Abstract
Cell-mediated immune responses are crucial in the protection against tuberculosis. In this study, we constructed DNA vaccines encoding cytotoxic T lymphocytes (CTL) and T helper cell (Th) epitopes of the 38-kDa lipoglycoprotein of Mycobacterium tuberculosis and analyzed and compared their immunogenicities with that of pXJ38, a DNA vaccine encoding the entire 38-kDa protein (X. Zhu, N. Venkataprasad, H. S. Thangaraj, M. Hill, M. Singh, J. Ivanyi, and H. M. Vordermeier, J. Immunol. 158:5921–5926, 1997). Plasmid DNAs encoding a CTL epitope, P3 (pP3), a Th epitope (vTh), or both the Th and the P3 epitopes (pThP3) were prepared and tested in C57BL6/J (H-2b) mice. Our results confirmed that DNA immunization with pXJ38 induces strong CD8+ CTL and Th1 responses (high gamma interferon [IFN-γ], low interleukin-4 [IL-4]). Coadministration of plasmid DNAs encoding a Th epitope with those encoding a CTL epitope (vTh+pP3) elicited both antigen-specific CD8+ CTL and Th1 responses. High levels of IFN-γ were secreted by spleen cells from all plasmid DNA-vaccinated mice after in vitro stimulation with the recombinant 38-kDa protein. Small or undetectable amounts of IL-4 were observed, which indicates the induction of a Th1-like response. Multiple-epitope vaccination by vTh+pP3 or pThP3 resulted in a broader Th1 response to peptide or epitopes than the single-epitope plasmid DNAs. Antigen-specific immunoglobulin G2a was only detected in sera from mice immunized with the plasmid pXJ38, and not in mice immunized with the epitope-based DNA vaccines. Thus, the absence of an antibody response after immunization with epitope plasmid DNAs and their ability to trigger only a specific cellular immune response may prove to be important advantages for a vaccine against tuberculosis.
Tuberculosis continues to affect about 30% of the world's population, particularly in developing countries, despite the existence of chemotherapeutic drugs and the widespread use of the Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine. Effective chemotherapeutic treatment takes a long time, is expensive, and is not available to people in the parts of the world where it is most needed. The situation is further complicated by the appearance of multidrug-resistant strains. BCG vaccination efficacy is controversial, and it seems to fail to protect adults against pulmonary tuberculosis (2, 3). These situations justify the need to develop better methods of tuberculosis prevention and therapy.
DNA technology has been used successfully in the vaccination of animal models against infection with viruses, bacteria, and parasites as well as in antitumor therapy and treatment of autoimmunity and allergies (34). Mycobacterium tuberculosis, the causative agent of tuberculosis, has received particular attention in this respect, and several of its proteins have been evaluated as DNA vaccines in experimental models. These include the 65-kDa heat shock protein (hsp65); the 36-kDa proline-rich antigens (32) Ag85A (13), Ag85B, and Ag85C (23); the 38-kDa protein (39); hsp70 and the 36- and 6-kDa proteins (21); MPT64 (23-kDa protein), Ag85B (30-kDa protein), and ESAT-6 (6-kDa protein) (14); and the phosphate-binding proteins PstS-1, PstS-2, and PstS-3 (31). CD4+ T-cell responses characterized by gamma interferon (IFN-γ) secretion, CD8+ cytotoxic T-lymphocyte (CTL) responses, and antibody secretion could generally be induced in these studies, and most reported protection after challenge with virulent M. tuberculosis or M. bovis BCG (13). This protection, however, was similar to or lower than that obtained with the BCG vaccine. Recently, DNA vaccination with hsp65 was used for tuberculosis therapy in mice and showed promising results for the elimination of persistent infection (22).
Epitope-based immunization has been shown to be protective in diverse models because of the induction-specific CTL responses it generates (15, 24, 28). The advantages of epitope immunization, compared to protein or organismal immunization, are that an immune response is elicited only against the protective epitope (avoidance of epitope drift in the case of viral infections) and that the required type of immune response is triggered (humoral versus cellular immunity). Examples of unwanted responses include the induction of antibodies in human immunodeficiency virus (20) or tuberculosis, which can promote infection in some cases (11). In addition, experiments with mice with the DNA vaccine encoding the 19-kDa lipoprotein of M. tuberculosis showed the induction of a nonprotective antibody-mediated immune response, rather than a T-cell response (8). Synthetic peptide vaccination has the disadvantage of inducing weak immune responses; it is generally difficult to elicit strong CTL responses, despite the use of all types of adjuvants. DNA vaccines encoding single or multiple epitopes can circumvent these disadvantages and have been shown to induce efficient cellular immunity in different models of viruses and tumors (5, 12, 33).
In order to evaluate the efficacy of epitope-based DNA vaccines against tuberculosis, we prepared DNA vaccines based on CTL (7) and Th cell (36) epitopes of the 38-kDa lipoglycoprotein of Mycobacterium tuberculosis and analyzed and compared their immunogenicities with that of the already described DNA vaccine pXJ38, which encodes the entire 38-kDa protein (39). We showed that the coadministration of plasmid DNAs encoding either a Th or CTL epitope (P3) induced antigen-specific CD8+ CTL and Th1 responses, which might play a major role in protection against tuberculosis. Moreover, these epitope-based DNA vaccines were unable to induce an antigen-specific humoral response. Antibodies against M. tuberculosis may be detrimental for protection against tuberculosis; therefore, epitope-based DNA vaccines may have an important advantage over other protein-based DNA vaccines for tuberculosis.
