC are shown in Figure 2, Figure 3, and Figure 4 and eFigures 1 through 6 in the Supplement. Areas suggestive of malignancy as well as benign structures within the healthy surrounding tissue could be observed in WF-OCT images and matched with fidelity within corresponding H&E slides by the trained reader. Benign tonsillar tissues and structures identified in these images included normal tonsillar lymphatic tissue (Figure 2A and Figure 3B), crypts (Figure 2A and Figure 4B), squamous epithelium (Figure 2B and Figure 4B), the submucosal layer (Figure 2B), skeletal muscle (Figure 2B and Figure 3A), a dilated lymphatic channel (Figure 2B), blood vessels (Figure 3C), and a lymph node (Figure 4B).
Figure 2. Representative Comparison of Images for Patient A.
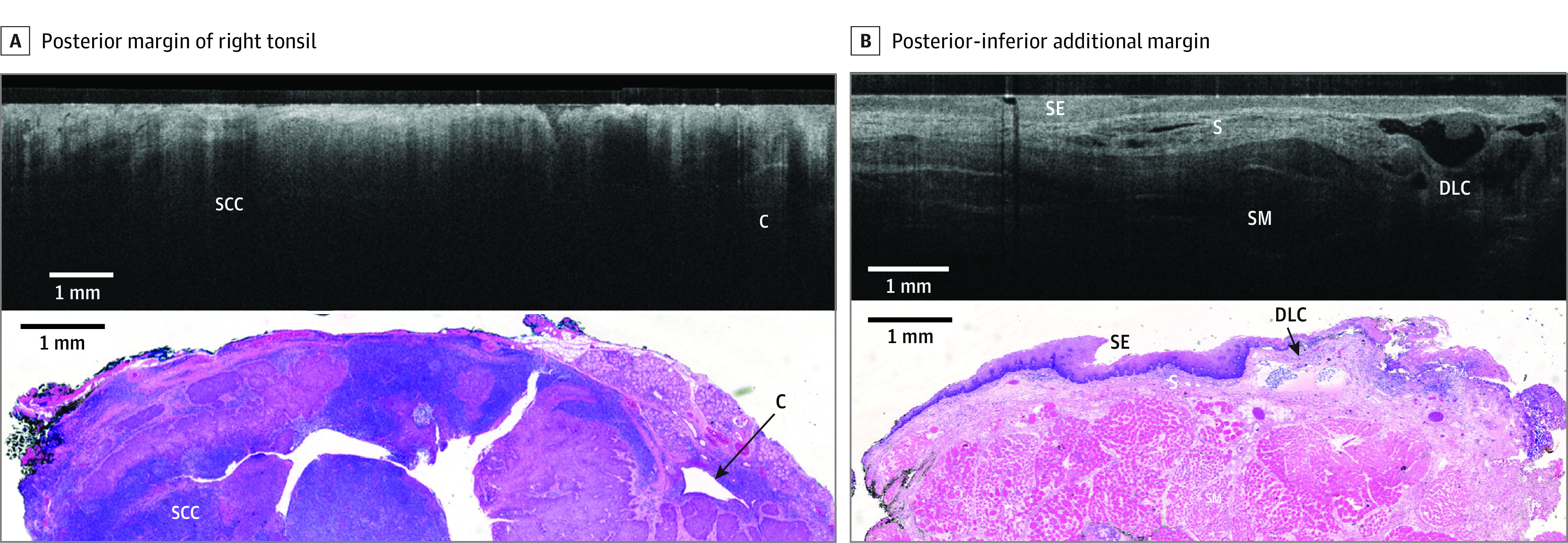
Comparison is shown between wide-field optical coherence tomography and permanent histology of invasive, moderately differentiated squamous cell carcinoma (SCC, p16 negative) with lymphatic invasion. A, The posterior margin of the right tonsil is shown, with wide-field optical coherence tomography in the top panel showing SCC as an area with decreased light penetrance depth compared with the adjacent normal tonsillar lymphatic tissue. In the bottom panel, a hematoxylin and eosin slide from the corresponding region is shown. A crypt (C) is also seen on the right side of the image. B, The posterior-inferior additional margin is shown, with wide-field optical coherence tomography in the top panel showing squamous epithelium (SE), submucosal layer (S), and skeletal muscle (SM). In the bottom panel, a hematoxylin and eosin slide from corresponding region is shown. A dilated lymphatic channel (DLC) is also seen on the right side of the image.
