Abstract
Background
The complex wound healing process involves activating and synchronizing intracellular, intercellular, and extracellular components. Adipose tissue is attracting attention to promote wound healing. Within subcutaneous adipose tissue, stromal vascular cells and their subsets release growth factors and cytokines critical for neovascularization and wound repair.
Objectives
This study evaluated human placental collagen/adipose‐derived stem cells (ADSCs) hydrogel for wound healing in rats.
Methods
In this study, ADSCs were harvested, cultured, and mixed with placental collagen. Twelve rats were used, and their backs were excised three times each. Group one received collagen/ADSCs, group two collagen, and group three non‐filled (control) excisions. The healing processes were assessed by histological analysis, taking photographs, and calculating the percentage of wound contraction in mentioned times.
Results
Histopathological analysis revealed that the content of fibroblasts, follicles of the hair, and angiogenesis in group one was significantly more than in other groups. Group one had a significant result compared with the collagen and control groups. In group one, significant wound healing and wound contraction were observed with 52% and 80% wound contraction at 7 and 14 days, respectively.
Conclusion
Collagen/ADSCs can be considered a suitable candidate hydrogel in wound healing with a high potential for enhancing wound repairing.
Keywords: ADSCs, collagen, hydrogel, placenta, Wound healing
This study evaluated human placental collagen/adipose‐derived stem cells (ADSCs) hydrogel for wound healing in rats. Histopathological analysis revealed that the content of fibroblasts, follicles of the hair, and angiogenesis in collagen/ADSCs group was significantly more than in other groups. Collagen/ADSCs group had a significant result compared to the collagen and control groups. In collagen/ADSCs group, significant wound healing and wound contraction were observed with 52%, and 80% wound contraction at 7 and 14 days, respectively. Collagen/ADSCs can be considered a suitable candidate hydrogel in wound healing with a high potential for enhancing wound repairing.
1. INTRODUCTION
Wound healing refers to restoring the normal function and morphology of tissue after injury. The initial phases of wound healing aim to regenerate the barriers to prevent fluid loss and infection. Moreover, the wound healing process involves angiogenesis and rehabilitation of normal blood and lymphatic flow (Tavakolizadeh et al., 2021, Vyas & Vasconez, 2014). The mechanical integrity of the injured tissue/organ is eventually restored as such. Routine wound healing has a predictable pattern comprising overlapping phases that accumulate certain cell populations and specific biochemical activities (Kaushal et al., 2006). It is also the third most common cause of death worldwide (Yadollahi, 2019). In some cases, traumatic injuries are so complex that a full recovery cannot be simply achieved by the conventional therapeutic modalities (Moradi et al., 2015, Vyas & Vasconez, 2014). Wound healing has been accelerated in several ways. Increasing knowledge of wound pathophysiology has led to biomedical advances and innovations for treating chronic and acute wounds (Vyas & Vasconez, 2014). A wound dressing protects the wound from the external environment. This facilitates and accelerates healing. Dressings such as hydrogels not only keep the skin moist but also contain a high water concentration. They are thus ideal for wound treatment. Hydrogels can be applied to exuding wounds and dry necrotic wounds. The dressings that create moist wound healing environments include films, hydrocolloids, foams, and hydrogels. Hydrogels are not only soft, malleable, and biocompatible, but they also have other unique features (Gupta et al., 2019, Khayatan et al., 2022). Recent literature studies have investigated the use of hydrogel as a wound healing scaffold. Biomaterial scaffolds are now commonly used to provide a suitable environment for directed cell proliferation and regeneration of specific cell types, tissues, or organs. Cells migrate and proliferate more when cells communicate with the extracellular matrix (Karami et al., 2019, Moradi et al., 2015). Tissue engineering relies heavily on scaffolds (Moradi et al., 2015). Collagen is a naturally occurring protein that maintains the structural integrity of cells and tissues. Myofibroblasts and fibroblasts produce skin collagen. In cutaneous tissue, tendons, and bone, collagen is arranged in fibrils constantly under tension and shear (Karami et al., 2019, Metcalfe & Ferguson, 2007, Shokrgozar et al., 2012). This protein is low immunogenic, excellent biocompatible, biodegradable, and enhances cellular adhesion, proliferation, and growth. Thus, it is a good candidate for tissue engineering scaffolds (Barzegar et al., 2022, Hakim et al., 2021, Moghaddam et al., 2022, Na et al., 2021, Tafazoli Moghadam et al., 2021, Yazdanian et al., 2021, 2022). In the dermis, fasciae, and tendons, scar tissue is mainly composed of type I collagen. A protein can also form transmembrane proteins or beaded structures (Metcalfe & Ferguson, 2007). This technique is highly challenging because both the characteristics of the scaffold and the release profile of the loaded medication can significantly affect the wound healing process and must be carefully adjusted and monitored (Cigna et al., 2009, Mohiti‐Asli et al., 2017). The adipose‐derived stem cells (ADSCs) are heterogeneous and are freshly isolated from the fat tissue. Their ability to differentiate into adipogenic, chondrogenic, and osteogenic lineages upon specific in vitro induction represents a relatively homogeneous subpopulation of mesenchymal stem cells (MSCs) (Hussain et al., 2022, Soudi et al., 2021, Trottier et al., 2008). Several processes involved in migration, inflammation, proliferating, and reducing scarring can be modulated by MSCs, contributing to the entire healing process of skin wounds. Several studies have shown that ADSCs and MSCs can accelerate cutaneous wound healing by increasing the migration and proliferation of fibroblasts and keratinocytes, collagen deposition, and macrophage polarization into myeloid M2 phenotypes. Physiologic wound healing is impaired in chronic wounds, making wound healing therapeutically efficacious and essential as a means of treating them (An et al., 2021). This study investigated the effect of human placental collagen/ADSCs on wound healing in rats.
