Key Points
Question
What is the effect of treatment for critically ill patients with COVID-19 on longer-term mortality, disability, and health-related quality of life?
Findings
In this bayesian adaptive randomized clinical platform trial that included 4869 critically ill patients with COVID-19, the probability was high that IL-6 receptor antagonists and antiplatelet agents improved survival at 6 months (posterior probabilities of superiority of >99.9% and 95.0%, respectively). Long-term outcomes were not improved with therapeutic anticoagulation (11.5%), convalescent plasma (54.7%), or lopinavir-ritonavir (31.9%) and were worsened with hydroxychloroquine (posterior probability of harm, 96.8%). Corticosteroids did not improve long-term outcomes, although enrollment had been terminated early in response to external evidence.
Meaning
Among critically ill patients with COVID-19 randomized to receive 1 or more therapeutic interventions, there was a high likelihood of improved 180-day mortality among patients treated with IL-6 receptor antagonists and antiplatelet agents.
Abstract
Importance
The longer-term effects of therapies for the treatment of critically ill patients with COVID-19 are unknown.
Objective
To determine the effect of multiple interventions for critically ill adults with COVID-19 on longer-term outcomes.
Design, Setting, and Participants
Prespecified secondary analysis of an ongoing adaptive platform trial (REMAP-CAP) testing interventions within multiple therapeutic domains in which 4869 critically ill adult patients with COVID-19 were enrolled between March 9, 2020, and June 22, 2021, from 197 sites in 14 countries. The final 180-day follow-up was completed on March 2, 2022.
Interventions
Patients were randomized to receive 1 or more interventions within 6 treatment domains: immune modulators (n = 2274), convalescent plasma (n = 2011), antiplatelet therapy (n = 1557), anticoagulation (n = 1033), antivirals (n = 726), and corticosteroids (n = 401).
Main Outcomes and Measures
The main outcome was survival through day 180, analyzed using a bayesian piecewise exponential model. A hazard ratio (HR) less than 1 represented improved survival (superiority), while an HR greater than 1 represented worsened survival (harm); futility was represented by a relative improvement less than 20% in outcome, shown by an HR greater than 0.83.
Results
Among 4869 randomized patients (mean age, 59.3 years; 1537 [32.1%] women), 4107 (84.3%) had known vital status and 2590 (63.1%) were alive at day 180. IL-6 receptor antagonists had a greater than 99.9% probability of improving 6-month survival (adjusted HR, 0.74 [95% credible interval {CrI}, 0.61-0.90]) and antiplatelet agents had a 95% probability of improving 6-month survival (adjusted HR, 0.85 [95% CrI, 0.71-1.03]) compared with the control, while the probability of trial-defined statistical futility (HR >0.83) was high for therapeutic anticoagulation (99.9%; HR, 1.13 [95% CrI, 0.93-1.42]), convalescent plasma (99.2%; HR, 0.99 [95% CrI, 0.86-1.14]), and lopinavir-ritonavir (96.6%; HR, 1.06 [95% CrI, 0.82-1.38]) and the probabilities of harm from hydroxychloroquine (96.9%; HR, 1.51 [95% CrI, 0.98-2.29]) and the combination of lopinavir-ritonavir and hydroxychloroquine (96.8%; HR, 1.61 [95% CrI, 0.97-2.67]) were high. The corticosteroid domain was stopped early prior to reaching a predefined statistical trigger; there was a 57.1% to 61.6% probability of improving 6-month survival across varying hydrocortisone dosing strategies.
Conclusions and Relevance
Among critically ill patients with COVID-19 randomized to receive 1 or more therapeutic interventions, treatment with an IL-6 receptor antagonist had a greater than 99.9% probability of improved 180-day mortality compared with patients randomized to the control, and treatment with an antiplatelet had a 95.0% probability of improved 180-day mortality compared with patients randomized to the control. Overall, when considered with previously reported short-term results, the findings indicate that initial in-hospital treatment effects were consistent for most therapies through 6 months.
This secondary analysis of an ongoing adaptive platform trial examines the effect of multiple interventions for critically ill adults with COVID-19 on longer-term outcomes.
Introduction
Randomized clinical trials in critically ill patients, including those with COVID-19, typically assess short-term outcomes, such as organ failure or 28-day mortality, with fewer published trials assessing whether treatments affect long-term survival and patient-centered outcomes, such as disability and health-related quality of life (HRQoL). Longer-term survival free of major disability with an acceptable HRQoL may be more important to patients than short-term survival.1,2 The World Health Organization and others have called for additional research on the effect of initial therapeutic interventions on longer-term outcomes.3
The Randomized Embedded Multifactorial Adaptive Platform for Community Acquired Pneumonia (REMAP-CAP) trial is an ongoing international, multicenter, randomized platform trial evaluating multiple treatments for patients with severe pneumonia in both pandemic and nonpandemic settings (NCT02735707) (Supplement 1).4 To date, the trial has reported results for 6 treatment domains for patients with COVID-19: corticosteroids, antiviral agents, immune modulators, immunoglobulin, anticoagulation, and antiplatelet therapy.5,6,7,8,9,10 The trial primary outcome for patients with COVID-19 is the composite of hospital survival and organ support provision up to 21 days. The trial reported benefit on this primary outcome for IL-6 receptor antagonists (immune modulators), but not for antiviral agents, anakinra (immune modulator), convalescent plasma (immunoglobulin), therapeutic anticoagulation (in critically ill patients), or antiplatelet therapies.6,7,8,9,10 The corticosteroid domain was closed early on the basis of external evidence, but a moderate probability of benefit was observed.5 Whether these initial treatment effects translate into effects on longer-term survival, disability, and HRQoL is unknown. The objective of this study was to report on the effects of these treatments on prespecified longer-term outcomes, including mortality, disability, and HRQoL, at 6 months for patients randomized to receive 1 or more treatments in the reported domains in the trial.
Methods
Trial Design and Oversight
The design of REMAP-CAP has been reported previously.4,5,6,7,8,9,10 In brief, patients are assessed for eligibility and potentially randomized to receive 1 or more interventions across multiple treatment domains. Domains encompass therapeutic areas and contain 2 or more interventions (including control). The trial enrolls both critically ill and non–critically ill patients in separate severity states and distinct group-specific effects are estimated. This report includes only critically ill patients because follow-up beyond 90 days in the REMAP-CAP trial does not include non–critically ill patients. The trial was approved by relevant regional ethics committees and conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Written or verbal informed consent, in accordance with local legislation, was obtained from patients or their substitute decision-maker.
Participants
Adult patients (≥18 years) admitted to an intensive care unit (ICU) with clinically suspected or microbiologically confirmed COVID-19 who were receiving respiratory or cardiovascular organ support were eligible for enrollment. Patients had to be enrolled within 48 hours of admission to the ICU. Exclusion criteria along with additional inclusion and exclusion criteria for each domain are shown in eTable 1 in Supplement 2.5,6,7,8,9,10 Patients were enrolled from 197 sites in 14 countries (eTable 2 in Supplement 2). In view of racial and ethnic differences in outcomes during the pandemic, this trial collected self-reported race and ethnicity information from either the participants or their surrogates via fixed categories appropriate to their region where ethical approval allowed. Collection of race and ethnicity data was not approved in Asia, Canada, and continental Europe.
