Abstract
Infection with Bordetella pertussis, the causative agent of pertussis (whooping cough) in humans, is followed by the production of antibodies of several isotypes, including immunoglobulin A (IgA). Little is known, however, about the role of IgA in immunity against pertussis. Therefore, we studied targeting of B. pertussis to the myeloid receptor for IgA, FcαRI (CD89), using either IgA purified from immune sera of pertussis patients or bispecific antibodies directed against B. pertussis and FcαRI (CD89 BsAb). Both IgA and CD89 BsAb facilitated FcαRI-mediated binding, phagocytosis, and bacterial killing by human polymorphonuclear leukocytes (PMNL) and PMNL originating from human FcαRI-transgenic mice. Importantly, FcαRI targeting resulted in enhanced bacterial clearance in lungs of transgenic mice. These data support the capacity of IgA to induce anti-B. pertussis effector functions via the myeloid IgA receptor, FcαRI. Increasing the amount of IgA antibodies induced by pertussis vaccines may result in higher vaccine efficacy.
The gram-negative bacterium Bordetella pertussis is the causative agent of pertussis (whooping cough). B. pertussis expresses various virulence factors, including adhesins and toxins, which all play a role in pathogenesis. B. pertussis colonizes the respiratory tract using adhesins specific for ciliated cells of the respiratory epithelium. Toxins are produced and are involved in disrupting host immune responses (21). The mechanisms underlying immunity to B. pertussis are incompletely understood. In murine infection models, protection against infection was obtained upon passive transfer of anti-B. pertussis antibodies (8, 10, 23). In addition, protective effects of T helper 1 cells (2, 16) and B cells (14) have been observed, indicating that antibodies, B cells, and T cells are involved in protective immunity.
Protection against bacterial infections depends on effector activities by phagocytic cells. Elimination of bacteria involves opsonization with antibodies and recognition by certain receptors that may result in phagocytosis, bacterial killing, and antigen presentation. Upon B. pertussis infection in humans, antibody levels rise, and high levels in acute-phase sera have been associated with a lower likelihood of acquiring pertussis (3, 5, 24). Anti-B. pertussis antibodies consist of different isotypes, including immunoglobulin A (IgA) (19, 37). B. pertussis is noninvasive and is found exclusively on mucosa of the respiratory tract. Since IgA represents the predominant antibody isotype at mucosal surfaces, a role for IgA in anti-B. pertussis mechanisms is possible.
IgA is generally believed to function by neutralizing and agglutinating pathogens or by preventing their attachment to mucosal surfaces (4, 12). The role of IgA, however, may be much broader because of effector functions induced by binding to IgA receptors. The prototypic IgA receptor (FcαRI [CD89]) is found exclusively on cells of the myeloid lineage: monocytes, macrophages, neutrophils, and eosinophils (13, 15, 17). Increasing evidence shows that FcαRI exhibits potent proinflammatory capacities. FcαRI cross-linking readily induces phagocytosis, degranulation, respiratory burst, antibody-dependent cellular cytotoxicity, and the release of proinflammatory cytokines (31).
The aim of the present study was to evaluate IgA-mediated effector functions against B. pertussis by studying the interaction of IgA-coated B. pertussis with human polymorphonuclear leukocytes (PMNL). In addition, experiments were performed with transgenic (Tg) mice expressing the human FcαRI (28). There is no known homologue of FcαRI in mice, and CD89-Tg mice have been used to study the in vivo role of human FcαRI (29). We demonstrate that anti-B. pertussis IgA exhibits bactericidal effector function via facilitation of binding, phagocytosis, and killing of B. pertussis involving FcαRI.
MATERIALS AND METHODS
Mice.
FcαRI (CD89) transgenic mice, were crossed with C57BL/6 mice, and experiments were performed with F1 generation Tg mice and nontransgenic (NTg) littermates. Similar to the situation in humans, FcαRI in these mice is constitutively expressed on PMNL and is inducible on macrophages (28). Both male and female mice were used at between 5 and 9 weeks of age. Mice were maintained under supervision of the institutes council for experiments on animals (DEC), according to Dutch legislation.
Bacterial strains and growth conditions.
B. pertussis strain B213 was used for the experiments and is a streptomycin-resistant derivative of strain Tohama. Bacteria were stored at −70°C, recovered by growth on Bordet Gengou (BG) agar plates supplemented with 30 μg of streptomycin per ml at 35°C for 3 days, and used for in vitro experiments. For infection of mice, strains were subsequently plated on BG plates without antibiotics, cultured for 3 days, and used for infection.