2. MATERIALS AND METHODS
2.1. Synthesis of hydrogel
A previous study had thoroughly described the collagen extraction process (Karami et al., 2019). Shortly, a general surgeon separates the human placenta from the abdomen. We then prepared the placenta by washing it with distilled water (4°C) and cutting it into pieces of no more than two centimeters on ice. The non‐collagenous proteins were removed by washing with 1 N Sodium hydroxide (refreshed every 2 h) and stirred for about 6 h. Next step, extra fat was removed with 10% butyl alcohol for 24 h (refreshed every 12 h) and filtered with a cheesecloth. Sodium chloride (pH = 7, 3 M) precipitated the collagen, which was centrifuged (5000 g, 30 min). In total, 2% acetic acid was used to dissolve the mass, which was then transferred to dialysis tubing, dissolved in distilled water for 5 days, and then freeze dried (Karami et al., 2019). Collagen was neutralized and stored at 4°C after immersion in hydrochloric acid (pH = 2).
2.2. Isolation of ADSCs
In each 250‐ml disposable centrifuge tube, 700 g of abdominal fat were harvested. Phosphate buffered saline (PBS) was used to wash the harvested adipose tissue twice then collagenase was added to digest at 37°C for 30 min while it was agitated every 5 min. ADSCs were collected as pellets after centrifuging for 5 min in four 50‐ml centrifuge tubes. The pellet was immersed in PBS after washing with saline twice to remove the residual enzyme. A 100 μm cell strainer and 1500 rpm centrifuge were used to filter and centrifuge ADSC suspension. The supernatant was discarded. Using a 40 μm cell strainer, the pellet was filtered again in saline.
2.3. Cell culture of ADSCs
Double‐rinse of PBS/gentamycin cleaned the ADSCs. In total, 10‐min centrifugation followed. The supernatant was discarded. After further suspension in complete Dulbecco's modified Eagle's medium (DMEM) (Sigma), the pellet was placed in T25 flasks (37°C, 5% CO2). A 90% confluence was observed after 3–4 days of refreshing the culture medium. Flow‐cytometry and morphological analysis was then performed on the ADSCs.
2.4. Flow‐cytometry analysis
In total, 80% confluence was achieved in six‐well plates using a complete DMEM. After that, trypsin was added with Ethylenediamine tetraacetic acid and PBS washed. We incubated them with fluorescently‐labeled CD 90, CD 34, CD 45, and CD 105 antibodies. Cells were processed for flow cytometry using BD FACSCalibur.
2.5. In vivo test
In the presence of a veterinarian, 12 male Wistar rats (180–200 g, 3–4 weeks) were kept at 65–75% humidity and 20–25°C. The general anesthesia was administered intramuscularly by administering a combination of 2% xylazine (10 mg/kg)and 10% ketamine (50 mg/kg). Then, we used a punch to make three circular incisions (8 mm in diameter) in lab animal under approved guidelines. The harvested collagen solution was mixed with 1 × 107 per ml. Then, the solution was incubated for 5 h to stabilize, and finally, the final solution was ready to be used in animal tests. The defected sites received injections of collagen/ADSCs: group one, collagen: group two, and unfilled: group three. In total, 3 mg collagen/ml and 1×105 ADSCs were injected over the wound. Lab animal care and use guidelines were followed for this study.
2.6. Macroscopic and microscopic analysis
Digital images were taken 7 and 14 days after the intervention in all three groups to examine the wound contraction macroscopically. To evaluate the percentage of wound contraction, we used the Image J software. Wound contraction was determined according to equation. In total, 7‐ and 14‐day‐old rats were sacrificed. For 48 h, each group of samples was fixed in 10% formalin. Gradually, increasing ethanol solutions between 80% and 100% are used to dehydrate the samples. A paraffin block was embedded in 5 mm sections stained with Hematoxylin and Eosin (Karami et al., 2019, Mosaddad et al., 2020, Ordeghan et al., 2022, Soufdoost et al., 2019, Tebyanian et al., 2017a, 2019, 2017b, Yazdanian et al., 2020). A light microscope was used to observe and photograph the samples. Tissue samples were counted for hair follicles, angiogenesis density assessed, and fibroblasts examined under a light microscope.
| (1) |
2.6.1. Statistical analysis
At a significance level of P<0.05, a non‐parametric Friedman test was used to compare the groups.
3. RESULTS
3.1. Isolation and culture of ADSCs cells
The ADSCs were isolated from human adipose tissue by centrifuging with PBS and filtering impurities. Then, the cells were cultured. Since mesenchymal cells are characterized by their adhesion to the surface, other cells were eliminated in rinsing and refreshing the culture medium. After four passages, the cells were relatively purified and then frozen. The cells’ stemness was determined by flow cytometry with CD 34, 45, 105, and 90 markers. The expression rate of each CD shows the stemness of MSCs. The flow‐cytometry graphs revealed that the cells were MSCs since they poorly expressed CD 34 and 45 and highly expressed CD 90 and 105 markers (Figure 1).
