Key Points
Question
Among patients with acute or subacute spine pain, does a multidisciplinary biopsychosocial intervention or an individualized postural therapy intervention improve disability and reduce health care spending?
Findings
In this cluster randomized clinical trial that included 2971 patients from 33 centers, both the biopsychosocial intervention and the postural therapy intervention, each compared with usual care, significantly reduced pain-related disability at 3 months. Compared with usual care at 1 year, the biopsychosocial intervention resulted in no significant difference in spine-related health care spending, and the postural therapy intervention significantly increased spine-related health care spending.
Meaning
Among patients with acute or subacute spine pain, both a biopsychosocial intervention and a postural therapy intervention resulted in modest statistically significant reductions in disability at 3 months compared with usual care; however, the biopsychosocial intervention resulted in no significant difference in spending and the postural therapy intervention resulted in greater spending at 1 year.
Abstract
Importance
Low back and neck pain are often self-limited, but health care spending remains high.
Objective
To evaluate the effects of 2 interventions that emphasize noninvasive care for spine pain.
Design, Setting, and Participants
Pragmatic, cluster, randomized clinical trial conducted at 33 centers in the US that enrolled 2971 participants with neck or back pain of 3 months’ duration or less (enrollment, June 2017 to March 2020; final follow-up, March 2021).
Interventions
Participants were randomized at the clinic-level to (1) usual care (n = 992); (2) a risk-stratified, multidisciplinary intervention (the identify, coordinate, and enhance [ICE] care model that combines physical therapy, health coach counseling, and consultation from a specialist in pain medicine or rehabilitation) (n = 829); or (3) individualized postural therapy (IPT), a postural therapy approach that combines physical therapy with building self-efficacy and self-management (n = 1150).
Main Outcomes and Measures
The primary outcomes were change in Oswestry Disability Index (ODI) score at 3 months (range, 0 [best] to 100 [worst]; minimal clinically important difference, 6) and spine-related health care spending at 1 year. A 2-sided significance threshold of .025 was used to define statistical significance.
Results
Among 2971 participants randomized (mean age, 51.7 years; 1792 women [60.3%]), 2733 (92%) finished the trial. Between baseline and 3-month follow-up, mean ODI scores changed from 31.2 to 15.4 for ICE, from 29.3 to 15.4 for IPT, and from 28.9 to 19.5 for usual care. At 3-month follow-up, absolute differences compared with usual care were −5.8 (95% CI, −7.7 to −3.9; P < .001) for ICE and −4.3 (95% CI, −5.9 to −2.6; P < .001) for IPT. Mean 12-month spending was $1448, $2528, and $1587 in the ICE, IPT, and usual care groups, respectively. Differences in spending compared with usual care were −$139 (risk ratio, 0.93 [95% CI, 0.87 to 0.997]; P = .04) for ICE and $941 (risk ratio, 1.40 [95% CI, 1.35 to 1.45]; P < .001) for IPT.
Conclusions and Relevance
Among patients with acute or subacute spine pain, a multidisciplinary biopsychosocial intervention or an individualized postural therapy intervention, each compared with usual care, resulted in small but statistically significant reductions in pain-related disability at 3 months. However, compared with usual care, the biopsychosocial intervention resulted in no significant difference in spine-related health care spending and the postural therapy intervention resulted in significantly greater spine-related health care spending at 1 year.
Trial Registration
ClinicalTrials.gov Identifier: NCT03083886
This cluster randomized clinical trial compares the efficacy of 2 interventions that emphasize noninvasive care for spine pain vs usual care in reducing pain-related disability and health care spending among patients with acute or subacute spine pain.
Introduction
Spine pain, defined as pain in the back or neck, accounted for more health spending than any other health condition in the US in 2016.1 Outcomes and health care spending for patients with spine pain could potentially be improved by interventions that increase self-efficacy, self-management, and coping skills.2 The identify, coordinate, and enhance (ICE) care model3 uses the STarT Back screening tool4 to select the appropriate intensity of an intervention that combines physical therapy, health coaches who provide counseling to mitigate patient “catastrophizing” of pain, and consultation to a patient’s primary care physician from a specialist in pain medicine or physiatry.3
Individualized postural therapy (IPT) is a specific technique that attempts to treat pain by realigning and rebalancing spinal muscles, emphasizing self-efficacy and self-management.5,6 It is delivered in a standardized fashion using the Egoscue Method at centers throughout the US and internationally.7
Neither the ICE care model nor IPT have been evaluated in randomized clinical trials. Accordingly, the Spine Pain Intervention to Enhance Care Quality and Reduce Expenditure (SPINE CARE) trial was designed to determine whether, compared with usual care, ICE and IPT would significantly reduce disability and spine-related health care spending.
Methods
Study Design
We conducted a 3-group pragmatic, open-label, cluster randomized clinical trial. The trial was approved by the institutional review boards at all participating institutions. All participants provided written or verbal informed consent.
The trial protocol is available in Supplement 1 and was previously published.8 Study enrollment began in June 2017 and ended March 31, 2020, because of slower than anticipated enrollment during the COVID-19 pandemic and funding limitations. Final follow-up occurred March 31, 2021.
Study Setting
This trial was conducted at 33 primary care clinics in the US, including 12 clinics affiliated with an academic medical center (Vanderbilt Medical Center; Nashville, Tennessee), 15 clinics in a community-based integrated delivery network (Honor Health; Phoenix, Arizona), and 6 privately owned clinics (Laguna Hills, North Hollywood, and Oxnard, California; and Houston, Texas) (Figure 1). Eligible practices provided primary care to adult patients with acute back and neck pain and were located within 30-minute driving distance of an Egoscue IPT clinic and within 30 minutes of a physical therapy clinic that could be trained in the ICE protocol. Clinics that had an existing comprehensive spine care practice model were excluded.
Figure 1. Participants Evaluated, Excluded, Randomized, and Analyzed in the SPINE CARE Trial.