Randomization
Patients were randomized via a centralized computer program with randomization ratios dependent on response-adaptive randomization and the number of interventions available at each site. The interventions within each domain, including timing and doses, have been reported previously.5,6,7,8,9,10 In brief, patients in the corticosteroid domain were randomized to receive a fixed 7-day course of intravenous hydrocortisone, a shock-dependent course, or no corticosteroid; patients in the immune modulation domain were randomized to receive tocilizumab, sarilumab (both IL-6 receptor antagonists), anakinra (an IL-1 receptor antagonist), interferon beta-1a, or no immune modulator; patients in the antiviral domain were randomized to receive lopinavir-ritonavir, hydroxychloroquine, the combination of both therapies, or no antiviral; patients in the immunoglobulin domain were randomized to receive 2 units of high-titer ABO-compatible convalescent plasma, delayed convalescent plasma (given if clinical deterioration occurs [only available in the US]), or no convalescent plasma; patients in the anticoagulation domain were randomized to receive therapeutic-dose anticoagulation with heparin or pharmacologic thromboprophylaxis in accordance with local usual care; and patients in the antiplatelet domain were randomized to receive aspirin, a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor), or no antiplatelet therapy. Patients could be randomized to receive additional interventions within other domains, depending on domains active at the site, patient eligibility, and consent (see http://www.remapcap.org).4 Other aspects of care were provided per each site’s standard of care.
Outcome Measures
The main outcome for this prespecified secondary analysis of longer-term outcomes was all-cause mortality within 6 months (180 days after randomization), a prespecified secondary outcome in the REMAP-CAP core protocol. This was modeled as a time-to-event outcome for this analysis. Additional secondary outcomes were prespecified in the core protocol and included day 90 mortality, HRQoL at 180 days measured using the 5-level EuroQol-5 Dimension (EQ-5D-5L) utility score and visual analog scale (VAS) score, and disability at 180 days measured using the 12-item World Health Organization Disability Assessment Schedule (WHODAS) 2.0. The EQ-5D-5L is a preference-based HRQoL instrument comprised of 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The EQ-5D-5L utility score is calculated from the individual response to each item and ranges from −0.593 (score of 0 is equivalent to death) to 1.00 (full health), with higher values indicating better health states.11 An EQ-5D-5L utility score of 0 was imputed for all patients known or imputed to be deceased at 180 days. For the EQ VAS, respondents are asked to indicate their present health state on a VAS ranging from the worst imaginable health state (score of 0) to the best imaginable health state (score of 100).12,13 The EQ-5D-5L utility score and EQ VAS score have no established minimal clinically important differences among critically ill patients. The WHODAS 2.0 covers 6 domains of functioning, with scores for each item ranging from 0 (no difficulty) to 4 (extreme difficulty) and a total score ranging from 0 to 48, with higher scores representing greater disability.14 The total score is divided by 48 and multiplied by 100 to convert to a percentage of maximum disability, with the percent score used to determine 5 mutually exclusive disability categories (from no disability to complete disability).15 The minimal clinically important difference for the WHODAS in critically ill patients is 10%.16
Ninety-day mortality was collected at all sites, whereas 180-day outcomes were collected only by a subset (174/197 [88.3%]) of sites. The decision to participate in the collection of longer-term outcomes was made regionally or locally reflecting funding, regulatory approval, and site resource availability. In the UK, the large numbers of recruited participants from January 5, 2021, to February 25, 2021, exceeded the capacity for extended follow-up of all patients within the required time window. Accordingly, at the time, a subset of participants was randomly selected not to be followed up. Overall, 587 of 787 survivors (74.6%) enrolled during this period were followed up through 6 months.
Survival status was assessed at 90 and 180 days and was determined using medical records or contact with the participant, their next of kin, or other health care professional. When a participant died, the date of death was recorded. Day 180 HRQoL and disability in survivors were collected by central trial staff or site staff by telephone with the patient or a proxy when the patient was not available. EQ-5D-5L utility scores were calculated using the crosswalk link function and the individual responses to the EQ-5D-5L descriptive system, using the UK time trade off value set.11 EQ-5D-5L and WHODAS scores were only included when the questionnaire was completed within the 12 weeks following day 180 (additional details in eMethods in Supplement 2).
Statistical Analysis
This study reports the analysis of longer-term secondary outcomes prespecified in the REMAP-CAP trial core protocol (Supplement 1). The analysis plan for evaluating these outcomes was finalized in a statistical analysis plan (Supplement 1) on March 10, 2022, prior to unblinding the 180-day outcomes. All patients were analyzed in the groups to which they were originally randomized. The full analysis set included all critically ill patients with suspected or proven COVID-19 randomized to an intervention in 1 or more of the 6 domains that have been closed and reported, excluding patients who withdrew consent. For consistency with the original analyses,5,6,7,8,9,10 the P2Y12 inhibitor and aspirin groups are reported as a pooled antiplatelet group and the tocilizumab and sarilumab groups are reported as a pooled IL-6 receptor antagonist group, given that both sets of interventions reached the prespecified definition of equivalence on organ support–free days in the original analysis. Due to the low number of patients randomized, the interferon beta-1a group within the immune modulation domain and the delayed convalescent plasma intervention within the immunoglobulin domain were not analyzed.
The primary analysis was performed using a bayesian piecewise exponential model. The underlying hazard rate was piecewise constant for each 15-day period up to day 90 and the 90-day period from day 90 to day 180. The prior distribution for each hazard rate was a γ distribution with 1 day of exposure and a mean equal to the total exposure (in days) divided by the total number of events. The primary model estimated treatment effects (log hazard ratios [HRs]) for each intervention relative to control within each domain with standard normal priors. The primary model included variables for each domain with each domain treatment as a category (with control interventions from each domain set as the referent) and was adjusted for location (site nested within country), patient age (categorized into 6 groups), sex, and time-period (2-week epochs). The posterior distributions of the interventions’ HRs were summarized with medians, 95% credible intervals (CrIs), and the probability that an intervention was superior to the control for that domain (ie, HR <1.0). Harm was defined as the probability the HR was greater than 1. Futility was defined as the probability that there was not more than a 20% relative improvement in outcome (HR >0.83). A prespecified interaction was modeled between antiplatelet therapy (pooled P2Y12 inhibitor and aspirin group) in the antiplatelet domain and therapeutic-dose heparin in the anticoagulation domain. Statistical thresholds based on posterior probabilities for superiority and harm were used for the primary outcome to determine trial stopping rules, but were not used to guide interpretation of other findings; rather, effect sizes along with posterior probabilities are presented for all analyses.
Ninety-day mortality was analyzed with a bayesian logistic regression model. The EQ-5D-5L utility score was analyzed with a 2-part/mixture model including 2 components: a continuous distribution of EQ-5D-5L utility scores for patients who survived to day 180 and a point mass at 0 for patients who died before day 180. The posterior distributions of the mean difference between treatment and control for EQ-5D-5L utility scores were summarized with medians, 95% CrIs, and the probability that an intervention was superior to the control for that domain (ie, a mean difference less than 0). Treatment effects were estimated for all patients, along with estimates for survivors only. The EQ VAS score was reported using descriptive statistics only. The WHODAS disability category was analyzed with a 2-part/mixture model including 2 components: an ordinal model of disability category for patients who survived to day 180 and the worst category of “death” for patients who died before day 180. Similarly, the posterior distributions of the interventions' odds ratio (OR) for WHODAS 2.0 disability category for survivors were summarized with medians, 95% CrIs, and the probability that an intervention was superior to the control for that domain (ie, OR <1). The EQ-5D-5L utility score and WHODAS category were multiply imputed as a function of the patient’s covariates for patients censored alive before day 180 or missing the HRQoL outcome. Sampling was from all patients enrolled in the timeframe and the sampling probability was independent of treatment assignment. The relationship between shorter- and longer-term end points was assessed by plotting the organ support–free days OR vs 180-day mortality HR for each intervention and reporting the coefficient of determination (R2).