Figure 3. Representative Comparison of Images for Patient B.
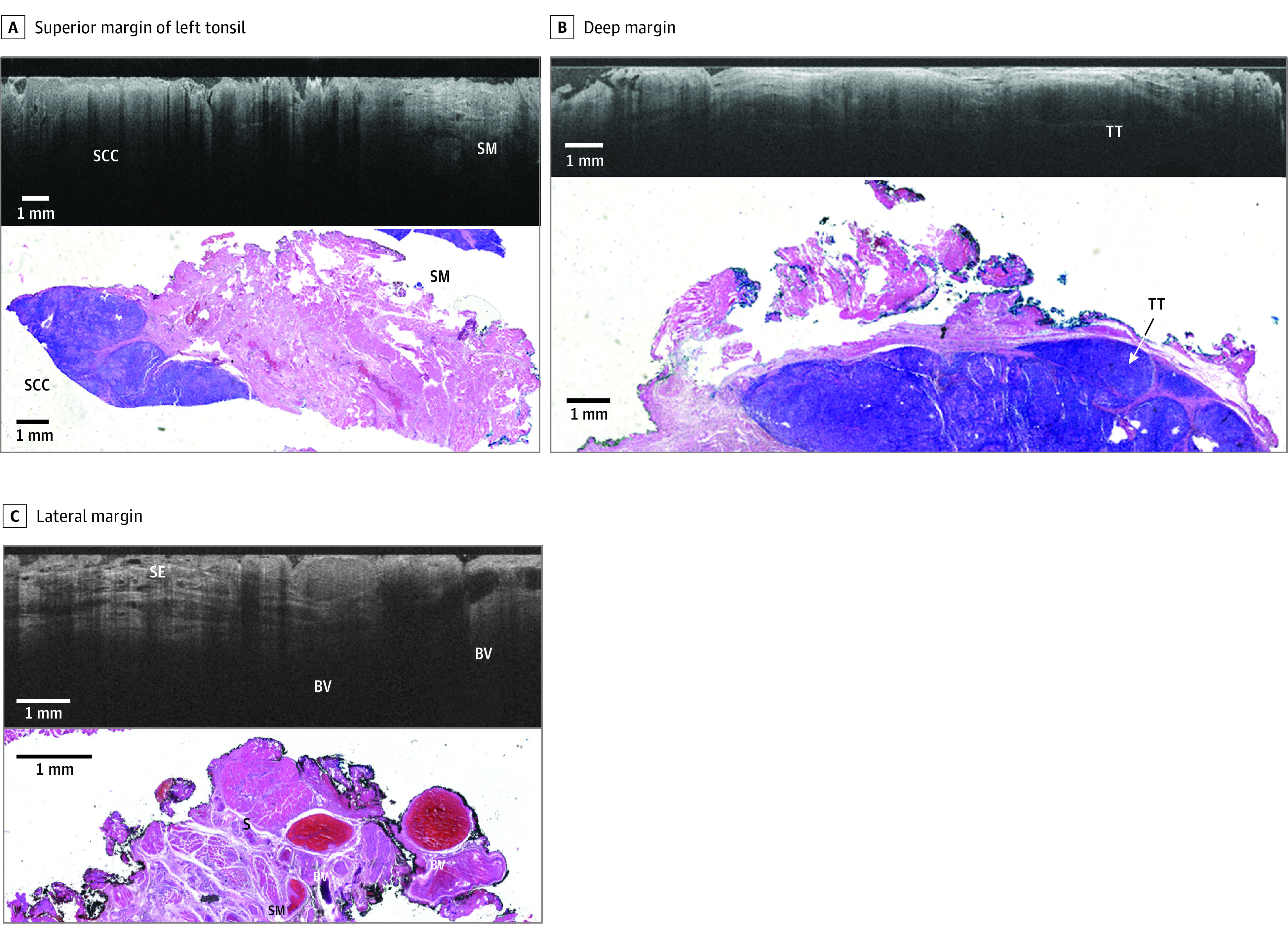
Comparison is shown between wide-field optical coherence tomography and permanent histology of human papillomavirus–related squamous cell carcinoma (SCC, p16 positive) with lymphatic invasion. A, The superior margin of the left tonsil is shown, with wide-field optical coherence tomography in the top panel showing SCC and skeletal muscle (SM). In the bottom panel, a hematoxylin and eosin slide from the corresponding region is shown. The optical coherence tomography optical slice and histology slide are perpendicular to one another. B, The deep margin is shown, with wide-field optical coherence tomography in the top panel showing tonsillar tissue (TT). In the bottom panel, a hematoxylin and eosin slide from the corresponding region is shown. The optical coherence tomography optical slice and histology slide are perpendicular to one another. C, The lateral margin is shown, with