Abstract
Background
Ticks are obligate hematophagous arthropods capable of transmitting a great variety of endemic and emerging pathogens causing diseases in animals and humans.
Objectives
The aim of this study was to investigate the presence of Bartonella spp., Rickettsia spp., Borrelia burgdorferi sensu lato (s.l.) and Anaplasma phagocytophilum in ticks collected from cattle in Benin and Togo.
Methods
Overall, 396 (148 males, 205 females and 43 nymphs) ticks were collected from cattle in 17 districts (Benin and Togo) between 2019 and 2020. Ticks were pooled into groups of 2–6 ticks per pool according to individual host, location, species and developmental stage. The DNA of each pool was extracted for molecular screening.
Results
PCR results revealed that 20 tick pools were positive for Bartonella spp. (Benin and Togo) and 23 tick pools positive for Rickettsia spp. (Benin), while all pools were negative for A. phagocytophilum and B. burgdorferi s.l. Sequence analysis of positive Rickettsia samples revealed the presence of Rickettsia aeschlimannii.
Conclusions
The present study highlights the presence of zoonotic agents in ticks collected from cattle in Benin and Togo. This information will raise awareness of tick‐borne diseases among physicians and veterinarians, stimulate further studies to monitor these pathogens, and advise on necessary measures to control the spread of these zoonoses.
Keywords: bacteria, public health, Rickettsia aeschlimannii, tick, West Africa
A total of 396 ticks were collected from cattle between 2019 and 2020, and divided in pools (two to six ticks per pool) for molecular screening. The present study highlights the presence of zoonotic agents in ticks collected from cattle in Benin and Togo.
1. INTRODUCTION
Ticks are known to be important disease vectors, capable of transmitting different pathogens such as protozoa, bacteria, and viruses to a wide range of hosts, including humans (Dantas‐Torres et al., 2012). The distribution of tick‐borne pathogens is influenced by a series of climatic and environmental factors, as well as density and diversity of the host species (Ogden, 2017; Wikel, 2018). Among the tick‐borne pathogens, Rickettsia, Anaplasma or Bartonella species are of high importance in cattle (Kasaija et al., 2021). Rickettsia spp. are obligate intracellular alpha‐proteobacteria, with a worldwide distribution and transmitted to humans and animals by various arthropod vectors, including ticks (Parola et al., 2013). They have a high host adaptability, and the infections are important causes of morbidity and mortality worldwide (Diop et al., 2019; Saravanan et al., 2020). Rickettsia spp. are considered emerging zoonotic pathogens that are responsible for conditions such as the spotted fevers and typhus (Bhengsri et al., 2016). The continuous identification of new species or genotypes of Rickettsia (Parola et al., 2013; Portillo et al., 2015) in ticks brings up new questions on their true diversity, ecology and biology and the role of climate change in their distribution (Dantas‐Torres et al., 2012). This study provides information on these bacteria in West Africa, including Benin and Togo.
Bartonella spp. (Rhizobiales) are intracellular Gram‐negative bacteria. About 30 Bartonella species were described in a great variety of domestic and wild mammals worldwide (Kosoy et al., 2012). Bartonella spp. cause endocarditis in humans and domestic animals, including cattle (Erol et al., 2013). Detection of Bartonella spp. in bats was reported from Ghana and Nigeria (Billeter et al., 2012; Kamani et al., 2014). It has also been detected in rodents and their ectoparasites in Nigeria (Kamani et al., 2013). Although several Bartonella spp. were identified in ticks, their vectorial competence is still under discussion (Telford & Wormser, 2010). Despite the potential public and veterinary health importance, no studies were conducted on these bacteria in Benin and Togo.
Borrelia burgdorferi sensu lato (s.l.) complex comprises more than 20 genospecies and was described worldwide in a great variety of animals and vectors including ticks (Margos et al., 2019). Borrelia burgdorferi s.l. is one of the most important tick‐borne zoonotic agents in humans (Lefeuvre et al., 2020). They are maintained in nature by the interaction that exists with its vector ticks vertebrate reservoir hosts. Borrelia burgdorferi s.l. is transmitted by hard ticks and has been identified in Europe (Marchant et al., 2017), America (Scott et al., 2018) and Asia (Pukhovskaya et al., 2019), but few studies reported this zoonotic agent in Africa (Chitanga et al., 2014). While there are some reports of the presence of B. burgdorferi s.l. in Northern Africa (Elhelw et al., 2021), in Western Africa, mainly B. crocidurae and other species responsible for relapsing fever than Lyme borreliosis have been detected (Margos et al., 2019).
Anaplasma phagocytophilum (Anaplasmataceae) is an obligate intracellular bacterium known as a zoonotic tick‐borne pathogen and poses significant public health importance (Dumler & Walker, 2001). Anaplasma phagocytophilum was reported in several species of wild and domestic mammals worldwide, causing the human granulocytic anaplasmosis (HGA). In animals, it is responsible for reduction of milk production, abortion and also death (Stuen, 2007). Anaplasma phagocytophilum has been reported in various countries from Africa including Morocco, Zimbabwe, Tunisia, Algeria and South Africa (El Hamiani Khatat et al., 2021; Kelly et al., 2014; Nakayima et al., 2014). Currently, very few confirmed cases of A. phagocytophilum infection were reported in West Africa (Djiba et al., 2013).