FIGURE 1.

Flow Cytometry graphs. The low expression of CD 34, 45, and the high expression of CD 90 and 105 markers of mesenchymal stem cells
3.2. Macroscopic results
Image J software was used to measure wound contraction using photographs were taken 7 and 14 days after sacrifice. The image shown in Figure 2 shows the wounds at a macro‐level. The collagen/ADSCs group recorded a greater wound healing rate than the other groups. At 7 days, wound contraction was 52%, 35%, and 13% in collagen/ADSCs, collagen, and control groups, respectively (Table 1). In the collagen/ADSCs group, wound healing occurred at 14 days, showing that these compounds enhance wound healing. Collagen/ADSCs exhibited remarkably higher wound contraction compared with collagen/ADSCs. After 14 days, wound contraction rates in collagen/ADSC, collagen, and control groups were 80%, 69%, and 33%, respectively.
FIGURE 2.
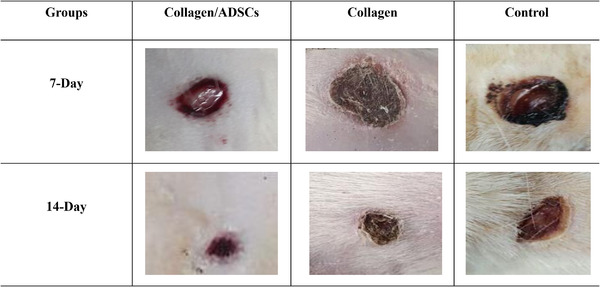
Macroscopic evaluation of the treated and untreated groups
TABLE 1.
The fibroblasts, vascularization, hair follicles contents, and percentage of wound contraction in treated and untreated groups
| Groups | Fibroblasts | Angiogenesis | Hair follicle | Wound contraction | |||||
|---|---|---|---|---|---|---|---|---|---|
| Days | 7 | 14 | 7 | 14 | 7 | 14 | 7 | 14 | |
| Collagen/ADSCs | 16250 | 26158 | 6.1 | 4.1 | 5 | 9 | 52% | 80% | |
| Collagen | 8954 | 13564 | 2.2 | 1.2 | 2 | 4 | 35% | 69% | |
| Control | 560 | 1875 | 0 | 0.6 | 0 | 0 | 13% | 33% | |
3.3. Microscopic results
After 7 days, wounds in the collagen/ADSCs group had significantly healed. Fibroblasts and hair follicles migrated into the wound site at 14 days, indicating that synthetic hydrogels accelerate wound healing and encourage migration of other factors (Figures 3, 4, 5). According to this finding, the collagen/ADSCs group had more fibroblasts, blood vessels, and hair follicles than the other groups in 7 days. Thus, collagen/ADSCs may play a critical role in wound healing. In 14 days, this process was elevated and indicated a positive reaction to the synthetic hydrogel and its role in enhancing wound healing (Table 1). At 14 days, the collagen/ADSCs group had a higher healing rate than at 7 days since collagen sheets formed. Cells migrated to the wound site, and angiogenesis increased at 14 days. This group also showed a more significant closure of the wound margins than the collagen group on day 14. Wound healing was observed within 7 days with collagen alone and minimal cell migration; however, after 14 days, more cells were migrated and greater angiogenesis occurred. The two groups were very similar in hair follicles and angiogenesis (Table 1), but the collagen/ADSC group had a higher number of fibroblasts. After 14 days, there was a significant increase in migration of cells to the wound site and angiogenesis in the collagen group (Figures 3, 4, 5). There was a healing in the connective tissue layer of the control group after 7 days, but the wound healing was no considerable. Collagen/ADSCs group healed wounds much faster at 14 days than 7 days. Fibroblasts, blood vessels, and hair follicles were significantly lower in collagen and control groups (Table 1).
FIGURE 3.

H and E staining evaluation of the collagen/ADSCs group (×40 magnification)
FIGURE 4.

H and E staining evaluation of the collagen group at 7 and 14 days (×40 magnification)
FIGURE 5.

