Key Points
Question
Does targeting MUC5AC with the NPC-1C antibody augment the antitumor activity of gemcitabine plus nab-paclitaxel as a second-line treatment for pancreatic cancer?
Findings
In this randomized phase 2 trial of 78 patients with advanced pancreatic cancer who previously progressed on first-line FOLFIRINOX, the addition of NPC-1C to second-line gemcitabine plus nab-paclitaxel did not prolong overall survival.
Meaning
Although NPC-1C did not enhance the efficacy of gemcitabine plus nab-paclitaxel in second-line advanced pancreatic cancer, this study establishes efficacy benchmarks, dose modification patterns, and characteristics associated with survival among patients receiving second-line gemcitabine plus nab-paclitaxel.
This randomized clinical trial investigates whether NPC-1C, an antibody directed against MUC5AC, might increase the efficacy of second-line gemcitabine and nab-paclitaxel in patients with advanced pancreatic ductal adenocarcinoma (PDAC).
Abstract
Importance
Treatment options are limited for patients with advanced pancreatic ductal adenocarcinoma (PDAC) beyond first-line 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX), with such individuals commonly being treated with gemcitabine and nab-paclitaxel.
Objective
To determine whether NPC-1C, an antibody directed against MUC5AC, might increase the efficacy of second-line gemcitabine and nab-paclitaxel in patients with advanced PDAC.
Design, Setting, and Participants
This multicenter, randomized phase II clinical trial enrolled patients with advanced PDAC between April 2014 and March 2017 whose disease had progressed on first-line FOLFIRINOX. Eligible patients had tumors with at least 20 MUC5AC staining by centralized immunohistochemistry review. Statistical analysis was performed from April to May 2022.
Interventions
Patients were randomly assigned to receive gemcitabine (1000 mg/m2) and nab-paclitaxel (125 mg/m2) administered intravenously on days 1, 8, and 15 of every 4-week cycle, with or without intravenous NPC-1C 1.5 mg/kg every 2 weeks.
Main Outcomes and Measures
The primary end point was overall survival (OS). Secondary end points were progression-free survival (PFS), objective response rate (ORR), and safety. Pretreatment clinical variables were explored with Cox proportional hazards analysis.
Results
A total of 78 patients (median [range] age, 62 [36-78] years; 32 [41%] women; 9 [12%] Black; 66 [85%] White) received second-line treatment with gemcitabine plus nab-paclitaxel (n = 40) or gemcitabine plus nab-paclitaxel and NPC-1C (n = 38). Median OS was 6.6 months (95% CI, 4.7-8.4 months) with gemcitabine plus nab-paclitaxel vs 5.0 months (95% CI, 3.3-6.5 months; P = .22) with gemcitabine plus nab-paclitaxel and NPC-1C. Median PFS was 2.7 months (95% CI, 1.9-4.1 months) with gemcitabine plus nab-paclitaxel vs 3.4 months (95% CI, 1.9-5.3 months; P = .80) with gemcitabine plus nab-paclitaxel and NPC-1C. The ORR was 3.1% (95% CI, 0.4%-19.7%) in the gemcitabine plus nab-paclitaxel and NPC-1C group and 2.9% (95% CI, 0.4%-18.7%) in the gemcitabine plus nab-paclitaxel group. No differences in toxicity were observed between groups, except that grade 3 or greater anemia occurred more frequently in patients treated with gemcitabine plus nab-paclitaxel and NPC-1C than gemcitabine plus nab-paclitaxel (39% [15 of 38] vs 10% [4 of 40]; P = .003). The frequency of chemotherapy dose reductions was similar in both groups (65% vs 74%; P = .47). Lower performance status, hypoalbuminemia, PDAC diagnosis less than or equal to 18 months before trial enrollment, lymphocyte-to-monocyte ratio less than 2.8, and CA19-9 greater than 2000 IU/mL were independently associated with poorer survival.
Conclusions and Relevance
In this randomized clinical trial of advanced PDAC, NPC-1C did not enhance the efficacy of gemcitabine/nab-paclitaxel. These data provide a benchmark for future trials investigating second-line treatment of PDAC.
Trial Registration
ClinicalTrials.gov Identifier: NCT01834235
Introduction
The aggressive biology of advanced pancreatic adenocarcinoma (PDAC), along with its limited sensitivity to cytotoxic chemotherapy, make systemic therapy for patients with advanced PDAC an enormous management challenge.1 Treatment options are limited and typically consist of 2 cytotoxic chemotherapy regimens that have modest efficacy. First-line chemotherapy options for patients with metastatic PDAC who have a good performance status are FOLFIRINOX (fluororuracil, leucovorin, irinotecan, and oxaliplatin) and gemcitabine plus nab-paclitaxel.2,3 After progression on first-line therapy, patients initially treated with gemcitabine plus nab-paclitaxel are usually offered a fluororuracil-based regimen, whereas patients initially treated with FOLFIRINOX are commonly treated with second-line gemcitabine plus nab-paclitaxel. Although clinical guidelines such as the National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) endorse second-line gemcitabine plus nab-paclitaxel, this recommendation is based on data obtained in the first-line setting, and there are limited prospective data evaluating the efficacy and tolerability of gemcitabine plus nab-paclitaxel in the second-line setting.4,5,6 This paucity of data poses a challenge for clinicians deciding on the appropriate dosing regimen for their patients and for investigators who need efficacy benchmarks for second-line studies involving gemcitabine plus nab-paclitaxel.
The therapeutic strategy of combining cytotoxic chemotherapy with immunogenic antibodies directed against cell-surface proteins has been successful in many malignant neoplasms, and identification of cell-surface targets for this purpose in the setting of pancreatic cancer is of great interest.7,8,9,10,11 The NPC-1C (NEO-102; ensituximab) chimeric IgG1 monoclonal antibody binds a cell-surface antigen found in an allogeneic tumor associated antigen (TAA)–based vaccine that showed preliminary signs of clinical efficacy against colorectal adenocarcinoma.12,13,14 NPC-1C targets an aberrantly glycosylated mucin, MUC5AC, which is produced by pancreatic and colorectal adenocarcinomas, but not by normal tissue.15,16 Preclinical analysis has revealed that exposure to NPC-1C induces antibody-dependent cellular cytotoxicity (ADCC) in MUC5AC-positive PDAC cell lines.17 MUC5AC plays a role in PDAC progression by enhancing its desmoplastic reaction and promoting metastatic spread.13,18,19 In an autochthonous mouse model of PDAC, MUC5AC deficiency impairs oncogenic progression of PDAC precursor lesions and decreases tumor formation.20 Similarly, treatment of murine PDAC models with NPC-1C delays PDAC tumor growth.17
A phase I clinical trial of NPC-1C monotherapy demonstrated a favorable toxicity profile with anemia being the most common grade 3 or 4 toxic effect.21 In an unpublished cohort of 5 patients with advanced PDAC, NPC-1C combined with gemcitabine was well-tolerated with no unexpected toxic effects. Notably, NPC-1C monotherapy showed encouraging signs of disease control as a single agent in colorectal cancer and PDAC patients with 31% having disease control at 8 weeks. We hypothesized that NPC-1C would enhance the activity of second-line gemcitabine plus nab-paclitaxel in patients with advanced PDAC. Here, we report the results of a randomized phase II trial evaluating the efficacy of second-line gemcitabine plus nab-paclitaxel and NPC-1C vs gemcitabine plus nab-paclitaxel alone in patients with advanced PDAC.
