Abstract
Simple Summary
Ibrutinib is an established standard of care in the first-line treatment of chronic lymphocytic leukemia. Since ibrutinib is given continuously, data on long-term treatment, outcomes, and safety are essential to inform clinical decision making. Here, we describe characteristics and outcomes of patients who received long-term treatment with ibrutinib for ≥5 years in the phase 3 RESONATE-2 study. More than half (58%) of the patients randomly assigned to receive ibrutinib in the RESONATE-2 study continued to benefit from ibrutinib treatment for ≥5 years, regardless of baseline characteristics. Among patients who were on ibrutinib treatment for ≥5 years, complete response rates improved over time through the 5 years. The safety profile of ibrutinib treatment for ≥5 years was consistent with previous reports and no new safety signals were identified. For patients who experienced adverse events, dose modification was effective in resolving adverse events, thereby facilitating continued treatment.
Abstract
Primary results from the phase 3 RESONATE-2 study demonstrated superior efficacy and tolerability with ibrutinib versus chlorambucil in patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Here, we describe characteristics and outcomes of patients who received ibrutinib treatment for ≥5 years in RESONATE-2. Patients aged ≥65 years with previously untreated CLL/SLL, without del(17p), were randomly assigned 1:1 to once-daily ibrutinib 420 mg until disease progression/unacceptable toxicity (n = 136) or chlorambucil 0.5–0.8 mg/kg for ≤12 cycles (n = 133). Baseline characteristics in ibrutinib-randomized patients (n = 136) were generally similar between patients on ibrutinib treatment for ≥5 years (n = 79) versus those on treatment for <5 years (n = 57). In patients on ibrutinib treatment for ≥5 years, complete response rates improved over time, reaching 42% by 5 years. Estimated 7-year progression-free survival and overall survival rates were 82% and 94%, respectively. Adverse events (AEs) led to dose reductions in 16/79 patients (20%); these AEs were resolved for 13/16 patients (81%). AEs led to dose holds (≥7 days) in 45/79 patients (57%); these AEs were resolved for 43/45 patients (96%). More than half (58%) of ibrutinib-randomized patients benefitted from ibrutinib treatment for ≥5 years regardless of baseline characteristics. Dose modification resolved AEs for most patients, thereby facilitating continued treatment.
Keywords: ibrutinib, chronic lymphocytic leukemia, long-term outcomes
1. Introduction
Ibrutinib is a once-daily oral Bruton tyrosine kinase (BTK) inhibitor that is approved as first-line treatment for patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) in the United States, Europe, and other countries. Ibrutinib has the longest follow-up of any targeted therapy across multiple randomized phase 3 studies and is the only therapy to date that has demonstrated a significant overall survival (OS) benefit compared with chemotherapy/chemoimmunotherapy in patients with previously untreated CLL/SLL [1,2,3,4]. Initial approval of ibrutinib in the first-line setting was supported by results from the primary analysis of the phase 3 RESONATE-2 study, which demonstrated that ibrutinib was superior to chlorambucil with respect to both efficacy and tolerability [5]. With up to 8 years of follow-up (median: 82.7 months; range, 0.1–96.6 months) in the RESONATE-2 study, the majority of ibrutinib-randomized patients remained progression-free; median progression-free survival (PFS) was not yet reached at the latest data cut [1].
Previous studies suggest that patients who continue treatment with single-agent ibrutinib experience better survival outcomes than patients who discontinue treatment within the first few years [6,7,8,9]. Additionally, real-world evidence suggests that dose management (dose reduction or temporary dose holds for up to 1–2 weeks) results in improvement in or resolution of adverse events (AEs) [10] without impacting disease outcomes [6,10,11,12,13,14,15,16,17,18]. Therefore, active management of AEs by dose modification might facilitate continued ibrutinib treatment and maximize clinical outcomes [12]. As of May 2022, the US prescribing information for ibrutinib includes updates to recommended dose modifications for AEs [19].
As it is the only BTK inhibitor with long-term follow-up for up to 8 years, we have the opportunity to examine efficacy and safety outcomes in patients with longer-term experience on ibrutinib treatment. Here, we describe characteristics and outcomes of patients who received treatment with ibrutinib for ≥5 years in the RESONATE-2 study.
2. Materials and Methods
2.1. Study Design and Patients
RESONATE-2 (PCYC-1115 [NCT01722487]/PCYC-1116 [NCT01724346]) is a multicenter, international, randomized, open-label, phase 3 study designed to compare the efficacy and safety of first-line treatment with ibrutinib versus chlorambucil in patients aged ≥65 years with previously untreated CLL/SLL who required therapy per the 2008 International Workshop on CLL (iwCLL) criteria [20]. Patients with del(17p) were excluded. Detailed methods were previously reported [5]. Briefly, eligible patients were randomly assigned in a 1:1 ratio to receive oral ibrutinib 420 mg once daily until occurrence of progressive disease or unacceptable toxicity, or chlorambucil 0.5 mg/kg, escalated to a maximum of 0.8 mg/kg as tolerated, on days 1 and 15 of each 28-day cycle for up to 12 cycles. After confirmation of progressive disease per iwCLL criteria [20,21], patients who were randomly assigned to the chlorambucil arm could cross over to second-line treatment with ibrutinib. Per protocol, ibrutinib was temporarily held for any unmanageable grade ≥ 3 AE that was considered by the investigator to be potentially related to the study’s treatment. Other AEs, including AEs of grade 2 in severity, could be managed with a one-level dose reduction of ibrutinib if the AE was considered to be potentially manageable by dose reduction as judged by the investigator.
This study was performed in accordance with International Conference on Harmonisation Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The study protocol was approved by institutional review boards of each participating institution, and all patients provided written informed consent before participation in the study. This study was registered with ClinicalTrials.gov, numbers NCT01722487 and NCT01724346.
2.2. Analysis
The current exploratory analysis evaluated baseline demographics and clinical characteristics, overall response rates (per iwCLL criteria [20,21]), PFS, OS, prevalence of AEs over time, and AEs leading to dose modifications (per protocol) for ibrutinib-randomized patients who were on ibrutinib treatment for ≥5 years. Since the study protocol provided flexibility for dose reductions based on an investigator’s judgment, additional analyses were performed to retrospectively determine the incidence of dose reductions due to AEs for which dose reductions are recommended in the recently updated US prescribing information (grade 2 cardiac failure, grade 3 cardiac arrhythmia, grade 3 or 4 nonhematologic AEs [excluding cardiac failure and cardiac arrhythmia], grade 3 or 4 neutropenia with infection or fever, and grade 4 hematologic AEs) [19].