H and E staining evaluation of the control group at 7 and 14 days (×40 magnification)
4. DISCUSSION
The process of healing a wound consists of several biological processes to restore the functionality and morphology of the damaged tissue (Kaushal et al., 2006). In recent years, tissue regeneration by tissue engineering approaches caused significant improvements in treatment quality and strategies (Zhang et al., 2022). An example of this is the use of nanotechnology‐based wound dressings that have revolutionized wound healing and tissue regeneration. As a result of these newer modalities, wounds heal rapidly, bacteria can be eradicated, and severe secondary complications of immunosuppression can be avoided (El‐Ashram et al., 2021). Tissue is composed of cells and extracellular matrix. Several approaches have been suggested to reconstruct the injured tissue (Porzionato et al., 2018). One approach is to provide a suitable scaffold to allow cell proliferation. Biodegradable polymer materials can best serve as scaffolds because they have high flexibility and optimal chemical and physical properties. Natural polymers are the most suitable type among the available polymers due to their excellent biocompatibility, favorable interactions, and optimal biodegradability. Under in vitro conditions, the cells are cultured on a polymer scaffold that serves as an artificial extracellular matrix; they are allowed adequate time and then implanted at the injury site (Fisher, 2006, Heydarkhan‐Hagvall et al., 2008, Yaszemski et al., 2003). A large amount of animal protein is collagen. As a biomechanical scaffold, collagen fibrils can be used to attach cells and entrap macromolecules, shape tissues, and maintain tissue integrity (Kittiphattanabawon et al., 2010). During the middle phases of wound healing, collagen‐based hydrogel‐treated wounds compare favorably with occlusive dressing alone; hydrogel‐treated wounds heal, neovascularize, and increase significantly faster (Williams et al., 2020). In diabetic wounds, synthetic scaffold membranes have increased reepithelialization, neovascularization, and cell proliferation. Nanocomposite scaffold treatment of diabetic rat wounds resulted in increased collagen synthesis, reduced wound size, and faster reepithelialization of wounds (Thangavel et al., 2018). A glutamate‐loaded chitosan hydrogel to diabetic rat wounds by Thangavel et al. (2017), and the hydrogel accelerated vascularization and macrophage recruitment. Based on a diabetic mouse model, acellular dermal matrix scaffolds induce neovascularization and collagen synthesis. ADSCs are clinically and biologically heterogeneous cells found in adipose tissue. Directly or in culture, ADSCs can be used to select and multiply an adhesive population. Detailed information on stromal vascular fraction and ADSCs has dramatically increased in recent years. In animal models, stromal vascular fraction cells and ADSCs are effective and safe. Clinical studies on stromal vascular fraction cells and ADSCs have been undertaken worldwide (Beahm et al., 2003, Jianying et al., 2014, Maharlooei et al., 2011, Tholpady et al., 2006, Xu et al., 2011). The phenotypic and functional traits of ADSCs are similar to those of MSCs. Typically, they can differentiate into multiple MSCs. In tissue regeneration and repair, ADSCs are an essential source of stem cells due to their high yields, proliferation, and low immunogenicity (Zhang et al., 2018). ADSCs play an essential role in wound healing. Scarring can be prevented and treated by decreasing inflammation, increasing angiogenesis, or reducing fibroblasts. Additionally, hydrogels derived from acellular porcine adipose tissue and porcine small intestinal mucosa may enhance ADSCs’ effects on skin scarring prevention (He et al., 2021).
In this study, ADSCs were isolated from adipose tissue and cultured and mixed with the collagen, which were derived from human. Mai et al. (2003) evaluated the efficacy of Sulbogin ointment (marketed under the brand name SuileTM) composed of borneol, bismuth subgallate, and Vaseline. They reported that it enhanced the closure of excision wounds in adult male Sprague‐Dawley rats. Bismuth subgallate is believed to enhance wound healing by inducing macrophages to release growth factors. Additionally, the Sulbogin ointment decreased wound surface area, enhanced granulation tissue formation, re‐epithelialized the wound, commenced collagen proliferation by activating fibroblasts, accelerated angiogenesis, and inhibited the formation of nitric oxide (Mai et al., 2003).
In this study, flow cytometry confirmed the stemness of the isolated cells. The graphs demonstrated that the cells had low expression CD34/45 and also, high expression CD90/105 which were confirmed to be MSCs. Then, the ADSCs/collagen hydrogel was prepared for animal test. Various synthetic methods can synthesize hydrogels that may be suitable for encapsulating ADSCs. By varying crosslinker concentrations and solid contents, either covalently or non‐covalently, it is possible to obtain tunable physical properties. However, it must be noted that those factors can have a detrimental effect on the cell‐matrix interaction. To achieve the best results In vivo, the ideal preparation methods must meet the following criteria: no introduction of a small molecule, mild crosslinking conditions, no toxic product, etc (Huang et al., 2017). Guo et al. (2018) have investigated the acceleration of ADSCs in wound healing of diabetic mice model. Their outcomes have exhibited that biomimetic collagen scaffolds containing murine ADSCs can improve cellular proliferation, and endothelial cell density and increase cell survival in mice wound models, which prepare relevant results to evaluate for translational therapy in human wound treatment. Kim et al. (2019) found that ADSCs administered intramuscularly, topically, or intravenously expedited the healing of mice with full‐thickness wounds. Intriguingly, ADSCs accelerate wound remodeling and increase the healing rate with improved closure. These findings of Kim et al. (2019) revealed that the administration method did not impact the efficacy of ADSCs on wound healing. Using freshly isolated adipose stromal vascular fraction cells, Klar et al. (2016) tested their 3D co‐culture model of a vascularized skin substitute. The cells were seeded on collagen hydrogel type I in vitro. Afterward, human keratinocytes were seeded on the hydrogels to create an epidermal substitute. Adipose tissue is an ideal cell source as it can be easily isolated and has abundant endothelial and mesenchymal cells. As a result of stromal vascular fractions from adipocytes, accelerated blood flow is promoted to skin substitutes in vitro and In vivo (Klar et al., 2016). In Shokrgozar et al.’s (2012) study, the stem cells were isolated from adipose tissue of rats and characterized with flow cytometry. Staining was used to test their differentiation ability. Using a carbodiimide‐based crosslinker, collagen and chitosan scaffolds were synthesized and crosslinked. Immunohistochemistry and polymerase chain reaction confirmed ADSCs and their differentiation into keratinocytes. Seeding ADSCs on scaffolds and implanting at wound sites were done. In the control group, the scaffold with no cells was implanted on the opposite side of the rat. According to histopathology, the scaffold‐cell side of the dermis and epidermis formed after 14 days (Shokrgozar et al., 2012). Using nuclear factor erythroid 2–related factor 2 (Nrf2) overexpressing ADSCs exosomes, Li et al. (2018) reported improved wound healing in 60 patients with diabetes mellitus, providing strong evidence that ADSCs exosomes are effective in wound healing. The collagen/ADSC group had significantly more wound healing than other groups. A significant wound contraction was observed in collagen/ADSCs versus collagen and controls. On the 14th day, wound contraction was highly significant. A significant difference was observed between collagen and ADSC wound healing. Collagen had more remarkable wound healing and contraction compared with the control group. The collagen/ADSCs group showed a high degree of wound healing at 7 days. On the 14th day, hair follicles were present at the wound site, suggesting that the hydrogel significantly induced fibroblast migration to the wound site and that additional factors were induced to migrate. After 14 days, the collagen/ADSC group showed a higher healing rate than on day 7. The wound contraction of the collagen group was more significant on day 14. At 14 days, the collagen/ADSCs hydrogel may enhance wound healing effectively, based on the current results.