Sensitivity analyses for the main outcome included excluding patients with negative SARS-CoV-2 test results, removing adjustments for assignments in other domains, and estimating independent effects of the 2 IL-6 receptor antagonist interventions and the 2 antiplatelet interventions. For secondary outcomes, sensitivity analyses included no imputation of missing EQ-5D-5L utility and WHODAS scores. Post hoc sensitivity analyses were conducted to evaluate the main outcome model and robustness to model assumptions, including the assumption of proportional hazards for treatment effects, the assumption of a parametric distribution of survival times (piecewise exponential), and the use of prior distributions in the bayesian framework (Model Evaluation Report in Supplement 2).
Two subgroup analyses were performed stratifying treatment effects on 180-day mortality based on the binary categories of receipt of invasive mechanical ventilation at baseline and preexisting immunosuppression. Further details of all analyses are provided in the statistical analysis plan in Supplement 1. Data management and summaries were created using R, version 3.6.0, with the primary analysis computed in R, version 4.1.2, using the rstan package, version 2.21.1 (R Foundation). Additional data management and analyses were performed in SQL 2016 and Stata, version 17.0.
Results
Enrollment and Participant Characteristics
A total of 4869 patients were enrolled from March 9, 2020, through June 24, 2021, to at least 1 of the 6 reported domains in the trial. Seventy-eight patients withdrew consent, resulting in 4791 patients being included in the analysis of longer-term outcomes (Figure 1). Of these patients, 473 were randomized at 19 sites that only participated in the 90-day follow-up, including all sites in Nepal, India, and the US. Details of recruitment and follow-up by country of enrollment are shown in eTable 2 in Supplement 2. The final 180-day follow up was completed on March 2, 2022. The baseline characteristics of the included patients are shown in Table. Baseline characteristics by intervention within each domain are shown in eTables 3 to 8 in Supplement 2.
Figure 1. Cohort Development in an Analysis of Longer-term Follow-up of Critically Ill Participants in the REMAP-CAP COVID-19 Clinical Trial.
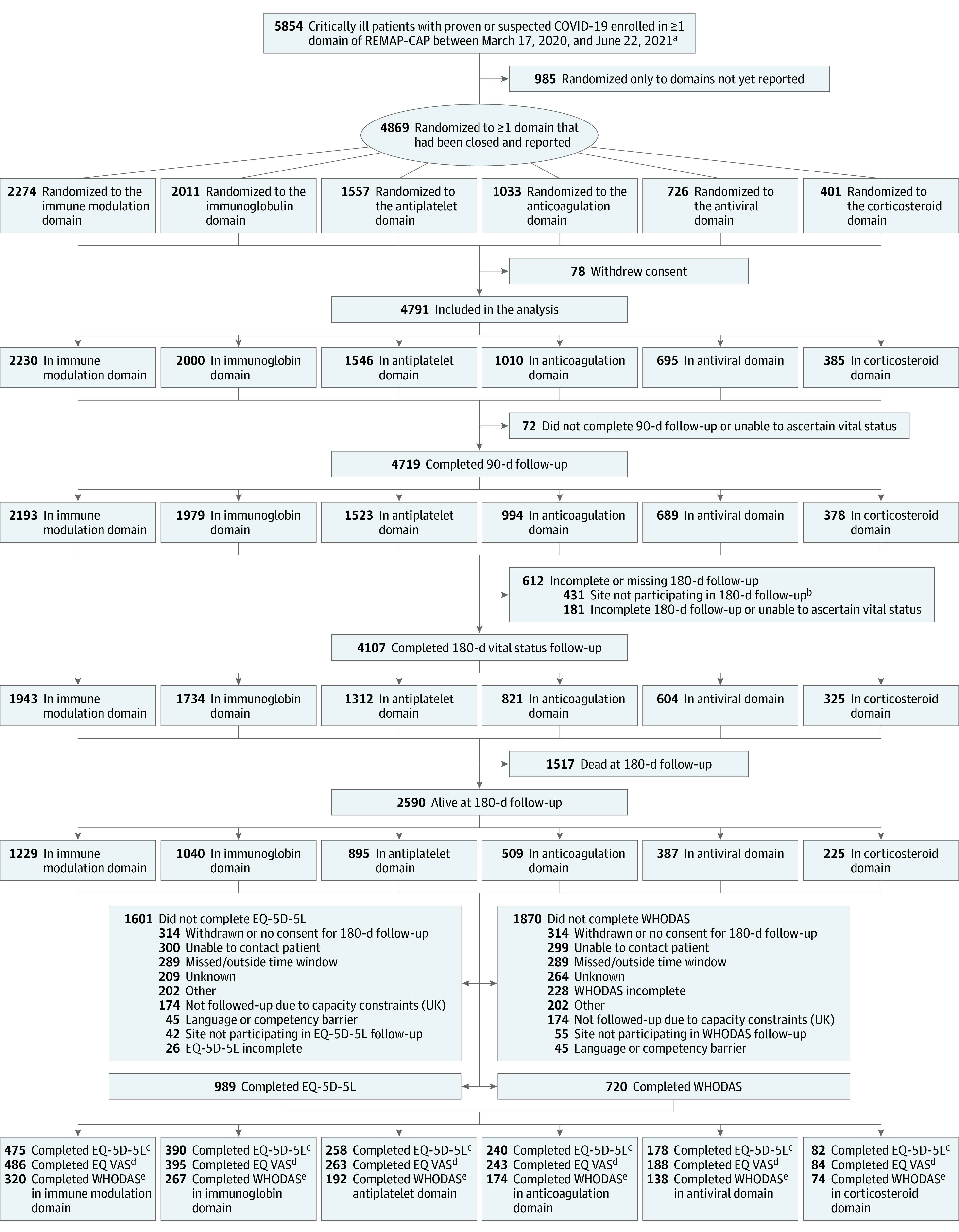
aAn additional 910 non–critically ill patients were enrolled in domains.
bAdditional 42 patients with unknown vital status at 90 d were from sites not participating in 180-d follow-up.
cQuality of life on the EQ-5D-5L; scores from −0.593 to 1.00 (full health).
dSelf-reported present health state, ranging from 0 (worst) to 100 (best).
eCovers 6 domains of functioning; 0 (no difficulty) to 4 (extreme difficulty).
Table. Baseline Characteristics of Patients in 1 or More Domains Included in the Analysis of Mortality at 180 Daysa.
| Characteristic | No./total No. (%) |
|---|---|
| No. | 4791 |
| Age, median (IQR) [No.], y | 60 (51-68) [n = 4790] |
| Male sex | 3253/4790 (67.9) |
| Female sex | 1537/4790 (32.1) |
| Race and ethnicitya | |
| Asian | 504/3557 (14.2) |
| Black | 160/3557 (4.5) |
| White | 2665/3557 (74.9) |
| Mixed | 69/3557 (1.9) |
| Otherb | 159/3557 (4.5) |
| Confirmed SARS-CoV2 infectionc | 4139/4764 (86.9) |
| BMI, median (IQR) | 30.5 (26.6-35.8) [n = 4317] |
| APACHE II score, median (IQR)d | 13 (8-19) [n = 4673] |
| Preexisting conditionse | |
| Diabetes | 1404/4788 (29.3) |
| Respiratory disease¤ | 1067/4788 (22.3) |
| Asthma/COPD | 897/4788 (18.7) |
| Other | 218/4788 (4.6) |
| Kidney disease | 370/4427 (8.4) |
| Severe cardiovascular disease | 376/4703 (8.0) |
| Immunosuppressive disease | 190/4788 (4.0) |
| Chronic immunosuppressive therapy | 175/4788 (3.7) |
| Time to enrolment, median (IQR) | |
| From hospital admission, d | 1.7 (0.9-3.3) |
| From ICU admission, h | 16.7 (9.1-22.5) |
| Acute respiratory support, No. (%) | |
| None/supplemental oxygen only | 7 (0.2) |
| High-flow nasal cannula | 1198 (25.0) |
| Noninvasive ventilation only | 1848 (38.6) |
| Invasive mechanical ventilation | 1746 (36.4) |
| ECMO | 4/4729 (0.1) |
| Vasopressor support | 1005 (21.0) |
| Acute physiology and laboratory values, median (IQR)f | |
| Pao2/Fio2 | 116 (89-158) [n = 4368] |
| C-reactive protein, μg/mL | 117 (65-192) [n = 3585] |
| Creatinine, mg/dL | 0.8 (0.7-1.1) [n = 4681] |
| Lactate, mmol/L | 1.3 (1.0-1.8) [n = 4205] |
| Platelets, ×109/L | 245 (183-314) [n = 4626] |
| Bilirubin, mg/dL | 0.5 (0.4-0.8) [n = 4443] |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; Fio2, fraction of inspired oxygen; Pao2, partial pressure of oxygen.
