Abstract
Simple Summary
There is no established standard second-line chemotherapy after modified FOLFIRINOX for unresectable pancreatic cancer. While gemcitabine plus nab-paclitaxel is often used as second-line chemotherapy after modified FOLFIRINOX, outcomes and prognostic factors of second-line gemcitabine plus nab-paclitaxel have been unclear. This study revealed that median overall survival (OS) and progression-free survival (PFS) of second-line gemcitabine plus nab-paclitaxel were 7.2 months and 3.6 months, respectively. Performance status, modified Glasgow prognostic score, and neutrophil-to-lymphocyte ratio were independently associated with overall survival. Our prognostic model using these parameters classifies patients into the good and poor prognosis groups. Median OS and PFS were longer in the good prognosis group than in the poor prognosis group. Efficacy of second-line gemcitabine plus nab-paclitaxel was notably limited, particularly in the poor prognosis group.
Abstract
Outcomes and prognostic factors of second-line gemcitabine plus nab-paclitaxel (GnP) after modified FOLFIRINOX (mFFX) for unresectable pancreatic cancer were unclear. We retrospectively analyzed consecutive patients with unresectable pancreatic cancer treated with GnP after first-line mFFX treatment between March 2015 and March 2022 at our hospital. A total of 103 patients were included. Median overall survival (OS) from the start of first-line and second-line treatments was 14.9 months and 7.2 months, respectively. Median progression-free survival (PFS) was 3.6 months. Performance status, modified Glasgow prognostic score, and neutrophil-to-lymphocyte ratio were independently associated with OS. Our prognostic model using these parameters classifies patients into good (n = 70) and poor (n = 33) prognosis groups. Median OS and PFS were longer in the good prognosis group than in the poor prognosis group (OS: 9.3 vs. 3.8 months, p < 0.01; PFS: 4.1 vs. 2.3 months, p < 0.01). Grade 3/4 adverse events were observed in 70.9% of patients, with neutropenia being the most frequent. While GnP as second-line treatment was well-tolerated, efficacy of second-line gemcitabine plus nab-paclitaxel was notably limited, particularly in the poor prognosis group.
Keywords: pancreatic cancer, second-line treatment, gemcitabine plus nab-paclitaxel
1. Introduction
Pancreatic cancer (PC) is unresectable at diagnosis in approximately 80% of cases, and its prognosis is poor [1]. Chemotherapy is widely used for the treatment of unresectable PC. Treatment outcomes of chemotherapy for unresectable PC have improved with the advent of FOLFIRINOX (FFX) and gemcitabine plus nab-paclitaxel (GnP) [2,3,4]. As superiority of one regimen over the other is still controversial, both FFX and GnP are positioned as standard first-line therapy [5].
There is no established standard of care for second-line chemotherapy in unresectable PC patients. After first-line GnP, the efficacy of nanoliposomal irinotecan plus fluorouracil and leucovorin and FFX as second-line chemotherapy has been reported [6,7,8]. After first-line FFX, gemcitabine-based therapy has been recommended as second-line treatment [9]. Since GnP has been reported to outperform gemcitabine monotherapy as first-line chemotherapy, GnP is often selected as second-line chemotherapy after FFX for patients in good general condition [3]. Most reports on GnP as second-line chemotherapy after FFX include small numbers of cases, and the prognostic factors of second-line GnP remain unclear [10,11,12,13,14,15,16,17,18]. Compared to FFX, a modified FOLFIRINOX (mFFX) regimen has fewer adverse events (AEs) and comparable efficacy, and mFFX has been widely used [19]. Therefore, a retrospective study was conducted to reveal outcomes and prognostic factors of second-line GnP after first-line mFFX.
2. Materials and Methods
2.1. Patients
We retrospectively analyzed consecutive patients diagnosed pathologically with unresectable PC, administered first-line mFFX treatment, and treated with GnP as second-line chemotherapy between March 2015 and March 2022 at our institution from a prospectively registered database.
2.2. Treatment
The regimen of GnP consisted of 125 mg/m2 nab-paclitaxel for 30 min, followed by 1000 mg/m2 gemcitabine for 30 min, on days 1, 8, and 15. Treatment cycles were repeated every 4 weeks. Prophylactic granulocyte colony stimulating factor was not used. In the event of AEs, dose reduction and/or delay were selected based on the discretion of the physician. The physician continued treatment until tumor progression, conversion surgery, intolerable AEs, patient refusal, or death. Third-line treatment was selected at the discretion of the physician.
2.3. Evaluations
Analyzed patient characteristics before second-line chemotherapy included age, sex, Eastern Cooperative Oncology Group performance status (PS), disease status (metastatic, locally advanced, or recurrent), tumor location (head or body/tail), metastatic site (liver, lung, distant lymph node, or peritoneum), and biliary drainage. Laboratory data including albumin, C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR) [20], carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA19-9) were evaluated. Modified albumin and CRP were used to determine the modified Glasgow prognostic score (mGPS): patients with both hypoalbuminemia (albumin <3.5 g/dL) and elevated CRP level (>1.0 mg/dL) were scored as 2; patients with only elevated CRP level without hypoalbuminemia were scored as 1; and patients without elevated CRP level were scored as 0 [21].