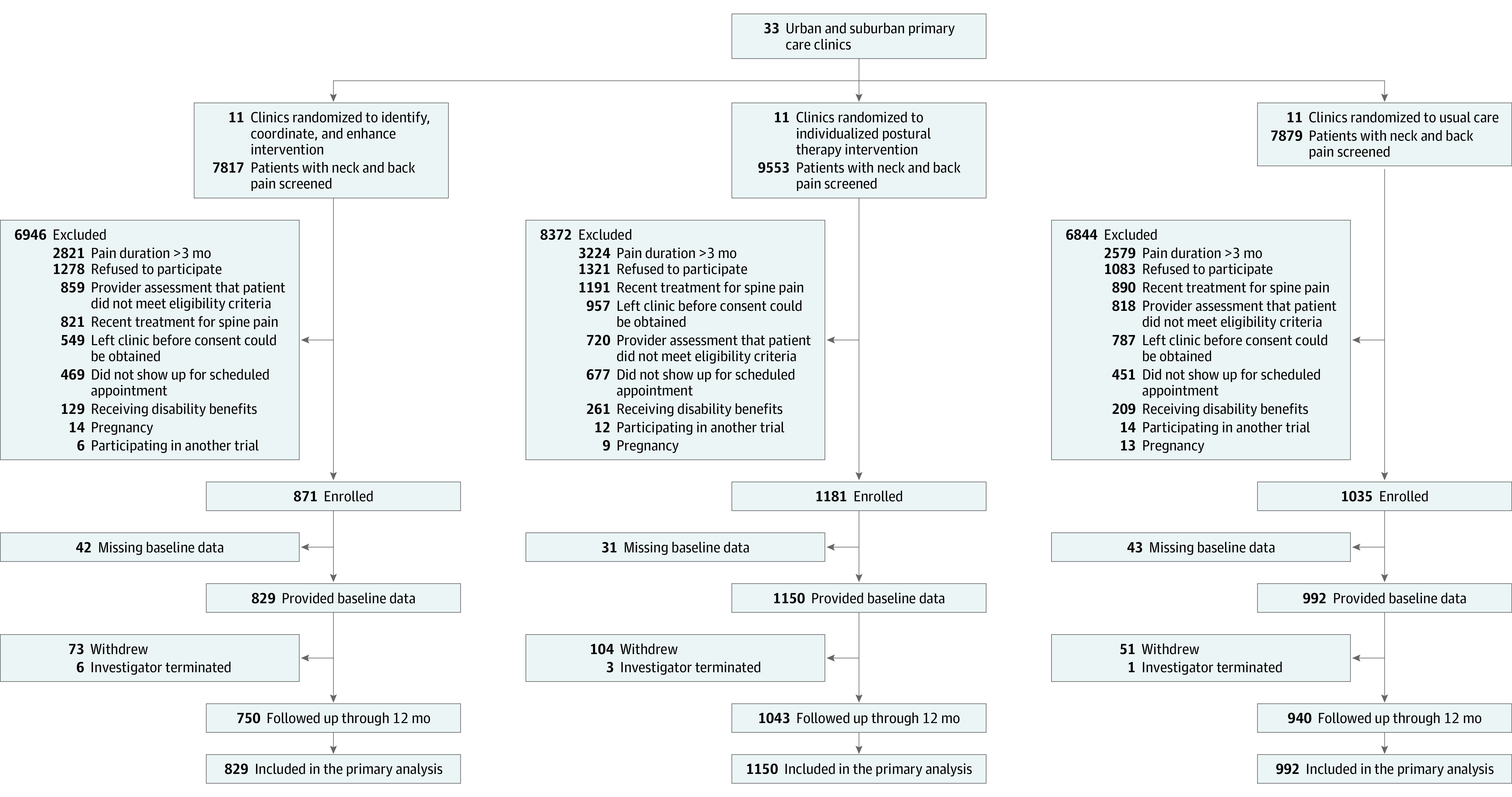
SPINE CARE indicates Spine Pain Intervention to Enhance Care Quality and Reduce Expenditure.
Eligible Participants and Enrollment
Individuals 18 years or older presenting to primary care with neck and/or back pain of 3 months’ duration or less were included. Exclusion criteria included absence of pain in the spine (eg, those with cervicogenic headache without neck pain); receipt of 7 or more consecutive days of narcotics, receipt of 6 or more physical therapy sessions or chiropractic care, acupuncture, postural therapy, or another spine therapy delivered by a trained primary clinician in the prior 3 months; current receipt of disability benefits; pregnancy; active cancer; and history of spine surgery, injections, or rhizotomy within the past 6 months or presence of fever, night sweats, unintentional weight loss, bowel or bladder dysfunction, neurologic weakness, or current intravenous drug use or other medical contraindications to study participation as determined by each patient’s clinician.
Potentially eligible participants were identified through electronic medical record review and were approached when they presented for a primary care visit. Because sociodemographic characteristics may influence access to health care resources and pain-related disability,9 participants were asked to self-report race and ethnicity using the following fixed categories: American Indian or Alaska Native, Asian, Black or African American, Hispanic, White, or other.
Randomization
Primary care practices were randomized in clusters to ICE, IPT, or usual care in a 1:1:1 ratio. Cluster randomization was used because the intervention involved clinic-level workflow changes and this approach minimized contamination between patients cared for by the same clinician or clinicians in the same practice.
Practices were stratified in blocks of 3 and randomized within the strata using a computer-generated random number. Different variables were used in each medical center to stratify randomization and account for differences across the medical centers. The 15 Honor Health clinics were stratified into 5 groups based on their number of potentially eligible participants. The 12 Vanderbilt clinics were stratified into 4 groups according to whether they provided walk-in care and were above or below the median distance to the local IPT clinic. Because the private clinics were from 2 distinct regions (California and Texas), the 6 sites were divided into 2 groups based on state.
Participants, staff, and treating physicians were not blind to group assignment.
Intervention Groups
In all 3 groups, patients did not pay for study visits, but paid standard co-payments according to their insurance carrier for services recommended by their primary care clinician.
ICE Care Model
The ICE care model3 included referral to physical therapy10 and motivational interviewing11 to prevent progression of acute pain to chronic pain by addressing modifiable biomedical, psychological, and social risk factors. The intervention was provided by a physical therapist, a “spine coach” and an “ICE MD.” The spine coach was a professional trained in motivational interviewing. The goals of coaching were to engage participants, provide acute spine pain self-care education, increase patients’ confidence in managing their own care, help patients adhere to physical therapy and prescribed activities, and promote self-efficacy. The ICE MD was a physician with specialty training in physiatry or pain management who advised primary care physicians about potentially beneficial diagnostic testing or therapeutic intervention.
The STarT Back Tool,4 a 9-item self-administered questionnaire, was designed to identify modifiable biomedical, psychological, and social risk factors to classify patients into low, medium, or high risk of developing chronic spine pain. Participants classified as low risk (STarT Back score ≤3) received 1 spine coach telephone consultation of approximately 30 minutes’ duration and 1 physical therapy visit of approximately 60 minutes’ duration. Physical therapy emphasized exercise and educated patients regarding activity modification. The spine coach helped participants with self-management and coping strategies for pain and promoted adherence to prescribed exercises. The ICE MD did not provide routine consultation on low-risk participants.
Participants categorized as medium and high risk (STarT Back score ≥4) received 3 spine coach calls (approximately 30 minutes) and 3 physical therapy visits (approximately 60 minutes). The spine coach focused on physical function, explaining that the patient’s experiences are like those of other patients and building self-management skills. Spine coach calls and physical therapy visits occurred every 1 to 2 weeks, with the goal of completing all visits within 6 weeks. The ICE MD provided a consultation by electronic health record message or telephone to the primary care physician of all participants at medium and high risk for spine pain.
