Key Points
Question
Is adolescents’ frequency of checking behaviors on 3 social media platforms (Facebook, Instagram, Snapchat) associated with longitudinal changes in functional brain development across adolescence.
Findings
In this cohort study of 169 sixth- and seventh-grade students, participants who engaged in habitual checking behaviors showed a distinct neurodevelopmental trajectory within regions of the brain comprising the affective salience, motivational, and cognitive control networks in response to anticipating social rewards and punishments compared with those who engaged in nonhabitual checking behaviors.
Meaning
These results suggest that habitual checking of social media in early adolescence may be longitudinally associated with changes in neural sensitivity to anticipation of social rewards and punishments, which could have implications for psychological adjustment.
Abstract
Importance
Social media platforms provide adolescents with unprecedented opportunities for social interactions during a critical developmental period when the brain is especially sensitive to social feedback.
Objective
To explore how adolescents’ frequency of checking behaviors on social media platforms is associated with longitudinal changes in functional brain development across adolescence.
Design, Setting, and Participants
A 3-year longitudinal cohort study of functional magnetic resonance imaging (fMRI) among sixth- and seventh-grade students recruited from 3 public middle schools in rural North Carolina.
Exposures
At wave 1, participants reported the frequency at which they checked Facebook, Instagram, and Snapchat.
Main Outcome or Measure
Neural responses to the Social Incentive Delay task when anticipating receiving social feedback, measured annually using fMRI for 3 years. Participants saw a cue that indicated whether the social feedback (adolescent faces with emotional expressions) would be a reward, punishment, or neutral; after a delay, a target appeared and students responded by pressing a button as quickly as possible; a display of social feedback depended on trial type and reaction time.
Results
Of 178 participants recruited at age 12 years, 169 participants (mean [SD] age, 12.89 [0.58] years; range, 11.93-14.52 years; 91 [53.8%] female; 38 [22.5%] Black, 60 [35.5%] Latinx, 50 [29.6%] White, 15 [8.9%] multiracial) met the inclusion criteria. Participants with habitual social media checking behaviors showed lower neural sensitivity to social anticipation at age 12 years compared with those with nonhabitual checking behaviors in the left amygdala, posterior insula (PI), and ventral striatum (VS; β, −0.22; 95% CI, −0.33 to −0.11), right amygdala (β, −0.19; 95% CI, −0.30 to −0.08), right anterior insula (AI; β, −0.23; 95% CI, −0.37 to −0.09), and left dorsolateral prefrontal cortex (DLPFC; β, −0.29; 95% CI, −0.44 to −0.14). Among those with habitual checking behaviors, there were longitudinal increases in the left amygdala/PI/VS (β, 0.11; 95% CI, 0.04 to 0.18), right amygdala (β, 0.09; 95% CI, 0.02 to 0.16), right AI (β, 0.15; 95% CI, 0.02 to 0.20), and left DLPFC (β, 0.19; 95% CI, 0.05 to 0.25) during social anticipation, whereas among those with nonhabitual checking behaviors, longitudinal decreases were seen in the left amygdala/PI/VS (β, −0.12; 95% CI, −0.19 to −0.06), right amygdala (β, −0.10; 95% CI, −0.17 to −0.03), right AI (β, −0.13; 95% CI, −0.22 to −0.04), and left DLPFC (β, −0.10, 95% CI, −0.22 to −0.03).
Conclusions and Relevance
The results of this cohort study suggest that social media checking behaviors in early adolescence may be associated with changes in the brain’s sensitivity to social rewards and punishments. Further research examining long-term associations between social media use, adolescent neural development, and psychological adjustment is needed to understand the effects of a ubiquitous influence on development for today’s adolescents.
This cohort study of middle school students in North Carolina examines whether the frequency of checking behaviors on 3 popular social media platforms (Facebook, Instagram, and Snapchat) is associated with trajectories of functional brain development across adolescence.
Introduction
In the span of a generation, social media has dramatically changed the landscape of adolescent development, providing unprecedented opportunities for social interactions around the clock. Social media provides a constant and unpredictable stream of social inputs to adolescents during a critical developmental period when the brain becomes especially sensitive to social rewards and punishments. Motivated by the anticipation of this social feedback, adolescents’ constant, habitual checking of social media may alter neurodevelopment, significantly changing the ways in which the adolescent brain responds to its environment.
Social media allows immediate access to social information at any time it is desired and is designed to hold users’ engagement by maximizing social rewards. “Likes,” notifications, and messages arrive unpredictably on a maximally powerful variable reinforcement schedule, conditioning individuals to check social media habitually in anticipation of this social feedback. With 78% of 13- to 17-year-olds reporting checking their devices at least hourly and 46% checking “almost constantly,” adolescents may be uniquely vulnerable to habitual checking behaviors.