Methods
Trial Design
This multi-institutional, open-label, randomized phase II clinical trial was approved by each site’s institutional review board and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines (Supplement 1).22 Thirteen clinical centers in the United States participated in the study. All participants provided written informed consent before participation. The study was registered at ClinicalTrials.gov (NCT01834235) and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.23 The study was industry-sponsored. The manuscript was written by the academic investigators and approved by the sponsor.
Study Population
Eligible patients had pathologically confirmed, locally advanced unresectable, or metastatic PDAC that progressed after primary therapy with FOLFIRINOX, a FOLFIRINOX-like regimen, or were intolerant of it. A FOLFIRINOX–like regimen was defined as fluororuracil/leucovorin or capecitabine combined with irinotecan, oxaliplatin, or both agents. Patients were eligible if their tumor stained with the NPC-1C antibody by at least 20% (tumor staining score) as determined by centralized immunohistochemical review.21 Patients needed to be at least 18 years of age, have measurable disease according to Response Evaluation Criteria in Solid Tumours (RECIST), version 1.1, and have an Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1. Key exclusion criteria included prior receipt of second-line therapy, known brain metastases, any major surgery within 4 weeks of enrollment, and higher than grade 2 ascites at the time of enrollment. Basic demographics, including self-reported race and ethnicity, were prospectively collected for each patient to determine generalizability.
Patients were randomized in a 1:1 ratio to groups receiving gemcitabine plus nab-paclitaxel combined with NPC-1C or gemcitabine plus nab-paclitaxel alone (eFigure 1 in Supplement 2). The randomization was generated by the Biostatistics and Data Management Section, Center for Cancer Research, National Cancer Institute.
Treatment Protocols
All patients initially received gemcitabine (1000 mg/m2) and nab-paclitaxel (125 mg/m2) administered intravenously (IV) on days 1, 8, and 15 of each 28-day cycle. Patients randomized to the experimental group also received NPC-1C (1.5 mg/kg) IV on days 1 and 15. Protocol therapy continued until disease progression, unacceptable toxic effects, or withdrawal of consent. Modifications of the dosing schedules of gemcitabine plus nab-paclitaxel were made at the investigators’ discretion and in accordance with the US Food and Drug Administration label. Two dose reductions of gemcitabine and nab-paclitaxel were allowed. Gemcitabine was reduced from 1000 mg/m2 to 800 mg/m2 and 600 mg/m2 while nab-paclitaxel was reduced from 125 mg/m2 to 100 mg/m2 and 75 mg/m2. When patients experienced toxic effects, investigators could elect to alter the gemcitabine plus nab-paclitaxel administration schedule or discontinue either gemcitabine or nab-paclitaxel. A schedule modification was defined as any time, regardless of the reason, that a patient did not receive an assigned administration of chemotherapy within a cycle.
Efficacy and Safety Assessments
The primary end point was overall survival (OS), defined as the time from start of treatment to death from any cause. Secondary end points were objective response rate (ORR), disease control rate, progression-free survival (PFS), and safety. PFS was defined as the time from treatment start to cancer progression or death, whichever occurred first. The ORR was determined according to RECIST version 1.1, and the disease control rate was defined as having at least a radiographic partial response or stable disease greater than or equal to 16 weeks.24 Radiological assessments occurred every 8 weeks. Adverse reactions were evaluated according to Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0. Following enrollment of the first 6 patients on the experimental group, a protocol-mandated halt to enrollment occurred so the safety of gemcitabine plus nab-paclitaxel and NPC-1C could be assessed.
Statistical Analysis
A sample size of 90 patients enabled the trial to have 80% power, at a 1-sided 10% significance level, to detect an increase in median OS from an anticipated 5 months in the gemcitabine plus nab-paclitaxel group to 8 months in the gemcitabine plus nab-paclitaxel and NPC-1C group. A log-rank test was applied to evaluate differences in PFS and OS between groups. The Kaplan-Meier method was used to assess PFS and OS with 95% CIs. A 2-sided P < .05 was considered statistically significant. Hazard ratios (HRs) indicating the treatment effect on OS within subgroups according to baseline characteristics were calculated and displayed in a forest plot. Differences in the treatment effect between subgroups were evaluated via interaction terms in Cox proportional hazards models.
A post hoc analysis was performed to examine whether any baseline or disease characteristics were associated with OS in univariate and multivariate analyses. We used traditional clinical and demographic factors including number of metastatic sites, pretreatment serum albumin level, performance status, curative-intent therapy with radiation and/or surgery, time from diagnosis to trial enrollment, and sites of metastatic disease.2,25,26 In addition, we used serological biomarkers of immune activation, such as an elevated neutrophil-to-lymphocyte ratio and decreased lymphocyte-to-monocyte ratio, that may have prognostic value in multiple malignant neoplasms, including pancreatic cancer.25,27,28,29,30,31,32,33 The neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio were calculated with cell counts obtained in the pretreatment complete blood count (CBC). The multivariable Cox proportional hazards regression model included forced variables (treatment group, age, sex, stage at trial entry, and performance status) and all optional clinical factors with P < .2 in univariate analysis with OS. Backward selection was used to remove optional clinical factors with P > .05 in the multivariable model. All analyses were performed in Stata version 17 (StataCorp), SAS version 9.4 (SAS Institute), or R version 4.0.3 (R Project for Statistical Computing).
Results
The baseline demographic and clinical characteristics are described in Table 1. Between April 2014 and March 2017, 230 patients were screened for eligibility. As part of eligibility prescreening, an immunohistochemical analysis was performed on archival tumor samples, and 130 patients (56.5%) met the eligibility criteria of having tumors with at least 20% of cells that bound NPC-1C. Eighty patients enrolled in the trial and were randomly assigned to gemcitabine plus nab-paclitaxel and NPC-1C (39 patients) or gemcitabine plus nab-paclitaxel (41 patients). One patient from each group withdrew or became ineligible for the trial before receiving therapy, so they were excluded from subsequent analyses (eFigure 1 in Supplement 2). Among the 78 treated patients (median [range] age, 62 [36-78] years; 32 [41%] women; 9 [12%] Black; 66 [85%] White), 35 patients (45%) had 20% to 40% of cells with positive NPC-1C tumor staining, 17 (22%) had 41% to 60% NPC-1C tumor staining, and 26 (33%) had 61% to 100% NPC-1C tumor staining (eTable 1 in Supplement 2).