In Africa, human tick‐borne diseases are underestimated and tick bites in humans go unreported due to the lack of awareness, knowledge of the risk of TBDs and the failure of the epidemiological surveillance system (N'koué Sambiéni et al., 2015; Rapp, 2014; Tutin, 2000). However, accurate identification of pathogens circulating between wild and domestic animals, ticks, and humans in a region is essential to facilitate diagnosis and treatment regimens, which depend on the pathogen involved. The lack of epidemiological knowledge on tick‐borne diseases induces confusion, with wrong diagnoses, and therefore, wrong treatment. The purpose of this study was to identify Bartonella spp., Rickettsia spp., B. burgdorferi s.l. and A. phagocytophilum in ticks collected from cattle in Benin and Togo (West Africa).
2. MATERIALS AND METHODS
2.1. Study area and tick collection
A total of 396 ticks were collected from cattle in 2019–2020 in Benin and Togo (Figure 1). Ticks were preserved in 70% ethanol and transported to the Department of Parasitology and Parasitic Diseases of the University of Agricultural Sciences and Veterinary Medicine of Cluj‐Napoca. Identification of tick species was done under a stereomicroscope by using morphological keys (Estrada‐Peña et al., 2004; J. J. Walker et al., 2000; A. R. Walker et al., 2003).
FIGURE 1.
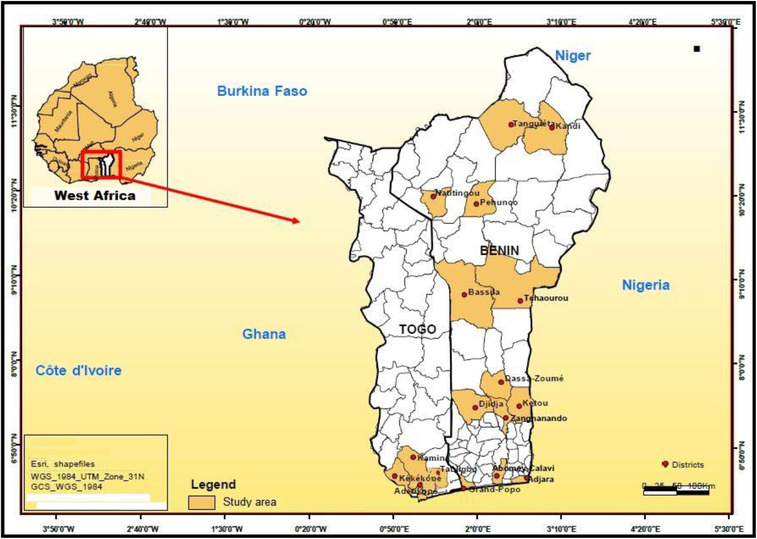
Geographical distribution of tick collection areas in Benin and Togo
2.2. Molecular assessment
Ticks were pooled into groups of 2–6 ticks per pool according to individual host, location, species and developmental stage. The DNA of each pool was extracted using ISOLATE II Genomic DNA Kit (Bioline Meridian Bioscience, Luckenwalde, Germany), according to the manufacturer's instructions and stored at −20°C.
Genomic DNA samples were screened for the presence of Bartonella spp., Rickettsia spp., B. burgdorferi s.l. and A. phagocytophilum by conventional and nested PCRs (Table 1), targeting the citrate synthase (gltA) gene of Rickettsia spp. and Bartonella spp., the flagellin B (flaB) gene of B. burgdorferi s.l. and the 16S rRNA gene of A. phagocytophilum.
TABLE 1.
Primers and polymerase chain reaction conditions used for the detection of pathogens in ticks
| Temperature and duration of: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Target species | Gene (∼amplicon length) | Forward and reverse primers (5'‐3') (Reference) | Initial denaturation | Denaturation | Annealing | Extension | Final extension | Number of cycles |
| Bartonella spp. | gltA (380–400 bp) |
bart781: GGG GAC CAG CTC ATG GTG G bart1137: AAT GCA AAA AGA ACA GTA AAC A (Norman et al., 1995) |
95°C, 5 m | 95°C, 30 s | 52°C, 30 s | 72°C, 30 s | 72°C, 5 m | 35 |
| Rickettsia spp. | gltA (381 bp) |
Rsfg877: GGG GGC CTG CTC ACG GCG G Rsfg1258: ATT GCA AAA AGT ACA GTG AAC A (Regnery et al., 1991) |
95°C, 2 m | 95°C, 30 s | 58°C, 30 s | 72°C, 30 s | 72°C, 2 m | 35 |
| B. burgdorferi s.l. (nested PCR) | flaB (650 bp) |
FlaLL:ACA TAT TCA GAT GCA GAC AGA GGT FlaRL: TGT TAG ACG TTA CCG ATA CTA ACG (Barbour et al., 1996) |
95°C, 5 m | 95°C, 30 s | 55°C, 30 s | 72°C, 45 s | 72°C, 5 m | 35 |
| flaB (350–400 bp) |
FlaLS: AAC AGC TGA AGA GCT TGG AAT G FlaRS: CGA TAA TCT TAC TAT TCA CTA GTT TC (Clark et al., 2013) |
95°C, 5 m | 95°C, 30 s | 59°C, 30 s | 72°C, 30 s | 72°C, 5 m | 35 | |
|
A. phagocytophilum (nested PCR) |
16S rRNA (945 bp) |
Ge3a: CAC ATG CAA GTC GAA CGG ATT ATT C Ge10r: TTC CGT TAA GAA GGA TCT AAT CTC C (Massung et al., 1998) |
95°C, 2 m | 94°C, 30 s | 60°C, 30 s | 72°C, 1 m | 72°C, 5 m | 40 |
| 16S rRNA (570 bp) |
Ge9f: AAC GGA TTA TTC TTT ATA GCT TGC T Ge2: GGC AGT ATT AAA AGC AGC TCC AGG (Massung et al., 1998) |
95°C, 2 m | 94°C, 30 s | 55°C, 30 s | 72°C, 1 m | 72°C, 5 m | 30 | |
Abbreviations: m, minute; s, second.