Baseline characteristics were also evaluated as potential predictors for remaining on treatment for ≥5 years using a multivariate logistic regression model including the following baseline characteristics: age group, sex, Eastern Cooperative Oncology Group performance status, Cumulative Illness Rating Scale score, creatinine clearance, TP53 mutation status, IGHV mutation status, del(11q) status, disease histology, bulky disease, β-2 microglobulin, Rai stage, any history of cytopenia, lactate dehydrogenase level, and geographic region. Additionally, PFS and OS were analyzed in subgroups of patients with and without dose reductions in the overall population of all ibrutinib-treated patients; these exploratory post hoc analyses were not powered for significance, and comparative statistics are provided for descriptive purposes only. PFS and OS were estimated using the Kaplan-Meier method.
3. Results
In RESONATE-2, 269 patients were randomly assigned to receive ibrutinib (n = 136) or chlorambucil (n = 133). Of the 136 patients in the ibrutinib arm, 79 (58%) received ibrutinib treatment for ≥5 years. Median follow-up duration for patients who were on ibrutinib treatment for ≥5 years (n = 79) was 89.2 months (range: 61.3–96.6). Of these 79 patients, 22 subsequently discontinued ibrutinib in years 5–6 (n = 9), 6–7 (n = 10), or 7–8 (n = 3); reasons for discontinuation in these 22 patients were progressive disease (n = 10), death (n = 4), AEs (n = 3), physician decision (n = 3), and patient withdrawal (n = 2).
3.1. Baseline Characteristics
Within ibrutinib-randomized patients in the intention-to-treat population (n = 136), baseline characteristics in the subset of patients who were on ibrutinib treatment for ≥5 years (n = 79) were generally similar to those in the subset of patients who were on ibrutinib treatment for <5 years (n = 57) (Table 1). Compared with the subset of patients who were on ibrutinib treatment for <5 years, the subset of patients who were on ibrutinib treatment for ≥5 years were more likely to be in the youngest age group (65–69 years; 37% vs. 19% of patients) and had a longer interval between initial diagnosis and initiation of study treatment (median 35 vs. 26 months).
Table 1.
Baseline characteristics.
| Characteristic | On Ibrutinib Treatment for <5 Years n = 57 |
On Ibrutinib Treatment for ≥5 Years n = 79 |
All Ibrutinib- Randomized Patients n = 136 |
|---|---|---|---|
| Median age, years (range) | 74 (65–89) | 71 (65–84) | 73 (65–89) |
| Age group, n (%) | |||
| 65–69 years | 11 (19) | 29 (37) | 40 (29) |
| 70–74 years | 21 (37) | 29 (37) | 50 (37) |
| 75–79 years | 11 (19) | 13 (16) | 24 (18) |
| ≥80 years | 14 (25) | 8 (10) | 22 (16) |
| Male sex, n (%) | 27 (47) | 49 (62) | 60 (44) |
| ECOG PS, n (%) | |||
| 0 | 27 (47) | 33 (42) | 60 (44) |
| 1 | 24 (42) | 41 (52) | 65 (48) |
| 2 | 6 (11) | 5 (6) | 11 (8) |
| Diagnosis, n (%) | |||
| CLL | 48 (84) | 75 (95) | 123 (90) |
| SLL | 9 (16) | 4 (5) | 13 (10) |
| Rai stage III/IV, n (%) | 23 (40) | 37 (47) | 60 (44) |
| CIRS score >6, n (%) | 16 (28) | 26 (33) | 42 (31) |
| CrCl <60 mL/min, n (%) | 28 (49) | 32 (41) | 60 (44) |
| Bulky disease ≥5 cm, n (%) | 25 (44) | 29 (37) | 54 (40) |
| LDH | |||
| Median, U/L (range) | 215 (65–1188) | 196 (52–514) | 199 (52–1188) |
| >250 U/L, n (%) | 21 (37) | 18 (23) | 39 (29) |
| Median β-2 microglobulin, mg/L (range) | 5 (2–20) | 4 (2–20) | 4 (2–20) |
| Median time from initial diagnosis, months (range) | 26 (1–162) | 35 (1–241) | 30 (1–241) |
| High-risk features, n (%) | 28 (49) | 45 (57) | 73 (54) |
| TP53 mutated | 4 (7) | 7 (9) | 11 (8) |
| del(11q) | 11 (19) | 18 (23) | 29 (21) |
| Unmutated IGHV | 22 (39) | 36 (46) | 58 (43) |
| Geographic region, n (%) | |||
| United States | 12 (21) | 19 (24) | 31 (23) |
| Europe | 32 (56) | 39 (49) | 71 (52) |
| Rest of world | 13 (23) | 21 (27) | 34 (25) |
CIRS, Cumulative Illness Rating Scale; CLL, chronic lymphocytic leukemia; CrCl, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; IGHV, immunoglobulin heavy chain variable region; LDH, lactate dehydrogenase; SLL, small lymphocytic lymphoma.
3.2. Predictors of Ibrutinib Treatment for ≥5 Years
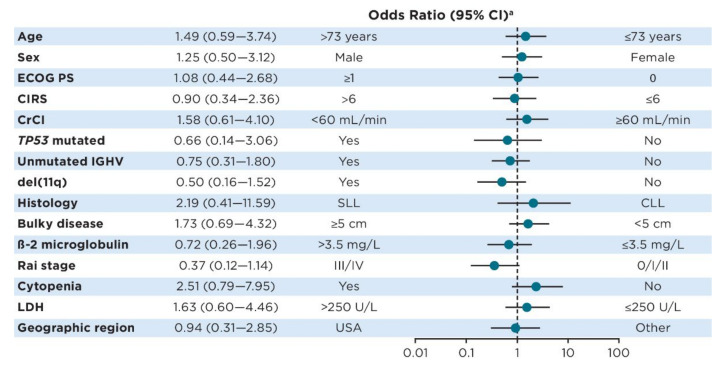
In multivariate analysis, several baseline characteristics showed a trend toward continuation of ibrutinib treatment for ≥5 years (age ≤ 73 years, female sex, creatinine clearance ≥60 mL/min, TP53 mutated, del(11q), CLL histology, absence of bulky disease [<5 cm], β-2 microglobulin >3.5 mg/L, Rai stage III/IV, absence of cytopenia, and lactate dehydrogenase ≤250 U/L), but none reached statistical significance (Figure 1).
Figure 1.
Forest plot of odds for continued ibrutinib treatment at 5 years according to baseline characteristics. a Odds ratios < 1 favor achievement of ≥5 years of ibrutinib treatment for the subgroup to the left of the plot, while odds ratios > 1 favor achievement of ≥5 years of ibrutinib treatment for the subgroup to the right of the plot. Abbreviations: CIRS, Cumulative Illness Rating Scale; CrCl, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; IGHV, immunoglobulin heavy chain variable region; LDH, lactate dehydrogenase.
