This randomized clinical trial examines the effects of 2 financial incentive strategies among patients with obesity living in low-income neighborhoods.
Key Points
Question
Is there a difference in weight-loss effectiveness of 2 financial incentive strategies using behavioral economic theory and a strategy of provision of weight-management resources?
Findings
In this randomized clinical trial, 668 patients with obesity living in low-income neighborhoods were randomized to 1 of 3 arms. At 6 months, the proportion losing at least 5% of baseline weight was 22.1% in the resources-only group, 39.0% in the goal-directed group, and 49.1% in the outcome-based incentive group; mean percentage of weight loss was similar in the incentive arms.
Meaning
Outcome-based and goal-directed financial incentives were more effective than resources only for weight loss in this low-income population.
Abstract
Importance
Financial incentives for weight management may increase use of evidence-based strategies while addressing obesity-related economic disparities in low-income populations.
Objective
To examine the effects of 2 financial incentive strategies developed using behavioral economic theory when added to provision of weight management resources.
Design, Setting, and Participants
Three-group, randomized clinical trial conducted from November 2017 to May 2021 at 3 hospital-based clinics in New York City, New York, and Los Angeles, California. A total of 1280 adults with obesity living in low-income neighborhoods were invited to participate, and 668 were enrolled.
Interventions
Participants were randomly assigned to goal-directed incentives, outcome-based incentives, or a resources-only group. The resources-only group participants were given a 1-year commercial weight-loss program membership, self-monitoring tools (digital scale, food journal, and physical activity monitor), health education, and monthly one-on-one check-in visits. The goal-directed group included resources and linked financial incentives to evidence-based weight-loss behaviors. The outcome-based arm included resources and linked financial incentives to percentage of weight loss. Participants in the incentive groups could earn up to $750.
Main Outcomes and Measures
Proportion of patients achieving 5% or greater weight loss at 6 months.
Results
The mean (SD) age of the 668 participants enrolled was 47.7 (12.4) years; 541 (81.0%) were women, 485 (72.6%) were Hispanic, and 99 (14.8%) were Black. The mean (SD) weight at enrollment was 98.96 (20.54) kg, and the mean body mass index (calculated as weight in kilograms divided by height in meters squared) was 37.95 (6.55). At 6 months, the adjusted proportion of patients who lost at least 5% of baseline weight was 22.1% in the resources-only group, 39.0% in the goal-directed group, and 49.1% in the outcome-based incentive group (difference, 10.08 percentage points [95% CI, 1.31-18.85] for outcome based vs goal directed; difference, 27.03 percentage points [95% CI, 18.20-35.86] and 16.95 percentage points [95% CI, 8.18-25.72] for outcome based or goal directed vs resources only, respectively). However, mean percentage of weight loss was similar in the incentive arms. Mean earned incentives was $440.44 in the goal-directed group and $303.56 in the outcome-based group, but incentives did not improve financial well-being.
Conclusions and Relevance
In this randomized clinical trial, outcome-based and goal-directed financial incentives were similarly effective, and both strategies were more effective than providing resources only for clinically significant weight loss in low-income populations with obesity. Future studies should evaluate cost-effectiveness and long-term outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT03157713
Introduction
More than 600 million adults worldwide have obesity. From 1999 to 2018, the prevalence of obesity among adults in the US rose from 30.5% to 42.4%, with a substantially higher percentage among racial and ethnic minority groups. Obesity also contributes to diabetes, heart disease, stroke, and cancer, and it is estimated that $147 billion (2008 dollars) are attributable to obesity-related illnesses in the US. Because individuals with obesity are more likely than individuals with normal weight to face social stigma, including employment discrimination and bias in educational settings, the increased prevalence of obesity among lower-income individuals exacerbates health and socioeconomic disparities.
Evidence-based strategies for weight loss include participating in a weight management program, self-monitoring weight and diet, and achieving physical activity goals. However, these strategies are underused. Behavioral economics, which combines concepts from economics and psychology, and financial incentives may be effective tools to address underuse, particularly when used in combination. However, it is unknown whether using these tools to target behavioral goals (goal-directed design) is more or less effective than strictly targeting weight loss (outcome-based design).
The objective of the Financial Incentives for Weight Reduction (FIReWoRk) randomized clinical trial was to compare the effectiveness of goal-directed vs outcome-based financial incentives on weight loss at 6 months among patients with obesity living in low-income neighborhoods, and to compare the use of these incentives with a strategy of provision of weight management resources only. We hypothesized that goal-directed incentives would lead to greater and more sustained weight loss than outcome-based incentives or the provision of behavior change resources alone. All patients were provided with weight management resources, including a 1-year commercial weight loss program membership, self-monitoring tools (digital scale, food journal, and wearable fitness tracker), health education, and monthly one-on-one check-in visits. The goal-directed strategy included resources and linked evidence-based behaviors with financial incentives. The outcome-based strategy included resources and linked percentage of weight loss with financial incentives.