Antibodies.
Sera of pertussis patients with high B. pertussis-specific IgA titers (measured by IgA enzyme-linked immunosorbent assay as described in reference 19) were pooled. IgA antibodies were subsequently purified using Affi-T columns (Biozym, Landgraaf, The Netherlands) and separated by size chromatography (Superdex 200; Pharmacia, Piscataway, N.J.). Fractions were analyzed by electrophoresis on sodium dodecyl sulfate–4 to 15% gradient gels (Phast gel; Pharmacia) and Coomassie brilliant blue staining. To exclude the presence of other isotypes, Western blot analyses were performed. Anti-FcαRI (A77; murine IgG1) was obtained from Medarex (Annandale, N.J.). FcαRI-blocking monoclonal antibody (2D11; murine IgG1) was a generous gift of G. van Zandbergen (18). Rabbits were immunized with pertussis whole-cell vaccine (RIVM, Bilthoven, The Netherlands) to generate polyclonal rabbit anti-B. pertussis IgG. Upon immunization, rabbits were boosted at 3 and 6 weeks. Sera were collected 7 weeks after primary immunization, and IgG was isolated using protein G columns (Pharmacia). CD89 bispecific antibodies (BsAb) with dual specificity for both B. pertussis and FcαRI were produced by chemical cross-linking as described previously (9). Briefly, F(ab′)2 fragments of polyclonal rabbit anti-B. pertussis antibodies were treated with sulfosuccinimidyl 4-(N-malmeimidomethyl)cyclohexane-1-carboxylate (sulfo-SMCC), which binds free lysines. The malmeimide groups of these F(ab′)2-SMCC fragments were reacted with equimolar concentrations of F(ab′) fragments of the A77 monoclonal antibody directed to FcαRI. BsAb were purified using Superdex 200 (Pharmacia) and analyzed by sodium dodecyl sulfate–8 to 18% gradient polyacrylamide gel electrophoresis (Pharmacia) after staining with Coomassie brilliant blue. Opsonization of B. pertussis was performed by incubation of bacteria with either IgA or CD89 BsAb for 30 min at 37°C. To assess IgA binding, bacteria were washed and further incubated with F(ab′)2 fragments of goat anti-human serum IgA (Jackson, West Grove, Pa.). To test (dual) specificity of CD89 BsAb, bacteria were incubated with either fluorescein isothiocyanate (FITC)-labeled F(ab′)2 fragments of goat anti-rabbit IgG antibodies (Jackson) or FITC-labeled F(ab′)2 fragments of goat anti-mouse IgG (Jackson). After washing, opsonization was quantified by flow cytometry on a FACScan (BD Biosciences, Europe).
PMNL isolation.
Human PMNL were isolated from heparinized blood using Ficoll-Histopaque (Sigma, St. Louis, Mo.) gradient centrifugation. PMNL were harvested, and erythrocytes were removed by hypotonic lysis. Cells were washed twice with RPMI 1640 medium supplemented with 10% fetal calf serum, counted, and used immediately.
Mouse PMNL were obtained as described previously (30). In short, mice were injected subcutaneously with 15 μg of pegylated granulocyte colony-stimulating factor (G-CSF) (Amgen, Thousand Oaks, Calif.) in 150 μl of phosphate-buffered saline. After 3 days, heparinized blood was drawn from the orbita plexus and erythrocytes were lysed by hypotonic treatment. The remaining leukocytes, consisting of ≈50% PMNL, ≈50% lymphocytes, and some monocytes, were washed three times in RPMI 1640–10% fetal calf serum prior to use.
Phagocytosis.