3.3. Efficacy in Patients on Ibrutinib Treatment for ≥5 Years
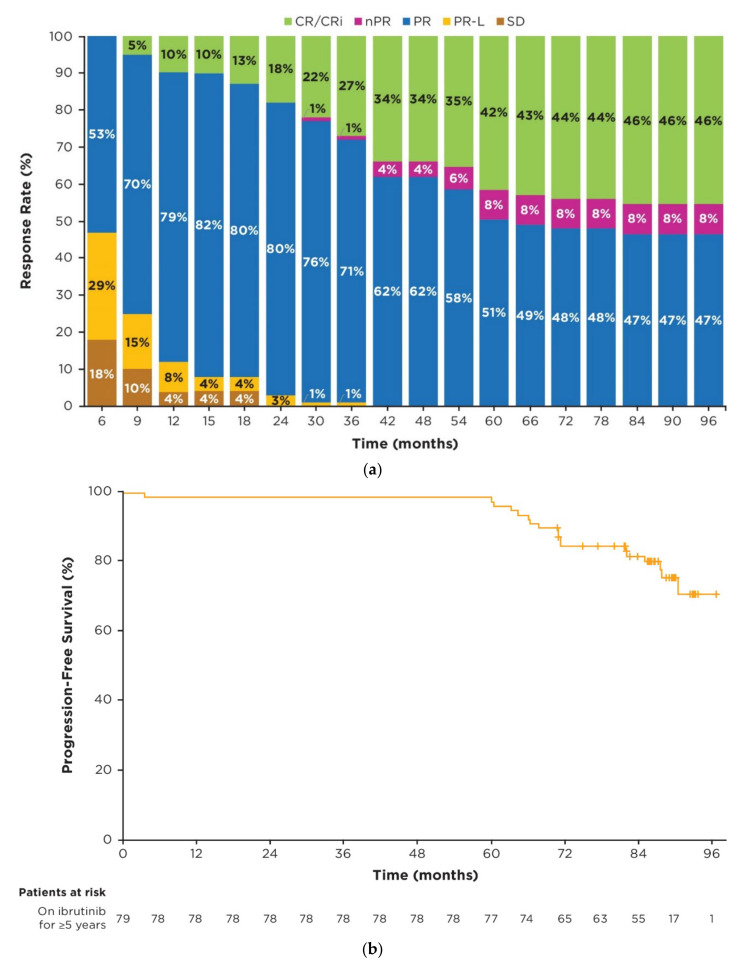
Responses deepened over time, as indicated by the improvement of complete response (CR) rates from 10% (8/79 patients) at 1 year to 42% (33/79 patients) by 5 years and 46% (36/79 patients) by 7 years (Figure 2a). In patients who were on ibrutinib treatment for ≥5 years, 23/79 (29%) had a documented response of partial response with lymphocytosis (PR-L); of these patients, 9/23 (39%) achieved a best response of partial response (PR), 1/23 (4%) achieved nodular PR (nPR), and 13/23 (57%) achieved CR. In the overall population of ibrutinib-randomized patients, 30/136 (22%) had a documented response of PR-L; of these patients, 13/30 (43%) achieved a best response of PR, 1/30 (3%) achieved nPR, and 15/30 (50%) achieved CR. In patients who were on ibrutinib treatment for ≥5 years, the median time to PR was 4.6 months (95% CI: 3.8–7.4), whereas the median time to CR was 32.3 months (95% CI: 19.7–37.7) for those patients achieving CR. With up to 8 years of follow-up, complete response was achieved in 44 patients in the overall population, 36 of whom received ibrutinib treatment for ≥5 years.
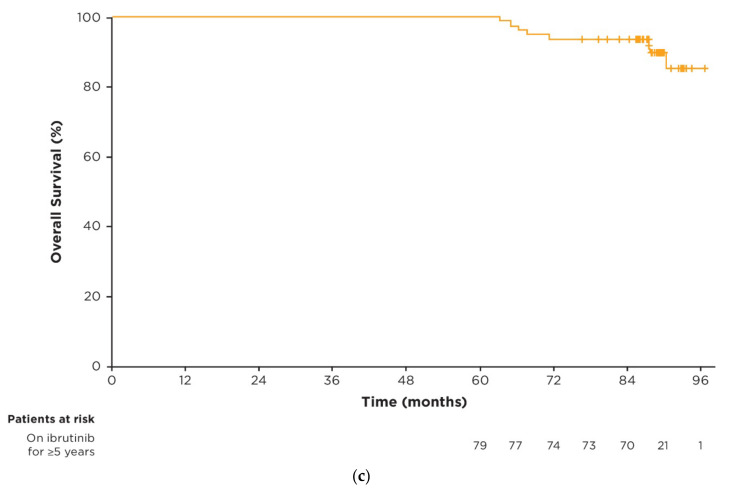
Figure 2.
Efficacy in patients on ibrutinib treatment for ≥5 years (n = 79). (a) Cumulative best response over time; (b) Kaplan–Meier curve of investigator-assessed PFS; (c) Kaplan–Meier curve of OS. Percentages of patients in each category of response may not add up to the overall proportion with a response due to rounding. Abbreviations: CR, complete response; CRi, complete response with incomplete bone marrow recovery; nPR, nodular partial response; OS, overall survival; PFS, progression-free survival; PR, partial response; PR-L, partial response with lymphocytosis; SD, stable disease.
In patients who were on ibrutinib treatment for ≥5 years, median PFS and OS were not yet reached; 7-year PFS and OS rates were 82% (95% CI: 71–89) and 94% (95% CI: 86–97), respectively (Figure 2b,c).