Table 1. Baseline Characteristics of Patients Who Started Protocol Treatment.
| Characteristic | No. (%) | |
|---|---|---|
| Gemcitabine/nab-paclitaxel/NPC-1C (n = 38) | Gemcitabine/nab-paclitaxel (n = 40) | |
| Age, median (range), y | 59 (36-78) | 63 (37-78) |
| Gender | ||
| Female | 11 (29) | 21 (52) |
| Male | 27 (71) | 19 (48) |
| Race | ||
| Black | 5 (13) | 4 (10) |
| White | 32 (84) | 34 (85) |
| Othera | 1 (3) | 2 (5) |
| Ethnicity | ||
| Hispanic or Latino | 4 (11) | 4 (10) |
| Not Hispanic or Latino | 33 (87) | 34 (85) |
| Otherb | 1 (3) | 2 (5) |
| Time from initial diagnosis, median (range), mo | 11.6 (2-58) | 12.6 (0.6-46) |
| Performance status | ||
| ECOG 0 | 12 (32) | 15 (38) |
| ECOG 1 | 26 (68) | 25 (62) |
| NPC-1C staining, median (range) | 50 (20-100) | 60 (20-100) |
| Baseline neuropathy | ||
| Present | 12 (32) | 23 (58) |
| Baseline diabetes | ||
| Present | 12 (32) | 11 (28) |
| Stage at trial entry | ||
| Locally advanced | 1 (3) | 3 (8) |
| Metastatic | 37 (97) | 37 (92) |
| Metastatic sites | ||
| Adrenal | 1 (3) | 1 (3) |
| Bone | 3 (8) | 0 |
| Liver | 30 (79) | 28 (70) |
| Lung | 7 (18) | 8 (20) |
| Lymph node | 8 (21) | 9 (23) |
| Peritoneal | 7 (18) | 11 (28) |
| CA19-9 level | ||
| Median (range), IU/mL | 2483, (0-678 278) | 1547, (0-45 842) |
| (<37 IU/mL) Normal | 7 (18) | 4 (10) |
| ULN–<59 × ULN | 11 (29) | 20 (50) |
| ≥59 × ULN (N, %) | 20 (53) | 16 (40) |
| Albumin, g/dL | ||
| Median, range | 3.8 (2.4-4.5) | 3.9 (2.8-4.8) |
| ≥3.4 g/dL | 30 (79) | 36 (90) |
| <3.4 g/dL | 8 (21) | 4 (10) |
| Prior therapy | ||
| Chemotherapy | 38 (100) | 40 (100) |
| Pancreatic surgery | 7 (18) | 13 (33) |
| Radiation | 8 (21) | 10 (25) |
SI conversion factor: To convert albumin levels to g/L, multiply by 10.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; g/dL, grams/deciliter; IU/mL, International Units/milliliter; ULN, upper limit of normal.
Other races were reported as “unknown.”
Other ethnicities were reported as “unknown.”
The baseline demographic and disease characteristics were reasonably balanced between treatment groups (Table 1). Locally advanced disease was rare in both groups (3% in gemcitabine plus nab-paclitaxel and NPC-1C group vs 8% in gemcitabine plus nab-paclitaxel alone group). The median (range) time from initial diagnosis was 12.3 (0.6-58) months. The gemcitabine plus nab-paclitaxel and NPC-1C group had fewer women and patients with peripheral neuropathy than the gemcitabine plus nab-paclitaxel group.
As of the data cutoff on September 30, 2019, all patients had discontinued study treatment or died. The most common reason for removal from the trial was progressive disease in patients receiving gemcitabine plus nab-paclitaxel and NPC-1C or gemcitabine plus nab-paclitaxel (68% vs 58%, P = .49). The median (range) treatment duration was 2.3 (0.4-8.4) months for the gemcitabine plus nab-paclitaxel group and 2.7 (0.3-16.5) months for the gemcitabine plus nab-paclitaxel and NPC-1C group (P = .53).
Efficacy
A preplanned interim futility analysis determined there was no benefit to combining NPC-1C with gemcitabine and nab-paclitaxel, and the trial was closed early (after 80 patients had enrolled) by the Data and Safety Monitoring Committee because of a lack of efficacy. The trial did not meet its primary end point as the addition of NPC-1C to gemcitabine plus nab-paclitaxel did not prolong OS. The median OS was 5.0 months (95% CI, 3.3-6.5 months) for patients in the gemcitabine plus nab-paclitaxel and NPC-1C group and 6.6 months (95% CI, 4.7-8.4 months) for those in the gemcitabine plus nab-paclitaxel group (log-rank P = .22). The HR for death on gemcitabine plus nab-paclitaxel and NPC-1C vs gemcitabine plus nab-paclitaxel was 1.34 (95% CI, 0.84-2.13; P = .22) (Figure 1A). The OS rates were 48.7% (95% CI, 32%-63.5%) for 6 months and 15.8% (95% CI, 6.2%-29.4%) for 12 months for the gemcitabine plus nab-paclitaxel and NPC-1C group vs 53.0% (95% CI, 36.2%-67.3%) for 6 months and 21.2% (95% CI, 10.0%-35.2%) for 12 months for the gemcitabine plus nab-paclitaxel group.
Figure 1. Kaplan-Meier Estimates of Overall Survival and Progression-Free Survival in Patients Treated With Gemcitabine Plus Nab-Paclitaxel and NPC-1C or Gemcitabine Plus Nab-Paclitaxel.
