Key Points
Question
What are the overall rates of follow-up colonoscopy (FU-CY) after a positive stool-based test result, and what factors are associated with FU-CY rates, including the early COVID-19 pandemic?
Findings
In this cohort study of 32 769 individuals from 39 different health care organizations, the overall FU-CY rate within 1 year of a positive stool-based test result was 56%. Race, ethnicity, insurance type, type of test (fecal immunochemical tests or multitarget stool DNA), health care organization, and the COVID-19 pandemic were significantly associated with these rates.
Meaning
Targeted interventions to improve overall FU-CY rates and to cover the backlog of colonoscopies from the peak COVID-19 months (March to June 2020) are necessary to achieve the full clinical benefits from stool-based colorectal cancer screening tests.
This cohort study evaluates follow-up colonoscopy rates among patients with positive stool-based test results across different health care organizations and patient-level characteristics.
Abstract
Importance
Noninvasive stool-based screening tests (SBTs) are effective alternatives to colonoscopy. However, a positive SBT result requires timely follow-up colonoscopy (FU-CY) to complete the colorectal cancer screening paradigm.
Objectives
To evaluate FU-CY rates after a positive SBT result and to assess the association of the early COVID-19 pandemic with FU-CY rates.
Design, Setting, and Participants
This mixed-methods cohort study included retrospective analysis of deidentified administrative claims and electronic health records data between June 1, 2015, and June 30, 2021, from the Optum Labs Data Warehouse and qualitative, semistructured interviews with clinicians from 5 health care organizations (HCOs). The study population included data from average-risk primary care patients aged 50 to 75 years with a positive SBT result between January 1, 2017, and June 30, 2020, at 39 HCOs.
Main Outcomes and Measures
The primary outcome was the FU-CY rate within 1 year of a positive SBT result according to patient age, sex, race, ethnicity, insurance type, Charlson Comorbidity Index (CCI), and prior SBT use.
Results
This cohort study included 32 769 individuals (16 929 [51.7%] female; mean [SD] age, 63.1 [7.1] years; 2092 [6.4%] of Black and 28 832 [88.0%] of White race; and 825 [2.5%] of Hispanic ethnicity). The FU-CY rates were 43.3% within 90 days of the positive SBT result, 51.4% within 180 days, and 56.1% within 360 days (n = 32 769). In interviews, clinicians were uniformly surprised by the low FU-CY rates. Rates varied by race, ethnicity, insurance type, presence of comorbidities, and SBT used. In the Cox proportional hazards regression model, the strongest positive association was with multitarget stool DNA use (hazard ratio, 1.63 [95% CI, 1.57-1.68] relative to fecal immunochemical tests; P < .001), and the strongest negative association was with the presence of comorbidities (hazard ratio, 0.64 [95% CI, 0.59-0.71] for a CCI of >4 relative to 0; P < .001). The early COVID-19 pandemic was associated with lower FU-CY rates.
Conclusions and Relevance
This study found that FU-CY rates after a positive SBT result for colorectal cancer screening were low among an average-risk population, with the median HCO achieving a 53.4% FU-CY rate within 1 year. Socioeconomic factors and the COVID-19 pandemic were associated with lower FU-CY rates, presenting opportunities for targeted intervention by clinicians and health care systems.
Introduction
Colonoscopy is the most common choice for colorectal cancer (CRC) screening in the US.1 However, logistical challenges, such as requiring bowel preparation, sedation, and transportation, may hinder colonoscopy completion rates.2 Offering patients and clinicians an opportunity to choose among various CRC screening modalities can improve screening adherence.3,4 Noncolonoscopy screening options include less invasive and more convenient at-home stool-based tests (SBTs), such as fecal immunochemical tests (FITs) and multitarget stool DNA (mt-sDNA) tests. Fecal immunochemical tests use antibodies to detect blood in the stool, whereas mt-sDNA tests detect blood using antibodies and identify DNA biomarkers associated with CRC. In recent years, particularly during the COVID-19 pandemic, these at-home SBTs had higher utilization rates.5
Although SBTs are a convenient alternative to colonoscopy for initial CRC screening, a positive SBT result must be followed by a follow-up colonoscopy (FU-CY). Failed (no FU-CY) or delayed (usually defined as >6 or >9 months) follow-up after a positive SBT result is associated with higher CRC risk and related complications.6,7,8,9 Despite evidence showing the need for timely follow-up after a positive SBT result, the reported FU-CY rates within community settings are low and vary substantially across different commercial, governmental, and nonprofit US health care settings. Reported rates range from 18% to 75% within 12 months of a positive SBT result, which is lower than the recommended rate of 80% and potentially fails to capitalize on the benefits of widespread at-home screenings.7,8,10,11,12,13,14,15 The recent COVID-19 pandemic has likely further influenced this screening paradigm, but no updated evaluation of FU-CY rates in the US exists to address this question.16 The current study evaluated FU-CY rates among patients with positive SBT results (FIT or mt-sDNA) across different health care organizations (HCOs) and patient-level characteristics and aimed to quantify the initial association of the COVID-19 pandemic with screening completion rates in a large national sample of US patients.
