Abstract
Background
Reverse zoonoses occur because of interactions between humans and animals. Homology of ACE‐2 cell receptors in different hosts and high mutation rate of SARS‐CoV‐2 enhance viral transmission among species.
Objectives
This study aimed to investigate spillover of SARS‐CoV‐2 between humans and companion animals.
Methods
A cross‐sectional study was constructed using nasopharyngeal/oropharyngeal swabs, serum and blood samples collected from 66 companion animals (33 cats and 33 dogs) that were in contact with SARS‐CoV‐2‐positive owners from December 2020 to March 2021. Swabs were screened by rRT‐PCR and some positive cases were confirmed by partial spike gene sequencing. Clinical pathology and pathological studies were also performed.
Results
Our findings revealed that 30% of cats (10/33) and 24% of dogs (8/33) were SARS‐CoV‐2 positive. While 33% of these animals were asymptomatic (6/18), 28% showed mild respiratory signs (5/18) and 39% displayed severe respiratory signs (7/18) including 4 dead cats 40% (4/10). Partial spike gene sequencing of 6 positive samples collected in December 2020 were identical to SARS‐CoV‐2 that was detected in humans in Egypt in that time frame. Clinical pathology findings revealed thrombocytopenia, lymphocytopenia, as well as elevated levels of D‐dimer, LDH, CRP, and ferritin. Post‐mortem and histopathological examinations illustrated multisystemic effects.
Conclusions
There is a potential occurrence of SARS‐CoV‐2 spillover between humans and pet animals.
Impacts
The present study highlighted the potential occurrence of SARS‐CoV‐2 spillover between humans and their companion animals.
Biosecurity measures should be applied to decrease spread of SARS‐CoV‐2 among humans and pet animals.
Keywords: companion animals, COVID‐19, SARS‐CoV‐2, spillover
Graphical abstract depicts SARS‐CoV‐2 spillover in humans and their pet animals with common symptoms. Besides, it illustrates number of SARS‐CoV‐2‐infected cats and dogs with severity of respiratory signs.
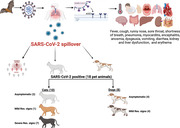
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) outbreak, triggered by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has been reported in late 2019 in Wuhan, China (Wu et al., 2020). SARS‐CoV‐2 has a positive‐sense single‐strand ribonucleic acid (+ss RNA) with an envelope (Zhu et al., 2020) and contains structural proteins (SPs) including spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins (Naqvi et al., 2020) and non‐structural proteins (NSPs) including proteases (NSP3 and NSP5), RNA‐dependent RNA polymerase (NSP12), helicase enzyme (NSP13), endoribonuclease enzyme (NSP15) and other NSPs (Acter et al., 2020). The spike glycoprotein initiates the viral infection via attaching to angiotensin‐converting enzyme‐2 (ACE2) cell receptors in many organs such as the lungs, kidneys, liver and brain (Hamming et al., 2004).
Cross‐species transmission of coronaviruses has been reported and some studies have revealed their ability to overcome species barriers (Zhou et al., 2020a). The high mutation rates (Lam et al., 2020) and the large size of genomic RNA enhance the emergence of new coronaviruses (Su et al., 2016). In addition, the ability of coronaviruses to interact with a broad range of ACE2 cell receptors in different species may increase the viral virulence (Su et al., 2016). Adaptation of coronaviruses to new hosts usually occurs via viral mutations (Bolles et al., 2011), changes that alter viral tropism and mutations that allow cross‐species transmission (Hoffmann et al., 2020; Zhou et al., 2020a).
Bats have been recognised as natural hosts for SARS‐CoV‐2 due to the high identity percentage (96.2%) between SARS‐CoV‐2, which was detected in humans and RaTG13 (bat coronavirus) (Zhou et al., 2020a). The latter finding raise number of enquiries about SARS‐CoV‐2 transmissibility to other species (Elaswad et al., 2020). The World Organisation for Animal Health (WOAH)/The Office International des Épizooties (OIE) has reported an emergence of 679 cases of SARS‐CoV‐2 infection in 24 animal species in 36 countries according to July 2022 report (WOAH/OIE, 2022). In particular, 360 cases in minks, 102 cases in cats, 92 cases in dogs, 2 cases in otters, 2 cases in pet ferrets, 5 cases in lions 12 cases in tigers, 3 cases in pumas, 2 cases in snow leopards, 2 cases in gorillas, 1 case in white‐tailed deer and 1 case in Amur leopard were documented (WOAH/OIE, 2021b). Furthermore, SARS‐CoV‐2 infection in cats has been reported in Brazil, Greece, Belgium, Switzerland, Japan, Canada (WOAH/OIE, 2020), France (Sailleau et al., 2020), Italy (Musso et al., 2020), Hong Kong (Barrs et al., 2020), Spain (Ruiz et al., 2021; Segalés et al., 2020), the United States (Hamer et al., 2021), the United (Hosie et al., 2021), and the Netherlands (Oreshkova et al., 2020). Moreover, several studies confirmed the presence of SARS‐CoV‐2 antibodies in cats that tested negative by rRT‐PCR, which revealed a previous viral exposure (Gaudreault et al., 2020; Halfmann et al., 2020; Shi et al., 2020). Additionally, it was shown that dogs are susceptible to SARS‐CoV‐2 infection as reported in the United States (Hamer et al., 2021), Hong Kong (Sit et al., 2020), and recently in Japan where a dog was tested positive for SARS‐CoV‐2 after hospitalisation of his owner in last September 2021 (WOAH/OIE, 2021c). Even though, an experimental infection of racoon dogs revealed their high susceptibility for SARS‐CoV‐2 infection and their ability to transmit the virus among the same species (Freuling et al., 2020; WOAH/OIE, 2021a), another study reported that SARS‐CoV‐2 shedding in dogs have a little to zero value (Shi et al., 2020).