Phagocytosis of opsonized B. pertussis was measured by a flow cytometric assay (22) with minor modifications. B. pertussis was labeled with PKH-26 (Sigma), a membrane marker with fluorescent characteristics in the phycoerythrin channel, according to the protocol provided by the manufacturer. PKH-26-labeled B. pertussis was opsonized with either polyclonal human anti-B. pertussis IgA (100 μg/ml) or CD89 BsAb (100 μg/ml) for 30 min at 37°C. Free antibodies were removed by washing, and bacteria were incubated with PMNL (mouse PMNL/B. pertussis ratio, 1:10; human PMNL/B. pertussis ratio, 1:100) for 30 min at 4°C to allow adherence. Unbound bacteria were removed by washing at 290 × g, and samples were split between two tubes and further incubated for 30 min at either 37°C (to allow phagocytosis) or 4°C (as a control for bacterial binding). In selected experiments, 4 μg of cytochalasin D (Sigma) was added to confirm that binding was followed by true phagocytosis. Phagocytosis was stopped by incubating PMNL on ice, and the cells were washed at 4°C with phosphate-buffered saline containing 0.1% sodium azide and 1% bovine serum albumin (Roche, Almere, The Netherlands). Remaining cell surface-bound bacteria were detected by incubation with FITC-conjugated F(ab′)2 fragments of goat anti-human serum IgA (α-chain specific; Jackson) (30 min, 4°C). Subsequently, samples were analyzed by flow cytometry. PKH-26 fluorescence served to determine the total amount of bacteria associated with PMNL. The decrease in FITC fluorescence between samples incubated at 4 and 37°C reflected phagocytosis, which was confirmed microscopically. Phagocytosis rates were calculated as described previously (22) as ΔFITC4–37°C/FITC4°C × PKH-26 and were expressed in arbitrary units. In selected experiments FcαRI was blocked by preincubating PMNL with monoclonal antibody 2D11 (10 μg/ml) for 20 min at 4°C prior to the attachment step.
B. pertussis kill assay.
Freshly grown B. pertussis was opsonized with CD89 BsAb (100 μg/ml), washed, and incubated with mouse PMNL (10:1) for 30 min at 4°C to allow adherence. To remove nonadherent bacteria, samples were washed extensively and were split among three tubes. One tube was used to determine the number of adherent bacteria by plating serial dilutions prepared in Verwey medium (32) on BG agar plates in triplicate. The remaining tubes were incubated at 37°C for 30 min to allow phagocytosis and killing. Subsequently, in the second tube, extracellular bacteria were killed by treatment with gentamicin (200 μg/ml) for 15 min, which killed >99% of the bacteria as determined in pilot experiments. The numbers of bacteria that survived were determined after washing (to remove gentamicin). PMNL were lysed in ice-cold distilled water (containing 1% saponin) for 10 min, and serial dilutions were prepared in Verwey medium and plated in triplicate on BG agar plates. Since not all bacteria that bind PMNL are phagocytosed, the last tube was used to quantify the percentage of phagocytosis by flow cytometry (phagocytosis assay described above), which was determined by the percent decrease in FITC fluorescence from samples incubated at 4°C (binding) compared to 37°C (binding and phagocytosis) (i.e., FITC fluorescence detects extracellular bacteria). To quantify the number of phagocytosed bacteria that were killed, numbers of intracellular bacteria (Nic) were calculated [Nic = (number of adherent bacteria/percent phagocytosis) × 100%], and numbers of killed bacteria were determined [Nic − number of viable bacteria at 30 min].
B. pertussis infection of mice.
B. pertussis (109/ml) was opsonized with 100 μg of polyclonal human IgA directed against B. pertussis per ml for 1 h at room temperature. Unbound antibodies were washed away, and mice were infected with opsonized or similarly treated nonopsonized bacteria. Intranasal infection of mice was performed as described previously (36). Briefly, mice were lightly anesthetized with ether, and 20 μl of inoculum (containing 107 B. pertussis organisms) was carefully placed on the top of each nostril and allowed to be inhaled. Prior to infection, the numbers of CFU in the inocula were determined by plating on BG plates. To assess bacterial colonization, groups of mice were killed by intramuscular injection of an overdose of pentobarbital sodium (Nembutal; Sanofi, Maassluis, The Netherlands) 2 days after infection. Lungs were excised and then homogenized using a blender in 900 μl of Verwey medium. Viable bacteria in homogenized organs were determined by plating serial dilutions on BG agar plates supplemented with 30 μg of streptomycin per ml.
Statistical analyses.
Means and standard deviations were calculated from log10-transformed numbers of CFU. Differences between various groups were assessed by two-tailed Student t tests with significance at a P value of <0.05.