The relative dose intensity (RDI) of gemcitabine and nab-paclitaxel was calculated as the actual dose intensity divided by the standard dose intensity for two courses after starting second-line treatment. Actual dose intensity was the actual dose divided by the actual period required to complete two courses of chemotherapy. Standard dose intensity was the standard total dose for two courses divided by eight weeks. Patients who did not complete two courses of chemotherapy were excluded from RDI calculations.
Follow-up computed tomography was performed every 8–12 weeks to assess efficacy of treatment. Treatment time and best response for mFFX as first-line chemotherapy were evaluated. CA19-9 was measured every month and CA19-9 reduction rate was evaluated by comparing the lowest CA19-9 level during second-line treatment with the CA19-9 level before the start of second-line treatment. The best responses for GnP as second-line chemotherapy were also evaluated. These analyses were performed in accordance with Response Evaluation Criteria in Solid Tumors, version 1.1 [22]. Categories and grades of AEs were evaluated according to Common Terminology Criteria for Adverse Events version 4.0 [23].
2.4. Statistical Analysis
Continuous variables are presented as medians (ranges) and were compared using the Mann–Whitney U test. Categorical variables are described as absolute numbers (proportions) and were analyzed using the χ2 test or Fisher’s exact test as appropriate. A p value < 0.05 was considered statistically significant. Progression-free survival (PFS) was measured from the first day of second-line chemotherapy to the time of disease progression, death, or last follow-up. Overall survival (OS) was measured from the first day of second-line chemotherapy to the time of death or last follow-up. Both PFS and OS were estimated using the Kaplan–Meier method and the Kaplan–Meier curves were compared by the log-rank test. To identify influencing factors for OS, the following variables were evaluated by univariate analysis: age (>70 years vs. ≤70 years), sex (male vs. female), PS (0 vs. 1/2), mGPS (0 vs. 1/2) and NLR (>3 vs. ≤3), CEA (>5 ng/mL vs. ≤5 ng/mL), CA19-9 (>37 U/mL vs. ≤37 U/mL), treatment duration of mFFX (≥4 months vs. <4 months), and best response of mFFX (complete response or partial response vs. stable disease or progressive disease). The Cox proportional hazards model was used to assess hazard ratios (HRs) and 95% confidence intervals (CIs) for OS. Factors in univariate analysis with p values < 0.05 were evaluated in multivariate analysis. The final analysis was conducted using follow-up data until 30 September 2022. All statistical analyses were performed with EZR ver. 1.40 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [24].
3. Results
3.1. Patient Characteristics
A total of 811 consecutive patients with unresectable PC received second-line chemotherapy between March 2015 and March 2022. Of these, 103 patients were treated with second-line GnP after mFFX. Patient characteristics are summarized in Table 1. Totally, 89 patients (86.4%) had metastatic PC. The most common metastatic site was the liver, observed in 60.2% of cases.
Table 1.
Patient characteristics.
| n = 103 | ||
|---|---|---|
| Age (years) | ||
| Median (range) | 67 (30–73) | |
| Sex, n (%) | ||
| Male | 58 (56.3) | |
| Female | 45 (43.7) | |
| PS, n (%) | ||
| 0 | 72 (69.9) | |
| 1 | 30 (29.1) | |
| 2 | 1 (1.0) | |
| Albumin (g/dL) | ||
| Median (range) | 3.5 (2.3–4.4) | |
| CRP (mg/dL) | ||
| Median (range) | 0.41 (0.01–17.2) | |
| CEA (ng/mL) | ||
| Median (range) | 7.9 (0.9–4061.8) | |
| CA19-9 (U/mL) | ||
| Median (range) | 1069.3 (2.0–50,000) | |
| mGPS, n (%) | ||
| Low (0) | 64 (62.1) | |
| High (1, 2) | 39 (37.9) | |
| NLR, n (%) | ||
| ≤3 | 60 (58.3) | |
| >3 | 43 (41.7) | |
| Disease status (%) | ||
| Metastatic | 89 (86.4) | |
| Locally advanced | 9 (8.7) | |
| Recurrent | 5 (4.9) | |
| Tumor location (%) | ||
| Head | 48 (49.0) | |
| Body and/or tail | 50 (51.0) | |
| Metastatic site, n (%) | ||
| Liver | 62 (60.2) | |
| Lung | 17 (16.5) | |
| Distant lymph node | 28 (27.2) | |
| Peritoneum | 35 (34.0) | |
| Biliary drainage, n (%) | 34 (33.0) | |
PS, performance status; CRP, C-reactive protein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; mGPS, modified Glasgow prognostic score; NLR, neutrophil–lymphocyte ratio.
Table 2 shows the results of first-line mFFX. The median treatment period of first-line mFFX was 6.8 (range: 0.7–30.1) months. Overall response rate (ORR) and disease control rate (DCR) of first-line mFFX were 26.2% and 69.9%, respectively. Reasons for discontinuing first-line treatment were tumor progression (98.1%) and intolerance (1.9%). Grades 2 and 3 peripheral neuropathy were observed in 24.3% and 1.0% of patients, respectively, at the end of first-line mFFX.