The PCR amplifications were performed in 25 μl reaction volume, containing 12.5μ1 Green PCR Mastermix (Rovalab GmBH, Teltow, Germany), 6.5 μl of ultra‐pure water, 1 μl (10 pmol/μl) of each primer (Table 1), and 4 μl of isolated DNA aliquot. One negative control (ultra‐pure water) as well as a positive control were included. For the nested PCR targeting the flaB gene of B. burgdorferi s.l. and 16S gene of A. phagocytophilum, a second reaction was performed in 25 μl reaction volume, containing 12.5μ1 Green PCR Mastermix (Rovalab GmBH), 9.5 μl of ultra‐pure water, 1 μl (10 pmol/μl) of each of the two primers (Table 1), and 1 μl aliquot of the first PCR reaction product.
The PCR was performed using the T1000™ Thermal Cycler (Bio‐Rad, London, UK). Amplification products were visualized by electrophoresis on 1.5% agarose gel stained with ECO Safe 20,000 × Nucleic Acid Staining Solution (Pacific Image Electronics, New Taipei, Taiwan) and their molecular weight was assessed by comparison to a molecular marker (O'GeneRuler™ 100 bp DNA Ladder, Thermo Fisher Scientific Inc., Waltham, MA, USA). PCR products were purified using the ISOLATE II PCR and Gel Kit (Bioline Meridian Bioscience) and sent for sequencing (Macrogen Europe, Amsterdam, the Netherlands).
2.3. Sequencing
All sequences were analyzed and edited using Geneious® 4.85 software. Basic Local Alignments Tool (BLAST) analyses (https://blast.ncbi.nlm.nih.gov) were conducted to compare all the obtained sequences with the ones deposited in the GenBank™ database.
3. RESULTS
3.1. Molecular detection
A total of 396 (148 males, 205 females and 43 nymphs) ticks were collected from cattle in 17 districts (Benin, Togo) (Figure 1) and divided into 96 pools. Overall, PCR results showed that 20 tick pools were positive for Bartonella spp. and 23 pools for Rickettsia spp.
In Benin, pools were positive for Bartonella spp. (25.39%) and Rickettsia spp. (30.15%). Bartonella spp. and Rickettsia spp. have been detected in Amblyomma variegatum, Hyalomma rufipes and Rhipicephalus microplus (Table 3). The PCR targeting the gltA gene allowed identifying Bartonella spp. and Rickettsia spp. in several districts in Benin, such as Abomey‐Calavi, Bassila Dassa‐Zoumè, Grand‐Popo, Kétou, Natitingou, Pehunco, Tchaourou and Zangnanando (Table 2).
TABLE 3.
Bartonella spp. and Rickettsia spp. detected in tick species
| Number of tested ticks | PCR positive pools | ||||||
|---|---|---|---|---|---|---|---|
| Country | Tick species | Male | Female | Nymph | Pools (n) | Bartonella spp. | Rickettsia spp. |
| Benin | Amblyomma variegatum | 73 | 62 | 12 | 29 (147) |
24.13 7/29 |
48.27 14/29 |
| Hyalomma rufipes | 13 | 8 | ‐ | 9 (21) |
33.33 3/9 |
33.33 3/9 |
|
| Rhipicephalus microplus | 15 | 77 | 31 | 25 (123) |
24 6/25 |
8 2/25 |
|
| Subtotal | 101 | 147 | 43 | 63 (291) |
25.39 16/63 |
30.15 19/63 |
|
| Togo | Amblyomma variegatum | 24 | 16 | ‐ | 8 (40) |
12.5 1/8 |
12.5 1/8 |
| Hyalomma truncatum | 6 | 5 | ‐ | 7 (11) | ‐ |
14.28 1/7 |
|
| Rhipicephalus annulatus | 2 | 6 | ‐ | 4 (5) | ‐ |
25 1/4 |
|
| Rhipicephalus decoloratus | ‐ | 3 | ‐ | 1 (3) | ‐ | ‐ | |
| Rhipicephalus lunulatus | ‐ | 3 | ‐ | 2 (3) | ‐ | ‐ | |
| Rhipicephalus microplus | 4 | 21 | ‐ | 5 (26) |
20 1/5 |
20 1/5 |
|
| Rhipicephalus muhsamae | ‐ | 2 | ‐ | 1 (2) |
100 1/1 |
‐ | |
| Rhipicephalus sanguineus | 8 | 2 | ‐ | 4 (10) | ‐ | ‐ | |
| Rhipicephalus sulcatus | 3 | ‐ | ‐ | 1 (3) |
100 1/1 |
‐ | |
| Subtotal | 47 | 58 | 0 | 33 (105) |
12.12 4/33 |
12.12 4/33 |
|
| Total |
20.83 20/96 |
23.95 23/96 |
|||||
TABLE 2.