5. CONCLUSION
Our study suggested that collagen/ADSC hydrogels may increased fibroblasts, hair follicles, and angiogenesis. The collagen/ADSC hydrogel significantly affected wound healing and contracture compared with other groups. More research is necessary to confirm our findings and this study provided a basic research for the clinical use of collagen/ADSCs hydrogel for wound healing.
AUTHOR CONTRIBUTIONS
Kamyar Abbasi: Investigation; Methodology; Project administration; Writing‐original draft; Writing‐review & editing. Artak Heboyan: Methodology; Writing‐original draft; Writing‐review & editing. Sadaf Fani‐Hanifeh: Methodology; Project administration; Supervision; Visualization; Writing‐original draft; Writing‐review & editing.
MA and SFH supervised this study and edited the manuscript; KA, ST, AH, MH, and RSS conducted the animal study and wrote the draft; AH helped in the data analysis; MA, SFH, and AH helped in the data analysis and also, edited the final manuscript; KA, MA, SFH, ST, and RSS helped in histopathology results.
CONFLICT OF INTEREST
The authors declare that they have no competing interests
CONSENT FOR PUBLICATION
Not applicable.
ANIMAL AND WELFARE ETHICS STATEMENT
This study protocol for experiments and animal caring was approved by the Ethical
Committee for Animal Research of Yakhteh Pajohan Pars (Private company), Tehran, Iran.
FUNDING
There is no financial support.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1059
ACKNOWLEDGEMENTS
The authors would like to thank guidance and advice from Animal Research of Yakhteh Pajohan Pars, Tehran, Iran.
Abbasi, K. , Tavakolizadeh, S. , Hadi, A. , Hosseini, M. , Soufdoost, R. S. , Heboyan, A. , Alam, M. , & Fani‐Hanifeh, S. (2023). The wound healing effect of collagen/adipose‐derived stem cells (ADSCs) hydrogel: In vivo study. Veterinary Medicine and Science, 9, 282–289. 10.1002/vms3.1059
Contributor Information
Mostafa Alam, Email: mostafa_alam1@yahoo.com.
Sadaf Fani‐Hanifeh, Email: sadaf.fn77@gmail.com.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- An, Y. , Lin, S. , Tan, X. , Zhu, S. , Nie, F. , Zhen, Y. , Gu, L. , Zhang, C. , Wang, B. , Wei, W. , Li, D. , & Wu, J. (2021). Exosomes from adipose‐derived stem cells and application to skin wound healing. Cell Proliferation, 54(3), e12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar, P. E. F. , Ranjbar, R. , Yazdanian, M. , Tahmasebi, E. , Alam, M. , Abbasi, K. , Tebyaniyan, H. , & Esmaeili Fard Barzegar, K. (2022). The current natural/chemical materials and innovative technologies in periodontal diseases therapy and regeneration: A narrative review. Materials Today Communications, 32, 104099. [Google Scholar]
- Beahm, E. K. , Walton, R. L. , & Patrick, C. W. (2003). Progress in adipose tissue construct development. Clinics in Plastic Surgery, 30(4), 547–558. [DOI] [PubMed] [Google Scholar]
- Cigna, E. , Tarallo, M. , Bistoni, G. , Anniboletti, T. , Trignano, E. , Tortorelli, G. , & Scuderi, N. (2009). Evaluation of polyurethane dressing with ibuprofen in the management of split‐thickness skin graft donor sites. In Vivo (Athens, Greece), 23(6), 983–986. [PubMed] [Google Scholar]
- El‐Ashram, S. , El‐Samad, L. M. , Basha, A. A. , & El Wakil, A. (2021). Naturally‐derived targeted therapy for wound healing: Beyond classical strategies. Pharmacological Research, 170, 105749. [DOI] [PubMed] [Google Scholar]
- Fisher, J. P. (2006). Tissue engineering. Taylor & Francis Group, New York. [Google Scholar]
- Guo, J. , Hu, H. , Gorecka, J. , Bai, H. , He, H. , Assi, R. , Isaji, T. , Wang, T. , Setia, O. , Lopes, L. , Gu, Y. , & Dardik, A. (2018). Adipose‐derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow‐derived cells. American Journal of Physiology‐Cell Physiology, 315(6), C885–C896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A. , Kowalczuk, M. , Heaselgrave, W. , Britland, S. T. , Martin, C. , & Radecka, I. (2019). The production and application of hydrogels for wound management: A review. European Polymer Journal, 111, 134–151. [Google Scholar]