MATERIALS AND METHODS
Mice.
Inbred C57BL/6 (H-2b) female mice were purchased from the Central Animal Laboratory of Utrecht University and used when 8 to 12 weeks old. The Ethics Committee on Animal Experimentation of Utrecht University approved these experiments.
Antigens and synthetic peptides.
The 38-kDa recombinant protein was purified to homogeneity as described previously (29). Tuberculin purified protein derivate (PPD) was obtained from Statens Serum Institute (Copenhagen Denmark). Peptides derived from the sequence of the 38-kDa protein were synthesized by R. van der Zee at the Peptide Center of the Research School of Infection and Immunity (Veterinary Faculty, Utrecht) or by D. L. Jue at the Core Facility of the Centers for Disease Control and Prevention (Atlanta, Ga.). The peptide amino acid sequences were as follows: P1 (amino acids [aa] 97 to 108), AGTVNIGASDAY(H-2Db); P3 (aa 166 to 175), IAALNPGVNL (H-2Db); P4 (aa 309 to 318), YPIINYEYAI(H-2Db); and Th (aa 70 to 84), PAFHERYPNVTITAQ (potential H-2Db motif, but anchor residues N and I not in the ideal position).
Genetic constructs.
pXJ38, a plasmid in which the gene coding the 38-kDa protein of M. tuberculosis was cloned into the expression vector pcDNA3, was a gift from X. Zhu and H. M. Vordermeier (VLA-Weybridge, TB Research Group, Surrey, United Kingdom) (39).
Two vectors were used for constructing the plasmids containing the various epitopes: pcDNA3.1+ and VR1012. Both vectors consist of a pUC18 backbone with the same cytomegalovirus (CMV) promoter. They differ in the kanamycin versus the ampicillin selection markers and in the polyadenylation site. In vivo and in vitro experiments revealed no differences between the two vectors in CTL induction, cytokine production (IFN-γ), or B-cell activation (polyclonal immunoglobulin M [IgM] production) in mouse spleen cells.
Three plasmids based on Th and CTL epitopes of the 38-kDa protein of M. tuberculosis were constructed. The nucleotide sequence corresponding to the epitopes was generated by using two overlapping oligonucleotides that served as both a primer and a template. All of the forward primers included a restriction site; a Kozak sequence (GCCGCCGCC), which enhances protein expression (18); the ATG start codon; and a part of the nucleotide sequence of the epitope. All of the reverse primers included a part of the nucleotide sequence of the epitope, the TAG stop codon, and a restriction site. Primers for the construction of pP3, encoding the previously described P3 CTL epitope (aa 166 to 175) (7), were as follows (nucleotide sequence corresponding to the epitope in boldface): sense, ATCCGGATCCGCCGCCGCCATGATCGCTGCGTCAACCCC; antisense, GGATCTCGAGCTACAGGTTCACGCCGGGGTTGAGCG. This construct was inserted into BamHI and XhoI sites of the eukaryotic expression vector pcDNA3.1+ (Invitrogen, San Diego, Calif.) downstream of the CMV promoter. The primers for the construction of vTh, encoding part of the previously described Th epitope (aa 71 to 81) (36), were as follows: sense, ATCCGTCGACGCCGCCGCCATGGCCTTTCACGAGAGG; antisense, GGATGGATCCCTAGATCGTGACGTTCGGATACCTCTCGTGA. This construct was inserted into SalI and BamHI sites of the eukaryotic expression vector VR1012 (Vical, Inc., San Diego, Calif.) downstream of the CMV promoter.
For the construction of pThP3, which contained the nucleotide sequences corresponding to the Th and CTL epitopes, the inserts encoding both epitopes were generated separately and then ligated into pcDNA3.1+. The Th insert was generated with the following primers: sense, ATCCGGATCCGCCGCCGCCATGGCCTTTCACGAGAG; antisense, GGATGAATTCGATCGTGACGTTCGGATACCTCTCG. The primers for the P3 insert were as follows: sense, ATCCGAATTCATCGCTGCGCTCAACCCCG; antisense, GGATCTCGAGCTACAGGTTCACGCCGGGGT. Th was inserted into BamHI and EcoRI sites of the pcDNA3.1+ vector, while P3 was inserted between EcoRI and XhoI sites, downstream of the CMV promoter. All plasmids were cloned into Escherichia coli DH5α, and the positive colonies were selected by PCR or restriction enzyme analysis. The nucleotide sequences of the inserts in all plasmids were confirmed by sequencing with the ABI Prism 377 (Perkin-Elmer, Norwalk, Conn.). The pcDNA3.1+ and VR1012 vectors, which do not encode any of the inserts, were used as controls (control vectors). Plasmid DNA was amplified in E. coli DH5α, purified with the Qiagen plasmid purification kit (Qiagen, Inc., Chatsworth, Calif.), redissolved in phosphate-buffered saline (PBS), and maintained at −20°C until use.
Cell lines and culture medium.