Methods
Study Design and Data Source
This mixed-methods cohort study included retrospective analyses of deidentified administrative claims and electronic health record (EHR) data between June 1, 2015, and June 30, 2021, from the Optum Labs Data Warehouse (OLDW) and qualitative interviews with health care professionals. The OLDW is a longitudinal, real-world data asset with deidentified administrative claims and EHR data, including EHR data on approximately 100 million lives. Study data were accessed using techniques compliant with the Health Insurance Portability and Accountability Act of 1996. This study was deemed exempt from institutional review board (IRB) approval and the need for informed consent by the WCG IRB because the research includes only interactions involving educational tests, survey procedures, interview procedures, or observations of public behavior. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies and the Standards for Reporting Qualitative Research (SRQR) reporting guideline for qualitative studies.
The EHR data were sourced from 39 of the 52 HCOs present in the OLDW resource. The remaining 13 were excluded because they contained no or little evidence of colonoscopy procedures (<100 total colonoscopies in the study period). The sensitivity analysis included adjudicated claims data available for a subset of patients in the EHR data set and linked using patient identifiers in the OLDW. The diagnosis and procedure codes used in this study are specified in eAppendixes 2 and 3 in Supplement 1.
The index date for each patient was defined as the date of the first positive SBT result (FIT or mt-sDNA) between January 1, 2017, and June 30, 2020. The follow-up period started the day after the index date, and patients were followed up for up to 1 year. Patients were censored from follow-up on the date of their last activity in the EHR or claim (eg, visit, procedure, or prescription), on the date of death, or at the end of the 1-year follow-up period, whichever came first. Colonoscopies were ascertained from procedure codes on an outbound billing claim or documentation in the EHR (eg, in a health maintenance table).
This study was primarily quantitative but included an exploratory qualitative analysis, including semistructured interviews with 7 clinicians from 5 HCOs conducted virtually, using a secure Zoom platform (Zoom Video Communications Inc). Participants were recruited via email from a pool of HCOs previously involved with the American Medical Group Association (AMGA) quality improvement initiatives. A common interview guide developed for this project (eAppendix 1 in Supplement 1) was used for each interview. The same set of 3 moderators (including E.L.C. and A.G.) participated in all interviews. All interviews were recorded and transcribed. Data reduction methods were used to summarize the interviews and organize the results.17
Study Population
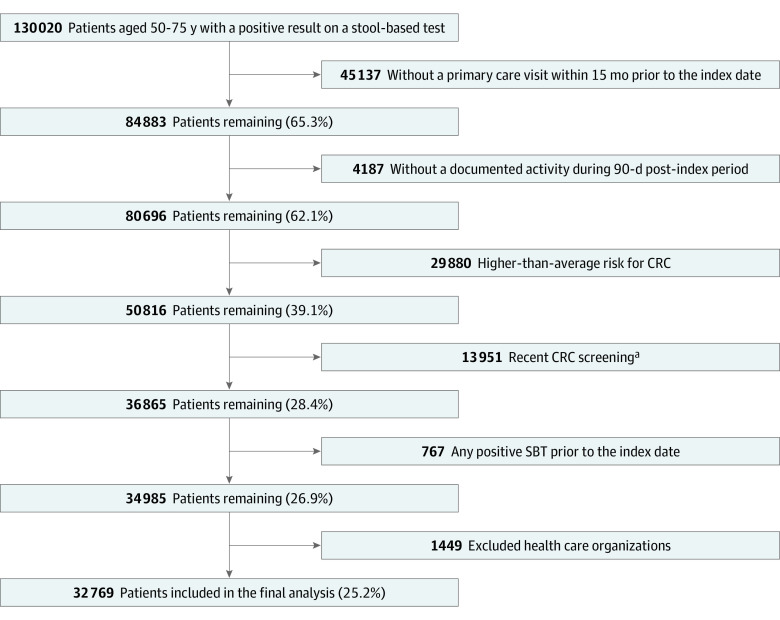
The study population included average-risk primary care patients aged 50 to 75 years (based on the US Preventive Services Task Force’s recommendations for CRC screening at the time of the study) with a documented positive SBT result between January 1, 2017, and June 30, 2020. Patients were included if they had a primary care visit within 15 months before the index date and had documented activity (eg, office visit, inpatient stay, prescription, or procedure) at least 90 days after the index date. This activity requirement ensured that patients were plausibly receiving ongoing care from the HCO and therefore had diagnoses and procedures included in the EHR data. Patients were excluded (Figure 1) if they were at higher-than-average risk for CRC (eAppendix 2 in Supplement 1). Patients were also excluded if they had more frequent screening (eg, colonoscopy <10 years, flexible sigmoidoscopy or computed tomographic colonography <5 years, mt-sDNA test <3 years, or FIT<9 months), a positive SBT result before the index date, or an inpatient hospital stay on the index date.