Although SARS‐CoV‐2 is a zoonotic virus and bats appear to be its natural hosts, till now, the identification of its intermediate reservoirs remains undetermined (Zhao et al., 2020). However, domestic and wild animals have the ability to be intermediate hosts; many studies are needed to clarify critical points surrounding this issue (Zhao et al., 2020). Hence, the OIE urgently recommends the reporting of SARS‐CoV‐2 in animals (WOAH/OIE, 2021b). For further investigation about the spillover and reverse zoonoses of SARS‐CoV‐2 between humans and their companion animals, we conducted molecular, clinical pathology and pathological investigations for some cats and dogs living in the same households with at least one COVID‐19‐positive human case.
2. METHODS
2.1. Sampling and epidemiological data collection
A cross‐sectional study was performed using 66 samples collected from 33 cats and 33 dogs from veterinary clinics in Gesr El‐Suez, Cairo, Egypt and shelters in El‐Saff, Giza, Egypt from December 2020 to March 2021. All tested companion animals were derived from homes having at least one SARS‐CoV‐2‐positive case by using antibody and/or antigen detection assays. Naso/oropharyngeal swabs were collected and placed into sterile tubes containing 2 ml of autoclaved phosphate buffer saline (PBS) to protect samples from nuclease enzymes. Then samples were placed in an icebox containing ice. Additionally, blood samples were collected and split into two groups, one group was mixed with 100 µl of ethylenediaminetetraacetic acid (EDTA) to prevent coagulation for complete blood count (CBC) and the other group was collected without EDTA to separate serum samples for the remaining clinical pathology studies as D‐dimer, lactate dehydrogenase (LDH), C‐reactive protein (CRP), and ferritin. Sampling was performed by veterinarians wearing personal protective equipment and applying strict biosecurity measures for sampling procedures and transportation. SARS‐CoV‐2 infection in humans and their pet animals, respiratory signs of owners along with those of their pet animals, and the workflow of this study were presented in Figure 1, Table 1 and Supplementary Table S1.
FIGURE 1.

SARS‐CoV‐2 infection in humans and their pet animals. (a) SARS‐CoV‐2 infection in humans and their pet animals with symptoms. (b) represents the distribution of the severity of clinical signs in owners and their pets with percentage. (c) The workflow of the presented study
TABLE 1.
SARS‐CoV‐2 infection in humans and their pet animals
| (a) | Cat | Dog | |||
|---|---|---|---|---|---|
| F | M | F | M | Total | |
| Clinic in Gesr El‐Suez, Cairo, Egypt | 25 | 4 | 4 | 9 | 42 |
| Shelter in El‐Saff, Giza, Egypt | 3 | 1 | 4 | 16 | 24 |
| Total | 33 | 33 | 66 | ||
| (b) | Severity of respiratory signs | Gender | Location | Age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic | Mild | Severe | F | M | Clinic | Shelter | <1 M | 1‐<6 M | 6‐<12 M | 1‐<2 Y | 2:3 Y | >3 Y | |
| COVID‐19 (+) | 6 | 5 | 7 | 11 | 7 | 16 | 2 | 1 | 1 | 1 | 8 | 4 | 3 |
| COVID‐19 (−) | 33 | 15 | 0 | 25 | 23 | 26 | 22 | 1 | 14 | 13 | 6 | 8 | 6 |
| Total | 39 | 20 | 7 | 36 | 30 | 42 | 24 | 2 | 15 | 14 | 14 | 12 | 9 |
Note: (a) Location, species and gender of the collected samples. (b) The severity of respiratory symptoms in owners and pet animals with numbers and percentage.
2.2. RNA extraction and one‐step real‐time RT‐PCR
The collected samples were immediately placed in automatic RNA extractor to decrease the biological hazard and obtain the highest yield of RNA. Viral RNA was extracted by using a MagPurix nucleic acid automatic extractor (Labgene Scientific, Switzerland, catalogue number: ZP01001) using the MagPurix® Viral Nucleic Acid Extraction Kit (Catalogue number: ZP02003), according to the manufacturer's instructions. Then, one‐step real‐time reverse‐transcription polymerase chain reaction (rRT‐PCR) was performed on the same day to protect the viral RNA from degradation. All RNAs were screened by rRT‐PCR to identify SARS‐CoV‐2‐positive samples using the Transgene multiplex SARS‐CoV‐2 kit (Transgene Biotech, catalogue number: DV101) that detected ORF1ab and nucleocapsid (N) genes, according to the manufacturer's instructions. PCR conditions were as follows: 10 min at 50°C and 30 s at 95°C, followed by 40 cycles of 5 s at 95°C and 30 s at 60°C.
2.3. Conventional RT‐PCR and amplification of partial spike gene
Some of December 2020 samples (6 samples) were picked randomly to perform partial spike gene sequencing as a confirmatory tool for diagnosis. The selected samples were re‐tested by conventional RT‐PCR, as a preparatory step for the partial spike sequencing, using partial spike gene‐specific primers (forward primer CCAGCAACTGTTTGTGGACC) as described previously (Ren et al., 2020) and reverse primer was designed in this study (TTGACTAGCTACACTACGTGCC) to amplify 510 bp in the spike gene at position from 23123 to 23632 in the reference genome. In this study, spike gene was selected for sequencing because it is the most variable and vital region in SARS‐CoV‐2 genome which plays a crucial role in viral attachment and entry (Fung & Liu, 2019). Partial spike gene amplification was carried out using the PrimeScript RT‐PCR Kit (Takara, California, USA, catalogue number: RR055A), according to the manufacturer's instructions. PCR conditions were as follows: 30 min at 50°C and 2 min at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at 50°C, and 1.5 min at 72°C, and a final extension of 10 min at 72°C.
2.4. Sequence, phylogenetic characterisation and identity matrix
DNA bands of the selected samples (6 samples) were purified using QIAquick gel extraction kit (Qiagen, catalogue number: 28706), according to the manufacturer's instructions. The purified PCR products were sequenced using the BigDye Terminators 3.1 Cycle sequencing Kit (Applied Biosystems, catalogue number: 4337454). Sequencing reactions were purified by using the Centri‐Sep spin columns (Applied Biosystems, catalogue number: CS‐901) and were analysed using a 3500 Genetic Analyser (Applied Biosystems, catalogue number: A30468). Nucleotide sequences were used to prepare multiple sequence alignments using BioEdit 7.2.5 (Hall, 1999). An unrooted maximum‐likelihood phylogenetic tree was constructed and visualised using MEGA 7 to display the evolutionary analysis (Kumar et al., 2016). The robustness of the tree topology was assessed using 1000 bootstrap replicates.