RESULTS
IgA enhances PMNL phagocytosis of B. pertussis
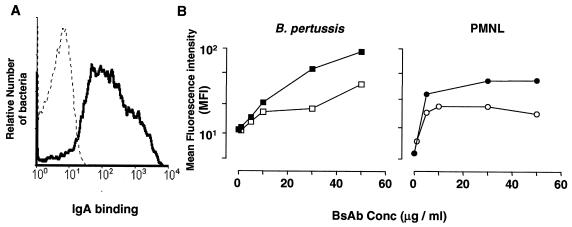
To study IgA-mediated cellular effector functions, we evaluated whether IgA antibodies facilitated binding and uptake of B. pertussis by PMNL. First, human anti-B. pertussis serum IgA was purified and its opsonic potential was investigated. B. pertussis cells were incubated with human IgA, and binding was visualized with FITC-conjugated secondary antibodies (α-chain specific). Purified anti-B. pertussis IgA bound B. pertussis efficiently as detected by flow cytometry (Fig. 1A). We next generated BsAb, consisting of one arm directed to B. pertussis and one directed to FcαRI (CD89 BsAb). The different origins of the two arms (mouse and rabbit) of CD89 BsAb enabled us to test their dual specificity. Isolated mouse PMNL and freshly grown B. pertussis were incubated with CD89 BsAb, and bound BsAb were stained with either FITC-conjugated anti-rabbit or anti-mouse immunoglobulin antibodies. CD89 BsAb bound effectively to both PMNL and B. pertussis and was recognized by both antirabbit and anti-mouse reagents (Fig. 1B).
FIG. 1.
Binding of IgA and BsAb to B. pertussis and PMNL. (A) B. pertussis was incubated with IgA, and binding was visualized by incubation with FITC-conjugated anti-human IgA (black line). FITC-conjugated anti-human IgA alone was used as a control (dotted line). Bacteria were gated based on their scatter characteristics. (B) B. pertussis and PMNL were incubated with CD89 BsAb directed against FcαRI. Binding was visualized with FITC-conjugated anti-rabbit IgG (□, ○) or FITC-conjugated anti-mouse IgG (▪, ●). Data are representative of those from three separate experiments. Conc, concentration.
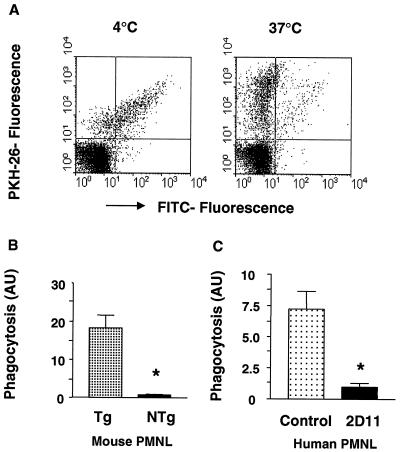
Phagocytosis of FcαRI-directed B. pertussis was analyzed using flow cytometry. B. pertussis was labeled with PKH-26, a red fluorescent marker detectable in the phycoerythrin channel, opsonized with human anti-B. pertussis IgA antibodies, and incubated with PMNL. In our flow cytometric assay, PKH-26 fluorescence reflects PMNL binding and phagocytosis of B. pertussis, whereas FITC fluorescence selectively assays nonphagocytosed (surface-bound) bacteria. Phagocytosis of IgA-opsonized B. pertussis was assessed using PMNL from human FcαRI-Tg mice and PMNL from NTg littermates (controls). IgA enhanced binding and subsequent phagocytosis of B. pertussis by Tg PMNL, as was reflected by decreased FITC fluorescence after a temperature shift from 4 to 37°C (Fig. 2A and B). In selected experiments, cytochalasin D was added during the assay to inhibit internalization. Phagocytosis was subsequently inhibited, which was indicated by residual high FITC fluorescence after incubation at 37°C (not shown). Phagocytosis was largely mediated by FcαRI, since NTg (control) PMNL bound IgA-opsonized B. pertussis less efficiently and attached bacteria were phagocytosed less well (Fig. 2B). Similar experiments were performed with human PMNL. Serum IgA promoted uptake and phagocytosis of B. pertussis by human PMNL, which was blocked by FcαRI-blocking antibody 2D11 (Fig. 2C). To prove phagocytosis to be truly triggered by IgA-FcαRI interactions, similar experiments were performed with CD89 BsAb-opsonized B. pertussis, yielding identical results (data not shown).
FIG. 2.