The brain undergoes significant structural and functional reorganization during adolescence. Neural regions involved in motivational relevance (eg, the ventral striatum; VS) and affective salience (eg, the amygdala and insula) become hyperactive, orienting teens to rewarding stimuli in their environment, particularly from peers. Adolescents’ habitual checking of social media may be exacerbating an already enhanced neural response to the anticipation of salient social feedback. Additionally, the motivational salience of social contexts may undermine adolescents’ ability to engage in cognitive control and, subsequently, to regulate their behaviors. Consequently, repeated exposure to digital social rewards (eg, notifications or likes) may increase neural reactivity to reward-related cues, reducing adolescents’ ability to resist urges to check social media.
The current study aimed to examine whether social media use is associated with longitudinal changes in functional brain development across adolescence, a developmental period characterized by peak social media use and heightened neural sensitivity to social feedback from peers. We hypothesized that checking social media habitually would make adolescents increasingly hypersensitive to social feedback anticipation and thus would be associated with longitudinal increases in neural activation, particularly within regions comprising the motivational (eg, VS), affective salience (eg, insula and amygdala), and cognitive control (eg, dorsolateral prefrontal cortex; DLPFC) networks. Conversely, we hypothesized that nonhabitual checking would be associated with longitudinal decreases in neural activation in the same brain regions. Given the limited research exploring longitudinal neural activation in relation to social media behaviors, we conducted exploratory whole-brain analyses to determine which brain regions showed the greatest differences in neural activation longitudinally. To our knowledge, results from this study would provide the first insight into how habitual social media behaviors may be altering adolescent brain development.
Methods
Participants
Participants were recruited from a larger, school-based study of 873 sixth- and seventh-grade students from 3 public rural middle schools in North Carolina to participate in a longitudinal functional magnetic resonance imaging (fMRI) study. We recruited 2 cohorts of participants at 12 to 13 years of age across 2 years of the study, leading to a sample size of 178 adolescents (148 students for cohort 1 and 30 for cohort 2). Of the recruited participants for cohort 1, 5 met exclusion criteria after consenting to the study and thus were excluded and not invited back for later waves (see the eMethods in the Supplement for exclusion criteria). Across all waves, 25 participants completed 1 time point, 36 completed 2 time points, and 112 completed 3 time points. All participants provided written informed consent or assent, and the University’s Institutional Review Board approved all aspects of the study. Race and ethnicity were self-reported by participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. For more information on study procedures, see the eMethods in the Supplement.
Self-reported Social Media Use
Participants reported frequency of checking at wave 1 only. For 3 popular social media platforms (Facebook, Instagram, and Snapchat), participants were asked how many times per day they checked each platform, with answers grouped into 8 numerical score categories (1, <1 time per day; 2, 1 time per day; 3, 2-3 times per day; 4, 4-5 times per day; 5, 6-10 times per day; 6, 11-15 times per day; 7, 16-20 times per day; 8, >20 times per day). We recoded participants’ scores to create an ordinal scale that captured social media checking frequency across a meaningful distribution that could be assessed quantitatively. A score of 1 was recoded to 0 and a score of 2 was recoded to 1. Scores between 3 and 7 were recoded to the average of the range of number of times checked; for example, if participants selected 6 for their Facebook use (ie, checked Facebook between 11 and 15 times per day), then their score was recoded to the average of 11 and 15 times, which in this case was 13 times checked. Reported scores of 8 (ie, checked >20 times per day) were recoded to 20 times checked. For each participant, the recoded checking behaviors on the 3 social media platforms were summed to create a total social media checking score that ranged from 0 to 54 (mean [SD] checking behaviors per day, 11.85 [15.39]).
Social Incentive Delay Task
At each wave, participants attended a brain imaging session during which they completed the Social Incentive Delay task while undergoing fMRI to measure neural responses when anticipating receiving social rewards and avoiding social punishments. On each trial, participants saw a cue (for 500 milliseconds) indicating whether the potential social feedback would be a reward, punishment, or neutral. After a variable delay (mean delay, 2000 milliseconds; range, 480-3900 milliseconds), a target appeared (for 300 milliseconds), at which point participants were instructed to respond by pressing a button as quickly as possible. The display of social feedback (for 1450 milliseconds) depended on the trial type and participants’ reaction time. In the social reward condition, happy faces were the outcome of a fast response (hit), and blurred faces were the outcome of a slow response (miss). In the social punishment condition, a hit earned a blurred face, and a miss earned an angry face. In the control condition, a blurred face was always the outcome for both hits and misses. Trials were presented in an event-related design, with reward, punishment, and neutral trials randomly ordered. Participants completed 2 rounds of the task, totaling 116 trials (48 reward, 48 punishment, and 20 neutral trials).