2.5. Clinical pathology studies
A complete blood count, including red blood cells, platelets, lymphocytes, eosinophils, monocytes, neutrophils and basophils, was performed using a VetScan HM5 analyser (Abaxis, Union City, CA, USA), according to the manufacturer's instructions. D‐dimer and LDH as fibrinolysis and clotting parameters, as well as CRP and ferritin as inflammatory parameters were obtained using a VetScan VS2 analyser (Abaxis), according to the manufacturer's instructions.
2.6. Statistical analysis
Complete blood count and other laboratory parameters data were analysed using PASW Statistics, Version 18.0 software (SPSS Inc., Chicago, IL, USA). Hematologic parameters of positive and negative animals were compared using independent sample t‐test and results were expressed as means ± standard error. A p‐value <0.05 was considered statistically significant.
2.7. Pathological studies
After owner consent, necropsy was performed for a cat. Tissue samples from the lungs, heart, intestine, liver, kidneys and spleen were tested SARS‐CoV‐2 positive by rRT‐PCR, they were trimmed and fixed in 10% neutral buffered formalin for 24 h. The fixed tissue samples were processed, cleared, and embedded in paraffin wax blocks and then sectioned with a manual microtome at a thickness of 5 µM. Tissue sections were stained with haematoxylin and eosin (HE) stain and covered with a cover slide (Bancroft & Gamble, 2008). The slides were photographed using an Olympus camera fixed on Leica light microscope.
3. RESULTS
3.1. One‐step real‐time RT‐PCR
According to the cycle threshold (Ct) values of the rRT‐PCR molecular detection of ORF1ab and N genes, 30% of cats (10/33) and 24% of dogs (8/33) were SARS‐CoV‐2 positive. Within these 18 positive cases, 6 pet animals were asymptomatic (6/18) (33%), 5 animals showed mild respiratory signs (5/18) (28%) and 7 pet animals displayed severe respiratory signs (7/18) (39%). Interestingly, only cats showed severe signs. From the latter severe cases, 4 female cats died (4/10) (40%). In contrast, 48 pet animals (23 cats and 25 dogs) were negative for COVID‐19 representing 70% (23/33) of the cats and 76% (25/33) of the tested dogs. While 69% (33/48) of these negative cases were asymptomatic, 31% (15/48) showed mild respiratory clinical signs. The rRT‐PCR results of pet animals in relation to the severity of their clinical signs are illustrated in Figure 2 and Supplementary Table S2.
FIGURE 2.

The rRT‐PCR results of the pet animals in relation to the severity of the clinical signs. (a) Cycle threshold (CT) values of rRT‐PCR for nucleocapsid (N) and ORF 1ab genes in COVID‐19‐positive companion animals that were in contact with positive owners. (b) The correlation between species and COVID‐19 infection. (c) The relationship between COVID‐19 infection and the severity of the respiratory signs. (d) COVID‐19 infection in correlation to death
3.2. Sequence, phylogenetic characterisation and identity matrix
Partial spike genes of some positive samples, that were collected in December 2020, were uploaded to the National Center for Biotechnology Information (NCBI) with accession numbers from MZ305405 to MZ305410. Phylogenetic analysis of these samples revealed a 99.7% identity with the Wuhan reference SARS‐CoV‐2 human isolate, and they were identical to SARS‐CoV‐2 that was detected in human samples in Egypt in that time frame. In particular, these samples showed high identity (100%) with Egypt_NRC1_2020| EPI_ISL/1315064|2020‐04‐26 and Egypt_MASRI‐C4‐029_2020| EPI_ISL/1165078|2020‐06‐28. The latter uploaded samples have high identity (99.9%) with previously detected SARS‐CoV‐2 in cats and dogs in other countries such as France and Italy like Cat_France_IDF‐53 2020|437349|2020‐04‐17 and Dog_Italy_399‐20BA_2020|730652|2020‐11‐04 as illustrated in Figure 3 and Supplementary Table S3.
FIGURE 3.

Molecular phylogenetic tree of the sequenced samples. The unrooted maximum likelihood tree depicts the correlation between the sequenced samples and SARS‐CoV‐2 found in humans and animals in which the related branches and taxa clustering together. Blue circles depict the partial spike gene sequence of SARS‐CoV‐2 detected in cats and dogs in the presented study and the green triangles display the circulated SARS‐CoV‐2 in Egypt at the same time frame
3.3. Clinical pathology findings
Based on the complete blood count, there was a significant decrease in platelet count (p‐value in cats was <0.0001* and 0.001* in dogs) and lymphocytes (p‐value in cats was <0.0001* and 0.007* in dogs). On contrast, a significant increase in monocytes was observed in SARS‐CoV‐2‐infected cats and dogs compared with negative groups (p‐value in cats was 0.010* and 0.015* in dogs) as demonstrated in the striped filled patterns in Figure 4, Supplementary Tables S4 and S5.
FIGURE 4.

Complete blood count (CBC) of SARS‐CoV‐2‐positive and ‐negative companion animals. (a) A significant thrombocytopenia in SARS‐CoV‐2‐infected cats and dogs compared with negative group in which (p‐value in cats was <0.0001* and 0.001* in dogs). (b) A significant lymphocytopenia with SARS‐CoV‐2 infection in which (p‐value in cats was <0.0001* and 0.007* in dogs). (c) A significant monocytosis with SARS‐CoV‐2 infection in which (p‐value in cats was 0.010* and 0.015* in dogs)
According to other laboratory parameters, there was a significant increase in inflammatory parameters (CRP and ferritin) and fibrinolysis and clotting factors (D‐dimer and LDH) in SARS‐CoV‐2‐infected cats and dogs compared with negative groups (p‐value was <0.0001* in cats and dogs for CRP, ferritin, D‐dimer and LDH) as listed in Table 2.
TABLE 2.