IPT
IPT was delivered using the Egoscue Method,7,12 consisting of a standardized evaluation and an individualized daily exercise program to improve spine alignment, muscle balance, coordination, and postural control.13 Each patient had an initial consultation lasting 1 to 1.5 hours and received up to 8 weekly IPT sessions either in person or via video call. After completing the IPT sessions, a summary progress report was sent to the primary care clinician.
Usual Care
Participants in the usual care group did not receive any interventions.
Primary Outcomes
The 2 primary outcomes were change in participant-level pain-related disability from baseline to 3 months using the Oswestry Disability Index (ODI) and spine-related cost of care at 1 year.14
The ODI ranges from 0 (best) to 100 (worst). The minimal clinically important difference (MCID) for ODI in patients with spine pain is 6 points.15 Resource utilization was measured using previously employed self-report checklists.15,16 Costs were estimated by applying unit costs from publicly available data sources.17,18,19,20
Secondary Outcomes
Secondary outcomes were change in ODI from baseline to 12 months, quality of life measured using the EuroQol 5-dimensional 5-level questionnaire (EQ-5D-5L)21,22,23 (range, 0-100; 100 = best; MCID, 5.3 to 10.5) and self-efficacy scale at 12 months using the “functioning” and “other symptom” subscales of Lorig et al24 (range, 0-100; 100 = best; no MCID defined).
Other Outcomes
We also collected the EQ-5D-5L dimensions of health (ie, mobility, self-care, usual activities, pain and discomfort, anxiety and depression), absenteeism (based on time lost from work or other usual activities associated with spine pain), and presenteeism (using the methods of van den Heuvel et al25). These outcomes are not reported in this article.
Sample Size Calculation
Statistical power was calculated based on the health care cost primary outcome because a larger sample size was required to attain statistical power for cost than for the ODI. Modeling studies estimated that ICE reduced spine-related spending by at least 20%.26,27,28 We assumed spine-related spending of $894 in the usual care group and an SD 1.25 times the mean.29 We assumed an intracluster correlation of 0.01 and 10% loss to follow-up. Similar assumptions were used for IPT due to absence of data on costs for IPT. Based on these assumptions, 3096 patients (1032 per group) were needed for 80% power to detect spending differences between either treatment group and usual care.
Analytic Plan
All changes to the final analytic protocol were made before study results were reviewed. Investigators and data analysts were unaware of outcome results until all follow-up data were obtained and the primary analytic strategies were finalized.
Means and frequencies of prerandomization variables were reported separately for each group. Differences between treatment groups were evaluated using standardized mean differences. Participants were analyzed according to their randomization group, regardless of adherence. Analyses for the 2 primary outcomes compared each treatment with usual care using multiple imputation to handle missing data and a Bonferroni-corrected 2-tailed type I error of .025. We performed 20 imputations with a fully conditional specification using Proc MI in SAS.30 Imputation was performed with the following prespecified variables: age, study group, study site, clinic, sex, race and ethnicity, body mass index, exercise frequency at baseline, education, employment status, smoking status, other medical conditions at baseline, number of medications used for spine pain at baseline, duration of pain at baseline, number of previous pain episodes, STarT Back score, baseline ODI, baseline self-efficacy, baseline EQ-5D-5L, and scores for patient-reported outcomes at every follow-up point (ODI, cost, Lorig et al self-efficacy scale, and EQ-5D-5L). Each imputed data set was analyzed separately using Proc GENMOD in SAS (with an identity link and normally distributed errors for ODI and a log link and Poisson-distributed errors for spine-related spending). Models adjusted for the correlation of participants within clinics (ie, the cluster randomized design) and included fixed effects for the delivery networks used for recruitment. Individual models were pooled using Proc MIANALYZE and adjusted for age and sex. Secondary analyses adjusted for covariates that had standardized mean differences greater than 0.1 at baseline despite randomization.
We conducted several prespecified sensitivity analyses. First, for pain-related disability, we evaluated the proportion of participants achieving a 6-point reduction in ODI from baseline to 3 months. These models used a logit link and binary distributed errors. Second, we conducted a complete-case analysis including individuals with no missing data. Third, because resource utilization and ODI were collected at multiple points, we evaluated cost and ODI data from each point using a repeated measures design. Fourth, because of the skewed nature of cost data, we repeated our analyses after replacing extreme cost values with the 95th percentile value for costs.
We evaluated the effect of treatment within prespecified subgroups including age, sex, STarT Back risk group, pain location, and whether this was the patient’s first pain episode. These analyses included terms interacting with the subgroup variable of interest and indicator terms for study group assignment. We considered treatment effects to differ by subgroup if the 2-tailed P value for the interaction term was less than .05.
Post hoc analyses evaluated spine-related spending after excluding the cost of the ICE and IPT interventions and the comparison of rates of outpatient visits, procedures, diagnostic testing, emergency department visits, hospitalization days, and medication use.
Prespecified analyses of secondary outcomes and post hoc analyses used the same methods as the primary ODI analyses, using 2-tailed P values with a type I error rate of .05. Because of the potential for type I error due to multiple comparisons, findings for secondary end points should be interpreted as exploratory.
All analyses used SAS version 9.4 (SAS Institute Inc).
Results
Because of slower than anticipated enrollment, funding limitations, and the onset of the COVID-19 pandemic, enrollment was stopped when 2971 patients had been randomized, comprising 94% of the target sample size and including 1792 females (60.3%), 71.5% patients who were White, and 56.5% with at least a college education (Figure 1, Table 1; eTable 1 in Supplement 2). Compared with participants in the ICE and IPT groups, those in usual care included a higher proportion of patients who were Black, unemployed, less than college educated, currently smoking, or reporting back pain as their presenting concern. ICE group participants had higher baseline STarT Back scores than IPT and usual care participants.