- Hakim, L. K. , Yazdanian, M. , Alam, M. , Abbasi, K. , Tebyaniyan, H. , Tahmasebi, E. , Khayatan, D. , Seifalian, A. , Ranjbar, R. , & Yazdanian, A. (2021). Biocompatible and biomaterials application in drug delivery system in oral cavity. Evidence‐Based Complementary and Alternative Medicine: eCAM, 2021, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, T. , Yang, J. , Liu, P. , Xu, L. , Lü, Q. , & Tan, Q. (2021). Research progress of adipose‐derived stem cells in skin scar prevention and treatment. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi = Zhongguo Xiufu Chongjian Waike Zazhi = Chinese Journal of Reparative and Reconstructive Surgery, 35(2), 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydarkhan‐Hagvall, S. , Schenke‐Layland, K. , Dhanasopon, A. P. , Rofail, F. , Smith, H. , Wu, B. M. , Shemin, R. , Beygui, R. E. , & Maclellan, W. R. (2008). Three‐dimensional electrospun ECM‐based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials, 29(19), 2907–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Q. , Zou, Y. , Arno, M. C. , Chen, S. , Wang, T. , Gao, J. , Dove, A. P. , & Du, J. (2017). Hydrogel scaffolds for differentiation of adipose‐derived stem cells. Chemical Society Reviews, 46(20), 6255–6275. [DOI] [PubMed] [Google Scholar]
- Hussain, A. , Tebyaniyan, H. , & Khayatan, D. (2022). The role of epigenetic in dental and oral regenerative medicine by different types of dental stem cells: A comprehensive overview. Stem Cells International, 2022, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianying, G. , Ninghua, L. , Xinrong, Y. , Zihao, F. , & Fazhi, Q. (2014). Adiposed‐derived stem cells seeded on PLCL/P123 eletrospun nanofibrous scaffold enhance wound healing. Biomedical Materials, 9(3), 035012. [DOI] [PubMed] [Google Scholar]
- Karami, A. , Tebyanian, H. , Barkhordari, A. , Motavallian, E. , Soufdoost, R. S. , & Nourani, M. R. (2019). Healing effects of ointment drug on full‐thickness wound. Comptes Rendus De L Academie Bulgare Des Sciences, 72(1), 123–129. [Google Scholar]
- Karami, A. , Tebyanian, H. , Soufdoost, R. S. , Motavallian, E. , Barkhordari, A. , & Nourani, M. R. (2019). Extraction and characterization of collagen with cost‐effective method from human placenta for biomedical applications. World Journal of Plastic Surgery, 8(3), 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal, M. , Kutty, N. G. , & Rao, C. M. (2006). Nitrooxyethylation reverses the healing‐suppressant effect of Ibuprofen. Mediators of Inflammation, 2006(4), 24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayatan, D. , Nilforoushzadeh, M. A. , Ahmadi Ashtiani, H. R. , & Hashemian, F. (2022). Effect of apple (Malus domestica) stem cells on UVB‐induced damage skin with anti‐inflammatory properties: An In vivo study. Advances in Materials Science and Engineering, 2022, 1. [Google Scholar]
- Kim, H. , Hyun, M. R. , & Kim, S. W. (2019). The effect of adipose‐derived stem cells on wound healing: Comparison of methods of application. Stem Cells International, 2019, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittiphattanabawon, P. , Benjakul, S. , Visessanguan, W. , Kishimura, H. , & Shahidi, F. (2010). Isolation and characterisation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum). Food Chemistry, 119(4), 1519–1526. [Google Scholar]
- Klar, A. S. , Guven, S. , Zimoch, J. , Zapiorkowska, N. A. , Biedermann, T. , Bottcher‐Haberzeth, S. , Meuli‐Simmen, C. , Martin, I. , Scherberich, A. , Reichmann, E. , & Meuli, M. (2016). Characterization of vasculogenic potential of human adipose‐derived endothelial cells in a three‐dimensional vascularized skin substitute. Pediatric Surgery International, 32(1), 17–27. 10.1007/s00383-015-3808-7 [DOI] [PubMed] [Google Scholar]
- Li, X. , Xie, X. , Lian, W. , Shi, R. , Han, S. , Zhang, H. , Lu, L. , & Li, M. (2018). Exosomes from adipose‐derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Experimental & Molecular Medicine, 50(4), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharlooei, M. K. , Bagheri, M. , Solhjou, Z. , Jahromi, B. M. , Akrami, M. , Rohani, L. , Monabati, A. , Noorafshan, A. , & Omrani, G. R. (2011). Adipose tissue derived mesenchymal stem cell (AD‐MSC) promotes skin wound healing in diabetic rats. Diabetes Research and Clinical Practice, 93(2), 228–234. [DOI] [PubMed] [Google Scholar]
- Mai, L.‐M. , Lin, C.‐Y. , Chen, C.‐Y. , & Tsai, Y.‐C. (2003). Synergistic effect of bismuth subgallate and borneol, the major components of Sulbogin, on the healing of skin wound. Biomaterials, 24(18), 3005–3012. [DOI] [PubMed] [Google Scholar]
