This cohort study evaluates the risk of first-trimester antipsychotic exposure with respect to congenital malformations, focusing on individual drugs and specific malformation subtypes.
Key Points
Question
Is prenatal antipsychotic exposure associated with increased risk of major congenital malformations?
Findings
In this cohort study of more than 26 000 antipsychotic-exposed pregnancies from 6 countries, prenatal exposure to individual antipsychotics was generally not associated with an increase in risk of major congenital malformations.
Meaning
Considering the evidence from both primary and sensitivity analyses, there was no indication that antipsychotics are major teratogens; the potential signals observed for oral clefts, gastroschisis, and certain brain anomalies will require confirmation as evidence continues to accumulate.
Abstract
Importance
Psychiatric disorders are common among female individuals of reproductive age. While antipsychotic medication use is increasing, the safety of such medications in pregnancy is an area with large evidence gaps.
Objective
To evaluate the risk of first-trimester antipsychotic exposure with respect to congenital malformations, focusing on individual drugs and specific malformation subtypes.
Design, Setting, and Participants
This cohort study used data from nationwide health registers from the 5 Nordic countries and the US and spanned 1996 to 2018. The Nordic cohort included all pregnancies resulting in singleton live-born infants, and the US cohort consisted of publicly insured mothers linked to their live-born infants nested in the nationwide Medicaid Analytic eXtract. Data were analyzed from November 2020 to April 2022.
Exposures
One or more first-trimester dispensing of any atypical, any typical, and individual antipsychotic drugs.
Main Outcomes and Measures
Any major congenital malformation and specific malformation subtypes previously suggested to be associated with antipsychotic exposure in utero: cardiovascular malformations, oral clefts, neural tube defects, hip dysplasia, limb reduction defects, anorectal atresia/stenosis, gastroschisis, hydrocephalus, other specific brain anomalies, and esophageal disorders. Propensity score stratification was used to control for potential confounders. Pooled adjusted estimates were calculated using indirect standardization.
Results
A total of 6 455 324 unexposed mothers (mean maternal age range across countries: 24-31 years), 21 751 mothers exposed to atypical antipsychotic drugs (mean age range, 26-31 years), and 6371 mothers exposed to typical antipsychotic drugs (mean age range, 27-32 years) were included in the study cohort. Prevalence of any major malformation was 2.7% (95% CI, 2.7%-2.8%) in unexposed infants, 4.3% (95% CI, 4.1%-4.6%) in infants with atypical antipsychotic drug exposure, and 3.1% (95% CI, 2.7%-3.5%) in infants with typical antipsychotic drug exposure in utero. Among the most prevalent exposure-outcome combinations, adjusted relative risks (aRR) were generally close to the null. One exception was olanzapine exposure and oral cleft (aRR, 2.1 [95% CI, 1.1-4.3]); however, estimates varied across sensitivity analyses. Among moderately prevalent combinations, increased risks were observed for gastroschisis and other specific brain anomalies after atypical antipsychotic exposure (aRR, 1.5 [95% CI, 0.8-2.6] and 1.9 [95% CI, 1.1-3.0]) and for cardiac malformations after chlorprothixene exposure (aRR, 1.6 [95% CI, 1.0-2.7]). While the association direction was consistent across sensitivity analyses, confidence intervals were wide, prohibiting firm conclusions.
Conclusions and Relevance
In this study, considering the evidence from primary and sensitivity analyses and inevitable statistical noise for very rare exposure-outcome combinations, in utero antipsychotic exposure generally was not meaningfully associated with an increased risk of malformations. The observed increased risks of oral clefts associated with olanzapine, gastroschisis, and other specific brain anomalies with atypical antipsychotics and cardiac malformations with chlorprothixene requires confirmation as evidence continues to accumulate.
Introduction
Psychiatric disorders are common among female individuals of reproductive age including pregnant individuals and those in the postpartum period.1,2,3 For female individuals with schizophrenia and bipolar disorder, antipsychotic medications are often the mainstay of treatment and the risk of recurrence of psychotic symptoms following discontinuation is high.4 Consequently, many patients require continued treatment during pregnancy. Further, antipsychotic medications are increasingly being used to manage depression and anxiety in patients refractory to other treatments.5 As pregnant individuals are excluded from most clinical therapeutic trials, the safety of antipsychotic medications in pregnancy is an area with large gaps in evidence.6 Health care professionals often lack the necessary information for evidence-based prescribing decisions and to counsel patients about the use of specific antipsychotics during pregnancy.
Teratogenicity is typically the principal concern in pregnancy for drug classes like antipsychotics that cross the placenta.7 The risk of congenital malformations following in utero exposure to antipsychotic medications has been evaluated in the context of spontaneous reporting systems, pregnancy exposure registries, case-control studies, and more recently cohort studies nested in health care utilization databases,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25 and several systematic reviews and meta-analyses exist.26,27,28,29,30,31,32,33,34,35,36,37,38,39 While the results generally point toward antipsychotics not being major teratogens, findings have been conflicting and safety signals have emerged for outcomes such as cardiovascular malformations11,14,16,17,20,22,25,28,38 and oral clefts.11,14,15,18,38 Given the small size of most studies, vast differences in study approach, differences in specific drugs and outcomes evaluated, and variable levels of confounding control, no coherent picture of the comparative safety of different antipsychotic medications has emerged from the literature.
The objective of this study was to evaluate the risk of first-trimester exposure to antipsychotic medications with respect to major congenital malformations, using a uniform study design and analytic approach across 6 countries and focusing on individual drugs and specific malformation subtypes that have been suggested to be associated with antipsychotic use in pregnancy.
In this article, the terms woman, mother, and maternal, which describe gender, are used throughout. However, our focus is on the biological sex, so the findings here should be taken to include people who do not identify as women but are pregnant or have given birth.