Task difficulty was standardized to a hit rate of approximately 50% for all participants by adjusting target duration to individual reaction times. Age-matched adolescent faces with emotional expressions of 24 ethnically diverse people (12 female) were used as reward and punishment stimuli. Photographs were taken from the National Institute of Mental Health Child Emotional Faces Picture Set. Participants were trained on the meaning of each cue and completed 12 practice trials prior to entering the scanner.
Statistical Analysis
fMRI Data Acquisition
Imaging data were collected using a 3-T Magnatom Prisma MRI scanner (Siemens Healthineers). For specific fMRI image acquisition parameters and preprocessing methods, see the eMethods in the Supplement. Individual level, fixed-effects analyses were estimated using the general linear model convolved with a canonical hemodynamic response function in Statistical Parametric Mapping software package SPM12 (Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology). The task was modeled as event related with 8 conditions, including 3 anticipation conditions (reward, punishment, and neutral), 2 outcome conditions for both reward (hit or miss) and punishment (hit or miss), and 1 outcome condition for neutral. Anticipation conditions were modeled as the onset of the cue and a duration of zero, and outcome conditions were modeled at the onset of the outcome with a duration of zero. Six motion parameters were modeled as nuisance regressors. Using the general linear model, linear contrast images comparing each of the conditions of interest were calculated for each individual. The primary contrasts of interest for this study were reward anticipation vs neutral anticipation and punishment anticipation vs neutral anticipation, given our supposition that checking behaviors on social media platforms is motivated by the anticipation of social feedback.
Longitudinal Whole-Brain Analyses
We conducted longitudinal whole-brain analyses using the 3dLMEr program (AFNI). This program allows for voxel-level whole-brain analysis of linear mixed effects (maximum likelihood, multilevel model). Missing data across waves were accounted for by using full information maximum likelihood, which provides an estimate of the value of a population parameter most likely to result in the observed data even in the presence of missing data. We modeled a 3-way interaction with age (minimum centered), condition (reward and punishment anticipation), and social media checking to assess whether age-related changes in neural activation during social anticipation differed as a function of the type of social anticipation (ie, reward vs punishment) and amount of social media checking behaviors. To correct for multiple comparisons, we conducted a Monte Carlo simulation using the 3dFWHMx and 3dClustSim programs (AFNI) and the group-level brain mask. Smoothness was estimated with the -acf option (-acf a, b, and c parameters 0.55, 4.61, 12.32), which used an average of individual-level autocorrelation function parameters (obtained using each participant’s residuals from the first-level model). This simulation indicated that a P < .05 familywise error corrected would be achieved with a voxelwise threshold of P < .001 and a minimum cluster size of 80 voxels. A 2-sided P < .01 indicated statistical significance.
To explore any significant whole-brain interactions and plot the trajectories, we extracted parameter estimates from significant clusters. Parameter estimates were fitted into a conditional linear trajectory model whereby these post hoc analyses allowed us to unpack the significant 3-way interaction between age, condition, and social media checking behavior. For plotting purposes, we categorized the total social media checking scores as high (>15; habitual), moderate (1-15), and low (0; nonhabitual). This allowed us to test whether trajectories of neural response differed as a function of anticipation type and amount of social media checking.
Results
After exclusions, the final sample size was 169 (mean [SD] age, 12.89 [0.58] years; range, 11.93-14.52 years; 91 [53.8%] female; 38 [22.5%] Black, 60 [35.5%] Latinx, 50 [29.6%] White, 15 [8.9%] multiracial [2 or more racial categories identified other Hispanic or Latinx], and 6 [3.6%] categorized as other [American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander]) collected across 3 waves; 136 participants completed wave 1 (mean [SD] age, 12.80 [0.52] years; range, 11.9-14.5 years; 71 [52.2%] female), 131 participants completed wave 2 (mean [SD] age, 13.7 [ 0.59] years; range, 12.4-15.4 years; 68 [51.9%] female), and 124 participants completed wave 3 (mean [SD] age, 14.70 [0.60] years; range, 13.4-16.3 years; 61 [49.2%] female). The mean (SD) time between waves 1 and 2 was 49.8 (3.9) weeks, and that between waves 2 and 3 was 52.9 (6.9) weeks. Retention was 81.1% from waves 1 to 2 and 85.3% from waves 2 to 3. Adolescents reported checking behaviors on 3 social media platforms at wave 1 only. For descriptive statistics regarding checking behaviors on all 3 platforms, see the eFigure in the Supplement. Checking behaviors within the 3 apps were recoded and summed for a total social media checking score, which ranged from 0 to 54 mean (SD) score, 11.85 (15.39).
