This cohort study analyzes how quickly novel immunotherapies are adopted after US Food and Drug Administration approval in different types of oncology practices.
Key Points
Question
How quickly have different types of oncology practices adopted novel immunotherapies?
Findings
In this cohort study of 71 659 Medicare claims from 1732 oncology practices, most practices adopted immunotherapy within 2 years of FDA approval, but adoption was not equal across practice types: adoption was lower at rural vs urban practices and small vs large practices. Adoption was similar at independent practices and nonacademic systems, but both had lower adoption than practices that were part of academic systems.
Meaning
Results of the study suggest that adoption of immunotherapy has been rapid but uneven across oncology practices.
Abstract
Importance
Immunotherapies reflect an important breakthrough in cancer treatment, substantially improving outcomes for patients with a variety of cancer types, yet little is known about which practices have adopted this novel therapy or the pace of adoption.
Objective
To assess adoption of immunotherapies across US oncology practices and examine variation in adoption by practice type.
Design, Setting, and Participants
This cohort study used data from Medicare fee-for-service beneficiaries undergoing 6-month chemotherapy episodes between 2010 and 2017. Data were analyzed January 19, 2021, to September 28, 2022, for patients with cancer types for which immunotherapy was approved by the US Food and Drug Administration (FDA) during the study period: melanoma, kidney cancer, lung cancer, and head and neck cancer.
Exposures
Oncology practice location (rural vs urban), affiliation type (academic system, nonacademic system, independent), and size (1 to 5 physicians vs 6 or more physicians).
Main Outcomes and Measures
The primary outcome was whether a practice adopted immunotherapy. Adoption rates for each practice type were estimated using multivariate linear models that adjusted for patient characteristics (age, sex, race and ethnicity, cancer type, Charlson Comorbidity Index, and median household income).
Results
Data included 71 659 episodes at 1732 oncology practices. Of these, 264 practices (15%) were rural, 900 (52%) were independent, and 492 (28%) had 1 to 5 physicians. Most practices adopted immunotherapy within 2 years of FDA approval, but there was substantial variation in adoption rates across practice types. After FDA approval, adoption of immunotherapy was 11 (95% CI, −16 to −6) percentage points lower at rural practices than urban practices and 27 (95% CI, −32 to −22) percentage points lower at practices with 1 to 5 physicians than practices with 6 or more physicians. Adoption rates were similar at independent practices and nonacademic systems; however, both practice types had lower adoption than academic systems (independent practice difference, −6 [95% CI, −9 to −3] percentage points; nonacademic systems difference, −9 [95% CI, −11 to −6] percentage points).
Conclusions and Relevance
In this cohort study of Medicare claims, practice characteristics, especially practice size and rural location, were associated with adoption of immunotherapy. These findings suggest that there may be geographic disparities in access to important innovations for treating patients with cancer.
Introduction
Adoption of proven novel therapies into clinical practice is a key factor underlying improvements in life expectancy.1,2,3 Yet adoption of new technologies is often slow and variable, creating disparities in health outcomes and access to care.4,5 Adoption of new therapies can require considerable financial investment and expertise, raising concern that some clinicians will fall behind in using valuable new technologies. These concerns are particularly acute for clinicians in small or rural practices, who tend to have fewer resources.6,7
Understanding variation in technology adoption is important in the context of cancer care because cancer is the second leading cause of death in the US and a substantial contributor to morbidity. In 2022, nearly 2 million individuals in the US were diagnosed with cancer, and more than 600 000 individuals died of cancer.8 Moreover, innovations in treatment have lowered the risk of cancer mortality but have also made cancer treatment increasingly complex.9,10 There is substantial heterogeneity in clinical expertise across oncologists,11 raising the possibility that some practices may be faster to adopt new technologies than others.
Oncology practices range from groups that are affiliated with academic or nonacademic systems to smaller, independent physicians’ offices in urban and rural settings. Oncologists in rural settings or smaller practices may have less specialized expertise, which could lead to slower adoption of novel therapies.12 They may also have less access to clinical trials, which increase information exchange and familiarity with new treatments.13,14 Moreover, lower patient volumes in smaller practices may limit practices’ ability to negotiate discounts on novel drugs, making their use less profitable. However, there is little understanding of which practices adopt innovative therapies.15
In this study, we examined the adoption of an important new treatment for patients with cancer: immune checkpoint inhibitors, or immunotherapies. Immunotherapies have improved survival in patients with cancers that were previously unresponsive to chemotherapy, revolutionizing care for many patients with advanced cancers.16,17,18,19,20,21 Between 2010 and 2021, the US Food and Drug Administration (FDA) approved immunotherapy for 19 cancer types.22 Although existing studies have documented immunotherapy use overall and across different types of patients, little is known about how adoption varies across practice types.13,23,24,25,26,27,28 On one hand, logistical and cost barriers to adopting immunotherapy are small compared with many other technologies, suggesting widespread adoption is possible. Immunotherapies are easy to administer, require no specialized equipment, and tend to have milder adverse effects than traditional chemotherapy. Moreover, advertising for immunotherapy has been substantial, suggesting lower informational barriers to adoption. On the other hand, immunotherapy adoption requires management of rare but serious, adverse effects (eg, pneumonitis, cardiac toxic effects), which may be less familiar to some clinicians.
We studied differential adoption of immunotherapy from 2010 to 2017 by practice location (rural vs urban), affiliation type (system vs independent), and size (by number of physicians). The aim of the study was to assess the adoption of immunotherapy before and after FDA approval in different practice types, focusing on patients with 4 types of cancers (melanoma, kidney cancer, lung cancer, head and neck cancer) for which immunotherapies were approved during the study period.