Bartonella spp. and Rickettsia spp. detected in different study sites
| Bartonella spp. | Rickettsia spp. | |||||||
|---|---|---|---|---|---|---|---|---|
| Country | District | Pools (n) | M | F | N | M | F | N |
| Benin | Abomey‐Calavi | 6 (26) | ‐ | 1/6 | ‐ | 1/6 | 1/6 | ‐ |
| Adjara | 4 (19) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Bassila | 3 (8) | ‐ | ‐ | ‐ | ‐ | 1/3 | ‐ | |
| Dassa‐Zoumè | 7 (36) | 1/7 | 2/7 | ‐ | 1/7 | 1/7 | ‐ | |
| Djidja | 4 (21) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Grand‐Popo | 6 (20) | 1/6 | 1/6 | ‐ | ‐ | 1/6 | ‐ | |
| Kandi | 4 (19) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Kétou | 3 (18) | ‐ | ‐ | ‐ | 1/3 | 1/3 | ‐ | |
| Natitingou | 4 (23) | 1/4 | 2/4 | ‐ | 1/4 | 2/4 | ‐ | |
| Pehunco | 4 (19) | 1/4 | 1/3 | ‐ | 1/4 | 3/4 | ‐ | |
| Tanguiéta | 5 (20) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Tchaourou | 6 (32) | ‐ | 1/6 | ‐ | 1/6 | ‐ | ||
| Zangnanando | 7 (30) | 1/7 | 1/7 | ‐ | 2/7 | 1/7 | ‐ | |
| Subtotal | 63 (291) | 5/63 | 9/63 | ‐ | 8/63 | 11/63 | ||
| Togo | Kamina | 10 (27) | ‐ | 1/10 | ‐ | ‐ | 1/1‐ | ‐ |
| Kékékopé | 6 (23) | ‐ | ‐ | ‐ | ‐ | 1/6 | ‐ | |
| Adétikopé | 9 (25) | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Tabligbo | 8 (30) | 3/8 | ‐ | ‐ | ‐ | 2/8 | ‐ | |
| Subtotal | 33 (105) | 3/33 | 1/33 | ‐ | ‐ | 4/33 | ‐ | |
| Total | 96/396 | 8/96 | 10/96 | ‐ | 8/96 | 15/96 | ‐ | |
In Togo, gltA rickettsial DNA was detected in four out of 33 tested tick pools, including A. variegatum, R. microplus, Rhipicephalus muhsamae, and Rhipicephalus sulcatus pools. The DNA of Rickettsia spp. was identified in A. variegatum, Hyalomma truncatum, Rhipicephalus annulatus and R. microplus (Table 3). Bartonella spp. were identified in Kamina and Tabligbo, while Rickettsia spp. were found in Kamina, Kékékopé and Tabligbo. All samples tested for B. burgdorferi s.l. and A. phagocytophilum in this study were negative.
3.2. Sequencing
The gltA gene sequences carried out from Bartonella spp. obtained from two pools of R. microplus in Benin were closely related to a Bartonella strain from China (98.23% and 90.76% identity) (accession No. KX354203). Analysis of the gltA gene from Rickettsia spp. revealed of sequences which had 97.29%–100% identity to various R ickettsia aeschlimannii sequences: accession No. MH267736, MH932014, MH932013; MH675642‐MH675648, MK608659‐MK608560; KJ663742; KY233219; KU961540 obtained from China, France, Italy, Lebanon and Crimean Peninsula. Rickettsia aeschlimannii was detected in seven tick pools including A. variegatum, R. microplus and H. rufipes collected in Benin.
4. DISCUSSION
Data on the distribution of tick‐borne pathogens are necessary for designing appropriate control measures and future approaches to more comprehensive surveillance (Gondard et al., 2017). Very few data exist on this topic in West Africa where a high diversity of tick species was recorded in Benin and Togo (Yessinou et al., 2022). Their presence and distribution can be explained by ecological and climatic conditions that are favourable or unfavourable for the development of ticks in certain countries. All these ticks identified in the study area are capable of playing a role in the transmission and/or maintenance of Bartonella spp. and Rickettsia spp. (Dantas‐Torres et al., 2012). In this study, five tick species collected from cattle were positive for the presence of Bartonella DNA, namely A. variegatum, R. microplus, H. rufipes, R. muhsamae, and R sulcatus. Several species of Bartonella have been reported in ticks, mammals and humans so far (Tsai et al., 2010; Vayssier‐Taussat et al., 2015). Our findings represent the first detection of Bartonella spp. in hard ticks in Benin and Togo. Bartonella spp. were previously also detected in rodents and ticks from Nigeria (Kamani et al., 2013).
Sequencing results also confirmed that A. variegatum, R. microplus and H. ruffipes were infected with R. aeschlimannii, a tick‐borne pathogen, reported in 1997 in Hyalomma marginatum ticks collected in Morocco (Beati et al., 1997). For the first time, the presence of R. aeschlimannii in ticks collected from cattle in Benin was shown in this study. In Senegal, Mediannikov et al. (2010) reported R. aeschlimannii in H. (m) rufipes, H. truncatum and Rhipicephalus evertsi evertsi ticks collected from cows, donkeys, sheep, goats and horses. Others studies in Niger, Mali, Nigeria Ivory‐Coast and Burkina‐Faso identified R. aeschlimannii in Hyalomma spp. (Ehounoud et al., 2016; Kamani et al., 2015; Parola et al., 2001; Tomassone et al., 2016), but also in Europe (Fernández Soto et al., 2003; Punda‐Polic et al., 2003). Hyalomma marginatum appears to serve as a vector but also as a reservoir of R. aeschlimannii (Parola et al., 2005). Rickettsia aeschlimannii was reported in other tick genera including Amblyomma, Dermacentor, Ixodes and Rhipicephalus (Orkun et al., 2014a; Rumer et al., 2011; Toma et al., 2014). This pathogen is implicated in several cases of fever in humans but also reported in several places in animals (Orkun et al., 2014b; Tosoni et al., 2016). The symptoms following an infection are similar to those of Mediterranean spotted fever caused by Rickettsia conorii (Rovery & Raoult, 2008). Raoult et al. (2002) reported cases of human infection caused by R. aeschlimannii in France.
