Abstract
Immunoglobulin binding proteins are one of several pathogenicity factors which have been associated with invasive disease caused by group A streptococci. The surface-bound M and M-like proteins of Streptococcus pyogenes are the most characterized of these immunoglobulin binding proteins, and in most cases they bind only a single antibody class. Here we report the identification of a novel non-M-type secreted protein, designated SibA (for secreted immunoglobulin binding protein from group A streptococcus), which binds all immunoglobulin G (IgG) subclasses, the Fc and Fab fragments, and also IgA and IgM. SibA has no significant sequence homology to any M-related proteins, is not found in the vir regulon, and contains none of the characteristic M-protein regions, such as the A or C repeats. Like M proteins, however, SibA does have relatively high levels of alanine, lysine, glutamic acid, leucine, and glycine. SibA and M proteins also share an alpha-helical N-terminal secondary structure which has been previously implicated in immunoglobulin binding in M proteins. Evidence presented here indicates that this is also the case for SibA. SibA also has regions of local similarity with other coiled-coil proteins such as Listeria monocytogenes P45 autolysin, human myosin heavy chain, macrogolgin, and Schistoma mansoni paramyosin, some of which are of potential significance since cross-reactive antibodies between myosin proteins and M proteins have been implicated in the development of the autoimmune sequelae of streptococcal disease.
The resurgence of severe group A streptococcal (GAS) disease has led to increased research efforts into the pathogenicity mechanisms of the causal bacterial species, Streptococcus pyogenes. This agent is responsible for a wide variety of diseases, ranging from uncomplicated pharyngitis (5) and impetigo (14) to severe life-threatening invasive conditions such as toxic shock-like syndrome, necrotizing fasciitis, and scarlet fever (30, 31). Complications of GAS infections can also lead to the poststreptococcal sequelae of rheumatic fever and glomerulonephritis (32). The exact mechanisms of GAS pathogenesis have not yet been fully elucidated; however, it has been demonstrated that these bacteria are capable of interacting with a large number of host cells and tissues types. This diversity has led to the speculation that the type of colonization event, e.g., adhesion and at times internalization, contributes directly to the various manifestations of disease (for reviews, see references 11, 12, and 21).
Immunoglobulin binding proteins are one of the pathogenicity factors which have been associated with invasive GAS disease (4, 23). The mechanism of their action is not fully understood; however, it is likely that they contribute to evasion of the host's immune defenses. Binding of both immunoglobulin A (IgA) and IgG or of IgG alone is found in all invasive-disease clinical isolates (24), whereas in septicemia and noninvasive throat strains, immunoglobulin binding is not a common characteristic (28). Nearly all S. pyogenes immunoglobulin binding proteins reported to date are of the surface-bound M protein family (for a review, see reference 28). All of these proteins, with the exception of Sir22, are limited to binding a single immunoglobulin class, whereas the aberrant Sir22 protein binds all IgG subclasses and also IgA (29).
We report here the identification of a novel non-M-type secreted protein from S. pyogenes, designated SibA (for secreted immunoglobulin binding protein from GAS), with an apparent molecular mass of 45 kDa which binds IgG, IgM, and IgA. Investigation revealed that >99% of GAS strains of various M types contained the encoding gene, and preliminary sequence analysis indicates that the DNA sequence is highly conserved. Sequence comparison searches demonstrated similarity to the N termini of predicted proteins from a number of other gram-positive bacteria; however, the variant predicted sequence for the remainder of the gene is a novel sequence.
MATERIALS AND METHODS
Bacterial strains and plasmids.
A total of 113 GAS isolates were used in this study. These strains were obtained from the Klinikum Aachen, Aachen, Germany (31 strains); Untersuchungsamt of Braunschweig, Braunschweig, Germany (6 strains); Royal Darwin Hospital, Darwin, Australia (40 strains); the German Microorganism Collection (Braunschweig, Germany) (4 strains); Untersuchungsamt of Giessen, Giessen, Germany (13 strains); the University of Minnesota, Minneapolis (6 strains); the Institute of Medical Microbiology, Muenster, Germany (11 strains); and the University of Siena, Siena, Italy (2 strains). All strains were typed and are representative of a broad spectrum of Vir types (10). Streptococci for PCR screening were grown at 37°C on 5% sheep blood agar plates. For liquid culture growth, S. pyogenes M1 strain A106 was incubated overnight in Todd-Hewitt broth (Oxoid) supplemented with 1% (wt/vol) yeast extract (Difco) (THY broth) at 37°C with shaking and subsequently harvested by centrifugation (4,000 × g for 15 min).