3.4. Prevalence of AEs over Time
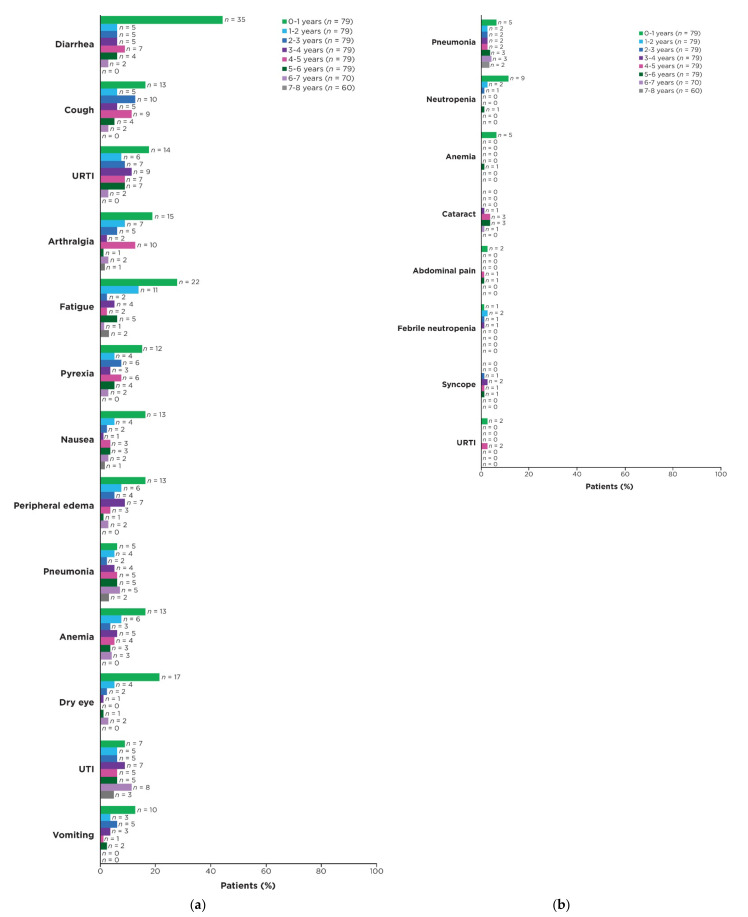
In patients who were on ibrutinib treatment for ≥5 years, the median duration of ibrutinib treatment was 89.2 months (range: 60.4–96.6). Median relative dose intensity of ibrutinib for these patients was 98% (range: 47–100). The most frequent AEs of any grade across the entire study period were diarrhea (42/79 patients; 53%), cough (34/79; 43%), and upper respiratory tract infection (33/79; 42%). Prevalence of the most frequent AEs of any grade and of grade ≥ 3 were generally highest in years 0–1 and decreased over time thereafter (Figure 3a,b). Prevalence of AEs of clinical interest of any grade over time are shown in Supplementary Figure S1.
Figure 3.
Prevalence of AEs over time in patients on ibrutinib treatment for ≥5 years. (a) Most frequent AEs of any grade (occurring in ≥25% of patients overall) by yearly interval; (b) Most frequent grade ≥ 3 AEs (occurring in ≥5% of patients overall) by yearly interval. Prevalence was determined by the proportion of patients with a given AE (existing event or new onset of an event) during each yearly interval. Multiple onsets of the same AE term within a specific yearly interval were counted once, and the same AE term continuing across several yearly intervals was counted in each of the intervals. Abbreviations: AE, adverse event; UTI, urinary tract infection; URTI, upper respiratory tract infection.
3.5. Dose Management with Ibrutinib Treatment
AEs led to dose reductions in 16/79 patients (20%) who were on ibrutinib treatment for ≥5 years and in 31/135 patients (23%) in the overall population of all ibrutinib-treated patients (Table 2). Most patients (12/16; 75%) experienced only one AE leading to dose reduction.
Table 2.
AEs leading to dose reductions per protocol.
| AEs Leading to Dose Reductions | On Ibrutinib Treatment for ≥5 Years n = 79 |
All Ibrutinib- Treated Patients n = 135 |
|---|---|---|
| Patients with any AE, n (%) | 16 (20) | 31 (23) |
| Median time from first dose reduction to discontinuation, months (range) | NR (8.4–87.7) | 36.1 (0.0–87.7) |
| Outcome of first AE leading to dose reduction, n/N (%) a | ||
| Initial AE resolved | 13/16 (81) | 28/31 (90) |
| No recurrence or recurred at lower grade | 10/16 (63) | 19/31 (61) |
| Recurred at same or higher grade | 6/16 (38) | 12/31 (39) c |
| First dose reduced to, n/N (%) a | ||
| 420 mg to 280 mg | 13/16 (81) | 27/31 (87) |
| 420 mg to 140 mg | 3/16 (19) | 4/31 (13) |
| AEs of interest by SOC, n (%) b | ||
| Infection | 4 (5) | 6 (4) |
| Hematologic | 3 (4) | 5 (4) |
| Dermatologic | 2 (3) | 4 (3) |
| Gastrointestinal | 1 (1) | 4 (3) |
| Cardiac | 1 (1) | 2 (1) |
| Injuries | 1 (1) | 2 (1) |
| Musculoskeletal | 1 (1) | 1 (1) |
| Neoplasms | 1 (1) | 1 (1) |
| Other | 4 (5) | 9 (7) |
| Grade of AE, n (%) b | ||
| Grade 1 | 6 (8) | 11 (8) |
| Grade 2 | 6 (8) | 10 (7) |
| Grade 3 | 5 (6) | 13 (10) |
| Grade 4 | 1 (1) | 2 (1) |
a Denominator is patients with dose reductions because of any AE. b The same patient may be counted in more than one category because of multiple AE events leading to dose reduction. c Of 12 AEs that recurred at same/higher grade at any point during treatment, 3/13 were infections, 2/13 were hematologic, 2/13 were cardiac, 1/13 was gastrointestinal, and 4/13 were other. Abbreviations: AE, adverse event; NR, not reached; SOC, system organ class.
Among patients who were on ibrutinib treatment for ≥5 years, the lowest ibrutinib dose for most patients with dose reductions was 280 mg once daily (10/16 patients). At data cutoff, 3/16 patients were receiving ibrutinib 420 mg once daily, 10/16 were receiving 280 mg once daily, and 3/16 were receiving 140 mg once daily. The median duration of treatment with ibrutinib at a reduced dose was not reached (range: 8.4–84.0+ months) for patients who were on ibrutinib treatment for ≥5 years compared with 36.1 months (range: 0.0–84.0+) in all ibrutinib-treated patients with dose reductions (n = 31).
Following dose reduction, 13/16 patients (81%) had a resolution of the initial AE. Three patients had AEs that were not resolved at data cutoff (grade 3 malignant lung neoplasm, grade 2 fatigue, and grade 1 contusion in 1 patient each). When considering the subset of AEs for which dose reductions are recommended in the updated ibrutinib US prescribing information (as of May 2022), such AEs led to dose reductions in 4/79 patients (5%) (Table 3). Among these patients, AEs did not recur or recurred at a lower grade in 3/4 patients; 1 patient had recurrence at the same grade AE 3 years after initial resolution (grade 3 atrial fibrillation), that resolved without further dose reduction.
Table 3.