Methods
Study Design
This was a 3-arm, randomized clinical trial conducted in 3 hospital-based clinics in New York City (Bellevue Hospital and NYU Langone Brooklyn) and Los Angeles (UCLA-Olive View). The protocol (Supplement 1) was approved by the institutional review boards of New York University School of Medicine, University of California Los Angeles (UCLA) David Geffen School of Medicine, and Olive View–UCLA Medical Center. All participants provided written informed consent. The protocol has been previously published and is also available at ClinicalTrials.gov (NCT03157713). This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients
Potential patients were recruited from primary care practices between November 2017 and March 2020 through electronic health record screenings with proactive outreach via mail with follow-up phone calls and physician referrals. The study population comprised adults with obesity (body mass index [BMI, calculated as weight in kilograms divided by height in meters squared] ≥30) who were aged 18 to 70 years, spoke English or Spanish, had seen a physician in the enrolling health clinics within the prior 2 years, had an active phone number, and lived in a qualifying census tract. A census tract was considered eligible if its median household income was less than approximately $40 000. This represented approximately the 40th percentile of income in both cities according to the 2015 American Community Survey. Participants self-reported race and ethnicity after being presented with several categories. We collected these data because they may influence the effect of behavioral interventions. A full list of exclusion criteria is available in the protocol, along with a detailed description of recruitment methods and study design.
Randomization and Masking
Randomization was stratified by study site and a participant’s self-reported preference for financial incentives structured as goal directed or outcome based, based on their response to a hypothetical vignette. We used block sizes of 4 or 6 at random using a random number generator in R (R Foundation for Statistical Computing; http://www.r-project.org) that was accessed centrally by study staff after they obtained informed consent and baseline measurements.
Procedures
At the initial study visit, all participants received a list of local weight management programs that met criteria for a high-intensity lifestyle intervention and a voucher for 1 year of WW Freestyle (formerly Weight Watchers). WW has been found to be an effective intervention for weight loss. We helped patients enroll in WW and identify Spanish-speaking groups when applicable. All participants also received self-monitoring instructions and tools, including a digital scale (Greater Goods Balance Bathroom Scale No. 039), a food journal (manufactured by BookFactory), and a Fitbit wearable fitness tracker (Alta HR or Inspire HR model). In addition, they were provided with education and materials on healthy eating and physical activity. Research staff advised all patients to attend WW at least twice per month, weigh themselves at least 3 days per week, maintain a paper- or app-based food diary at least 5 days per week, and accumulate at least 75 physical activity minutes (moderate to vigorous) per week (which increased to 150 minutes per week after 3 months to approximate physical activity guidelines). We verified these activities using documentation from weight management programs, reviews of journal entries, and review of fitness tracker metrics at monthly check-in visits.
A detailed incentive schedule, description of behavioral economic enhancements, and other procedures are described in eMethods in Supplement 2. Participants randomized to goal-directed incentives could also earn up to $750 over 6 months for participating in evidence-based weight loss therapies. They received a onetime incentive of $150 for registering and attending at least half of the weekly weight management program sessions for 1 month, as verified with documentation. They continued to receive $60 monthly thereafter for attending at least half of the weekly program sessions. In the first 6 months, they received up to $30 per month for using their food journal at least 5 days per week and recording their body weight at least 3 days per week. In the first 3 months, they also received up to $20 per month for achieving 75 minutes of physical activity per week, as verified by fitness tracker data. From month 4 to month 6, the physical activity goal increased to 150 minutes per week.
Outcomes
The primary outcome was percentage of patients who achieved a 5% or greater reduction in baseline weight at 6 months, an amount considered clinically significant for adults with a BMI in the overweight/obese range based on clinical guidelines to reduce cardiometabolic risk. We chose to measure the main outcome at 6 months because this time point corresponds with when maximum weight loss occurs for lifestyle change. Other weight-related outcomes included change in weight from baseline, change in waist circumference, and change in BMI at 6 and 12 months and change in percentage of patients achieving 5% or greater reduction in weight at 12 months. We assessed use of evidence-based weight loss behaviors, including weight management program attendance, physical activity frequency, and adherence to self-monitoring of diet and weight. We assessed the mean dollar amount that patients received and changes in intrinsic and extrinsic motivation. We also assessed whether patients randomized to an intervention arm congruent with their preferences (goal directed or outcome based) exhibited more weight loss than patients randomized to an incongruent intervention.
We also note that while the primary outcome was correctly reported on NIH Reporter (which reflects the research grant application) and in our protocol manuscript, it was incorrectly listed on ClinicalTrials.gov due to a clerical error. This error was corrected in September 2021.
Statistical Analyses
Outcomes were analyzed using a generalized mixed-effect model for repeated measures to account for missing data. We used linear mixed effects models as our primary model for the binary outcome of 5% reduction in baseline weight. Models included fixed effects of treatment group, time and treatment–time interaction, study site, and incentive preference. Random effects included residuals and a random intercept for participant. We conducted subgroup analysis by including interaction terms between subgroup variables, time, and treatment group. The P values were all from model estimations; all tests were 2-tailed, with a .05 significance level. All analyses followed the intention-to-treat principle and were performed using Stata (version 16; StataCorp LLC) and R (www.r-project.org).