EL-4 (H-2b), a thymoma cell line, was a gift from P. van Koten (Department of Immunology, Veterinary Faculty, Utrecht University, The Netherlands); and EX-38 (H-2b), an EL-4 cell line transfected with the gene encoding an M. tuberculosis 38-kDa protein, was a gift from X. Zhu and H. M. Vordermeier (VLA-Weybridge, TB Research Group, Surrey, United Kingdom) (39). All cells were grown in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% heat-inactivated fetal calf serum, 10 μg of gentamicin per ml, 2 mM glutamine, 1.7 g of NaHCO3, and 50 μM β-mercaptoethanol (IMDM-complete). The transfected cell line, EX-38, was grown in IMDM-complete with G418 at a concentration of 1 mg/ml.
Immunization procedures.
Mice were injected three times biweekly with 100 μg of the plasmid DNA, pXJ38, vTh, pP3, pThP3, or a mixture of vTh and pP3 (vTh+pP3) (50 μg of vTh plus 50 μg of pP3) or with control DNA vectors (PcDNA3.1+ or VR1012) in the quadriceps muscle. Blood samples were collected from the retroorbital plexus, and spleens were isolated 2 weeks after the last injection.
In vitro expansion of antigen-specific CTL.
After spleen isolation, cell suspensions were prepared as described previously (38), and the erythrocytes were lysed by hypotonic shock and washed twice with IMDM. Spleen cells (30 × 106) from in vivo-primed mice were cocultured with mitomycin (Sigma Chemical Co., St. Louis, Mo.)-treated EX-38 cells (1.5 × 106) in a 10-ml cell suspension in IMDM-complete for 5 to 6 days at 37°C in 5% CO2. Ten to 30 U of recombinant interleukin-2 (IL-2) per ml (Roche Diagnostics, Mannheim, Germany) was added after 48 h of culture.
51Cr release assay.
Cells were collected from the in vitro culture and washed once with IMDM-complete. Viable cytotoxic effector cells were separated from dead cells by centrifugation with a Lympholyte-M density gradient (820 × g for 10 min), washed twice, and resuspended in RPMI 1640 supplemented with 5% fetal calf serum (CTL medium) at a concentration of 4 × 106 cells/ml. EX-38 or EL-4 target cells (2 × 106) were incubated for 1 h at 37°C with 100 μCi of 51Cr (Amersham, Life Science, United Kingdom). After being washed three times, 5 × 103 target cells/100 μl were added to 100 μl of various numbers of effector cells that had been plated in 96-well U-bottom plates. The plates were then centrifuged for 1 to 2 min at 200 × g and incubated for 4 to 6 h at 37°C in 5% CO2. After incubation, 100 μl of supernatant was collected and counted in a gamma counter. The specific lysis (percentage of cytotoxicity) was calculated with the equation % Lysis = [(sample − spontaneous release)/(total release − spontaneous release)] × 100%.
In the antibody-blocking experiments, either anti-mouse CD4 monoclonal antibody (MAb) (clone GK1.5) or anti-mouse CD8 MAb (clone 53–6.7) (Pharmingen, San Diego, Calif.) was added (5 μg/well) to the effector cells 30 min before the 51Cr-labeled EX-38 cells were added (39).
In vitro cytokine production and cytokine determination by ELISA.
Pools of spleen cell suspensions (5 × 106 cells) from groups of mice immunized with DNA constructs (PxJ38, ThP3, Th, and Th+P3 or plasmid alone) were cultured in IMDM-complete in 24-well plates in the presence of 20 μg of PPD per ml, 20 μg of recombinant 38-kDa protein per ml, or 50 μg of 38-kDa peptides (P1, P3, P4, and Th) per ml at 37°C in 5% CO2. Supernates were harvested after 72 h, aliquoted, and stored at −20°C until assayed for specific cytokines. IL-4 and IFN-γ contents were measured with a commercial enzyme-linked immunosorbent assay (ELISA) kit (Genzyme Co, Cambridge, Mass.) according to the manufacturer's protocol. Results are expressed as mean cytokine level of duplicate wells after subtracting the spontaneous release of the cytokine by unstimulated cells.
Detection of IgG1 and IgG2a by ELISA.
Recombinant 38-kDa protein was dissolved in 0.1 M carbonate-bicarbonate buffer (pH 9.6), while peptide P3 or Th was dissolved in PBS at a concentration of 10 μg/ml. Both were then dispensed into 96-well polyvinyl microtiter plates (100 μl/well) (MaxiSorp F96; Nunc, Roskilde, Denmark). Plates coated with the 38-kDa protein were incubated for 1 h at 37°C, followed by overnight incubation at 4°C; plates coated with peptide P3 or Th were incubated overnight at 37°C. Free binding sites were blocked with 3% gelatin (Merck) in PBS for 1 h at 37°C. Serial dilutions of sera were prepared in PBS–0.05% Tween 20 1% (wt/vol) milk (Protifar), dispensed into ELISA plates, and incubated for 2 h at 37°C. After incubation, the plates were washed three times with PBS–0.05% Tween 20. Then, 50 μl of either biotin-conjugated mouse anti-mouse IgG2a (Igh-1b) or biotin-conjugated mouse anti-mouse IgG1 (Igh-4b) (Pharmingen) in PBS–0.05% Tween 20 3% (wt/vol) milk (Protifar) was added. After a 1-h incubation at 37°C, the plates were washed again three times and incubated with 50 μl of horseradish peroxidase-conjugated streptavidin for 15 min at 37°C. After a final washing, 50 μl of substrate (0.1 M NaAc–tetramethyl benzidine–30% H2O2 at 10,000:400:1 [vol/vol/vol]) was added. The reaction was stopped after 20 to 30 min with 100 μl of 1 M H2SO4, and the plates were read in a Titertek Multiskan MCC/340 at 450 nm. The titers were defined as the reciprocal dilution that gave an optical density of 0.5 at 450 nm.