Methods
Data and Study Sample
This cohort study used Medicare claims data (inpatient, outpatient, carrier, durable medical equipment, hospice, and Part D event files) from 2010 to 2017 for a random 20% sample of fee-for-service beneficiaries. We characterized episodes of care for patients with cancer who received infused or oral chemotherapy, following a similar approach to the Oncology Care Model.29,30 Specifically, episodes were triggered by a Part B or D chemotherapy claim (cytotoxic chemotherapy, biologic therapy, or immunotherapy) with an associated claim for an outpatient visit. The outpatient visit claim was required to have a cancer diagnosis code, which was used to assign cancer type (eAppendix in the Supplement).31 We studied patients who had 1 of 4 cancer types with FDA approvals for immunotherapy between 2011 and 2016, allowing us to observe adoption patterns for at least 1 year before and after the approval for each cancer type: melanoma (first approval in 2011), kidney cancer (2015), lung cancer (2015), and head and neck cancer (2016) (eTables 1 and 2 in the Supplement). The study was approved by the institutional review boards of Harvard Medical School and the National Bureau of Economic Research, and informed consent was waived because only deidentified data were used. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Episodes included all treatment within 6 months of the first chemotherapy claim. Patients were eligible for multiple episodes; subsequent episodes began with the first chemotherapy claim after the end of the 6-month episode. The study population included beneficiaries who were 65 years or older and continuously enrolled in Medicare Parts A and B throughout the 6-month episode (or until death or hospice enrollment).
Practice Attribution and Characteristics
Episodes were attributed to the oncology practice that delivered the plurality of outpatient medical oncology visits (eAppendix in the Supplement).32 We restricted the population to practices with 5 or more episodes with the cancer types of interest during the study period. These practices accounted for 96% of episodes.
We categorized practices along 3 dimensions. First, practices were classified as urban or rural based on 2010 Rural-Urban Commuting Area codes for each practice’s zip code (eAppendix in the Supplement).33 Second, we categorized practices as independent or system-affiliated and further categorized systems as academic or nonacademic (eAppendix in the Supplement). Third, we categorized practices as large or small according to the number of physicians billing under the practice. Large practices had 6 or more total physicians; small practices had 1 to 5 total physicians, reflecting the bottom quartile of number of physicians per practice (eFigure 1 in the Supplement).
Immunotherapy Use
The primary outcome was whether a practice adopted immunotherapy. To begin, we characterized each episode according to the presence of any immunotherapy treatment. Immunotherapy use was defined using Current Procedural Terminology (CPT) billing codes (eTable 1 in the Supplement). Permanent billing codes for new immunotherapies became available 6 to 18 months after the first FDA approval. Before these codes were available, practitioners used temporary CPT codes (C codes) for hospital-based infusions or nonspecific CPT codes (eg, J9999: “Not otherwise classified, antineoplastic drugs”) for non–hospital-based infusions. Then we assessed whether each practice had adopted immunotherapy based on the episodes attributed to that practice. Practice-level adoption corresponded with the start of the first attributed episode with immunotherapy treatment, coded as 1 for practices with any immunotherapy use in current or past periods and 0 otherwise.
Statistical Analyses
Data were analyzed January 19, 2021, to September 28, 2022. We plotted patterns in immunotherapy adoption before and after FDA approval. Time since first FDA approval was measured in 6-month intervals, such that time 0 included the month of the first FDA approval and the 5 subsequent months. Episodes were assigned to time intervals according to the date of their initial chemotherapy treatment, even if immunotherapy was only administered later in the episode. We plotted patterns in immunotherapy adoption separately by cancer type and by practice type: rural vs urban, independent vs system affiliated, and small vs large.
We used multivariate linear regression to measure differential adoption of immunotherapy across practice types. Analyses were run at the practice-time level and were weighted by the number of episodes attributed to a practice in each period. The model used data from the years after FDA approval and included 3 key explanatory variables: rural practice location, system affiliation type, and small practice size; these variables measured the mean percentage point differences in adoption across practice types in the postapproval period. The model also adjusted for the mix of patients at each practice in each time period, including percentage of patients aged 65 to 74 years, 75 to 84 years, and 85 years or older; percentage of patients whose documented sex was female; percentage of patients by race and ethnicity; percentage of patients with each cancer type; percentage of patients with a Charlson Comorbidity Index of 0, 1, 2, or 3 or more (Klabunde modification)34; and share of patients in zip codes with median household incomes of less than $40 000, $40 000 to $69 999, and $70 000 or more. We used the Medicare Research Triangle Institute race variable,35 which classifies race and ethnicity based on Social Security Administration records and an algorithm to identify additional Hispanic and Asian beneficiaries. Race and ethnicity categories included Hispanic, non-Hispanic Black, non-Hispanic White, and other race (including Asian or Pacific Islander, American Indian or Alaska Native, and other or unknown race; 0.9% of episodes had unknown race). We excluded episodes if they had missing values for any covariate in the regression (0.8% of episodes). The SEs were clustered at the practice level.
In supplemental analyses, we measured differential adoption across practice types over time, rather than overall differences in the postapproval period. In this specification, we assessed the interaction of the 3 key explanatory variables (rural practice location, system affiliation type, and small practice size) with time since FDA approval, measured in 6-month increments. Although our main model estimated mean differences in adoption across practice types in the postapproval period, this supplementary model traced the shape of immunotherapy adoption curves separately for different practice types. We also conducted several sensitivity analyses. First, we redefined immunotherapy use based only on permanent CPT codes (vs including temporary or nonspecific codes in our main model). Second, we repeated the analyses using practices with 20 or more episodes during the study period (vs 5 in the main analysis). Third, we reestimated our model at the episode level rather than the practice-time level. The dependent variable was the probability that an episode was overseen by a practice that had adopted immunotherapy. This specification allowed us to control for clinical risk factors at the patient level rather than mean patient characteristics at the practice-time level. Fourth, we redefined adoption as the first time period in which immunotherapy was used for 10% of cumulative episodes at the practice since FDA approval (vs any immunotherapy use in the main model), following work by Keating et al15 (eAppendix in the Supplement). Fifth, we estimated adoption separately by cancer type. Finally, we modeled adoption by allowing practice characteristics to have interaction effects, assessing the interaction of rural location with 3 types of practices: small independent, large independent, and system. We did not assess for an interaction between system and size because systems are large by definition. We grouped academic and nonacademic systems because there are few academic systems in rural areas (eFigure 2 in the Supplement).