Results
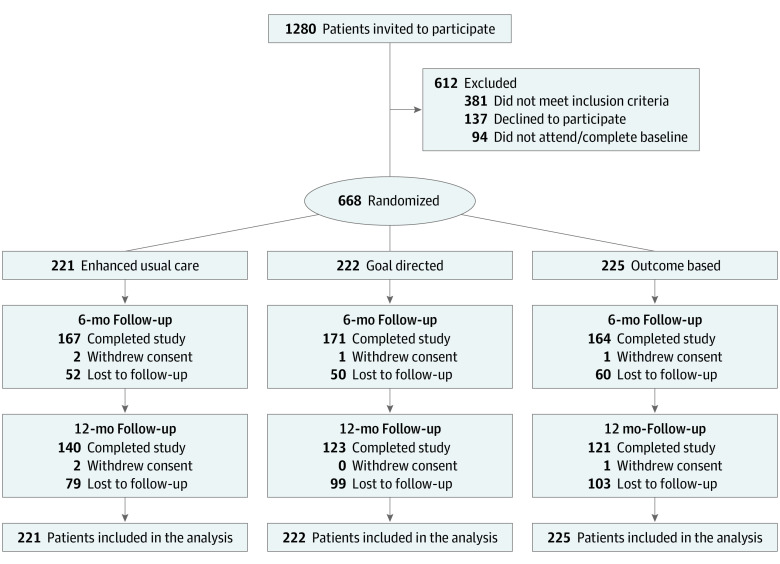
Between November 2017 and March 2020, a total of 1280 patients were invited to participate in the study, and 668 underwent randomization (Figure 1). The mean (SD) age of patients was 47.69 (12.43) years; 541 were women (81.0%), 99 were Black (14.8%), 485 were Hispanic (72.6%), and 231 (34.6%) were Spanish-speaking without English proficiency (Table 1). The mean (SD) weight was 98.96 (20.54) kg, and mean (SD) BMI was 37.95 (6.55). The median (IQR) census tract household income was $34 622 ($29 702-$38 696). The mean (SD) financial well-being score was 58.55 (10.15) (0-100 scale range), and mean (SD) intrinsic motivation scores were 3.17 (0.78) for weight loss (0-4 scale range) and 1.45 (1.51) for self-monitoring (0-4 scale range).
Figure 1. CONSORT Diagram.
Table 1. Characteristics of Study Participants.
| Characteristic | No. (%) | Standardized mean difference | ||||
|---|---|---|---|---|---|---|
| Resources only (n = 221) | Goal directed (n = 222) | Outcome based (n = 225) | Goal directed vs resources only | Outcome based vs resources only | Goal directed vs outcome based | |
| Age, mean (SD), y | 47.84 (12.04) | 48.23 (12.11) | 47.03 (13.12) | 0.033 | 0.064 | 0.095 |
| Gender | ||||||
| Female | 168 (76.0) | 187 (84.2) | 186 (82.7) | 0.221 | 0.184 | 0.042 |
| Male | 52 (23.5) | 35 (15.8) | 39 (17.3) | |||
| Nonbinary | 1 (0.5) | 0 | 0 | |||
| Baseline anthropomorphic measures, mean (SD) | ||||||
| Weight, kg | 98.44 (19.78) | 98.12 (19.76) | 100.31 (21.99) | 0016 | 0.089 | 0.104 |
| BMI | 37.83 (6.38) | 37.68 (6.27) | 38.34 (6.99) | 0.023 | 0.077 | 0.100 |
| Waist circumference, cm | 113.26 (14.81) | 112.88 (13.68) | 113.81 (15.07) | 0.027 | 0.037 | 0.065 |
| Race and ethnicity | ||||||
| Hispanic | 160 (72.4) | 157 (70.7) | 168 (74.7) | 0.255 | 0.177 | 0.203 |
| Non-Hispanic | ||||||
| Black | 28 (12.7) | 43 (19.4) | 28 (12.4) | |||
| White | 12 (5.4) | 12 (5.4) | 17 (7.6) | |||
| Othera | 21 (9.5) | 10 (4.5) | 12 (5.3) | |||
| Spanish-speaking, not proficient in English | 76 (34.4) | 73 (32.9) | 82 (36.4) | 0.032 | 0.046 | 0.079 |
| Education | ||||||
| High school or less | 125 (56.6) | 126 (56.8) | 132 (58.7) | 0.041 | 0.139 | 0.177 |
| Some college | 45 (20.4) | 48 (21.6) | 34 (15.1) | |||
| College graduate | 51 (23.1) | 48 (21.6) | 58 (25.8) | |||
| Median household income of census tract, $ | 33 804 | 34 413 | 35 000 | 0.133 | 0.121 | 0.015 |
| Marital status | ||||||
| Married | 89 (40.3) | 86 (38.7) | 77 (34.2) | 0.031 | 0.122 | 0.091 |
| Not married | 132 (59.7) | 136 (61.3) | 147 (65.3) | |||
| Preferences for incentive design | ||||||
| Goal directed | 132 (59.7) | 133 (59.9) | 137 (60.9) | 0.004 | 0.024 | 0.020 |
| Outcome based | 89 (40.3) | 89 (40.1) | 88 (39.1) | |||
| Health insurance | ||||||
| Private | 19 (8.6) | 15 (6.8) | 19 (8.4) | 0.180 | 0.252 | 0.192 |
| Medicare | 17 (7.7) | 23 (10.4) | 22 (9.8) | |||
| Medicaid | 124 (56.1) | 127 (57.2) | 143 (63.6) | |||
| Other | 19 (8.6) | 25 (11.3) | 17 (7.6) | |||
| Uninsured/unknown | 42 (19.0) | 32 (14.4) | 24 (10.7) | |||
| Intrinsic motivation, mean (SD) | ||||||
| For weight loss TSRQ score | 3.20 (0.79) | 3.18 (0.75) | 3.14 (0.80) | 0.024 | 0.071 | 0.049 |
| For monitoring diet and activity TSRQ score | 1.41 (1.50) | 1.69 (1.56) | 1.27 (1.43) | 0.183 | 0.097 | 0.282 |
| Financial well-being score, mean (SD) | 59.71 (10.30) | 57.63 (10.46) | 58.33 (9.61) | 0.201 | 0.139 | 0.069 |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; TSRQ, Treatment Self-Regulation Questionnaire.