Statistical analysis.
Differences between the ability of the plasmid DNA vaccines to induce CTL were analyzed with Student's t test. P < 0.05 was considered to be significant.
RESULTS
CTL induction by DNA vaccination.
C57BL/6 mice were injected intramuscularly three times biweekly with plasmid DNA encoding either the P3 CTL epitope (pP3), the Th epitope (vTh), both the Th and the CTL epitopes (pThP3), a mixture of vTh and pP3 (vTh+pP3), the positive control (pXJ38), or a control vector. Mice were tested 2 weeks after the last injection. Cytotoxic T-cell responses were determined with a classical 51Cr release assay, after in vitro restimulation, against the target cell line EX-38, which expresses the 38-kDa protein. EL-4 was used as a negative control. DNA immunization with pXJ38 resulted in strong CTL responses (specific lysis at a target-to-effector [T:E] ratio of 1:80; 50% ± 12%), which were significantly above the background level. Specific CTL induction was also observed after immunization with the vTh+pP3 mixture, which was significantly higher than the background level (T:E ratio of 1:80, P < 0.025; T:E ratio of 1:40, P < 0.05) (Table 1). Neither the plasmids encoding the single CTL epitope pP3 nor the plasmids with the insert corresponding to the Th and CTL epitope (pThP3) induced any specific CTL above the levels induced by the control vector. The lysis observed with EL-4 was below 4%.
TABLE 1.
CTL responses after immunization with plasmid DNA
| Immunogen | % Specific lysis at T:E ratioa
|
||
|---|---|---|---|
| 1:80 | 1:40 | 1:20 | |
| pXJ38 | 50 ± 12*** | 44 ± 15*** | 36 ± 16*** |
| Control vector | 15 ± 8 | 11 ± 6 | 6 ± 3 |
| pP3 | 12 ± 2 | 8 ± 1 | 7 ± 4 |
| Control vector | 18 ± 11 | 13 ± 9 | 7 ± 4 |
| vTh | 18 ± 6 | 12 ± 4 | 9 ± 3 |
| Control vector | 14 ± 5 | 10 ± 5 | 6 ± 3 |
| vTh+pP3 | 24 ± 10** | 16 ± 8* | 9 ± 5 |
| Control vector | 12 ± 5 | 7 ± 4 | 5 ± 3 |
| pThP3 | 12 ± 6 | 10 ± 3 | 6 ± 4 |
| Control vector | 12 ± 6 | 7 ± 4 | 5 ± 3 |
CTL responses (percentage of specific lysis) measured after in vitro restimulation of the in vivo-primed spleen cells with mitomycin-treated EX-38 cells are shown. The effector cells (E) were incubated with EX-38 target cells (T) in a classical 51C release assay. Results are presented as means ± standard deviations of three to eight independent experiments. Lysis of EL-4 cells (T) as the negative control was <4%. Comparisons were statistically significant compared to control vector in the same experiments at P < 0.0005 (***), P < 0.025 (**), P < 0.05 (*) according to Student's t-test.
DNA vaccines induce CD8+ cytotoxic T cells.
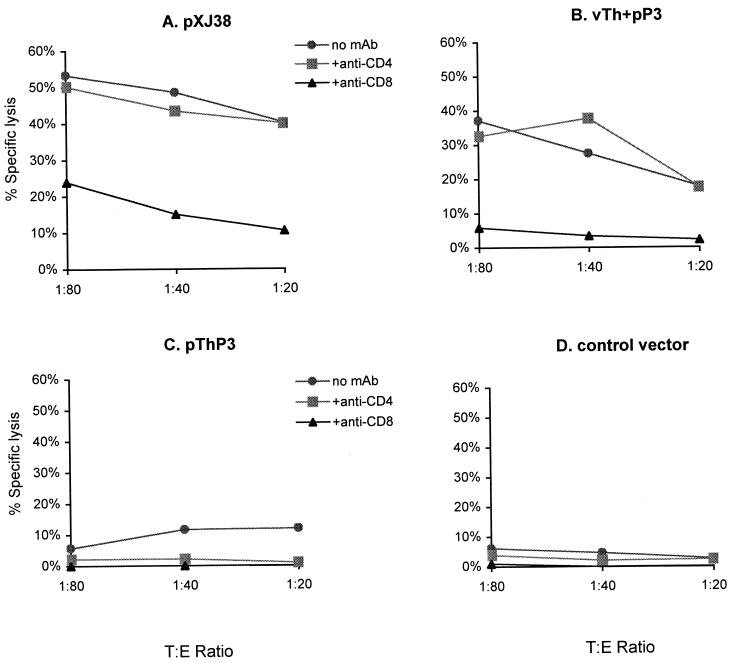
In order to determine which effector T-cell population was primed by vaccination with plasmid DNAs, blocking experiments with anti-CD8 and anti-CD4 MAb were performed. Figure 1A and B show that the specific lysis of EX-38 cells was only blocked by the anti-CD8 MAb, not by the anti-CD4 MAb, demonstrating that the effector-specific CTL induced by pXJ38 and vTh+pP3 were CD8+ T cells.