New tick‐borne pathogens infecting humans have increased in recent years, thus playing an important role in public health. In sub‐Saharan Africa, R. aeschlimannii is known as a potentially important pathogen and was identified in humans, animals and ticks (Parola et al., 2013). In these regions, febrile illnesses in breeders, farmers and animal health professionals may be caused by rickettsiosis (Moumouni et al., 2016). Mediannikov et al. (2010) showed that tick‐borne rickettsioses are among the causes of acute nonmalarial febrile diseases. Traditionally, in West Africa, almost all febrile conditions are generally considered to be linked to malaria and are treated as such without further investigations, but other tick‐borne pathogens might be involved. Thus, clinical manifestations of Rickettsioses should be considered in the differential diagnosis of the malaria in patients mainly in rural areas or locations of ruminants rearing.
It should also be noted that the identification of pathogen DNA in a tick does not imply that it is necessarily a biological vector. Studies must be conducted to prove the vector competence of the tick species. The growth of the world population, the transformation of natural habitats, the global changes and the practices of use of the fauna are some of the factors that modify and facilitate the interactions between wild and anthropic environments (Cable et al., 2017). These growing contacts between wildlife and domestic animals, humans and ticks gradually promote the exchange of pathogens that can have harmful health consequences on the animals and humans.
5. CONCLUSION
This study reports the identification and distribution of pathogens in hard ticks collected from cattle in West Africa. These results suggest the need to include bartonellosis and rickettsiosis among the causes of febrile illnesses among breeders, para‐veterinarians, veterinarians and travellers in West Africa. Doctors should also consider these tick‐borne illnesses as a differential diagnostic with malaria. On the other hand, the role of domestic and wild animals in the epidemiology of diseases transmitted by ticks requires further investigation.
AUTHOR CONTRIBUTIONS
Conceptualization, data curation, formal analysis, investigation, methodology, and writing—original draft: Roland Eric Yessinou. Conceptualization, formal analysis, and methodology: Cristina Daniela Cazan. Conceptualization, formal analysis, and methodology: Luciana Cătălina Panait. Investigation: Eyabana Mollong. Conceptualization and methodology: Abel S. Biguezoton. Conceptualization, methodology, and writing—original draft: Sarah Irène Bonnet. Conceptualization: Souaïbou Farougou. Conceptualization, Data curation, Methodology: Martin H. Groschup. Funding acquisition, Supervision, Writing – review & editing: Andrei Daniel Mihalca.
[Correction added on 21 December 2022, after first online publication: The Author contributions section was updated.]
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
No ethical approval was required, as this study does not involve clinical trials or experimental procedures. The cattle's are still alive and used for milk and meat production. This study did not involve endangered or protected species.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1022.
ACKNOWLEDGEMENT
We are grateful to the Francophony University Agency (AUF) for Postdoctoral Fellowship EUGEN IONESCU and Department of Parasitology and Parasitic Diseases, Faculty of Veterinary Medicine, University of Agricultural Sciences and Veterinary Medicine of Cluj‐Napoca, Romania for supporting this research.
Yessinou, R. E. , Cazan, C. D. , Panait, L. C. , Mollong, E. , Biguezoton, A. S. , Bonnet, S. I. , Farougou, S. , Groschup, M. H. , & Mihalca, A. D. (2023). New geographical records for tick‐borne pathogens in ticks collected from cattle in Benin and Togo. Veterinary Medicine and Science, 9, 345–352. 10.1002/vms3.1022
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Barbour, A. G. , Maupin, G. O. , Teltow, G. J. , Carter, C. J. , & Piesman, J. (1996). Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: Possible agent of a Lyme disease‐like illness. Journal of Infectious Diseases, 173, 403–409. [DOI] [PubMed] [Google Scholar]
- Beati, L. , Meskini, M. , Thiers, B. , & Raoult, D. (1997). Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. International Journal of Systematic and Evolutionary Microbiology, 47, 548–554. [DOI] [PubMed] [Google Scholar]
- Bhengsri, S. , Baggett, H. C. , Edouard, S. , Dowell, S. F. , Dasch, G. A. , Fisk, T. L. , Raoult, D. , & Parola, P. (2016). Sennetsu neorickettsiosis, spotted fever group, and typhus group rickettsioses in three provinces in Thailand. American Journal of Tropical Medicine and Hygiene, 95, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter, S. A. , Hayman, D. T. S. , Peel, A. J. , Baker, K. , Wood, J. L. N. , Cunningham, A. , Suu‐Ire, R. , Dittmar, K. , & Kosoy, M. Y. (2012). Bartonellaspecies in bat flies (Diptera: Nycteribiidae) from western Africa. Parasitology, 139(3), 324–329. 10.1017/s0031182011002113 [DOI] [PubMed] [Google Scholar]