The other race and ethnicity category comprises participants who reported being Afro-Caribbean, Arabic, Armenian, Asian, Caribbean American, Greek, other Hispanic, Indian, Indian Asian, Irish German, Jewish, Mestizo, Middle Eastern, Native American, West Indian, or mixed race.
During follow-up, weight was recorded for 498 participants (74.6%) at 6 months and for 364 (54.5%) at 12 months. Participants of younger age, Hispanic ethnicity, with other or unknown insurance, and in New York sites were more likely to have missing data at 6 months (eTable 1 in Supplement 2). At the primary outcome time point of 6 months, the adjusted proportion of patients who lost at least 5% of their baseline weight was 22.1% in the resources-only group, 39.0% in the goal-directed group, and 49.1% in the outcome-based incentive group (difference, 10.08 percentage points [95% CI, 1.31-18.85] for outcome based vs goal directed; difference, 27.03 percentage points [95% CI, 18.20-35.86] and 16.95 percentage points [95% CI, 8.18-25.72] for outcome based or goal directed vs resources only, respectively) (Table 2).
Table 2. Weight Loss by Group at 6 and 12 Months.
| Measure | Mean (95% CI) | Goal directed vs resources only | Outcome based vs resources only | Goal directed vs outcome based | |||||
|---|---|---|---|---|---|---|---|---|---|
| Resources only | Goal directed | Outcome based | P value | 95% CI | P value | 95% CI | P value | 95% CI | |
| Total weight loss, kg | |||||||||
| 6 mo | −2.21 (−2.95 to −1.47) | −4.47 (−5.21 to −3.74) | −4.79 (−5.53 to −4.05) | <.001 | −3.30 to −1.22 | <.001 | −3.62 to −1.53 | .55 | −1.35 to 0.72 |
| 12 mo | −2.74 (−3.51 to −1.97) | −5.43 (−6.22 to −4.63) | −4.61 (−5.42 to −3.81) | <.001 | −3.80 to −1.58 | .001 | −2.99 to −0.76 | .16 | −0.32 to 1.95 |
| Percentage of weight loss, % | |||||||||
| 6 mo | −2.25 (−2.93 to −1.58) | −4.58 (−5.25 to −3.91) | −4.74 (−5.42 to −4.07) | <.001 | −3.28 to −1.37 | <.001 | −3.45 to −1.54 | .73 | −1.12 to 0.78 |
| 12 mo | −2.68 (−3.39 to −1.97) | −5.33 (−6.06 to −4.60) | −4.48 (−5.22 to −3.74) | <.001 | −3.67 to −1.64 | <.001 | −2.83 to −0.78 | .11 | −0.19 to 1.89 |
| Proportion at least 5% below baseline weight, % | |||||||||
| 6 mo | 22.06 (15.81 to 28.31) | 39.01 (32.84 to 45.18) | 49.09 (42.84 to 55.33) | <.001 | 8.18 to 25.72 | <.001 | 18.20 to 35.86 | .02 | 1.31 to 18.85 |
| 12 mo | 31.26 (24.61 to 37.91) | 41.91 (34.99 to 48.84) | 41.40 (34.36 to 48.44) | .03 | 1.06 to 20.25 | .04 | 0.46 to 19.82 | .99 | −10.38 to 9.35 |
| Change in waist circumference, cm | |||||||||
| 6 mo | −2.90 (−3.81 to −1.98) | −5.08 (−6.00 to −4.16) | −4.37 (−5.29 to −3.44) | <.001 | −3.48 to −0.89 | .03 | −2.77 to −0.17 | .28 | −0.59 to 2.02 |
| 12 mo | −2.70 (−3.80 to −1.60) | −6.14 (−7.28 to −5.01) | −4.66 (−5.82 to −3.49) | <.001 | −5.02 to −1.87 | .02 | −3.56 to −0.36 | .07 | −0.14 to 3.11 |
The proportion of participants whose weight at 12 months was at least 5% below their baseline weight was 31.3% in the resources-only group, 41.9% in the goal-directed incentive group, and 41.4% in the outcome-based incentive group (difference, −0.52 percentage points [95% CI, −10.38 to 9.35] for outcome based vs goal directed; difference, 10.14 percentage points [95% CI, 0.46-19.82] and 10.66 percentage points [95% CI, 1.06-20.25] for outcome based or goal directed vs resources only, respectively).