Figure 1. Sample Attrition.
CRC indicates colorectal cancer.
aDefined as a colonoscopy within 10 years prior to the index date, flexible sigmoidoscopy or computed tomography colonography within 5 years prior to the index date, a multitarget stool DNA test within 3 years prior to the index date, or a fecal immunochemical test within 9 months prior to the index date.
Data Reporting
The primary outcome was the FU-CY rate after a positive SBT result according to patient age, sex, race, ethnicity, insurance type, Charlson Comorbidity Index (CCI), and prior SBT use. Race and ethnicity are based on EHR data and reflect patient self-reports. We included data on patient race and ethnicity to identify potential disparities in FU-CY and to provide context for how our results may match demographically with the larger US population. Patients with no documented evidence (diagnostic or procedure code) of a colonoscopy were censored on death, last documented activity, or 365 days after the index date. The probability of having received an FU-CY was calculated using the Kaplan-Meier method. Mean rates of follow-up and 95% CIs were estimated at 90, 180, and 360 days from the index date. In a sensitivity analysis, this analysis was repeated on a data set restricted to only patients with both EHR and adjudicated claims data, which captures colonoscopies performed outside the HCO where the index event occurred. A multivariable Cox proportional hazards regression model was used to estimate the hazard ratios (HRs) and 95% CIs associated with patient-level covariates.
To assess the association of the early COVID-19 pandemic with FU-CY rates, patients with index dates during January to May 2019 and January to May 2020 were compared. Patients were clustered by month of the index date, allowing for a comparison of the evolving pandemic’s association over time. Because the timing of the pandemic was different for each cluster and not proportional across the compared years, a proportional hazards comparison was not appropriate. Instead, each cluster was evaluated using the Kaplan-Meier method, stratified by index year. Cumulative follow-up rates were compared across years at 90-, 180-, and 360-day intervals from the index date using the log-rank test.18
Statistical Analysis
R software, version 4.1.0 (R Foundation for Statistical Computing) was used for data processing and analysis. Survival analyses were conducted using the survival package. A priori significance levels were set at a 2-sided P < .05, and 2-sided tests were used for all comparisons.
Results
Patient Characteristics
We identified 32 769 eligible patients (16 929 [51.7%] female and 15 840 [48.3%] male; 469 [1.4%] Asian, 2092 [6.4%] Black, 28 832 [88.0%] White, and 1376 [4.2%] of unknown race; 825 [2.5%] Hispanic, 30 013 [91.6%] non-Hispanic, and 1931 [5.1%] of unknown ethnicity; mean [SD] age, 63.1 [7.1] years) with a positive SBT result between June 1, 2017, and June 30, 2020 (Table 1; Figure 1). The number of patients per HCO ranged from 58 to 9806, with a median count of 425. Patient sociodemographic and clinical characteristics can be found in Table 1.
Table 1. Patient Demographic and Clinical Characteristics.
| Characteristics at index date | No. (%) |
|---|---|
| Overall | 32 769 (100) |
| Age, y | |
| 50-59 | 10 874 (33.2) |
| 60-69 | 14 463 (44.1) |
| 70-75 | 7432 (22.7) |
| Sex | |
| Female | 16 929 (51.7) |
| Male | 15 840 (48.3) |
| Race | |
| Asian | 469 (1.4) |
| Black | 2092 (6.4) |
| White | 28 832 (88.0) |
| Unknown | 1376 (4.2) |
| Ethnicity | |
| Hispanic | 825 (2.5) |
| Non-Hispanic | 30 013 (91.6) |
| Unknown | 1931 (5.9) |
| Insurance type | |
| Commercial | 22 100 (67.4) |
| Medicaid | 1924 (5.9) |
| Medicare | 7760 (23.7) |
| Other | 524 (1.6) |
| Unknown | 461 (1.4) |
| Smoking status | |
| Never | 10 949 (33.4) |
| Current | 7236 (22.1) |
| Not currently | 2161 (6.6) |
| Previously | 9802 (29.9) |
| Unknown | 2621 (8.0) |
| Up-to-date SBT | 5581 (17.0) |
| Index year | |
| 2017 | 8408 (25.7) |
| 2018 | 9738 (29.7) |
| 2019 | 11 729 (35.8) |
| 2020 | 2894 (8.8) |
| mt-sDNA index test | 13 597 (41.5) |
| CCI levels | |
| 0 | 18 648 (56.9) |
| 1-2 | 9792 (29.9) |
| 3-4 | 2927 (8.9) |
| ≥5 | 1402 (4.3) |
Abbreviations: CCI, Charlson Comorbidity Index; mt-sDNA, multitarget stool DNA; SBT, stool-based test.