FIG. 1.
Induction of CD8+ CTL following immunization with various plasmid DNAs. (A) pXJ38. (B) vTh+pP3. (C) pThP3. (D) Control vector. The percentage of specific lysis was measured after in vitro restimulation of the in vivo-primed cells with mitomycin-treated EX-38 cells. Effector cells (E) were incubated with EX-38 cells (target cells [T]) in a classical 51Cr release assay. C57BL/6 mice were immunized intramuscularly three times biweekly with 100 μg of plasmid DNA. Spleens were isolated from groups of two mice 2 weeks after the last injection, and spleen cells were pooled. CTL responses were blocked with anti-CD4 or anti-CD8 MAb. Results are representative of four independent experiments.
Th1 type of immune responses following DNA vaccination.
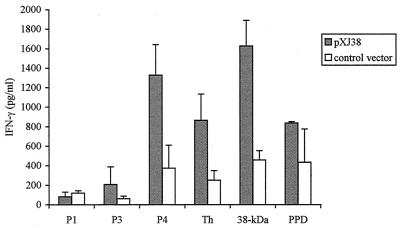
To investigate the cytokine profile induced by the different plasmid DNAs, we measured the amount of antigen-specific IL-4 and IFN-γ produced by spleen cells from plasmid DNA-immunized mice. The spleens were collected 2 weeks after the last injection; spleen cells were restimulated in vitro with the peptides derived from the 38-kDa protein, the recombinant 38-kDa protein, or PPD. High levels of IFN-γ were secreted by spleen cells from all plasmid DNA-vaccinated mice after in vitro stimulation with the recombinant 38-kDa protein (Fig. 2 and Table 2). IL-4 production was low or not even detectable for all of the plasmid DNAs tested (<10 pg/ml) (data not shown). The cells from pXJ38-vaccinated mice recognized peptides Th, P4, and, to a lesser extent, P3, but not peptide P1 (Fig. 2). The multiple-epitope vaccination with either vTh+pP3 or pThP3 resulted in a broader and stronger induction of IFN-γ upon in vitro stimulation with antigens than the single-epitope plasmid DNA vTh or pP3 (Table 2). Surprisingly, the vTh+pP3 and pThP3 DNA vaccines also primed cells that produced IFN-γ upon stimulation with P4 peptide. PPD only stimulated IFN-γ production in spleen cells from mice that were immunized with plasmid DNAs encoding more than one epitope (pXJ38, vTh+P3, and pThP3). In general, none of the construct-primed cells produced IFN-γ upon stimulation with peptide P1 or P3.
FIG. 2.
IFN-γ secreted by pXJ38-primed cells. Amounts of IFN-γ were measured in supernatants from spleen cell cultures of mice vaccinated with pXJ38 or control vector and restimulated in vitro with 50 μg of the peptide P1, P3, or P4 per ml; with 20 μg of the recombinant 38-kDa protein per ml; or with 20 μg of PPD per ml. C57BL/6 mice were immunized intramuscularly three times biweekly with 100 μg of plasmid DNA. Spleens were isolated from groups of two mice 2 weeks after the last injection, and spleen cells were pooled. Cytokine production by the unstimulated cells was subtracted from that by the stimulated cells in each experiment. Results are presented as means ± standard errors of two to five independent experiments.
TABLE 2.
Specific IFN-γ secretion after vaccination with plasmid DNA
| Immunogen | Antigen for in vitro stimulationa
|
|||||
|---|---|---|---|---|---|---|
| P1 | P3 | P4 | Th | 38-kDa | PPD | |
| pXJ38 | − | + | +++ | +++ | ++++ | +++ |
| pP3 | − | − | − | + | ++++ | − |
| vTh | − | − | − | +/− | ++ | − |
| vTh+pP3 | + | +/− | +++ | − | ++++ | +++ |
| pThP3 | − | − | ++ | − | +++ | ++ |
Specific IFN-γ secretion was measured as IFN-γ of spleen cells of mice immunized with immunogen −IFN-γ secretion of spleen cells of mice immunized with control vector. Five million spleen cells from DNA-immunized mice were incubated with the indicated antigens for 72 h. −, <50 pg/ml +/−, 50 to 100 pg/ml; +, 100 to 250 pg/ml; ++, 250 to 500 pg/ml; +++, 500 to 1,000 pg/ml; ++++, 1,000 to 1,500 pg/ml.
T-cell epitope-based DNA vaccines do not elicit anti-38-kDa antibodies.
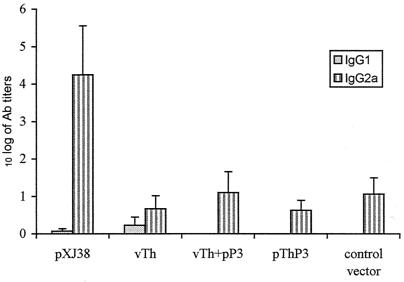
To investigate the antigen-specific humoral response after immunization with the different plasmid DNAs, we measured anti-38-kDa antibodies in the sera of mice vaccinated with the plasmid pXJ38, vTh, pThP3, or vTh+pP3 or with a control vector. The IgG1 and IgG2a titers were determined 2 weeks after the last DNA injection. High titers of specific IgG2a were only detected in the sera from mice immunized with the plasmid pXJ38; IgG2a antibody levels in the sera of all the other DNA-immunized mice were not significantly different from those detected in the sera of mice injected with a control vector (Fig. 3). The IgG1 titers in the sera of all tested mice were either very low (pXJ38 and vTh) or undetectable. Sera of the DNA-vaccinated mice were also tested in a peptide-based ELISA to examine the presence of anti-Th or anti-P3 antibodies; neither IgG1 nor IgG2a was detected (data not shown).