Table 2.
Treatment outcomes and adverse events of first-line mFFX.
| n = 103 | ||
|---|---|---|
| First-line treatment period (months) | ||
| Median (range) | 6.8 (0.7–30.1) | |
| Complete response, n (%) | 0 (0) | |
| Partial response, n (%) | 27 (26.2) | |
| Stable disease, n (%) | 45 (43.7) | |
| Progressive disease, n (%) | 31 (30.1) | |
| Overall response rate, % | 26.2% | |
| Disease control rate, % | 69.9% | |
| Reason for discontinuing first-line treatment, n (%) | ||
| Tumor progression | 101 (98.1) | |
| Intolerance | 2 (1.9) | |
| Peripheral neuropathy at the end of first-line mFFX, n (%) | ||
| Grade 1 | 77 (74.7) | |
| Grade 2 | 25 (24.3) | |
| Grade 3 | 1 (1.0) |
mFFX, modified FOLFIRINOX.
3.2. Treatment Outcomes of Second-Line Chemotherapy
Ninety-one patients (88.3%) received two or more courses of second-line treatment. Median RDIs of gemcitabine and nab-paclitaxel were both 76.2% (range: 30.0–100%). The dose of gemcitabine or nab-paclitaxel was reduced in 83.5% (76/91) within two courses. The most common reason for dose reduction was hematologic adverse events, followed by fatigue and peripheral neuropathy.
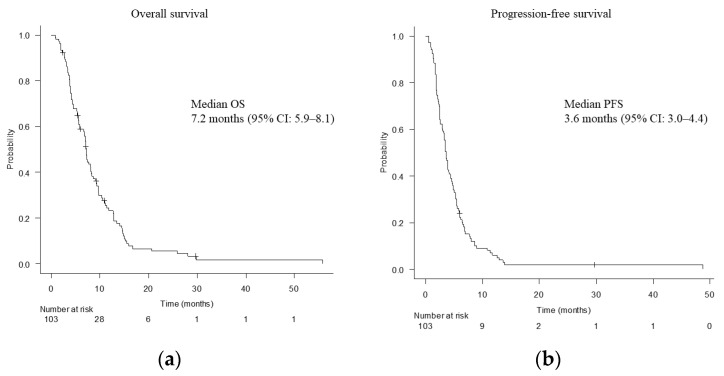
The median follow-up period was 7.2 (range: 0.9–56.6) months. Median OS was 7.2 months (95% CI, 5.9–8.1 months) (Figure 1a). Median PFS was 3.6 months (95% CI, 3.0–4.4 months) (Figure 1b). The ORR and DCR were 7.8% and 63.1%, respectively (Table 3). CA19-9 reduction rate exceeded 50% in 40 patients (38.8%).
Figure 1.
Overall survival (a) and progression-free survival (b). OS, overall survival; CI, confidence interval; PFS, progression-free survival.
Table 3.
Outcomes of second-line gemcitabine plus nab-paclitaxel.
| n = 103 | ||
|---|---|---|
| Complete response, n (%) | 0 (0) | |
| Partial response, n (%) | 8 (7.8) | |
| Stable disease, n (%) | 57 (55.3) | |
| Progressive disease, n (%) | 35 (34.0) | |
| Not evaluable, n (%) | 3 (2.9) | |
| Overall response rate, % | 7.8 | |
| Disease control rate, % | 63.1 | |
| Completion rate of 2 courses, % | 88.3 | |
| RDI, %, Median (range) | ||
| Gemcitabine | 76.2 (30.0–100) | |
| nab-paclitaxel | 76.2 (30.0–100) | |
| Reason for reduction within 2 courses, n (%) | 76 (83.5) | |
| Hematologic adverse event | 47 (51.6) | |
| Fatigue | 11 (12.1) | |
| Peripheral neuropathy | 8 (8.8) | |
| AST/ALT increased | 5 (5.5) | |
| Ascites | 3 (3.3) | |
| Gastrointestinal symptoms | 2 (2.2) | |
RDI, relative dose intensity; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
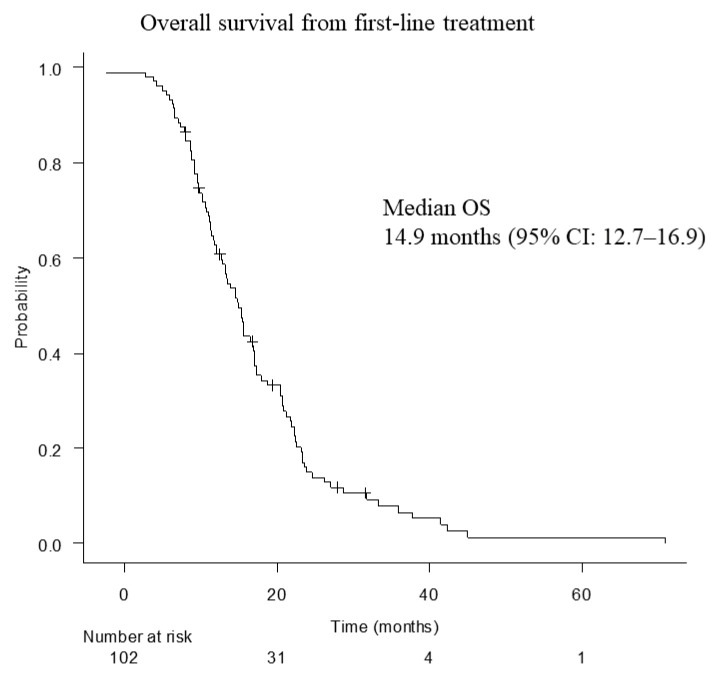
Median OS from the start of first-line mFFX was 14.9 months (12.7–16.9 months) (Figure 2).