Rates of FU-CY Over Time
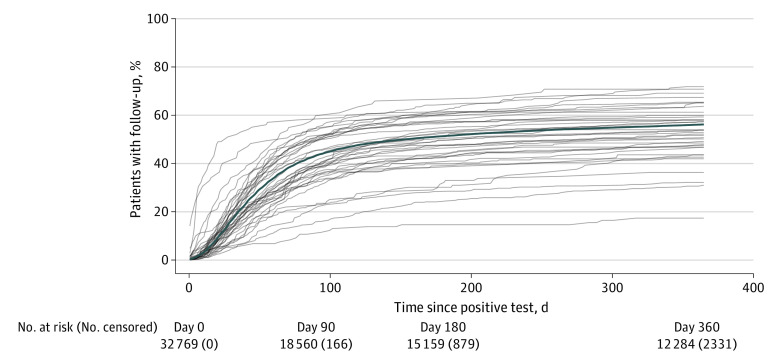
Across all patients, 56.1% (95% CI, 55.6%-56.6%) had FU-CY within 360 days (Figure 2). The largest fraction of these patients received an FU-CY within 90 days (43.2%; 95% CI, 42.7%-43.8%), and 51.4% (95% CI, 50.8%-51.9%) had FU-CY within 180 days. Rates of FU-CY varied widely across the HCOs, with a median 360-day follow-up rate of 53.4% (range, 17.4%-71.8%). In a sensitivity analysis, we found that FU-CY rates were approximately 10% to 13% higher when evaluating a subset of patients with adjudicated claims data (n = 3474) (eTable 1 in Supplement 1). This difference was largest for the 360-day FU-CY rate, which in this subpopulation was 68.6% (95% CI, 67.1%-70.2%), 12.5% greater than observed in EHR data only.
Figure 2. Time-to-Event Curves for Follow-up Colonoscopy.
A Cox proportional hazards regression model was used to study the association of patient-level covariates, controlling for HCO identity (Table 2; eTable 2 in Supplement 1; unadjusted FU-CY rates are reported in eTable 3 in Supplement 1). Compared with White patients, the hazard ratio (HR) of follow-up was significantly lower for Black patients (HR, 0.85; 95% CI, 0.80-0.91) and for Asian patients (HR, 0.79; 95% CI, 0.69-0.91). Patients with commercial insurance were significantly more likely to have a FU-CY than patients with Medicare (HR, 0.95; 95% CI, 0.91-0.99) or Medicaid (HR, 0.79, 95% CI, 0.73-0.85). A recent history of SBT (9 months to 2 years for FIT and 3-4 years for mt-sDNA) use was associated with slightly higher FU-CY rates (HR, 1.12; 95% CI, 1.08-1.16), whereas the use of mt-sDNA was associated with much higher rates (HR 1.63; 95% CI, 1.57-1.68). Finally, the higher the mortality risk as measured by the CCI, the less likely a patient was to receive a FU-CY, with the lowest HR for patients with a CCI of 5 or greater (HR, 0.64; 95% CI, 0.59-0.71). No significant association was found between age, sex, or ethnicity and FU-CY rates. None of these factors were influenced in a sensitivity analysis that excluded patients within 6 months of the beginning of the COVID-19 period (eTable 4 in Supplement 1).
Table 2. Cox Proportional Hazard Model Coefficientsa.
| Characteristic at index date | HR (95% CI) |
|---|---|
| Age, y | |
| 50-59 | 1 [Reference] |
| 60-69 | 0.97 (0.94-1.01) |
| 70-75 | 0.97 (0.93-1.01) |
| Sex | |
| Female | 1 [Reference] |
| Male | 0.99 (0.96-1.02) |
| Race | |
| Asian | 0.79 (0.69-0.91) |
| Black | 0.85 (0.80-0.91) |
| White | 1 [Reference] |
| Unknown | 0.93 (0.86-1.02) |
| Ethnicity | |
| Hispanic | 0.94 (0.84-1.04) |
| Non-Hispanic | 1 [Reference] |
| Unknown | 0.96 (0.88-1.04) |
| Insurance type | |
| Commercial | 1 [Reference] |
| Medicaid | 0.79 (0.73-0.85) |
| Medicare | 0.95 (0.91-0.99) |
| Other | 0.87 (0.76-0.99) |
| Unknown | 0.67 (0.58-0.77) |
| Smoking status | |
| Never smoked | 1 [Reference] |
| Current smoker | 0.87 (0.84-0.91) |
| Not currently smoking | 1.03 (0.97-1.10) |
| Previously smoked | 0.97 (0.94-1.01) |
| Prior SBT use | |
| No | 1 [Reference] |
| Yes | 1.12 (1.08-1.16) |
| Index year | |
| 2017 | 1 [Reference] |
| 2018 | 1.00 (0.96-1.05) |
| 2019 | 0.90 (0.86-0.93) |
| 2020 | 0.72 (0.68-0.77) |
| SBT type | |
| FIT | 1 [Reference] |
| mt-sDNA | 1.63 (1.57-1.68) |
| CCI levels | |
| 0 | 1 [Reference] |
| 1-2 | 0.89 (0.87-0.92) |
| 3-4 | 0.72 (0.68-0.77) |
| 5+ | 0.64 (0.59-0.71) |
Abbreviations: CCI, Charlson Comorbidity Index; FIT, fecal immunochemical tests; HCO, health care organizations; HR, hazard ratio; mt-sDNA, multitarget stool DNA; SBT, stool-based test.