wide-field optical coherence tomography in the top panel showing blood vessels (BVs). In the bottom panel, a hematoxylin and eosin slide from the corresponding region is shown.
Figure 4. Representative Comparison of Images for Patient C.
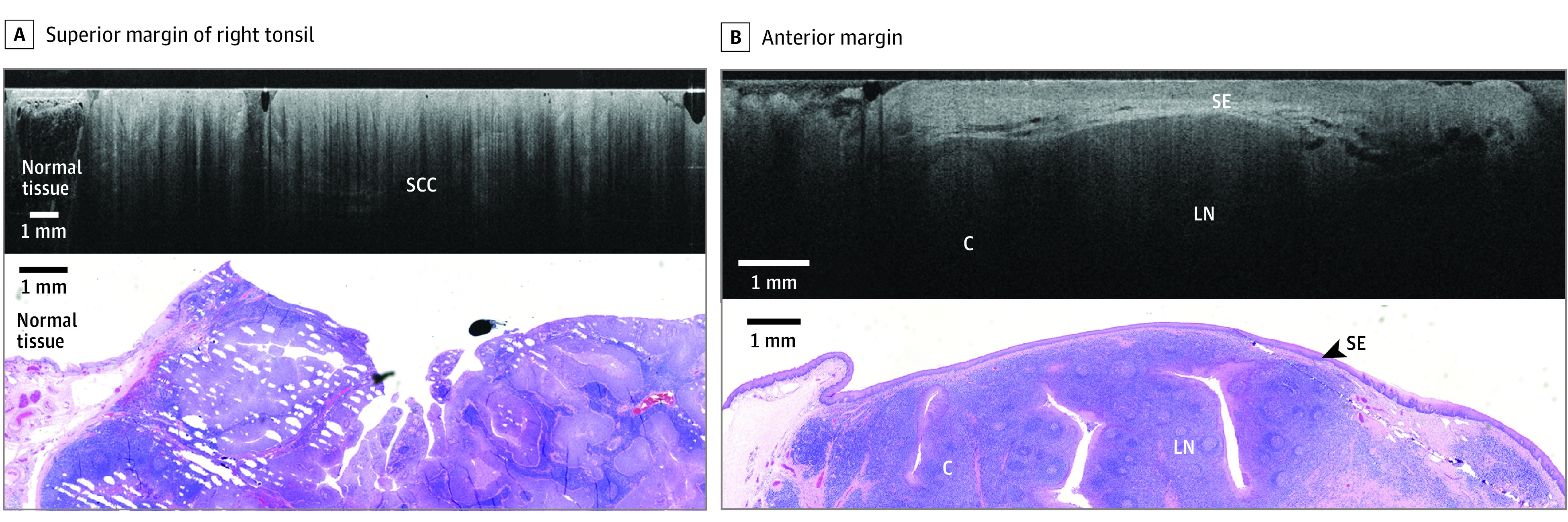
Comparison is shown between wide-field optical coherence tomography and permanent histology of invasive human papillomavirus–related squamous cell carcinoma exhibiting an exophytic papillary component. A, The superior margin of the right tonsil is shown, with wide-field optical coherence tomography in the top panel showing squamous cell carcinoma. In the bottom panel, a hematoxylin and eosin slide from the corresponding region is shown. B, The anterior margin is shown, with wide-field optical coherence tomography of the superior margin showing squamous epithelium (SE), lymph node (LN), and a crypt (C) in the top panel. In the bottom panel, a hematoxylin and eosin slide from the corresponding region is shown.