Using 3dLMEr to model longitudinal whole-brain changes in sensitivity to social anticipation, there was not a 3-way interaction between type of social anticipation, age, and social media checking behavior, so we collapsed social reward and social punishment. We found significant 2-way interactions between age and social media checking behaviors in several regions, including the posterior insula (PI; x, 34; y, 6; z, −4), the left amygdala (x, −26; y, −2; z, −12), the VS (x, −24; y, 14; z, −4), the right amygdala (x, 22; y, 4; z, −18), anterior insula (AI; x, 36; y, 22; z, −4), and the DLPFC (x, 42; y, −42; z, 28) (Table). Of particular interest were the left amygdala extending into the PI and VS (Figure 1A), the right amygdala (Figure 2A), right AI (Figure 3A), and left DLPFC (Figure 4A). Significant 2-way interactions between age and social media checking behaviors were found in similar brain regions when receiving social feedback (eTable in the Supplement).
Table. Age-Related Neural Changes as a Function of Social Media Checking During Anticipation of Social Feedback.
| Anatomical region | MNI coordinatesa | t statistic | Cluster size, voxelsb | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Posterior insula | 34 | 6 | −4 | 46.1 | 2038 |
| Left amygdala | −26 | −2 | −12 | 46.1 | 2038 |
| Ventral striatum | −24 | 14 | −4 | 46.1 | 2038 |
| Orbitofrontal cortex | −20 | −8 | −18 | 38.3 | 1027 |
| Right amygdala | 22 | 4 | −18 | 38.3 | 1027 |
| Cerebellum | 0 | 80 | −32 | 30.3 | 736 |
| Thalamus | 12 | 26 | 0 | 35.3 | 640 |
| Frontal operculum | −56 | −14 | −8 | 31.8 | 606 |
| Anterior insula | 36 | 22 | −4 | 31.8 | 606 |
| Middle cingulate cortex | −18 | 38 | 38 | 41.4 | 534 |
| Dorsolateral prefrontal cortex | 42 | −42 | 28 | 44.1 | 501 |
| Globus pallidus | 34 | 2 | 66 | 27.1 | 456 |
| Inferior temporal gyrus | −58 | 38 | −22 | 45.9 | 374 |
| Postcentral gyrus | 54 | 16 | 28 | 31.1 | 369 |
| Hippocampus | −32 | 8 | −32 | 26 | 235 |
| Somatosensory area | −60 | 8 | 38 | 26.5 | 179 |
| Superior temporal gyrus | 60 | 32 | 12 | 26.3 | 177 |
| Supramarginal gyrus | 44 | 36 | 28 | 25.3 | 153 |
| Cerebellar vermis | −4 | 50 | −6 | 26.8 | 143 |
| Intraparietal sulcus | −22 | 88 | 16 | 19.1 | 116 |
| Supplementary motor area | −8 | −4 | 44 | 20.7 | 114 |
| Inferior parietal lobule | −52 | 42 | 56 | 27.5 | 106 |
| Cuneus | 12 | 86 | 34 | 23.4 | 102 |
| Anterior inferior parietal lobule | −50 | 34 | 38 | 23.9 | 102 |
| Intraparietal sulcus | −38 | 58 | 50 | 20.5 | 91 |
Abbreviation: MNI, Montreal Neurological Institute.
Values are the MNI coordinates to regions of the brain that changed significantly over age.
Clusters that survived cluster-extent threshold correction when modeling longitudinal whole-brain changes in sensitivity to social anticipation as a function of social media checking behaviors, assessed using the 3dLMEr program (AFNI). Multiple brain regions may lie within the same brain cluster.
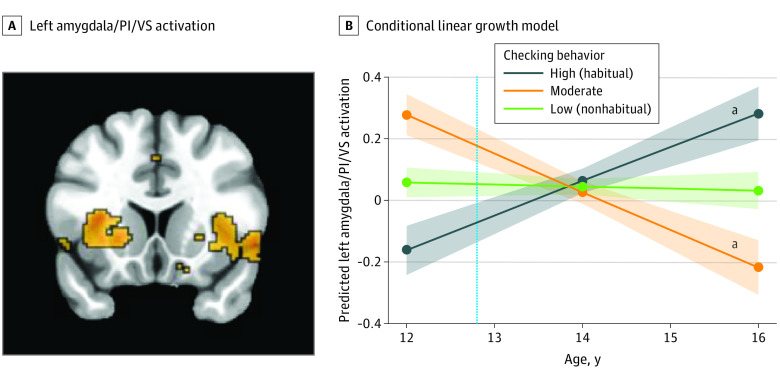
Figure 1. Functional Activation in the Left Amygdala, Posterior Insula (PI), and Ventral Striatum (VS) During the Anticipation of Social Feedback.