Identification of immunoglobulin binding protein.
In a previous study (25) Streptococcus agalactiae was grown overnight in THY broth at 37°C with shaking. The cells were subsequently removed by centrifugation (4,000 × g for 15 min), and the proteins in the supernatant were resolved by polyacrylamide gel electrophoresis (PAGE) and subsequently tested for immunoglobulin binding using Western blotting. A secreted immunoglobulin binding protein was identified, and N-terminal protein sequencing gave partial sequence information which was compared with the S. pyogenes genome sequence database at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) using the TBLASTN program (2). An open reading frame was identified, and PCR primers were designed to amplify the gene from S. pyogenes strain A106, which was subsequently cloned in a His tag expression vector (Qiagen); the plasmid was designated pBG1.
Construction of subclones.
The sibA sequence was searched using the Simple Modular Architecture Research Tool (http://smart.embl-heidelberg.de/smart/show_motifs.pl). Areas with predicted secondary structures were noted, and a series of primers for subclones was designed. The relevant PCR products were amplified from the parental S. pyogenes A106 strain and were ligated into a cloning vector (pCR2.1 TOPO [Invitrogen]). The sibA gene fragments were subsequently cloned in a His tag expression vector (Qiagen), and the plasmids were designated pKF21 to pKF23.
Recombinant protein purification techniques.
The recombinant protein was expressed in pQE32 (Qiagen), which encodes a six-histidine tag fused to the C terminus of the recombinant protein. Purification was carried out using a nickel affinity column to bind the histidine tag. The protocol used was for either native or denatured protein purification as described by the manufacturer. The eluted protein was subsequently dialyzed against phosphate-buffered saline (PBS) overnight at 4°C.
DNA techniques.
Agarose gel electrophoresis and other routine DNA techniques were carried out essentially as described by Sambrook et al. (26). DNA was isolated using commercially available kits from Qiagen according to the manufacturer's instructions. DNA sequencing was carried out using the Applied Biosystems ABI-Prism sequencing kit as described by the manufacturer. Structural characteristics of the predicted protein were determined using the following programs available on the Internet: tmpred, Paircoil score, ProtParam (http://www.expasy.ch/cgi-bin/protparam), meta-signalp, and 2zip-results (http://www.dfkz-heideberg.de/tbi).
PCR.
Ten bacterial colonies were treated using InstaGene (Bio-Rad) matrix to remove PCR inhibitors according to the manufacturer's instructions. A-2 μl aliquot of the prepared sample was used as the template in subsequent PCRs. PCR was performed with primers pBG1-F (5′-TGCCACACGAGCTCGTGAGGATTTAAGTACTA-3′), pBG1-R (5′-AAAAGAGCTCAAGCTTCTCTCAGAACTATT-3′), pKF20-F (5′-GGAGCGGAGGATTTAAGTACTAAGA-3′), pKF20-R (5′-GCAACAATTTGACTTGTTAGAGCCT-3′), pKF21-F (5′-GGAGCGGAGGATTTAAGTACTAAGA-3′), pKF21-R (5′-AGCTTGCTTTTCTTCAAGGGA-3′), pKF22-F (5′-GGAGCGGAGGATTTAAGTACTAAGA-3′), and pKF22-R (5′-CTTCAGTAGCAGATGCTAATTGGAG-3′).
For all PCRs the following conditions were used: initial denaturing at 94°C for 2 min followed by a 32-cycle run with 94°C for 30 s (denaturing), 60°C for 30 s (annealing), and 72°C for 90 s (extension). A final step of 72°C for 4 min ensured that amplification of the PCR products was complete. In the event of a variant-sized product being amplified, a series of eight internal primers was designed over the entire length and used to confirm sibA identity.
Southern hybridization dot blotting.
A digoxigenin PCR labeling kit (Roche) was used to label the entire sibA gene, and screening of S. pyogenes by colony hybridization was carried out according to the manufacturer's instructions with the following modifications. Cells from 3 ml of an overnight culture were pelleted and washed with PBS before resuspension in colony Taq buffer (200 mM Tris, 20 mM MgCl2, 250 mM KCl, 0.5% Tween 20, and 1 mg of gelatin per ml). Bacteria were heat killed by boiling for 15 min. One microliter of this suspension was spotted onto a nylon membrane (polyvinylidene difluoride), and cells were then treated as per the Roche colony hybridization method.
PAGE and Western blotting analysis.