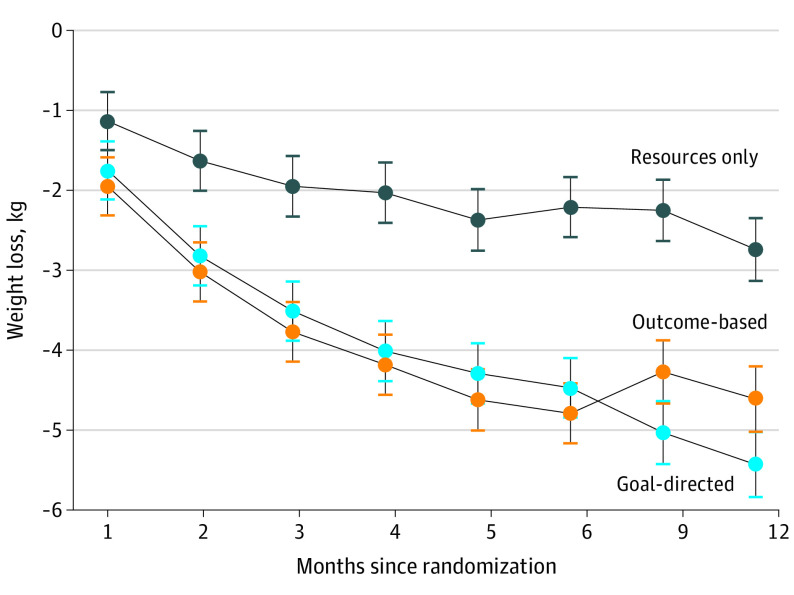
The mean (SE) change in weight from baseline at 6 months was −2.21 (0.38) kg in the resources-only group, −4.47 (0.37) kg in the group receiving goal-directed incentives, and −4.79 (0.38) kg in the group receiving outcome-based incentives (Figure 2). At 12 months, the corresponding changes were −2.74 (0.39) kg in the resources-only group, −5.43 (0.41) kg in the group receiving goal-directed incentives, and −4.61 (0.41) kg in the group receiving outcome-based incentives. At 6 months and 12 months, mean weight loss between the financial incentive groups was not significantly different, but participants in each group lost significantly more weight than participants in the resources-only group. Waist circumference was also significantly lower in the goal-directed and outcome-based incentive groups at 6 months and 12 months compared with the resources-only group.
Figure 2. Mean Weight Change by Randomization Group.
Subgroup analyses showed no difference between outcomes before vs after COVID-19 emergency declarations were issued and no difference between men and women. For race and ethnicity, we did see higher incentive effects for Hispanic participants compared with Black participants (eTable 2 in the Supplement 2).
Table 3 shows verified rates of use of evidence-based weight loss therapies during the first 6 months of follow-up. In the goal-directed group, the adjusted percentage of participants who enrolled in WW was 83.4%, and the percentage of enrollees who actively participated for at least 1 month was 81.2%, compared with an enrollment rate of 61.2% and an active participation rate of 49.6% in the resources-only group. Participants in the goal-directed group also had higher rates of physical activity, food diary use, and self-monitoring of weight than participants in the resources-only group (Table 3). There were no substantial differences in use of evidence-based weight loss therapies between the resources-only group and the outcome-based group.
Table 3. Use of Evidence-Based Weight Loss Therapies at 6 and 12 Monthsa.
| Measure | No./total No. (%) | Goal directed vs resources only | Outcome based vs resources only | Goal directed vs outcome based | |||||
|---|---|---|---|---|---|---|---|---|---|
| Resources only | Goal directed | Outcome based | P value | 95% CI | P value | 95% CI | P value | 95% CI | |
| Enrollment in weight loss program | |||||||||
| 6 mob | 121/199 (60.89) | 165/199 (82.83) | 110/203 (54.19) | <.001 | 12.99 to 30.88 | .14 | −15.60 to 2.20 | <.001 | −37.54 to −19.74 |
| 12 moc | 1/156 (0.67) | 1/142 (0.67) | 0/141 | >.99 | −1.53 to 1.54 | .39 | −2.21 to 0.86 | .40 | −2.25 to 0.89 |
| Active participation in weight loss programd | |||||||||
| 6 mo | 60/121 (49.59) | 134/165 (81.21) | 66/110 (60.00) | <.001 | 20.91 to 42.34 | .11 | −2.36 to 23.19 | <.001 | 10.29 to 32.14 |
| 12 mo | 25/122 (20.49) | 49/166 (29.52) | 22/110 (20.00) | .08 | −0.95 to 18.90 | .93 | −10.84 to 9.86 | .08 | −0.68 to 19.72 |
| At least 75 or 150 min of physical activity per weeke | |||||||||
| 6 mo | 52/163 (32.53) | 80/168 (46.69) | 53/161 (31.61) | <.001 | 4.00 to 24.32 | .86 | −11.17 to 9.34 | .003 | −25.27 to −4.89 |
| 12 mo | 37/125 (27.32) | 34/119 (23.51) | 31/114 (26.71) | .51 | −15.15 to 7.53 | .92 | −12.07 to 10.83 | .54 | −8.34 to 14.73 |
| Active food diary usef | |||||||||
| 6 mo | 81/124 (60.78) | 137/155 (85.01) | 89/121 (67.42) | <.001 | 14.27 to 34.19 | .21 | −3.83 to 17.11 | <.001 | −27.61 to −7.56 |
| 12 mo | 43/89 (44.76) | 44/85 (48.84) | 31/83 (33.43) | .51 | −7.97 to 16.12 | .07 | −23.47 to 0.81 | .01 | −27.63 to −3.19 |
| Active self-monitoring of weightg | |||||||||
| 6 mo | 80/144 (53.46) | 127/164 (76.62) | 86/147 (56.23) | <.001 | 12.89 to 33.43 | .61 | −7.74 to 13.29 | <.001 | −30.61 to −10.16 |
| 12 mo | 43/115 (32.48) | 50/101 (46.77) | 36/104 (31.82) | .02 | 2.28 to 26.29 | .91 | −12.60 to 11.27 | .02 | −27.22 to −2.68 |
All percentages are adjusted except for active participation in weight loss program (verified by study team). Sample size is less than total study enrollment because data on use of evidence-based therapies were not collected from all patients.