- Cable, J. , Barber, I. , Boag, B. , Ellison, A. R. , Morgan, E. R. , Murray, K. , Pascoe, E. L. , Sait, S. M. , Wilson, A. J. , & Booth, M. (2017). Global change, parasite transmission and disease control: Lessons from ecology. Philosophical Transactions of The Royal Society B Biological Sciences, 372, 20160088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitanga, S. , Gaff, H. , & Mukaratirwa, S. (2014). Tick‐borne pathogens of potential zoonotic importance in the southern African Region. Journal of the South African Veterinary Association, 85, 01–03. [DOI] [PubMed] [Google Scholar]
- Clark, K. L. , Leydet, B. , & Hartman, S. (2013). Lyme borreliosis in human patients in Florida and Georgia, USA. International Journal of Medical Sciences, 10, 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas‐Torres, F. , Chomel, B. B. , & Otranto, D. (2012). Ticks and tick‐borne diseases: A One Health perspective. Trends in Parasitology, 28, 437–446. [DOI] [PubMed] [Google Scholar]
- Diop, A. , Raoult, D. , & Fournier, P.‐E. (2019). Paradoxical evolution of rickettsial genomes. Ticks and Tick‐borne Diseases, 10, 462–469. [DOI] [PubMed] [Google Scholar]
- Djiba, M. L. , Mediannikov, O. , Mbengue, M. , Thiongane, Y. , Molez, J.‐F. , Seck, M. T. , Fenollar, F. , Raoult, D. , & Ndiaye, M. (2013). Survey of Anaplasmataceae bacteria in sheep from Senegal. Tropical Animal Health and Production, 45, 1557–1561. [DOI] [PubMed] [Google Scholar]
- Dumler, J. S. , & Walker, D. H. (2001). Tick‐borne ehrlichioses. The Lancet Infectious Diseases, 1, 21–28.11871406 [Google Scholar]
- Ehounoud, C. B. , Yao, K. P. , Dahmani, M. , Achi, Y. L. , Amanzougaghene, N. , Kacou N'Douba, A. , N'Guessan, J. D. , Raoult, D. , Fenollar, F. , & Mediannikov, O. (2016). Multiple pathogens including potential new species in tick vectors in Côte d'Ivoire. PLoS Neglected Tropical Diseases, 10, e0004367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hamiani Khatat, S. , Daminet, S. , Duchateau, L. , Elhachimi, L. , Kachani, M. , & Sahibi, H. (2021). Epidemiological and clinicopathological features of Anaplasma phagocytophilum infection in dogs: A systematic review. Frontiers in Veterinary Science, 8, 686644. 10.3389/fvets.2021.686644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhelw, R. , Elhariri, M. , Hamza, D. , Abuowarda, M. , Ismael, E. , & Farag, H. (2021). Evidence of the presence of Borrelia burgdorferi in dogs and associated ticks in Egypt. BMC Veterinary Research, 17, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol, E. , Jackson, C. , Bai, Y. , Sells, S. , Locke, S. , & Kosoy, M. (2013). Bartonella bovis isolated from a cow with endocarditis. Journal of Veterinary Diagnostic Investigation, 25, 288–290. [DOI] [PubMed] [Google Scholar]
- Estrada‐Peña, A. , Bouattour, A. , Camicas, J. L. , & Walker, A. R. (2004). Ticks of domestic animals in the Mediterranean region: A guide to identification of species. University of Zaragoza. [Google Scholar]
- Fernández Soto, P. , Grandes, A. E. , & Pérez Sánchez, R. (2003). Rickettsia aeschlimannii in Spain: Molecular evidence in Hyalomma marginatum and five other tick species that feed on humans. Emerging Infectious Diseases, 9(7), 889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondard, M. , Cabezas‐Cruz, A. , Charles, R. A. , Vayssier‐Taussat, M. , Albina, E. , & Moutailler, S. (2017). Ticks and tick‐borne pathogens of the Caribbean: Current understanding and future directions for more comprehensive surveillance. Frontiers in Cellular and Infection Microbiology, 7, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamani, J. , Baneth, G. , Apanaskevich, D. A. , Mumcuoglu, K. Y. , & Harrus, S. (2015). Molecular detection of Rickettsia aeschlimannii in Hyalomma spp. ticks from camels (Camelus dromedarius) in Nigeria, West Africa. Medical and Veterinary Entomology, 29, 205–209. 10.1111/mve.12094 [DOI] [PubMed] [Google Scholar]
- Kamani, J. , Morick, D. , Mumcuoglu, K. Y. , & Harrus, S. (2013). Prevalence and diversity of Bartonella species in commensal rodents and ectoparasites from Nigeria, West Africa. PLoS Neglected Tropical Diseases, 7, e2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaija, P. D. , Estrada‐Peña, A. , Contreras, M. , Kirunda, H. , & de la Fuente, J. (2021). Cattle ticks and tick‐borne diseases: A review of Uganda's situation. Ticks and Tick‐borne Diseases, 12(5), 101756. [DOI] [PubMed] [Google Scholar]
- Kelly, P. , Marabini, L. , Dutlow, K. , Zhang, J. , Loftis, A. , & Wang, C. (2014). Molecular detection of tick‐borne pathogens in captive wild felids, Zimbabwe. Parasites & Vectors, 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy, M. , Hayman, D. T. , & Chan, K. ‐ S. (2012). Bartonella bacteria in nature: Where does population variability end and a species start? Infection, Genetics and Evolution, 12, 894–904. [DOI] [PubMed] [Google Scholar]