Model also included adjustment for HCO identity (coefficients in eTable 1 in Supplement 1).
Association of Early COVID-19 Pandemic With FU-CY Rates
A total of 2894 patients had their index test result between January 1 and June 30, 2020. These patients had lower FU-CY rates than patients in any of the other study years (Figure 3A), although this reduction was not proportional due to the time-varying impact of COVID-19, making a Cox proportional hazards model inappropriate (Figure 3B). We therefore compared the FU-CY rates for each of these patient subgroups at 90, 180, and 360 days from the index using the Kaplan-Meier method described previously (eTable 5 in Supplement 1).
Figure 3. Impact of the Early COVID-19 Pandemic on Follow-up Colonoscopy Rates.
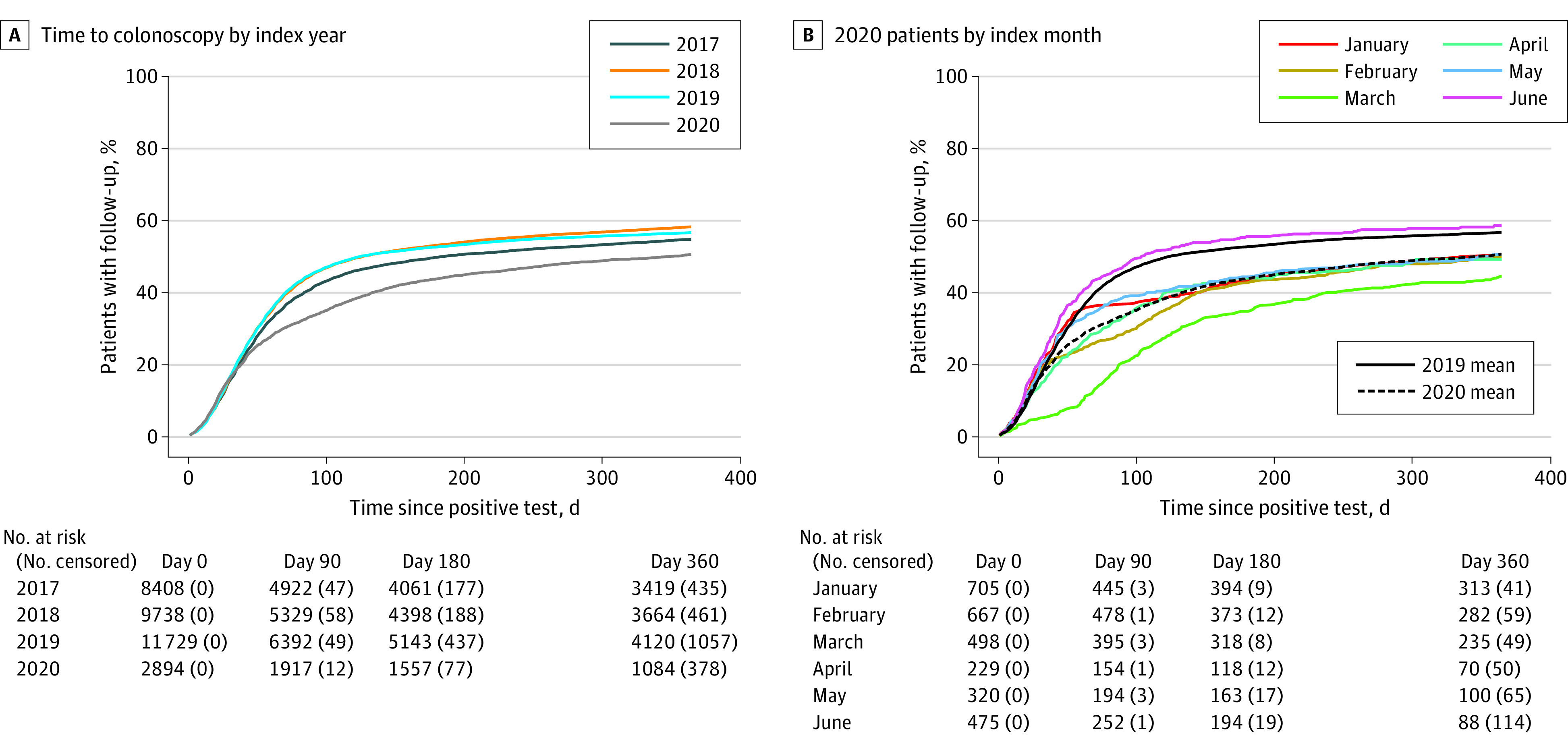
The FU-CY rates were significantly lower in 2020 compared with 2019 across most index months, particularly for the 90- and 180-day time points. Patients with an index result in March 2020 had only 44.0% of patients receiving FU-CY within 360 days compared with 55.9% among patients indexed in March 2019. Patients indexed in June 2020 did not have significantly different FU-CY rates compared with patients indexed in June 2019 across any of the compared follow-up periods.