IgA induces B. pertussis phagocytosis by PMNL. (A) Mouse PMNL Tg for the human IgA receptor were incubated with PKH-26-labeled and IgA-opsonized B. pertussis at 4°C for 30 min. Nonadherent bacteria were removed, and PMNL were further incubated for 30 min at either 4 or 37°C to allow phagocytosis. Subsequently, surface-bound B. pertussis cells were detected by incubation with FITC-conjugated F(ab′)2 fragments. Viable PMNL were gated based on their scatter characteristics, and a reduction of FITC fluorescence reflects uptake of B. pertussis by PMNL. (B) Phagocytosis was expressed in arbitrary units (AU). (C) Phagocytosis of IgA-opsonized B. pertussis by human PMNL in the absence (control) or presence of FcαRI-blocking antibody 2D11. Data are representative of those from at least three individual experiments and are depicted as means and standard errors of the means. ∗, P < 0.05.
Bactericidal activity mediated via FcαRI.
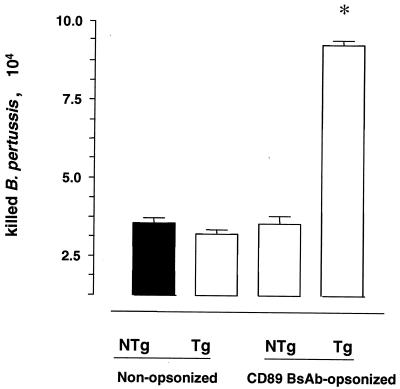
We next investigated whether phagocytosis via FcαRI induces PMNL-mediated bacterial killing. Tg and NTg PMNL were allowed to internalize nonopsonized or CD89 BsAb-opsonized B. pertussis, and numbers of killed bacteria were determined. Both Tg and NTg PMNL killed B. pertussis, but FcαRI targeting significantly increased the numbers of killed bacteria (Fig. 3).
FIG. 3.
Bactericidal activity mediated by mouse PMNL Tg for the human FcαRI. FcαRI-Tg and NTg mouse PMNL were incubated with nonopsonized B. pertussis or B. pertussis opsonized with CD89 BsAb. Numbers of killed bacteria per 105 PMNL were determined after 30 min. Bars represent means and standard deviations of killed bacteria from one representative experiment out of three. ∗, P < 0.05.
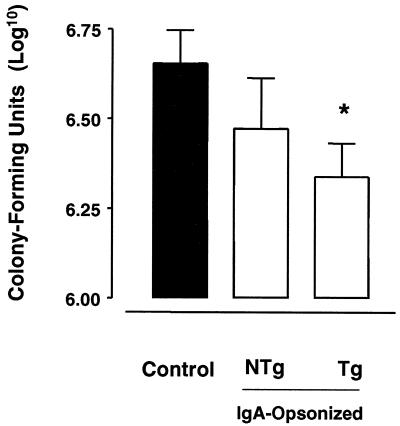
Anti-B. pertussis activity via FcαRI was also analyzed in vivo by infecting both Tg and NTg mice with B. pertussis, either nonopsonized or opsonized with IgA. In the infection model, decreased numbers of viable B. pertussis organisms in murine airways reflect protection against bacterial infection (36). Upon IgA opsonization, the numbers of viable B. pertussis organisms in lungs of Tg mice were significantly decreased compared to those in lungs of mice infected with nonopsonized bacteria. A decrease in colonization of lungs of NTg mice was also observed but was not significant (Fig. 4).
FIG. 4.
In vivo targeting to human FcαRI enhances B. pertussis clearance in FcαRI-Tg mice. Tg and NTg mice were infected intranasally with B. pertussis that was either nonopsonized or opsonized with serum IgA. The control group, consisting of both Tg and NTg mice, was infected with nonopsonized bacteria. Two days after infection, lungs were excised and homogenized, and the numbers of viable bacteria were determined. Each bar represents the mean number of log10 CFU from at least 12 mice tested in two individual experiments. Error bars indicate standard errors of the means. ∗, P < 0.05.
DISCUSSION
In spite of high vaccination coverage in developed countries, the incidence of B. pertussis infections appears to be rising (6). Research into the basis of immunity may lead to the development of more effective vaccines. In humans, infection is followed by the production of antibodies of several isotypes, including IgA (19, 37). In this study, anti-B. pertussis IgA was shown to be capable of inducing bactericidal effects by facilitating binding, phagocytosis, and killing of B. pertussis via the myeloid IgA receptor, FcαRI (CD89).