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Identify, coordinate, and enhance (n = 829) | Individualized postural therapy (n = 1150) | Usual care (n = 992) | |
| Age, mean (SD), y | 50.9 (16.0) | 52.6 (16.0) | 51.2 (16.0) |
| Sex | |||
| Female | 514 (62.0) | 688 (59.8) | 590 (59.5) |
| Male | 315 (38.0) | 462 (40.2) | 402 (40.5) |
| Race and ethnicitya | |||
| American Indian or Alaska Native | 7 (0.8) | 13 (1.1) | 11 (1.1) |
| Asian | 19 (2.3) | 43 (3.7) | 22 (2.2) |
| Black or African American | 80 (9.7) | 120 (10.4) | 207 (20.9) |
| Hispanic | 131 (15.8) | 112 (9.7) | 108 (10.9) |
| White | 604 (72.9) | 868 (75.5) | 651 (65.6) |
| Otherb | 10 (1.2) | 15 (1.3) | 9 (0.9) |
| Employed | 582 (70.2) | 771 (67.0) | 633 (63.8) |
| Education level | n = 827 | n = 1150 | n = 990 |
| High school or less | 139 (16.8) | 152 (13.2) | 277 (28.0) |
| Some college | 192 (23.2) | 296 (25.7) | 236 (23.8) |
| College or graduate degree | 496 (60.0) | 702 (61.0) | 477 (48.2) |
| BMI, median (IQR) | 28.4 (25.0-33.7) | 28.7 (25.0-33.7) | 29.4 (25.1-34.6) |
| Current smoker | 61 (7.4) | 92 (8.0) | 120 (12.1) |
| Medical historya | |||
| Depression/anxiety | 241 (29.1) | 340 (29.6) | 259 (26.1) |
| Osteoarthritis | 98 (11.8) | 121 (10.5) | 101 (10.2) |
| Diabetes | 78 (9.5) | 138 (12.0) | 119 (12.0) |
| No. of previous back and neck pain episodesc | |||
| None | 271 (32.7) | 343 (29.8) | 332 (33.5) |
| 1-2 | 189 (22.8) | 252 (21.9) | 228 (23.0) |
| 3-4 | 116 (14.0) | 179 (15.6) | 128 (12.9) |
| 5-6 | 54 (6.5) | 80 (7.0) | 70 (7.1) |
| >6 | 199 (24.0) | 296 (25.7) | 234 (23.6) |
| Chief concern | |||
| Back pain | 574 (69.2) | 811 (70.5) | 760 (76.6) |
| Back and neck pain | 71 (8.6) | 110 (9.6) | 92 (9.3) |
| Neck pain | 78 (9.4) | 114 (9.9) | 96 (9.7) |
| Otherd | 106 (12.8) | 115 (10.0) | 44 (4.4) |
| Medications taken for spine paina | |||
| NSAIDs | 366 (44.2) | 489 (42.5) | 492 (49.6) |
| Muscle relaxers | 168 (20.3) | 206 (17.9) | 185 (18.7) |
| Steroids | 61 (7.4) | 48 (4.2) | 57 (5.8) |
| Opioids | 29 (3.5) | 39 (3.4) | 61 (6.2) |
| Baseline STarT Back score, mean (SD)e | 4.9 (2.2) | 4.6 (2.2) | 4.5 (2.4) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NSAIDs, nonsteroidal anti-inflammatory drugs.
Participants were allowed to select multiple categories. As a result, the total percentages for each category do not add up to 100%.
The “other” category was one of the fixed categories used during data collection.
Participants self-reported whether they had previously ever had back or spine pain at any point.
Patients could have presented with other symptoms (eg, hip, arm, leg, or head pain) and were included in the trial if these symptoms were attributable to a back or neck origin.
The STartT Back tool identifies modifiable biomedical, psychological, and social risk factors to classify patients based on their risk of developing chronic pain. Scores are based on patient self-report and range from 0 (lowest risk) to 9 (highest risk).
Participants in the ICE group had a mean (SD) of 1.67 (1.25) physical therapy visits and 1.90 (1.13) spine coach consultations (eTable 2 in Supplement 2). IPT group participants attended a mean (SD) of 5.5 (3.3) IPT visits.
Primary Outcomes
Change in mean ODI scores from baseline to 3-month follow-up were 31.2 to 15.4 for the ICE group, 29.3 to 15.4 for IPT, and 28.9 to 19.5 for usual care (Table 2, Figure 2). ICE and IPT participants had significantly greater improvements in ODI scores at 3 months compared with those in the usual care group (between-group differences: ICE group, −5.8 [95% CI, −7.7 to −3.9], P < .001; IPT group, −4.3 [95% CI, −5.9 to −2.6], P < .001).
Table 2. Primary and Secondary Outcomes.
| Mean (SD) | Between-group change | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICE | IPT | Usual care | ICE v usual care | IPT v usual care | |||||||||
| Baseline | Follow-upa | Difference | Baseline | Follow-upa | Difference | Baseline | Follow-upa | Difference | Effect estimate (95% CI)b | P value | Effect estimate (95% CI)b | P value | |
| Primary outcomes | |||||||||||||
| Change in ODI at 3 moc | 31.2 (16.9) | 15.4 (13.0) | −15.9 (16.2) | 29.3 (16.1) | 15.4 (13.7) | −13.9 (15.9) | 28.9 (17.4) | 19.5 (16.2) | −9.4 (18.3) | −5.8 (−7.7 to −3.9) | <.001d | −4.3 (−5.9 to −2.6) | <.001d |
| Total 12-mo spine-related health care spending, $ | 1448 (2756) | 2528 (2756) | 1587 (5774) | 0.93 (0.87 to 0.997) | .04d | 1.40 (1.35 to 1.45) | <.001d | ||||||
| Secondary outcomes | |||||||||||||
| Change in ODI at 12 mo | 31.2 (16.9) | 13.6 (13.0) | −17.7 (16.4) | 29.3 (16.1) | 13.0 (13.8) | −16.3 (16.1) | 28.9 (17.4) | 19.7 (17.8) | −9.2 (18.9) | −7.5 (−9.4 to −5.7) | <.001 | −6.7 (−8.4 to −5.0) | <.001 |
| EQ-5D-5L visual analog scale at 12 moe | 69.4 (18.7) | 78.4 (14.4) | 8.9 (7.7 to 10.2) | 68.7 (18.2) | 79.2 (14.2) | 10.5 (9.4 to 11.6) | 69.7 (19.0) | 72.9 (18.0) | 3.2 (1.8 to 4.5) | 4.7 (2.6 to 6.8) | <.001 | 6.0 (4.1 to 7.9) | <.001 |
| Lorig et al self-efficacy at 12 mof | |||||||||||||
| Functioning subscale | 82.9 (17.2) | 90.3 (12.6) | 7.4 (6.4 to 8.4) | 82.4 (18.7) | 88.7 (14.3) | 6.4 (5.4 to 7.3) | 82.5 (18.5) | 81.5 (20.3) | −1.0 (−2.3 to 0.3) | 6.8 (5.0 to 8.7) | <.001 | 6.7 (5.0 to 8.4) | <.001 |
| Other symptom subscale | 72.7 (20.8) | 83.4 (15.9) | 10.7 (9.4 to 12.1) | 71.5 (21.1) | 84.7 (16.1) | 13.2 (12.1 to 14.3) | 74.1 (21.5) | 77.6 (20.9) | 3.5 (2.1 to 5.0) | 4.0 (1.9 to 6.2) | <.001 | 6.3 (4.2 to 8.3) | <.001 |
Abbreviations: BMI, body mass index; EQ-5D-5L, EuroQol 5-dimensional 5-level; ICE, identify, coordinate, and enhance; IPT, individualized postural therapy; MCID, minimal clinically important difference; ODI, Oswestry Disability Index.