Baseline characteristics by intervention in each of the six domains can be found in eTables 3-8 in Supplement 1. Percentages may not sum to 100 because of rounding.
Self-reported via fixed categories. Data collection was not approved in Asia, Canada, and continental Europe. “Other” includes “other ethnic group” and those who declined to respond or were not asked by registration personnel.
SARS-CoV2 infection was confirmed by respiratory tract polymerase chain reaction test.
Measures the severity of illness based on age, medical history, and physiological variables. Scores range from 0 to 71; higher numbers represent greater risk of death. The median score of 12 is typical for patients with COVID-19 admitted to intensive care units (ICUs).
Kidney disease was determined from the most recent stable serum creatinine level prior to this hospital admission, except in patients who were receiving dialysis. Abnormal kidney function was defined as a creatinine level of 130 μmol/L or greater (1.5 mg/dL) for men or 100 μmol/L or greater (1.1 mg/dL) for women not previously receiving dialysis. Cardiovascular disease was defined as New York Heart Association class IV symptoms. Immunosuppression was defined by the receipt of recent chemotherapy, radiation, high-dose or long-term steroid treatment, or presence of immunosuppressive disease.
Laboratory results available when captured for clinical care. Although specific laboratory cut-offs may vary, ranges for the following variables are generally considered normal: Pao2/Fio2 ratio ≥400; creatinine <1.2 mg/dL; platelets ≥150 ×109/L; and bilirubin <1.2 mg/dL.
Main Outcome
Of the 4318 patients randomized at sites participating in 180-day follow-up, 180-day mortality status was available for 4107 patients (95.1%). Patients with known 180-day mortality status differed from those with unknown status; patients with unknown status were younger, had higher frequencies of noninvasive ventilation receipt at baseline, had lower frequencies of invasive mechanical ventilation and vasopressor use at baseline, had lower Acute Physiology and Chronic Health Evaluation II scores, and were less likely to have prevalent asthma or chronic obstructive pulmonary disease, immunosuppressive disease, or be receiving chronic immunosuppressive therapy (eTable 9 in Supplement 2). Of patients with known 180-day mortality status, 1517 (36.9%) died (Figure 2 and Figure 3). Of patients who died by day 180, a total of 91 of 1516 deaths (6.0%) occurred between hospital discharge and day 180. The pooled IL-6 receptor antagonists and antiplatelet treatment groups each had a high probability of benefit (>99.9% and 95.0%, respectively) compared with their control groups. The probability of benefit for fixed-dose corticosteroids and shock-dependent corticosteroids compared with no corticosteroids was 61.6% and 57.1%, respectively. The probability of trial-defined statistical futility was high for therapeutic anticoagulation (in critically ill patients) (99.9%; HR, 1.13 [95% CrI, 0.93-1.42]), convalescent plasma (99.2%; HR, 0.99 [95% CrI, 0.86-1.14]), and lopinavir-ritonavir (96.6%; HR, 1.06 [95% CrI, 0.82-1.38]). Hydroxychloroquine and its combination with lopinavir-ritonavir had a high probability of harm (96.9%; HR, 1.51 [95% CrI, 0.98-2.29] for hydroxychloroquine and 99.5%; HR, 1.61 [95% CrI, 0.97-2.67] for combination) (Figure 3 and eFigure 1 in Supplement 2). Results of the sensitivity analyses for patients with negative COVID-19 test results and in domain-specific populations were consistent with the primary analysis (eTables 10-12 and eFigure 2 in Supplement 2). Post hoc sensitivity analyses showed the results were robust to model assumptions, including the proportional hazards assumption (eFigures 3-4 and Model Evaluation Report in Supplement 2). Across interventions there was a strong association (R2 = 0.84) between the ORs for the composite of in-hospital mortality and organ support–free days to day 21 (primary outcome in the trial) and the reciprocals of 180-day mortality HRs (eFigure 5 in Supplement 2).
Figure 2. Kaplan-Meier Curves for Mortality Through 180 Days.
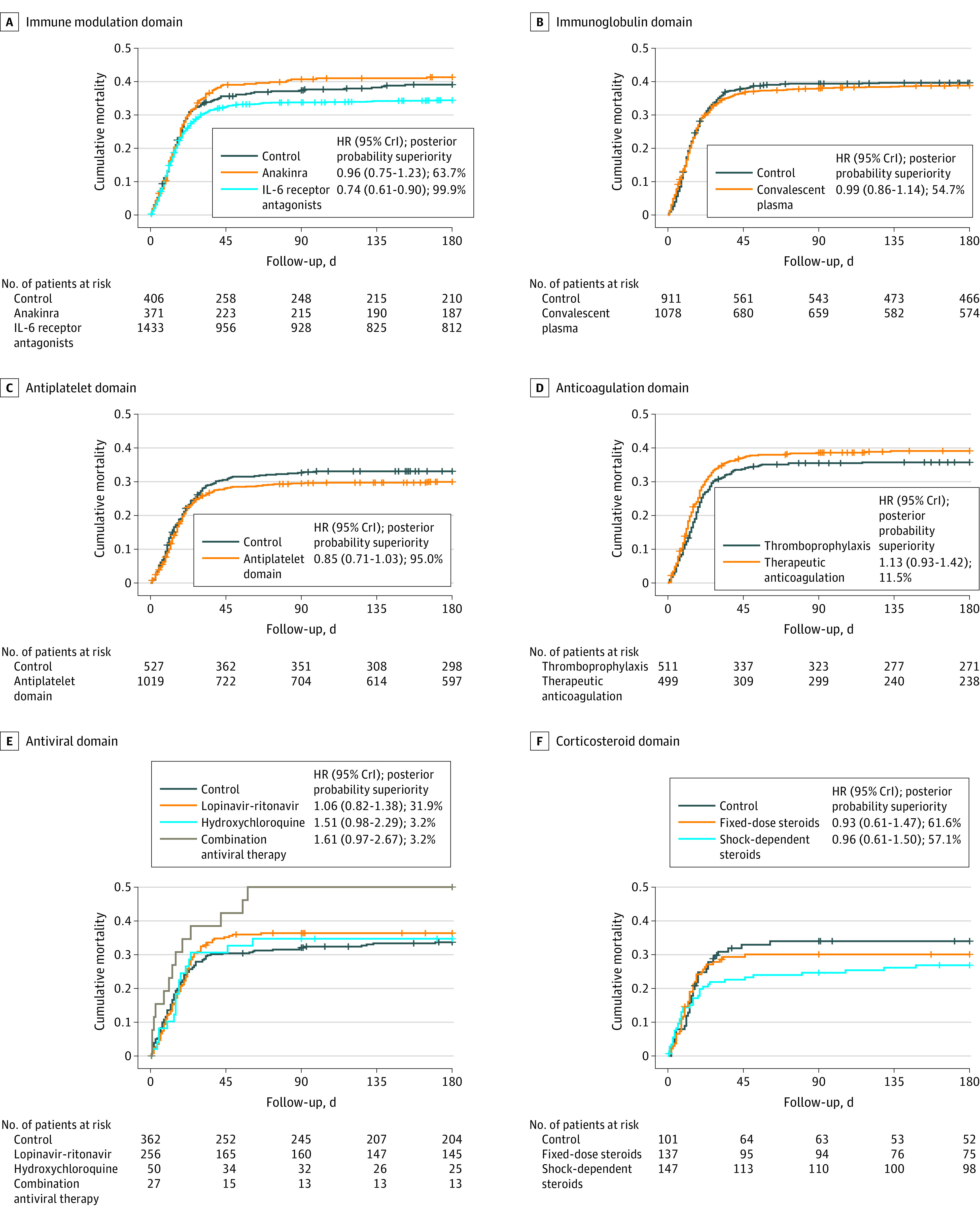
The probability of superiority of each active intervention to control for 180-day mortality is reported from the fully adjusted bayesian model (adjusting for other treatments from other domains, site, time, sex, and age). Censored participants are indicated with vertical tick marks. CrI indicates credible interval.