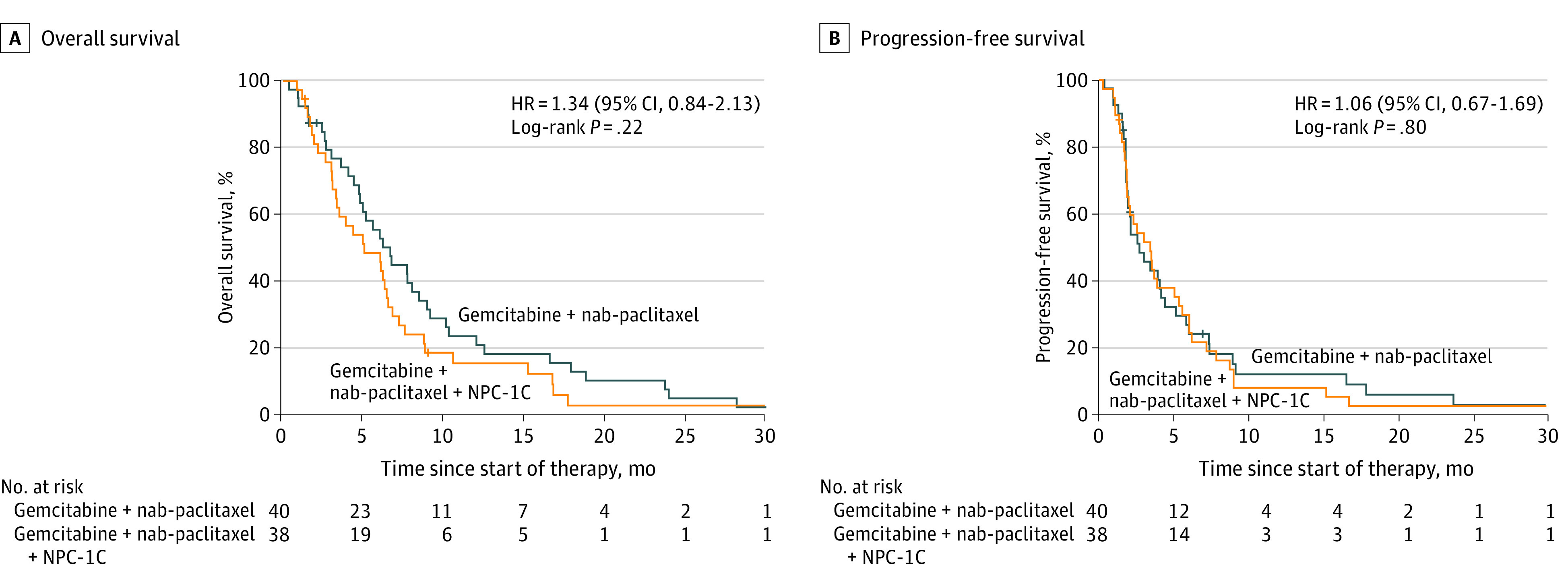
The median PFS was 3.5 months (95% CI, 2.0-5.6 months) for the gemcitabine plus nab-paclitaxel and NPC-1C group and 2.7 months (95% CI, 1.9-4.1 months) for the gemcitabine plus nab-paclitaxel group (log-rank P = .80). The HR for progression after gemcitabine plus nab-paclitaxel and NPC-1C vs gemcitabine plus nab-paclitaxel was 1.04 (95% CI, 0.66-1.66; P = .80) (Figure 1B). The PFS was 32.5% (95% CI, 18.3%-47.6%) for 6 months and 8.1% (95% CI, 2.1%-19.6%) for 12 months for the gemcitabine plus nab-paclitaxel and NPC-1C group vs 24.2% (95% CI, 12.1%-38.6%) for 6 months and 13.5% (95% CI, 4.9%-26.3%) for 12 months for the gemcitabine plus nab-paclitaxel group.
Similarly, no significant difference in ORR was observed between groups (n = 66 evaluable patients). One patient in each group had a confirmed objective response. The ORR was 3.1% (95% CI, 0.4%-19.7%) in the gemcitabine plus nab-paclitaxel and NPC-1C group (n = 32) and 2.9% (95% CI, 0.4%-18.7%) in the gemcitabine plus nab-paclitaxel group (n = 34) (eTable 2 in Supplement 2). There was also no difference in the disease control rate, defined as having at least a radiographic partial response or stable disease greater than or equal to 16 weeks, between the 2 treatment groups. The disease control rate was 28.1% (95% CI, 15.1%-46.2%) in the gemcitabine plus nab-paclitaxel and NPC-1C group and 23.5% (95% CI, 12.1%-40.8%) in the gemcitabine plus nab-paclitaxel group (P = .78).
A post hoc subgroup analysis examining multiple demographic and baseline disease characteristics, including age, ECOG performance status, staging, number of sites of metastases, and NPC-1C staining score, did not identify any subgroup of patients with significantly prolonged OS among patients treated with gemcitabine plus nab-paclitaxel and NPC-1C compared with patients treated with gemcitabine plus nab-paclitaxel alone (eFigure 2 in Supplement 2).
Safety
The most common grade 3 or 4 adverse events observed were toxic effects typically reported for gemcitabine plus nab-paclitaxel: myelosuppression, fatigue, liver function test abnormalities, and neuropathy (Table 2; and eTable 3 and 4 in Supplement 2). Treatment-associated grade 3 or 4 anemia was observed more frequently in patients receiving gemcitabine plus nab-paclitaxel and NPC-1C (39%) than in those in the gemcitabine/nab-paclitaxel group (39% [15/38] vs 10% [4/40]; P = .003) (Table 2). No other significant differences in toxic effects were observed between treatment groups (eTable 4 in Supplement 2). Adverse events resulted in the discontinuation of protocol therapy in 7.9% (95% CI, 2.5%-22.5%) of patients receiving gemcitabine plus nab-paclitaxel and NPC-1C and 17.5% (95% CI, 8.4%-33.0%) of patients receiving gemcitabine plus nab-paclitaxel (P = .31). Notably, no treatment-associated grade 5 events occurred in either group.
Table 2. Most Common Grade 3 or 4 Adverse Events Possibly, Probably, or Definitely Associated With Protocol Treatment.
| All grade ≥3 events | No. (%) | P valuea | ||
|---|---|---|---|---|
| All patients | Gemcitabine/nab-paclitaxel/NPC-1C (n = 38) | Gemcitabine/nab-paclitaxel (n = 40) | ||
| Anemia | 19 (24) | 15 (39) | 4 (10) | .003 |
| Fatigue | 6 (8) | 5 (13) | 1 (3) | .10 |
| Liver function testb | 8 (10) | 6 (16) | 2 (5) | .15 |
| Lymphopenia | 6 (8) | 3 (8) | 3 (8) | >.99 |
| Neutropenia | 26 (33) | 14 (37) | 12 (30) | .63 |
| Peripheral neuropathy | 4 (5) | 1 (3) | 3 (8) | .62 |
| Thrombocytopenia | 20 (26) | 12 (32) | 8 (20) | .30 |
P value comparing gemcitabine plus nab-paclitaxel and NPC-1C vs gemcitabine plus nab-paclitaxel.
Liver function test defined as an abnormality in aspartate transaminase, alanine transaminase, or total bilirubin.