Qualitative Interviews With Clinicians
In the qualitative interviews, 100% of clinicians indicated that they were unaware of low FU-CY rates. Only 1 of the organizations represented in an interview tracked FU-CY rates after SBTs, although all tracked initial screening rates (consistent with current quality measure guidelines). When asked about barriers to FU-CY, clinicians cited both patient discomfort (with colonoscopy preparation and procedure) and organizational barriers (eg, clinician not alerted to positive test result). When asked about the significant discrepancy between FIT and mt-sDNA FU-CY rates, clinicians again cited patient attitudes (eg, believing the mt-sDNA result was more urgent or valid) as well as the potential for overscreening with FITs.
Discussion
In this large, multicenter cohort study, only 56.1% of patients had a recommended FU-CY within approximately 1 year of a positive SBT result. This rate is far off the follow-up target of 80% recommended by the US Multi-Society Task Force on CRC,15 and even the best performing HCOs in our sample did not achieve this target. In line with a previous study,7 we found significant variability across HCOs, as well as important patient-level factors that were associated with rates of FU-CY. Additionally, we found a significant decrease in follow-up during the early COVID-19 pandemic, suggesting a backlog of patients with positive SBT results that must be addressed. In qualitative interviews carried out to contextualize these findings, we found that awareness of FU-CY rates was low, suggesting a need for transparent reporting of FU-CY rates within organizations.
In a sensitivity analysis, we observed higher FU-CY in adjudicated insurance claims data available for a subpopulation of patients, suggesting that a fraction of patients received FU-CY that were not documented in the EHR (eTable 1 in Supplement 1). This was encouraging because it indicated that the lack of follow-up may not be as low as indicated by EHR data alone. However, this claims population did not include Medicaid, traditional Medicare, or uninsured patients and only included data from a single national insurer. As we found that commercially insured patients had the highest FU-CY rates (Table 2), this sensitivity analysis likely represents an upper bound on FU-CY rates. Even in the most optimistic interpretation of these results, this difference was not large enough to account for the entire care gap among patients who did not receive a FU-CY. These results indicate a significant need for increased FU-CYs rather than simply a failure to document these colonoscopies in the EHR.
After adjusting for HCO identity, we identified multiple disparities in FU-CY by racial and socioeconomic status. African American and Asian patients had significantly lower FU-CY rates after a positive SBT than White patients. Several studies19,20,21,22 have shown significantly lower CRC screening uptake rates and higher CRC-related mortality rates among Black patients compared with the White population. The observed FU-CY rates after a positive result on SBT were also lower among Black individuals.
In our study, the type of SBT was significantly associated with FU-CY rates, with mt-sDNA recipients having a much higher FU-CY rate after a positive SBT result compared with FIT recipients. This finding is consistent with previous work23 that found patients with a positive mt-sDNA result had higher rates of follow-up within 6 months compared with FIT recipients (71.5% vs 46.7%). Another retrospective study conducted using deidentified administrative claims data from January 1, 2006, to June 30, 2020, for commercially insured and Medicare Advantage enrollees also reported a significantly higher follow-up colonoscopy rate among patients with positive mt-sDNA vs those with positive FIT (72% vs 46%).24 The mt-sDNA test is supported by a patient navigation program for the initial screening test and notification to the ordering physician of positive mt-sDNA results.25 Outreach efforts may play a role in improving the overall FU-CY completion rates among those with positive mt-sDNA tests.26,27 Other factors may contribute to different follow-up rates by test type, eg, patients placing a higher value on a “DNA” test, as reported in the clinician interviews. These and other factors highlighted by the interviews (eg, organization-level barriers to follow-up) require further research to understand what factors underly the large variability in FU-CY rates.
Finally, our study showed a significant decline in FU-CY rates during the early COVID-19 pandemic. This decrease was largest among patients who received a result prior to the beginning of pandemic-related disruptions in the US (approximately mid-March 2020), suggesting that the initial stages of the pandemic were more disruptive than subsequent months. In fact, patients who received a positive SBT result in June followed up at a higher overall rate than the 2019 average (Figure 2B), though the absolute number of patients in this subpopulation was small. A similar study found that time to FU-CY after FIT was slower in the early months of the pandemic before recovering around May 2020, though this was not in a US population.16 These results highlight a potential backlog of patients who did not follow up during the early stages of the COVID-19 pandemic. Further work is needed to determine whether FU-CY rates recovered quickly after these initial pandemic-related disruptions.