AEs leading to dose reductions per USPI recommendations a.
| AEs Leading to Dose Reductions | On Ibrutinib Treatment for ≥5 Years n = 79 |
All Ibrutinib- Treated Patients n = 135 |
|---|---|---|
| Patients with any AE, n (%) | 4 (5) | 11 (8) |
| Median time from first dose reduction to discontinuation, months (range) | 36.1 (8.4–56.0+) | 32.9 (0.0–56.0+) |
| First dose reduced to, n/N (%) b | ||
| 420 mg to 280 mg | 3/4 (75) | 9/11 (82) |
| 420 mg to 140 mg | 1/4 (25) | 1/11 (9) |
| 280 mg to 140 mg | 0/4 (0) | 1/11 (9) |
| Outcome of first AE leading to dose reduction, n/N (%) b | ||
| Initial AE resolved | 3/4 (75) | 10/11 (91) |
| No recurrence or recurred at lower grade | 3/4 (75) | 7/11 (64) |
| Recurred at same or higher grade | 1/4 (25) | 4/11 (36) c |
a AEs for which dose reductions are recommended in the ibrutinib USPI (grade 2 cardiac failure, grade 3 cardiac arrhythmia, grade 3 or 4 nonhematologic AEs [excluding cardiac failure and cardiac arrhythmia], grade 3 or 4 neutropenia with infection or fever, and grade 4 hematologic AEs). b Denominator is patients with dose reductions because of AEs per recommendations in the ibrutinib USPI. c Four patients had AEs that recurred at the same grade (grade 3 atrial fibrillation that recurred after 3 years [n = 1], grade 3 pleural effusion that recurred after 2 years [n = 1], grade 3 diarrhea that recurred after 4 days [n = 1], and grade 3 headache that recurred after 1 week [n = 1]). Abbreviations: AE, adverse event; USPI, US prescribing information.
Patients who were on ibrutinib treatment for <5 years (n = 56) experienced similar rates of AEs leading to dose reduction (15/56; 27%). Most common reasons for dose reduction by system organ class in this subgroup were hematologic (n = 3), cardiac (n = 3), and dermatologic (n = 3). Dose reductions were more common in response to grade 3 AEs (n = 8); however, 100% of AEs (15/15) were initially resolved. Six patients (40%) experienced a recurrence of their AE at the same or higher grade.
AEs led to dose holds of ≥7 days in 45/79 patients (57%) who were on ibrutinib treatment for ≥5 years and in 79/135 patients (59%) in the overall population of all ibrutinib-treated patients (Table 4).
Table 4.
AEs leading to dose holds ≥7 days.
| AEs Leading to Dose Holds ≥7 Days | On Ibrutinib Treatment for ≥5 Years n = 79 |
All Ibrutinib- Treated Patients n = 135 |
|---|---|---|
| Patients with any AE, n (%) | 45 (57) | 79 (59) |
| Number of dose holds per patient, n (%) | ||
| 1 | 20 (25) | 35 (26) |
| ≥ 2 | 25 (32) | 44 (33) |
| Dose restarted after dose hold per patient, n/N (%) a,b | ||
| 420 mg | 42/45 (93) | 65/79 (82) |
| 280 mg | 8/45 (18) | 21/79 (27) |
| 140 mg | 5/45 (11) | 8/79 (10) |
| Other | 0 | 7/79 (9) |
| AEs leading to dose hold ≥7 days by SOC, n (%) a | ||
| Infection | 20 (25) | 28 (21) |
| Neoplasms | 8 (10) | 13 (10) |
| Eye disorders | 7 (9) | 10 (7) |
| Dermatologic | 6 (8) | 12 (9) |
| Gastrointestinal | 5 (6) | 10 (7) |
| Injuries | 5 (6) | 9 (7) |
| Hematologic | 4 (5) | 9 (7) |
| Musculoskeletal | 2 (3) | 4 (3) |
| Cardiac | 1 (1) | 6 (4) |
| Other | 13 (16) | 23 (17) |
| AEs leading to dose hold ≥7 days by grade, n (%) a | ||
| Grade 1 | 6 (8) | 10 (7) |
| Grade 2 | 25 (32) | 38 (28) |
| Grade 3 | 30 (38) | 49 (36) |
| Grade 4 | 5 (6) | 12 (9) |
| AE resolution, n/N (%) b | ||
| AE resolved with dose hold(s) | 43/45 (96) | 75/79 (95) |
| Any AE not resolved | 2/45 (4) | 12/79 (15) |
a The same patient may be counted in more than one category because of multiple AE events leading to dose holds; b Denominator is patients with dose holds ≥7 days because of any AE. Abbreviations: AE, adverse event; SOC, system organ class.
Among patients who were on ibrutinib treatment for ≥5 years, ibrutinib was restarted at 420 mg once daily after dose holds of ≥7 days for most patients (42/45 patients). Following a dose hold of ≥7 days, 43/45 patients (96%) had resolution of the initial AE.
Among patients who were on ibrutinib for ≥5 years, the frequency of AEs leading to dose reductions was highest in years 0–2 and lower in subsequent years, whereas the frequency of AEs leading to dose holds of ≥7 days remained relatively consistent across the first 6 years of treatment (Supplementary Figure S2).
3.6. Exploratory Post Hoc Analysis of Outcomes in Patients with Dose Reductions
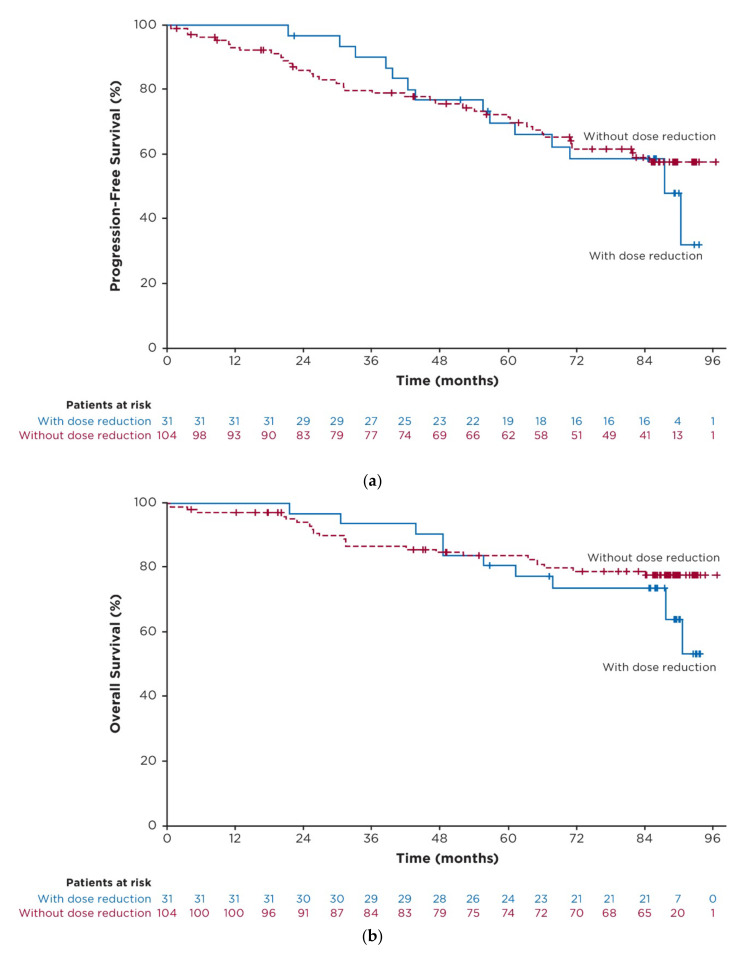
In the overall population of all ibrutinib-treated patients, median PFS for patients who had dose reductions (n = 31) was 87.7 months (95% CI: 56.9–NE) and was not reached (95% CI: 81.9–NE) for those without dose reductions (n = 104) (hazard ratio 0.96 [95% CI: 0.50–1.84]; p = 0.9011; Figure 4a). Estimated 7-year PFS rates were 59% (95% CI: 39–74) and 59% (95% CI: 48–68) for patients with and without dose reductions, respectively. With up to 8 years of follow-up, median OS was not reached in either group (hazard ratio 1.28 [95% CI: 0.58–2.83]; p = 0.5363; Figure 4b); estimated 7-year OS rates were 74% (95% CI: 54–86) and 79% (95% CI: 69–86) in patients with and without dose reductions, respectively.