- Metcalfe, A. D. , & Ferguson, M. W. J. (2007). Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. Journal of the Royal Society, Interface, 4(14), 413–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam, A. , Ranjbar, R. , Yazdanian, M. , Tahmasebi, E. , Alam, M. , Abbasi, K. , Hosseini, Z. S. , & Tebyaniyan, H. (2022). The current antimicrobial and antibiofilm activities of synthetic/herbal/biomaterials in dental application. BioMed Research International, 2022, 1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mohiti‐Asli, M. , Saha, S. , Murphy, S. V. , Gracz, H. , Pourdeyhimi, B. , Atala, A. , & Loboa, E. G. (2017). Ibuprofen loaded PLA nanofibrous scaffolds increase proliferation of human skin cells in vitro and promote healing of full thickness incision wounds in vivo. Journal of Biomedical Materials Research Part B, Applied Biomaterials, 105(2), 327–339. [DOI] [PubMed] [Google Scholar]
- Moradi, M. , Hood, B. , Moradi, M. , & Atala, A. (2015). The potential role of regenerative medicine in the management of traumatic patients. Journal of Injury and Violence Research, 7(1), 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaddad, S. A. , Yazdanian, M. , Tebyanian, H. , Tahmasebi, E. , Yazdanian, A. , Seifalian, A. , & Tavakolizadeh, M. (2020). Fabrication and properties of developed collagen/strontium‐doped Bioglass scaffolds for bone tissue engineering. Journal of Materials Research and Technology, 9(6), 14799–14817. [Google Scholar]
- Na, K.‐S. , Fernandes‐Cunha, G. M. , Varela, I. B. , Lee, H. J. , Seo, Y. A. , & Myung, D. (2021). Effect of mesenchymal stromal cells encapsulated within polyethylene glycol‐collagen hydrogels formed in situ on alkali‐burned corneas in an ex vivo organ culture model. Cytotherapy, 23(6), 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordeghan, A. N. , Khayatan, D. , Saki, M. R. , Alam, M. , Abbasi, K. , Shirvani, H. , Yazdanian, M. , Soufdoost, R. S. , Raad, H. T. , Karami, A. , & Tebyaniyan, H. (2022). The wound healing effect of nanoclay, collagen, and tadalafil in diabetic rats: An In vivo study. Advances in Materials Science and Engineering, 2022, 1. [Google Scholar]
- Porzionato, A. , Stocco, E. , Barbon, S. , Grandi, F. , Macchi, V. , & De Caro, R. (2018). Tissue‐engineered grafts from human decellularized extracellular matrices: A systematic review and future perspectives. International Journal of Molecular Sciences, 19(12), 4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokrgozar, M. A. , Fattahi, M. , Bonakdar, S. , Ragerdi Kashani, I. , Majidi, M. , Haghighipour, N. , Bayati, V. , Sanati, H. , & Saeedi, S. N. (2012). Healing potential of mesenchymal stem cells cultured on a collagen‐based scaffold for skin regeneration. Iranian Biomedical Journal, 16(2), 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudi, A. , Yazdanian, M. , Ranjbar, R. , Tebyanian, H. , Yazdanian, A. , Tahmasebi, E. , Keshvad, A. , & Seifalian, A. (2021). Role and application of stem cells in dental regeneration: A comprehensive overview. EXCLI Journal, 20, 454–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufdoost, R. S. , Yazdanian, M. , Tahmasebi, E. , Yazdanian, A. , Tebyanian, H. , Karami, A. , Nourani, M. R. , & Panahi, Y. (2019). In vitro and in vivo evaluation of novel Tadalafil/β‐TCP/Collagen scaffold for bone regeneration: A rabbit critical‐size calvarial defect study. Biocybernetics and Biomedical Engineering, 39(3), 789–796. [Google Scholar]
- Tafazoli Moghadam, E. , Yazdanian, M. , Alam, M. , Tebyanian, H. , Tafazoli, A. , Tahmasebi, E. , Ranjbar, R. , Yazdanian, A. , & Seifalian, A. (2021). Current natural bioactive materials in bone and tooth regeneration in dentistry: A comprehensive overview. Journal of Materials Research and Technology, 13, 2078–2114. [Google Scholar]
- Tavakolizadeh, M. , Pourjavadi, A. , Ansari, M. , Tebyanian, H. , Seyyed Tabaei, S. J. , Atarod, M. , Rabiee, N. , Bagherzadeh, M. , & Varma, R. S. (2021). An environmentally friendly wound dressing based on a self‐healing, extensible and compressible antibacterial hydrogel. Green Chemistry, 23, 1312–1329. [Google Scholar]
- Tebyanian, H. , Karami, A. , Motavallian, E. , Aslani, J. , Samadikuchaksaraei, A. , Arjmand, B. , & Nourani, M. R. (2017a). Histologic analyses of different concentrations of TritonX‐100 and Sodium dodecyl sulfate detergent in lung decellularization. Cellular and Molecular Biology, 63(7), 46–51. [DOI] [PubMed] [Google Scholar]