In summary, at-home stool-based testing offers a useful tool to supplement routine colonoscopy and reach a wider patient population. Nonetheless, it is important to emphasize that all positive SBTs must be followed by a timely colonoscopy to complete the screening paradigm. Not completing a colonoscopy after a positive SBT is associated with a significantly higher risk of CRC complications and mortality, and the recommended optimum time for FU-CY completion is earlier than 6 to 9 months after a positive SBT.6,8,9,28,29 The fact that few health systems reported tracking FU-CY rates in our qualitative interviews but are tracking initial screening rates, coupled with the disparity in initial screening vs follow-up colonoscopy rates, suggests the need for a quality performance measure of FU-CY. Current evidence suggests that while colonoscopy completion rates were as high as 90% in randomized clinical trials,30 the reported rates in community-based real-world studies ranged from 30% to 65% after a positive FIT test12 and between 70% to 96% after a positive mt-sDNA test.23,24,31 Our study confirms that these low rates of FU-CY are persistent in a broad national sample and finds that a lack of follow-up after a positive SBT was exacerbated by the COVID-19 pandemic. Addressing this care gap can reduce CRC mortality by improving early detection rates.
Limitations
This study has some limitations. As this study relied on EHR data and outbound billing claims from HCOs, only colonoscopies documented in the EHR were counted in the FU-CY rate, and a sensitivity analysis found that this may underestimate the FU-CY rate by 10% to 13% (eTable 1 in Supplement 1). Identification of patients at average risk and screening-specific SBTs was done algorithmically using EHR data, and this may have resulted in the inclusion of some patients who may not have met criteria for CRC screening or FU-CY when examined clinically. Factors such as race and ethnicity may be absent for some patients or HCOs or may be inaccurately documented, leading to potential unobservable and unmeasured confounding. Finally, although the clinical data used in this study were sourced from a diverse set of HCOs across the US, these HCOs were a convenience sample based on their relationship with the company managing our data resource (Optum Labs), which may introduce selection bias if these HCOs differed systematically from other US health care organizations. Similarly, results from this analysis may not be broadly generalizable to other populations outside the US or within other care delivery systems (eg, the Veterans Health Administration).
Conclusions
Successful screening for CRC requires timely colonoscopy after positive SBTs. This cohort study found that rates of FU-CY were low among an average-risk population, with a median FU-CY rate of 53.4% across 39 HCOs. These rates were not consistent across patient demographic categories, indicating potentially addressable health disparities. A significant decline in completion of screening with FU-CY during the early COVID-19 pandemic warrants prioritizing screening backlogs, given that the long delays in follow-up care may lead to worse CRC outcomes.
eTable 1. Sensitivity Analysis Using Adjudicated Claims Data
eTable 2. Coefficients of HCO Identity for Cox Model
eTable 3. Unadjusted FU-CY Rates for Select Characteristics
eTable 4. Cox Proportional Hazard Sensitivity Analysis Excluding COVID Period
eTable 5. Comparison of Index Years 2019 and 2020 Assessing COVID-19 Impact
eAppendix 1. Exclusionary ICD-9 and ICD-10 Codes for Patients Not at Above-Average Risk
eAppendix 2. Colonoscopy Procedure Codes
eAppendix 3. AMGA Colorectal Cancer Screening and Follow-up Interview Guide
Data Sharing Statement
References
- 1.Issa IA, Noureddine M. Colorectal cancer screening: an updated review of the available options. World J Gastroenterol. 2017;23(28):5086-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson KW, Barry MJ, Mangione CM, et al. ; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi: 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 3.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575-582. doi: 10.1001/archinternmed.2012.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang PS, Wheat CL, Abhat A, et al. Adherence to competing strategies for colorectal cancer screening over 3 years. Am J Gastroenterol. 2016;111(1):105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beshara A, Ahoroni M, Comanester D, et al. Association between time to colonoscopy after a positive guaiac fecal test result and risk of colorectal cancer and advanced stage disease at diagnosis. Int J Cancer. 2020;146(6):1532-1540. [DOI] [PubMed] [Google Scholar]