Inflammatory parameters, fibrinolysis and clotting factors for SARS‐CoV‐2 (positive and negative) cat and dog samples
| Sample ID | Species | Clinical pathology (means ± SE) | |||
|---|---|---|---|---|---|
| CRP (up to 6 mg/dl) | Ferritin (13–200 ng/ml) | LDH (up to 300 micro/L) | D‐Dimer (0.5–1) | ||
| Cats (n = 14) | Cat+ | 7.95 ± 0.62 a | 229.76 ± 12.88 a | 380.20 ± 24.40 a | 1.34 ± 0.08 a |
| Cat– | 1.45 ± 0.22 b | 27.30 ± 2.14 b | 136.50 ± 6.89 b | 0.67 ± 0.07 b | |
| p | <0.0001* | ||||
| Dogs (n = 14) | Dog+ | 9.38 ± 0.77 a | 232.13 ± 9.65 a | 376.13 ± 16.95 a | 1.55 ± 0.12 a |
| Dog– | 1.50 ± 0.20 b | 30.13 ± 1.49 b | 146.00 ± 6.41 b | 0.62 ± 0.05 b | |
| p | <0.0001* | ||||
Note: The table displays CRP, ferritin, LDH and D‐dimer of the examined animals along with their standard normal values.
a,bDifferent superscripts in the same column indicate significance at p < 0.05.
SE: standard error.
3.4. Pathological studies
Post‐mortem lesions and histopathological images of a cat case with COVID‐19 showed generalised multisystemic effects in different organs (Figures 5 and 6). The respiratory pathologies were remarkable, and severe fibrinous pneumonia in addition to epithelial hyperplasia of the bronchioles was observed. Moreover, congestion and thrombosis of the pulmonary blood vessels were noted (Figure 5a–d). Cardiac lesions included necrotic changes in the muscle bundles and oedema (Figure 5e). Lesions in the digestive system included intestinal villi necrosis and fusion (Figure 5f) hepatic congestion, centrilobular necrosis and portal vein thrombosis (Figure 6a–c). Renal histopathological examination revealed severe acute tubular injury with minimal glomerular changes. The interstitial tissue showed inflammatory infiltrates (Figure 6d–f). Depletion of lymphoid tissue in the spleen was evident (Figure 6g and h).
FIGURE 5.

Pathological findings of lung, heart and intestine of COVID‐19‐positive cat. Microphotographs of tissue sections from lung, heart and intestine of COVID‐19 naturally infected cat: (a) The bronchial epithelium is hyperplastic leading to corrugations and severe peri bronchial oedema (arrow). Pulmonary blood vessels are severely congested. The alveoli are obliterated with diffuse fibrinous exudate and inflammatory cells (HE, scale bar 200 µm). (b) Hyperplasia of the epithelial lining of bronchioles. The alveoli are filled with diffuse fibrinous exudate mixed with inflammatory cells. Pulmonary blood vessels are severely congested (HE, scale bar 200 µm). (c) Pulmonary blood vessel shows severe vasculitis and thickening of its wall with severe congestion and thrombosis (arrow) (HE, scale bar 100 µm). (d) The bronchiolar lumen is filled with inflammatory cells and cell debris (star), peribronchiolar blood vessels are congested. The alveoli are filled with fibrinous exudate and inflammatory cells infiltrations (HE, scale bar 100 µm). (e) Heart shows focal fragmentation and necrosis of some muscle bundles and oedema (arrow) (HE, scale bar 200 µm). (f) Intestinal villi are suffering from moderate degree of necrosis and fusion. The epithelial lining of the intestinal glands contains intracytoplasmic inclusion bodies (arrows) (HE, scale bar 50 µm)
FIGURE 6.

Pathological findings of liver, kidney, and spleen of COVID‐19‐positive cat. Microphotographs of tissue sections from liver, kidney and spleen of COVID‐19 naturally infected cat: (a) Severe multifocal centrilobular necrosis in the liver (arrow) and congestion of portal vein (HE, scale bar 200 µm). (b) Congestion of the hepatic central vein (arrow) and centrilobular necrosis with dilatation of the hepatic sinusoids (HE, scale bar 100 µm). (c) Large thrombus blocks the portal vein with presence of fibrosis around the bile duct (arrow). Some hepatocytes are necrosed (HE, scale bar 100 µm). (d) Kidney shows severe and diffuse necrosis of the renal tubules and severe perivascular oedema around renal blood vessels (arrow) (HE, scale bar 200 µm). (e) Renal tubules are completely necrosed with presence of focal infiltrates of inflammatory cells (arrows). Shrinkage of some glomeruli is noted (HE, scale bar 200 µm). (f) Renal tubules are completely necrosed with sloughing of the whole epithelial lining (arrows) and presence of inflammatory cells infiltrations (HE, scale bar 100 µm). (g) Spleen is showing moderate multifocal lymphoid depletion (HE, scale bar 200 µm). (h) Focal lymphoid tissue depletion and lymphocytolysis in the spleen (arrow) (HE, scale bar 100 µm)
4. DISCUSSION
Detection of SARS‐CoV‐2 in cats and dogs has generated concerns about its transmission from humans to their pets (Goletic et al., 2022). Beside the viral detection in companion animals, it was reported in wild, zoo and farm animals which has evoked questions about the viral zoonotic and reverse zoonotic transmissibility (Goraichuk et al., 2021). Although wide range of COVID‐19's clinical signs that makes its differential diagnosis challenging, anosmia and dysgeusia are significant predictors in humans (Roland et al., 2020). In contrast, in dogs and cats, many viruses cause respiratory signs (canine respiratory coronavirus, influenza, parainfluenza, distemper, herpesvirus, adenovirus and pnemovirus in dogs, whereas feline infectious peritonitis virus, influenza, calicivirus and herpesvirus in cats) and others cause gastrointestinal illnesses (canine enteric coronavirus, parvovirus, distemper, norovirus, rotavirus, adenovirus, astrovirus and calicivirus in dogs, while feline coronavirus, rotavirus and panleukopenia in cats) (Sykes, 2015). The latter diseases have common signs such as fever, lethargy, respiratory distress, and/or gastrointestinal disturbance, but none of these signs are precise enough to be included in the COVID‐19 differential diagnosis. Therefore, this study is based on case history of pet animals that were in contact with their SARS‐CoV‐2‐positive owners, molecular diagnosis by rRT‐PCR, confirmatory partial spike sequence, clinical, pathological findings besides the clinical signs to investigate SARS‐CoV‐2 spillover between humans and their pet animals.