Adipose tissue, connective tissue, and cysts were also observed. In the tongue, squamous metaplasia and dysplasia were identified. In buccal tissues, benign structures included minor salivary glands and salivary ducts.
Discussion
This nonrandomized, investigator-initiated qualitative study evaluated the feasibility of using WF-OCT as an adjunct imaging modality for the visualization of microstructures at the margin of excised oral cavity or oropharyngeal tissue specimens. We found that the imaging resolution was sufficient to allow identification of specific, pertinent microarchitectural features within WF-OCT images of benign and diseased tissue and that these features could be observed on permanent histology slides from the corresponding location within the specimen. The brief elapsed time necessary to scan the excised specimen (approximately 1-2 minutes per margin) would not have been associated with changes in the timing of the procedure and was not associated with the integrity of the specimens for downstream pathology.
Involvement of deep soft-tissue margins represents a major problem in oropharyngeal cancer.38,39,40 Current intraoperative practices to increase the likelihood of gaining local control include visual gross examination and palpation of the surgical specimen and resection cavity, as well as FSA. While FSA was previously found to be associated with reduced positive surgical margins and decreased re-excision rates,6 the associated time needed for processing and assessment can add as much as 30 minutes to the surgical procedure, requires a pathologist to be on call during the surgery, and is not readily available outside large academic centers. In addition, FSA is a destructive method, so it can be performed on only a small subsection of the primary specimen. It is thus subject to sampling bias, and results of FSA cannot be compared directly with those of permanent histology.5,40
Other avenues of development for real-time, nondestructive intraoperative margin analysis have focused on adapting various forms of confocal microscopy, fluorescence microscopy, and imaging of tissue autofluorescence, fluorescent nuclear contrast agents, or immunofluorescent probes targeted against specific biomarkers.41 While study is ongoing, to date there is insufficient evidence that any 1 of these techniques has sufficient sensitivity and specificity for widespread clinical utility in rapid intraoperative margin assessment.
Significant effort has gone into attempting to develop OCT for this purpose, as well, but handheld probe designs have been insufficient given that they provide low-power images of narrow bands of tissue, which lack sufficient resolution at a clinically relevant depth. The nature of images produced by WF-OCT is very different; WF-OCT is performed ex vivo on a flatbed scanner and has optical sectioning capability similar to that of confocal microscopy. In our study, it rapidly provided high-resolution (approximately 15 μm), en bloc imaging of specimen margins. A surgeon or other trained reader could view and interpret these images before a specimen is sent to pathology. We also found WF-OCT to be flexible and readily adaptable to surgical and pathology workflows.
Optical coherence tomography imaging is widely used for ophthalmologic and endovascular imaging. The use of OCT has also been investigated in numerous other tissues, including breast, lung, cardiac, genitourinary tract, gastrointestinal tract, and head and neck tissues.19 However, the use of WF-OCT is currently principally under study as an adjunct tool for detecting tumor-involved margins in real time during breast-conserving surgery.34,35 To our knowledge, this is the first reported study of WF-OCT in the oral cavity and oropharynx.