For the primary outcomes, follow-up is 3 months for ODI and 12 months for spine-related spending. For the secondary outcomes, follow-up is 12 months for all outcomes. For the post hoc outcome, follow-up is 12 months.
The effect estimate for ODI at 3 months, ODI at 12 months, EQ-5D-5L, and the Lorig et al self-efficacy scales are absolute differences. The effect estimate for spine-related health spending are risk ratios.
ODI scores capture pain-related disability based on patient self-report. ODI scores range from 0 (best) to 100 (worst). The MCID for ODI in patients with spine pain is 6 points.15
A 2-sided significance threshold of .025 was used to define statistical significance for the 2 primary outcomes.
EQ-5D-5L visual analog scale scores measure health-related quality of life. Scores range from 0 to 100, with 100 indicating the best score. The MCID for the EQ-5D-5L visual analog scale ranges from 5.3 to 10.5.21,22,23
The Lorig et al self-efficacy scale items ask individuals how certain they are that they can perform certain tasks or manage their symptoms. Scores range from 0 to 100, with 100 indicating the best score. The MCID for the Lorig et al self-efficacy scale has not been established.
Figure 2. Baseline, 3-Month Follow-up, and Change in Oswestry Disability Index Score at 3 Months Among Participants With Acute and Subacute Back and Neck Pain.
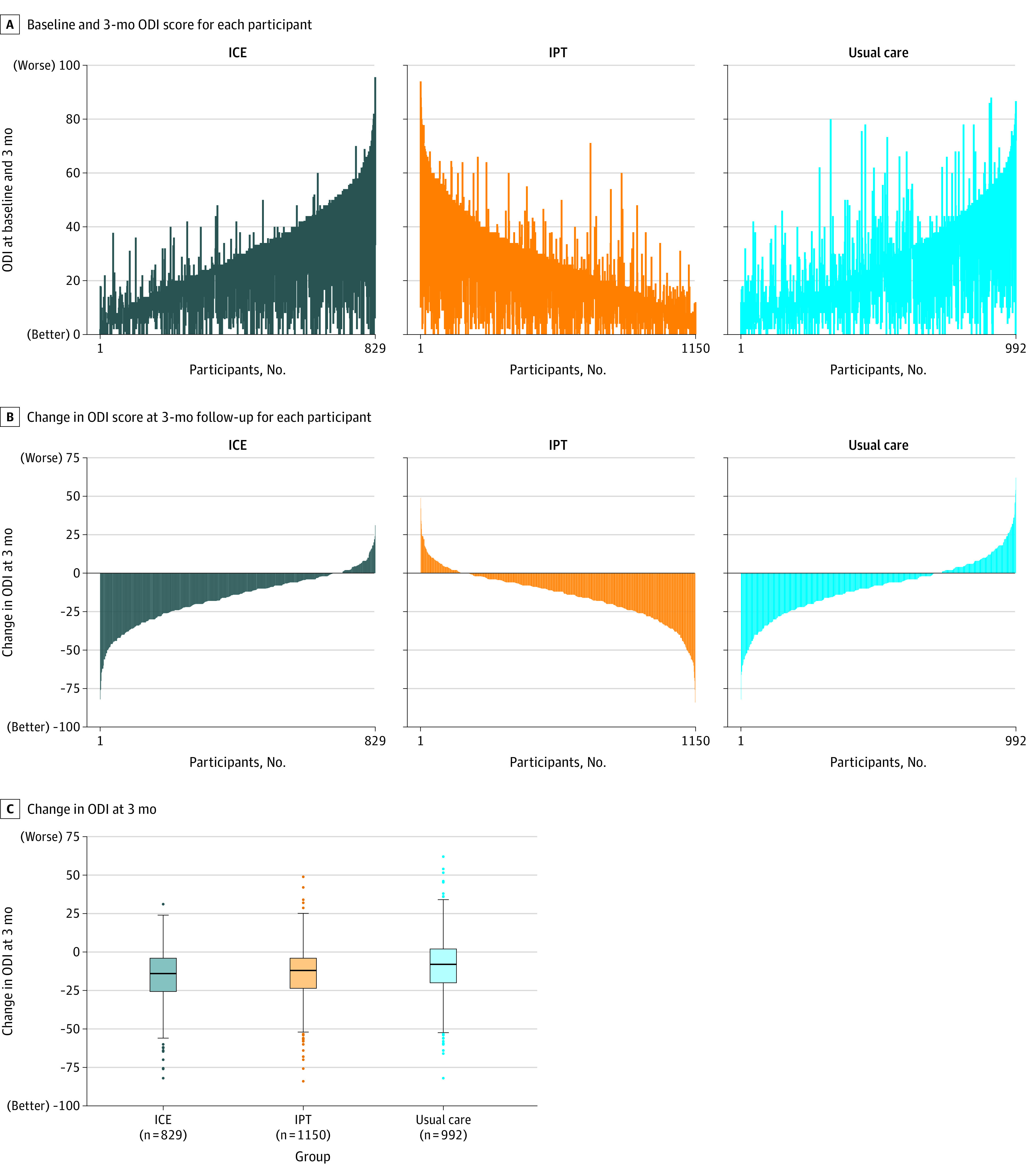
ICE indicates identify, coordinate, and enhance; IPT, individualized postural therapy; and ODI, Oswestry Disability Index.
A, Each vertical line represents an individual participant, with participants ordered by baseline value and the vertical line extending up (deterioration) or down (improvement) to the 3-month value.
B, Vertical lines extending down denote the degree of improvement in ODI score at 3-month follow-up. Vertical lines extending up denote the degree of decline in ODI score.
C, Each box ranges from the 25th (top) to 75th (bottom) percentile of the distribution with the black horizontal line signifying the median. The whiskers extend to the furthest points that are within the 1.5 × IQR of the box (the upper and lower adjacent values). The solid circles beyond the whiskers represent more extreme values.
Mean spine-related spending at 12-month follow-up was $1448, $2528, and $1587 in the ICE, IPT, and usual care groups, respectively. Compared with usual care, total spine-related health care spending for ICE did not meet the prespecified statistically significant threshold of P < .025 (difference, −$139; risk ratio, 0.93 [95% CI, 0.87 to 0.997]; P = .04) and spending for IPT was significantly increased (difference, $941; risk ratio, 1.40 [95% CI, 1.35 to 1.45]; P < .001) (Figure 3; eFigure 1 in Supplement 2).
Figure 3. Total Spine-Related Health Care Spending at 12 Months Among Participants With Acute and Subacute Back and Neck Pain.