- 7.Chubak J, Garcia MP, Burnett-Hartman AN, et al. ; PROSPR consortium . Time to colonoscopy after positive fecal blood test in four US health care systems. Cancer Epidemiol Biomarkers Prev. 2016;25(2):344-350. doi: 10.1158/1055-9965.EPI-15-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631-1641. doi: 10.1001/jama.2017.3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forbes N, Hilsden RJ, Martel M, et al. Association between time to colonoscopy after positive fecal testing and colorectal cancer outcomes: a systematic review. Clin Gastroenterol Hepatol. 2021;19(7):1344-1354.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharti B, May FFP, Nodora J, et al. Diagnostic colonoscopy completion after abnormal fecal immunochemical testing and quality of tests used at 8 Federally Qualified Health Centers in Southern California: Opportunities for improving screening outcomes. Cancer. 2019;125(23):4203-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gingold-Belfer R, Leibovitzh H, Boltin D, et al. The compliance rate for the second diagnostic evaluation after a positive fecal occult blood test: a systematic review and meta-analysis. United European Gastroenterol J. 2019;7(3):424-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green BB, Baldwin LM, West II, Schwartz M, Coronado GD. Low rates of colonoscopy follow-up after a positive fecal immunochemical test in a Medicaid health plan delivered mailed colorectal cancer screening program. J Prim Care Community Health. Published online September 10, 2020. doi: 10.1177/2150132720958525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net system. Am J Gastroenterol. 2017;112(2):375-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor EA, Nielson CM, Petrik AF, Green BB, Coronado GD. Prospective cohort study of predictors of follow-up diagnostic colonoscopy from a pragmatic trial of FIT screening. Sci Rep. 2020;10(1):2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;152(5):1217-1237.e3. [DOI] [PubMed] [Google Scholar]
- 16.Kortlever TL, de Jonge L, Wisse PHA, et al. The national FIT-based colorectal cancer screening program in the Netherlands during the COVID-19 pandemic. Prev Med. 2021;151:106643. doi: 10.1016/j.ypmed.2021.106643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen M. The SAGE Encyclopedia of Communication Research Methods. Vol. 4. SAGE Publications; 2017. doi: 10.4135/9781483381411 [DOI] [Google Scholar]
- 18.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & Sons; 2022. [Google Scholar]
- 19.Jackson CS, Oman M, Patel AM, Vega KJ. Health disparities in colorectal cancer among racial and ethnic minorities in the United States. J Gastrointest Oncol. 2016;7(suppl 1):S32-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May FP, Yang L, Corona E, Glenn BA, Bastani R. Disparities in colorectal cancer screening in the United States before and after implementation of the Affordable Care Act. Clin Gastroenterol Hepatol. 2020;18(8):1796-1804.e2. [DOI] [PubMed] [Google Scholar]
- 21.Warren Andersen S, Blot WJ, Lipworth L, Steinwandel M, Murff HJ, Zheng W. Association of race and socioeconomic status with colorectal cancer screening, colorectal cancer risk, and mortality in southern US adults. JAMA Netw Open. 2019;2(12):e1917995. doi: 10.1001/jamanetworkopen.2019.17995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White PM, Itzkowitz SH. Barriers driving racial disparities in colorectal cancer screening in African Americans. Curr Gastroenterol Rep. 2020;22(8):41. [DOI] [PubMed] [Google Scholar]
- 23.Cooper GS, Grimes A, Werner J, Cao S, Fu P, Stange KC. Barriers to follow-up colonoscopy after positive FIT or multitarget stool DNA testing. J Am Board Fam Med. 2021;34(1):61-69. [DOI] [PubMed] [Google Scholar]
- 24.Austin G, Kowalkowski H, Guo Y, et al. Patterns of initial colorectal cancer screenings after turning 50 years old and follow-up rates of colonoscopy after positive stool-based testing among the average-risk population. Curr Med Res Opin. Published online August 26, 2022. doi: 10.1080/03007995.2022.2116172 [DOI] [PubMed] [Google Scholar]
- 25.Weiser E, Parks PD, Swartz RK, et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening: Real-world data from a large cohort of older adults. J Med Screen. 2021;28(1):18-24. doi: 10.1177/0969141320903756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlitz JJ, Fendrick AM, Bhatt J, et al. Cost-effectiveness of outreach strategies for stool-based colorectal cancer screening in a Medicaid population. Popul Health Manag. 2022;25(3):343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idos GE, Bonner JD, Haghighat S, et al. Bridging the gap: patient navigation increases colonoscopy follow-up after abnormal FIT. Clin Transl Gastroenterol. 2021;12(2):e00307. doi: 10.14309/ctg.0000000000000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YC, Hsu CY, Chen SL, et al. Effects of screening and universal healthcare on long-term colorectal cancer mortality. Int J Epidemiol. 2019;48(2):538-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi: 10.1053/j.gastro.2021.01.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467-1471. [DOI] [PubMed] [Google Scholar]
- 31.Eckmann JD, Ebner DW, Kisiel JB. Multi-target stool DNA testing for colorectal cancer screening: emerging learning on real-world performance. Curr Treat Options Gastroenterol. Published online January 21, 2020. doi: 10.1007/s11938-020-00271-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Sensitivity Analysis Using Adjudicated Claims Data
eTable 2. Coefficients of HCO Identity for Cox Model
eTable 3. Unadjusted FU-CY Rates for Select Characteristics
eTable 4. Cox Proportional Hazard Sensitivity Analysis Excluding COVID Period
eTable 5. Comparison of Index Years 2019 and 2020 Assessing COVID-19 Impact
eAppendix 1. Exclusionary ICD-9 and ICD-10 Codes for Patients Not at Above-Average Risk
eAppendix 2. Colonoscopy Procedure Codes
eAppendix 3. AMGA Colorectal Cancer Screening and Follow-up Interview Guide
Data Sharing Statement