Human PMNL bound and phagocytosed IgA-opsonized B. pertussis, and both processes were inhibitable by blocking FcαRI. This indicated that IgA-induced phagocytosis is largely mediated by FcαRI. Tg mouse PMNL expressing human FcαRI also exhibited potent IgA-mediated phagocytosis. FcαRI-mediated phagocytosis resulted in enhanced killing of B. pertussis by PMNL. The enhanced phagocytosis of mouse PMNL relative to human cells (Fig. 2) is likely attributable to the treatment with G-CSF prior to mouse PMNL isolation, which is known to stimulate FcαRI function (35). Although both Tg and NTg PMNL bound IgA-opsonized B. pertussis, binding and phagocytosis by Tg PMNL was clearly enhanced. B. pertussis binding to NTg PMNL is most likely mediated by B. pertussis virulence factors that interact directly with phagocyte receptors such as CR3 (20) and VLA-5 (11).
To prove that IgA-mediated effects were truly attributable to interaction with FcαRI, experiments were performed with both IgA and CD89 BsAb. The advantage of CD89 BsAb is that they recognize FcαRI outside its ligand-binding domain, which enables direct bacterial targeting to FcαRI. All in vitro experiments were performed with both IgA and CD89 BsAb-opsonized bacteria, yielding similar results, which demonstrated that the IgA-mediated effects depend on FcαRI triggering.
More importantly, IgA-mediated anti-B. pertussis activity was also observed in a murine pertussis infection model. Previously, high IgA titers in sera of human pertussis patients younger than 1 year of age were found to correlate with reduced duration of positive pertussis culture and PCR in throat samples (26). These findings pointed to bactericidal effects of anti-B. pertussis IgA in humans. Indeed, in our Tg mouse model, IgA opsonization of B. pertussis prior to infection resulted in increased bacterial clearance in lungs that was attributable to FcαRI interaction.
The IgA used in our work was purified from immune sera of B. pertussis patients that were collected relatively soon after infection. Serum IgA consists mainly of IgA1, and in the upper respiratory tract IgA1 also represents the main antibody isotype (4). However, in contrast to serum IgA, mucosal secretory IgA is in a large part dimeric, containing the J chain and secretory component. Although secretory IgA is capable of interacting with FcαRI, the types of functions initiated by serum and secretory IgAs may be different (4).
A recent study reported that serum opsonization of B. pertussis inhibited phagocytosis by PMNL compared to no opsonization (33). Our data indicate that purified IgA antibodies are able to increase PMNL binding and phagocytosis of B. pertussis. Our phagocytosis assay, however, differs from that used by Weingart et al. (33) in that we used PMNL in suspension rather than as adherent cells. Second, adenylate cyclase toxin was reported to be the virulence factor responsible for inhibition of opsonized B. pertussis phagocytosis (34). Our antibodies may (partly) consist of adenylate cyclase toxin-neutralizing antibodies, resulting in efficient phagocytosis in the present study.
A recent trial with pertussis vaccines in The Netherlands showed that boosting of 4-year-old children with the Dutch whole-cell pertussis vaccine induced anti-B. pertussis-specific serum IgA, in contrast to boosting with acellular vaccines (1). Our findings demonstrating IgA to be capable of inducing anti-B. pertussis activity may be important in the evaluation of vaccines. For years IgA has been considered to play a passive, “noninflammatory” role in immunity; by blocking microbial interaction with host tissue, it may prevent cell damage and inflammation. However, IgA proved to be very effective in inducing cellular immune functions via FcαRI expressed on myeloid cells. A number of recent studies have already reported IgA-mediated phagocytosis of different microorganisms and tumor cells (7, 25, 27, 30). This study documents an important role for IgA in anti-B. pertussis activity and shows, for the first time, IgA-mediated bactericidal activity in vivo.
ACKNOWLEDGMENTS
We thank Bert Elvers for assembling the immune sera of pertussis patients, Jeff Andresen for kindly providing G-CSF, and Ger van Zandbergen for kindly providing FcαRI-blocking antibody 2D11.
Sandra M. M. Hellwig and Annemiek B. van Spriel contributed equally to this study.