Validating the technology specifically for head and neck is important because breast tissue is relatively homogenous compared with the multipart structures of the oral cavity and oropharynx, and disease manifests differently. For example, transitional, premalignant, or in situ disease patterns common to breast tissue are not generally relevant to head and neck cancers; head and neck SCC manifests as starkly different from normal tissue, with little to no architectural transitional morphology. With WF-OCT, we found that epithelium was readily distinguishable from submucosal and muscular layers of the tongue, with a clear and well-delineated transition. This suggests that WF-OCT may therefore be especially useful for analysis of head and neck SCC tumor margins given that they penetrate deeper tissue layers.
Margin assessment is critical at the posterior deep margin of the tonsils and the base of the tongue, and here, every millimeter of tissue is critical to outcomes. In these cases, better margin management may be associated with complete local control, de-escalation of treatment, and the possibility of surgical cure, especially for HPV-associated head and neck SCC. For the population of individuals with HPV who are young and otherwise healthy and who do not smoke, a surgical cure for HPV-associated oral cancer may yield meaningful long-term benefits in health, quality of life, and economic measures that may more than offset the incremental cost of the technology.
More rigorous studies will be necessary to validate the clinical utility and performance of WF-OCT in intraoperative margin assessment during head and neck procedures. However, based on our experience, we hypothesize that WF-OCT imaging may have a place as an adjunct tool with which to collect valuable image data regarding the deep margin during the time in which the surgeon is performing gross assessment of the resection bed. The WF-OCT workflow may be associated with reductions in certain limitations of FSA (eg, sampling bias and sample destruction), but this study was not designed to evaluate superiority or noninferiority. Furthermore, we do not envision WF-OCT as a substitute for standard pathology; it is intended as an adjunct intraoperative imaging modality with data that may serve to give the surgeon an additional level of confidence in the completeness of margin resection during primary surgery.
Limitations
This study has several limitations. It was a device feasibility study with no formal control group and was not designed to make direct comparisons between WF-OCT and other emerging technologies for intraoperative margin assessment, nor was it designed to measure performance. However, it was designed as a prospective study, surgeons were blinded to image assessment at the time of surgery, and at least some portion of the study population formed a self-control group when healthy and diseased contralateral tonsils were removed.
A clinically relevant limitation of OCT in general is that it has a maximum light-penetration depth in biological tissue of 1 to 2 mm. While that depth is generally sufficient for margin assessment in breast oncology,29 tumors of the oral cavity are typically defined as negative if the margin is clear to a depth of 5 mm or more.42 At present, OCT is not capable of assessing tissue microarchitecture at that depth unless multiple scans are acquired from different specimen aspects. This is an area for further study and refinement.
As with other methods of margin analysis, artifacts caused by surgical electrocauterization during transoral robotic surgery present a potential technical limitation because they may complicate image interpretation at the deep margin. Ultrasonic or other low-temperature dissection methods may eventually solve the problem of cautery artifacts, but no such method has yet reached SOC. Further work is needed to solve the problem of margin destruction during electrosurgical dissection.
We also identified a workflow limitation related to standardization of margin orientation and specimen processing. Specifically, we found that specimens were often being serially sectioned along a different axis than the direction of OCT optical slices (eg, sectioned posterior to anterior, while OCT was scanned inferior to superior). This impaired the ability of pathologists to make direct comparisons between WF-OCT images and corresponding histology in some cases. Simple communication and workflow alignment were sufficient to remedy the issue and demonstrate the adaptability of technology and team. Future studies using this technology should consider this workflow detail during study design.
Conclusions
The results of this qualitative study suggest that specimens may be scanned safely with WF-OCT during a procedure. We found that this process was not associated with impaired results of downstream final pathology and that microarchitectural features observed in WF-OCT images of benign and malignant tissue could be located with fidelity within corresponding permanent histology slides.