Samples were resolved using a sodium dodecyl sulfate–12% polyacrylamide separating gel according to the method described by Laemmli et al. (17) with a 10-min sample boiling step prior to loading. Proteins were transferred semidry to Immobilon-P (Millipore) and subsequently blocked with 5% (wt/vol) skim milk in PBS (pH 7.4) for 1 h at room temperature. Membranes were then rinsed in PBS and probed for 1 h at room temperature using rabbit polyclonal antiserum raised against purified SibA protein (raised commercially by Eugenetic, Herstal, Belgium) diluted 1/1,000 with PBS. The membranes were then washed. The second antibody used was peroxidase-labeled swine anti-rabbit immunoglobulin antibody diluted 1/1,000 in PBS and incubated for 1 h at room temperature. Membranes were subsequently washed, and bound immunoglobulins was visualized using 4-chlor-1-naphthol (Sigma).
ELISA.
Ninety-six-well assay plates (Greiner) were coated overnight at 4°C with 1 ng of SibA recombinant protein per well diluted in PBS. After overnight coating, the plates were washed five times with PBS and wells were blocked using 2.5% (wt/vol) bovine serum albumin in PBS for 1 h at 37°C. The plates were then washed as previously, and the immunoglobulins were added at 100 ng per well diluted in PBS before the plates were incubated for 1 h at 37°C. Human IgG subclasses and fragments were obtained from Sigma, and peroxidase-labeled isotypes were obtained from Jackson ImmunoResearch. Following incubation, the enzyme-linked immunosorbent assay (ELISA) plates were washed and rabbit anti-human serum proteins (Jackson ImmunoResearch) diluted 1/1,000 in PBS were added to the Fc, Fab, and IgG subclass wells. The isotype wells did not require a second antibody step due to the immunoglobulins being peroxidase labeled, and these wells received PBS. The plate was subsequently incubated for 1 h at 37°C. Plates were washed, and the reactions were developed using ABTS [2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid)] in 0.1 M citrate-phosphate buffer (pH 4.35) containing 0.01% H2O2. The optical density at 405 nm was determined after 1 h of incubation at room temperature.
RESULTS
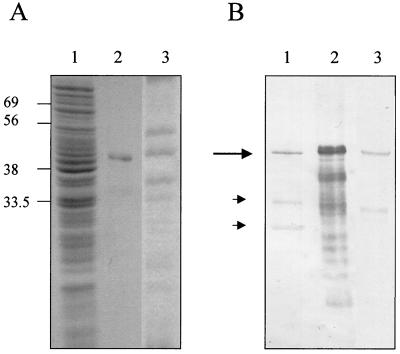
Polyclonal antisera raised against recombinant SibA identified a native protein with an apparent molecular mass of approximately 45 kDa in both the S. pyogenes whole-cell protein fraction and overnight-growth culture supernatant (Fig. 1B, lanes 1 and 3). There are a number of smaller reactive bands visible in the recombinant fraction (Fig. 1B, lane 2), which are most likely breakdown products. It is possible, however, that these smaller reactive bands seen in S. pyogenes whole-cell and supernatant fractions are breakdown products or discrete cross-reactive S. pyogenes proteins. Rabbit prebleed sera did not react against the 45-kDa protein under the same conditions. Despite the fact that SibA is secreted, surface localization of SibA was detected by immunogold electron microscopy using gold-labeled rabbit polyclonal antiserum raised against the recombinant protein (results not shown).
FIG. 1.
Expression and identification of S. pyogenes SibA protein. (A) Coomassie brilliant blue-stained 10% polyacrylamide gel. Lane 1, S. pyogenes A106 whole-cell extract; lane 2, recombinant SibA purified from Escherichia coli JM109; lane 3, supernatant from S. pyogenes A106 overnight-growth culture. (B) Western blot analysis of the equivalent protein samples described for panel A using rabbit anti-SibA antiserum. Positions of molecular mass markers are shown (in kilodaltons) on the left of panel A. The 45-kDa SibA protein is indicated with a large arrow; the smaller arrows indicate smaller reactive proteins.
DNA sequence and characteristics of sibA.
