Key Points
Question
Are antiepileptic drugs (AEDs) associated with increased risk of developing Parkinson disease (PD)?
Findings
In this case-control study of 1433 individuals with a Hospital Episode Statistics–coded diagnosis of PD and 8598 controls in the UK Biobank, prescription of an AED was associated with an increased risk of subsequent PD.
Meaning
The findings of this study suggest an association between certain AEDs and PD; the relative contribution of epilepsy and AEDs should be further examined in light of these findings.
Abstract
Importance
Recent studies have highlighted an association between epilepsy and Parkinson disease (PD). The role of antiepileptic drugs (AEDs) has not been explored.
Objective
To investigate the association between AEDs and incident PD.
Design, Setting, and Participants
This nested case-control study started collecting data from the UK Biobank (UKB) in 2006, and data were extracted on June 30, 2021. Individuals with linked primary care prescription data were included. Cases were defined as individuals with a Hospital Episode Statistics (HES)–coded diagnosis of PD. Controls were matched 6:1 for age, sex, race and ethnicity, and socioeconomic status. Prescription records were searched for AEDs prescribed prior to diagnosis of PD. The UKB is a longitudinal cohort study with more than 500 000 participants; 45% of individuals in the UKB have linked primary care prescription data. Participants living in the UK aged between 40 and 69 years were recruited to the UKB between 2006 and 2010. All participants with UKB-linked primary care prescription data (n = 222 106) were eligible for enrollment in the study. Individuals with only a self-reported PD diagnosis or missing data for the matching variables were excluded. In total, 1477 individuals were excluded; 49 were excluded due to having only self-reported PD, and 1428 were excluded due to missing data.
Exposures
Exposure to AEDs (carbamazepine, lamotrigine, levetiracetam, and sodium valproate) was defined using routinely collected prescription data derived from primary care.
Main Outcomes and Measures
Odds ratios and 95% CIs were calculated using adjusted logistic regression models for individuals prescribed AEDs before the first date of HES-coded diagnosis of PD.
Results
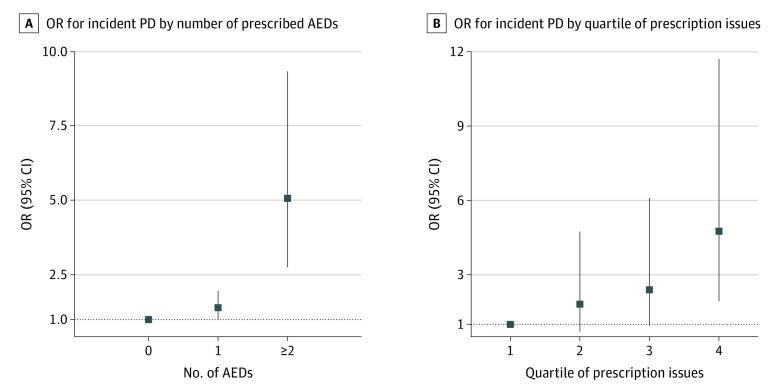
In this case-control study, there were 1433 individuals with an HES-coded PD diagnosis (cases) and 8598 controls in the analysis. Of the 1433 individuals, 873 (60.9%) were male, 1397 (97.5%) had their race and ethnicity recorded as White, and their median age was 71 years (IQR, 65-75 years). An association was found between AED prescriptions and incident PD (odds ratio, 1.80; 95% CI, 1.35-2.40). There was a trend for a greater number of prescription issues and multiple AEDs being associated with a greater risk of PD.
Conclusions and Relevance
This study, the first to systematically look at PD risk in individuals prescribed the most common AEDs, to our knowledge, found evidence of an association between AEDs and incident PD. With the recent literature demonstrating an association between epilepsy and PD, this study provides further insights.
This case-control study investigates the association between antiepileptic drugs and incident Parkinson disease.
Introduction
There is evidence for an association between Parkinson disease (PD) and epilepsy.1,2,3 Recent observational studies have also established a temporal association between epilepsy and incident PD.3,4 The mechanism underlying this association remains unclear.