Figure 2.
Overall survival from first-line treatment. OS, overall survival; CI, confidence interval.
3.3. Prognostic Factors
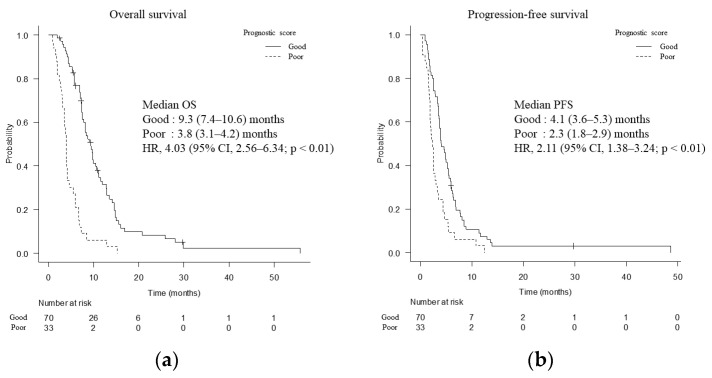
Univariate and multivariate analyses of factors affecting OS before the start of second-line chemotherapy are shown in Table 4. Multivariate analysis showed that PS = 0, mGPS = 0, and NLR ≤ 3 were independent prognostic factors. A prognostic index was created which assigns one point to each of the above variables. Patients with a score of 2 or 3 were included in the good prognosis group (n = 70), while those with a score of 0 or 1 were included in the poor prognosis group (n = 33). Median OS was longer in the good prognosis group (9.3 months; 95% CI, 7.4–10.6 months) than in the poor prognosis group (3.8 months; 95% CI, 3.1–4.2 months) (HR, 4.03; 95% CI, 2.56–6.34; p < 0.01) (Figure 3a). Median PFS was 4.1 months (95% CI, 3.6–5.3 months) in the good prognosis group and 2.3 months (95% CI, 1.8–2.9 months) in the poor prognosis group (HR, 2.11; 95% CI, 1.38–3.24; p < 0.01) (Figure 3b).
Table 4.
Factors influencing overall survival.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Age | 0.40 | ||||
| >70 | 0.78 (0.44–1.39) | ||||
| Sex | 0.35 | ||||
| Male | 1.22 (0.81–1.84) | ||||
| PS | <0.01 | <0.01 | |||
| 0 | 0.44 (0.28–0.68) | 0.44 (0.27–0.71) | |||
| mGPS | <0.01 | <0.01 | |||
| 0 | 0.30 (0.19–0.47) | 0.41 (0.26–0.66) | |||
| NLR | <0.01 | <0.01 | |||
| ≤3 | 0.48 (0.32–0.73) | 0.51 (0.32–0.80) | |||
| CEA | 0.12 | ||||
| ≤5 | 0.71 (0.45–1.10) | ||||
| CA19-9 | 0.78 | ||||
| >37 | 1.07 (0.66–1.73) | ||||
| Treatment duration of mFFX | 0.14 | ||||
| ≥4 months | 0.73 (0.48–1.11) | ||||
| Best response of mFFX | 0.73 | ||||
| PR | 0.92 (0.58–1.46) | ||||
HR, hazard ratio; CI, confidence interval; PS, performance status; mGPS, modified Glasgow prognostic score; NLR, neutrophil-to-lymphocyte ratio; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; mFFX, modified FOLFIRINOX.
Figure 3.
Overall survival (a) and progression-free survival (b) divided by prognostic factors. OS, overall survival; HR, hazard ratio; CI, confidence interval; PFS, progression-free survival.
After the start of second-line GnP, CA19-9 reduction of more than 50% was significantly associated with OS (9.8 months with CA19-9 reduction of more than 50% vs. 5.6 months without CA19-9 reduction of more than 50%, p < 0.01). When comparing OS of patients with stable disease and progressive disease, significantly better OS was observed in the former (9.3 vs. 4.0 months, p < 0.01).
3.4. Adverse Events
Treatment-related AEs are shown in Table 5. No patient died of treatment-related causes. Grade 3/4 AEs were observed in 70.9% of the patients. Among grade 3/4 AEs, neutropenia occurred most frequently (46.6%). Peripheral neuropathy of all grades was observed in 86.4% of the patients, while grade 3/4 peripheral neuropathy was observed in only one patient.
Table 5.