A, Left amygdala (x, −26; y, −2; Z, −12)/PI (x, 34; y, 6; z, −4)/VS (x, −24; y, 14; z, −4) activation during social anticipation differed significantly across time, as a function of social media checking behaviors. B, The dotted vertical line indicates the mean age of participants when they reported the number of checks at wave 1. The shaded areas represent the spread of the data based on the SE of each group.
aP < .05.
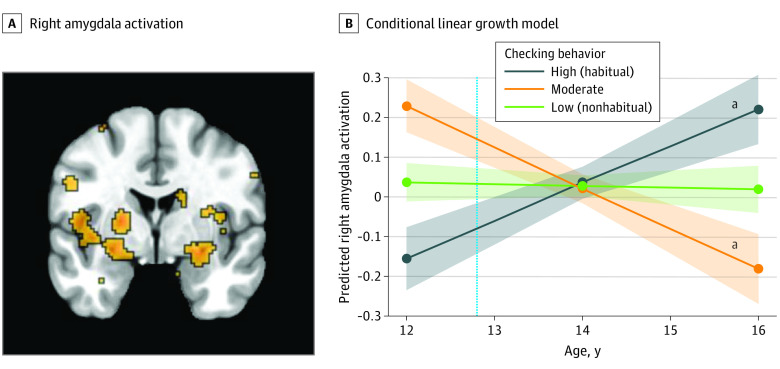
Figure 2. Functional Activation in the Right Amygdala During the Anticipation of Social Feedback.
A, Right amygdala (x, 22; y, 4; z, −18) activation during social anticipation significantly differed across time as a function of social media checking behaviors. B, The dotted vertical line indicates the mean age of participants when they reported the number of checks at wave 1. The shaded areas represent the spread of the data based on the SE of each group.
aP < .05.
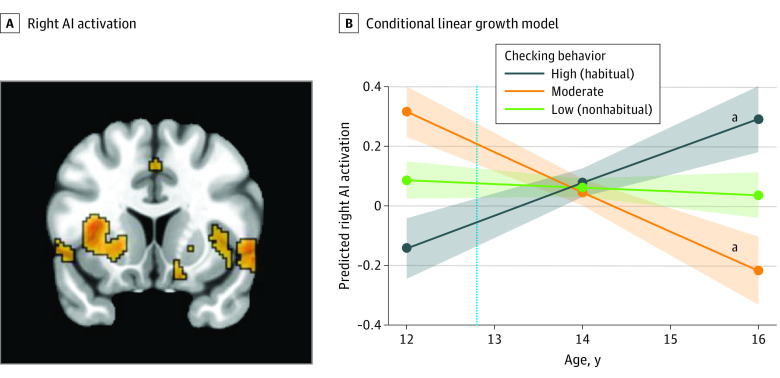
Figure 3. Functional Activation in the Right Anterior Insula (AI) During the Anticipation of Social Feedback.
A, Right AI (x, 36; y, 22; z, −4) activation during social anticipation significantly differed across time as a function of social media checking behaviors. B, The dotted vertical line indicates the mean age of participants when they reported the number of checks at wave 1. The shaded areas represent the spread of the data based on the SE of each group.
aP < .05.
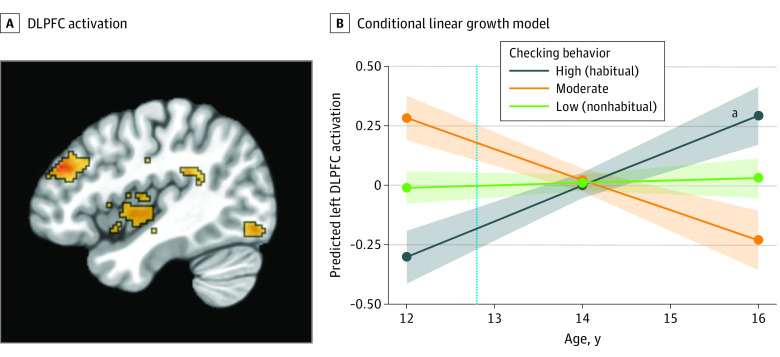
Figure 4. Functional Activation in the Left Dorsolateral Prefrontal Cortex (DLPFC) During the Anticipation of Social Feedback.