Our findings further support the probability of SARS‐CoV‐2 spillover between humans and companion animals which aligned with recent studies in Bosnia and Herzegovina (Goletic et al., 2022), the United States (Newman et al., 2020) and Hong Kong (Sit et al., 2020). Even though only a partial spike gene was sequenced in this study, still 6 positive samples collected in December 2020 were identical to SARS‐CoV‐2 that was detected in human in Egypt during the same timeline that agreed with previous studies in Bosnia and Herzegovina (Goletic et al., 2022), France (Sailleau et al., 2020), China (Barrs et al., 2020), and the United Kingdom (Hosie et al., 2021). We noticed that in our study SARS‐CoV‐2 infection rate in cats is higher than that in dogs, which agreed with previous studies (Barrs et al., 2020; Hamdy et al., 2022; Sit et al., 2020). Cats are more susceptible to SARS‐CoV‐2 than dogs (Hamdy et al., 2022; Hamer et al., 2021), which may be because of the high similarity of ACE2 receptor configuration in cats and humans (Hamdy et al., 2022; Hamer et al., 2021; Patterson et al., 2020; Ruiz et al., 2021; Sailleau et al., 2020). Besides, the viral persistence in cats (21 days) (Hamer et al., 2021) is longer than that in dogs (13 days) (Shi et al., 2020; Sit et al., 2020). In addition, it was reported that cats can shed the virus horizontally among feline species up to 6 days post‐infection, while no shedding has been reported in dogs (Hamer et al., 2021).
The rationale behind clinical pathology parameters selection is as currently, there is no gold standard diagnostic test for COVID‐19, and its perfect diagnosis depends on sampling time in correlation with symptom onset. For instance, in the early stage of infection, viral RNA can be detected easily by rRT‐PCR, while in the late‐stage CT chest scan and laboratory findings can be a confirmatory tool. Therefore, combination of different diagnostic techniques could facilitate the perfect interpretation (Laura et al., 2021). In our study, we have combined molecular diagnosis with laboratory findings to provide a deep insight into the laboratory picture of SARS‐CoV‐2 infection in pet animals. Clinical pathology parameters were selected based on the prevalent laboratory findings of COVID‐19 (Zhang et al., 2020; Zhou et al., 2020b). The obtained platelets and lymphocytes values were significantly decreased as compared to the normal values, that range from 300,000/µl to 800,000/µl and from 27% to 36% in cats and from 200,000/µl to 500,000/µl and from 8% to 21% in dogs, respectively (Fielder, 2021).
Our clinical findings revealed a significant thrombocytopenia in SARS‐CoV‐2‐infected cats and dogs compared with negative group. This thrombocytopenia may be due to pulmonary embolism and/or systemic thrombosis (Harrison et al., 2021). Additionally, we detected a significant lymphocytopenia with SARS‐CoV‐2 infection. The latter lymphocytopenia has several hypotheses as some researchers linked its occurrence to lymphocyte apoptosis due to the cytokine storm (Terpos et al., 2020). Another study suggests that SARS‐CoV‐2 has the ability to promote systemic inflammation and causes direct neutralisation in lymph nodes and spleen inducing lymphocytopenia (Xiang et al., 2021). Furthermore, monocytosis was significantly detected in the infected group that aligned with a previous study (Nazarullah et al., 2020). Additionally, inflammatory parameters (CRP and ferritin) and clotting parameters (D‐dimer and LDH) were significantly increased as compared to the normal values (Latimer, 2011).
These elevation was in agreement with the prevalent laboratory finding of COVID‐19 (Zhang et al., 2020; Zhou et al., 2020b). Furthermore, lymphocytopenia, high levels of D‐dimer, LDH, increased CRP, and elevated ferritin levels have been linked to the severity in companion animals, as some (n = 4) of these cats died, which is consistent with previous studies in humans (Zhang et al., 2020; Zhou et al., 2020b).
SARS‐CoV‐2‐positive animals in this study have a wide range of clinical signs (asymptomatic, mild and severe). Asymptomatic positive animals aligned with a previous paper which mentioned that cats can be SARS‐CoV‐2 infected without manifesting any clinical signs and act as a reservoir for healthy cats (Gaudreault et al., 2020). Positive animals with mild signs agreed with a recent study that reported mild gastrointestinal and respiratory problems in SARS‐CoV‐2‐positive cats and dogs (Goletic et al., 2022). Infection with severe SARS‐CoV‐2 in cats induced multisystemic affections in different organs as shown in our pathological results. Cats are highly susceptible to SARS‐CoV‐2 infection as shown by the manifestation of inflammatory lesions in the respiratory system (Shi et al., 2020). During the early course of infection, lesions are mainly observed in the upper respiratory tract. However, at later stages of infection, the lesions are more predominant in the pulmonary tissue, with the presence of inflammatory infiltrates in the alveolar lumina (Bosco‐Lauth et al., 2020). These lesions resemble the pathological picture of COVID‐19 in humans. The enteric and hepatic lesions are attributed to the tropism of SARS‐COV2 to the digestive system (Shi et al., 2020) and its ability to replicate in the enterocytes and to reach the liver via the portal vein. This was evident from necrosis of the intestinal villi and portal vein thrombosis. Cardiac lesions included necrotic changes in the muscle bundles and oedema. The COVID‐19 associated lesions in the hearts of cats and dogs were first reported during infection with Alpha variant of SARS‐COV2 (Ferasin et al., 2021). Renal histopathological examination revealed severe acute tubular injury with minimal glomerular changes. Indeed, acute tubular injury was the main finding in the majority of human cases with acute kidney injury–associated with COVID‐19 infections (Santoriello et al., 2020; Sharma et al., 2021; Su et al., 2020). Nonetheless, this is the first study to report renal lesions in SARS‐COV2‐infected cats.
5. CONCLUSIONS
In summary, the present study highlights SARS‐CoV‐2 spillover and reverse zoonoses occurrence. Hence, biosecurity measures should be applied to prevent the spread of SARS‐CoV‐2 among humans and companion animals.