These findings suggest that formal clinical studies investigating use of WF-OCT for intraoperative analysis of deep margins in head and neck surgery may be warranted. Other potential areas for study include the development of a comprehensive WF-OCT image atlas of benign and abnormal microstructures observed in excised specimens of the oral cavity and oropharynx, evaluation of the use of artificial intelligence tools currently in development to aid in detection of suspicious regions of interest, and a direct comparison of WF-OCT with FSA or specimen x-ray for intraoperative margin analysis.
eTable. Lesion Tissue Origin
eFigure 1. Left Buccal Tissue
eFigure 2. Base of Tongue, View 1
eFigure 3. Base of Tongue, View 2
eFigure 4. Left Lateral Tongue
eFigure 5. Right Lateral Tongue, View 1
eFigure 6. Right Lateral Tongue, View 2
References
- 1.Jesse RH, Sugarbaker EV. Squamous cell carcinoma of the oropharynx: why we fail. Am J Surg. 1976;132(4):435-438. doi: 10.1016/0002-9610(76)90314-7 [DOI] [PubMed] [Google Scholar]
- 2.Jäckel MC, Ambrosch P, Martin A, Steiner W. Impact of re-resection for inadequate margins on the prognosis of upper aerodigestive tract cancer treated by laser microsurgery. Laryngoscope. 2007;117(2):350-356. doi: 10.1097/01.mlg.0000251165.48830.89 [DOI] [PubMed] [Google Scholar]
- 3.Kurita H, Nakanishi Y, Nishizawa R, et al. Impact of different surgical margin conditions on local recurrence of oral squamous cell carcinoma. Oral Oncol. 2010;46(11):814-817. doi: 10.1016/j.oraloncology.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 4.Slootweg PJ, Hordijk GJ, Schade Y, van Es RJ, Koole R. Treatment failure and margin status in head and neck cancer: a critical view on the potential value of molecular pathology. Oral Oncol. 2002;38(5):500-503. doi: 10.1016/S1368-8375(01)00092-6 [DOI] [PubMed] [Google Scholar]
- 5.Smits RW, Koljenović S, Hardillo JA, et al. Resection margins in oral cancer surgery: room for improvement. Head Neck. 2016;38(suppl 1):E2197-E2203. doi: 10.1002/hed.24075 [DOI] [PubMed] [Google Scholar]
- 6.Horwich P, MacKay C, Bullock M, et al. Specimen oriented intraoperative margin assessment in oral cavity and oropharyngeal squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2021;50(1):37. doi: 10.1186/s40463-021-00501-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joo YH, Cho KJ, Kim GJ, Kim MS. Prognostic impact of resection margin involvement in surgically managed HPV-positive tonsil cancer. Oral Oncol. 2020;108:104806. doi: 10.1016/j.oraloncology.2020.104806 [DOI] [PubMed] [Google Scholar]
- 8.Molony P, Kharytaniuk N, Boyle S, et al. Impact of positive margins on outcomes of oropharyngeal squamous cell carcinoma according to p16 status. Head Neck. 2017;39(8):1680-1688. doi: 10.1002/hed.24824 [DOI] [PubMed] [Google Scholar]
- 9.Nieberler M, Häußler P, Kesting MR, et al. Clinical impact of intraoperative cytological assessment of bone resection margins in patients with head and neck carcinoma. Ann Surg Oncol. 2016;23(11):3579-3586. doi: 10.1245/s10434-016-5208-1 [DOI] [PubMed] [Google Scholar]
- 10.Woolgar JA, Rogers S, West CR, Errington RD, Brown JS, Vaughan ED. Survival and patterns of recurrence in 200 oral cancer patients treated by radical surgery and neck dissection. Oral Oncol. 1999;35(3):257-265. doi: 10.1016/S1368-8375(98)00113-4 [DOI] [PubMed] [Google Scholar]