A, DLPFC (x, 42; y, −42; z, 28) activation during social anticipation significantly differed across time, as a function of social media checking behaviors. B, The dotted vertical line indicates the mean age of participants when they reported the number of checks at wave 1. The shaded areas represent the spread of the data based on the SE of each group.
aP < .05.
We extracted parameter estimates from each participant at each wave from the significant clusters in order to unpack the 2-way interaction. We ran post hoc conditional linear growth models to compare the trajectories of adolescents who engaged in low (nonhabitual; n = 79), moderate (n = 34), or high (habitual; n = 56) social media checking behaviors. Participants with high (habitual) checking behaviors showed a lower neural sensitivity to social anticipation at age 12 years (ie, the intercept) compared with those with low (nonhabitual) checking behaviors in the left amygdala/PI/VS (β, −0.22; 95% CI, −0.33 to −0.11 [Figure 1B]), right amygdala (β, −0.19; 95% CI, −0.30 to −0.08 [Figure 2B]), right AI (β, −0.23; 95% CI, −0.37 to −0.09 [Figure 3B]), and left DLPFC (β, −0.29; 95% CI, −0.44 to −0.14 [Figure 4B]). Here, β values refer to the main result of checking behavior at age 12 years where negative values indicate lower neural activation with higher checking behavior.
Developmentally, participants with high checking behaviors at age 12 years showed longitudinal increases (ie, the linear slope) in neural sensitivity in the left amygdala/PI/VS (β, 0.11; 95% CI, 0.04 to 0.18 [Figure 1B]), right amygdala (β, 0.09; 95% CI, 0.02 to 0.16 [Figure 2B]), right AI (β, 0.15; 95% CI, 0.02 to 0.20 [Figure 3B]), and left DLPFC (β, 0.19; 95% CI, 0.05 to 0.25 [Figure 4B]). Participants with low checking behaviors showed significant longitudinal decreases in neural sensitivity in the left amygdala/PI/VS (β, −0.12; 95% CI, −0.19 to −0.06 [Figure 1B]), right amygdala (β, −0.10; 95% CI, −0.17 to −0.03 [Figure 2B]), right AI (β, −0.13; 95% CI, −0.22 to −0.04 [Figure 3B]), and small decreases in the left DLPFC (β, −0.10, 95% CI, −0.22 to −0.03 [Figure 4B]). Here, β represents the age-related change in neural activation for each group. Results suggest that trajectories of neural sensitivity to anticipation of social feedback for habitual and nonhabitual checkers are inversely related.
Discussion
This cohort study examined whether early adolescents’ frequency of checking behaviors on 3 popular social media platforms (Facebook, Instagram, and Snapchat) was associated with trajectories of functional brain development across adolescence. Adolescents who engaged in high (habitual) checking behaviors showed distinct neural trajectories when anticipating social feedback compared with those who engaged in moderate or low (nonhabitual) checking behaviors, suggesting that habitual social media checking early in adolescence is associated with divergent brain development over time.
We found that 12-year-old adolescents showed different neural patterns based on their social media checking behavior. While participants with habitual checking behaviors demonstrated hypoactivation of the amygdala, PI, VS, and DLPFC in response to anticipation of social feedback, those with nonhabitual behaviors demonstrated hyperactivation in these same brain regions. Interestingly, these patterns diverged across development, with those with habitual behaviors showing longitudinal increases in activation in these regions and those with nonhabitual behaviors showing longitudinal decreases in activation.
Longitudinal decreases in neural activation among participants with nonhabitual checking behaviors may indicate a developmentally normative decreasing sensitivity to social anticipation. Indeed, prior research has found that in response to social anticipation, early adolescents show an initial hypersensitivity, followed by a decrease in activation of the PI and VS, brain regions associated with salience and motivation, respectively. Additionally, activation of the DLPFC during inhibitory control normatively decreases across adolescence. Decreasing DLPFC activation observed among nonhabitual checkers may indicate that these adolescents are better able to control impulsive or habitual behaviors, such as checking social media, and thus recruit prefrontal cortical regions less over time. In contrast, those with habitual checking behaviors showed longitudinal increases in neural activation in the amygdala, VS, PI, and DLPFC. Research has shown that with constant reinforcement, dopaminergic neurons within salience-related brain regions (ie, the VS, PI, and amygdala) become increasingly responsive to social feedback, and the enhanced value of rewards in the salience and motivation networks may override inhibitory control exerted by the PFC and cause a positive-feedback loop. The observed increase in DLPFC activation may indicate that more effort is required for cognitive control when anticipating social feedback.