- Lefeuvre, B. , Cantero, P. , Ehret‐Sabatier, L. , Lenormand, C. , Barthel, C. , Po, C. , Parveen, N. , Grillon, A. , Jaulhac, B. , & Boulanger, N. (2020). Effects of topical corticosteroids and lidocaine on Borrelia burgdorferi sensu lato in mouse skin: Potential impact to human clinical trials. Scientific Reports, 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant, A. , Coupanec, A. L. , Joly, C. , Perthame, E. , Sertour, N. , Garnier, M. , Godard, V. , Ferquel, E. , & Choumet, V. (2017). Infection of Ixodes ricinus by Borrelia burgdorferi sensu lato in peri‐urban forests of France. PLoS One, 12, e0183543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos, G. , Fingerle, V. , & Reynolds, S. (2019). Borrelia bavariensis: Vector switch, niche invasion, and geographical spread of a tick‐borne bacterial parasite. Frontiers in Ecology and Evolution, 7(401), 1–20. [Google Scholar]
- Massung, R. F. , Slater, K. , Owens, J. H. , Nicholson, W. L. , Mather, T. N. , Solberg, V. B. , & Olson, J. G. (1998). Nested PCR assay for detection of granulocytic ehrlichiae. Journal of Clinical Microbiology, 36, 1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediannikov, O. , Diatta, G. , Fenollar, F. , Sokhna, C. , Trape, J.‐F. , & Raoult, D. (2010). Tick‐borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Neglected Tropical Diseases, 4, e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moumouni, P. F. A. , Terkawi, M. A. , Jirapattharasate, C. , Cao, S. , Liu, M. , Nakao, R. , Umemiya‐Shirafuji, R. , Yokoyama, N. , Sugimoto, C. , & Fujisaki, K. (2016). Molecular detection of spotted fever group rickettsiae in Amblyomma variegatum ticks from Benin. Ticks and Tick‐borne Diseases, 7, 828–833. [DOI] [PubMed] [Google Scholar]
- Nakayima, J. , Hayashida, K. , Nakao, R. , Ishii, A. , Ogawa, H. , Nakamura, I. , Moonga, L. , Hang'ombe, B. M. , Mweene, A. S. , & Thomas, Y. (2014). Detection and characterization of zoonotic pathogens of free‐ranging non‐human primates from Zambia. Parasites & Vectors, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'koué Sambiéni, E. , Danko, N. , & Ridde, V. (2015). La Fièvre Hémorragique à Virus Lassa au Bénin en 2014 en contexte d'Ebola : une épidémie révélatrice de la faiblesse du système sanitaire. Anthropologie et Santé, 11, 16. 10.4000/anthropologiesante.1772 [DOI] [Google Scholar]
- Norman, A. F. , Regnery, R. , Jameson, P. , Greene, C. , & Krause, D. C. (1995). Differentiation of Bartonella‐like isolates at the species level by PCR‐restriction fragment length polymorphism in the citrate synthase gene. Journal of Clinical Microbiology, 33, 1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden, N. H. (2017). Climate change and vector‐borne diseases of public health significance. FEMS Microbiology Letters, 364, fnx186. [DOI] [PubMed] [Google Scholar]
- Orkun, Ö. , Karaer, Z. , Çakmak, A. , & Nalbantoğlu, S. (2014a). Spotted fever group rickettsiae in ticks in Turkey. Ticks and Tick‐borne Diseases, 5, 213–218. 10.1016/j.ttbdis.2012.11.018 [DOI] [PubMed] [Google Scholar]
- Orkun, Ö. , Karaer, Z. , Çakmak, A. , & Nalbantoğlu, S. (2014b). Identification of tick‐borne pathogens in ticks feeding on humans in Turkey. PLoS Neglected Tropical Diseases, 8, e3067. 10.1371/journal.pntd.0003067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola, P. , Inokuma, H. , Camicas, J.‐L. , Brouqui, P. , & Raoult, D. (2001). Detection and identification of spotted fever group Rickettsiae and Ehrlichiae in African ticks. Emerging Infectious Diseases, 7, 1014–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola, P. , Paddock, C. D. , & Raoult, D. (2005). Tick‐borne rickettsioses around the world: Emerging diseases challenging old concepts. Clinical Microbiology Reviews, 18, 719–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola, P. , Paddock, C. D. , Socolovschi, C. , Labruna, M. B. , Mediannikov, O. , Kernif, T. , Abdad, M. Y. , Stenos, J. , Bitam, I. , & Fournier, P.‐E. (2013). Update on tick‐borne rickettsioses around the world: A geographic approach. Clinical Microbiology Reviews, 26, 657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo, A. , Santibáñez, S. , García‐Álvarez, L. , Palomar, A. M. , & Oteo, J. A. (2015). Rickettsioses in Europe. Microbes and Infection, 17, 834–838. [DOI] [PubMed] [Google Scholar]
- Pukhovskaya, N. M. , Morozova, O. V. , Vysochina, N. P. , Belozerova, N. B. , & Ivanov, L. I. (2019). Prevalence of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in ixodid ticks in the Far East of Russia. International Journal for Parasitology: Parasites and Wildlife, 8, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punda‐Polic, V. , Petrovec, M. , Trilar, T. , Duh, D. , Bradaric, N. , Klismanic, Z. , & Avsic‐Zupanc, T. (2003). Detection and identification of spotted fever group rickettsiae in ticks collected in southern Croatia. Ticks and tick‐borne pathogens (pp.169–176). Springer. [DOI] [PubMed] [Google Scholar]