We reported 95% CIs for all estimates. Statistical analyses were conducted using Stata, version 17 (StataCorp LLC).
Results
Analyses included 71 659 episodes across 1732 practices. Of these, 264 practices (15%) were rural, 900 practices (52%) were independent, and 492 practices (28%) had 1 to 5 physicians (Table 1). Rural practices had higher shares of non-Hispanic White patients than urban practices (4634 of 5109 [91%] vs 56 115 of 66 550 [84%]) and higher shares of patients living in zip codes with median household incomes of less than $40 000 (1950 of 5109 [38%] vs 12 467 of 66 550 [19%]). Other differences across practice types were more modest.
Table 1. Characteristics of Oncology Practices and Episodes.
| Characteristic | No (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| All practices | Practice location | Practice affiliation type | Practice size by No. of physicians | |||||
| Rural | Urban | Independent | Nonacademic system | Academic system | Small (1-5 physicians) | Large (≥6 physicians) | ||
| Characteristics of practices | ||||||||
| Practices | 1732 | 264 (15) | 1468 (85) | 900 (52) | 693 (40) | 139 (8) | 492 (28) | 1240 (72) |
| Beneficiariesa | 42 660 | 3298 (8) | 39 490 (93) | 20 857 (49) | 14 590 (34) | 8200 (19) | 4599 (11) | 38 409 (90) |
| Episodes | 71 659 | 5109 (7) | 66 550 (93) | 35 236 (49) | 23 220 (32) | 13 203 (18) | 7693 (11) | 63 966 (89) |
| Episodes per practice | 41 | 19 | 45 | 39 | 34 | 95 | 16 | 52 |
| Beneficiaries per practice | 25 | 12 | 27 | 23 | 21 | 59 | 9 | 31 |
| Episodes per beneficiary | 1.7 | 1.5 | 1.7 | 1.7 | 1.6 | 1.6 | 1.7 | 1.7 |
| Characteristics of episodes across practicesb | ||||||||
| Sex | ||||||||
| Male | 33 433/71 659 (47) | 2342/5109 (46) | 31 091/66 550 (47) | 16 241/35 236 (46) | 10 851/23 220 (47) | 6341/13 203 (48)c | 3606/7693 (47) | 29 827/63 966 (47) |
| Female | 38 226/71 659 (53) | 2767/5109 (54) | 35 459/66 550 (53) | 18 995/35 236 (54) | 12 369/23 220 (53) | 6862/13 203 (52)c | 4087/7693 (53) | 34 139/63 966 (53) |
| Mean (SD) age, y | 74 (6) | 74 (6) | 74 (6) | 74 (6) | 74 (6) | 73 (6) | 75 (6) | 74 (6) |
| Age, y | ||||||||
| 65-74 | 40 913/71 659 (57) | 2991/5109 (59) | 37 922/66 550 (57) | 19 527/35 236 (55) | 13 326/23 220 (57)c | 8060/13 203 (61)c | 4124/7693 (54) | 36 789/63 966 (58)c |
| 75-84 | 26 120/71 659 (36) | 1856/5109 (36) | 24 264/66 550 (36) | 13 256/35 236 (38) | 8429/23 220 (36)c | 4435/13 203 (34)c | 2962/7693 (39) | 23 158/63 966 (36)c |
| ≥85 | 4626/71 659 (6) | 262/5109 (5) | 4364/66 550 (7)c | 2453/35 236 (7) | 1465/23 220 (6) | 708/13 203 (5)c | 607/7693 (8) | 4019/63 966 (6)c |
| Race and ethnicity | ||||||||
| Hispanic | 2433/71 659 (3) | 61/5109 (1) | 2372/66 550 (4)c | 1358/35 236 (4) | 555/23 220 (2)c | 520/13 203 (4) | 354/7693 (5) | 2079/63 966 (3)c |
| Non-Hispanic Black | 5239/71 659 (7) | 275/5109 (5) | 4964/66 550 (7)c | 2395/35 236 (7) | 1545/23 220 (7) | 1299/13 203 (10)c | 662/7693 (9) | 4577/63 966 (7) |
| Non-Hispanic White | 60 749/71 659 (85) | 4634/5109 (91) | 56 115/66 550 (84)c | 29 993/35 236 (85) | 20 201/23 220 (87)c | 10 555/13 203 (80)c | 6242/7693 (81) | 54 507/63 966 (85)c |
| Otherd | 3238/71 659 (5) | 139/5109 (3) | 3099/66 550 (5)c | 1490/35 236 (4) | 919/23 220 (4) | 829/13 203 (6)c | 435/7693 (6) | 2803/63 966 (4) |
| Charlson Comorbidity Indexe | ||||||||
| 0 | 13 076/71 659 (18) | 818/5109 (16) | 12 258/66 550 (18)c | 6395/35 236 (18) | 3908/23 220 (17)c | 2773/13 203 (21)c | 1242/7693 (16) | 11 834/63 966 (19)c |
| 1 | 18362/71 659 (26) | 1340/5109 (26) | 17 022/66 550 (26) | 8955/35 236 (25) | 6017/23 220 (26) | 3390/13 203 (26) | 1948/7693 (25) | 16 414/63 966 (26) |
| 2 | 15 158/71 659 (21) | 1159/5109 (23) | 13 999/66 550 (21)c | 7583/35 236 (22) | 4957/23 220 (21) | 2618/13 203 (20)c | 1697/7693 (22) | 13 461/63 966 (21) |
| ≥3 | 25 063/71 659 (35) | 1792/5109 (35) | 23 271/66 550 (35) | 12 303/35 236 (35) | 8338/23 220 (36) | 4422/13 203 (33) | 2806/7693 (36) | 22 257/63 966 (35) |