Our findings suggest that checking behaviors on social media in early adolescence may tune the brain’s sensitivity to potential social rewards and punishments. Whereas individuals with habitual checking behaviors showed initial hypoactivation but increasing sensitivity to potential social cues over time, those with nonhabitual checking behaviors showed initial hyperactivation and decreasing sensitivity over time. Two primary theories contend over whether hypo- or hyperresponsivity to rewards is more associated with behavior. The hyperresponsive theory posits that adolescent reward-associated behaviors are associated with greater activation of the ventral-striatal dopamine circuit. Consequently, adolescents would experience an increased dopaminergic release in response to social feedback and rewards, which further encourages high-reward behaviors. Indeed, compared with children and adults, adolescents show higher activation in the reward system when receiving rewards. In contrast, the hyporesponsive theory posits that adolescent reward-seeking behaviors may be associated with a deficit in the activity of brain regions associated with motivation. This theory argues that repeated exposure to a social reward downregulates dopamine receptors and production, which results in decreased sensitivity of reward circuits. Studies suggest that, as adolescents experience fewer or less intense positive feelings from previously rewarding stimuli, they are driven to pursue new appetitive reinforcements through increases in reward-seeking behaviors, which increases activity in dopamine-related circuitry. Indeed, relative to adults, adolescents show less engagement of the VS in anticipation of rewards. While for some individuals with habitual checking behaviors, an initial hyposensitivity to potential social rewards and punishments followed by hypersensitivity may contribute to checking behaviors on social media becoming compulsive and problematic, for others, this change in sensitivity may reflect an adaptive behavior that allows them to better navigate their increasingly digital environment.
Limitations
This study has limitations. Notably, because differences in neural trajectories already existed between participants with habitual and nonhabitual checking behaviors at the start of the study, it is difficult to determine whether social media use prior to data collection caused these distinct neural trajectories or preexisting differences in neural activation placed some youth at risk for more habitual checking behaviors. Future studies should explore the neurodevelopmental trajectories of social feedback responsiveness from an earlier age to uncover causal pathways behind this association. Moreover, examination of social media checking behaviors across time is needed to further elucidate associations with development. Finally, future work should examine functional connectivity to explore how affective salience, motivational, and cognitive control networks coactivate and function at a network level.
Conclusions
Adolescent social media use has proliferated extensively in the past decade. This longitudinal cohort study suggests that social media behaviors in early adolescence may be associated with changes in adolescents’ neural development, specifically neural sensitivity to potential social feedback. Further research examining long-term prospective associations between social media use, adolescent neural development, and psychological adjustment is needed to understand the effects of a ubiquitous influence on development for today’s adolescents.
eMethods. Study Procedures
eTable. Age-Related Neural Changes as a Function of Social Media Checking During Social Feedback
eFigure. Descriptive Statistics of Social Media Checking Behaviors
References
- 1.Nesi J, Choukas-Bradley S, Prinstein MJ. Transformation of adolescent peer relations in the social media context: part 1-a theoretical framework and application to dyadic peer relationships. Clin Child Fam Psychol Rev. 2018;21(3):267-294. doi: 10.1007/s10567-018-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somerville LH. Special issue on the teenage brain: sensitivity to social evaluation. Curr Dir Psychol Sci. 2013;22(2):121-127. doi: 10.1177/0963721413476512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odgers CL, Jensen MR. Annual research review: adolescent mental health in the digital age: facts, fears, and future directions. J Child Psychol Psychiatry. 2020;61(3):336-348. doi: 10.1111/jcpp.13190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prinstein MJ, Nesi J, Telzer EH. Commentary: an updated agenda for the study of digital media use and adolescent development—future directions following Odgers & Jensen (2020). J Child Psychol Psychiatry. 2020;61(3):349-352. doi: 10.1111/jcpp.13219 [DOI] [PubMed] [Google Scholar]
- 5.Griffiths M. Adolescent social networking: how do social media operators facilitate habitual use? Education and Health. 2018;36(3):66-69. [Google Scholar]
- 6.Rideout V, Fox S. Digital health practices, social media use, and mental well-being among teens and young adults in the U.S. July 1, 2018. Accessed March 20, 2022. https://digitalcommons.psjhealth.org/publications/1093