AUTHOR CONTRIBUTIONS
Conceptualization, MH, AE‐D, NH, MS, and HH; methodology, MH, AE‐D, NH, MS, and HH; software, MH; validation, MH, AE‐D, NH, MS, NL, MS, and HH; formal analysis, MH; investigation, MH, AE‐D, NH, MS, NL, MS, and HH; resources, MH, AE‐D, NH, MS, MS, and HH; data curation, MH, and HH; writing original draft preparation, MH, MS, and HH; writingreview and editing, MH, MS, and HH; visualization, MH; supervision, AE‐D, NH, MS, and HH; project administration, MH, NH, and HH; funding acquisition, MH. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests.
ETHICAL APPROVAL
The protocol and procedures employed were ethically reviewed and approved by the Animal Health Research Institute Animal Care Committees (No. 11429).
Supporting information
Tables S1–S5
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Elshaimaa Ismael, Assistant Professor of Veterinary Hygiene & Management, Faculty of Veterinary Medicine, Cairo University for her support in the statistical analysis.
Hamdy, M. E. , El‐Deeb, A. H. , Hagag, N. M. , Shahein, M. A. , Liyanage, N. P. M. , Shalaan, M. , & Hussein, H. A. (2023). SARS‐CoV‐2 infection of companion animals in Egypt and its risk of spillover. Veterinary Medicine and Science, 9, 13–24. 10.1002/vms3.1029
DATA AVAILABILITY STATEMENT
Data available in article supplementary material.
REFERENCES
- Acter, T. , Uddin, N. , Das, J. , Akhter, A. , Choudhury, T. R. , & Kim, S. (2020). Evolution of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) as coronavirus disease 2019 (COVID‐19) pandemic: A global health emergency. Science of the Total Environment, 730, 138996. 10.1016/j.scitotenv.2020.138996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft, J. D. , & Gamble, M. (2008). Theory and practice of histological techniques. Elsevier Health Sciences. [Google Scholar]
- Barrs, V. R. , Peiris, M. , Tam, K. W. , Law, P. Y. , Brackman, C. J. , To, E. M. , Yu, V. Y. , Chu, D. K. , Perera, R. A. , & Sit, T. H. (2020). SARS‐CoV‐2 in quarantined domestic cats from COVID‐19 households or close contacts, Hong Kong, China. Emerging Infectious Diseases, 26(12), 3071–3074. 10.3201/eid2612.202786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles, M. , Donaldson, E. , & Baric, R. (2011). SARS‐CoV and emergent coronaviruses: Viral determinants of interspecies transmission. Current Opinion in Virology, 1(6), 624–634. 10.1016/j.coviro.2011.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco‐Lauth, A. M. , Hartwig, A. E. , Porter, S. M. , Gordy, P. W. , Nehring, M. , Byas, A. D. , VandeWoude, S. , Ragan, I. K. , Maison, R. M. , & Bowen, R. A. (2020). Experimental infection of domestic dogs and cats with SARS‐CoV‐2: Pathogenesis, transmission, and response to reexposure in cats. Proceedings of the National Academy of Sciences, 117(42), 26382–26388. 10.1073/pnas.2013102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaswad, A. , Fawzy, M. , Basiouni, S. , & Shehata, A. A. (2020). Mutational spectra of SARS‐CoV‐2 isolated from animals. PeerJ, 8, e10609. 10.7717/peerj.10609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferasin, L. , Fritz, M. , Ferasin, H. , Becquart, P. , Corbet, S. , Ar Gouilh, M. , Legros, V. , & Leroy, E. M. (2021). Infection with SARS‐CoV‐2 variant B. 1.1. 7 detected in a group of dogs and cats with suspected myocarditis. Veterinary Record, 189(9), 1–9. 10.1002/vetr.944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielder, S. E. (2021). MSD veterinary manual. Retrieved from https://www.msdvetmanual.com/special‐subjects/reference‐guides/hematologic‐reference‐ranges
- Freuling, C. M. , Breithaupt, A. , Müller, T. , Sehl, J. , Balkema‐Buschmann, A. , Rissmann, M. , Klein, A. , Wylezich, C. , Höper, D. , & Wernike, K. (2020). Susceptibility of raccoon dogs for experimental SARS‐CoV‐2 infection. Emerging Infectious Diseases, 26(12), 2982. 10.3201/eid2612.203733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, T. S. , & Liu, D. X. (2019). Human coronavirus: Host‐pathogen interaction. Annual Review of Microbiology, 73, 529–557. 10.1146/annurev-micro-020518-115759 [DOI] [PubMed] [Google Scholar]
- Gaudreault, N. N. , Trujillo, J. D. , Carossino, M. , Meekins, D. A. , Morozov, I. , Madden, D. W. , Indran, S. V. , Bold, D. , Balaraman, V. , & Kwon, T. (2020). SARS‐CoV‐2 infection, disease and transmission in domestic cats. Emerging Microbes & Infections, 9(1), 2322–2332. 10.1080/22221751.2020.1833687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goletic, S. , Goletic, T. , Softic, A. , Zahirovic, A. , Rukavina, D. , Kavazovic, A. , Omeragic, J. , Umihanic, S. , & Hukic, M. (2022). The evidence of SARS‐CoV‐2 human‐to‐pets transmission in household settings in Bosnia and Herzegovina. Frontiers in Genetics, 13, 839205. 10.3389/fgene.2022.839205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraichuk, I. V. , Arefiev, V. , Stegniy, B. T. , & Gerilovych, A. P. (2021). Zoonotic and reverse zoonotic transmissibility of SARS‐CoV‐2. Virus Research, 302, 198473. 10.1016/j.virusres.2021.198473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann, P. J. , Hatta, M. , Chiba, S. , Maemura, T. , Fan, S. , Takeda, M. , Kinoshita, N. , Hattori, S.‐I. , Sakai‐Tagawa, Y. , & Iwatsuki‐Horimoto, K. (2020). Transmission of SARS‐CoV‐2 in domestic cats. New England Journal of Medicine, 383(6), 592–594. 10.1056/NEJMc2013400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, T. (1999). BioEdit: A user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Paper presented at the Nucleic Acids Symp. Ser.