Figure 3. Mortality at 180 Days.
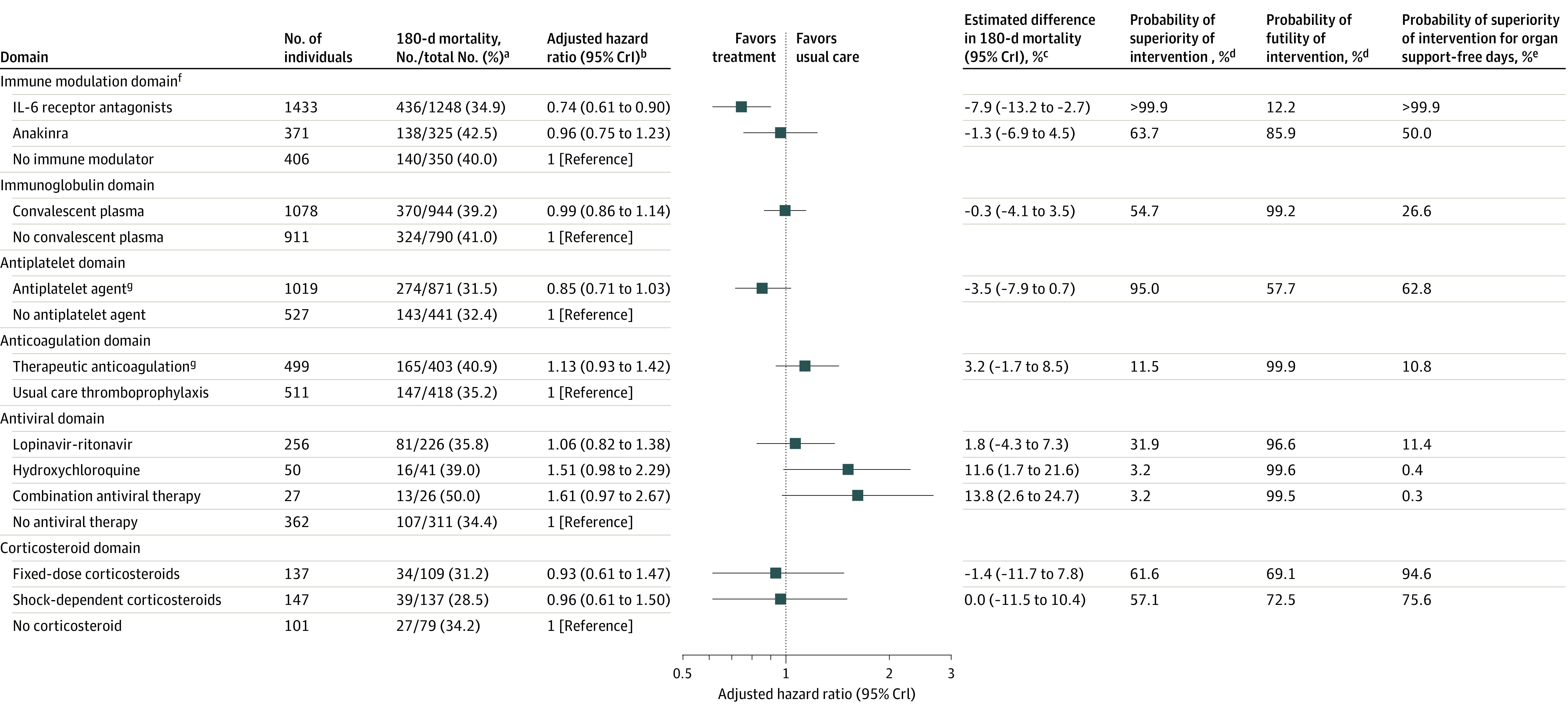
aDue to censoring, reported 180-day mortality rates are restricted to patients at sites participating in 180-day follow-up with known 180-day vital status. The Kaplan-Meier curves include additional exposure and events from patients who were censored before day 180 or enrolled at sites that did not participate in 180-day follow-up.
bHazard ratios <1 indicate improved survival and hazard ratios >1 indicate worsened survival.
cThe difference in 180-day mortality is determined from the 180-day mortality rates which are estimated from the primary analysis model. For each domain, day 180 mortality rates are estimated for the population of patients randomized within that domain based on their baseline covariates and the estimated model parameters. For each patient within the domain population, separate survival curves are predicted assuming the patient received each intervention within the domain. The mean of the survival curves was taken across patients to summarize the mean survival for each intervention within the domain population.
dThe probability of superiority (hazard ratio <1) and futility (hazard ratio >0.83) is computed from a bayesian piecewise exponential model using the posterior distribution.
eOrgan support–free days are a composite ordinal scale consisting of survival to hospital discharge and days free of organ support to day 21. Probabilities may differ from those presented in the original trial reports for each domain due to changes in patient consent.
fDomains are ordered based on the total number of patients enrolled in the domain from largest to smallest.
gA total of 35 patients within the antiplatelet and anticoagulation domains were randomized to the prespecified combination of therapeutic anticoagulation and an antiplatelet agent. The combination effect provides the effect of giving both therapeutic anticoagulation and antiplatelet interventions together in combination (relative to giving control in both domains). This is estimated by multiplying the hazard ratio for antiplatelet, therapeutic anticoagulation and the interaction effect for antiplatelet and therapeutic anticoagulation. The hazard ratio for the combination effect is 1.34 (95% credible interval [CrI], 0.82-2.23) with a probability of superiority of 11.6%. The hazard ratio for the therapeutic anticoagulation/antiplatelet interaction is 1.39 (95% CrI, 0.87-2.19).
Secondary Outcomes
The ORs for 90-day mortality are shown in eTable 13 and eFigure 6 in Supplement 2. Results were consistent with the 180-day mortality analysis.
Of the 2589 survivors at sites participating in follow-up, the EQ VAS was available for 1009 patients (39.0%), the EQ-5D-5L utility score was available for 989 patients (38.2%), and the WHODAS 2.0 score was available for 720 patients (27.8%) (Figure 1). Patients without EQ-5D-5L utility and WHODAS scores differed from those with scores available; patients missing scores were younger, received less high-flow nasal oxygen and more noninvasive and invasive ventilation, and were less likely to have immunosuppressive disease or be receiving chronic immunosuppressive therapy (eTables 14 and 15 in Supplement 2).