Defined as participant enrolling in a weight loss program at any time point between baseline and 6 months.
Defined as participant enrolling in a weight loss program at any time point between 6 and 12 months.
Defined as participant attending at least 2 sessions per month or 50% or more sessions monthly, whichever is greater, at any time point between baseline and 6 months.
Defined as meeting physical activity goal for at least 4 weeks at month 6 or 12.
Defined as using the food diary on at least 5 days per week for at least 4 weeks at month 6 or 12.
Defined as self-weighing at home and recording weight on at least 3 days per week for at least 4 weeks at month 6 or 12.
The mean (SD) earned financial incentives was $440.44 ($281.76) in the goal-directed group and $303.56 ($290.03) in the outcome-based group. The adjusted mean (SE) intrinsic motivation scores for weight loss at 6 months were similar: 3.4 (0.1) in the resources-only group, 3.4 (0.1) in the goal-directed incentive group, and 3.4 (0.1) in the outcome-based incentive group. The corresponding intrinsic motivation scores for self-monitoring were 3.0 (0.1) in the resources-only group, 3.3 (0.1) in the group receiving goal-directed incentives, and 3.0 (0.1) in the group receiving outcome-based incentives. The intrinsic motivation score for self-monitoring was higher in the goal-directed group compared with the resources-only group (P = .03) but not the outcome-based group (P = .81). Being randomized to an intervention arm congruent with the participant’s preferences (goal directed or outcome based) was not associated with an incremental effect on weight loss compared with randomization to an incongruent intervention.
The adjusted mean (SE) financial well-being score at 6 months was 59.91 (0.81) in the resources-only group, 60.89 (0.80) in the group receiving goal-directed incentives, and 60.19 (0.82) in the group receiving outcome-based incentives. There was no significant difference between groups.
The sensitivity analyses for the primary end point at 6 and 12 months to address missing data are provided in eTable 3 in Supplement 2. Compared with the main analysis, both sensitivity analyses showed similar trends at 6 months. The group differences were largely attenuated at 12 months.
There were no significant differences between groups with regard to potentially dangerous weight management behaviors. Adverse events were reported by 49 participants (7.3%) with 13 (5.9%), 17 (7.6%), and 18 (8.1%) in the goal-directed, outcome-based, and resources-only arms, respectively. There were 8 inpatient hospitalizations during the study period, with similar numbers in each arm, that were unrelated to study participation. There was 1 suicide attempt and 1 new disability reported. We learned of 1 death in the outcome-based arm that was unrelated to study participation.
Discussion
This randomized clinical trial demonstrated the effectiveness of large financial incentive strategies for weight loss that encourage use of evidence-based therapies or weight loss targets and incorporate behavioral economics. A relatively high proportion of patients in all 3 study groups lost clinically significant amounts of weight at the 6-month primary time point, and many were able to maintain weight loss at 12-month follow-up. Outcome-based and goal-directed financial incentives were similarly effective for weight loss, and more patients in both financial incentive groups lost at least 5% of baseline weight than patients in the resources-only group. A greater proportion of participants in the outcome-based group exceeded the 5% weight loss threshold, but many of these participants did so marginally, so mean weight loss was similar in the 2 financial incentive groups.
Goal-directed incentives were more effective than outcome-based incentives and resources only to promote enrollment and participation in a weight loss program. However, despite higher program participation, total weight loss at 6 months in the goal-directed group was comparable to that in the outcome-based group. The reasons are unclear but may relate to differences in patient response to incentives that directly target a 5% weight loss outcome, in contrast to incentives that indirectly target the same outcome by incentivizing evidence-based activities in the outcome’s pathway. Notably, differences in 5% weight loss between goal-directed and outcome-based incentives that were present at 6 months were no longer present at 12 months. Longer follow-up would have provided additional insight on whether the initial use of goal-directed incentives resulted in more sustainable weight loss.
Despite using large incentives, financial incentives did not reduce intrinsic motivation for weight loss or self-monitoring. In addition, we found no evidence that patients enrolled in a financial incentive group experienced reductions in financial distress.
Clinical trials of financial incentives for weight loss have generally structured incentives to reward either weight loss alone or weight loss combined with engagement in evidence-based behaviors, such as physical activity or self-monitoring. It has been unknown whether a goal-directed or outcome-based financial incentive strategy was more effective at promoting weight loss. For instance, one 8-week study randomized 30 adults with overweight or obesity and reported that goal-directed and outcome-based interventions yielded comparable amounts of weight loss. Patients in both groups lost more weight than patients randomized to the control group. A second 16-week study randomized 703 adults with overweight or obesity and reported that goal-directed and outcome-based interventions did not result in greater weight loss than a control intervention.
While the effectiveness of a financial incentive strategy depends on multiple aspects of its design, including the size of incentives used, whether behavioral economics is incorporated, and what specific outcomes are incentivized, we provide evidence that offering comparable total attainable incentive amounts in goal-directed or outcome-based designs generally yields more favorable weight loss outcomes at 6 months among patients receiving outcome-based designs. It is also noteworthy that the present study does not inform any general conclusions about the comparative effectiveness of goal-directed vs outcome-based incentives, particularly since details of the reward structure will vary across different health programs.