REFERENCES
- 1.Berbers G, Lafeber A B, Labadie B, Vermeer-de Bondt P E, Bolscher D J A, Plantinga A D. A randomized controlled study with whole-cell or acellular pertussis vaccines in combination with regular DT-IPV vaccine and a new poliomyelitis (IPV-Vero) component in children 4 years of age in The Netherlands. Report no. 105000001. Bilthoven, The Netherlands: National Institute of Health and the Environment; 1999. [Google Scholar]
- 2.Brady M T, Mahon B P, Mills K H G. Pertussis infection and vaccination induces Th1 cells. Immunol Today. 1998;19:534. doi: 10.1016/s0167-5699(98)01359-0. [DOI] [PubMed] [Google Scholar]
- 3.Cherry J D, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine. 1998;16:1901–1906. doi: 10.1016/s0264-410x(98)00226-6. [DOI] [PubMed] [Google Scholar]
- 4.Childers N K, Bruce M G, McGhee J R. Molecular mechanisms of immunoglobulin A defense. Annu Rev Microbiol. 1989;43:503–536. doi: 10.1146/annurev.mi.43.100189.002443. [DOI] [PubMed] [Google Scholar]
- 5.Deen J L, Mink C M, Cherry J D, et al. Household contact study of Bordetella pertussis infections. Clin Infect Dis. 1995;21:1211–1219. doi: 10.1093/clinids/21.5.1211. [DOI] [PubMed] [Google Scholar]
- 6.de Melker H E, Conyn van Spaendonck M A, Rumke H C, van Wijngaarden J K, Mooi F R, Schellekens J F. Pertussis in The Netherlands: an outbreak despite high levels of immunization with whole-cell vaccine. Emerg Infect Dis. 1997;3:175–178. doi: 10.3201/eid0302.970211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deo Y M, Sundarapandiyan K, Keler T, Wallace P K, Graziano R F. Bispecific molecules directed to the Fc receptor for IgA (Fc alpha RI, CD89) and tumor antigens efficiently promote cell-mediated cytotoxicity of tumor targets in whole blood. J Immunol. 1998;160:1677–1686. [PubMed] [Google Scholar]
- 8.Dolby J, Dolby D, Bronne S C. The effects of humoral, cellular and non-specific immunity on intracerebral Bordetella pertussis infections in mice. J Hyg. 1975;74:85–102. doi: 10.1017/s002217240004674x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanger M W. Bispecific antibodies. Heidelberg, Germany: Springer-Verlag; 1995. pp. 1–26. [Google Scholar]
- 10.Halperin S, Issekutz T, Kasina Modulation of Bordetella pertussis infection with monoclonal antibodies to pertussis toxin. J Infect Dis. 1991;163:355–361. doi: 10.1093/infdis/163.2.355. [DOI] [PubMed] [Google Scholar]
- 11.Hazenbos W L W, van den Berg B M, Geuijen C W, Mooi F R, van Furth R. Binding of FimD on Bordetella pertussis to very late antigen-5 on monocytes activates complement receptor type 3 via protein tyrosine kinases. J Immunol. 1995;155:3972–3978. [PubMed] [Google Scholar]
- 12.Kerr M A. The structure and function of human IgA. Biochem J. 1990;271:285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr M A, Mazengera R L, Stewart W W. Structure and function of immunoglobulin A receptors on phagocytic cells. Biochem Soc Trans. 1990;18:215–217. doi: 10.1042/bst0180215. [DOI] [PubMed] [Google Scholar]
- 14.Leef M, Elkins K L, Barbic J, Shahin R D. Protective immunity to Bordetella pertussis requires both B cells and CD4(+) T cells for key functions other than specific antibody production. J Exp Med. 2000;191:1841–1852. doi: 10.1084/jem.191.11.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazengera R L, Kerr M A. The specificity of the IgA receptor purified from human neutrophils. Biochem J. 1990;272:159–165. doi: 10.1042/bj2720159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills K, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton H C, van Egmond M, van de Winkel J G J. Structure and function of human IgA Fc receptors (Fc alpha R) Crit Rev Immunol. 1996;16:423–440. [PubMed] [Google Scholar]
- 18.Morton H C, van Zandbergen G, van Kooten C, Howard C J, van de Winkel J G J, Brandtzaeg P. Immunoglobulin-binding sites of human Fc alpha RI (CD89) and bovine Fc gamma 2R are located in their membrane-distal extracellular domains. J Exp Med. 1999;189:1715–1722. doi: 10.1084/jem.189.11.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagel J, Poot Scholtens E J. Serum IgA antibody to Bordetella pertussis as an indicator of infection. J Med Microbiol. 1983;16:417–426. doi: 10.1099/00222615-16-4-417. [DOI] [PubMed] [Google Scholar]