Dose and Treatment Schedule Modifications
Dose modifications of gemcitabine plus nab-paclitaxel were common in both groups, and no differences were observed in the frequency of dose or schedule modifications in either group (eTable 5 in Supplement 2). Chemotherapy dose reductions occurred at least once during the treatment course in 28 of the 38 patients (74%) receiving gemcitabine plus nab-paclitaxel and NPC-1C and 26 of the 40 patients (65%) receiving gemcitabine plus nab-paclitaxel (P = .47) (eTable 6 in Supplement 2). Chemotherapy schedule modifications occurred at least once during the treatment course in 29 of the 38 patients (76%) in gemcitabine plus nab-paclitaxel and NPC-1C group and 26 of the 40 patients (65%) in the gemcitabine plus nab-paclitaxel group (P = .33) (eTable 7 in Supplement 2). By the end of cycle 1, 30 of 38 patients (78.9%) receiving gemcitabine plus nab-paclitaxel and NPC-1C and 25 of 40 patients (62.5%) receiving gemcitabine plus nab-paclitaxel had undergone either a dose reduction or schedule modification (P = .14). Chemotherapy dose reductions in cycle 1 occurred in 20 of 38 patients (52.6%) receiving gemcitabine plus nab-paclitaxel and NPC-1C and 16 of 40 patients (40.0%) on gemcitabine plus nab-paclitaxel (P = .33) (eTable 6 in Supplement 2). Schedule modifications during cycle 1 occurred in 20 of 38 patients (52.6%) receiving gemcitabine plus nab-paclitaxel and NPC-1C and 14 of 40 patients (35.0%) on gemcitabine plus nab-paclitaxel (P = .03) (eTable 7 in Supplement 2).
In an exploratory analysis, we combined the patients in both groups to understand how clinicians adjust therapy in advanced cycles. In the third cycle, 31 of the 38 patients (81.6%) remaining in the trial had undergone a chemotherapy dose reduction (52.6% [20 of 38]) and/or schedule modification (57.9% [22 of 38]). Of those patients, 14 (37%) received full dose gemcitabine plus nab-paclitaxel, 10 (26%) received gemcitabine 800 mg/m2 and/or nab-paclitaxel 100 mg/m2, and 14 (37%) received doses below either gemcitabine 800 mg/m2 or nab-paclitaxel 100 mg/m2. Additionally, in cycle 3, 8 patients (21%) received chemotherapy every other week, and 9 patients (24%) received chemotherapy for 2 weeks on followed by 2 weeks off.
Clinical Features Associated With Survival
Given the limited data on factors associated with survival for patients with advanced PDAC treated with second-line gemcitabine plus nab-paclitaxel, we performed a post hoc analysis to examine whether any baseline clinical or demographic factors were associated with OS. Because we observed no statistically significant differences in efficacy outcomes in both groups, to increase statistical power, our analysis included all patients treated in the trial. Albumin less than 3.4 g/dL (to convert to grams per liter, multiply by 10), diabetes, presence of liver metastases, 2 or more metastatic sites of disease, lymphocyte-to-monocyte ratio less than 2.8, neutrophil-to-lymphocyte ratio greater than 5, platelet-to-lymphocyte ratio at least 180, PDAC diagnosis less than or equal to 18 months before trial enrollment, surgery and/or radiation, neuropathy, and CA19-9 greater than 2000 IU/mL were included in the initial multivariable model based on the results of the univariate analysis (eTable 8 in Supplement 2). In the final multivariable analysis model, lower performance status (HR, 3.92; 95% CI, 1.51-10.13; P = .005), albumin less than 3.4 g/dL (HR, 2.94; 95% CI, 1.15-7.52; P = .02), lymphocyte-to-monocyte ratio less than 2.8 (HR, 3.83; 95% CI, 1.57-9.30; P = .003), PDAC diagnosis less than or equal to 18 months before trial enrollment (HR, 2.77; 95% CI, 1.30-5.88; P = .008), and CA19-9 greater than 2000 IU/mL (HR, 3.38; 95% CI, 1.46-7.81; P = .004) were independently associated with OS (Table 3).
Table 3. Multivariable Analysis Model of Features Associated With Overall Survival.
| Baseline characteristic | Reference | Comparator | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|
| Age, y | <50 | ≥50 | 0.51 (0.17-1.49) | .22 |
| Albumin <3.4 g/dL | No | Yes | 2.94 (1.15-7.52) | .02 |
| CA19-9 > 2000 IU/mL | No | Yes | 3.38 (1.46-7.81) | .004 |
| ECOG performance status | 0 | 1 | 3.92 (1.51-10.13) | .005 |
| Lymphocyte-to-monocyte ratio <2.8 | No | Yes | 3.83 (1.57-9.30) | .003 |
| Sex | Male | Female | 1.64 (0.78-3.44) | .19 |
| Stage | Locally advanced | Metastatic | 1.29 (0.40-4.14) | .67 |
| Time from diagnosis to trial treatment | >18 mos | ≤18 mos | 2.77 (1.30-5.88) | .008 |
| Treatment | Gemcitabine + nab-paclitaxel | Gemcitabine + nab-paclitaxel + NPC-1C | 1.27 (0.65-2.47) | .48 |
SI conversion factor: To convert albumin levels to g/L, multiply by 10.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IU/mL, International Unit/milliliter; g/dL, grams/deciliter.
Having identified clinical and demographic factors that were associated with prognosis, we next examined the prognosis associated with these factors among patients with multiple high-risk features. The median OS was 8.0 months (95% CI, 6.0-12.0 months) for patients with 2 or fewer factors but only 4.3 months (95% CI, 2.6-5.6 months; P < .001) for patients with 3 to 5 factors (Figure 2; eFigure 3 in Supplement 2).
Figure 2. Kaplan-Meier Estimates of Overall Survival, Stratified According to Patient Characteristics.