Methods
Data Sources and Study Cohorts
This study was conducted by the International Pregnancy Safety Study (InPress) Consortium, a collaboration among research groups from the 5 Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden) and the US with access to high-quality health care databases and registers.40,41 Use of the Nordic data was approved by applicable ethics review boards or data providing authorities (eAppendix 1 in the Supplement). Use of the US data was approved by the Brigham and Women’s Hospital Institutional Review Board, which granted a waiver of informed consent. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The Nordic cohort included all pregnancies resulting in singleton live-born infants (4 531 396 pregnancies), with countries contributing data from different time periods depending on access to the nationwide health registers (Denmark: 1997-2017; Finland: 1996-2016; Iceland: 2004-2017; Norway: 2005-2018; Sweden: 2006-2016) (see eAppendix 1 in the Supplement for details on the Nordic cohort creation). The US cohort consisted of publicly insured mothers linked to their live-born infants nested in the nationwide Medicaid Analytic eXtract (2000-2014). The creation of this linked cohort has been previously described.42 Mothers aged 12 to 55 years were required to have Medicaid coverage from at least 3 months prior to pregnancy to 1 month after delivery; infants were required to have Medicaid coverage from birth until 3 months after birth, unless they died before that. The US source cohort included 2 074 159 pregnancies. For the US cohort, race and ethnicity was determined on the basis of information submitted to the Centers for Medicare & Medicaid Services by individual states, which was based on information that had been collected and coded from Medicaid applications. Pregnancies with a fetal chromosomal abnormality (10 487 in the Nordic cohort and 3668 in the US cohort) or with exposure to a known teratogenic medication (14 244 in the Nordic cohort and 11 867 in the US cohort) were excluded (Figure 1).
Figure 1. Cohort Creation and Antipsychotic Drug Exposure During the First Trimester of Pregnancies in the Nordic Databases and the US Medicaid Analytic eXtract.
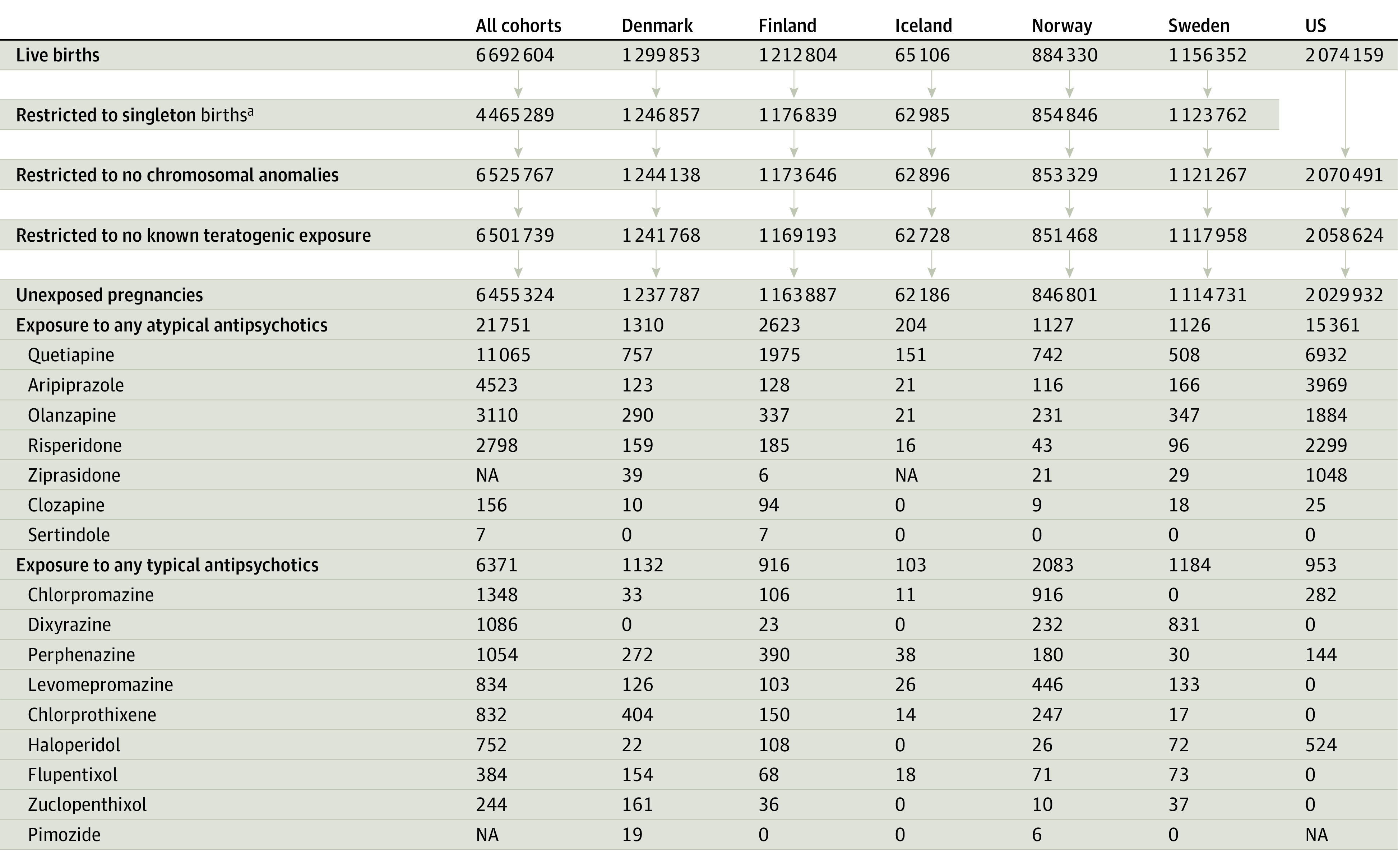
aRestriction to singleton births was only applied within the Nordic cohorts. In the US data, multiples remained in the study cohort, with multiple gestation being included as a covariate in the propensity score. NA indicates not available (numbers suppressed due to country-/data-specific suppression policy).
Antipsychotic Exposure
Exposure to atypical and typical antipsychotics was defined based on filling 1 or more prescriptions of the respective drug class during the first trimester, the period for organogenesis. Individual antipsychotics were considered if they were available in at least 4 Nordic countries during the study period (Figure 1). Women were considered unexposed if they did not fill any antipsychotic prescriptions during the 3 months prior to pregnancy until the end of the first trimester (eTable 1 in the Supplement).
Congenital Malformations
Aside from major congenital malformations overall, we considered specific malformation subtypes that had previously been described as potentially associated with prenatal antipsychotic exposure: cardiovascular malformations,11,14,16,17,20,22,25,28,38 oral clefts,11,14,15,18,38 neural tube defects,15 hip dysplasia,14,30 limb reduction defects,33 anorectal atresia/stenosis,11,18,30 gastroschisis,11,14 hydrocephalus,15,24 other specific brain anomalies,24,38 and esophageal disorders18,38 (eTables 2-3 in the Supplement).
Covariates
A broad range of (proxies for) potential confounders was considered, including demographic factors, treatment indications and other mental disorders, other maternal/obstetrical conditions, lifestyle behaviors, other prescription medication exposures, and health care utilization metrics as potential markers of the overall comorbidity burden or severity of mental illness (eTable 4 in the Supplement).