It is plausible that antiepileptic drugs (AEDs) may account for some or all of the apparent association between epilepsy and PD. Various AEDs list movement disorders (such as parkinsonism, postural tremor, and dystonia) as possible adverse events, but the association between AEDs and PD has not been well studied.5 It remains unclear whether AEDs may partly explain recently reported associations between epilepsy and PD. We used the UK Biobank (UKB) and linked primary care medication data to investigate the association between AED prescriptions and incident PD.
Methods
Cohort
The UKB is a large cohort study that includes data on more than 500 000 participants from the UK. The methods of data collection have been described elsewhere.6 In 2019, the UKB released linked primary care data for 45% of its participants. This included prescription data in the form of Read version 2, the British National Formulary, and the NHS Dictionary of medicines and devices codes. Where available, drug names and quantities were also provided.
Exposure and Outcome Definitions
We conducted a nested case-control study in the UKB. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. We defined PD cases as individuals with a Hospital Episode Statistics (HES) (field identification [ID] 41270, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code G20). Controls were matched to year of birth (field ID 34), sex (field ID 31), socioeconomic class quartiles measured using the Townsend deprivation index (field ID 189), and race and ethnicity (field ID 21000). Risk factors for PD may be associated with race and ethnicity; therefore, we controlled for race and ethnicity in this study. Race and ethnicity were self-reported by participants. Participants were asked “What is your ethnic group?” Options were White, mixed, Asian or Asian British, Black or Black British, Chinese, other ethnic group, do not know, or prefer not to answer. Sequential questions further clasifying race and ethnicity were then asked.
Individuals with a self-reported PD diagnosis but no HES diagnostic code were excluded from the primary analysis. Six controls were matched for each case. Date of diagnosis was set to the first date an HES PD code was found in hospital records. This was used as an index date for cases. Controls were assigned an index date set to the date of diagnosis of their matched case.
Medications were searched for using Read version 2 codes and drug names and descriptions (eTable 1 in the Supplement). The first prescription issue date was used as the date of starting an AED. Prescriptions after the index date were excluded from the analysis. Individuals were divided into quartiles based on the number of prescription issues for all AEDs, with those in the first quartile with the fewest issues and those in the fourth quartile with the most issues. We searched for the 4 most commonly prescribed AEDs in the UK (sodium valproate, lamotrigine, carbamazepine, and levetiracetam).7 We also conducted a wider search of AEDs in the cohort (eTable 2 in the Supplement).
Sensitivity analyses were performed. We excluded prescriptions issued within 1-, 2-, and 5-year windows prior to the index date. The association with self-reported PD (field ID 20002) was also studied. For this analysis, all individuals with a self-reported PD diagnosis were included; individuals with an HES PD code but no self-reported PD diagnosis were excluded. The self-reported date of diagnosis (field ID 20008) was used as the date of diagnosis. We also conducted a further sensitivity analysis with a more stringent definition of PD; HES diagnosis and 2 or more prescriptions for PD medications (levodopa, dopamine receptor agonists, and monoamine oxidase B inhibitors).
Informed written consent was obtained from all participants on enrollment in the UKB. Participants were free to withdraw their consent at any time, at which time their data were censored and excluded from future analysis. The UKB has approval from the North West Multicentre Research Ethics Committee.
Statistical Analysis
Statistical analysis was performed in R, version 3.6.1 (R Project for Statistical Computing). R scripts used in this study are available at GitHub.8 Logistic regression models, adjusting for age, sex, and Townsend deprivation index, were used to calculate odds ratios (ORs) and 95% CIs. A second logistic regression model was built adjusting for age, sex, Townsend deprivation index, and HES epilepsy diagnosis to investigate epilepsy as a potential confounding factor. Individuals with missing data for matching variables were excluded from the analysis.