| Median household incomef | ||||||||
| <$40 000 | 14 417/71 659 (20) | 1950/5109 (38) | 12 467/66 550 (19)c | 7540/35 236 (21) | 4710/23 220 (20) | 2167/13 203 (16)c | 1792/7693 (23) | 12 625/63 966 (20)c |
| $40 000-$69 999 | 39 407/71 659 (55) | 3021/5109 (59) | 36 386/66 550 (55)c | 19 158/35 236 (54) | 13 714/23 220 (59)c | 6535/13 203 (49)c | 4180/7693 (54) | 35 227/63 966 (55) |
| ≥$70 000 | 17 835/71 659 (25) | 138/5109 (3) | 17 697/66 550 (27)c | 8538/35 236 (24) | 4796/23 220 (21)c | 4501/13 203 (34)c | 1721/7693 (22) | 16 114/63 966 (25) |
| Cancer type | ||||||||
| Melanoma | 2022/71 659 (3) | 101/5109 (2) | 1921/66 550 (3) | 1037/35 236 (3) | 430/23 220 (2)c | 555/13 203 (4)c | 268/7693 (3) | 1754/63 966 (3) |
| Lung cancer | 58 737/71 659 (82) | 4284/5109 (84) | 54 453/66 550 (82)c | 29 416/35 236 (83) | 19 319/23 220 (83) | 10 002/13 203 (76)c | 6544/7693 (85) | 52 193/63 966 (82)c |
| Kidney cancer | 5352/71 659 (7) | 318/5109 (6) | 5034/66 550 (8)c | 2377/35 236 (7) | 1708/23 220 (7) | 1267/13 203 (10)c | 460/7693 (6) | 4892/63 966 (8)c |
| Head and neck cancer | 5548/71 659 (8) | 406/5109 (8) | 5142/66 550 (8) | 2406/35 236 (7) | 1763/23 220 (8)c | 1379/13 203 (10)c | 421/7693 (5) | 5127/63 966 (8)c |
Beneficiaries can have multiple episodes and may appear in multiple columns; therefore, numbers may not sum to the totals.
Mean practice characteristics are based on episodes attributed to each practice. Episodes were triggered by a Part B or D chemotherapy claim (17% Part D), following the Oncology Care Model.
Difference between practices is statistically significant at P < .05 (rural vs urban, independent vs system, or small vs large).
Other races and ethnicities include Asian or Pacific Islander, American Indian or Alaska Native, and other or unknown race and ethnicity.
Zero indicates no Charlson comorbid conditions; a greater score indicates an increasing number or severity of comorbid conditions.
This was calculated as the share of patients in zip codes with median household incomes in each category.
There was rapid adoption of immunotherapy after FDA approval for each cancer type studied (Figure 1). Figure 1A plots adoption rates by calendar time, whereas Figure 1B plots adoption rates by time since FDA approval. Within 6 months of FDA approval, a weighted 9% of practices with melanoma episodes had adopted immunotherapy for at least 1 patient (Figure 1B, 0 on the horizontal axis). Adoption within 6 months of approval reached a weighted 51% for practices with lung episodes, 62% with kidney episodes, and 56% with head and neck cancer episodes. By 2 years after approval, adoption reached a weighted 76% of practices with melanoma episodes, 95% with lung episodes, and 81% with kidney episodes. Head and neck cancer episodes were not observed 2 years after approval given the 2016 approval date. For some cancer types, immunotherapy adoption began before FDA approval, in part reflecting off-label use of drugs that had been approved for other cancers. Because we assigned episodes to time periods according to their start dates, episodes that began before FDA approval but ended afterward could also contribute to use in the first period before approval.
Figure 1. Immunotherapy Adoption Before and After US Food and Drug Administration (FDA) Approval by Cancer Type.
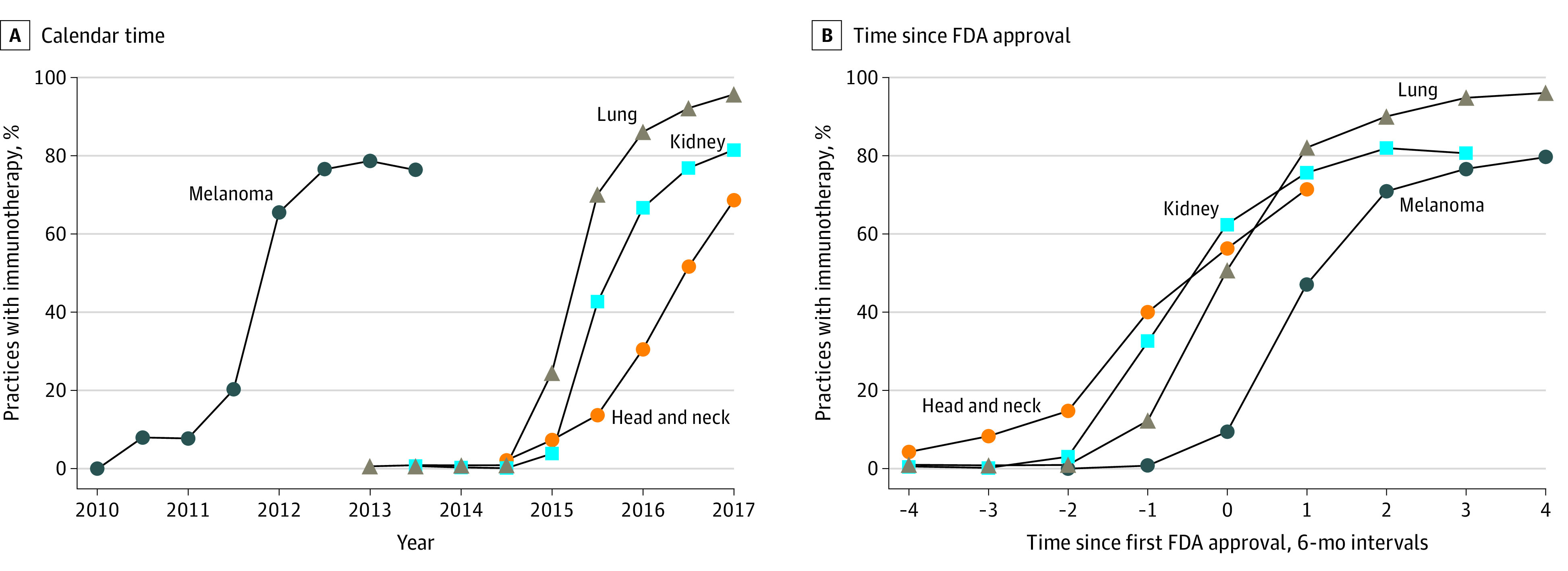
Immunotherapy adoption rates are plotted for each cancer type over calendar time (A) and in the periods before and after FDA approval (B). Because FDA approval dates can occur in the middle of 6-month calendar periods (eg, immunotherapy was approved for melanoma in March 2011), adoption curves can have different shapes across panels. Kidney cancer and head and neck cancer episodes were observed for shorter time periods after FDA approval given the later approval dates for immunotherapy use.