Figure 4.
Efficacy in patients with and without dose reductions because of AEs in the overall population of ibrutinib-treated patients. (a) Kaplan-Meier curve of investigator-assessed PFS; (b) Kaplan-Meier curve of OS. Abbreviations: AE, adverse event; OS, overall survival; PFS, progression-free survival.
3.7. Concomitant Medications
Concomitant medications of clinical interest in patients who were on ibrutinib treatment for ≥5 years are shown in Supplementary Table S1. Anticoagulants and antiplatelet agents were frequently used during the treatment period (33% and 65%, respectively), as were antihypertensive medications, including agents acting on the renin-angiotensin system (61%), beta-blocking agents (46%), calcium channel blockers (35%), and other antihypertensives (10%). Overall, 67% of patients received medications to treat acid-related disorders, including proton pump inhibitors in 58% of patients.
4. Discussion
Results of the current analysis demonstrate that more than half of patients with previously untreated CLL/SLL were able to receive treatment with single-agent ibrutinib for ≥5 years. While real-world studies have suggested an increased risk of discontinuation of targeted therapies in patients with older age, higher comorbidity burden, higher tumor burden, and/or worse performance status at baseline [13,22,23], no individual baseline characteristics were identified as significant predictors for continuation of long-term ibrutinib treatment in the current study.
Among patients who were on ibrutinib treatment for ≥5 years, responses deepened over time. This subgroup of patients had a higher CR rate over the course of the study (46%) relative to the overall population of ibrutinib-randomized patients (34%) [1], suggesting that patients with favorable responses may be more likely to continue on ibrutinib treatment. In line with this, a higher PFS rate at 7 years was seen in patients who remained on long-term ibrutinib treatment for ≥5 years (82%) relative to the overall ibrutinib-randomized population (59%) [1]. These findings are consistent with those of previous studies, suggesting that continuation of ibrutinib treatment is associated with improved efficacy outcomes [6,7,8].
Safety results in patients who were on ibrutinib treatment for ≥5 years were consistent with those seen in the overall population of ibrutinib-treated patients, including incidences of AEs of clinical interest (hypertension, atrial fibrillation, and major hemorrhage) [1]. AEs generally decreased over time with continued ibrutinib treatment, and no new safety signals emerged in patients who received ibrutinib treatment for ≥5 years. Treatment with ibrutinib was well tolerated irrespective of the frequent use of concomitant antithrombotics, antihypertensives, and acid-reducing agents. Since AEs are the most common reason for discontinuation of ibrutinib in the first-line setting [1,23,24,25,26,27,28], optimization of AE management is crucial to enabling patients to remain on long-term therapy. In the subgroup of patients who were on ibrutinib treatment for ≥5 years, active management of AEs with dose reductions or dose holds was associated with AE resolution in the majority (>80%) of patients. Additionally, dose reductions helped to prevent recurrence or worsening of AEs for most patients, facilitating continued benefit from ibrutinib treatment.
In the current study, disease assessments were performed at regularly scheduled intervals based on iwCLL criteria [20,21]. With up to 8 years of follow-up in the RESONATE-2 study, PFS and OS were similar between patients with and without dose reductions in the overall population of ibrutinib-randomized patients. Patients who had dose reductions received reduced doses of ibrutinib for extended periods of time (median of 3 years in all ibrutinib-treated patients with dose reductions). Together, these results suggest that patients experiencing AEs leading to dose reduction continue to benefit from ibrutinib at the reduced dose. While two real-world studies found significantly worse PFS in patients receiving ibrutinib at reduced doses (<420 mg once daily), this finding from RESONATE-2 is consistent with several other studies that have found no significant difference in efficacy outcomes between patients with dose reductions due to AEs compared to patients without dose reductions [10,12,13,14,15,16,29,30].
5. Conclusions
Regardless of demographic and disease characteristics at baseline, more than half (58%) of the patients randomly assigned to the ibrutinib arm in the RESONATE-2 study continued to benefit from ibrutinib treatment for ≥5 years. With up to 8 years of follow-up, the subset of patients who received ibrutinib treatment for ≥5 years experienced sustained efficacy benefits as evidenced by improved depth of response over time and high PFS rates. For patients who received ibrutinib treatment for ≥5 years, the safety profile was consistent with previous reports of long-term ibrutinib treatment and no new or unexpected AEs were observed. Dose modification (dose reduction or dose hold) was effective in resolving AEs for most patients, likely facilitating continuation of ibrutinib treatment.
Acknowledgments
We thank the patients who participated in the study and their supportive families, as well as the investigators and clinical research staff from the study centers. Editorial support was provided by Melanie Sweetlove, and funded by Pharmacyclics LLC, an AbbVie Company.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers15020507/s1: Figure S1, Adverse events of clinical interest of any grade by yearly interval; Figure S2, AEs leading to dose modifications over time in patients on long-term ibrutinib treatment for ≥5 years; Table S1, Concomitant medications of clinical interest in patients on long-term ibrutinib treatment for ≥5 years.