- Tebyanian, H. , Karami, A. , Motavallian, E. , Aslani, J. , Samadikuchaksaraei, A. , Arjmand, B. , & Nourani, M. R. (2017b). A comparative study of rat lung decellularization by chemical detergents for lung tissue engineering. Open Access Macedonian Journal of Medical Sciences, 5(7), 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebyanian, H. , Karami, A. , Motavallian, E. , Samadikuchaksaraei, A. , Arjmand, B. , & Nourani, M. R. (2019). Rat lung decellularization using chemical detergents for lung tissue engineering. Biotechnic & Histochemistry, 94(3), 214–222. [DOI] [PubMed] [Google Scholar]
- Thangavel, P. , Kannan, R. , Ramachandran, B. , Moorthy, G. , Suguna, L. , & Muthuvijayan, V. (2018). Development of reduced graphene oxide (rGO)‐isabgol nanocomposite dressings for enhanced vascularization and accelerated wound healing in normal and diabetic rats. Journal of Colloid & Interface Science, 517, 251–264. [DOI] [PubMed] [Google Scholar]
- Thangavel, P. , Ramachandran, B. , Chakraborty, S. , Kannan, R. , Lonchin, S. , & Muthuvijayan, V. (2017). Accelerated healing of diabetic wounds treated with L‐Glutamic acid loaded hydrogels through enhanced collagen deposition and angiogenesis: An in vivo study. Scientific Reports, 7(1), 10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholpady, S. S. , Llull, R. , Ogle, R. C. , Rubin, J. P. , Futrell, J. W. , & Katz, A. J. (2006). Adipose tissue: Stem cells and beyond. Clinics in Plastic Surgery, 33(1), 55–62. [DOI] [PubMed] [Google Scholar]
- Trottier, V. R. , Marceau‐Fortier, G. , Germain, L. , Vincent, C. , & Fradette, J. (2008). IFATS collection: Using human adipose‐derived stem/stromal cells for the production of new skin substitutes. Stem Cells, 26(10), 2713–2723. [DOI] [PubMed] [Google Scholar]
- Vyas, K. S. , & Vasconez, H. C. (2014). Wound healing: Biologics, skin substitutes, biomembranes and scaffolds. Healthcare (Basel, Switzerland), 2(3), 356–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, T. , Sotelo Leon, D. , Kaizawa, Y. , Wang, Z. , Leyden, J. , Chang, J. , & Fox, P. M. (2020). A human‐derived, collagen‐rich hydrogel augments wound healing in a diabetic animal model. Annals of Plastic Surgery, 85(3), 290–294. [DOI] [PubMed] [Google Scholar]
- Xu, W. , Hu, R. , Fan, E. , & Han, D. (2011). Adipose‐derived mesenchymal stem cells in collagen—hyaluronic acid gel composite scaffolds for vocal fold regeneration. Annals of Otology, Rhinology & Laryngology, 120(2), 123–130. [DOI] [PubMed] [Google Scholar]
- Yadollahi, M. (2019). A study of mortality risk factors among trauma referrals to trauma center, Shiraz, Iran, 2017. Chinese Journal of Traumatology = Chung‐Hua Chuang Shang Tsa Chih, 22(4), 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaszemski, M. J. , Trantolo, D. J. , Lewandrowski, K.‐U. , Hasirci, V. , Altobelli, D. E. , & Wise, D. L. . Tissue engineering and novel delivery systems. CRC Press, Boca Raton, 2003. [Google Scholar]
- Yazdanian, M. , Arefi, A. H. , Alam, M. , Abbasi, K. , Tebyaniyan, H. , Tahmasebi, E. , Ranjbar, R. , Seifalian, A. , & Rahbar, M. (2021). Decellularized and biological scaffolds in dental and craniofacial tissue engineering: A comprehensive overview. Journal of Materials Research and Technology, 15, 1217–1251. [Google Scholar]
- Yazdanian, M. , Rostamzadeh, P. , Rahbar, M. , Alam, M. , Abbasi, K. , Tahmasebi, E. , Tebyaniyan, H. , Ranjbar, R. , Seifalian, A. , & Yazdanian, A. (2022). The potential application of green‐synthesized metal nanoparticles in dentistry: A comprehensive review. Bioinorganic Chemistry and Applications, 2022, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanian, M. , Tabesh, H. , Houshmand, B. , Tebyanian, H. , Soufdoost, R. S. , Tahmasebi, E. , Karami, A. , & Ghullame, S. (2020). Fabrication and properties of βTCP/Zeolite/Gelatin scaffold as developed scaffold in bone regeneration: In vitro and in vivo studies. Biocybernetics and Biomedical Engineering, 40(4), 1626–1637. [Google Scholar]
- Zhang, W. , Bai, X. , Zhao, B. , Li, Y. , Zhang, Y. , Li, Z. , Wang, X. , Luo, L. , Han, F. , Zhang, J. , Han, S. , Cai, W. , Su, L. , Tao, K. , Shi, J. , & Hu, D. (2018). Cell‐free therapy based on adipose tissue stem cell‐derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Experimental Cell Research, 370(2), 333–342. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Chen, X. , Hong, H. , Hu, R. , Liu, J. , & Liu, C. (2022). Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioactive Materials, 10, 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