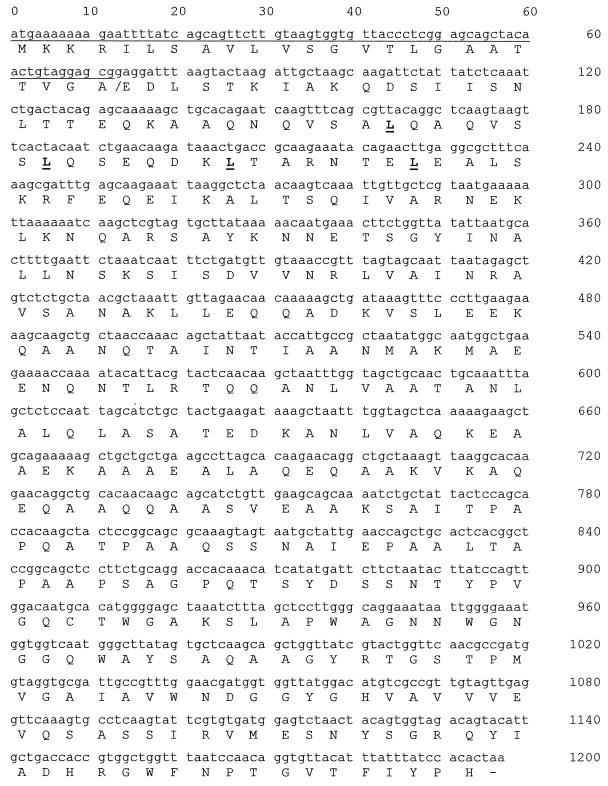
DNA sequencing of the sibA gene revealed an 1,197-bp open reading frame coding for a 398-amino-acid protein (Fig. 2). The predicted protein is rich in alanine (19.35%) and exhibits features typical of secreted streptococcal proteins. At the N terminus is a putative 21-amino-acid signal peptide which is predicted to be transmembrane spanning with the C terminus external to the cell; this region ends with a putative signal cleavage sequence, VGA-ED. The predicted molecular mass of the secreted component is approximately 32 kDa. There are no predicted membrane-anchoring sequences, confirming SibA as a secreted protein.
FIG. 2.
Nucleotide sequence of the cloned 1,197-bp S. pyogenes sibA gene. Predicted amino acids are shown as single letters under the DNA sequence. The sequence encoding the putative signal peptide is underlined, and the corresponding cleavage site is indicated by a slash in the amino acid sequence. Leucine residues predicted to be involved in the formation of a leucine zipper structure are shown in boldface and underlined.
The secreted peptide is predicted to have two alpha-helical regions spanning amino acids 24 to 106 and 192 to 256, relative to the precursor SibA with the signal peptide, separated by a region of low complexity. Each of the alpha-helical regions is predicted to become a coiled coil, with the first of these regions containing repeats corresponding to a basic-region leucine zipper (bZip) structure (Fig. 2). The C terminus (amino acids 259 to 398) has a predicted extended-sheet secondary structure and possesses an unusual amino acid composition which is low in lysine (1 of 21 amino acids) but rich in glycine (16 of 17 amino acids) and proline (12 of 12 amino acids) relative to the rest of the protein.
Sequence comparison to predicted open reading frames in known DNA sequences deposited in GenBank revealed some significant similarity to open reading frames in other gram-positive bacterial species. None of the BLAST hit results showed similarity to the entire SibA protein, but rather they showed similarity to either the N or C terminus (Fig. 3). Four hits against the N-terminal region were above 50% similarity, and all represented the corresponding regions in their respective proteins. The strongest similarity was with a putative secreted Streptococcus mutans protein of unknown function. The other three hits were with known secreted proteins, i.e., Lactococcus lactis usp45 (33), Enterococcus faecium P54 (9), and the Listeria monocytogenes autolysin P45 (24). There was some similarity between the predicted SibA N terminus and the streptococcal M-related proteins enn4 (GenPept accession no. 383763), emm1 (311758), and fcrA (311760). This homology was restricted to the secretory signal sequence and the immediate N terminus and was never above 45% similarity. The C terminus had homology to other C-terminal domains found in the Staphylococcus carnosus SceB precursor (GenPept accession no. 2735506), Staphylococcus aureus ORF1 (1340128) and TraG (3676441), and Streptococcus thermophilus transfer complex (6782409) proteins (Fig. 3). There is also a high similarity in this region to an open reading frame immediately downstream of the comAB transporter complex genes of Streptococcus gordonii (identical, 58%; conserved, 73%; and gaps, 6%). This open reading frame is in the reverse orientation to the transporter genes reported previously (19) and is incomplete at the 5′ end; this region also has the stop codon conserved relative to the SibA stop codon. As mentioned in Materials and Methods, an analogous protein, designated PcsB, is present in S. agalactiae (25), and although these predicted proteins have a low amino acid identity (55%), these proteins are considered analogous due to the remarkable conservation of the domains.