Author Contributions
Conceptualization, J.A.W. and M.J.; methodology, J.A.W. and M.J.; software, V.G.; validation, V.G., E.H. and M.J.; formal analysis, V.G.; investigation, J.A.W., P.M.B., T.J.K., J.C.B., I.E.A., P.G. and J.A.B.; resources, J.A.W., P.M.B., T.J.K., J.C.B., I.E.A., P.G. and J.A.B.; data curation, V.G.; writing—original draft preparation, J.A.W. and M.J.; writing—review and editing, J.A.W., P.M.B., T.J.K., J.C.B., I.E.A., P.G., V.G., E.H., M.J. and J.A.B.; visualization, J.A.W. and M.J.; supervision, M.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the Institutional Review Boards or Independent Ethics Committees of each participating institution.
Informed Consent Statement
Informed consent was obtained from all patients involved in the study.
Data Availability Statement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Conflicts of Interest
J.A.W.: consulting/advisory role with AbbVie, AstraZeneca, ArQule, BeiGene, Genentech, Janssen, Loxo, Newave, and Pharmacyclics LLC, an AbbVie Company; research funding from AbbVie, Loxo, Karyopharm, MorphoSys, Schrodinger, and Verastem; P.M.B.: consulting/advisory role with AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, MEI Pharma, Merck, MorphoSys, Pharmacyclics LLC, an AbbVie Company, Seattle Genetics, and TG Therapeutics; research funding from AstraZeneca and TG Therapeutics; T.J.K.: consulting/advisory role with AbbVie, Celgene, Genentech-Roche, Gilead, and Pharmacyclics LLC, an AbbVie Company; research funding from AbbVie, Genentech-Roche, Oncternal Therapeutics, and Pharmacyclics LLC, an AbbVie Company; J.C.B.: honoraria from Janssen; consulting/advisory role for BeiGene, AbbVie, MEI, and AstraZeneca; research funding from Oncternal Therapeutics, Velosbio/Merck, and Pharmacyclics LLC, an AbbVie Company; I.E.A.: no conflicts of interest; P.G.: honoraria from and consulting/advisory role with AbbVie, AstraZeneca, ArQule/Merck Sharp & Dohme, Celgene/Juno/Bristol Myers Squibb, Janssen, Loxo/Lilly, MEI Pharma and Roche; research funding from AbbVie, AstraZeneca, Janssen, and Sunesis; V.G.: employment with Everest Clinical Research; other relationship with AbbVie; E.H.: employment with Pharmacyclics LLC, an AbbVie Company; stock or other ownership with AbbVie; M.J.: employment and stock or other ownership with Pharmacyclics LLC, an AbbVie Company (self) and Gilead Sciences (family member); J.A.B.: honoraria from Gilead, Janssen, Novartis, TG Therapeutics, and Pharmacyclics LLC, an AbbVie Company; consulting/advisory role and speakers bureau for BeiGene, Gilead, Janssen, TG Therapeutics, and Pharmacyclics LLC, an AbbVie Company; research funding from AstraZeneca, BeiGene, and Pharmacyclics LLC; an AbbVie Company; travel/accommodations/expenses from Gilead, Janssen, Novartis, TG Therapeutics, and Pharmacyclics LLC; an AbbVie Company. This study was sponsored by Pharmacyclics LLC, an AbbVie Company. The sponsor was involved in study design, data analysis, data interpretation, writing/review of the manuscript, and the decision to publish the results. The sponsor had no role in data collection.
Funding Statement
This research was funded by Pharmacyclics LLC, an AbbVie Company.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Barr P.M., Owen C., Robak T., Tedeschi A., Bairey O., Burger J.A., Hillmen P., Coutre S.E., Dearden C., Grosicki S., et al. Up to 8-year follow-up from RESONATE-2: First-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:3440–3450. doi: 10.1182/bloodadvances.2021006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shanafelt T.D., Wang X.V., Kay N.E., Hanson C.A., O’Brien S., Barrientos J., Jelinek D.F., Braggio E., Leis J.F., Zhang C.C., et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019;381:432–443. doi: 10.1056/NEJMoa1817073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno C., Greil R., Demirkan F., Tedeschi A., Anz B., Larratt L., Simkovic M., Samoilova O., Novak J., Ben-Yehuda D., et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:43–56. doi: 10.1016/S1470-2045(18)30788-5. [DOI] [PubMed] [Google Scholar]
- 4.Woyach J.A., Ruppert A.S., Heerema N.A., Zhao W., Booth A.M., Ding W., Bartlett N.L., Brander D.M., Barr P.M., Rogers K., et al. Long-term results of alliance A041202 show continued advantage of Ibrutinib-based regimens compared with Bendamustine plus Rituximab (BR) chemoimmunotherapy; Proceedings of the 2021 ASH Meeting and Exhibition; Atlanta, GA, USA. 10–14 December 2021. [Google Scholar]
- 5.Burger J.A., Tedeschi A., Barr P.M., Robak T., Owen C., Ghia P., Bairey O., Hillmen P., Bartlett N.L., Li J., et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UK CLL Forum. Follows G.A. UK CLL forum 5 year update on 315 relapsed refractory CLL patients treated with ibrutinib in 66 UK and Ireland centres; Proceedings of the 61st ASH Annual Meeting & Exposition; Orlando, FL, USA,. 7–10 December 2019. [Google Scholar]
- 7.Akhtar O.S., Torka P., Bhat S.A., Hare R., Sait S.N.J., Block A.W., Hernandez-Ilizaliturri F.J. Disease Progression on Ibrutinib Therapy Is Associated with a Poor Clinical Outcome in Chronic Lymphocytic Leukemia (CLL) Patients Managed in Standard Clinical Practice. Blood. 2017;130:5350. [Google Scholar]
- 8.Ysebaert L., Quinquenel A., Bijou F., Ferrant E., Michallet A.S. Overall survival benefit of symptom monitoring in real-world patients with chronic lymphocytic leukaemia treated with ibrutinib: A FiLO group study. Eur. J. Cancer. 2020;135:170–172. doi: 10.1016/j.ejca.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Sharman J.P., Black-Shinn J.L., Clark J., Bitman B. Understanding Ibrutinib Treatment Discontinuation Patterns for Chronic Lymphocytic Leukemia. Blood. 2017;130:4060. doi: 10.1182/blood.V130.Suppl_1.4060.4060. [DOI] [Google Scholar]
- 10.Akhtar O.S., Attwood K., Lund I., Hare R., Hernandez-Ilizaliturri F.J., Torka P. Dose reductions in ibrutinib therapy are not associated with inferior outcomes in patients with chronic lymphocytic leukemia (CLL) Leuk. Lymphoma. 2019;60:1–6. doi: 10.1080/10428194.2018.1554862. [DOI] [PubMed] [Google Scholar]