Immunotherapy adoption differed across practice types (Figure 2; Table 2; eTable 3 in the Supplement). In adjusted analyses, mean adoption among rural practices was 11 (95% CI, −16 to −6) percentage points lower than in urban practices in the years after FDA approval. Differences in adoption were larger immediately after FDA approval, with rural practices catching up to urban practices over time (eg, 13 [95% CI, 4-22] percentage point gain 2 years after approval, relative to immediately after approval; Figure 2; eTable 3 in the Supplement). Practice size was also associated with immunotherapy adoption. Small practices were 27 (95% CI, −32 to −22) percentage points less likely to adopt immunotherapy after FDA approval, with smaller differences later in the study period. Independent practices and nonacademic systems had similar adoption patterns, but both had lower adoption compared with academic systems (vs independent, −6 [95% CI, −9 to −3] percentage points; vs nonacademic systems, −9 [95% CI, −11 to −6] percentage points).
Figure 2. Immunotherapy Adoption Before and After US Food and Drug Administration (FDA) Approval by Practice Type.
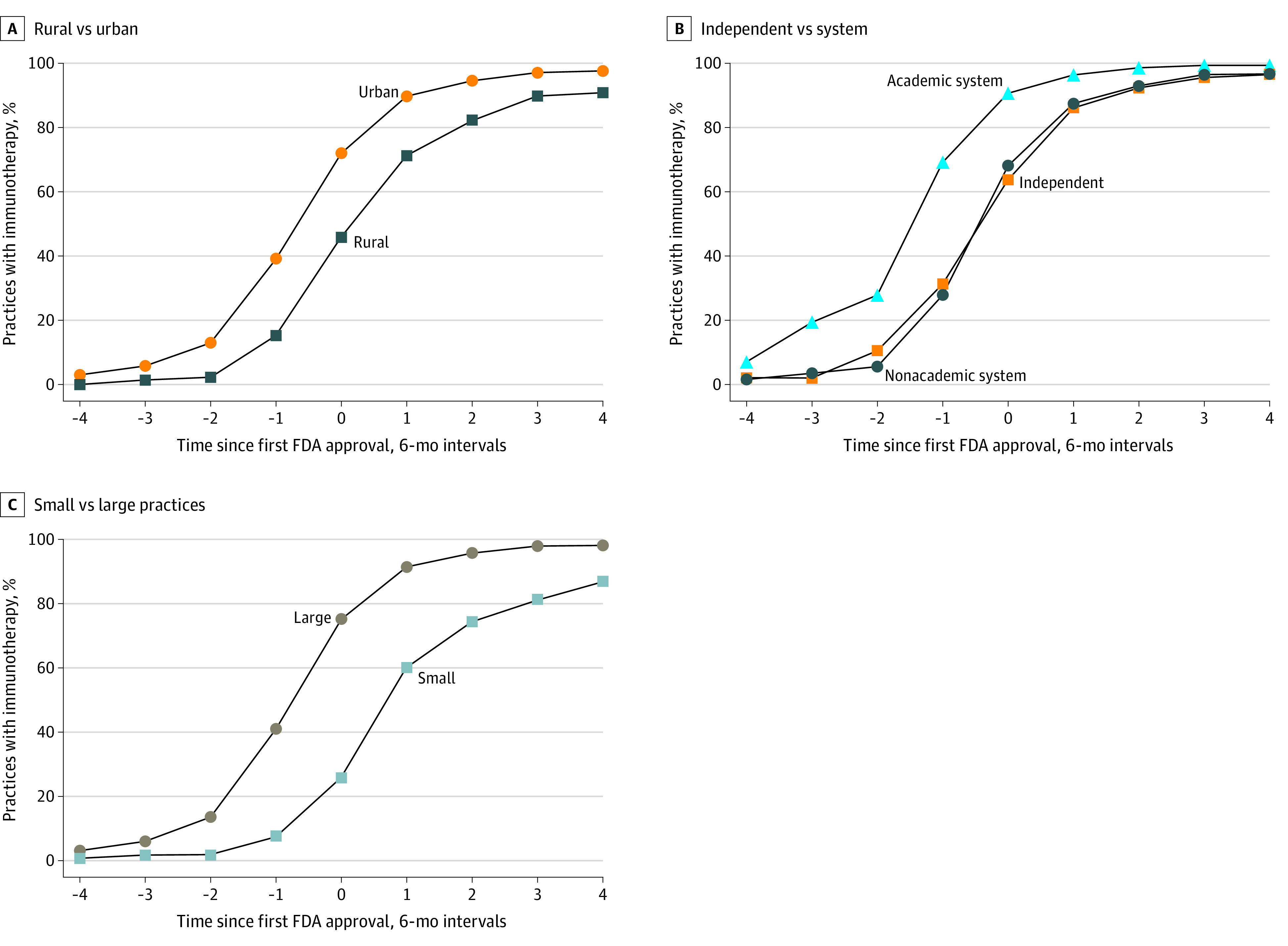
Immunotherapy adoption rates are plotted by practice type before and after FDA approval. Because some immunotherapy indications were approved near the end of the study period in 2017, not all cancer types are included in each time period (eg, immunotherapy was approved for head and neck cancer in 2016, allowing for 1 year of postapproval observation). C, Large practices included 6 or more physicians; small practices included 1 to 5 physicians.