FIG. 3.
Predicted sequence similarity for the SibA protein aligned against high-scoring domains of proteins deposited in GenBank. The upper diagram represents the secondary structure of the SibA protein. Domains with amino acid similarity are indicated below the diagram. The protein name, GenPept accession number, and similarity (+ve) (including conserved residues) are given to the side of the corresponding domain.
Immunoglobulin binding.
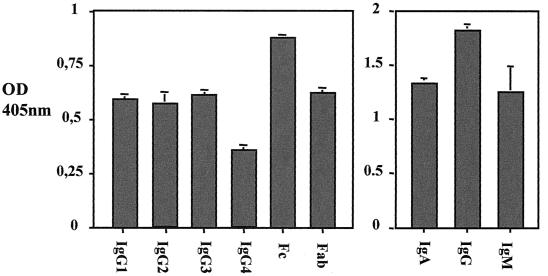
Recombinant SibA protein demonstrated the ability to bind human IgA, IgG, and IgM isotypes in an ELISA when SibA was bound to the plate (Fig. 4). The protein bound all human IgG subclasses and displayed the ability to bind both the Fc and Fab fragments of human IgG, with a preference for the Fc fragment (Fig. 4). Under the same ELISA conditions, detection of IgG binding is reduced to the background blank level (optical density at 405 nm of 0.05) when 3 ng of IgG is added (Fig. 5). In similar ELISAs SibA was demonstrated not to bind other serum proteins such as plasminogen, collagen, or fibronectin (results not shown).
FIG. 4.
Graph representing ELISA results for S. pyogenes SibA immunoglobulin binding. (Left) SibA protein immunoglobulin binding with IgG subclasses 1 to 4 and IgG antibody fragments. (Right) SibA immunoglobulin binding with IgA, IgG, and IgM isotypes. The optical density (OD) (405 nm) is indicated on the vertical axis. Graphs are blanked against a PBS control which had no binding protein added. Error bars indicate standard deviations.
FIG. 5.
Graph representing ELISA results showing the titration of S. pyogenes SibA binding to human IgG. The optical density (OD) (405 nm) is indicated on the vertical axis. Graphs are blanked against a PBS control which had no binding protein added. Error bars indicate standard deviations.
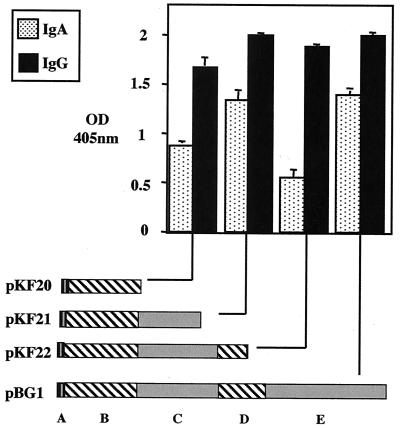
It is apparent from the subcloned truncated recombinant proteins (pKF20 to -22) that immunoglobulin binding is associated with the N terminus of SibA (Fig. 6). This region corresponds to a predicted coiled-coil region containing a putative bZip-like structure (pKF20). Binding of IgG and IgA is strongest when the intervening noncoiled region is present (pKF21); the level of binding for this region is the same as that for the full-length recombinant protein (pBG1). The binding of subclones remains relatively constant for all of the subclones; however, IgA binding is reduced when the region corresponding to the second predicted coiled coil is expressed (pKF22).
FIG. 6.
Graph representing ELISA results for the immunoglobulin binding by the respective indicated subclones, pKF20 to -22, expressing truncated SibA. The domains expressed by the subclones are a predicted coiled-coil region containing the bZip-like domain (A), a region of low complexity (B), predicted coiled-coil region 2 (C), and a proline-rich extended-sheet domain (D). The optical density (OD) (405 nm) is indicated on the vertical axis. Graphs were blanked against a PBS control which had no binding protein added. Error bars indicate standard deviations.
Epidemiology.
To determine the distribution and frequency of the SibA gene within the S. pyogenes population, 113 strains of diverse Vir types collected from a wide geographical range were screened using PCR and confirmed by Southern hybridization. Only a single negative strain was detected. Four strains produced an additional PCR product approximately 800 bp larger. DNA sequencing showed that the size variation was due to a duplication event involving a stretch of DNA encompassing ≈450 bp upstream of sibA and the first 25% of the gene. This results in a truncated sibA gene tandemly arranged upstream of a full-length sibA, with ≈450 bp lying between. Both open reading frames have promoter and ribosome binding sequences; however, it is unknown if both proteins are expressed in these strains.