Analyses
Baseline characteristics were compared between women treated with the antipsychotic drug/class of interest and those without any antipsychotic exposure, using standardized differences (for calculation, see footnote in Figure 2). An absolute standardized difference greater than 0.1 was considered evidence of imbalance.43 Distribution of characteristics and balance were assessed separately for all Nordic countries combined and the US.
Figure 2. Standardized Differences of Pregnancies With Atypical or Typical Antipsychotic (AP) Exposure vs No AP Exposure During the First Trimester (Unadjusted and Propensity Score Weighted).

Not all standardized differences for each covariate and country shown due to small cell size suppression policy.
aOther/unknown: American Indian or Alaska Native, Asian or Pacific Islander, Native Hawaiian or Other Pacific Islander, Hispanic or Latino and ≥1 races, >1 race, and unknown. Hispanic: Hispanic or Latino with no race information available. Other/unknown: Hispanic or Latino with ≥1 races.
bOther psychoactive drugs include benzodiazepines, barbiturates, anxiolytics, and other hypnotics.
cEstimated as (Xexp-Xref)/√((sexp2+sref2)/2). X represents the sample mean and s2 the sample variance of the covariate in the AP exposed and reference group.
Analyses were conducted separately for each country, with results being pooled as described here. For each exposure-outcome combination, we calculated absolute risks and unadjusted relative risks (RRs) with their 95% CIs. The pooled risks and unadjusted RRs were estimated by summing the number of outcomes and the number of pregnancies across countries for each exposure (for detailed information on the RR calculation, see eAppendix 2 in the Supplement).
A propensity score approach was used to control for potential confounders.44 A separate propensity score was estimated for each exposure and country using a logistic regression model (eTable 5 in the Supplement). Observations from the nonoverlapping regions of the propensity score distribution were trimmed, and equally sized propensity score strata based on the distribution among the exposed were created, requiring a minimum of 3 exposed pregnancies per stratum and 50 strata or fewer. Unexposed pregnancies were weighted using the distribution of the exposed pregnancies among propensity score strata and country-specific adjusted RRs (aRR) were estimated using a log-binomial model with a weight statement. This propensity score fine stratification with weighting approach has been shown to be particularly helpful when the exposure is infrequent.45 Results were pooled across propensity score strata and country using an indirect standardization approach (eAppendix 2 in the Supplement).
Several sensitivity analyses were conducted to test the robustness of the main results. First, to evaluate the potential association of exposure misclassification with outcomes, we redefined exposure as having 2 or more dispensing of the drug/class of interest during the first trimester, the assumption being that if a woman refilled her prescription, she was probably consuming the medication. Second, to mitigate potential residual confounding by mental disorders, we restricted our analysis to women with 1 or more recorded diagnoses for psychosis, bipolar disorder, depression, schizophrenia, anxiety, attention-deficit/hyperactivity disorder, or other psychiatric disorders (hereafter referred to as mental health conditions). Third, we restricted our analysis to women with exposure to only the drug/class of interest (monotherapy).
All analyses were conducted using SAS version 9.4 (SAS Institute). Precision around the estimates of risks and RRs is provided using 95% CIs. No adjustments were made for multiple comparisons. Interpretation of the results was based on the strength of the adjusted risk estimates, regardless of whether the 95% CI included the null, and the degree to which the upper limit of the 95% CI suggested low compatibility between the data and a strong adverse reaction. While focusing on specific drugs and malformation subtypes is critical to avoid missing signals due to the fallacy of class action teratogenesis46 and lumping of etiologically unrelated defects,47 it inevitably leads to numerous causal contrasts being evaluated (increasing the risk of chance findings) and sparse data challenges. We therefore present results from multiple exposure-outcome combinations in a way that conveys the levels of confidence in the findings. Data were analyzed from November 2020 to April 2022.
Results
Cohort Characteristics
This cohort included 6 455 324 unexposed pregnancies (mean maternal age range across countries, 24-31 years), 21 751 pregnancies with atypical antipsychotic exposure (mean age range, 26-31 years), and 6371 with typical antipsychotic exposure (mean age range, 27-32 years) (Table). The most commonly dispensed atypical antipsychotics were quetiapine (n = 11 065), aripiprazole (n = 4523), and olanzapine (n = 3110); the most common typical antipsychotics were chlorpromazine (n = 1348), dixyrazine (n = 1086, Nordic countries only), and perphenazine (n = 1054) (Figure 1).
Table. Selected Cohort Characteristics of Pregnancies with Atypical Antipsychotic Exposure, Typical Antipsychotic Exposure, and No Antipsychotic Exposure During the First Trimester, Separately for Nordic Countries and the US Medicaid Analytic eXtract (Unadjusted).