Adverse events of gemcitabine plus nab-paclitaxel.
| Adverse Events | n = 103 | ||
|---|---|---|---|
| All Grades | Grade ≥ 3 | ||
| Hematologic adverse events, n (%) | |||
| Anemia | 102 (99.0) | 30 (29.1) | |
| Neutropenia | 80 (77.7) | 48 (46.6) | |
| Thrombocytopenia | 93 (90.3) | 38 (36.9) | |
| Non-hematologic adverse events, n (%) | |||
| Febrile neutropenia | 1 (1.0) | 1 (1.0) | |
| Nausea/vomiting | 53 (51.5) | 0 (0) | |
| Anorexia | 15 (14.6) | 0 (0) | |
| Diarrhea | 41 (39.8) | 0 (0) | |
| Constipation | 65 (63.1) | 0 (0) | |
| Stomatitis | 21 (20.4) | 0 (0) | |
| Alopecia | 91 (88.3) | 0 (0) | |
| Eruption | 23 (22.3) | 0 (0) | |
| Skin hyperpigmentation | 7 (6.8) | 0 (0) | |
| Fatigue | 93 (90.3) | 2 (1.9) | |
| Peripheral neuropathy | 89 (86.4) | 1 (1.0) | |
| Hypertension | 3 (2.9) | 0 (0) | |
| AST/ALT increased | 91 (88.3) | 6 (5.8) | |
| Creatinine increased | 16 (15.5) | 0 (0) | |
| Interstitial pneumonia | 2 (1.9) | 2 (1.9) | |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
3.5. Subsequent Treatment
Treatment after second-line chemotherapy was introduced to 51.0% of cases. The most common third-line treatment was S-1 (32.3%), followed by nanoliposomal irinotecan plus fluorouracil and leucovorin (6.3%). Two patients received conversion surgery, both of whom had liver metastases (Table 6).
Table 6.
Subsequent treatment after second-line gemcitabine plus nab-paclitaxel.
| n = 49/96 (51.0%) * | |
|---|---|
| S-1 | 31 (32.3%) |
| Nanoliposomal irinotecan plus fluorouracil and leucovorin | 6 (6.3%) |
| Erlotinib plus gemcitabine | 3 (3.1%) |
| S-1 with radiation | 2 (2.1%) |
| mFFX | 2 (2.1%) |
| Conversion surgery | 2 (2.1%) |
| Gemcitabine plus S-1 | 1 (1.0%) |
| Clinical trial | 1 (1.0%) |
| Folk medicine | 1 (1.0%) |
| Second-line treatment continued after disease progression | 2 |
| Best supportive care | 47 |
| Transferred to another hospital | 5 |
mFFX, modified FOLFIRINOX. * Patients continuing second-line treatment after disease progression or transferred to another hospital were excluded.
4. Discussion
In this study, we evaluated the efficacy and AEs of GnP as second-line treatment after mFFX in a real-world setting. Second-line GnP achieved median OS of 7.2 months and median PFS of 3.6 months. Although the dose of gemcitabine and/or nab-paclitaxel was reduced in 83.5% of cases within two courses, GnP had an acceptable safety profile. Independent prognostic factors relating to OS were PS, mGPS, and NLR, which were used to classify the patients into the good and poor prognosis groups. Median OS and PFS were 9.3 months and 4.1 months in the good prognosis group, respectively, and 3.8 months and 2.3 months in the poor prognosis group, respectively. Efficacy of second-line GnP in the poor prognosis group was limited.
There is no established standard of second-line chemotherapy for unresectable PC after first-line mFFX. As gemcitabine-based therapy is recommended as second-line treatment after first-line mFFX [9], GnP is often selected for patients in good general condition, while gemcitabine monotherapy is selected for those in poor general condition. Median OS and PFS of second-line GnP have been reported to be 5.4–15.6 months and 2.8–5.8 months, respectively [10,11,12,13,14,15,16,17,18] (Table 7). The outcomes of this study were comparable to previous reports. On the other hand, median PFS of GnP as first-line treatment has been reported to be 5.0–8.4 months [3,5,25,26,27]. Outcomes of second-line GnP may be worse than those of first-line GnP because of poor general condition, high tumor volume, and resistance to chemotherapy. While ORR in this study (7.8%) was lower than that of previous reports, CA19-9 reduction exceeded 50% in 38.8% of patients. Although many patients did not achieve tumor shrinkage, the OS and PFS in this study were comparable to those in previous reports because a certain number of patients had more than a 50% reduction in CA19-9.
Table 7.
Previous studies on second-line gemcitabine plus nab-paclitaxel.
| Author | Year | Number of Patients |
ORR (%) | DCR (%) | Median PFS (Months) |
Median OS (Months) |
|---|---|---|---|---|---|---|
| Zhang Y et al. [10] | 2015 | 28 | 18 | 46 | NA | 5.4 |
| Portal A et al. [11] | 2015 | 57 | 17.5 | 58 | 5.1 | 8.8 |
| Dadi N et al. [12] | 2017 | 47 | NA | NA | 2.8 | 7.5 |
| Nguyen KT, et al. [13] | 2017 | 30 | NA | NA | 3.7 | 12.4 |
| Zhang H et al. [14] | 2018 | 30 | NA | NA | 3.6 | 5.7 |
| Mita N et al. [15] | 2019 | 30 | 13.3 | NA | 3.8 | 7.6 |
| Chae H et al. [16] | 2020 | 102 | 8.5 | 73.6 | 4.6 | 9.8 |
| Hayuka K et al. [17] | 2021 | 25 | 12 | 96 | 5.3 | 15.6 |
| Huh G et al. [18] | 2021 | 40 | 15 | 87.5 | 5.8 | 9.9 |
| Present study | 103 | 7.8 | 63.1 | 3.6 | 7.2 |
OS, overall survival; PFS, progression-free survival; ORR, overall response rate; DCR, disease control rate; NA, not applicable.