- 20.Relman D, Tuomanen E, Falkow S, Golenbock D, Saukkonen K, Wright S. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 21.Relman D A. Bordetella pertussis: determinants of virulence. In: Moss J, et al., editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Dekker; 1995. pp. 367–405. [Google Scholar]
- 22.Rodriguez M E, van der Pol W L, Sanders L A, van de Winkel J G J. Crucial role of FcgammaRIIa (CD32) in assessment of functional anti-Streptococcus pneumoniae antibody activity in human sera. J Infect Dis. 1999;179:423–433. doi: 10.1086/314603. [DOI] [PubMed] [Google Scholar]
- 23.Sato H, Sato Y. Protective activities in mice of monoclonal antibodies against pertussis toxin. Infect Immun. 1990;58:3369–3374. doi: 10.1128/iai.58.10.3369-3374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storsaeter J, Hallander H O, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16:1907–1916. doi: 10.1016/s0264-410x(98)00227-8. [DOI] [PubMed] [Google Scholar]
- 25.van der Pol W, Vidarsson G, Vile H A, van de Winkel J G J, Rodriguez M E. Pneumococcal capsular polysaccharide-specific IgA triggers efficient neutrophil effector functions via Fc alpha RI (CD89) J Infect Dis. 2000;182:1139–1145. doi: 10.1086/315825. [DOI] [PubMed] [Google Scholar]
- 26.van der Zee A, Agterberg C, Peeters M, Mooi F, Schellekens J. A clinical validation of Bordetella pertussis and Bordetella parapertussis polymerase chain reaction: comparison with culture and serology using samples from patients with suspected whooping cough from a highly immunized population. J Infect Dis. 1996;174:89–96. doi: 10.1093/infdis/174.1.89. [DOI] [PubMed] [Google Scholar]
- 27.van Egmond M, van Vuuren A J, van de Winkel J G J. The human Fc receptor for IgA (Fc alpha RI, CD89) on transgenic peritoneal macrophages triggers phagocytosis and tumor cell lysis. Immunol Lett. 1999;68:83–87. doi: 10.1016/s0165-2478(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 28.van Egmond M, van Vuuren A J, Morton H C, et al. Human immunoglobulin A receptor (Fc alpha RI, CD89) function in transgenic mice requires both FcR gamma chain and CR3 (CD11b/CD18) Blood. 1999;93:4387–4394. [PubMed] [Google Scholar]
- 29.van Egmond M, van Garderen E, van Spriel A B, et al. FcalphaRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med. 2000;6:680–685. doi: 10.1038/76261. [DOI] [PubMed] [Google Scholar]
- 30.van Spriel A B, van den Herik Oudijk I E, van Sorge N M, Vile H A, van Strijp J A, van de Winkel J G J. Effective phagocytosis and killing of Candida albicans via targeting FcgammaRI (CD64) or FcalphaRI (CD89) on neutrophils. J Infect Dis. 1999;179:661–669. doi: 10.1086/314643. [DOI] [PubMed] [Google Scholar]
- 31.van Spriel, A. B., and J. G. J. van de Winkel. CD89 (FcαRI) review. Encycloped. Mol. Med., in press.
- 32.Verweij T S S. A simplified liquid culture medium for growth of Haemophilus pertussis. J Bacteriol. 1949;58:127–134. [PMC free article] [PubMed] [Google Scholar]
- 33.Weingart C L, Broitman Maduro G, Dean G, Newman S, Peppler M, Weiss A A. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 1999;67:4264–4267. doi: 10.1128/iai.67.8.4264-4267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weingart C L, Mobberley-Schuman P S, Hewlett E L, Gray M C, Weiss A A. Neutralizing antibodies to adenylate cyclase toxin promote phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun. 2000;68:7152–7155. doi: 10.1128/iai.68.12.7152-7155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisbart R H, Kacena A, Schuh A, Golde D W. GM-CSF induces human neutrophil IgA-mediated phagocytosis by an IgA Fc receptor activation mechanism. Nature. 1988;32:647–648. doi: 10.1038/332647a0. [DOI] [PubMed] [Google Scholar]
- 36.Willems R J L, Kamerbeek J, Geuijen C A W, et al. The efficacy of a whole cell pertussis vaccine and fimbriae against Bordetella pertussis and Bordetella parapertussis infections in a respiratory mouse model. Vaccine. 1998;16:410–416. doi: 10.1016/s0264-410x(97)80919-x. [DOI] [PubMed] [Google Scholar]
- 37.Zackrisson G, Lagergard T, Trollfors B, Krantz I. Immunoglobulin A antibodies to pertussis toxin and filamentous hemagglutinin in saliva from patients with pertussis. J Clin Microbiol. 1990;28:1502–1505. doi: 10.1128/jcm.28.7.1502-1505.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]