Results
Demographic Characteristics
There were 222 106 individuals in the UKB with linked primary care medication data. In total, 1477 individuals were excluded; 49 were excluded due to having only self-reported PD, and 1428 were excluded due to missing data for matching variables. Of 1443 individuals with an HES-coded PD diagnosis, 1433 had complete data for year of birth, sex, Townsend deprivation index, and race and ethnicity. There were 8598 matched controls. The median age at PD diagnosis was 71 years (IQR, 65-75 years). Of the 1433 participants with PD (cases) 873 (60.9%) were male, and 1397 (97.5%) had their race and ethnicity recorded as White (eTable 3 in the Supplement).
In this nested case-control cohort study, there were 62 individuals (4.3%) with an AED prescription prior to their date of PD diagnosis. In the control group, there were 211 individuals (2.5%) prescribed an AED before the index date (eTable 4 in the Supplement). In the cases, 63 (4.4%) had an epilepsy diagnosis compared with 113 (1%) of the controls. Of the individuals with 2 or more issues of a PD medication, 96% had an HES-coded PD diagnosis. The remaining 4% had a self-reported PD diagnosis.
Association Between AED and PD
There was evidence of an association of lamotrigine, levetiracetam, and sodium valproate with PD, with weaker evidence for carbamazepine (Table). The OR for PD following prescription of any AED was 1.80 (95% CI, 1.35-2.40). The odds of incident PD were higher among individuals prescribed more than 1 AED and among individuals with higher numbers of issues (Figure). The number of prescriptions issued for AEDs ranged from 1 to 1354, with a median of 10. Evidence of an association remained between sodium valproate and PD in the model adjusting for age, sex, Townsend deprivation index, and epilepsy (eTable 5 in the Supplement).
Table. ORs of Antiepileptic Drugs and Their Association With PD.
| Medication | Cases (n = 1433) | Controls (n = 8598) | OR for PD (95% CI) | P valuea |
|---|---|---|---|---|
| Any antiepileptic drug | 62 | 211 | 1.80 (1.35-2.40) | 6.93 × 10−5 |
| Carbamazepine | 32 | 135 | 1.43 (0.97-2.11) | .07 |
| Lamotrigine | 15 | 32 | 2.83 (1.53-5.25) | 9.29 × 10−4 |
| Levetiracetam | 12 | 24 | 3.02 (1.51-6.05) | 1.85 × 10−3 |
| Sodium valproate | 30 | 48 | 3.82 (2.41-6.05) | 1.17 × 10−8 |
Abbreviations: OR, odds ratio; PD, Parkinson disease.
Asymptotic P values were calculated from the z statistic.
Figure. Forest Plot of Odds Ratios (ORs) of Number of Different Antiepileptic Drugs (AEDs) and Number of AEDs Issues for Parkinson Disease (PD).
Sensitivity Analyses
Excluding prescriptions issued 1, 2, and 5 years before the date of PD diagnosis did not alter the strength of any association between individual AEDs and PD except for carbamazepine at 1 year (eTable 6 in the Supplement). There were 410 individuals with a self-reported PD diagnostic code. As with HES-coded PD, being prescribed an AED was associated with an increased risk of an incident self-reported PD diagnosis (OR, 2.23; 95% CI, 1.11-4.48). Of those with a PD diagnosis, 913 of 1433 individuals (63.7%) had a record of 2 or more issues of a PD medication. With this more stringent definition of PD, strong evidence of an association remained for sodium valproate (eTable 7 in the Supplement).
Discussion
Using linked prescription records and health care data from UKB, we found an association between AED use and incident PD. We used a nested case-control design to identify 1433 PD cases and 8598 matched controls. The magnitude of the association increased with the number of discrete AEDs prescribed and the number of prescription issues. Having multiple discrete AEDs or multiple prescription issues over time is a useful proxy for long-term exposure to AEDs in the absence of accurate information on duration of medication use. On an individual drug level, we observed associations of the use of lamotrigine, levetiracetam, and sodium valproate with PD. The association between sodium valproate and incident PD was most robust and remained even after adjusting for epilepsy diagnosis.
These findings are consistent with previous reports of an association between epilepsy and PD.1,2,3,4 One explanation for the association between epilepsy and PD is that medications prescribed to treat epilepsy may increase PD risk.