DISCUSSION
It has been widely reported that GAS are capable of binding immunoglobulins on their surface via anchored cell surface proteins (3, 6, 18, 28). All S. pyogenes immunoglobulin binding proteins reported to date, with the exception of the human IgG binding SfbI protein (20), are M-related proteins. This paper describes a non-M-related streptococcal protein which is highly conserved between strains and which binds all IgG subclasses, the Fc and Fab regions, and also IgA and IgM. SibA is not an M-related protein, although it does share an alpha-helical N-terminal secondary structure and has the characteristic relatively high levels of alanine, lysine, glutamic acid, leucine, and glycine (8). SibA has no significant homology to any M-related proteins, is not found in the vir regulon, and contains none of the characteristic M-like protein regions, such as the A or C repeats (22). SibA has regions of local similarity with other coiled-coil proteins such as human myosin heavy chain, macrogolgin, and Schistoma mansoni paramyosin, another characteristic shared with the M-related proteins, although regions of local similarity vary between the two streptococcal protein classes. Of potential significance is that cross-reactive antibodies between myosin proteins have been implicated in the development of autoimmune disease, one of the many sequelae of streptococcal disease (7).
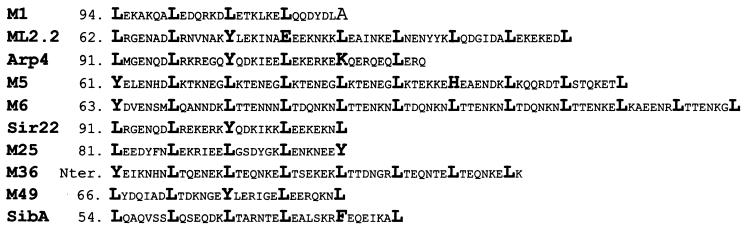
The subclone immunoglobulin binding experiment supports the M-protein evidence that both IgA binding (4, 28) and IgG binding (29) are effected by N-terminal coiled coil regions. The M-like protein consensus sequence, ALXGENXDLR, suggested by Bessen (4) to be important in IgA binding is not present in SibA; however, an interesting pattern in this N terminus is apparent. SibA contains the predicted coiled-coil leucine zipper-like arrangement. This heptad periodicity with leucine in position a is also seen in a number of reported immunoglobulin binding M and M-like proteins (Sir22, Arp4, ML2.2, and M1) and also in the M proteins of S. pyogenes strains reported to bind immunoglobulins (M5, M6, M25, M36, and M49). All of these M and M-like proteins, with the exception of M1 and to a lesser degree ML2.2, have an aromatic tyrosine residue substitution in position a in the heptad arrangement immediately before, or within, the region of heptad periodicity (Fig. 7). In SibA there is no tyrosine residue associated with this region; instead phenylalanine, which is also aromatic and has properties similar to those of tyrosine, is present in the corresponding position.
FIG. 7.
Amino acid alignment comparing the heptad periodicity regions from various M and M-like proteins against SibA. Those amino acids present in heptad position a are shown in boldface. Numbers to the left of the alignment represent amino acids from the unprocessed protein.
There is no direct evidence that this heptad periodicity or the presence of the aromatic residue is directly linked to IgA binding in SibA; however, the following evidence from the M proteins strongly implicates the necessity of this secondary structure. Nonimmune binding of Arp4 (1) and Sir22 (15) to IgA requires noncovalent dimer formation, indicating that the coiled-coil structure is essential; furthermore, homodimer formation has long been associated with the leucine zipper motif (13, 16). All recombinant SibA proteins described in this study form complexes which can be visualized by PAGE under mild denaturing conditions. This dimerization and immunoglobulin binding is most probably due to the first coiled-coil domain expressed in the pKF20 subclone, which contains the predicted bZip motif. The coiled-coil secondary structure is widely reported to be stabilized by flanking regions. This may explain the increased immunoglobulin binding when the region of low complexity downstream of the first predicted coiled-coil sequence is expressed in the recombinant clone pKF21.
Like the M and M-like proteins of GAS, SibA is found both associated with the surface and secreted, with a preference for the latter. From examination of the various domains identified in the predicted protein sequence of sibA, it is very likely that SibA will also prove to share the multifunctional nature of the M and M-like proteins (6).