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Nordic countries | US | |||||
| Antipsychotic | Unexposed | Antipsychotic | Unexposed | |||
| Atypical | Typical | Atypical | Typical | |||
| Total | 6390 | 5418 | 4 425 392 | 15 361 | 953 | 2 029 932 |
| Demographic factor | ||||||
| Maternal age, y | ||||||
| ≤24 | 1303 (20.39) | 801 (15.07)a | 579 025 (13.08) | 6912 (44.99) | 366 (38.41) | 1 146 914 (56.50) |
| 25-34 | 3450 (53.99) | 3118 (58.66)a | 2 870 762 (64.87) | 4210 (27.41) | 288 (30.22) | 498 404 (24.55) |
| ≥35 | 1624 (25.41) | 1389 (26.13)a | 971 682 (21.96) | 1578 (10.28) | 120 (12.59) | 134 268 (6.61) |
| Race and ethnicityb,c | ||||||
| Black | NA | NA | NA | 3450 (22.46) | 373 (39.14) | 673 523 (33.18) |
| Hispanic | NA | NA | NA | 799 (5.20) | 47 (4.93) | 293 282 (14.45) |
| White | NA | NA | NA | 9643 (62.78) | 439 (46.07) | 820 321 (40.41) |
| Other/unknown | NA | NA | NA | 1469 (9.56) | 94 (9.86) | 242 806 (11.96) |
| Mother born outside of Nordic countriesd | 787 (12.32) | 1181 (21.80) | 675 999 (15.28) | NA | NA | NA |
| Mental/neurologic conditions | ||||||
| Bipolar disorder | 878 (13.74) | 136 (2.51) | 4025 (0.09) | 6313 (41.10) | 313 (32.84) | 24 636 (1.21) |
| Psychosisa | 314 (5.08) | 150 (2.77) | 735 (0.02) | 807 (5.25) | 135 (14.17) | 3131 (0.15) |
| Depression | 3648 (57.09) | 1756 (32.41) | 136 955 (3.09) | 6894 (44.88) | 328 (34.42) | 119 256 (5.87) |
| Schizophreniaa | 606 (9.80) | 371 (6.98) | 879 (0.02) | 1085 (7.06) | 191 (20.04) | 1673 (0.08) |
| Anxiety | 1133 (17.73) | 416 (7.68) | 36 374 (0.82) | 4461 (29.04) | 221 (23.19) | 76 108 (3.75) |
| ADHD | 287 (4.49) | 83 (1.84) | 7802 (0.18) | 1063 (6.92) | 25 (2.62) | 13 217 (0.65) |
| Other psychiatric disorders | 4283 (67.03) | 1761 (32.50) | 113 749 (2.57) | 1509 (9.82) | 85 (8.92) | 22 849 (1.13) |
| Other maternal/obstetrical conditions | ||||||
| Epilepsy/convulsions | 68 (1.06) | 34 (0.77) | 9241 (0.21) | 457 (2.98) | 31 (3.25) | 12 263 (0.60) |
| Migraine/headache | 188 (3.04) | 186 (3.50) | 54 884 (1.24) | 2503 (16.29) | 162 (17.00) | 149 058 (7.34) |
| Sleep disorder | 1362 (21.31) | 793 (14.64) | 42 988 (0.97) | 762 (4.96) | 41 (4.30) | 15 028 (0.74) |
| Pregestational | ||||||
| Hypertension | 45 (0.73) | 17 (0.83) | 14 147 (0.32) | 861 (5.61) | 69 (7.24) | 48 660 (2.40) |
| Diabetes | 128 (2.00) | 69 (1.30) | 41 204 (0.93) | 538 (3.50) | 60 (6.30) | 33 853 (1.67) |
| Multiple gestationc | NA | NA | NA | 266 (1.73) | 12 (1.26) | 27 852 (1.37) |
| Lifestyle behavior | ||||||
| Tobacco use | 2283 (35.73) | 1342 (24.77) | 484 673 (10.95) | 1991 (12.96) | 126 (13.22) | 83 069 (4.09) |
| Alcohol dependence/use disorder | 291 (4.55) | 141 (2.60) | 7260 (0.16) | 862 (5.61) | 77 (8.08) | 13 633 (0.67) |
| Other substance use disorder | 518 (8.11) | 227 (4.19) | 9870 (0.22) | 2405 (15.66) | 146 (15.32) | 38 885 (1.92) |
| Other prescription medication exposure | ||||||
| Anticonvulsants | 1049 (16.42) | 372 (6.87) | 18 248 (0.41) | 3888 (25.31) | 178 (18.68) | 33 128 (1.63) |
| Antidepressants | 3648 (57.09) | 1756 (32.41) | 136 955 (3.09) | 11 007 (71.66) | 525 (55.09) | 168 442 (8.30) |
| Antidiabetics | 128 (2.00) | 69 (1.30) | 41 204 (0.93) | 470 (3.06) | 46 (4.83) | 28 968 (1.43) |
| Antihypertensives | 342 (5.35) | 183 (3.44) | 44 174 (1.00) | 1682 (10.95) | 87 (9.13) | 58 951 (2.90) |
| Prescription opioids | 667 (10.44) | 558 (10.30) | 100 056 (2.26) | 6872 (44.74) | 374 (39.24) | 456 709 (22.50) |
| Stimulants | 272 (4.26) | 73 (1.62) | 7484 (0.17) | 1215 (7.91) | 29 (3.04) | 11 697 (0.58) |
| Triptans | 188 (3.04) | 186 (3.50) | 54 884 (1.24) | 600 (3.91) | 28 (2.94) | 21 502 (1.06) |
| Other psychoactive drugse | 2112 (33.05) | 1266 (23.37) | 74 380 (1.68) | 7290 (47.46) | 415 (43.55) | 145 508 (7.17) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; NA, not available.
Informaton for Iceland was not included due to small cell size suppression policy.
Race and ethnicity were determined on the basis of information submitted to the Centers for Medicare & Medicaid Services by individual states, which was based on information that had been collected and coded from Medicaid applications. Race and ethnicity category other/unknown includes the following races and ethnicities: American Indian or Alaska Native, Asian or Pacific Islander, Native Hawaiian or Other Pacific Islander, Hispanic or Latino and 1 or more races, more than 1 race, and unknown. The category Hispanic includes Hispanic or Latino with no race information available, whereas other/unknown includes Hispanic or Latino with 1 or more races.
Available for US cohort only.
Available for Nordic cohorts only.
Other psychoactive drugs include benzodiazepines, barbiturates, anxiolytics, and other hypnotics.
Compared with unexposed individuals, women with antipsychotic exposure were more likely to be White (race and ethnicity available for US cohort only and also included Black and Hispanic individuals and other/unknown race and ethnicity), to have a higher burden of comorbidities, to be dispensed other medications, and to have more unhealthy lifestyle behaviors (eg, tobacco, alcohol, or other substance use) (Table, Figure 2, eTable 6 in the Supplement). These imbalances of baseline characteristics between exposed and unexposed groups were generally observed across cohorts and for each individual antipsychotic agent. After propensity score weighting, characteristics were largely balanced between exposed and unexposed groups (Figure 2 and eTable 7 in the Supplement). Most women were exposed to only 1 type of antipsychotic drug during the first trimester (eTable 8 in the Supplement).
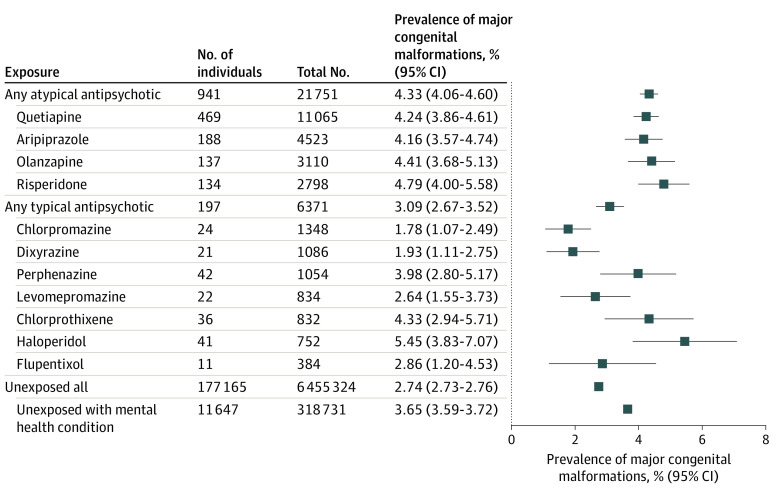
The risk of having an infant diagnosed with any major congenital malformation was 2.7% (2.7%-2.8%) (pooled absolute risk) among all unexposed women and 3.7% (95% CI, 3.6%-3.7%) when restricted to unexposed women with a mental health condition. The risk of any major congenital malformation was 4.3% (95% CI, 4.1%-4.6%) among atypical antipsychotic exposed and 3.1% (95% CI, 2.7%-3.5%) among typical antipsychotic exposed, with risks ranging from 1.8% (95% CI, 1.1%-2.5%) to 5.5% (95% CI, 3.8%-7.1%), depending on the specific antipsychotic drug (Figure 3).