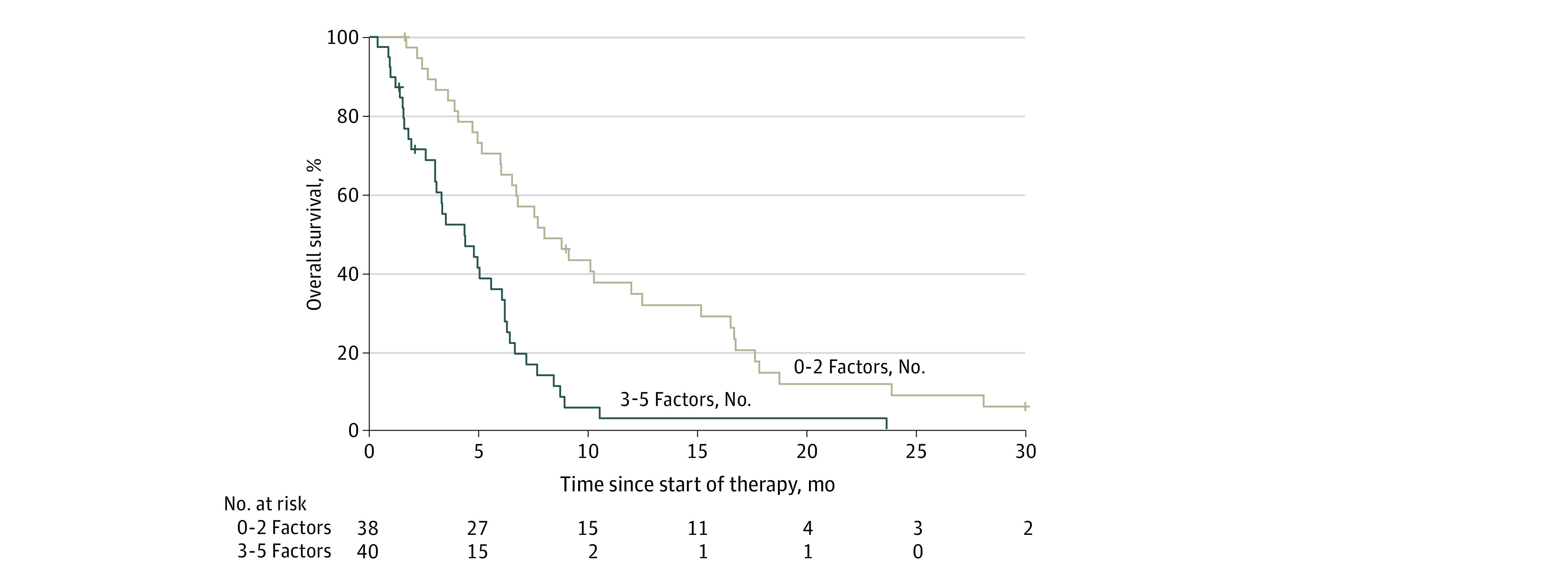
Kaplan-Meier estimates of overall survival stratified according to presence of factors independently associated with survival on multivariable analysis (0-2 factors vs 3-5 factors). Factors included albumin less than 3.4 g/dL (to convert to grams per liter, multiply by 10), CA19-9 greater than 2000 IU/mL, lower performance status, lymphocyte-to-monocyte ratio less than 2.8, and PDAC diagnosis less than or equal to 18 months before trial enrollment.
Discussion
Gemcitabine plus nab-paclitaxel is commonly used as second-line therapy in patients with PDAC who have previously been treated with first-line FOLFIRINOX. This randomized clinical trial found that the addition of NPC-1C, an anti-MUC5AC monoclonal antibody, to second-line gemcitabine plus nab-paclitaxel did not improve OS, PFS, and ORR over gemcitabine plus nab-paclitaxel alone. These disappointing results highlight the challenges of using therapeutics reliant on ADCC in tumors with an immunosuppressive microenvironment, such as PDAC.34,35
Although many patients receive second-line gemcitabine plus nab-paclitaxel in routine clinical practice, few prospective studies have investigated second-line gemcitabine plus nab-paclitaxel after disease progression on FOLFIRINOX.36,37 This trial establishes important efficacy benchmarks for gemcitabine plus nab-paclitaxel as a second-line therapy for PDAC. The median OS for patients receiving second-line gemcitabine plus nab-paclitaxel alone was 6.6 months (95% CI, 4.7-8.4 months), the median PFS was 2.7 months (95% CI, 1.9-4.1 months), and the ORR was 2.9% (95% CI, 0.4%-18.7%). In agreement with our study, the Trybeca-1 phase III trial demonstrated that patients with PDAC treated with second-line gemcitabine/nab-paclitaxel had a median OS of 6.9 months and median PFS of 3.5 months.38 Notably, survival outcomes with second-line gemcitabine plus nab-paclitaxel are similar to those with fluororuracil-based second-line regimens observed in phase III clinical trials with a median PFS of 2 to 3 months and median OS of 6 to 7 months.39,40,41,42,43 The consistently modest efficacy of second-line chemotherapy in PDAC, regardless of regimen, demonstrates how PDAC becomes increasingly resistant to cytotoxic chemotherapy. The diminishing returns from cytotoxic chemotherapy emphasize the urgent need to develop other therapeutic strategies such as targeted and/or immunotherapeutic approaches.44 Future efforts targeting cell surface antigens in PDAC, such as MUC5AC or other mucins, could consider leveraging the increased cytotoxicity observed with third-generation antibody drug conjugates.45,46 The favorable toxicity profile of NPC-1C suggests the feasibility of anti-MUC5AC antibody drug conjugates.
The limited efficacy of second-line gemcitabine plus nab-paclitaxel presents a substantial challenge to clinicians discussing prognosis with their patients and to investigators designing future trials of second-line gemcitabine plus nab-paclitaxel in PDAC. We sought to identify clinical and demographic factors associated with survival among for patients with PDAC receiving second-line gemcitabine plus nab-paclitaxel. Using an exploratory multivariate analysis, we observed that reduced performance status (ECOG of 1 vs 0), hypoalbuminemia, PDAC diagnosis less than or equal to 18 months before trial enrollment, lymphocyte-to-monocyte ratio less than 2.8, and elevated CA19-9 level greater than 2000 IU/mL were independently associated with increased risk of death, in agreement with prior findings.2,25,26,29,32,33,36,47,48,49,50,51 Strikingly, though, there was a significant survival difference dependent upon the number of risk factors: patients with PDAC with 3 or more risk factors had a median OS of 4.3 months (95% CI, 2.6-5.6 months), which was significantly less than those patients with 2 or fewer risk factors (8.0 months [95% CI, 6.0-12.0 months]) (P < .001).
A challenge in using gemcitabine plus nab-paclitaxel as a second-line therapy is that the dosing schedule of this regimen was developed for PDAC patients in the first-line setting. Unlike most patients treated with first-line gemcitabine plus nab-paclitaxel, patients receiving second-line gemcitabine plus nab-paclitaxel had received first-line FOLFIRINOX which causes multiple cumulative toxic effects including myelosuppression and neuropathy. Unsurprisingly, patients in our study required frequent modifications of the gemcitabine plus nab-paclitaxel regimen. The high frequency of dose and schedule modifications suggest that clinicians should expect to tailor the gemcitabine plus nab-paclitaxel regimen when it is used as second-line therapy. Additionally, it suggests that future investigations of second-line gemcitabine plus nab-paclitaxel should consider modified dosing schedules.
Limitations
There are some limitations that may affect generalizability of the efficacy benchmarks and dose modification patterns of this study. One challenge in generalizing these data to all patients with PDAC receiving second-line gemcitabine plus nab-paclitaxel is that the patients in this study were required to meet strict protocol eligibility and consequently may reflect a fitter population than that routinely seen in clinical practice. Hence, the survival benchmarks and dose modification patterns reported here may be different compared with routine clinical practice. In addition, it is important to emphasize that patients in this study had MUC5AC expressing tumors (at least 20% of cells with NPC-1C staining by immunohistochemistry). The effects of MUC5AC positivity on prognosis and sensitivity to gemcitabine plus nab-paclitaxel is unknown, potentially affecting generalizability of our findings to the full population of patients with advanced PDAC. It also should be noted that the multivariate analysis of clinical and demographic factors associated with OS was conducted post hoc. However, other investigators have observed similar results.2,25,26,28,32,42,49,51 Finally, although enrolling an additional 10 patients would have been unlikely to affect the outcomes, the trial closed early for futility, thus slightly reducing the statistical power of the outcome comparisons.