FIG. 3.
Specific 38-kDa protein antibodies following immunization with various plasmid DNAs. The sera of mice were tested in an ELISA for 38-kDa protein-specific IgG1 and IgG2a antibodies 2 weeks after the last (third) immunization. The results are expressed as log10 antibody titers (mean ± standard error; n = 4). The serum antibody titers of nonimmunized control mice were subtracted from those of the test groups. Results are representative of at least two different experiments.
DISCUSSION
DNA vaccines encoding various proteins of Mycobacterium tuberculosis have been tested successfully in animal models (13, 14, 21, 22, 32, 39). So far, however, the protection achieved with those vaccines has been similar to or lower than that elicited by BCG vaccination. The future success of DNA vaccines for tuberculosis might reside in the fact that they are effective inducers of cellular immune responses mediated by the induction of IFN-γ-secreting cells and CD8+ CTL. These types of responses have been shown to be crucial for protection against tuberculosis in both humans (25, 26, 30, 37) and animals (6, 9, 10).
DNA vaccines encoding single or multiple epitopes have been shown to induce cellular immunity in different models of viruses and tumors (5, 12, 33). Thus, the use of multiple-epitope DNA vaccines may be a suitable approach to improve the current DNA vaccines for various diseases. Since this approach has not yet been evaluated for tuberculosis, we constructed DNA vaccines based on cytotoxic and Th epitopes of the 38-kDa lipoglycoprotein of M. tuberculosis and analyzed and compared their immunogenicity with that of pXJ38, a DNA vaccine encoding the entire 38-kDa protein (39).
The ability of epitope-based DNA vaccines to induce CTL was studied with the cell line EX-38, which expresses the entire 38-kDa protein. The vTh+pP3 combination was able to induce antigen-specific CTL, demonstrating that vaccination with vTh+pP3 primes CD8+ CTL, which can recognize the naturally processed 38-kDa protein. The percentage of lysis induced by the vTh+pP3 combination was half that observed with the whole-protein DNA vaccine (Table 1). However, since the 38-kDa protein has more CTL epitopes and the target cell line used expresses the entire 38-kDa protein, it is not surprising that pXJ38 induced higher levels of CTL.
It is noteworthy that only vTh+pP3 efficiently primed CD8+ CTL. One plausible explanation for this is that only with this combination of plasmid DNAs does good cooperation between Th and CTL functions exist. Possibly, the Th and CTL epitopes, generated after vTh+pP3 injection, are efficiently presented by the same antigen-presenting cell (APC) in the same compartment of the immune system or by the same APC, a situation that is known to lead to an effective CTL priming (1). The failure of the pThP3 construct to induce CTL might be a consequence of the inadequate presentation of the epitopes, since both epitopes are now expressed within one peptide, and this peptide might be processed differently by the APC. Furthermore, CTL effector cells can act alone when lysing target cells, while their differentiation from naive CD8+ T cells is dependent on CD4+ helper cells (1, 4, 16). IFN-γ and IL-12 are known to be important in promoting a Th1 response and enhancing cytotoxicity during the priming and maturation of lymphocytes (35). In contrast to the other epitope vaccines used in this study, the coadministration of vTh and pP3 plasmid DNAs (vTh+pP3) contributed more nonmethylated CpG motifs (20% more), which are known to induce Th1 cytokines such as IFN-γ and IL-12 (17, 27). The failure of pP3 and pThP3 to induce CD8+ CTL can be partially explained by the inadequate antigen presentation by APC or the wrong cytokine milieu and, consequently, lack of priming of CTLs. However, other factors, like the deficient transcription and expression of the epitopes after injection (which needs further investigation), cannot be excluded. The inclusion of reporter genes in plasmid DNAs may reveal whether minigenes in the plasmids are being transcripted and the corresponding peptides expressed.
After in vitro stimulation with the recombinant 38-kDa protein, spleen cells from mice vaccinated with either pXJ38, pP3, vTh, pThP3, or vTh+pP3 secreted high levels of IFN-γ (Table 2). Small amounts or no IL-4 was detected. This suggests the induction of a Th1 type of response by all DNA plasmids tested. These results also demonstrated that epitope-based DNA vaccines primed cells that recognize the epitopes (peptides) generated from the entire 38-kDa protein after natural processing. In addition, pXJ38-, vTh+P3-, and pThP3-primed cells were also stimulated by culture filtrates of M. tuberculosis (PPD) (Table 2).