Figure 3. Pooled Absolute Risk With 95% CIs of Major Malformations Overall Among Live-born Infants With and Without Antipsychotic Exposure in Utero.
Prevalence of major congenital malformations among ziprasidone-, zuclopenthixol-, and clozapine-exposed pregnancies is not presented because the number of cases had to be suppressed due to country-/data-specific small cell suppression policies. Prevalence of major congenital malformations among pimozide- and sertindole-exposed pregnancies was zero and is therefore not shown.
RR of Malformations
Figure 4 presents the pooled unadjusted RRs (Figure 4A) and adjusted RRs (Figure 4B) from the main analyses. Exposures (rows) and outcomes (columns) are sorted by prevalence in the cohorts. To facilitate interpretation, the figure is divided into 3 segments. The first segment represents exposure-outcome combinations with a substantial amount of information, represented by the area to the left of the blue dotted line (>100 exposed individuals, outcome prevalence ≥0.5 per 1000 unexposed infants). The second segment includes combinations with a limited amount of information, represented by the area to the right of the orange dashed line. The third segment includes combinations with a moderate amount of information and is visually represented by the area between both lines. Cells are shaded based on the upper limit of the 95% CI with darker shading corresponding with higher upper limits, indicating the data are compatible with a large increased risk.
Figure 4. Pooled Unadjusted Relative Risk and Propensity Score—Weighted Standardized Morbidity Ratio (SMR), Separately for Each Exposure-Outcome Contrast, Sorted by Prevalence of Exposure and Outcome in the Nordic and US Cohorts.

Rows are sorted by the most common to least common antipsychotic exposures and columns are sorted by the most common to least common outcomes among the unexposed. The outcome order was determined based on the absolute risk among the unexposed as observed in the US cohort (absolute risk for Nordic cohorts was not available for some outcomes due to country-specific cell suppression policies). Drugs in red belong to the atypical antipsychotic class and drugs in black to the typical antipsychotic class. The blue dotted line connects antipsychotic drugs with ≥100 exposed individuals and malformations with a prevalence of ≥0.5 per 1000 infants among the unexposed (as observed in the US cohort). The orange dashed line connects the least frequent antipsychotic with the least frequent malformation. Cells containing zeros are those with no outcomes of interest among the exposed.
For combinations with a substantial amount of information, unadjusted estimates ranged from 0.5 (95% CI, 0.3-0.8) to 2.1 (95% CI, 1.0-4.1). After adjustment, results shifted substantially toward the null for all combinations (range [95% CI]: 0.7 [0.2-2.1] to 1.2 [0.9-1.6]), the only exception being olanzapine and oral cleft, with an aRR of 2.1 (95% CI, 1.1-4.3) (Figure 4 and eTable 9 in the Supplement).
In contrast, combinations with a limited amount of information mostly consisted of either zeros due to no events among the exposed or very high unadjusted RRs (up to 17.5) and corresponding upper limits of the 95% CI (>10), all associated with very few exposed events. Adjustment did not attenuate these results and in most instances strengthened the associations (Figure 4 and eTable 9 in the Supplement).
For combinations supported by a moderate amount of information, adjusted risk estimates were generally, although not uniformly, compatible with a null finding, particularly for the more common outcomes (eg, any major congenital malformation, cardiovascular malformations). The largest elevations in the point estimates for risk were observed for those combinations closest to the lower triangle, indicating fewer data informed the estimates resulting in very wide CIs. For example, the aRR was 2.4 (95% CI, 0.3-17.4) for perphenazine and the risk of hydrocephaly was 2.2 (95% CI, 0.3-15.7) for ziprasidone and gastroschisis and 2.0 (95% CI, 0.7-6.2) for olanzapine and anorectal atresia/stenosis.
Sensitivity Analyses
Risk estimates for olanzapine and oral clefts ranged from an aRR of 1.1 (95% CI, 0.4-2.8) when restricting to monotherapy to 2.6 (95% CI, 1.2-5.4) when requiring a diagnosis of a mental health condition for both the exposed and the reference group (eTable 9 in the Supplement).
Sensitivity analyses for combinations in the lower triangle are provided for transparency but are not considered informative because they are based on such limited information and are therefore not interpreted (eTable 9 in the Supplement).
Finally, among combinations supported by a moderate amount of information, a few potential safety signals emerged for which the direction of the association was consistent across sensitivity analyses. We focus our interpretation on those informed by at least 10 exposed events. The aRRs ranged from 1.3 to 2.1 (with corresponding upper limits of the 95% CI from 2.6 to 4.0) for atypical antipsychotics and the risk of gastroschisis, from 1.9 to 3.9 (upper limit of the 95% CI, 3.0 to 6.4) for atypical antipsychotics and other specific brain anomalies, and from 1.5 to 2.1 (upper limit of the 95% CI, 2.6 to 8.5) for chlorprothixene and cardiac malformations across main and sensitivity analyses (eTable 9 in the Supplement). The associations with atypical antipsychotics appeared to be associated with quetiapine (eTable 9 in the Supplement).
Discussion
Based on this large cohort study including more than 26 000 exposed pregnancies from 6 countries, using a common design and analytic approach, we found no consistent safety signals for the contrasts supported by a substantial amount of information, suggesting that antipsychotics are not major teratogens. For exposure-outcome combinations where less information was available, we observed some elevated point estimates, but these associations were imprecisely estimated as reflected by the width of the CIs.
In an earlier study using the US Medicaid cohort with data from 2000-2010, we reported a small increase in the risk of major congenital malformations overall (aRR, 1.26; 95% CI, 1.02-1.56) observed with risperidone (1566 exposed pregnancies), which was associated with cardiovascular malformations (RR, 1.26; 95% CI, 0.88-1.81).20 We viewed this finding as a potential safety signal that required follow-up in other studies because no apparent biological mechanism could readily explain this outcome.48 The signal was not replicated in the present study, which used a similar approach, and included an additional 1232 risperidone-exposed pregnancies for a total of 2798. These findings highlight the importance of confirmatory studies and of continued monitoring of the safety of medications during pregnancy as evidence accumulates over time.