- 11.Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V; EHNS Executive Board. Electronic address: secretariat@ehns.org; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org; ESTRO Executive Board. Electronic address: info@estro.org . Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1462-1475. doi: 10.1016/j.annonc.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 12.Miles BA, Posner MR, Gupta V, et al. De-escalated adjuvant therapy after transoral robotic surgery for human papillomavirus-related oropharyngeal carcinoma: the Sinai Robotic Surgery (SIRS) trial. Oncologist. 2021;26(6):504-513. doi: 10.1002/onco.13742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanvetyanon T, Padhya T, McCaffrey J, et al. Postoperative concurrent chemotherapy and radiotherapy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2015;37(6):840-845. doi: 10.1002/hed.23684 [DOI] [PubMed] [Google Scholar]
- 14.Hinni ML, Ferlito A, Brandwein-Gensler MS, et al. Surgical margins in head and neck cancer: a contemporary review. Head Neck. 2013;35(9):1362-1370. doi: 10.1002/hed.23110 [DOI] [PubMed] [Google Scholar]
- 15.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178-1181. doi: 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drexler W, Fujimoto JG, eds. Optical Coherence Tomography: Technology and Applications. Springer International Publishing Switzerland; 2015. doi: 10.1007/978-3-319-06419-2 [DOI] [Google Scholar]
- 17.Gentile E, Maio C, Romano A, Laino L, Lucchese A. The potential role of in vivo optical coherence tomography for evaluating oral soft tissue: a systematic review. J Oral Pathol Med. 2017;46(10):864-876. doi: 10.1111/jop.12589 [DOI] [PubMed] [Google Scholar]
- 18.Vakoc BJ, Fukumura D, Jain RK, Bouma BE. Cancer imaging by optical coherence tomography: preclinical progress and clinical potential. Nat Rev Cancer. 2012;12(5):363-368. doi: 10.1038/nrc3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Xu Y, Boppart SA. Review of optical coherence tomography in oncology. J Biomed Opt. 2017;22(12):1-23. doi: 10.1117/1.JBO.22.12.121711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuman JS, Puliafito CA, Fujimoto JG, Duker JS. Optical Coherence Tomography of Ocular Diseases. 3rd ed. Slack; 2012. [Google Scholar]
- 21.Albrecht M, Schnabel C, Mueller J, Golde J, Koch E, Walther J. In vivo endoscopic optical coherence tomography of the healthy human oral mucosa: qualitative and quantitative image analysis. Diagnostics (Basel). 2020;10(10):10. doi: 10.3390/diagnostics10100827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamdoon Z, Jerjes W, McKenzie G, Jay A, Hopper C. Optical coherence tomography in the assessment of oral squamous cell carcinoma resection margins. Photodiagnosis Photodyn Ther. 2016;13:211-217. doi: 10.1016/j.pdpdt.2015.07.170 [DOI] [PubMed] [Google Scholar]
- 23.Obade AY, Pandarathodiyil AK, Oo AL, Warnakulasuriya S, Ramanathan A. Application of optical coherence tomography to study the structural features of oral mucosa in biopsy tissues of oral dysplasia and carcinomas. Clin Oral Investig. 2021;25(9):5411-5419. doi: 10.1007/s00784-021-03849-0 [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein M, Hu AC, Chung PS, et al. Intraoperative use of optical coherence tomography to differentiate normal and diseased thyroid and parathyroid tissues from lymph node and fat. Lasers Med Sci. 2021;36(2):269-278. doi: 10.1007/s10103-020-03024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walther J, Schnabel C, Tetschke F, et al. In vivo imaging in the oral cavity by endoscopic optical coherence tomography. J Biomed Opt. 2018;23(7):1-13. doi: 10.1117/1.JBO.23.7.071207 [DOI] [PubMed] [Google Scholar]
- 26.Yang N, Boudoux C, De Montigny E, et al. Rapid head and neck tissue identification in thyroid and parathyroid surgery using optical coherence tomography. Head Neck. 2019;41(12):4171-4180. doi: 10.1002/hed.25972 [DOI] [PubMed] [Google Scholar]
- 27.Erickson-Bhatt SJ, Nolan RM, Shemonski ND, et al. Real-time imaging of the resection bed using a handheld probe to reduce incidence of microscopic positive margins in cancer surgery. Cancer Res. 2015;75(18):3706-3712. doi: 10.1158/0008-5472.CAN-15-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karni T, Pappo I, Sandbank J, et al. A device for real-time, intraoperative margin assessment in breast-conservation surgery. Am J Surg. 2007;194(4):467-473. doi: 10.1016/j.amjsurg.2007.06.013 [DOI] [PubMed] [Google Scholar]