In the overall cohort, the median (IQR) EQ-5D-5L utility score in survivors was 0.74 (0.55-0.88) (n = 989). The median (IQR) EQ VAS score was 75 (50-85) (n = 1009). The percentages of patients reporting problems in each domain and the EQ VAS scores are shown in eTables 16 and 17 in Supplement 2. Among survivors, the adjusted mean difference in the EQ-5D-5L utility score in the pooled antiplatelet group was 0.08 (95% CrI, 0.00-0.15) units higher compared with the control, with a posterior probability of superiority of 97.4%; among all patients (survivors and nonsurvivors), the adjusted mean difference was 0.08 (95% CrI, 0.02-0.13), with a probability of superiority of 99.6% (Figure 4). Among all patients, the adjusted mean difference in the pooled IL-6 receptor antagonists was 0.08 (95% CrI, 0.02-0.13) units higher compared with the control, with a posterior probability of 99.5%. The mean EQ-5D-5L utility score in the lopinavir-ritonavir group was lower than the control group, with a posterior probability of harm of 98.7% among survivors and 98.4% among all patients. The mean difference in EQ-5D-5L utility scores between each remaining intervention and their control group are shown in Figure 4 and eFigure 7 in Supplement 2.
Figure 4. Health-Related Quality of Life at 180 Daysa.

aResults for the EQ visual analog scale and the World Health Organization Disability Assessment Schedule (WHODAS) 2.0 are available in eTables 17-19 in Supplement 2.
bThe probability of superiority and adjusted mean difference are computed from the posterior distribution of a bayesian 2-part/mixture model that multiply imputes 5-level EuroQol-5 Dimension (EQ-5D-5L) utility scores using patients’ baseline covariates for patients censored alive before 6 months and patients known to be alive at 6 months with unknown health-related quality of life. For patients who were censored before 6 months, first 6-month mortality outcomes are multiply imputed from the piecewise exponential component of the bayesian 2-part/mixture model. For patients who were known or imputed to be alive at 6 months, a value of EQ-5D-5L is multiply imputed from the continuous component of the 2-part/mixture model. For patients who were imputed as dead by 6 months, EQ-5D-5L was set to 0 and they did not contribute to the analysis of EQ-5D-5L in survivors. In this analysis, 4307 of 4791 patients (90%) had known survival status at 6 months and a mortality outcome was multiply imputed for the remaining 484 patients (10%). Of the 2590 patients known to be alive at 6 months, 852 (33%) had a known EQ-5D-5L utility score and the remaining 1738 (67%) were imputed.
cDomains are ordered based on the total number of patients enrolled in the domain from largest to smallest.
Disability in survivors is shown in eTables 18 and 19 in Supplement 2. A total of 273 of 720 survivors (37.9%) had moderate, severe, or complete disability at day 180. The 95% CrIs for the ORs for WHODAS disability category were wide, with intervals crossing 1 for all interventions (eFigure 8 in Supplement 2). Among these interventions, pooled IL-6 receptor antagonists had a 92.6% probability and anakinra had 90.8% probability of reducing disability, and lopinavir-ritonavir had a 91.7% probability of worsening disability.
Results for the sensitivity analyses of the EQ-5D-5L utility score and WHODAS 2.0 score without imputation for missing scores were consistent with the imputed analysis (eTables 20 and 21 in Supplement 2).
Subgroup Analyses
Treatment effects on 180-day mortality did not meaningfully vary by invasive mechanical ventilation status at baseline (eTables 22 and 23 and eFigure 9 in Supplement 2). In patients with immunodeficiency at baseline, therapeutic anticoagulation had a posterior probability of worsened 180-day mortality of 98.8% and antiplatelet therapy had a posterior probability of worsened 180-day mortality of 92.4% (eTable 24 and 25 and eFigure 10 in Supplement 2).
Discussion
In this prespecified secondary analysis of a bayesian adaptive randomized clinical platform trial that included 4791 critically ill patients with COVID-19, there was a greater than 99.9% probability that IL-6 receptor antagonists and a 95.0% probability that antiplatelet agents improved survival through 6 months. In contrast, survival was not improved with therapeutic anticoagulation, convalescent plasma, or lopinavir-ritonavir, and the probability that survival was worsened with hydroxychloroquine alone or in combination with lopinavir-ritonavir was 96.8%. Corticosteroids did not confer a high probability of improved longer-term survival, but enrollment in this domain was closed early in response to external evidence that may limit statistical power to detect any potential effect. Although the majority of deaths occurred early, 6% of all deaths occurred between hospital discharge and day 180.
Interventions are delivered in critically ill patients with the goal of increasing long-term survival as well as improving HRQoL and functional status in survivors. However most clinical trials in critically ill patients have evaluated shorter-term outcomes, many of which may not be patient-centered.17 Longer-term trajectories after critical illness, including acute respiratory distress syndrome and sepsis, are characterized by frequent rehospitalization, sustained impairments in HRQoL and functional status, an excess hazard of mortality, and exacerbation of chronic comorbidities that may persist for years after initial hospitalization.18,19,20,21,22,23 Patients who initially survive face later mortality and morbidity hazards that may offset potential benefits of treatment. Accordingly, the effect of many treatments administered in the ICU on long-term outcomes after critical illness—including COVID-19—remain uncertain. Although the REMAP-CAP trial evaluated a hospital-based primary outcome reflecting survival to discharge and, in survivors, receipt of ICU-level organ support, the protocol included the collection of longer-term (6-month) outcomes reflecting survival, HRQoL, and disability. Overall, there was a strong correlation between the trial primary outcome and 6-month outcomes.
There was broad variation in 6-month HRQoL and disability scores. The majority of survivors reported favorable HRQoL and functional status, although approximately 1 in 3 patients had at least moderate disability that persisted through 6 months (although participants’ baseline disability was not known). IL-6 receptor antagonists, which improved organ support–free days, also had a greater than 99.9% probability of improving survival over 6 months compared with no immune modulator. Furthermore, among survivors, there was an 87.0% probability of improved HRQoL and 92.6% probability of reduced disability, suggesting that improvement in survival was not occurring at the expense of poor-quality survival. Although antiplatelet agents did not improve the outcome of organ support–free days, they showed a 92.3% probability of improved 90-day survival, 95.0% probability of improved 180-day survival, and a 97.4% probability of improved HRQoL in survivors at 6 months. When death and HRQoL were evaluated as a composite outcome, the probability of superiority was 99.6%.
Although there was a 93% probability that fixed-dose hydrocortisone improved organ support–free days, no early or late effect on survival was observed. Other treatments that did not improve organ support–free days, including therapeutic anticoagulation, convalescent plasma, lopinavir-ritonavir, and hydroxychloroquine, were similarly ineffective in improving longer-term outcomes. Overall, there was a strong correlation between organ support–free days and longer-term outcomes in critically ill patients with COVID-19.
To the best of our knowledge, this is the largest trial that has reported on the effect of treatments for COVID-19 on longer-term mortality, HRQoL, and disability in critically ill patients. The HRQoL and disability outcomes in this trial are similar to observational cohort reports of critically ill survivors of COVID-1924,25,26 and similar to both observational and trial cohorts of critically ill patients prior to the pandemic.24,27,28 Although there does not appear to be large effects of ICU-based treatments on either HRQoL or disability, it is encouraging that overall increases in longer-term survival are not accompanied by a decrement in HRQoL or increased disability. There is a theoretical risk that a treatment that saves lives among critically ill patients may convert nonsurvivors to survivors who have poor quality of survival. A recent systematic review evaluating HRQoL in randomized trials reporting a survival benefit in critically ill patients found that only 2.9% of studies that reported a significant reduction in mortality also reported on HRQoL.29 Of those trials reporting HRQoL, results were inconsistent, with 2 studies reporting improvements in both survival and HRQoL, 3 reporting no difference in HRQoL in survivors, and 2 reporting lower HRQoL in survivors.29 The present study demonstrates that the improved survival associated with IL-6 receptor antagonists and antiplatelet agents does not result in a lower HRQoL in survivors. Given the global effect of COVID-19, small differences in mortality or other patient-centered outcomes, such as HRQoL and disability, can result in important clinical and health economic benefits at the population level, particularly in a global pandemic.