The signals that emerged from this study should be viewed through that same lens. Among those exposure-outcome combinations with a substantial amount of information, we observed a potential association between olanzapine and oral clefts (2.1; 95% CI, 1.1-4.3). No prior study included a sufficiently large number of olanzapine-exposed pregnancies to be able to evaluate the risk of oral clefts with some confidence given the expected prevalence of about 1 in 690 births.49 Using data from the Finnish registers, Ellfolk and colleagues21 reported an RR of 1.3 (95% CI, 0.1-21.6), which was imprecisely estimated based on 413 olanzapine-exposed pregnancies only. The mean placental passage ratio for olanzapine has been reported to be slightly higher than for other antipsychotics, but it is highly variable between patients.7 While the inconsistency of the safety signal across sensitivity analyses supports a noncausal explanation, it will be helpful to reevaluate this association in future studies.
Previous reports on the potential associations between atypical antipsychotics and gastroschisis and brain anomalies were based on small case series,11,14,15,24,38 and to our knowledge, no study has previously reported a potential association between chlorprothixene and cardiac malformations. While our findings were estimated imprecisely, the point estimates were consistently elevated across sensitivity analyses, indicating that continued monitoring of these associations would be prudent.
Strengths and Limitations
The strengths of this study include its large size, enabling us to assess the risk of specific malformations associated with individual antipsychotics, independent ascertainment of exposure and outcome, rich information for confounding adjustment, and no risk of recall bias. While analyses were conducted locally in a distributed data network approach, the harmonized study design and analyses governed by a common protocol resulted in comparable country-specific estimates that could be pooled in a meaningful way.
Nevertheless, we faced some challenges that deserve further comment. To inform clinical practice and avoid missing potential signals, it is important to consider the etiologic heterogeneity of congenital anomalies and focus on individual drugs and specific malformations. This resulted in 198 comparisons (18 exposure groups × 11 outcomes) for the main analyses alone. Each of the 3 sensitivity analyses contributed up to an additional 198 estimates. While we do not advocate the use of P < .05 (or a confidence interval that does not encompass the null value) to interpret findings,50,51 it is important to recognize that for the main analyses, we would expect 10 of the associations to meet this conventional significance threshold by chance alone even if in truth none of the antipsychotics were associated with an increased risk of congenital malformations. To avoid an inadvertent focus on significance testing, which can lead to erroneous conclusions, we centered the summary presentation of results on the point estimate (which is the estimate most consistent with the data) and the upper limit of the 95% CI to indicate compatibility between the data and a strong adverse reaction.
Second, whenever evaluating uncommon drugs and rare outcomes, the first observed event among the exposed group will per definition result in a large RR. This is apparent in Figure 4 where the lower right triangle consists of either combinations with no events among the exposed group or with high RRs driven by just 1 or at most a few events. This challenge is not unique to this study. For example, exposure pregnancy registries, which are typically characterized by small numbers, also face this issue. In this context, the rule of 3 has been used before: follow-up of a signal occurs once 3 specific defects are reported for a specific exposure. Rather than using a threshold based on the number of events observed, we opted instead to account for the amount of information that contributed to the finding by organizing results by the number of exposed pregnancies and the number of outcomes in both the presentation and interpretation of the results.
Finally, to protect patient confidentiality, several of the data holders impose disclosure limitations to avoid the release of information that can be used to identify individuals. Consequently, we were not able to share the absolute number of events if numbers were less than 5 in the Nordic countries or less than 11 in the US. Such restrictions complicate the sharing of results especially when the focus is on uncommon exposures and rare outcomes.
Conclusions
In conclusion, robust data on embryo fetal risk associated with antipsychotic medication use in pregnancy are needed to inform prescribing decisions and counsel patients about treatment during pregnancy. To be most clinically useful, such data must focus on individual drugs and specific teratogenic effects. While epidemiologic studies provide the only means of obtaining reliable quantitative estimates regarding the risk of congenital anomalies in an exposed pregnancy, spurious associations can occur especially in the context of sparse data and a large number of comparisons. If we are to better communicate risk and research findings, it is imperative that we provide a comprehensive picture of the comparative safety of different treatment alternatives and transparently convey our level of confidence in the findings. We presented one possible way of doing so, allowing us to conclude that despite the inevitable noise in the data, taken as a whole, the findings offer assurance that antipsychotics are not major teratogens.
eAppendix 1. Description of Nordic Data
eAppendix 2. Calculation of Pooled Relative Risk and Corresponding 95% Confidence Interval Using an Indirect Standardization Approach
eTable 1. Antipsychotic Drugs Considered
eTable 2. Definition of Malformations of Interest in Nordic Countries
eTable 3. Definition of Malformations of Interest in US Medicaid Analytic eXtract
eTable 4. Definition and Assessment Period of Covariates of Interest
eTable 5. Countries Contributing to the Main Adjusted Analyses
eTable 6. Cohort Characteristics of Pregnancies with Atypical Antipsychotic Exposure, Typical Antipsychotic Exposure and No Antipsychotic Exposure During the First Trimester, Separately for Nordic Countries and the US Medicaid Analytic eXtract (Unadjusted)
eTable 7. Cohort Characteristics of Pregnancies with Atypical Antipsychotic Exposure, Typical Antipsychotic Exposure and No Antipsychotic Exposure During the First Trimester, Separately for Nordic Countries and the US Medicaid Analytic eXtract (Propensity Score Weighted)
eTable 8. Number of Pregnancies with Antipsychotic Exposure Based on ≥1 Dispensing of the Drug of Interest Irrespective of Exposure to Other Antipsychotic Drugs, and Number of Pregnancies Exposed to Only one Antipsychotic Drug (Monotherapy) During the First Trimester, Separately for Nordic Countries and the US Medicaid Analytic eXtract
eTable 9. Pooled Risk Estimates of Malformations of Interest in Infants With and Without Prenatal Exposure to Antipsychotic Drugs of Interest During the First Trimester (Main and Sensitivity Analyses)
eReferences.