- Hamdy, M. E. , El‐Deeb, A. H. , Hagag, N. M. , Shahein, M. A. , Alaidi, O. , & Hussein, H. A. (2022). Mutations of the SARS‐CoV‐2 spike glycoprotein detected in cats and their effect on its structure and function. Frontiers in Cellular and Infection Microbiology, 12, 629–646. 10.3389/fcimb.2022.875123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, S. A. , Pauvolid‐Corrêa, A. , Zecca, I. B. , Davila, E. , Auckland, L. D. , Roundy, C. M. , Tang, W. , Torchetti, M. K. , Killian, M. L. , & Jenkins‐Moore, M. (2021). SARS‐CoV‐2 infections and viral isolations among serially tested cats and dogs in households with infected owners in Texas, USA. Viruses, 13(5), 938. 10.3390/v13050938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. , Lely, A. , Navis, G. J. , & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland, 203(2), 631–637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, S. R. , Klassen, J. R. , Bridgewood, C. , Scarsbrook, A. , Marzo‐Ortega, H. , & McGonagle, D. (2021). Chest pain mimicking pulmonary embolism may be a common presentation of COVID‐19 in ambulant patients without other typical features of infection. Journal of Internal Medicine, 290(2), 349–358. 10.1111/joim.13267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N.‐H. , & Nitsche, A. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e278. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie, M. J. , Epifano, I. , Herder, V. , Orton, R. J. , Stevenson, A. , Johnson, N. , MacDonald, E. , Dunbar, D. , McDonald, M. , & Howie, F. (2021). Detection of SARS‐CoV‐2 in respiratory samples from cats in the UK associated with human‐to‐cat transmission. Veterinary Record, 188(8), 1–9. 10.1002/vetr.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, T. T.‐Y. , Jia, N. , Zhang, Y.‐W. , Shum, M. H.‐H. , Jiang, J.‐F. , Zhu, H.‐C. , Tong, Y.‐G. , Shi, Y.‐X. , Ni, X.‐B. , & Liao, Y.‐S. (2020). Identifying SARS‐CoV‐2‐related coronaviruses in Malayan pangolins. Nature, 583(7815), 282–285. 10.1038/s41586-020-2169-0 [DOI] [PubMed] [Google Scholar]
- Latimer, K. S. (2011). Duncan and Prasse's veterinary laboratory medicine: Clinical pathology. John Wiley & Sons. [Google Scholar]
- Laura, C. , Géraldine, D. M. , Yves, L. , Valeska, L. , Amber, L. , Els, V. V. , & Chloé, W. T. (2021). Factsheet COVID‐19 disease (SARS‐CoV‐2 virus), Version 13 Sciensano. Retrieved from https://covid‐19.sciensano.be/sites/default/files/Covid19/factsheetENG.pdf
- Musso, N. , Costantino, A. , La Spina, S. , Finocchiaro, A. , Andronico, F. , Stracquadanio, S. , Liotta, L. , Visalli, R. , & Emmanuele, G. (2020). New SARS‐CoV‐2 infection detected in an Italian pet cat by RT‐qPCR from deep pharyngeal swab. Pathogens, 9(9), 746. 10.3390/pathogens9090746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi, A. A. T. , Fatima, K. , Mohammad, T. , Fatima, U. , Singh, I. K. , Singh, A. , Atif, S. M. , Hariprasad, G. , Hasan, G. M. , & Hassan, M. I. (2020). Insights into SARS‐CoV‐2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochimica et Biophysica Acta (BBA)‐Molecular Basis of Disease, 1866(10), 165878. 10.1016/j.bbadis.2020.165878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarullah, A. , Liang, C. , Villarreal, A. , Higgins, R. A. , & Mais, D. D. (2020). Peripheral blood examination findings in SARS‐CoV‐2 infection. American Journal of Clinical Pathology, 154(3), 319–329. 10.1093/ajcp/aqaa108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, A. , Smith, D. , Ghai, R. R. , Wallace, R. M. , Torchetti, M. K. , Loiacono, C. , Murrell, L. S. , Carpenter, A. , Moroff, S. , & Rooney, J. A. (2020). First reported cases of SARS‐CoV‐2 infection in companion animals—New York, March–April 2020. Morbidity and Mortality Weekly Report, 69(23), 710. 10.15585/mmwr.mm6923e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova, N. , Molenaar, R. J. , Vreman, S. , Harders, F. , Munnink, B. B. O. , Hakze‐van Der Honing, R. W. , Gerhards, N. , Tolsma, P. , Bouwstra, R. , & Sikkema, R. S. (2020). SARS‐CoV‐2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance, 25(23), 2001005. 10.2807/1560-7917.ES.2020.25.23.2001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, E. I. , Elia, G. , Grassi, A. , Giordano, A. , Desario, C. , Medardo, M. , Smith, S. L. , Anderson, E. R. , Prince, T. , & Patterson, G. T. (2020). Evidence of exposure to SARS‐CoV‐2 in cats and dogs from households in Italy. Nature Communications, 11(1), 1–5. 10.1038/s41467-020-20097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, L.‐L. , Wang, Y.‐M. , Wu, Z.‐Q. , Xiang, Z.‐C. , Guo, L. , Xu, T. , Jiang, Y.‐Z. , Xiong, Y. , Li, Y.‐J. , & Li, X.‐W. (2020). Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chinese Medical Journal, 133(09), 1015–1024. 10.1097/CM9.0000000000000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland, L. T. , Gurrola, J. G. , Loftus, P. A. , Cheung, S. W. , & Chang, J. L. (2020). Smell and taste symptom‐based predictive model for COVID‐19 diagnosis. International Forum of Allergy & Rhinology, 10(7), 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz, I. , Portillo, A. , Palomar, A. M. , Santibáñez, S. , Santibáñez, P. , Cervera, C. , & Oteo, J. A. (2021). Detection of SARS‐CoV‐2 in pets living with COVID‐19 owners diagnosed during the COVID‐19 lockdown in Spain: A case of an asymptomatic cat with SARS‐CoV‐2 in Europe. Transboundary and Emerging Diseases, 68(2), 973–976. 