Limitations
This study has several limitations. First, the trial used an open-label design, although the mortality outcome is at a lower risk for bias. Second, the collection of outcomes beyond day 90 was not mandated, although 6-month survival status was available for 85.7% of patients. Not all regions and sites collected HRQoL and disability outcomes and there was a substantial amount of missing data for these outcomes. Bayesian multiple imputation techniques were used to account for missing data, and sensitivity analyses without imputation did not meaningfully change the results. Third, HRQoL and disability scores could not be collected at baseline while patients were critically ill, and therefore this trial could not assure balance across randomization groups or evaluate change in score over time. As such, it is possible that differences in HRQoL or disability may have been due to imbalances in these measures at baseline, although randomization and temporal and site adjustment in the models would have been expected to balance these across the treatment groups. Fourth, the results relate to the effectiveness of interventions on prior variants of COVID-19 and prior to widespread availability of vaccination, and the effectiveness on newer variants and vaccinated patients is unknown. Further study is needed to determine whether the observed effects are sustained through different waves of the pandemic.
Conclusions
Among critically ill patients with COVID-19 randomized to receive 1 or more therapeutic intervention, treatment with an IL-6 receptor antagonist had a greater than 99.9% probability of improved 180-day mortality compared with patients randomized to the control and treatment with an antiplatelet agent had a 95.0% probability of improved 180-day mortality compared with patients randomized to the control. Overall, when considered with previously reported short-term results, the findings indicate that initial in-hospital treatment effects were consistent for most therapies.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Protocol
Brief introduction to explain the protocol structure given modular nature of ongoing platform trial
REMAP-CAP Core Protocol (Version 3.0, 10th July 2019, the Original Version - predating any Covid-19 screening and inclusion)
Pandemic Appendix to Core (PAtC) protocol (Final Version 2.0, 18th May 2020 including summary of changes from version 1.1 and Original Version 1.1, 12th February 2020)
REMAP-COVID Core Protocol (Version 1.0, 27th March 2020)
Statistical Analysis Plan for the secondary analysis of long-term survival and disability in relation to randomized treatments in patients with suspected of proven COVID-19 in the REMAP-CAP trial (Version 1.1, 10th March 2022)
eMethods
eTable 1. Platform and Domain-Specific Inclusion and Exclusion Criteria
eTable 2. Patients and Follow-up by Country of Enrolment
eTable 3. Baseline Characteristics of Patients in the Immune Modulation Domain
eTable 4. Baseline Characteristics of Patients in the Immunoglobulin Domain
eTable 5. Baseline Characteristics of Patients in the Antiplatelet Domain
eTable 6. Baseline Characteristics of Patients in the Anticoagulation Domain
eTable 7. Baseline Characteristics of Patients in the Antiviral Domain
eTable 8. Baseline Characteristics of Patients in the Corticosteroid Domain
eTable 9. Baseline Characteristics of Survivors With and Without Known Day 180 vital Status
eTable 10. Sensitivity Analysis of Day 180 Mortality in Non-Negative COVID-19 Population
eTable 11. Sensitivity Analysis of Day 180 Mortality With Independent Effects for Interleukin-6 Receptor Antagonists and Antiplatelet Interventions
eTable 12. Sensitivity Analysis of Day 180 Mortality Within Domain-Specific Populations
eTable 13. Day 90 Mortality
eTable 14. Baseline Characteristics of Survivors With and Without EQ-5D-5L Utility Scores
eTable 15. Baseline Characteristics of Survivors With and Without WHODAS Scores
eTable 16. Day 180 EQ-5D-5L Results
eTable 17. Day 180 EQ VAS Results
eTable 18. Day 180 Disability Categories
eTable 19. Day 180 Disability
eTable 20. Sensitivity Analysis of EQ-5D-5L Utility Score Without Imputation
eTable 21. Sensitivity Analysis of WHODAS Disability Category Without Imputation
eTable 22. Day 180 Mortality in Patients Mechanically Ventilated at Baseline
eTable 23. Day 180 Mortality in Patients Not Mechanically Ventilated at Baseline
eTable 24. Day 180 Mortality in Immune Deficient Patients
eTable 25. Day 180 Mortality in Non-Immune Deficient Patients
eFigure 1. Forest Plot of Day 180 Mortality Hazard Ratios in Unblinded ITT Population
eFigure 2. Forest Plot of Day 180 Mortality Hazard Ratios in Sensitivity Analyses
eFigure 3. Fitted Kaplan-Meier Curves for Mortality Through Day 180 With Proportional Hazard Ratios, Adjusting for Covariates
eFigure 4. Fitted Kaplan-Meier Curves for Mortality Through Day 180 With Time-Varying Hazards, Adjusting for Covariates
eFigure 5. Association Between OSFD Odds Ratios and the Reciprocal of Day 180 Mortality Hazard Ratios
eFigure 6. Forest Plot of Day 90 Mortality Odds Ratios in Unblinded ITT Population
eFigure 7. Forest Plot of Expected Effect on EQ-5D-5L Utility Score in All Patients (assuming patients who died within 180 days have a utility score of 0)
eFigure 8. Forest Plot of the Odds Ratio on WHODAS Disability Category in Survivors in Unblinded ITT Analysis
eFigure 9. Forest Plot of Day 180 Mortality Hazard Ratios in Unblinded ITT Population Comparing Mechanical Ventilation Status at Baseline
eFigure 10. Forest Plot of Day 180 Mortality Hazard Ratios in Unblinded ITT Population Comparing Immune Deficient Status at Baseline
Model Evaluation Report
Nonauthor collaborators
Data Sharing Statement
References
- 1.Auriemma CL, Harhay MO, Haines KJ, Barg FK, Halpern SD, Lyon SM. What matters to patients and their families during and after critical illness: a qualitative study. Am J Crit Care. 2021;30(1):11-20. doi: 10.4037/ajcc2021398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashem MD, Nallagangula A, Nalamalapu S, et al. Patient outcomes after critical illness: a systematic review of qualitative studies following hospital discharge. Crit Care. 2016;20(1):345. doi: 10.1186/s13054-016-1516-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical Management of COVID-19: Interim Guidance. World Health Organization ; 2020. Accessed March 11, 2022. https://apps.who.int/iris/handle/10665/332196
- 4.Angus DC, Berry S, Lewis RJ, et al. The REMAP-CAP (randomized embedded multifactorial adaptive platform for community-acquired pneumonia) study: rationale and design. Ann Am Thorac Soc. 2020;17(7):879-891. doi: 10.1513/AnnalsATS.202003-192SD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Derde L, Al-Beidh F, et al. ; Writing Committee for the REMAP-CAP Investigators . Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317-1329. doi: 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabi YM, Gordon AC, Derde LPG, et al. ; REMAP-CAP Investigators . Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial. Intensive Care Med. 2021;47(8):867-886. doi: 10.1007/s00134-021-06448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradbury CA, Lawler PR, Stanworth SJ, et al. ; REMAP-CAP Writing Committee for the REMAP-CAP Investigators . Effect of antiplatelet therapy on survival and organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2022;327(13):1247-1259. doi: 10.1001/jama.2022.2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goligher EC, Bradbury CA, McVerry BJ, et al. ; REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators . Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385(9):777-789. doi: 10.1056/NEJMoa2103417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators . Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491-1502. doi: 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estcourt LJ, Turgeon AF, McQuilten ZK, et al. ; Writing Committee for the REMAP-CAP Investigators . Effect of convalescent plasma on organ support-free days in critically ill patients with covid-19: a randomized clinical trial. JAMA. 2021;326(17):1690-1702. doi: 10.1001/jama.2021.18178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. University of York; 1995. [Google Scholar]