- 29.Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Ann Surg Oncol. 2016;23(12):3801-3810. doi: 10.1245/s10434-016-5449-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridgway JM, Armstrong WB, Guo S, et al. In vivo optical coherence tomography of the human oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 2006;132(10):1074-1081. doi: 10.1001/archotol.132.10.1074 [DOI] [PubMed] [Google Scholar]
- 31.Wong BJ, Jackson RP, Guo S, et al. In vivo optical coherence tomography of the human larynx: normative and benign pathology in 82 patients. Laryngoscope. 2005;115(11):1904-1911. doi: 10.1097/01.MLG.0000181465.17744.BE [DOI] [PubMed] [Google Scholar]
- 32.Yemul KS, Zysk AM, Richardson AL, Tangella KV, Jacobs LK. Interpretation of optical coherence tomography images for breast tissue assessment. Surg Innov. 2019;26(1):50-56. doi: 10.1177/1553350618803245 [DOI] [PubMed] [Google Scholar]
- 33.Zysk AM, Chen K, Gabrielson E, et al. Intraoperative assessment of final margins with a handheld optical imaging probe during breast-conserving surgery may reduce the reoperation rate: results of a multicenter study. Ann Surg Oncol. 2015;22(10):3356-3362. doi: 10.1245/s10434-015-4665-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha R, Friedlander LC, Hibshoosh H, et al. Optical coherence tomography: a novel imaging method for post-lumpectomy breast margin assessment-a multi-reader study. Acad Radiol. 2018;25(3):279-287. doi: 10.1016/j.acra.2017.09.018 [DOI] [PubMed] [Google Scholar]
- 35.Schmidt H, Connolly C, Jaffer S, et al. Evaluation of surgically excised breast tissue microstructure using wide-field optical coherence tomography. Breast J. 2020;26(5):917-923. doi: 10.1111/tbj.13663 [DOI] [PubMed] [Google Scholar]
- 36.Lancaster GA, Thabane L. Guidelines for reporting non-randomised pilot and feasibility studies. Pilot Feasibility Stud. 2019;5:114. doi: 10.1186/s40814-019-0499-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang T, Brewer M, Zhu Q. An overview of optical coherence tomography for ovarian tissue imaging and characterization. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(1):1-16. doi: 10.1002/wnan.1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geltzeiler M, Doerfler S, Turner M, et al. Transoral robotic surgery for management of cervical unknown primary squamous cell carcinoma: updates on efficacy, surgical technique and margin status. Oral Oncol. 2017;66:9-13. doi: 10.1016/j.oraloncology.2016.12.033 [DOI] [PubMed] [Google Scholar]
- 39.Woolgar JA, Triantafyllou A. A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol. 2005;41(10):1034-1043. doi: 10.1016/j.oraloncology.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 40.Mannelli G, Comini LV, Piazza C. Surgical margins in oral squamous cell cancer: intraoperative evaluation and prognostic impact. Curr Opin Otolaryngol Head Neck Surg. 2019;27(2):98-103. doi: 10.1097/MOO.0000000000000516 [DOI] [PubMed] [Google Scholar]
- 41.Miles BA. How close are we to real time optical margin control in head and neck oncologic surgery. Cancer Cell Microenviron. 2016;3:e1305. [Google Scholar]
- 42.Moore EJ, Van Abel KM, Price DL, et al. Transoral robotic surgery for oropharyngeal carcinoma: surgical margins and oncologic outcomes. Head Neck. 2018;40(4):747-755. doi: 10.1002/hed.25055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Lesion Tissue Origin
eFigure 1. Left Buccal Tissue
eFigure 2. Base of Tongue, View 1
eFigure 3. Base of Tongue, View 2
eFigure 4. Left Lateral Tongue
eFigure 5. Right Lateral Tongue, View 1
eFigure 6. Right Lateral Tongue, View 2