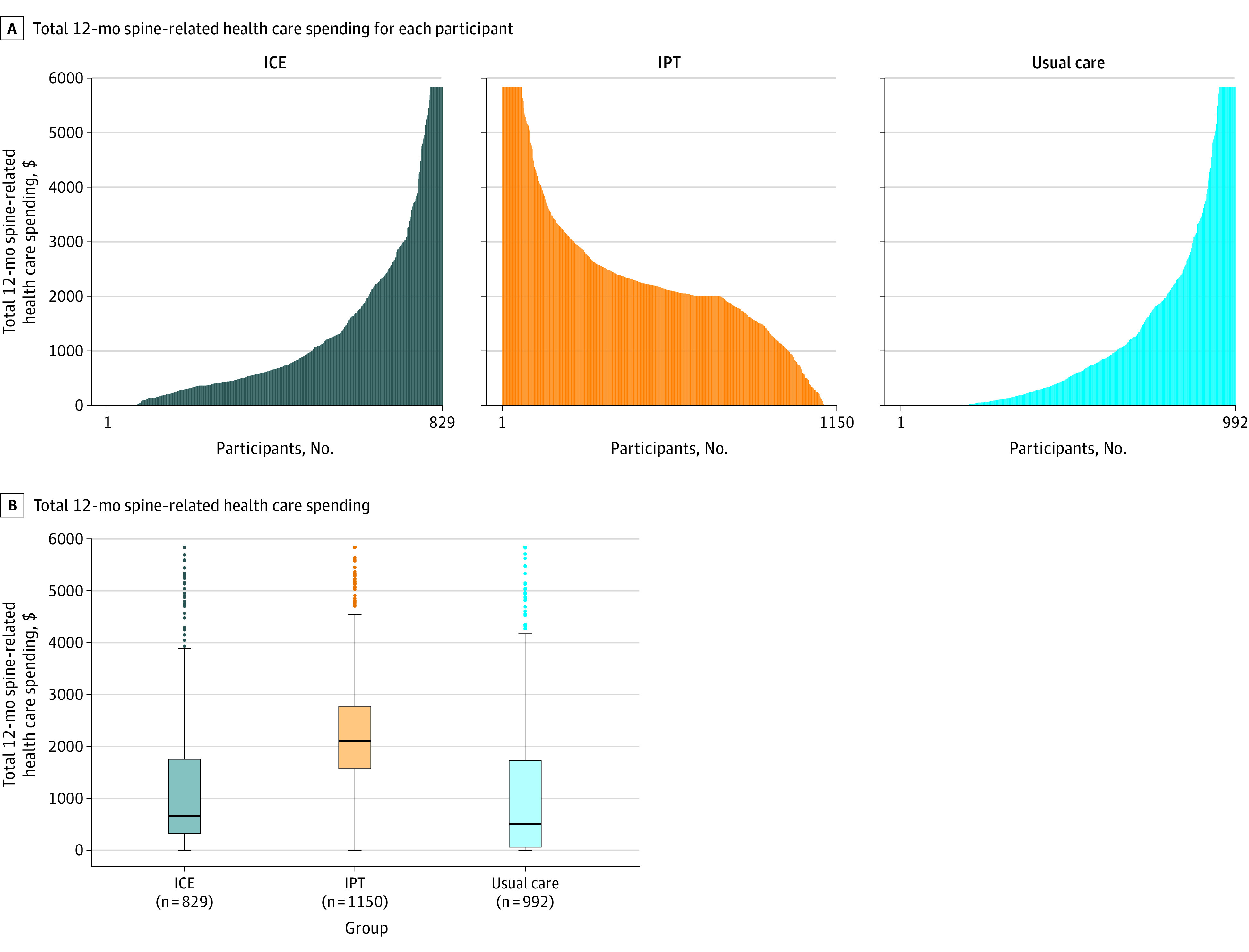
ICE indicates identify, coordinate, and enhance; and IPT, individualized postural therapy.
A, Each vertical line represents the cumulative spine-related over 12 months of follow-up for each participant. Values are winsorized at the 95th percentile for the graphical representation. This 95th percentile threshold was $5837 and winsorization was done for 31 patients in the ICE group, 68 patients in the IPT group, and 49 patients in the usual care group.
B, Each box ranges from the 25th (top) to 75th (bottom) percentile of the distribution with the black horizontal line signifying the median. The whiskers extend to the furthest points that are within the 1.5 × IQR of the box (the upper and lower adjacent values). The solid circles beyond the whiskers are outliers. Values are winsorized at the 95 percentile for the graphical representation.
ODI results were missing at 3 months for 25.5% of participants, and spending data were missing at all time points for 12.4% of participants; complete case analyses yielded similar results to the primary analyses (eTables 3 and 4 in Supplement 2).
Secondary Outcomes
Changes in ODI scores from baseline to 12 months were 31.2 to 13.6, 29.3 to 13.0, and 28.9 to 19.7 in the ICE, IPT, and usual care groups, respectively (Table 2). Compared with usual care, ODI at 12 months was significantly reduced in the ICE (−7.5 points [95% CI, −9.4 to −5.7]; P < .001) and IPT (−6.7 points [95% CI, −8.4 to −5.0]; P < .001) groups (Table 2; eFigure 2 and eTable 5 in Supplement 2).
Changes in EQ-5D-5L visual analog scores from baseline to 12 months were 69.4 to 78.4, 68.7 to 79.2, and 69.7 to 72.9 in the ICE, IPT, and usual care groups, respectively. Compared with usual care, 12-month changes in the EQ-5D-5L visual analog scale scores were 4.7 for ICE (95% CI, 2.6 to 6.8) and 6.0 for IPT (95% CI, 4.1 to 7.9) (Table 2; eFigure 3 in Supplement 2).
Changes in the Lorig et al24 self-efficacy functioning subscale scores from baseline to 12 months were 82.9 to 90.3, 82.4 to 88.7, and 82.5 to 81.5 in the ICE, IPT, and usual care groups, respectively. Compared with usual care, differences at 12 months in this measure were 6.8 (95% CI, 5.0 to 8.7) for ICE and 6.7 (95% CI, 5.0 to 8.4) for IPT (Table 2; eFigure 4 in Supplement 2). Changes in Lorig et al24 other symptom subscale scores from baseline to 12-month follow-up were 72.7 to 83.4, 71.5 to 84.7, and 74.1 to 77.6 in the ICE, IPT, and usual care groups, respectively. Compared with usual care, differences at 12 months in this measure were 4.0 (95% CI, 1.9 to 6.2) for ICE and 6.3 (95% CI, 4.2 to 8.3) for IPT (Table 2; eFigure 5 in Supplement 2).
Additional Analyses
Compared with usual care, the effect of the interventions on pain-related disability did not vary significantly across the following prespecified subgroups: age, sex, back pain location, STarT Back pain score, or whether the patient was presenting with a first episode of back pain (eTable 6 in Supplement 2). For health care costs, the intervention effects varied for some subgroups (eTable 7 in Supplement 2).