- 7.Vogels EA. Gelles-Watnick R, Massarat N. Teens, social media and technology 2022. Pew Research Center. August 10, 2022. Accessed August 18, 2022. https://www.pewresearch.org/internet/2022/08/10/teens-social-media-and-technology-2022/
- 8.Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 2014;65:187-207. doi: 10.1146/annurev-psych-010213-115202 [DOI] [PubMed] [Google Scholar]
- 9.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62-77. doi: 10.1016/j.dr.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JR, Asarnow RF, Sabb FW, et al. A unique adolescent response to reward prediction errors. Nat Neurosci. 2010;13(6):669-671. doi: 10.1038/nn.2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279-1291. doi: 10.1016/j.neuroimage.2004.12.038 [DOI] [PubMed] [Google Scholar]
- 12.Galván A, McGlennen KM. Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. J Cogn Neurosci. 2013;25(2):284-296. doi: 10.1162/jocn_a_00326 [DOI] [PubMed] [Google Scholar]
- 13.Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, Crone EA. What motivates the adolescent? brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20(1):61-69. doi: 10.1093/cercor/bhp078 [DOI] [PubMed] [Google Scholar]
- 14.Galván A. The teenage brain: sensitivity to rewards. Curr Dir Psychol Sci. 2013;22(2):88-93. doi: 10.1177/0963721413480859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev. 2008;28(1):78-106. doi: 10.1016/j.dr.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veissière SPL, Stendel M. Hypernatural monitoring: a social rehearsal account of smartphone addiction. Front Psychol. 2018;9:141. doi: 10.3389/fpsyg.2018.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihssen N, Wadsley M. A reward and incentive-sensitization perspective on compulsive use of social networking sites—wanting but not liking predicts checking frequency and problematic use behavior. Addict Behav. 2021;116:106808. doi: 10.1016/j.addbeh.2020.106808 [DOI] [PubMed] [Google Scholar]
- 18.Lenhart A, Purcell K, Smith A, Zickuhr K. Social media and mobile internet use among teens and young adults: millennials. Pew internet and American Life Project. 2010. Accessed August 18, 2022. https://eric.ed.gov/?id=ED525056
- 19.Cremers HR, Veer IM, Spinhoven P, Rombouts SARB, Roelofs K. Neural sensitivity to social reward and punishment anticipation in social anxiety disorder. Front Behav Neurosci. 2015;8:439. https://www.frontiersin.org/articles/10.3389/fnbeh.2014.00439. doi: 10.3389/fnbeh.2014.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spreckelmeyer KN, Krach S, Kohls G, et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc Cogn Affect Neurosci. 2009;4(2):158-165. doi: 10.1093/scan/nsn051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013;73:176-190. doi: 10.1016/j.neuroimage.2013.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen R. Missing data imputation versus full information maximum likelihood with second-level dependencies. Struct Equ Modeling. 2011;18(4):649-662. doi: 10.1080/10705511.2011.607721 [DOI] [Google Scholar]
- 23.Ward HA, Riederer SJ, Grimm RC, et al. Prospective multiaxial motion correction for fMRI. Mag Reson Med. 2000;43(3):459–469. doi: [DOI] [PubMed] [Google Scholar]
- 24.Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885-6892. doi: 10.1523/JNEUROSCI.1062-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunther Moor B, van Leijenhorst L, Rombouts SARB, Crone EA, Van der Molen MW. Do you like me? neural correlates of social evaluation and developmental trajectories. Soc Neurosci. 2010;5(5-6):461-482. doi: 10.1080/17470910903526155 [DOI] [PubMed] [Google Scholar]
- 26.Van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51(1):345-355. doi: 10.1016/j.neuroimage.2010.02.038 [DOI] [PubMed] [Google Scholar]
- 27.Vrtička P, Sander D, Anderson B, Badoud D, Eliez S, Debbané M. Social feedback processing from early to late adolescence: influence of sex, age, and attachment style. Brain Behav. 2014;4(5):703-720. doi: 10.1002/brb3.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci. 2013;33(46):18109-18124. doi: 10.1523/JNEUROSCI.1741-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815-834. doi: 10.1016/j.neuron.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24(8):1793-1802. doi: 10.1523/JNEUROSCI.4862-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galván A. Neural systems underlying reward and approach behaviors in childhood and adolescence. Curr Top Behav Neurosci. 2014;16:167-188. doi: 10.1007/978-3-662-45758-0_240 [DOI] [PubMed] [Google Scholar]
- 32.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041-1052. doi: 10.1176/appi.ajp.160.6.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blum K, Sheridan PJ, Wood RC, et al. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89(7):396-400. doi: 10.1177/014107689608900711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108(37):15037-15042. doi: 10.1073/pnas.1010654108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417-463. doi: 10.1016/S0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- 36.Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Study Procedures
eTable. Age-Related Neural Changes as a Function of Social Media Checking During Social Feedback
eFigure. Descriptive Statistics of Social Media Checking Behaviors