Table 2. Association Between Practice Characteristics and Immunotherapy Adoption After US Food and Drug Administration Approval.
| Characteristic | Practices with immunotherapy adoption, % (n = 1309)a | Percentage point difference (95% CI) | |
|---|---|---|---|
| Unadjusted | Adjustedb | ||
| Practice location | |||
| Rural | 74 | −15 (−20 to −10) | −11 (−16 to −6) |
| Urban | 89 | Reference | Reference |
| Practice affiliation | |||
| Independent | 85 | −11 (−15 to −7) | −6 (−9 to −3) |
| Nonacademic system | 88 | −9 (−11 to −7) | −9 (−11 to −6) |
| Academic system | 97 | Reference | Reference |
| Practice size | |||
| Small (1-5 physicians) | 62 | −29 (−33 to −25) | −27 (−32 to −22) |
| Large (≥6 physicians) | 91 | Reference | Reference |
Percentages are weighted by the number of episodes at each practice in each time period.
This column contains estimates from a single regression model that includes the 3 key explanatory variables: rural practice location, system affiliation type, and small practice size. Estimates represent the mean difference in adoption rates across practice types in the years after US Food and Drug Administration approval. The model also adjusts for the mix of patients at each practice, including cancer type, age, sex, race and ethnicity, Charlson Comorbidity Index, and median household income in the patient’s zip code of residence.
Sensitivity analyses supported the main results. Robustness checks that (1) used only permanent codes to define immunotherapy, (2) restricted the analysis to practices with 20 or more episodes, (3) ran episode-level analysis, and (4) redefined adoption as immunotherapy use among 10% of episodes all found differences in adoption by practice location and size, as in our main model (eg, for rural vs urban practices, robustness model 1 found differential adoption of −12 [95% CI, −17 to −7] percentage points; model 2, −5 [95% CI, −11 to 0] percentage points; model 3, −11 [95% CI, −16 to −6] percentage points; and model 4, −7 [95% CI, −13 to −2] percentage points; eTable 4 in the Supplement). In addition, we drew similar conclusions when we stratified the analysis by cancer type (eg, for small vs large practices, for melanoma episodes, we found differential adoption of −11 [95% CI, −24 to 2] percentage points; lung cancer episodes, −30 [95% CI, −34 to −25] percentage points; kidney cancer episodes, −23 [95% CI, −35 to −12] percentage points; and head and neck cancer episodes, −9 [95% CI, −17 to −1] percentage points; eTable 5 in the Supplement) and modeled interaction effects of practice characteristics (eFigure 3 in the Supplement); rural practices had lower adoption rates, except among small practices, where adoption was uniformly low across locations (eg, for rural vs urban practices, among system-affiliated practices, adoption was 13 [95% CI, −26 to −1] percentage points lower). Large practices and systems had higher adoption than small practices, especially in urban areas (eg, for large independent vs small independent practices, among urban practices, adoption was 29 [95% CI, 24-35] percentage points higher).
Discussion
In this cohort analysis of Medicare claims, we documented 2 key findings about the adoption of novel immunotherapies across oncology practices. First, most oncology practices adopted immunotherapy soon after FDA approval for all cancer types studied. Second, adoption of immunotherapy was not equal across practice types. Rural practices were slower to adopt immunotherapy, as were small practices. In contrast, we found similar adoption across independent practices and practices affiliated with nonacademic systems; however, both types of practices had less adoption than practices affiliated with academic systems.
Our findings contribute to a nascent literature on practice-level variation in adoption of novel cancer drugs.15 Consistent with existing work on adoption of bevacizumab15—one of the first widely used biologic cancer therapies—we found evidence that nonclinical factors, such as practice type, may alter use of new technologies. However, there were 2 notable differences between our findings and the work on bevacizumab. First, we found more complete adoption of immunotherapies 2 years after FDA approval (75%-95% of practices adopting immunotherapies across cancer types) compared with bevacizumab (20%-50% of practices adopting therapies across cancer types). Second, the investigation of bevacizumab found faster adoption among independent vs academic practices. A key difference between bevacizumab and immunotherapies is that the benefits of bevacizumab are modest compared with those of immunotherapies. One explanation for the higher adoption of immunotherapy overall and at academic systems is that oncologists use clinical evidence when deciding whether to adopt new therapies, especially oncologists in academic systems.14
This analysis also has implications for geographic variations in treatment patterns, which have been well documented.2,4,5,36 Findings of the present study suggest a possible mechanism: small or rural practices fall behind in terms of adopting new technologies. This finding is consistent with other research reporting lower adoption of high-cost technologies at rural or small hospitals—such as intensive care units, cardiac catheterization labs, robotic surgery, and electronic health records—and higher adoption at academic hospitals.6,37,38,39 Compared with these technologies, barriers to immunotherapy adoption are likely low, because immunotherapy use requires little specialized training and equipment. Nevertheless, there were persistent differences in immunotherapy adoption patterns across practices. The shape of the adoption curves was similar for smaller and rural practices compared with larger and urban practices, but the start of adoption was later; this pattern suggests that interventions focusing on initial use may be particularly important to address differences in adoption.