Conclusions
This randomized clinical trial found that the addition of NPC-1C to second-line gemcitabine plus nab-paclitaxel did not improve survival outcomes. Data from this study clearly demonstrated that frequent dosing and schedule modifications of gemcitabine plus nab-paclitaxel are needed when this regimen is used in the second-line setting for the treatment of advanced PDAC. This trial establishes efficacy benchmarks of second-line gemcitabine plus nab-paclitaxel and identifies features that may aid in the design of future clinical trials.
Trial Protocol
eTable 1. NPC-1C Staining Score
eTable 2. Best Radiological Response According to RECIST Criteria
eTable 3. Frequency of Adverse Events by Grade That Were Possibly, Probably, or Definitely Related to Protocol Treatment
eTable 4. Grade 3 or 4 Adverse Events Possibly, Probably, or Definitely Associated with Protocol Treatment
eTable 5. Relative Treatment Dose Intensity (RTDI)
eTable 6. Chemotherapy Dose Reductions
eTable 7. Modifications of Chemotherapy Administration Schedule
eTable 8. Univariate Analysis of Prognostic Features Associated with Overall Survival
eFigure 1. CONSORT Diagram for Patient Disposition
eFigure 2. Subgroup Analysis of Demographic and Baseline Disease Characteristics in Relation to Overall Survival
eFigure 3. Overall Survival of Patients Based on Presence of Prognostic Factors
Data Sharing Statement
References:
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. ; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup . FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh G, Lee HS, Choi JH, et al. Gemcitabine plus Nab-paclitaxel as a second-line treatment following FOLFIRINOX failure in advanced pancreatic cancer: a multicenter, single-arm, open-label, phase 2 trial. Ther Adv Med Oncol. 2021;13:17588359211056179. doi: 10.1177/17588359211056179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pijnappel EN, Dijksterhuis WPM, van der Geest LG, et al. ; Dutch Pancreatic Cancer Group . First- and second-line palliative systemic treatment outcomes in a real-world metastatic pancreatic cancer cohort. J Natl Compr Canc Netw. 2021;20(5):443-450.e3. doi: 10.6004/jnccn.2021.7028 [DOI] [PubMed] [Google Scholar]
- 6.Portal A, Pernot S, Tougeron D, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer. 2015;113(7):989-995. doi: 10.1038/bjc.2015.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Innocenti F, Yazdani A, Rashid N, et al. Tumor Immunogenomic features determine outcomes in patients with metastatic colorectal cancer treated with standard-of-care combinations of bevacizumab and cetuximab. Clin Cancer Res. 2022;28(8):1690-1700. doi: 10.1158/1078-0432.CCR-21-3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang Y-J, Van Cutsem E, Feyereislova A, et al. ; ToGA Trial Investigators . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 9.Sahin U, Türeci Ö, Manikhas G, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32(5):609-619. doi: 10.1016/j.annonc.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 10.Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022;386(4):351-363. doi: 10.1056/NEJMoa2115304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wainberg ZA, Enzinger PC, Kang Y-K, et al. Randomized double-blind placebo-controlled phase 2 study of bemarituzumab combined with modified FOLFOX6 (mFOLFOX6) in first-line (1L) treatment of advanced gastric/gastroesophageal junction adenocarcinoma (FIGHT). J Clin Oncol. 2021;39(3)(suppl):160. doi: 10.1200/JCO.2021.39.3_suppl.160 [DOI] [Google Scholar]
- 12.Hollinshead A, Elias EG, Arlen M, Buda B, Mosley M, Scherrer J. Specific active immunotherapy in patients with adenocarcinoma of the colon utilizing tumor-associated antigens (TAA). a phase I clinical trial. Cancer. 1985;56(3):480-489. doi: [DOI] [PubMed] [Google Scholar]
- 13.Kageyama-Yahara N, Yamamichi N, Takahashi Y, et al. Gli regulates MUC5AC transcription in human gastrointestinal cells. PLoS One. 2014;9(8):e106106. doi: 10.1371/journal.pone.0106106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King J, Bouvet M, Singh G, Williams J. Improving theranostics in pancreatic cancer. J Surg Oncol. 2017;116(1):104-113. doi: 10.1002/jso.24625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004;122(1):61-69. doi: 10.1309/9R6673QEC06D86Y4 [DOI] [PubMed] [Google Scholar]
- 16.Luka J, Arlen PM, Bristol A. Development of a serum biomarker assay that differentiates tumor-associated MUC5AC (NPC-1C ANTIGEN) from normal MUC5AC. J Biomed Biotechnol. 2011;2011:934757. doi: 10.1155/2011/934757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel SP, Bristol A, Saric O, et al. Anti-tumor activity of a novel monoclonal antibody, NPC-1C, optimized for recognition of tumor antigen MUC5AC variant in preclinical models. Cancer Immunol Immunother. 2013;62(6):1011-1019. doi: 10.1007/s00262-013-1420-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manne A, Esnakula A, Abushahin L, Tsung A. Understanding the clinical impact of MUC5AC expression on pancreatic ductal adenocarcinoma. Cancers (Basel). 2021;13(12):3059. doi: 10.3390/cancers13123059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamazoe S, Tanaka H, Sawada T, et al. RNA interference suppression of mucin 5AC (MUC5AC) reduces the adhesive and invasive capacity of human pancreatic cancer cells. J Exp Clin Cancer Res. 2010;29(1):53. doi: 10.1186/1756-9966-29-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganguly K, Krishn SR, Rachagani S, et al. Secretory mucin 5AC promotes neoplastic progression by augmenting KLF4-mediated pancreatic cancer cell stemness. Cancer Res. 2021;81(1):91-102. doi: 10.1158/0008-5472.CAN-20-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beg MS, Azad NS, Patel SP, et al. A phase 1 dose-escalation study of NEO-102 in patients with refractory colon and pancreatic cancer. Cancer Chemother Pharmacol. 2016;78(3):577-584. doi: 10.1007/s00280-016-3108-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 23.Schulz KF, Altman DG, Moher D, Group C; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726-732. doi: 10.7326/0003-4819-152-11-201006010-00232 [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 25.Stocken DD, Hassan AB, Altman DG, et al. Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer. 2008;99(6):883-893. doi: 10.1038/sj.bjc.6604568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. 2015;20(2):143-150. doi: 10.1634/theoncologist.2014-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sierzega M, Lenart M, Rutkowska M, et al. Preoperative neutrophil-lymphocyte and lymphocyte-monocyte ratios reflect immune cell population rearrangement in resectable pancreatic cancer. Ann Surg Oncol. 2017;24(3):808-815. doi: 10.1245/s10434-016-5634-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang-Gillam A, Hubner RA, Siveke JT, et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: final overall survival analysis and characteristics of long-term survivors. Eur J Cancer. 2019;108:78-87. doi: 10.1016/j.ejca.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 29.Goldstein D, El-Maraghi RH, Hammel P, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107(2):dju413. doi: 10.1093/jnci/dju413 [DOI] [PubMed] [Google Scholar]