Prognostic factors after first-line treatment suggested by previous reports include PS, CEA level, CA19-9 level, PFS of first-line treatment, and a systemic inflammation-based prognostic score [7,28,29,30,31,32]. Multivariate analysis indicated that good PS, low mGPS, and low NLR were independent predictors of longer survival in this study. We created an original prognostic score by combining the prognostic factors identified in this study. Patients were classified into the good and poor prognosis groups, with a significant difference in median OS (9.3 months in good prognosis group vs. 3.8 months in poor prognosis group, p < 0.01). As median OS in the poor prognosis group was only 3.8 months, appropriate management including informed consent of end-of-life care should be given along with chemotherapy. Although a significant difference in PFS was also observed, the difference was less than two months (4.1 months in the good prognosis group vs. 2.3 months in the poor prognosis group, p < 0.01). It should be noted that components of our prognostic score may simply be associated with survival regardless of chemotherapy, instead of being predictors of the efficacy of second-line GnP.
Both mFFX and GnP are known to cause peripheral neuropathy. The frequency of grade 3/4 peripheral neuropathy of first-line mFFX and GnP was reported to be 1.8–9.0% and 2.8–17.0%, respectively [2,3,5]. The frequency of grade 3/4 peripheral neuropathy of second-line mFFX after GnP was reported to be 1.2–5.1% [8,33]. The reason for the low frequency of peripheral neuropathy in second-line mFFX might be due to appropriate dose reduction of nab-paclitaxel in first-line treatment and oxaliplatin in second-line treatment. In this study, grade 3 peripheral neuropathy at the start of second-line GnP was observed in only one patient, and no new patients developed grade 3/4 peripheral neuropathy during second-line GnP. The reason for the small number of patients with grade 3/4 peripheral neuropathy may be because 83.5% of patients had a reduced nab-paclitaxel dose and RDI of nab-paclitaxel was 76.2% within the first two courses of second-line GnP. Appropriate dose reduction before the development of grade 3/4 peripheral neuropathy may allow second-line GnP to be continued safely.
There are several limitations. First, this study was a retrospective cohort study conducted at a single center with no control group. Second, subsequent treatment was not standardized. Third, administration of therapeutic agents for peripheral neuropathy was not evaluated. Fourth, included patients had a mixture of metastatic, locally advanced, and recurrent PC.
5. Conclusions
In conclusion, GnP as second-line treatment was well-tolerated. Efficacy of second-line GnP was limited, particularly in the poor prognosis group.
Abbreviations
| AEs | adverse events |
| CA 19-9 | carbohydrate antigen 19-9 |
| CEA | carcinoembryonic antigen |
| CI | confidence interval |
| CRP | C-reactive protein |
| DCR | disease control rate |
| FFX | FOLFIRINOX |
| GnP | gemcitabine plus nab-paclitaxel |
| GPS | Glasgow prognostic score |
| HR | hazard ratio |
| mFFX | modified FOLFIRINOX |
| mGPS | modified Glasgow prognostic score |
| NLR | neutrophil-to-lymphocyte ratio |
| ORR | overall response rate |
| OS | overall survival |
| PC | pancreatic cancer |
| PFS | progression-free survival |
| PS | performance status |
| RDI | relative dose intensity |
Author Contributions
Conceptualization, T.M. and T.S.; Data curation, T.M.; Formal analysis, T.M.; Investigation, T.M. and T.T.; Methodology, T.M.; Project administration, T.S.; Resources, T.M., T.I., M.Y., H.N., T.F., A.K. and M.M.; Software, T.M.; Supervision, T.S., M.O. and N.S.; Validation, T.M. and T.S.; Visualization, T.M.; Writing—original draft, T.M.; Writing—review and editing, T.M., T.S., T.T., T.O. and T.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Cancer Institute Hospital, Japanese Foundation for Cancer Research (approval number: 2022-GB-099 and date of approval: 18 November 2022).
Informed Consent Statement
Informed consent for participation in the study was waived because of the retrospective nature of the study, and patients were given opportunities to opt out from the study.
Data Availability Statement
The datasets used and/or analyzed in the study are available from the corresponding author on reasonable request.