The analogous PcsB protein in S. agalactiae has been identified to be involved in cell wall separation (25). Insertional inactivation of the gene revealed that the encoded protein was linked to the formation of the cell septum and was also involved in cell separation after division. This protein's actual biochemical function is unknown, and it is unlikely that the protein is an autolysin, as no enzymatic activity for this protein has been demonstrated. No published study describes which domains of PcsB are responsible for the effects demonstrated in the insertional mutant, making comparison to the analogous domain in SibA impossible. Currently an insertional SibA mutant is being developed to see if this protein influences GAS cell division. The functions of all other proteins sharing similarities to regions of SibA, with the exception of P45, have yet to be elucidated and therefore provide no indication as to the overall biological function of this secreted immunoglobulin binding protein. P45 has peptidoglycan lytic activity, with a functional domain in the N terminus with a single cysteine residue (27). The similarity between P45 and SibA, however, is in the N-terminal region, and although a cysteine residue is situated in the SibA C terminus, the surrounding residues are not conserved with the P45 active domain (27).
SibA is an immunoglobulin binding protein that is present in 112 of 113 GAS strains tested. The conservation of this gene implicates it as an important, if not essential, gene. As is the case for all other GAS immunoglobulin binding proteins, the actual role of this binding activity in pathogenesis is unclear (6). Immunoglobulin binding is a function of the N-terminal region, and presently the biological activities of the other domains have not been elucidated. However, the similarity to S. agalactiae PcsB protein indicates that SibA may possess other activities in these domains, making this GAS secreted protein an interesting multifunctional protein worthy of further investigation.
ACKNOWLEDGMENTS
We thank and acknowledge the Streptococcal Genome Sequencing Project, funded by USPHS/NIH (grant AI38406), and the following people involved in that project: B. A. Roe, S. P. Linn, L. Song, X. Yuan, S. Clifton, R. E. McLaughlin, M. McShan, and J. Ferretti. A special thanks goes to Rebecca Towers for careful proofreading of the manuscript and helpful discussion.
REFERENCES
- 1.Akerstrom B, Lindahl G, Bjorck L, Lindqvist A. Protein Arp and protein H from group A streptococci. Ig binding and dimerisation are regulated by temperature. J Immunol. 1992;148:3238–3243. [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Bessen D, Fischetti V A. A human IgG receptor of group A streptococci is associated with tissue site of infection and streptococcal class. J Infect Dis. 1990;161:747–754. doi: 10.1093/infdis/161.4.747. [DOI] [PubMed] [Google Scholar]
- 4.Bessen D E. Localization of immunoglobulin A-binding sites within M or M-like proteins of group A streptococci. Infect Immun. 1994;62:1968–1974. doi: 10.1128/iai.62.5.1968-1974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartwright K. Group A streptococcal infections in humans. Soc Appl Bacteriol Symp Ser. 1997;26:52S–61S. [PubMed] [Google Scholar]
- 6.Cleary P, Retnoningrum D. Group A streptococcal immunoglobulin-binding proteins: adhesins, molecular mimicry or sensory proteins? Trends Microbiol. 1994;2:131–136. doi: 10.1016/0966-842x(94)90600-9. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham M W, Antone S M, Gulizia J M, McManus B M, Fischetti V A, Gauntt C J. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses and human cardiac myosin. Proc Natl Acad Sci USA. 1992;89:1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frithz E, Heden L O, Lindahl G. Extensive sequence homology between IgA receptor and M proteins in Streptococcus pyogenes. Mol Microbiol. 1989;3:1111–1119. doi: 10.1111/j.1365-2958.1989.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 9.Fürst P, Mosch H U, Solioz M. A protein of unusual composition from Enterococcus faecium. Nucleic Acids Res. 1989;17:6724. doi: 10.1093/nar/17.16.6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardiner D L, Hartas J, Currie B, Mathews D J, Sriprakash K S. Vir typing: a long-PCR typing method for group A streptococci. PCR Methods Appl. 1995;4:228–293. doi: 10.1101/gr.4.5.288. [DOI] [PubMed] [Google Scholar]