The change in weight and waist circumference over time appeared to diverge between the goal-directed and outcome-based groups after 6 months, with participants in the goal-directed group demonstrating relatively larger reductions from baseline, as shown in Table 2 and Figure 2. While this divergence was not significant, it highlights the possibility that the 2 financial incentive strategies may yield different long-term weight loss outcomes. In this study, weight and waist circumference measures at 12 months tended to be more favorable with goal-directed incentives rather than outcome-based incentives, and patients in the goal-directed group also reported higher rates of weight and diet self-monitoring. Related to this, more research is needed to assess how differences in adoption of evidence-based behaviors may influence weight loss outcomes when financial incentives are incorporated.
One major criticism of financial incentive interventions for behavior change is that they may crowd out intrinsic motivation, thereby adversely affecting patient health or well-being. Some researchers have argued that the overall risk of “crowd out” is small because levels of intrinsic motivation for evidence-based weight loss behaviors tend to be low, leaving little motivation at risk for crowd out. The possibility that losing weight or successfully participating in evidence-based therapies may itself increase self-efficacy further complicates the intrinsic-extrinsic motivation dynamic in the context of weight loss. For example, attainment of behavioral goals precedes weight loss outcomes, thereby providing earlier opportunities for success, which may increase self-efficacy and intrinsic motivation for weight management. Our finding that participants in the goal-directed group reported higher levels of intrinsic motivation provides some support for this hypothesis.
One of the study objectives was to examine whether financial well-being could be improved by enrolling low-income patients and linking them to relatively large incentives. While the mean earnings in the incentive groups ranged from $300 to $400, we did not detect a difference in financial well-being scores. It is possible that participants living in low-income neighborhoods may require considerably larger incentives to relieve financial distress.
Limitations
Study limitations include uncertainty about the generalizability of the findings to populations living in higher-income neighborhoods, along with populations that do not primarily comprise racial and ethnic minority groups and women. These individuals may be less responsive to incentives comparable in size to those we used in this study. A component of our intervention was providing all participants with free access to a commercial weight loss program. This limits external validity, since these programs can be expensive, and free access may not be available outside of a clinical trial. Another limitation is that we stopped enrollment early due to the COVID-19 pandemic and restrictions placed on research activities. Because the study provided patients with portable scales they could use at home, we were able to leverage video technology to collect data on weight loss during the pandemic. A sensitivity analysis that excluded weight loss measures obtained outside of the clinical site (ie, at participants’ homes during the COVID-19 pandemic) was performed. This sensitivity analysis yielded similar findings to the main results. In addition, even though all participants had similar follow-up visit schedules, participants in the financial incentive groups received incentive-specific feedback and may have therefore received more intensive human interaction. This may have affected the results. The subgroup analysis was not planned a priori, and interpretation of results is limited by the small sample size of many of the subgroups.
In addition, the weight loss program we offered was not tailored to Hispanic and Spanish-speaking populations. This may partially explain why patients did not lose more weight in the goal-directed arm despite reporting higher rates of WW attendance. However, we attempted to connect patients to studio sessions with Spanish-speaking WW facilitators. Future studies should explore using goal-directed financial incentives for culturally tailored programs. The low rates of follow-up at 12 months are also a limitation, although we accounted for loss to follow-up in our analysis. In addition, because the interventions were complex, we are unable to determine the effectiveness of individual components. We also did not measure body fat, which would have provided further insights into differences in waist circumstance between the outcome-based and goal-directed financial incentive groups.
Conclusions
In this randomized clinical trial, financial incentive strategies that incorporated behavioral economics and targeted outcome-based interventions were similarly effective to strategies with a goal-directed design for 5% weight loss at 6 months in low-income patient populations with obesity, and both were more effective than providing resources only. Furthermore, the amount of weight lost by these patients was substantial, including patients in the resources-only group. Future work should address cost-effectiveness of these strategies, long-term outcomes, and effectiveness when disseminated to clinical and community settings outside of clinical trials.
FIReWoRk Protocol and Statistical Analysis
eMethods.