References
- 1.Farr SL, Bitsko RH, Hayes DK, Dietz PM. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-9. doi: 10.1016/j.ajog.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 2.Ko JY, Farr SL, Dietz PM, Robbins CL. Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005-2009. J Womens Health (Larchmt). 2012;21(8):830-836. doi: 10.1089/jwh.2011.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Hara MW. Postpartum depression: what we know. J Clin Psychol. 2009;65(12):1258-1269. doi: 10.1002/jclp.20644 [DOI] [PubMed] [Google Scholar]
- 4.Edinoff AN, Sathivadivel N, McNeil SE, et al. Antipsychotic use in pregnancy: patient mental health challenges, teratogenicity, pregnancy complications, and postnatal risks. Neurol Int. 2022;14(1):62-74. doi: 10.3390/neurolint14010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeroff CB. Use of atypical antipsychotics in refractory depression and anxiety. J Clin Psychiatry. 2005;66(suppl 8):13-21. [PubMed] [Google Scholar]
- 6.Wisner KL. The last therapeutic orphan: the pregnant woman. Am J Psychiatry. 2012;169(6):554-556. doi: 10.1176/appi.ajp.2012.12030367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newport DJ, Calamaras MR, DeVane CL, et al. Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164(8):1214-1220. doi: 10.1176/appi.ajp.2007.06111886 [DOI] [PubMed] [Google Scholar]
- 8.Ballester-Gracia I, Pérez-Almarcha M, Galvez-Llompart A, Hernandez-Viadel M. Use of long acting injectable aripiprazole before and through pregnancy in bipolar disorder: a case report. BMC Pharmacol Toxicol. 2019;20(1):52. doi: 10.1186/s40360-019-0330-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tényi T, Nagy Á, Herold R, Fekete S. Extended release quetiapine fumarate and pregnancy. Neuropsychopharmacol Hung. 2013;15(1):49-50. [PubMed] [Google Scholar]
- 10.Widschwendter CG, Hofer A. Aripiprazole use in early pregnancy: a case report. Pharmacopsychiatry. 2012;45(7):299-300. doi: 10.1055/s-0032-1312591 [DOI] [PubMed] [Google Scholar]
- 11.Anderson KN, Ailes EC, Lind JN, et al. ; National Birth Defects Prevention Study . Atypical antipsychotic use during pregnancy and birth defect risk: National Birth Defects Prevention Study, 1997-2011. Schizophr Res. 2020;215:81-88. doi: 10.1016/j.schres.2019.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry. 2005;66(3):317-322. doi: 10.4088/JCP.v66n0307 [DOI] [PubMed] [Google Scholar]
- 13.Freeman MP, Viguera AC, Góez-Mogollón L, et al. Reproductive safety of aripiprazole: data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Arch Womens Ment Health. 2021;24(4):659-667. doi: 10.1007/s00737-021-01115-6 [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni J, Worsley R, Gilbert H, et al. A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One. 2014;9(5):e94788. doi: 10.1371/journal.pone.0094788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4):444-449. doi: 10.4088/JCP.v66n0406 [DOI] [PubMed] [Google Scholar]
- 16.Cohen LS, Góez-Mogollón L, Sosinsky AZ, et al. Risk of major malformations in infants following first-trimester exposure to quetiapine. Am J Psychiatry. 2018;175(12):1225-1231. doi: 10.1176/appi.ajp.2018.18010098 [DOI] [PubMed] [Google Scholar]
- 17.Källén B, Borg N, Reis M. The use of central nervous system active drugs during pregnancy. Pharmaceuticals (Basel). 2013;6(10):1221-1286. doi: 10.3390/ph6101221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montastruc F, Salvo F, Arnaud M, Bégaud B, Pariente A. Signal of gastrointestinal congenital malformations with antipsychotics after minimising competition bias: a disproportionality analysis using data from Vigibase(®). Drug Saf. 2016;39(7):689-696. doi: 10.1007/s40264-016-0413-1 [DOI] [PubMed] [Google Scholar]
- 19.Peng M, Gao K, Ding Y, et al. Effects of prenatal exposure to atypical antipsychotics on postnatal development and growth of infants: a case-controlled, prospective study. Psychopharmacology (Berl). 2013;228(4):577-584. doi: 10.1007/s00213-013-3060-6 [DOI] [PubMed] [Google Scholar]
- 20.Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938-946. doi: 10.1001/jamapsychiatry.2016.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellfolk M, Leinonen MK, Gissler M, Kiuru-Kuhlefelt S, Saastamoinen L, Malm H. Second-generation antipsychotic use during pregnancy and risk of congenital malformations. Eur J Clin Pharmacol. 2021;77(11):1737-1745. doi: 10.1007/s00228-021-03169-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habermann F, Fritzsche J, Fuhlbrück F, et al. Atypical antipsychotic drugs and pregnancy outcome: a prospective, cohort study. J Clin Psychopharmacol. 2013;33(4):453-462. doi: 10.1097/JCP.0b013e318295fe12 [DOI] [PubMed] [Google Scholar]
- 23.Petersen I, Sammon CJ, McCrea RL, et al. Risks associated with antipsychotic treatment in pregnancy: comparative cohort studies based on electronic health records. Schizophr Res. 2016;176(2-3):349-356. doi: 10.1016/j.schres.2016.07.023 [DOI] [PubMed] [Google Scholar]
- 24.Wichman CL. Atypical antipsychotic use in pregnancy: a retrospective review. Arch Womens Ment Health. 2009;12(1):53-57. doi: 10.1007/s00737-008-0044-3 [DOI] [PubMed] [Google Scholar]
- 25.Sadowski A, Todorow M, Yazdani Brojeni P, Koren G, Nulman I. Pregnancy outcomes following maternal exposure to second-generation antipsychotics given with other psychotropic drugs: a cohort study. BMJ Open. 2013;3(7):e003062. doi: 10.1136/bmjopen-2013-003062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gentile S, Fusco ML. Schizophrenia and motherhood. Psychiatry Clin Neurosci. 2019;73(7):376-385. doi: 10.1111/pcn.12856 [DOI] [PubMed] [Google Scholar]
- 27.Damkier P, Videbech P.. The safety of second-generation antipsychotics during pregnancy: a clinically focused review. CNS Drugs. 2018;32(4):351-366. doi: 10.1007/s40263-018-0517-5 [DOI] [PubMed] [Google Scholar]
- 28.Thomson M, Sharma V. Weighing the risks: the management of bipolar disorder during pregnancy. Curr Psychiatry Rep. 2018;20(3):20. doi: 10.1007/s11920-018-0882-2 [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother. 2015;16(9):1335-1345. doi: 10.1517/14656566.2015.1041501 [DOI] [PubMed] [Google Scholar]