- 11.Hanski E, Fogg G, Tovi A, Okada N, Burstein I, Caparon M. Molecular analysis of Streptococcus pyogenes adhesion. Methods Enzymol. 1995;253:269–305. doi: 10.1016/s0076-6879(95)53025-8. [DOI] [PubMed] [Google Scholar]
- 12.Hasty D L, Courtney H S. Group A streptococcal adhesion. All of the theories are correct. Adv Exp Med Biol. 1996;408:81–94. [PubMed] [Google Scholar]
- 13.Hodges R S. Boehringer Mannheim award lecture 1995. De novo design of alpha-helical proteins: basic research to medical applications. Biochem Cell Biol. 1996;74:133–154. doi: 10.1139/o96-015. [DOI] [PubMed] [Google Scholar]
- 14.Hogan P. Paediatric dermatology. Impetigo. Aust Fam Physician. 1998;27:735–736. [PubMed] [Google Scholar]
- 15.Johnsson E, Areschoug T, Mestecky J, Lindahl G. An IgA-binding peptide derived from streptococcal surface protein. J Biol Chem. 1999;21:14521–14524. doi: 10.1074/jbc.274.21.14521. [DOI] [PubMed] [Google Scholar]
- 16.Kammerer R A. Alpha-helical coiled-coil oligomerization domains in extracellular proteins. Matrix Biol. 1997;15:555–565. doi: 10.1016/s0945-053x(97)90031-7. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Lindahl G, Stenberg L. Binding of IgA and/or IgG is a common property among clinical isolates of group A streptococci. Epidemiol Infect. 1990;105:87–93. doi: 10.1017/s0950268800047683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunsford R D, London J. Natural genetic elements in Streptococcus gordonii: comX imparts spontaneous competence in strain wicky. J Bacteriol. 1996;178:5831–5835. doi: 10.1128/jb.178.19.5831-5835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medina E, Molinari G, Rohde M, Haase B, Chhatwal G S, Guzmán C A. Fc-mediated nonspecific binding between fibronectin-binding protein I of Streptococcus pyogenes and human immunoglobulins. J Immunol. 1999;163:3396–3402. [PubMed] [Google Scholar]
- 21.Molinari G, Chhatwal G S. Streptococcal invasion. Curr Opin Microbiol. 1999;2:56–61. doi: 10.1016/s1369-5274(99)80010-1. [DOI] [PubMed] [Google Scholar]
- 22.O'Toole P, Stenberg L, Rissler M, Lindahl G. Two major classes in the M protein family in group A streptococci. Proc Natl Acad Sci USA. 1992;89:8661–8665. doi: 10.1073/pnas.89.18.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raeder R, Boyle M D. Distinct profiles of immunoglobulin G-binding-protein expression by invasive serotype M1 isolates of Streptococcus pyogenes. Clin Diagn Lab Immunol. 1995;2:478–483. doi: 10.1128/cdli.2.4.478-483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raeder R, Boyle M D. Analysis of immunoglobulin G-binding-protein expression by invasive isolates of Streptococcus pyogenes. Clin Diagn Lab Immunol. 1995;2:484–486. doi: 10.1128/cdli.2.4.484-486.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinscheid D J, Gottschalk B, Schubert A, Eikmanns B J, Chhatwal G S. Identification and molecular analysis of PcsB, a protein required for cell wall separation of group B streptococcus. J Bacteriol. 2001;183:1175–1183. doi: 10.1128/JB.183.4.1175-1183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Schubert K, Bichlmaier A M, Mager E, Wolff K, Ruhland G, Feidler F. P45, an extracellular 45 kDa protein of Listeria monocytogenes with similarity to protein P60 and exhibiting peptidoglycan lytic activity. Arch Microbiol. 2000;173:21–28. doi: 10.1007/s002030050003. [DOI] [PubMed] [Google Scholar]
- 28.Stenberg L. Genetics and biochemistry of group A streptococcal cell surface proteins with special reference to immunoglobulin A-binding proteins. Lund, Sweden: Lund University Press; 1994. [Google Scholar]
- 29.Stenberg L, O'Toole P W, Mestecky J, Lindahl G. Molecular characterisation of protein Sir, a streptococcal cell surface protein that binds both immunoglobulin A and immunoglobulin G. J Biol Chem. 1994;269:13458–13464. [PubMed] [Google Scholar]
- 30.Stevens D L. Invasive group A streptococcus infections. Clin Infect Dis. 1992;14:2–11. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 31.Stevens D L. Invasive group A streptococcal disease. Infect Agents Dis. 1996;5:157–166. [PubMed] [Google Scholar]
- 32.Stollerman G H. Rheumatic fever. Lancet. 1997;349:935–942. doi: 10.1016/S0140-6736(96)06364-7. [DOI] [PubMed] [Google Scholar]
- 33.van Asseldonk M, Rutten G, Oteman M, Siezen R J, de Vos W M, Simons G. Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis subsp. lactis MG1363. Gene. 1990;95:155–160. doi: 10.1016/0378-1119(90)90428-t. [DOI] [PubMed] [Google Scholar]