At 3-month follow-up, compared with usual care, the ICE and IPT groups had higher rates of attaining a 6-point reduction in ODI (70% for ICE, 67% for IPT, and 58% for usual care) (ICE group odds ratio, 1.75 [95% CI, 1.44 to 2.12]; IPT group odds ratio, 1.54 [95% CI, 1.29 to 1.84) (eFigure 6 in Supplement 2).
Post Hoc Analyses
Analyses of health care costs that excluded the cost of the ICE and IPT interventions are presented in eTable 8 and eFigure 7 in Supplement 2. Associations of the 2 interventions on types of resource utilization are in eTable 9 in Supplement 2.
Discussion
Among patients with acute or subacute spine pain, ICE or IPT, compared with usual care, each resulted in small but statistically significant reductions in pain-related disability at 3 months. However, compared with usual care, ICE did not significantly improve spine-related health care spending and IPT significantly increased spine-related health care spending at 1 year. Both interventions improved health-related quality of life and self-efficacy.
Prior randomized trials that evaluated interventions similar to the ICE intervention yielded mixed results. First, Hill et al2 tested a STarT Back–stratified intervention in the UK that included “psychologically-informed” physical therapy for 1573 individuals with spine pain of any duration who had consulted a primary care clinician and found that the intervention significantly reduced disability measured on the Roland and Morris Disability Questionnaire by 1.7 points (score range, 0 to 24), although the magnitude of the effect was below the minimum clinically important difference. Second, in the MATCH cluster randomized clinical trial of 1701 patients with low back pain, an intervention similar to that tested by Hill et al was not effective for improving back pain physical function and pain severity compared with usual primary care.31 Third, the TARGET randomized trial that included 2300 patients with low back pain of less than 3 months’ duration presenting to primary care reported no effect of psychologically informed physical therapy on development of chronic low back pain or self-reported disability at 6 months.32
In contrast to this study, the MATCH and TARGET clinical trials used electronic health record alerts to encourage primary care physicians to prescribe risk-appropriate care, but these electronic health record tools were used by physicians for only approximately half of intervention patients. This may explain the negative results of the MATCH and TARGET clinical trials. In contrast, most potentially eligible patients in the current trial were identified in advance of a clinic visit and were referred for the study intervention by clinic staff. While use of clinic staff likely increases intervention costs, in the current clinical trial, 74% of ICE participants attended at least 1 physical therapy visit and 84% had at least 1 spine coach consultation. Similar rates of protocol adherence were observed in IPT. Another potentially important feature of the current clinical trial was that even patients at low risk for developing chronic spine pain were referred for the intervention. For the ICE intervention, this feature of our intervention resulted in all patients receiving motivational interviewing by trained health coaches. In contrast, prior clinical trials used only physical therapists for the interventions.
ICE had no significant effect on spine-related health care spending. Similarly, the trial by Hill et al2 reported no significant effect of the intervention on health care costs in the UK. Spine-related spending for patients receiving IPT was significantly higher than for usual care. Whether the cost of the ICE and IPT interventions are justified by their benefits will require a formal cost-effectiveness analysis.
Limitations
This study has several limitations. First, there was no allocation concealment. Second, there were differences at baseline in characteristics of participants in the usual care group compared with those in the 2 intervention groups. These baseline imbalances may have contributed to the differences in outcomes between each intervention and usual care. Third, participants were not blinded to their assigned intervention. This is a particularly important limitation for self-reported outcomes, such as the ODI. Fourth, health care utilization data were collected by self-report, which is subject to recall bias and other sources of inaccuracy.33 Fifth, a lower proportion of individuals allocated to ICE and IPT agreed to participate than in usual care. This may have influenced results if only highly motivated people were inclined to participate in the ICE and IPT interventions. Sixth, there was no attention control group. Seventh, while subgroup analyses by age were prespecified, the age categories for these subgroup analyses were not prespecified.
Conclusions
Among patients with acute or subacute spine pain, a multidisciplinary biopsychosocial intervention or an individualized postural therapy intervention, each compared with usual care, resulted in small but statistically significant reductions in pain-related disability at 3 months. However, compared with usual care, the biopsychosocial intervention resulted in no significant difference in spine-related health care spending and the postural therapy intervention resulted in significantly greater spine-related health care spending at 1 year.
Trial Protocol
eTable 1. Characteristics of the included clinics
eTable 2. Use of interventions by treatment group
eTable 3. Pain-related disability sensitivity analyses
eTable 4. Spine-related spending sensitivity analyses
eTable 5. Clinical outcomes by treatment group at all time points
eTable 6. Subgroup analyses for change in Oswestry Disability Index from baseline to 3 months
eTable 7. Subgroup analyses for change in total spine-related health spending from baseline to 12 months
eTable 8. 12-month resource utilization with and without intervention costs
eTable 9. 12-month resource utilization by study arm
eFigure 1. Cumulative spine-related health care spending over the 12 months after enrollment
eFigure 2. Oswestry Disability Index scores at baseline, 1.5 months, 3 months, 6 months and 12 months after enrollment
eFigure 3. EQ-5D visual analogue scale scores at baseline, 1.5 months, 3 months, 6 months and 12 months after enrollment
eFigure 4. Lorig Functioning subscale scores at baseline, 1.5 months, 3 months, 6 months and 12 months after enrollment
eFigure 5. Lorig Other Symptom subscale scores at baseline, 1.5 months, 3 months, 6 months and 12 months after enrollment
eFigure 6. Proportion of individuals achieving 6-point reduction in ODI from baseline to 3-months
eFigure 7. Cumulative spine-related health care spending excluding intervention costs over the 12 months after enrollment
Data Sharing Statement
References
- 1.Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996-2016. JAMA. 2020;323(9):863-884. doi: 10.1001/jama.2020.0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill JC, Whitehurst DG, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378(9802):1560-1571. doi: 10.1016/S0140-6736(11)60937-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood A, Matula SR, Huan L, Linos E, Platchek T, Milstein A. Improving the value of medical care for patients with back pain. Pain Med. 2019;20(4):664-667. doi: 10.1093/pm/pnx049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill JC, Dunn KM, Lewis M, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59(5):632-641. doi: 10.1002/art.23563 [DOI] [PubMed] [Google Scholar]
- 5.Pillastrini P, de Lima E Sá Resende F, Banchelli F, et al. Effectiveness of global postural re-education in patients with chronic nonspecific neck pain: randomized controlled trial. Phys Ther. 2016;96(9):1408-1416. doi: 10.2522/ptj.20150501 [DOI] [PubMed] [Google Scholar]
- 6.Kudchadkar G, Gurudut P, Welling A. Comparative effect of mat pilates and egoscue exercises in asymptomatic individuals with lumbar hyperlordosis: a randomized controlled trial. Indian J Phys Ther Research. 2019;1(2):79-88. doi: 10.4103/ijptr.ijptr_38_19 [DOI] [Google Scholar]
- 7.Egoscue Inc . The Egoscue Method. Accessed November 16, 2016. https://www.egoscue.com/
- 8.Choudhry NK, Fontanet CP, Ghazinouri R, et al. Design of the Spine Pain Intervention to Enhance Care Quality and Reduce Expenditure Trial (SPINE CARE) study: methods and lessons from a multi-site pragmatic cluster randomized controlled trial. Contemp Clin Trials. 2021;111:106602. doi: 10.1016/j.cct.2021.106602 [DOI] [PubMed] [Google Scholar]
- 9.Licciardone JC, Ganta S, Goehring L, Wallace K, Pu R. Analysis of the patient-physician relationship, race, and pain control and physical function among adults with chronic low back pain. JAMA Netw Open. 2022;5(6):e2216270. doi: 10.1001/jamanetworkopen.2022.16270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz JM, Lane E, McFadden M, et al. Physical therapy referral from primary care for acute back pain with sciatica: a randomized controlled trial. Ann Intern Med. 2021;174(1):8-17. doi: 10.7326/M20-4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey AJ, Lee J, Small JW, et al. Mechanisms of motivational interviewing: a conceptual framework to guide practice and research. Prev Sci. 2021;2(6):689-700. doi: 10.1007/s11121-020-01139-x [DOI] [PubMed] [Google Scholar]
- 12.Egoscue P. The Egoscue Method of Health Through Motion. William Morrow Paperbacks; 1993. [Google Scholar]
- 13.Rencher NR. The acute effects of whole-body corrective exercise on postural alignment. Brigham Young University; 2014.