Conflicts of Interest
T.S. received honoraria from Taiho Pharmaceutical and Yakult. M.O. received honoraria from Taiho Pharmaceutical, Yakult, MSD, Ono Pharmaceutical, Nihon Servier, Bayer, and Pfizer. N.S. received research funding from Taiho Pharmaceutical, Eisai, Chugai Pharmaceutical, and Takeda Pharmaceutical.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Park W., Chawla A., O’Reilly E.M. Pancreatic Cancer: A Review. JAMA. 2021;326:851–862. doi: 10.1001/jama.2021.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J.J., Gourgou-Bourgade S., de la Fouchardiere C., et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasaki T., Kanata R., Yamada I., Matsuyama M., Ozaka M., Sasahira N. Improvement of Treatment Outcomes for Metastatic Pancreatic Cancer: A Real-world Data Analysis. In Vivo. 2019;33:271–276. doi: 10.21873/invivo.11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mie T., Sasaki T., Takeda T., Fukuda K., Furukawa T., Yamada Y., Kasuga A., Matsuyama M., Ozaka M., Sasahira N. Comparison of Treatment Outcomes Between Gemcitabine With Nab-Paclitaxel and Modified FOLFIRINOX for First-Line Chemotherapy in Metastatic and Recurrent Pancreatic Cancer: Propensity Score Matching. Pancreas. 2021;50:595–601. doi: 10.1097/MPA.0000000000001801. [DOI] [PubMed] [Google Scholar]
- 6.Wang-Gillam A., Li C.P., Bodoky G., Dean A., Shan Y.S., Jameson G., Macarulla T., Lee K.H., Cunningham D., Blanc J.F., et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): Aglobal, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 7.Sawada M., Kasuga A., Mie T., Furukawa T., Taniguchi T., Fukuda K., Yamada Y., Takeda T., Kanata R., Matsuyama M., et al. Modified FOLFIRINOX as a second-line therapy following gemcitabine plus nab-paclitaxel therapy in metastatic pancreatic cancer. BMC Cancer. 2020;20:449. doi: 10.1186/s12885-020-06945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mie T., Sasasaki T., Okamoto T., Takeda T., Mori C., Furukawa T., Kasuga A., Matsuyama M., Ozaka M., Sasahira N. Treatment outcomes of nanoliposomal irinotecan as second-line chemotherapy after gemcitabine and nab-paclitaxel in metastatic and recurrent pancreatic cancer. Jpn. J. Clin. Oncol. 2022;52:1399–1407. doi: 10.1093/jjco/hyac145. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology Pancreatic Adenocarcinoma Version 2. 25 February 2021. [(accessed on 16 October 2022)]. Available online: https://www2.tri-kobe.org/nccn/guideline/pancreas/english/pancreatic.pdf.
- 10.Zhang Y., Hochster H., Stein S., Lacy J. Gemcitabine plus nab-paclitaxel for advanced pancreatic cancer after first-line FOLFIRINOX: Single institution retrospective review of efficacy and toxicity. Exp. Hematol. Oncol. 2015;4:29. doi: 10.1186/s40164-015-0025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portal A., Pernot S., Tougeron D., Arbaud C., Bidault A.T., de la Fouchardière C., Hammel P., Lecomte T., Dréanic J., Coriat R., et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: An AGEO prospective multicentre cohort. Br. J. Cancer. 2015;113:989–995. doi: 10.1038/bjc.2015.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dadi N., Stanley M., Shahda S., O’Neil B.H., Sehdev A. Impact of Nab-Paclitaxel-based Second-line Chemotherapy in Metastatic Pancreatic Cancer. Anticancer Res. 2017;37:5533–5539. doi: 10.21873/anticanres.11985. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen K.T., Kalyan A., Beasley H.S., Singhi A.D., Sun W., Zeh H.J., Normolle D., Bahary N. Gemcitabine/nab-paclitaxel as second-line therapy following FOLFIRINOX in metastatic/advanced pancreatic cancer-retrospective analysis of response. J. Gastrointest. Oncol. 2017;8:556–565. doi: 10.21037/jgo.2017.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H., Kellett C., Lambert P., Kim C.A. Efficacy and Tolerability of Second-line Nab-paclitaxel and Gemcitabine After Failure of First-line FOLFIRINOX for Advanced Pancreas Cancer: A Single-institution Experience. Clin. Colorectal. Cancer. 2018;17:e451–e456. doi: 10.1016/j.clcc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Mita N., Iwashita T., Uemura S., Yoshida K., Iwasa Y., Ando N., Iwata K., Okuno M., Mukai T., Shimizu M. Second-Line Gemcitabine Plus Nab-Paclitaxel for Patients with Unresectable Advanced Pancreatic Cancer after First-Line FOLFIRINOX Failure. J. Clin. Med. 2019;8:761. doi: 10.3390/jcm8060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chae H., Heong H., Cheon J., Chon H.J., Ryu J., Kim I.H., Kang M.J., Jeong J.H., Ryoo B.Y., Kime K.P., et al. Efficacy and safety of second-line nab-paclitaxel plus gemcitabine after progression on FOLFIRINOX for unresectable or metastatic pancreatic ductal adenocarcinoma: Multicenter retrospective analysis. Ther. Adv. Med. Oncol. 2020;12:1–8. doi: 10.1177/1758835920923424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayuka K., Okuyama H., Murakami A., Okita Y., Nishiuchi T., Okano K., Suzuki Y., Tsuji A. Gemcitabine Plus Nab-Paclitaxel as Second-Line Chemotherapy following FOLFIRINOX in Patients with Unresectable Pancreatic Cancer: A Single-Institution, Retrospective Analysis. Chemotherapy. 2021;66:58–64. doi: 10.1159/000517244. [DOI] [PubMed] [Google Scholar]