The epitope specificity of IFN-γ-producing cells generated by DNA-plasmid vaccination was also analyzed in more detail (Table 2). The Th peptide and the CTL peptides (H-2Db) P4 and, to a lesser extent, P3 triggered IFN-γ secretion from cells primed with pXJ38. These results are in agreement with those of Zhu et al. (39), except for peptide P4, which was not studied. Zhu and colleagues also showed that the peptide (aa 65 to 83) that contains our Th peptide (aa 70 to 84) was recognized not only after DNA immunization with pXJ38, but also after natural infection with M. tuberculosis (39). This observation indicates that our peptide (aa 70 to 84) is a good protective epitope to include in a future DNA vaccine. The unexpected observation that P4 stimulated IFN-γ secretion from cells primed with pThP3 and vTh+pP3 plasmids can be explained by cross-reactivity, probably due to similarities between the P4 and Th and P3 peptides (i.e., YP, AI, and AL sequences [see sequences in Materials and Methods]). These sequences are close to or include the binding sites of the peptide to major histocompatibility complex (MHC) class I molecules, which results in CTL induction. Thus, when P4 is processed and presented to MHC molecules, the P4 might be presented in a very similar configuration to the peptides Th and P3, which will permit recognition by Th- and or P3-specific T cells. These T cells are probably CTLs producing IFN-γ. However, the processing and presentation of P4 can also occur via MHC class II, due to those similarities, with subsequent activation of CD4+, which can produce the IFN-γ. In conclusion, P4 seems to behave like a promiscuous epitope, being recognized in the context of multiple MHC alleles.
The multiple-epitope DNA plasmids gave stronger and broader Th1 responses than the single-epitope DNA vaccines and were comparable to the ones induced by the plasmid encoding the entire protein (Table 2). Slight differences were also observed between pThP3 and vTh+pP3, the latter being more efficient in inducing cells that secrete more IFN-γ and recognize additional peptide epitopes. The superior ability of multiple-epitope plasmids to induce IFN-γ-secreting cells might be related to their higher nonmethylated CpG content, which is known to induce IFN-γ and IL-12 (17, 27). These cytokines are required for the efficient induction of a Th1 type of immune response. Improvement of the cellular immune response and protection by the coadministration of different plasmids have also been achieved by other researchers. Lee et al. (19), for example, observed an increase in antigen-specific CTL induction, T-cell proliferation, and specific antibody secretion after coadministration of a plasmid encoding the hemagglutinin of influenza A virus with the empty vector DNA.
Investigation of the antigen-specific humoral response after immunization with different plasmid DNAs did not indicate any increase in the anti-38-kDa antibodies in the sera of mice vaccinated with plasmid vTh, pThP3, or vTh+pP3 (compared with control vector). Such antibodies were only detected in the sera of mice vaccinated with the plasmid encoding the entire 38-kDa protein, pXJ38. These results are in agreement with those obtained by other researchers with DNA vaccines encoding entire protein antigens from M. tuberculosis (39).
In conclusion, we were able to induce strong antigen-specific CD8+ CTL, Th1 responses, and IgG2a antibody after immunization with pXJ38, which confirmed previous results by Zhu et al. (39). We also found that the coadministration of vTh and pP3 resulted in the induction of antigen-specific CD8+ CTL and Th1 responses. Both can play a key role in protection against M. tuberculosis. Moreover, the absence of an antigen-specific antibody response after immunization with the epitope-based DNA vaccines provides important advantages compared with whole-protein-based DNA vaccines for tuberculosis. Thus, the development of epitope-based DNA vaccines should be considered when designing prophylactic and therapeutic vaccines to use against M. tuberculosis infection in humans.
ACKNOWLEDGMENTS
This work was supported by grant PRAXIS XXI BD/2698/94 from Fundação para a Ciência e a Tecnologia, Portugal.
We thank Hans M. Vordermeier and X. Zhu (Veterinary Laboratories Agency, Surrey, United Kingdom) for providing the EX-38 cell line and the pXJ38 plasmid, Danny L. Jue (Centers for Disease Control and Prevention, Atlanta, Ga.) and Ruurd van der Zee (Institute of Infectious Diseases and Immunology, Faculty of Veterinary Medicine, Utrecht, The Netherlands) for synthesizing the peptides, and Theo Harmsen for technical assistance.