- 12.EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 13.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ustün TB, Chatterji S, Kostanjsek N, et al. ; WHO/NIH Joint Project . Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ. 2010;88(11):815-823. doi: 10.2471/BLT.09.067231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ustun TB, Kostanjesek N, Chatterji S, Rehm J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule (WHODAS 2.0). World Health Organization; 2012. [Google Scholar]
- 16.Higgins AM, Serpa Neto A, Bailey M, et al. The psychometric properties and minimal clinically important difference for disability assessment using WHODAS 2.0 in critically ill patients. Crit Care and Resuscitation. 2021;23(1):103-112. doi: 10.51893/2021.1.oa10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudry S, Messika J, Ricard JD, et al. Patient-important outcomes in randomized controlled trials in critically ill patients: a systematic review. Ann Intensive Care. 2017;7(1):28. doi: 10.1186/s13613-017-0243-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herridge MS, Cheung AM, Tansey CM, et al. ; Canadian Critical Care Trials Group . One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683-693. doi: 10.1056/NEJMoa022450 [DOI] [PubMed] [Google Scholar]
- 19.Herridge MS, Tansey CM, Matté A, et al. ; Canadian Critical Care Trials Group . Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293-1304. doi: 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 20.Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319(1):62-75. doi: 10.1001/jama.2017.17687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. doi: 10.1136/bmj.i2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankar-Hari M, Saha R, Wilson J, et al. Rate and risk factors for rehospitalisation in sepsis survivors: systematic review and meta-analysis. Intensive Care Med. 2020;46(4):619-636. doi: 10.1007/s00134-019-05908-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosyakovsky LB, Angriman F, Katz E, et al. Association between sepsis survivorship and long-term cardiovascular outcomes in adults: a systematic review and meta-analysis. Intensive Care Med. 2021;47(9):931-942. doi: 10.1007/s00134-021-06479-y [DOI] [PubMed] [Google Scholar]
- 24.Hodgson CL, Higgins AM, Bailey MJ, et al. ; COVID-Recovery Study Investigators and the ANZICS Clinical Trials Group . Comparison of 6-month outcomes of survivors of COVID-19 versus non-COVID-19 critical illness. Am J Respir Crit Care Med. 2022;205(10):1159-1168. doi: 10.1164/rccm.202110-2335OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McPeake J, Shaw M, MacTavish P, et al. Long-term outcomes following severe COVID-19 infection: a propensity matched cohort study. BMJ Open Respir Res. 2021;8(1):e001080. doi: 10.1136/bmjresp-2021-001080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weihe S, Mortensen CB, Haase N, et al. Long-term cognitive and functional status in Danish ICU patients with COVID-19. Acta Anaesthesiol Scand. 2022;66(8):978-986. doi: 10.1111/aas.14108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown SM, Wilson E, Presson AP, et al. ; with the National Institutes of Health NHLBI ARDS Network . Predictors of 6-month health utility outcomes in survivors of acute respiratory distress syndrome. Thorax. 2017;72(4):311-317. doi: 10.1136/thoraxjnl-2016-208560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins AM, Neto AS, Bailey M, et al. ; PREDICT Study Investigators . Predictors of death and new disability after critical illness: a multicentre prospective cohort study. Intensive Care Med. 2021;47(7):772-781. doi: 10.1007/s00134-021-06438-7 [DOI] [PubMed] [Google Scholar]
- 29.Pallanch O, Ortalda A, Pelosi P, et al. Effects on health-related quality of life of interventions affecting survival in critically ill patients: a systematic review. Crit Care. 2022;26(1):126. doi: 10.1186/s13054-022-03993-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol
Brief introduction to explain the protocol structure given modular nature of ongoing platform trial
REMAP-CAP Core Protocol (Version 3.0, 10th July 2019, the Original Version - predating any Covid-19 screening and inclusion)
Pandemic Appendix to Core (PAtC) protocol (Final Version 2.0, 18th May 2020 including summary of changes from version 1.1 and Original Version 1.1, 12th February 2020)
REMAP-COVID Core Protocol (Version 1.0, 27th March 2020)
Statistical Analysis Plan for the secondary analysis of long-term survival and disability in relation to randomized treatments in patients with suspected of proven COVID-19 in the REMAP-CAP trial (Version 1.1, 10th March 2022)
eMethods
eTable 1. Platform and Domain-Specific Inclusion and Exclusion Criteria
eTable 2. Patients and Follow-up by Country of Enrolment
eTable 3. Baseline Characteristics of Patients in the Immune Modulation Domain
eTable 4. Baseline Characteristics of Patients in the Immunoglobulin Domain
eTable 5. Baseline Characteristics of Patients in the Antiplatelet Domain
eTable 6. Baseline Characteristics of Patients in the Anticoagulation Domain
eTable 7. Baseline Characteristics of Patients in the Antiviral Domain
eTable 8. Baseline Characteristics of Patients in the Corticosteroid Domain
eTable 9. Baseline Characteristics of Survivors With and Without Known Day 180 vital Status
eTable 10. Sensitivity Analysis of Day 180 Mortality in Non-Negative COVID-19 Population
eTable 11. Sensitivity Analysis of Day 180 Mortality With Independent Effects for Interleukin-6 Receptor Antagonists and Antiplatelet Interventions
eTable 12. Sensitivity Analysis of Day 180 Mortality Within Domain-Specific Populations
eTable 13. Day 90 Mortality
eTable 14. Baseline Characteristics of Survivors With and Without EQ-5D-5L Utility Scores
eTable 15. Baseline Characteristics of Survivors With and Without WHODAS Scores
eTable 16. Day 180 EQ-5D-5L Results
eTable 17. Day 180 EQ VAS Results
eTable 18. Day 180 Disability Categories
eTable 19. Day 180 Disability
eTable 20. Sensitivity Analysis of EQ-5D-5L Utility Score Without Imputation
eTable 21. Sensitivity Analysis of WHODAS Disability Category Without Imputation
eTable 22. Day 180 Mortality in Patients Mechanically Ventilated at Baseline
eTable 23. Day 180 Mortality in Patients Not Mechanically Ventilated at Baseline
eTable 24. Day 180 Mortality in Immune Deficient Patients
eTable 25. Day 180 Mortality in Non-Immune Deficient Patients
eFigure 1. Forest Plot of Day 180 Mortality Hazard Ratios in Unblinded ITT Population
eFigure 2. Forest Plot of Day 180 Mortality Hazard Ratios in Sensitivity Analyses
eFigure 3. Fitted Kaplan-Meier Curves for Mortality Through Day 180 With Proportional Hazard Ratios, Adjusting for Covariates
eFigure 4. Fitted Kaplan-Meier Curves for Mortality Through Day 180 With Time-Varying Hazards, Adjusting for Covariates
eFigure 5. Association Between OSFD Odds Ratios and the Reciprocal of Day 180 Mortality Hazard Ratios
eFigure 6. Forest Plot of Day 90 Mortality Odds Ratios in Unblinded ITT Population
eFigure 7. Forest Plot of Expected Effect on EQ-5D-5L Utility Score in All Patients (assuming patients who died within 180 days have a utility score of 0)
eFigure 8. Forest Plot of the Odds Ratio on WHODAS Disability Category in Survivors in Unblinded ITT Analysis
eFigure 9. Forest Plot of Day 180 Mortality Hazard Ratios in Unblinded ITT Population Comparing Mechanical Ventilation Status at Baseline
eFigure 10. Forest Plot of Day 180 Mortality Hazard Ratios in Unblinded ITT Population Comparing Immune Deficient Status at Baseline
Model Evaluation Report
Nonauthor collaborators
Data Sharing Statement