- 18.Huh G., Lee H.S., Choi J.H., Lee S.H., Paik W.H., Ryu J.K., Kim Y.T., Bang S., Lee E.S. Gemcitabine plus Nab-paclitaxel as a second-line treatment following FOLFIRINOX failure in advanced pancreatic cancer: A multicenter, single-arm, open-label, phase 2 trial. Ther. Adv. Med. Oncol. 2021;13:1–10. doi: 10.1177/17588359211056179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozaka M., Ishii H., Sato T., Ueno M., Ikeda M., Uesugi K., Sata N., Miyashita K., Mizuno N., Tsuji K., et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother. Pharmacol. 2018;81:1017–1023. doi: 10.1007/s00280-018-3577-9. [DOI] [PubMed] [Google Scholar]
- 20.Stoz M., Gerger A., Eisner F., Szkandera J., Loibner H., Ress A.L., Kornprat P., AlZoughbi W., Seggewies F.S., Lackner C., et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br. J. Cancer. 2013;109:416–421. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMillan D.C., Crozie J.E., Canna K., Angerson W.J., McAedle C.S. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int. J. Colorectal. Dis. 2007;22:881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) 6th ed. National Cancer Institute; Bethesda, MD, USA: 2020. [(accessed on 16 October 2022)]. Available online: http://www.meddramsso.com. [Google Scholar]
- 24.Kanda Y. Investigation of the freely available easy-to-use software ‘ezr’ for medical statistics. Bone Marrow. Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Javed M.A., Beyer G., Le N., Vinci A., Wong H., Palmer D., Morgan R.D., Lamara A., Hubner R.A., Valle J.W., et al. Impact of intensified chemotherapy in metastatic pancreatic ductal adenocarcinoma (PDAC) in clinical routine in Europe. Pancreatology. 2019;19:97–104. doi: 10.1016/j.pan.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Williet N., Saint A., Pointet A.L., Tougeron D., Pernot S., Pozet A., Bechade D., Trouilloud I., Lourenco N., Hautefeuille V., et al. Folfirinox versus gemcitabine/nabpaclitaxel as first-line therapy in patients with metastatic pancreatic cancer: A comparative propensity score study. Therap. Adv. Gastroenterol. 2019;12:1756284819878660. doi: 10.1177/1756284819878660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho I.R., Kang H., Jo J.H., Lee H.S., Chung M.J., Park J.Y., Park S.W., Song S.Y., An C., Park M.S., et al. FOLFIRINOX vs gemcitabine/nab-paclitaxel for treatment of metastatic pancreatic cancer: Single-center cohort study. World J. Gastrointest. Oncol. 2020;12:182–194. doi: 10.4251/wjgo.v12.i2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiorean E.G., Von Hoff D.D., Tabernero J., El-Maraghi R., Ma W.W., Reni M., Harris M., Whorf R., Liu H., Li J.S., et al. Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br. J. Cancer. 2016;115:188–194. doi: 10.1038/bjc.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinn M., Dälken L., Striefler J.K., Bischoff S., Schweitzer N., Pelzer U., Dörken B., Riess H., Stieler J.M. Second-Line Treatment in Pancreatic Cancer Patients: Who Profits?--Results From the CONKO Study Group. Pancreas. 2016;45:601–605. doi: 10.1097/MPA.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 30.Ekström A., Brun E., Eberhard J., Segerlantz M. Second-line palliative chemotherapy, survival, and prognostic factors in patients with advanced pancreatic cancer. Acta Oncol. 2021;60:1580–1588. doi: 10.1080/0284186X.2021.1973680. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez-Sainz L., Viñal D., Villamayor J., Marinez-Perez D., Garcia-Cuesta J.A., Ghanem I., Custodio A., Feliu J. Prognostic factors in advanced pancreatic ductal adenocarcinoma patients-receiving second-line treatment: A single institution experience. Clin. Transl Oncol. 2021;23:1838–1846. doi: 10.1007/s12094-021-02589-7. [DOI] [PubMed] [Google Scholar]
- 32.Mie T., Sasaki T., Takeda T., Okamoto T., Mori C., Furukawa T., Yamada Y., Kasuga A., Matsuyama M., Ozaka M., et al. Treatment outcomes of erlotinib plus gemcitabine as late-line chemotherapy in unresectable pancreatic cancer. Jpn. J. Clin. Oncol. 2021;51:1416–1422. doi: 10.1093/jjco/hyab091. [DOI] [PubMed] [Google Scholar]
- 33.Tezuka S., Ueno M., Kobayashi S., Hamaguchi T., Yamachika Y., Oishi R., Nagashima S., Fukushima T., Morimoto M., Shin M. Nal-IRI/5-FU/LV versus modified FOLFIRINOX and FOLFIRI as second-line chemotherapy for unresectable pancreatic cancer: A single center retrospective study. Pancreatology. 2022;22:789–796. doi: 10.1016/j.pan.2022.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the study are available from the corresponding author on reasonable request.