eTable 1. Characteristics of Study Participants, by Having 6-Month Visit Missing or Not
eTable 2. Subgroup Analysis at 6-Months and 12-Months, Adjusted Values with Model Estimation in R
eTable 3. Sensitivity Analysis to Address Uncertainty Caused by Missing Data
Data Sharing Statement
References
- 1.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10. doi: 10.1016/j.metabol.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;360(360):1-8. [PubMed] [Google Scholar]
- 3.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009;28(5):w822-w831. doi: 10.1377/hlthaff.28.5.w822 [DOI] [PubMed] [Google Scholar]
- 4.Puhl RM, Heuer CA. Obesity stigma: important considerations for public health. Am J Public Health. 2010;100(6):1019-1028. doi: 10.2105/AJPH.2009.159491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen MD, Ryan DH, Apovian CM, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25)(suppl 2):S102-S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy J. Gallup’s Health and Healthcare survey: Americans’ effort to lose weight still trails desire. 2014. Accessed January 22, 2016. https://www.gallup.com/poll/179771/americans-effort-lose-weight-trails-desire.aspx
- 7.Kahwati LC, Lance TX, Jones KR, Kinsinger LS. RE-AIM evaluation of the Veterans Health Administration’s MOVE! Weight Management Program. Transl Behav Med. 2011;1(4):551-560. doi: 10.1007/s13142-011-0077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleich SN, Pickett-Blakely O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient Educ Couns. 2011;82(1):123-129. doi: 10.1016/j.pec.2010.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladapo JA, Prochaska JJ. Paying smokers to quit: does it work? should we do it? J Am Coll Cardiol. 2016;68(8):786-788. doi: 10.1016/j.jacc.2016.04.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631-2637. doi: 10.1001/jama.2008.804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kullgren JT, Troxel AB, Loewenstein G, et al. Individual- versus group-based financial incentives for weight loss: a randomized, controlled trial. Ann Intern Med. 2013;158(7):505-514. doi: 10.7326/0003-4819-158-7-201304020-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilger M, Shah M, Tan NC, et al. Process- and outcome-based financial incentives to improve self-management and glycemic control in people with type 2 diabetes in Singapore: a randomized controlled trial. Patient. 2021;14(5):555-567. doi: 10.1007/s40271-020-00491-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong Y, Trentadue TP, Shrestha S, Losina E, Collins JE. Financial incentives for objectively-measured physical activity or weight loss in adults with chronic health conditions: a meta-analysis. PLoS One. 2018;13(9):e0203939. doi: 10.1371/journal.pone.0203939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VanEpps EM, Troxel AB, Villamil E, et al. Effect of process- and outcome-based financial incentives on weight loss among prediabetic New York Medicaid patients: a randomized clinical trial. Am J Health Promot. 2019;33(3):372-380. doi: 10.1177/0890117118783594 [DOI] [PubMed] [Google Scholar]
- 15.Barleen NA, Marzec ML, Boerger NL, Moloney DP, Zimmerman EM, Dobro J. Outcome-based and participation-based wellness incentives: impacts on program participation and achievement of health improvement targets. J Occup Environ Med. 2017;59(3):304-312. doi: 10.1097/JOM.0000000000000965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jay M, Orstad SL, Wali S, et al. Goal-directed versus outcome-based financial incentives for weight loss among low-income patients with obesity: rationale and design of the Financial Incentives foR Weight Reduction (FIReWoRk) randomised controlled trial. BMJ Open. 2019;9(4):e025278. doi: 10.1136/bmjopen-2018-025278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Census Bureau . American Community Survey, Table B19013; generated using the Center for Enterprise Dissemination Services and Consumer Innovation platform. Accessed on November 1, 2022. https://data.census.gov/cedsci/
- 18.Morris E, Jebb SA, Oke J, et al. Effect of weight loss on cardiometabolic risk: observational analysis of two randomised controlled trials of community weight-loss programmes. Br J Gen Pract. 2021;71(705):e312-e319. doi: 10.3399/bjgp20X714113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gudzune KA, Clark JM. Role of commercial weight-loss programs in medical management of obesity. Endocrinol Metab Clin North Am. 2020;49(2):275-287. doi: 10.1016/j.ecl.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 20.Ahern AL, Wheeler GM, Aveyard P, et al. Extended and standard duration weight-loss programme referrals for adults in primary care (WRAP): a randomised controlled trial. Lancet. 2017;389(10085):2214-2225. doi: 10.1016/S0140-6736(17)30647-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrero DG, Palmer KN, Phillips EO, Miller-Kovach K, Foster GD, Saha CK. Comparison of commercial and self-initiated weight loss programs in people with prediabetes: a randomized control trial. Am J Public Health. 2016;106(5):949-956. doi: 10.2105/AJPH.2015.303035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries HJ, Kooiman TJM, van Ittersum MW, van Brussel M, de Groot M. Do activity monitors increase physical activity in adults with overweight or obesity? a systematic review and meta-analysis. Obesity (Silver Spring). 2016;24(10):2078-2091. doi: 10.1002/oby.21619 [DOI] [PubMed] [Google Scholar]
- 23.Buchner D, Bishop J, Brown D, et al. 2008 Physical activity guidelines for Americans. Accessed November 21, 2021. https://health.gov/paguidelines/pdf/paguide.pdf
- 24.Ratliff JC, Palmese LB, Tonizzo KM, Chwastiak L, Tek C. Contingency management for the treatment of antipsychotic-induced weight gain: a randomized controlled pilot study. Obes Facts. 2012;5(6):919-927. doi: 10.1159/000345975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105-115. doi: 10.1037/h0030644 [DOI] [Google Scholar]
- 26.Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627-668. doi: 10.1037/0033-2909.125.6.627 [DOI] [PubMed] [Google Scholar]
- 27.Promberger M, Marteau TM. When do financial incentives reduce intrinsic motivation? comparing behaviors studied in psychological and economic literatures. Health Psychol. 2013;32(9):950-957. doi: 10.1037/a0032727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodenheimer T, Handley MA. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns. 2009;76(2):174-180. doi: 10.1016/j.pec.2009.06.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIReWoRk Protocol and Statistical Analysis
eMethods.
eTable 1. Characteristics of Study Participants, by Having 6-Month Visit Missing or Not
eTable 2. Subgroup Analysis at 6-Months and 12-Months, Adjusted Values with Model Estimation in R
eTable 3. Sensitivity Analysis to Address Uncertainty Caused by Missing Data
Data Sharing Statement