- 14.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25(22):2940-2952. doi: 10.1097/00007632-200011150-00017 [DOI] [PubMed] [Google Scholar]
- 15.Fritz JM, Magel JS, McFadden M, et al. Early physical therapy vs usual care in patients with recent-onset low back pain: a randomized clinical trial. JAMA. 2015;314(14):1459-1467. doi: 10.1001/jama.2015.11648 [DOI] [PubMed] [Google Scholar]
- 16.Goossens ME, Rutten-van Mölken MP, Vlaeyen JW, van der Linden SM. The cost diary: a method to measure direct and indirect costs in cost-effectiveness research. J Clin Epidemiol. 2000;53(7):688-695. doi: 10.1016/S0895-4356(99)00177-8 [DOI] [PubMed] [Google Scholar]
- 17.Ivanova JI, Birnbaum HG, Schiller M, Kantor E, Johnstone BM, Swindle RW. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J. 2011;11(7):622-632. doi: 10.1016/j.spinee.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicaid Services . Physician Fee Schedule look-up tool. Accessed February 10, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search/overview
- 19.Kaiser Family Foundation . Hospital adjusted expenses per inpatient day. Accessed February 10, 2021. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
- 20.GoodRx . Accessed February 10, 2021. https://www.goodrx.com/
- 21.EuroQol Group . EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 22.Hu X, Jing M, Zhang M, Yang P, Yan X. Responsiveness and minimal clinically important difference of the EQ-5D-5L in cervical intraepithelial neoplasia: a longitudinal study. Health Qual Life Outcomes. 2020;18(1):324. doi: 10.1186/s12955-020-01578-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soer R, Reneman MF, Speijer BL, Coppes MH, Vroomen PC. Clinimetric properties of the EuroQol-5D in patients with chronic low back pain. Spine J. 2012;12(11):1035-1039. doi: 10.1016/j.spinee.2012.10.030 [DOI] [PubMed] [Google Scholar]
- 24.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32(1):37-44. doi: 10.1002/anr.1780320107 [DOI] [PubMed] [Google Scholar]
- 25.van den Heuvel SG, Geuskens GA, Hooftman WE, Koppes LL, van den Bossche SN. Productivity loss at work: health-related and work-related factors. J Occup Rehabil. 2010;20(3):331-339. doi: 10.1007/s10926-009-9219-7 [DOI] [PubMed] [Google Scholar]
- 26.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8-20. doi: 10.1016/j.spinee.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 27.Luo X, Pietrobon R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine (Phila Pa 1976). 2004;29(1):79-86. doi: 10.1097/01.BRS.0000105527.13866.0F [DOI] [PubMed] [Google Scholar]
- 28.Veroff DR, Ochoa-Arvelo T, Venator B. A randomized study of telephonic care support in populations at risk for musculoskeletal preference-sensitive surgeries. BMC Med Inform Decis Mak. 2013;13:21. doi: 10.1186/1472-6947-13-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritz JM, Kim M, Magel JS, Asche CV. Cost-effectiveness of primary care management with or without early physical therapy for acute low back pain: economic evaluation of a randomized clinical trial. Spine (Phila Pa 1976). 2017;42(5):285-290. doi: 10.1097/BRS.0000000000001729 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res. 2015;4(3):287-295. doi: 10.6000/1929-6029.2015.04.03.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherkin D, Balderson B, Wellman R, et al. Effect of low back pain risk-stratification strategy on patient outcomes and care processes: the MATCH randomized trial in primary care. J Gen Intern Med. 2018;33(8):1324-1336. doi: 10.1007/s11606-018-4468-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delitto A, Patterson CG, Stevans JM, et al. Stratified care to prevent chronic low back pain in high-risk patients: the TARGET trial: a multi-site pragmatic cluster randomized trial. EClinicalMedicine. 2021;34:100795. doi: 10.1016/j.eclinm.2021.100795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev. 2006;63(2):217-235. doi: 10.1177/1077558705285298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Characteristics of the included clinics
eTable 2. Use of interventions by treatment group
eTable 3. Pain-related disability sensitivity analyses
eTable 4. Spine-related spending sensitivity analyses
eTable 5. Clinical outcomes by treatment group at all time points
eTable 6. Subgroup analyses for change in Oswestry Disability Index from baseline to 3 months
eTable 7. Subgroup analyses for change in total spine-related health spending from baseline to 12 months
eTable 8. 12-month resource utilization with and without intervention costs
eTable 9. 12-month resource utilization by study arm
eFigure 1. Cumulative spine-related health care spending over the 12 months after enrollment
eFigure 2. Oswestry Disability Index scores at baseline, 1.5 months, 3 months, 6 months and 12 months after enrollment
eFigure 3. EQ-5D visual analogue scale scores at baseline, 1.5 months, 3 months, 6 months and 12 months after enrollment
eFigure 4. Lorig Functioning subscale scores at baseline, 1.5 months, 3 months, 6 months and 12 months after enrollment
eFigure 5. Lorig Other Symptom subscale scores at baseline, 1.5 months, 3 months, 6 months and 12 months after enrollment
eFigure 6. Proportion of individuals achieving 6-point reduction in ODI from baseline to 3-months
eFigure 7. Cumulative spine-related health care spending excluding intervention costs over the 12 months after enrollment
Data Sharing Statement