Limitations
This study has limitations. First, as with any observational study, unmeasured differences in the patients served by different practices may be associated with observed differences in adoption rates. Some practices may be less likely to treat patients with advanced-stage cancers, for example, those for whom immunotherapy was first approved and few alternative treatment options are available. Because Medicare claims lack clinical details, such as cancer stage and line of therapy, we could not identify which patients were eligible for immunotherapies based on the specific FDA indications. Unobserved patient differences could cause us to either understate or overstate differences in adoption across practice types. Previous work, for example, has found that patients in rural areas tend to be diagnosed with more advanced cancers than patients in urban areas, which could facilitate higher adoption rates among rural practices unless such patients are disproportionately referred to academic or urban practices.40,41
Second, there may be measurement error in the adoption variable. We inferred adoption based on immunotherapy use among a 20% random sample of fee-for-service Medicare beneficiaries; it is therefore possible that we did not capture a practice’s first dose, although we do not expect this to alter our conclusions. Moreover, if practices used immunotherapy for patients with commercial insurance or Medicare Advantage, but not fee-for-service beneficiaries, the analysis would understate adoption levels and potentially mismeasure differential adoption across practice types. Medicare Advantage penetration is higher in urban vs rural areas, for example, and some large practices have a financial stake in a Medicare Advantage plan.42,43 In addition, we defined adoption based on any use of immunotherapy within a practice. This approach will overstate adoption if a practice experiments with immunotherapy but does not continue to use the drug, although the results of the present study were robust to using a stricter definition of adoption. In addition, we used nonspecific billing codes to capture immunotherapy use in non–hospital-based practices before permanent billing codes were available. However, we would expect use of nonspecific codes to bias toward greater use of immunotherapies in smaller practices, which we did not observe, and the findings were robust to using only permanent billing codes.
Third, our ability to draw conclusions about adoption before FDA approval was limited. In particular, we found higher preapproval adoption for cancer types with later approvals. This pattern could reflect increased comfort with immunotherapy over time or the immediate availability of permanent billing codes for cancer types with later approvals. In addition, we could not capture immunotherapy use during clinical trials that preceded FDA approval and the availability of billing codes. Nevertheless, engagement in such clinical trials may have contributed to the faster adoption we observed in larger or academic practices.
Conclusions
In this cohort study, adoption of immunotherapy by oncology practices occurred rapidly after FDA approval. However, adoption varied across practice types, with lower adoption at rural practices and small practices and higher adoption at academic systems. A natural question is whether immunotherapy adoption has changed the productivity of oncology care. Future work could usefully measure the benefits and costs of adoption across practice types, which was not observable in the data used in this study.
eAppendix. Study Population, Practice Attribution, and Practice Characteristics
eTable 1. Approved Immunotherapy Treatments Through 2016 and CPT Codes
eTable 2. Approved Immunotherapy Treatments by Cancer Type and Year
eTable 3. Full Model Results for the Association Between Practice Characteristics and Immunotherapy Adoption After FDA Approval
eTable 4. Sensitivity Checks for Association Between Practice Characteristics and Immunotherapy Adoption
eTable 5. Immunotherapy Adoption by Practice and Cancer Type After FDA Approval, Stratified by Cancer Type
eFigure 1. Distribution of Episode Volume Across Practice, by Practice Size Quartile
eFigure 2. Distribution of Practice Size and System Affiliation Type by Practice Location
eFigure 3. Predicted Rate of Immunotherapy Adoption by Practice Type
eReferences
References
- 1.Cutler DM, Rosen AB, Vijan S. The value of medical spending in the United States, 1960-2000. N Engl J Med. 2006;355(9):920-927. doi: 10.1056/NEJMsa054744 [DOI] [PubMed] [Google Scholar]
- 2.Skinner J, Staiger D. Technology diffusion and productivity growth in health care. Rev Econ Stat. 2015;97(5):951-964. doi: 10.1162/REST_a_00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard DH, Chernew ME, Abdelgawad T, Smith GL, Sollano J, Grabowski DC. New anticancer drugs associated with large increases in costs and life expectancy. Health Aff (Millwood). 2016;35(9):1581-1587. doi: 10.1377/hlthaff.2016.0286 [DOI] [PubMed] [Google Scholar]
- 4.Nattinger AB, Gottlieb MS, Veum J, Yahnke D, Goodwin JS. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326(17):1102-1107. doi: 10.1056/NEJM199204233261702 [DOI] [PubMed] [Google Scholar]
- 5.Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992;326(17):1097-1101. doi: 10.1056/NEJM199204233261701 [DOI] [PubMed] [Google Scholar]
- 6.Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306(1):45-52. doi: 10.1001/jama.2011.902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DesRoches CM, Campbell EG, Rao SR, et al. Electronic health records in ambulatory care—a national survey of physicians. N Engl J Med. 2008;359(1):50-60. doi: 10.1056/NEJMsa0802005 [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 9.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640-649. doi: 10.1056/NEJMoa1916623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 11.Vandergrift JL, Gray BM, Barnhart BJ, Lynn LA, Lipner RS. Opportunities for maintenance of certification to better reflect scope of practice among medical oncologists. JCO Oncol Pract. 2020;16(8):e641-e648. doi: 10.1200/JOP.19.00651 [DOI] [PubMed] [Google Scholar]
- 12.Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U.S. Cancer. 2008;112(4):909-918. doi: 10.1002/cncr.23229 [DOI] [PubMed] [Google Scholar]