- Raoult, D. , Fournier, P.‐E. , Abboud, P. , & Caron, F. (2002). First documented human Rickettsia aeschlimannii infection. Emerging Infectious Diseases, 8, 748, 10.3201/eid0807.010480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp, C. (2014). Epidémie à virus Ebola en Afrique de l'Ouest: réalités et perspectives. Médecine Santé Trop, 24, 229–231. [DOI] [PubMed] [Google Scholar]
- Regnery, R. L. , Spruill, C. L. , & Plikaytis, B. D. (1991). Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. Journal of Bacteriology, 173, 1576–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovery, C. , & Raoult, D. (2008). Mediterranean spotted fever. Infectious Disease Clinics of North America, 22, 515–530. 10.1016/j.idc.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Rumer, L. , Graser, E. , Hillebrand, T. , Talaska, T. , Dautel, H. , Mediannikov, O. , Roy‐Chowdhury, P. , Sheshukova, O. , Mantke, O. D. , & Niedrig, M. (2011). Rickettsia aeschlimannii in Hyalomma marginatum ticks, Germany. Emerging Infectious Diseases, 17, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan, P. , Nagaraj, M. V. , & Somashekhar, C. (2020). Rickettsial fever in tertiary care hospital in rural Bengaluru: Clinical profile and complications. Indian Journal of Maternal & Child Health, 7, 385–387. [Google Scholar]
- Scott, J. D. , Clark, K. L. , Foley, J. E. , Bierman, B. C. , & Durden, L. A. (2018). Far‐reaching dispersal of Borrelia burgdorferi sensu lato‐infected blacklegged ticks by migratory songbirds in Canada. Healthcare. Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuen, S. (2007). Anaplasma phagocytophilum—The most widespread tick‐borne infection in animals in Europe. Veterinary Research Communications, 31, 79–84. [DOI] [PubMed] [Google Scholar]
- Telford III, S. R. , & Wormser, G. P. (2010). Bartonella spp. transmission by ticks not established. Emerging Infectious Diseases, 16, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma, L. , Mancini, F. , Di Luca, M. , Cecere, J. G. , Bianchi, R. , Khoury, C. , Quarchioni, E. , Manzia, F. , Rezza, G. , & Ciervo, A. (2014). Detection of microbial agents in ticks collected from migratory birds in central Italy. Vector‐Borne and Zoonotic Diseases, 14, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassone, L. , De Meneghi, D. , Adakal, H. , Rodighiero, P. , Pressi, G. , & Grego, E. (2016). Detection of Rickettsia aeschlimannii and Rickettsia africae in ixodid ticks from Burkina Faso and Somali Region of Ethiopia by new real‐time PCR assays. Ticks and Tick‐borne Diseases, 7, 1082–1088. [DOI] [PubMed] [Google Scholar]
- Tosoni, A. , Mirijello, A. , Ciervo, A. , Mancini, F. , Rezza, G. , Damiano, F. , & Addolorato, G. (2016). Human Rickettsia aeschlimannii infection: First case with acute hepatitis and review. European Review for Medical and Pharmacological Sciences, 20, 2630–2633. [PubMed] [Google Scholar]
- Tsai, Y.‐L. , Chuang, S.‐T. , Chang, C.‐C. , Kass, P. H. , & Chomel, B. B. (2010). Bartonella species in small mammals and their ectoparasites in Taiwan. American Journal of Tropical Medicine and Hygiene, 83, 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutin, C. E. G. (2000). Ecologie et organisation sociale des primates de la forêt tropicale africaine: aide à la compréhension de la transmission des retrovirus. Bulletin de la Société de Pathologie Exotique, 93, 3–157. [PubMed] [Google Scholar]
- Vayssier‐Taussat, M. , Kazimirova, M. , Hubalek, Z. , Hornok, S. , Farkas, R. , Cosson, J.‐F. , Bonnet, S. , Vourch, G. , Gasqui, P. , Mihalca, A. D. , Plantard, O. , Silaghi, C. , Cutler, S. , & Rizzoli, A. (2015). Emerging horizons for tick‐borne pathogens: From the ‘one pathogen–one disease’ vision to the pathobiome paradigm. Future Microbiology, 10, 2033–2043. 10.2217/fmb.15.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, A. R. , Bouattour, A. A. , Camicas, J. L. , & Estrada‐Pena, I. G. (2003). Ticks of domestic animals in Africa: A guide to identification of species. Bioscience Reports Edinburgh. [Google Scholar]
- Walker, J. B. , Keirans, J. E. , & Horak, I. G. (2000). The genus Rhipicephalus (Acari, Ixodidae): A guide to the brown ticks of the world. Tropical Animal Health and Production, 32, 417. [Google Scholar]
- Wikel, S. K. (2018). Ticks and tick‐borne infections: Complex ecology, agents, and host interactions. Journal of Veterinary Science, 5, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yessinou, R. E. , Cazan, C. D. , Bonnet, S. I. , Farougou, S. , & Mihalca, A. D. (2022). Geographical distribution of hard ticks (Acari:Ixodidae) and tick‐host associations in Benin, Burkina‐Faso, Ivory‐Coast and Togo. Acta Tropica, 232, 106510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