- 30.Pointer DT Jr, Roife D, Powers BD, et al. Neutrophil to lymphocyte ratio, not platelet to lymphocyte or lymphocyte to monocyte ratio, is predictive of patient survival after resection of early-stage pancreatic ductal adenocarcinoma. BMC Cancer. 2020;20(1):750. doi: 10.1186/s12885-020-07182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue P, Hang J, Huang W, et al. Validation of lymphocyte-to-monocyte ratio as a prognostic factor in advanced pancreatic cancer: an East Asian cohort study of 2 countries. Pancreas. 2017;46(8):1011-1017. doi: 10.1097/MPA.0000000000000891 [DOI] [PubMed] [Google Scholar]
- 32.Ma LX, Espin-Garcia O, Wang Y, et al. Comparison of systematic inflammatory prognostic scores in patients with advanced pancreatic adenocarcinoma. J Clin Oncol. 2022;40(16)(suppl):4149. doi: 10.1200/JCO.2022.40.16_suppl.4149 [DOI] [Google Scholar]
- 33.Ma LX, Holzapfel NT, Wang Y, et al. Prognostic ability of the Gustave Roussy Immune Score for patients with advanced pancreatic adenocarcinoma. J Clin Oncol. 2022;40(4)(suppl):469. doi: 10.1200/JCO.2022.40.4_suppl.469 [DOI] [Google Scholar]
- 34.Bear AS, Vonderheide RH, O’Hara MH. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell. 2020;38(6):788-802. doi: 10.1016/j.ccell.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balachandran VP, Beatty GL, Dougan SK. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology. 2019;156(7):2056-2072. doi: 10.1053/j.gastro.2018.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsang ES, Wong HL, Wang Y, et al. Outcomes and characteristics of patients receiving second-line therapy for advanced pancreatic cancer. Am J Clin Oncol. 2019;42(2):196-201. doi: 10.1097/COC.0000000000000500 [DOI] [PubMed] [Google Scholar]
- 37.Mita N, Iwashita T, Uemura S, et al. Second-line gemcitabine plus nab-paclitaxel for patients with unresectable advanced pancreatic cancer after first-line FOLFIRINOX failure. J Clin Med. 2019;8(6):761. doi: 10.3390/jcm8060761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammel P, El-Hariry I, Macarulla T, et al. Trybeca-1: a randomized, phase 3 study of eryaspase in combination with chemotherapy versus chemotherapy alone as second-line treatment in patients with advanced pancreatic adenocarcinoma (NCT03665441). J Clin Oncol. 2022;40(4)(suppl):518. doi: 10.1200/JCO.2022.40.4_suppl.518 [DOI] [Google Scholar]
- 39.Hecht JR, Lonardi S, Bendell J, et al. Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J Clin Oncol. 2021;39(10):1108-1118. doi: 10.1200/JCO.20.02232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung V, McDonough S, Philip PA, et al. Effect of selumetinib and MK-2206 vs oxaliplatin and fluorouracil in patients with metastatic pancreatic cancer after prior therapy: SWOG S1115 study randomized clinical trial. JAMA Oncol. 2017;3(4):516-522. doi: 10.1001/jamaoncol.2016.5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill S, Ko YJ, Cripps C, et al. PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol. 2016;34(32):3914-3920. doi: 10.1200/JCO.2016.68.5776 [DOI] [PubMed] [Google Scholar]
- 42.Wang-Gillam A, Li C-P, Bodoky G, et al. ; NAPOLI-1 Study Group . Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545-557. doi: 10.1016/S0140-6736(15)00986-1 [DOI] [PubMed] [Google Scholar]
- 43.Chiorean EG, Guthrie KA, Philip PA, et al. Randomized phase II study of PARP inhibitor ABT-888 (veliparib) with modified FOLFIRI versus FOLFIRI as second-line treatment of metastatic pancreatic cancer: SWOG S1513. Clin Cancer Res. 2021;27(23):6314-6322. doi: 10.1158/1078-0432.CCR-21-1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosein AN, Dougan SK, Aguirre AJ, Maitra A. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat Cancer. 2022;3(3):272-286. doi: 10.1038/s43018-022-00349-2 [DOI] [PubMed] [Google Scholar]
- 45.Shah A, Rauth S, Aithal A, et al. The current landscape of antibody-based therapies in solid malignancies. Theranostics. 2021;11(3):1493-1512. doi: 10.7150/thno.52614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315-337. doi: 10.1038/nrd.2016.268 [DOI] [PubMed] [Google Scholar]
- 47.Yuan C, Rubinson DA, Qian ZR, et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. J Clin Oncol. 2015;33(1):29-35. doi: 10.1200/JCO.2014.57.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan C, Kim J, Wang QL, et al. ; PanScan/PanC4 I-III Consortium . The age-dependent association of risk factors with pancreatic cancer. Ann Oncol. 2022;33(7):693-701. doi: 10.1016/j.annonc.2022.03.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiorean EG, Von Hoff DD, Reni M, et al. CA19-9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol. 2016;27(4):654-660. doi: 10.1093/annonc/mdw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee K, Bang K, Yoo C, et al. Clinical outcomes of second-line chemotherapy after progression on nab-paclitaxel plus gemcitabine in patients with metastatic pancreatic adenocarcinoma. Cancer Res Treat. 2020;52(1):254-262. doi: 10.4143/crt.2019.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiorean EG, Von Hoff DD, Tabernero J, et al. Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br J Cancer. 2016;115(2):188-194. doi: 10.1038/bjc.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. NPC-1C Staining Score
eTable 2. Best Radiological Response According to RECIST Criteria
eTable 3. Frequency of Adverse Events by Grade That Were Possibly, Probably, or Definitely Related to Protocol Treatment
eTable 4. Grade 3 or 4 Adverse Events Possibly, Probably, or Definitely Associated with Protocol Treatment
eTable 5. Relative Treatment Dose Intensity (RTDI)
eTable 6. Chemotherapy Dose Reductions
eTable 7. Modifications of Chemotherapy Administration Schedule
eTable 8. Univariate Analysis of Prognostic Features Associated with Overall Survival
eFigure 1. CONSORT Diagram for Patient Disposition
eFigure 2. Subgroup Analysis of Demographic and Baseline Disease Characteristics in Relation to Overall Survival
eFigure 3. Overall Survival of Patients Based on Presence of Prognostic Factors
Data Sharing Statement