It is plausible that AEDs are associated with drug-induced parkinsonism, which is misdiagnosed (or misrecorded) as idiopathic PD. We tried to mitigate the risk of misclassification in our analysis by using stringent definitions of incident PD incorporating multiple sources of diagnostic codes and prescription of PD treatments. Furthermore, to exclude cases of transient drug-induced parkinsonism, which may abate on cessation of the drug, we excluded AED prescriptions within 1, 2, and 5 years of the PD diagnosis date. Although this analysis would not remove individuals with tardive parkinsonism, this condition is relatively rare with AED use and is unlikely to be a major source of bias.9 The latter analysis also reduces the possibility of reverse causation, in which some patients with PD may have been treated with selected AEDs for early mood or neuropsychiatric symptoms.
Studies have shown that AEDs have the potential to interfere in dopamine pathways. Both carbamazepine and sodium valproate are associated with downregulation of dopamine receptors and dopamine insensitivity.10,11 While this may explain drug-induced parkinsonism, it is likely that other factors may contribute to PD pathogenesis. In a case series with extended follow-up, Dal and Whyte12 found that patients who initially experienced remission of drug-induced parkinsonism symptoms after stopping AED treatment later developed PD. This may suggest that these patients had subclinical PD or were at risk of PD. While we are not aware of prospective data to support or refute this observation, it is supported by postmortem studies showing that individuals with drug-induced parkinsonism have reduced levels of homovanillic acid and dopamine in the striatum.13 It has also been observed that individuals taking levetiracetam were at higher risk of psychotropic adverse effects if they had genetic variants associated with decreased dopamine activity.14
Limitations
A major limitation of the study is that epilepsy is a common reason for admission to the hospital. In HES data, ascertainment of PD may contribute to the observed associations simply because patients with epilepsy had been admitted to the hospital more than patients without epilepsy. Our study was likely to be underpowered to detect effects in some of our sensitivity analysis. In particular, further work in larger cohorts is needed to fully assess the effects of AEDs on individuals without epilepsy. Although we studied the 4 most commonly prescribed AEDs in the UK, these findings cannot be generalized to other AEDs. Other limitations of this study include the generalizability of the UKB cohort to a wider UK population (although it should be noted that the prevalence of epilepsy in the control group closely matched that in the UK more generally15), that medication data are available only for roughly 45% of the UKB cohort, and that data quality and missingness meant that overall lifetime dose exposure was difficult to determine.
Conclusion
To the best of our knowledge, this is the first observational study to investigate a range of AEDs and their association with incident PD. As such, it sets the scene and highlights the need for further work to corroborate our findings in other large data sets because these findings could have important implications for clinical decision-making. The underlying reasons for an association between AEDs and PD should be further explored.
eTable 1. Medication Search Terms
eTable 2. Frequency of Cases Prescribed an Antiepileptic Drug
eTable 3. Demographics of Cases and Controls
eTable 4. Frequency of Prescribed Medications
eTable 5. Odds Ratios of Antiepileptic Drugs for Parkinson’s Disease in Model Adjusting for Epilepsy Diagnosis
eTable 6. Sensitivity Analysis Excluding Prescriptions Issued 1, 2 and 5 Years Before Parkinson’s Disease Diagnosis
eTable 7. Sensitivity Analysis With Parkinson’s Disease Defined as Hospital Episode Statistic and Two or More Prescriptions for Parkinson’s Disease Medications
References