- 30.Cuomo A, Goracci A, Fagiolini A. Aripiprazole use during pregnancy, peripartum and lactation: a systematic literature search and review to inform clinical practice. J Affect Disord. 2018;228:229-237. doi: 10.1016/j.jad.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 31.Mehta TM, Van Lieshout RJ. A review of the safety of clozapine during pregnancy and lactation. Arch Womens Ment Health. 2017;20(1):1-9. doi: 10.1007/s00737-016-0670-0 [DOI] [PubMed] [Google Scholar]
- 32.Ennis ZN, Damkier P. Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations: a systematic review. Basic Clin Pharmacol Toxicol. 2015;116(4):315-320. doi: 10.1111/bcpt.12372 [DOI] [PubMed] [Google Scholar]
- 33.Coughlin CG, Blackwell KA, Bartley C, Hay M, Yonkers KA, Bloch MH. Obstetric and neonatal outcomes after antipsychotic medication exposure in pregnancy. Obstet Gynecol. 2015;125(5):1224-1235. doi: 10.1097/AOG.0000000000000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Brauer R, Man KKC, Alfageh B, Mongkhon P, Wong ICK. Prenatal exposure to antipsychotic agents and the risk of congenital malformations in children: a systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87(11):4101-4123. doi: 10.1111/bcp.14839 [DOI] [PubMed] [Google Scholar]
- 35.Andrade C. Major congenital malformations associated with exposure to second-generation antipsychotic drugs during pregnancy. J Clin Psychiatry. 2021;82(5):21f14252. doi: 10.4088/JCP.21f14252 [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan DL, Byatt N, Dossett EC. Long-acting injectable antipsychotic medications in pregnancy: a review. J Acad Consult Liaison Psychiatry. 2022;63(1):53-60. doi: 10.1016/j.jaclp.2021.08.011 [DOI] [PubMed] [Google Scholar]
- 37.Betcher HK, Montiel C, Clark CT. Use of antipsychotic drugs during pregnancy. Curr Treat Options Psychiatry. 2019;6(1):17-31. doi: 10.1007/s40501-019-0165-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coppola D, Russo LJ, Kwarta RF Jr, Varughese R, Schmider J. Evaluating the postmarketing experience of risperidone use during pregnancy: pregnancy and neonatal outcomes. Drug Saf. 2007;30(3):247-264. doi: 10.2165/00002018-200730030-00006 [DOI] [PubMed] [Google Scholar]
- 39.Patton SW, Misri S, Corral MR, Perry KF, Kuan AJ. Antipsychotic medication during pregnancy and lactation in women with schizophrenia: evaluating the risk. Can J Psychiatry. 2002;47(10):959-965. doi: 10.1177/070674370204701008 [DOI] [PubMed] [Google Scholar]
- 40.Bateman BT, Heide-Jørgensen U, Einarsdóttir K, et al. β-Blocker use in pregnancy and the risk for congenital malformations: an international cohort study. Ann Intern Med. 2018;169(10):665-673. doi: 10.7326/M18-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huybrechts KF, Bröms G, Christensen LB, et al. Association between methylphenidate and amphetamine use in pregnancy and risk of congenital malformations: a cohort study from the international pregnancy safety study consortium. JAMA Psychiatry. 2018;75(2):167-175. doi: 10.1001/jamapsychiatry.2017.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One. 2013;8(6):e67405. doi: 10.1371/journal.pone.0067405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55. [Google Scholar]
- 45.Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology. 2017;28(2):249-257. doi: 10.1097/EDE.0000000000000595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horton D, Hernandez-Diaz S, Lasky T, Huybrechts K. Research on the effects of medications in pregnancy and in children. In: Strom B, Kimmel S, Hennessy S, eds. Pharmacoepidemiology. 6th ed. Wiley-Blackwell; 2019:1208. doi: 10.1002/9781119413431.ch22 [DOI] [Google Scholar]
- 47.Khoury MJ, Moore CA, James LM, Cordero JF. The interaction between dysmorphology and epidemiology: methodologic issues of lumping and splitting. Teratology. 1992;45(2):133-138. doi: 10.1002/tera.1420450206 [DOI] [PubMed] [Google Scholar]
- 48.Wisner KL, Jeong H, Chambers C. Use of antipsychotics during pregnancy: pregnant women get sick-sick women get pregnant. JAMA Psychiatry. 2016;73(9):901-903. doi: 10.1001/jamapsychiatry.2016.1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mai CT, Cassell CH, Meyer RE, et al. ; National Birth Defects Prevention Network . Birth defects data from population-based birth defects surveillance programs in the United States, 2007 to 2011: highlighting orofacial clefts. Birth Defects Res A Clin Mol Teratol. 2014;100(11):895-904. doi: 10.1002/bdra.23329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wasserstein RL, Lazar NA. The ASA statement on P values: context, process, and purpose. Am Stat. 2016;70(2):129-133. doi: 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- 51.Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567(7748):305-307. doi: 10.1038/d41586-019-00857-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Description of Nordic Data
eAppendix 2. Calculation of Pooled Relative Risk and Corresponding 95% Confidence Interval Using an Indirect Standardization Approach
eTable 1. Antipsychotic Drugs Considered
eTable 2. Definition of Malformations of Interest in Nordic Countries
eTable 3. Definition of Malformations of Interest in US Medicaid Analytic eXtract
eTable 4. Definition and Assessment Period of Covariates of Interest
eTable 5. Countries Contributing to the Main Adjusted Analyses
eTable 6. Cohort Characteristics of Pregnancies with Atypical Antipsychotic Exposure, Typical Antipsychotic Exposure and No Antipsychotic Exposure During the First Trimester, Separately for Nordic Countries and the US Medicaid Analytic eXtract (Unadjusted)
eTable 7. Cohort Characteristics of Pregnancies with Atypical Antipsychotic Exposure, Typical Antipsychotic Exposure and No Antipsychotic Exposure During the First Trimester, Separately for Nordic Countries and the US Medicaid Analytic eXtract (Propensity Score Weighted)
eTable 8. Number of Pregnancies with Antipsychotic Exposure Based on ≥1 Dispensing of the Drug of Interest Irrespective of Exposure to Other Antipsychotic Drugs, and Number of Pregnancies Exposed to Only one Antipsychotic Drug (Monotherapy) During the First Trimester, Separately for Nordic Countries and the US Medicaid Analytic eXtract
eTable 9. Pooled Risk Estimates of Malformations of Interest in Infants With and Without Prenatal Exposure to Antipsychotic Drugs of Interest During the First Trimester (Main and Sensitivity Analyses)
eReferences.