- 11.Winqvist M., Andersson P.O., Asklid A., Karlsson K., Karlsson C., Lauri B., Lundin J., Mattsson M., Norin S., Sandstedt A., et al. Long-term real-world results of ibrutinib therapy in patients with relapsed or refractory chronic lymphocytic leukemia: 30-month follow up of the Swedish compassionate use cohort. Haematologica. 2019;104:e208–e210. doi: 10.3324/haematol.2018.198820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mato A.R., Timlin C., Ujjani C., Skarbnik A., Howlett C., Banerjee R., Nabhan C., Schuster S.J. Comparable outcomes in chronic lymphocytic leukaemia (CLL) patients treated with reduced-dose ibrutinib: Results from a multi-centre study. Br. J. Haematol. 2018;181:259–261. doi: 10.1111/bjh.14540. [DOI] [PubMed] [Google Scholar]
- 13.Gordon M.J., Churnetski M., Alqahtani H., Rivera X., Kittai A., Amrock S.M., James S., Hoff S., Manda S., Spurgeon S.E., et al. Comorbidities predict inferior outcomes in chronic lymphocytic leukemia treated with ibrutinib. Cancer. 2018;124:3192–3200. doi: 10.1002/cncr.31554. [DOI] [PubMed] [Google Scholar]
- 14.Winqvist M., Asklid A., Andersson P.O., Karlsson K., Karlsson C., Lauri B., Lundin J., Mattsson M., Norin S., Sandstedt A., et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: Data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica. 2016;101:1573–1580. doi: 10.3324/haematol.2016.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhodes J., Barr P.M., Ujjani C.S., Nabhan C., Tam C.S., Jacobs R., Lansigan F., Hill B.T., Brander D.M., Shadman M., et al. The Impact of Front-Line Ibrutinib Dose Reduction and Interruption on Outcomes in Chronic Lymphocytic Leukemia (CLL) Patients. Blood. 2017;130:4313. [Google Scholar]
- 16.Dmitrieva E., Nikitin E., Rimashevskaya E., Ptushkin V. Poor adherence to ibrutinib is not associated with adverse outcome in relapsed refreactory CLL patients; Proceedings of the EHA 2020 Virtual Meeting; Virtual. 19–20 November 2020. [Google Scholar]
- 17.Ahn I.E., Basumallik N., Tian X., Soto S., Wiestner A. Clinically indicated ibrutinib dose interruptions and reductions do not compromise long-term outcomes in CLL. Blood. 2019;133:2452–2455. doi: 10.1182/blood.2019896688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barr P.M., Brown J.R., Hillmen P., O’Brien S., Barrientos J.C., Reddy N.M., Coutre S., Mulligan S.P., Jaeger U., Furman R.R., et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood. 2017;129:2612–2615. doi: 10.1182/blood-2016-12-737346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pharmacyclics LLC . IMBRUVICA® (Ibruztinib) Prescribing Information. Pharmacyclics LLC; Sunnyvale, CA, USA: 2022. [Google Scholar]
- 20.Hallek M., Cheson B.D., Catovsky D., Caligaris-Cappio F., Dighiero G., Döhner H., Hillmen P., Keating M.J., Montserrat E., Rai K.R., et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallek M., Cheson B., Catovsky D., Caligaris-Cappio F., Dighiero G., Dohner H. Response assessment in chronic lymphocytic leukemia treated with novel agents causing an increase of peripheral blood lymphocytes. Blood. 2012;119:5348. [Google Scholar]
- 22.Lu X., Huang Q., Wu L., Emond B., Forbes S.P., Hilts A., Liu S., Lafeuille M.-H., Lefebvre P., Rogers K.A. HSR22-153: Real-World Time to Discontinuation of First-Line Venetoclax + Obinutuzumab in Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. J. Nat. Comp. Cancer Net. 2022;20:HSR22-153. doi: 10.6004/jnccn.2021.7275. [DOI] [Google Scholar]
- 23.Frei C.R., Le H., McHugh D., Ryan K., Jones X., Galley S., Franklin K., Baus C.J., Tavera J., Janania-Martinez M., et al. Outcomes in chronic lymphocytic leukemia patients on novel agents in the US Veterans Health Administration System. Leuk. Lymphoma. 2021;62:1664–1673. doi: 10.1080/10428194.2021.1876863. [DOI] [PubMed] [Google Scholar]
- 24.Mato A.R., Nabhan C., Thompson M.C., Lamanna N., Brander D.M., Hill B., Howlett C., Skarbnik A., Cheson B.D., Zent C., et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: A real-world analysis. Haematologica. 2018;103:874–879. doi: 10.3324/haematol.2017.182907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrisqueta P., Loscertales J., Terol M.J., Ramírez Payer Á., Ortiz M., Pérez I., Cuellar-García C., Fernández de la Mata M., Rodríguez A., Lario A., et al. Real-world characteristics and outcome of patients treated with single-agent ibrutinib for chronic lymphocytic leukemia in spain (IBRORS-LLC Study) Clin. Lymphoma Myeloma Leuk. 2021;21:e985–e999. doi: 10.1016/j.clml.2021.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Mato A.R., Allan J.N., Pagel J.M., Brander D.M., Hill B.T., Cheson B.D., Furman R.R., Lamanna N., Tam C.S., Jacobs R., et al. Front-Line Ibrutinib Therapy for Chronic Lymphocytic Leukemia (CLL) in the Real World: Responses, Toxicity, Outcomes and Subsequent Therapies. Blood. 2017;130:3011. [Google Scholar]
- 27.Hou J.Z., Ryan K., Du S., Fang B., Marks S., Page R., Peng E., Szymanski K., Winters S., Le H. Real-world ibrutinib dose reductions, holds and discontinuations in chronic lymphocytic leukemia. Future Oncol. 2021;17:4959–4969. doi: 10.2217/fon-2021-0964. [DOI] [PubMed] [Google Scholar]
- 28.Jain P., Thompson P.A., Keating M., Estrov Z., Ferrajoli A., Jain N., Kantarjian H., Burger J.A., O’Brien S., Wierda W.G. Long-term outcomes for patients with chronic lymphocytic leukemia who discontinue ibrutinib. Cancer. 2017;123:2268–2273. doi: 10.1002/cncr.30596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.UK CLL Forum Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: A UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101:1563–1572. doi: 10.3324/haematol.2016.147900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams A.M., Baran A.M., Casulo C., Reagan P., Friedberg J.W., Helber M., Moore J., Baloga E., Zent C.S., Barr P.M. Ibrutinib Dose Adherence and Therapeutic Efficacy in Non-Hodgkin Lymphoma: A Single-Center Experience. Clin. Lymphoma Myeloma Leuk. 2019;19:41–47. doi: 10.1016/j.clml.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.