- 1.Gruntz K, Bloechliger M, Becker C, et al. Parkinson disease and the risk of epileptic seizures. Ann Neurol. 2018;83(2):363-374. doi: 10.1002/ana.25157 [DOI] [PubMed] [Google Scholar]
- 2.Heilbron K, Noyce AJ, Fontanillas P, Alipanahi B, Nalls MA, Cannon P; 23andMe Research Team . The Parkinson’s phenome-traits associated with Parkinson’s disease in a broadly phenotyped cohort. NPJ Parkinsons Dis. 2019;5:4. doi: 10.1038/s41531-019-0077-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonet C, Bestwick J, Jitlal M, et al. Assessment of risk factors and early presentations of Parkinson disease in primary care in a diverse UK population. JAMA Neurol. 2022;79(4):359-369. doi: 10.1001/jamaneurol.2022.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs BM, Belete D, Bestwick J, et al. Parkinson’s disease determinants, prediction and gene-environment interactions in the UK Biobank. J Neurol Neurosurg Psychiatry. 2020;91(10):1046-1054. doi: 10.1136/jnnp-2020-323646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datapharm Ltd. Electronic Medicines Compendium. Accessed November 17, 2022. https://www.medicines.org.uk/emc/about-the-emc#gref
- 6.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell G, Logan J, Kiri V, Borghs S. Trends in antiepileptic drug treatment and effectiveness in clinical practice in England from 2003 to 2016: a retrospective cohort study using electronic medical records. BMJ Open. 2019;9(12):e032551. doi: 10.1136/bmjopen-2019-032551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Association-Between-Antiepileptic-Drugs-and-Incident-Parkinson-Disease-in-UK-Biobank. GitHub, Inc. Accessed November 19, 2022. https://github.com/Daniel-Belete/Association-Between-Antiepileptic-Drugs-and-Incident-Parkinson-Disease-in-UK-Biobank
- 9.Bolu A, Garip B, Öznur T, Uzun Ö. Case of risperidone-induced tardive parkinsonism. Psychiatry Clin Neurosci. 2019;73(5):285-286. doi: 10.1111/pcn.12837 [DOI] [PubMed] [Google Scholar]
- 10.Basselin M, Chang L, Chen M, Bell JM, Rapoport SI. Chronic carbamazepine administration attenuates dopamine D2-like receptor-initiated signaling via arachidonic acid in rat brain. Neurochem Res. 2008;33(7):1373-1383. doi: 10.1007/s11064-008-9595-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramadan E, Basselin M, Taha AY, et al. Chronic valproate treatment blocks D2-like receptor-mediated brain signaling via arachidonic acid in rats. Neuropharmacology. 2011;61(8):1256-1264. doi: 10.1016/j.neuropharm.2011.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dal S, Whyte S. Valproate-induced parkinsonism ‘an early warning’: case reports and review of literature. J Neurol Neurosurg Psychiatry. 2019;90(A):12. doi: 10.1136/jnnp-2019-anzan.32 [DOI] [Google Scholar]
- 13.Rajput AH, Rozdilsky B, Hornykiewicz O, Shannak K, Lee T, Seeman P. Reversible drug-induced parkinsonism. Clinicopathologic study of two cases. Arch Neurol. 1982;39(10):644-646. doi: 10.1001/archneur.1982.00510220042009 [DOI] [PubMed] [Google Scholar]
- 14.Helmstaedter C, Mihov Y, Toliat MR, et al. Genetic variation in dopaminergic activity is associated with the risk for psychiatric side effects of levetiracetam. Epilepsia. 2013;54(1):36-44. doi: 10.1111/j.1528-1167.2012.03603.x [DOI] [PubMed] [Google Scholar]
- 15.Ridsdale L, Charlton J, Ashworth M, Richardson MP, Gulliford MC. Epilepsy mortality and risk factors for death in epilepsy: a population-based study. Br J Gen Pract. 2011;61(586):e271-e278. doi: 10.3399/bjgp11X572463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Medication Search Terms
eTable 2. Frequency of Cases Prescribed an Antiepileptic Drug
eTable 3. Demographics of Cases and Controls
eTable 4. Frequency of Prescribed Medications
eTable 5. Odds Ratios of Antiepileptic Drugs for Parkinson’s Disease in Model Adjusting for Epilepsy Diagnosis
eTable 6. Sensitivity Analysis Excluding Prescriptions Issued 1, 2 and 5 Years Before Parkinson’s Disease Diagnosis
eTable 7. Sensitivity Analysis With Parkinson’s Disease Defined as Hospital Episode Statistic and Two or More Prescriptions for Parkinson’s Disease Medications