10.1111/tbed.13803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailleau, C. , Dumarest, M. , Vanhomwegen, J. , Delaplace, M. , Caro, V. , Kwasiborski, A. , Hourdel, V. , Chevaillier, P. , Barbarino, A. , & Comtet, L. (2020). First detection and genome sequencing of SARS‐CoV‐2 in an infected cat in France. Transboundary and Emerging Diseases, 67(6), 2324–2328. 10.1111/tbed.13659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoriello, D. , Khairallah, P. , Bomback, A. S. , Xu, K. , Kudose, S. , Batal, I. , Barasch, J. , Radhakrishnan, J. , D'Agati, V. , & Markowitz, G. (2020). Postmortem kidney pathology findings in patients with COVID‐19. Journal of the American Society of Nephrology, 31(9), 2158–2167. 10.1681/ASN.2020050744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalés, J. , Puig, M. , Rodon, J. , Avila‐Nieto, C. , Carrillo, J. , Cantero, G. , Terrón, M. T. , Cruz, S. , Parera, M. , & Noguera‐Julián, M. (2020). Detection of SARS‐CoV‐2 in a cat owned by a COVID‐19−affected patient in Spain. Proceedings of the National Academy of Sciences, 117(40), 24790–24793. 10.1073/pnas.2010817117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, P. , Ng, J. H. , Bijol, V. , Jhaveri, K. D. , & Wanchoo, R. (2021). Pathology of COVID‐19‐associated acute kidney injury. Clinical Kidney Journal, 14(Supplement 1), i30–i39. 10.1093/ckj/sfab003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , Yang, H. , Wang, C. , Huang, B. , Liu, R. , He, X. , Shuai, L. , & Sun, Z. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science, 368(6494), 1016–1020. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit, T. H. , Brackman, C. J. , Ip, S. M. , Tam, K. W. , Law, P. Y. , To, E. M. , Yu, V. Y. , Sims, L. D. , Tsang, D. N. , & Chu, D. K. (2020). Infection of dogs with SARS‐CoV‐2. Nature, 586(7831), 776–778. 10.1038/s41586-020-2334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, S. , Wong, G. , Shi, W. , Liu, J. , Lai, A. C. , Zhou, J. , Liu, W. , Bi, Y. , & Gao, G. F. (2016). Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends in Microbiology, 24(6), 490–502. 10.1016/j.tim.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, H. , Yang, M. , Wan, C. , Yi, L.‐X. , Tang, F. , Zhu, H.‐Y. , Yi, F. , Yang, H.‐C. , Fogo, A. B. , & Nie, X. (2020). Renal histopathological analysis of 26 postmortem findings of patients with COVID‐19 in China. Kidney International, 98(1), 219–227. 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes, J. E. (2015). Small animal critical care medicine, part X: Infectious disorders, chapter 96 – Viral infections (2nd ed.). Elsevier. [Google Scholar]
- Terpos, E. , Ntanasis‐Stathopoulos, I. , Elalamy, I. , Kastritis, E. , Sergentanis, T. N. , Politou, M. , Psaltopoulou, T. , Gerotziafas, G. , & Dimopoulos, M. A. (2020). Hematological findings and complications of COVID‐19. American Journal of Hematology, 95(7), 834–847. 10.1002/ajh.25829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOAH/OIE . (2020, 28 August 2020). World Organisation for Animal Health, Events in Animals. Retrieved from https://www.oie.int/en/scientific‐expertise/specific‐information‐and‐recommendations/questions‐and‐answers‐on‐2019novel‐coronavirus/events‐in‐animals/
- WOAH/OIE . (2021a, 10th December 2021). SARS‐CoV‐2 in animals 2021 fact sheet. Retrieved from https://www.oie.int/app/uploads/2021/11/en‐factsheet‐sars‐cov‐2‐20211025.pdf
- WOAH/OIE . (2021b, 8 December 2021). SARS‐COV‐2 in animals situation report 5 till 30 September. Retrieved from https://www.oie.int/app/uploads/2021/10/sars‐cov‐2‐situation‐report‐5.pdf
- WOAH/OIE . (2021c, 8th December 2021). SARS‐CoV‐2 in animals situation report 6 till 31‐10‐2021. Retrieved from https://www.oie.int/app/uploads/2021/11/sars‐cov‐2‐situation‐report‐6.pdf
- WOAH/OIE . (2022, 8 September 2022). SARS‐CoV‐2 in animals situtaion report 15 till 31 July. Retrieved from https://www.woah.org/app/uploads/2022/08/sars‐cov‐2‐situation‐report‐15.pdf
- Wu, F. , Zhao, S. , Yu, B. , Chen, Y.‐M. , Wang, W. , Song, Z.‐G. , Hu, Y. , Tao, Z.‐W. , Tian, J.‐H. , & Pei, Y.‐Y. (2020). A new coronavirus associated with human respiratory disease in China. Nature, 579(7798), 265–269. 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Q. , Feng, Z. , Diao, B. , Tu, C. , Qiao, Q. , Yang, H. , Zhang, Y. , Wang, G. , Wang, H. , & Wang, C. (2021). SARS‐CoV‐2 induces lymphocytopenia by promoting inflammation and decimates secondary lymphoid organs. Frontiers in Immunology, 12, 1292. 10.3389/fimmu.2021.661052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z.‐L. , Hou, Y.‐L. , Li, D.‐T. , & Li, F.‐Z. (2020). Laboratory findings of COVID‐19: A systematic review and meta‐analysis. Scandinavian Journal of Clinical and Laboratory Investigation, 80(6), 441–447. 10.1080/00365513.2020.1768587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Cui, W. , & Tian, B.‐P. (2020). The potential intermediate hosts for SARS‐CoV‐2. Frontiers in Microbiology, 11, 2400. 10.3389/fmicb.2020.580137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. , Si, H.‐R. , Zhu, Y. , Li, B. , & Huang, C.‐L. (2020a). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , Xiang, J. , Wang, Y. , Song, B. , & Gu, X. (2020b). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. The Lancet, 395(10229), 1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N. , Zhang, D. , Wang, W. , Li, X. , Yang, B. , Song, J. , Zhao, X. , Huang, B. , Shi, W. , & Lu, R. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 382, 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Data Availability Statement
Data available in article supplementary material.