REFERENCES
- 1.Bennett S R, Carbone F R, Karamalis F, Miller J F A P, Heath W R. Induction of CD8 cytotoxic T lymphocyte response by cross-priming requires cognate CD4 help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom R B, Murray C J L. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 3.Bloom B R, Mckinney J D. The death and resurrection of tuberculosis. Nat Med. 1999;5:872–874. doi: 10.1038/11309. [DOI] [PubMed] [Google Scholar]
- 4.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciernik I F, Berzofsky J A, Carbone D P. Induction of cytotoxic T lymphocytes and antitumor immunity with DNA vaccines expressing single T cell epitopes. J Immunol. 1996;156:2369–2375. [PubMed] [Google Scholar]
- 6.Cooper A M, Dalto D K, Stewart T A, Griffin J P, Russel D G, Orme I M. Disseminated tuberculosis in interferon-γ gene disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Fonseca D P A J, Joosten D, van der Zee R, Jue D L, Singh M, Vordermeier H M, Snippe H, Verheul A F M. Identification of new cytotoxic T-cell epitopes on the 38-kilodalton lipoglycoprotein of Mycobacterium tuberculosis by using lipopeptides. Infect Immun. 1998;66:3190–3197. doi: 10.1128/iai.66.7.3190-3197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erb K J, Kirman J, Woodfield L, Wilson T, Collins D M, Watson J D, LeGros G. Identification of potential CD8+ T-cell epitopes of the 19 kDa and AhpC proteins from Mycobacterium tuberculosis. No evidence for CD8+ T-cell priming against the identified peptides after DNA-vaccination of mice. Vaccine. 1998;16:692–697. doi: 10.1016/s0264-410x(97)00253-3. [DOI] [PubMed] [Google Scholar]
- 9.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11:514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanke T, Schneider J, Gilbert S C, Hill A V S, McMichael A. DNA multi-CTL epitope vaccines for HIV and Plasmodium falciparum: immunogenicity in mice. Vaccine. 1998;16:426–435. doi: 10.1016/s0264-410x(97)00296-x. [DOI] [PubMed] [Google Scholar]
- 13.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, van Vooren J, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 14.Kamath A T, Feng C G, Macdonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kast W M, Roux L, Curren J, Blom H J, Voordouw A C, Meloen R H, Kolakofsky D, Melief C J. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci USA. 1991;88:2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keene J A, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocyte. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987;196:947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- 19.Lee S W, Sung Y C. Immuno-stimulatory effects of bacterial-derived plasmids depend on the nature of the antigen in intramuscular DNA inoculations. Immunology. 1998;94:285–289. doi: 10.1046/j.1365-2567.1998.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy J A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowrie D B, Silva C L, Colston M J, Ragno S, Tascon R E. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine. 1997;15:834–838. doi: 10.1016/s0264-410x(97)00073-x. [DOI] [PubMed] [Google Scholar]
- 22.Lowrie D B, Tascon R E, Bonato V L D, Lima V M F, Faccioli L H, Stavropoulos E, Colston M J, Hewinson R G, Moelling K, Silva C L. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 23.Lozes E, Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Vandenbussche P, van Vooren J, Drowart A, Ulmer J B, Liu M A. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine. 1997;15:830–833. doi: 10.1016/s0264-410x(96)00274-5. [DOI] [PubMed] [Google Scholar]
- 24.Minev B R, McFarland B J, Spiess P J, Rosenberg S A, Restifo N P. Insertion signal sequence fused to minimal peptides elicits specific CD8+ T-cell responses and prolongs survival of thymoma-bearing mice. Cancer Res. 1994;54:4155–4161. [PMC free article] [PubMed] [Google Scholar]
- 25.Ottenhoff T H M, Mutis T. Role of cytotoxic cells in the protective immunity against and immunopathology of intracellular infections. Eur J Clin Investig. 1995;25:371–377. doi: 10.1111/j.1365-2362.1995.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 26.Rees A, Scoging A, Mehlert A, Young D B, Ivanyi J. Specificity of proliferative response of human CD8 clones to mycobacterial antigens. Eur J Immunol. 1988;18:1881–1887. doi: 10.1002/eji.1830181203. [DOI] [PubMed] [Google Scholar]
- 27.Roman M, Martin-Orozco E, Goodman J S, Nguyen M D, Sato Y, Ronaghy A, Kornbluth R S, Richman D D, Carson D A, Raz E. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 28.Schultz M, Zinkernagel R M, Hengartner H. Peptide-induced anti-viral protection by cytotoxic T cells. Proc Natl Acad Sci USA. 1991;88:991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh M, Andersen A B, McCarthy J E G, Rohde M, Shutte H, Sanders E, Timmis K N. The Mycobacterium tuberculosis 38 kDa protein antigen: hyperexpression of the gene in Escherichia coli and purification and characterization of the recombinant product. Gene. 1992;117:53–60. doi: 10.1016/0378-1119(92)90489-c. [DOI] [PubMed] [Google Scholar]
- 30.Surcel H, Troye-Blomberg M, Paulie S, Andersson G, Moreno C, Pasvol G, Ivanyi J. Th1/Th2 profiles in tuberculosis based on proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–176. [PMC free article] [PubMed] [Google Scholar]
- 31.Tanghe A, Lefevre P, Denis O, D'Souza S, Braibant M, Lozes E, Singh M, Montgomery D L, Content J, Huygen K. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J Immunol. 1999;162:1113–1119. [PubMed] [Google Scholar]
- 32.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 33.Thomson S A, Sherritt M A, Medveczky J, Elliott S L, Moss D J, Fernando G J, Brown L E, Suhrbier A. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160:1717–1723. [PubMed] [Google Scholar]
- 34.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri G. Immunobiology of interleukin-12. Immunol Res. 1998;17:269–278. doi: 10.1007/BF02786451. [DOI] [PubMed] [Google Scholar]
- 36.Vordermeier H M, Harris D P, Moreno C, Singh M, Ivanyi J. The nature of the immunogen determines the specificity of antibodies and T cells to selected peptides of the 38-kDa mycobacterial antigen. Int Immunol. 1995;4:559–566. doi: 10.1093/intimm/7.4.559. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson R J, Zhu X, Wilkinson K A, Lalvani A, Ivanyi J, Pasvol G, Vordermeier H M. 38 000 MW antigen-specific major histocompatibility complex class I restricted interferon-γ-secreting CD8+ T cells in healthy contacts of tuberculosis. Immunology. 1998;95:585–590. doi: 10.1046/j.1365-2567.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X, Berg L, Abdel Motal U M, Jondal M. In vivo primary induction of virus-specific CTL by immunization with 9-mer synthetic peptides. J Immunol Methods. 1992;153:193–200. doi: 10.1016/0022-1759(92)90322-k. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]