- 13.Krimphove MJ, Tully KH, Friedlander DF, et al. Adoption of immunotherapy in the community for patients diagnosed with metastatic melanoma. J Immunother Cancer. 2019;7(1):289. doi: 10.1186/s40425-019-0782-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agha L, Molitor D. The local influence of pioneer investigators on technology adoption: evidence from new cancer drugs. Rev Econ Stat. 2018;100(1):29-44. doi: 10.1162/REST_a_00670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating NL, Huskamp HA, Schrag D, et al. Diffusion of bevacizumab across oncology practices: an observational study. Med Care. 2018;56(1):69-77. doi: 10.1097/MLR.0000000000000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 17.Mok TSK, Wu YL, Kudaba I, et al. ; KEYNOTE-042 Investigators . Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non–small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi: 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 18.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non–small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937. doi: 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 19.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39(21):2339-2349. doi: 10.1200/JCO.21.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ascierto PA, Long GV, Robert C, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2019;5(2):187-194. doi: 10.1001/jamaoncol.2018.4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33(10):1191-1196. doi: 10.1200/JCO.2014.56.6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Research Institute . FDA approval timeline of active immunotherapies. 2022. Accessed September 28, 2022. https://www.cancerresearch.org/en-us/scientists/immuno-oncology-landscape/fda-approval-timeline-of-active-immunotherapies
- 23.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. doi: 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parikh RB, Adamson BJS, Khozin S, et al. Association between FDA label restriction and immunotherapy and chemotherapy use in bladder cancer. JAMA. 2019;322(12):1209-1211. doi: 10.1001/jama.2019.10650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connor JM, Fessele KL, Steiner J, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4(8):e180798. doi: 10.1001/jamaoncol.2018.0798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moyers JT, Patel A, Shih W, Nagaraj G. Association of sociodemographic factors with immunotherapy receipt for metastatic melanoma in the US. JAMA Netw Open. 2020;3(9):e2015656. doi: 10.1001/jamanetworkopen.2020.15656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youn B, Wilson IB, Mor V, Trikalinos NA, Dahabreh IJ. Population-level changes in outcomes and Medicare cost following the introduction of new cancer therapies. Health Serv Res. 2021;56(3):486-496. doi: 10.1111/1475-6773.13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raphael J, Richard L, Lam M, et al. Utilization of immunotherapy in patients with cancer treated in routine care settings: a population-based study using health administrative data. Oncologist. 2022;27(8):675-684. doi: 10.1093/oncolo/oyac085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Medicare & Medicaid Services . Evaluation of the Oncology Care Model: performance periods 1-3. 2020. Accessed September 28, 2022. https://innovation.cms.gov/data-and-reports/2020/ocm-evaluation-annual-report-2
- 30.Keating NL, Jhatakia S, Brooks GA, et al. ; Oncology Care Model Evaluation Team . Association of participation in the Oncology Care Model With Medicare payments, utilization, care delivery, and quality outcomes. JAMA. 2021;326(18):1829-1839. doi: 10.1001/jama.2021.17642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Medicare & Medicaid Services . OCM performance-based payment methodology. Published February 2020. Accessed September 28, 2022. https://innovation.cms.gov/files/x/ocm-pp3beyond-pymmeth.pdf
- 32.Gondi S, Wright AA, Landrum MB, et al. Assessment of patient attribution to care from medical oncologists, surgeons, or radiation oncologists after newly diagnosed cancer. JAMA Netw Open. 2021;4(5):e218055. doi: 10.1001/jamanetworkopen.2021.8055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United States Department of Agriculture . Documentation: 2010 rural-urban commuting area (RUCA) codes. Updated August 17, 2020. Accessed September 28, 2022. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/
- 34.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258-1267. doi: 10.1016/S0895-4356(00)00256-0 [DOI] [PubMed] [Google Scholar]
- 35.Research Data Assistance Center . Research Triangle Institute (RTI) race code. Accessed September 28, 2022. https://resdac.org/cms-data/variables/research-triangle-institute-rti-race-code
- 36.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138(4):273-287. doi: 10.7326/0003-4819-138-4-200302180-00006 [DOI] [PubMed] [Google Scholar]
- 37.Adler-Milstein J, DesRoches CM, Kralovec P, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015;34(12):2174-2180. doi: 10.1377/hlthaff.2015.0992 [DOI] [PubMed] [Google Scholar]
- 38.Horowitz J, Hsuan C, Nichols A. The role of hospital and market characteristics in invasive cardiac service diffusion. Rev Ind Organ. 2018;53:81-115. doi: 10.1007/s11151-018-9625-0 [DOI] [Google Scholar]
- 39.Horn D, Sacarny A, Zhou A. Technology adoption and market allocation: the case of robotic surgery. J Health Econ. 2022;86:102672. doi: 10.1016/j.jhealeco.2022.102672 [DOI] [PubMed] [Google Scholar]
- 40.Zahnd WE, Fogleman AJ, Jenkins WD. Rural-urban disparities in stage of diagnosis among cancers with preventive opportunities. Am J Prev Med. 2018;54(5):688-698. doi: 10.1016/j.amepre.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 41.Chow CJ, Al-Refaie WB, Abraham A, et al. Does patient rurality predict quality colon cancer care? A population-based study. Dis Colon Rectum. 2015;58(4):415-422. doi: 10.1097/DCR.0000000000000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shrestha M, Ullrich F, Mueller K. Medicare Advantage enrollment update 2021. RUPRI Center for Rural Health Policy Analysis. September 2021. Accessed September 28, 2022. https://rupri.public-health.uiowa.edu/publications/policybriefs/2021/Medicare%20Advantage%20Enrollment%20Update%202021.pdf
- 43.Welch WP, Bindman AB. Town and gown differences among the 100 largest medical groups in the United States. Acad Med. 2016;91(7):1007-1014. doi: 10.1097/ACM.0000000000001240 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Study Population, Practice Attribution, and Practice Characteristics
eTable 1. Approved Immunotherapy Treatments Through 2016 and CPT Codes
eTable 2. Approved Immunotherapy Treatments by Cancer Type and Year
eTable 3. Full Model Results for the Association Between Practice Characteristics and Immunotherapy Adoption After FDA Approval
eTable 4. Sensitivity Checks for Association Between Practice Characteristics and Immunotherapy Adoption
eTable 5. Immunotherapy Adoption by Practice and Cancer Type After FDA Approval, Stratified by Cancer Type
eFigure 1. Distribution of Episode Volume Across Practice, by Practice Size Quartile
eFigure 2. Distribution of Practice Size and System Affiliation Type by Practice Location
eFigure 3. Predicted Rate of Immunotherapy Adoption by Practice Type
eReferences


