Key Points
Question
Does baseline frailty modify the benefits of physical rehabilitation intervention among older patients with acute decompensated heart failure (ADHF)?
Findings
In this prespecified secondary analysis of the REHAB-HF trial including 337 participants, older patients with ADHF with worse baseline frailty status had more robust and significant improvement in physical function in response to an innovative, early, transitional, tailored, multidomain physical rehabilitation intervention.
Meaning
These findings suggest that frail patients with ADHF may derive greater benefit from early physical rehabilitation intervention.
This prespecified secondary analysis of the REHAB-HF trial evaluates whether baseline frailty modified the benefits of the physical rehabilitation intervention among older patients with acute decompensated heart failure (HF).
Abstract
Importance
Frailty is common among older patients with acute decompensated heart failure (ADHF) and is associated with worse quality of life (QOL) and a higher risk of clinical events. Frailty can also limit recovery and response to interventions. In the Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial, a 3-month innovative, early, transitional, tailored, multidomain physical rehabilitation intervention improved physical function and QOL (vs usual care) in older patients with ADHF.
Objective
To evaluate whether baseline frailty modified the benefits of the physical rehabilitation intervention among patients with ADHF enrolled in the REHAB-HF trial and to assess the association between changes in frailty with the risk of adverse clinical outcomes on follow-up.
Design, Setting, and Participants
This prespecified secondary analysis of the REHAB-HF trial, a multicenter randomized clinical trial, included 337 patients 60 years and older hospitalized for ADHF. Patients were enrolled from September 17, 2014, through September 19, 2019. Participants were stratified across baseline frailty strata as assessed using modified Fried criteria. Data were analyzed from July 2021 to September 2022.
Interventions
Physical rehabilitation intervention or attention control.
Main Outcomes and Measures
Primary outcome was the Short Physical Performance Battery (SPPB) score at 3 months. Clinical outcomes included all-cause hospitalization or mortality at 6 months.
Results
This prespecified secondary analysis included 337 participants; 181 (53.7%) were female, 167 (49.6%) were Black, and the mean (SD) age was 72 (8) years. A total of 192 (57.0%) were frail and 145 (43.0%) were prefrail at baseline. A significant interaction was observed between baseline frailty status and the treatment arm for the primary trial end point of overall SPPB score, with a 2.6-fold larger improvement in SPPB with intervention among frail patients (2.1; 95% CI, 1.3-2.9) vs prefrail patients (0.8; 95% CI, −0.1 to 1.6; P for interaction = .03). Trends consistently favored a larger intervention effect size, with significant improvement among frail vs prefrail participants for 6-minute walk distance, QOL, and the geriatric depression score, but interactions did not achieve significance.
Conclusions and Relevance
In this prespecified secondary analysis of the REHAB-HF trial, patients with ADHF with worse baseline frailty status had a more significant improvement in physical function in response to an innovative, early, transitional, tailored, multidomain physical rehabilitation intervention than those who were prefrail.
Trial Registration
Clinical Trials.gov Identifier: NCT02196038
Introduction
Frailty, a complex syndrome of accelerated decline in physiologic reserve with aging that results in greater susceptibility to stressors, is common in older patients hospitalized with heart failure (HF) and associated with worse functional status, poor quality of life (QOL), and a high risk of adverse clinical events.1,2 Thus, there is a need for effective strategies to address frailty and its associated clinical risk and functional impairment in patients with HF.
Frailty is often considered a limiting factor to recovery from acute stressors and achieving expected physiologic benefits from physical rehabilitation interventions.3 Supervised exercise training has been shown to improve physical function and exercise capacity in patients with chronic HF.4 However, most exercise trials in HF trials have excluded hospitalized patients with acute decompensated HF (ADHF) and focused on aerobic exercise as the key domain for intervention. Furthermore, these trials did not assess frailty at the time of enrollment and often excluded those at the highest risk of frailty, including older individuals and those with a high burden of functional impairment and comorbidities.4,5 Earlier evidence among frail community-dwelling older persons has been conflicting as to whether frailty limits the improvement in physical function in response to physical rehabilitation interventions.3,6,7 Thus, there is uncertainty regarding the effect of frailty on responsiveness to physical rehabilitation interventions, particularly those targeting impairments in multiple functional domains. This is particularly relevant among older patients with ADHF who have a high burden of frailty and broad and severe impairments in multiple domains of physical function.8,9,10
In the Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial, an innovative, early, transitional, tailored, progressive, multidomain physical rehabilitation intervention was associated with significant, clinically meaningful improvements in physical function status, 6-minute walk distance (6MWD), and QOL in older patients with ADHF.11 Notably, the REHAB-HF population had a high proportion of frail participants, and it is unknown whether the baseline frailty burden modified the treatment benefits of the intervention in this cohort of older patients with ADHF.12 Accordingly, this prespecified secondary analysis of the REHAB-HF trial evaluated the relationship of baseline frailty status with the treatment effects of the multidomain physical rehabilitation intervention. As an exploratory analysis, we also assessed the association between longitudinal frailty changes and the risk of downstream adverse clinical events.
Methods
Study Design and Population
The details of the REHAB-HF trial design,13 the study intervention,14 and primary results have been published previously.11 The trial protocol appears in Supplement 1 and the statistical analysis plan in Supplement 2. Briefly, the REHAB-HF trial was a multicenter, attention-controlled randomized clinical trial of a 12-week early, progressive physical rehabilitation intervention in patients 60 years and older hospitalized for ADHF. Key inclusion criteria were independence with basic activities of daily living, ambulatory at the time of enrollment, and expected to be discharged home. The primary trial consort diagram is shown in eFigure 1 in Supplement 3. Exclusion criteria included end-stage HF, end-stage kidney disease with dialysis, significant dementia, or inability to participate in the REHAB-HF intervention. This study received institutional review board approval from all participating sites. Participants provided written informed consent prior to study enrollment.
Study Intervention
The study intervention was an innovative, early, transitional, tailored, progressive multidomain physical rehabilitation intervention developed for frail, older patients with ADHF. The intervention was initiated in the hospital, when feasible, and continued as soon as possible after hospital discharge in the ambulatory setting 3 days per week for 60 minutes for 36 sessions over 12 weeks. The intervention focused on 4 physical-function domains (strength, balance, mobility, and endurance) and continually progressed based on the participant’s performance. After 3 months of facility-based rehabilitation, patients were transitioned to the independent maintenance phase for months 4 through 6. The individualized exercise program during the maintenance phase included primarily walking and functional strength exercises that were safe to perform at home and required no special equipment. High rates of participant retention and adherence to the intervention sessions were maintained under the supervision of a dedicated committee.11,15
Assessment of Frailty
The Fried phenotype model was used to assess baseline frailty across 5 domains: unintentional weight loss over the past year, self-reported exhaustion, grip strength, slowness assessed by gait speed, and low physical activity assessed by the Short Form-12 Physical Composite Score.12,16,17 At the baseline visit, patients were categorized as frail if they met 3 or more criteria, prefrail if they met 1 or 2 criteria, and nonfrail if they met none of the criteria. Change in frailty from baseline to 3 months was assessed as the difference in the number of Fried criteria present at the 2 visits, where a decrease in frailty score is an improvement. For this comparison, the weight loss criterion was excluded because of the challenges of ascertaining weight changes due to changes in fluid status among patients with HF.
Outcomes of Interest
Consistent with the primary trial end point, the primary outcome for this analysis was the Short Physical Performance Battery (SPPB) score at 3-month follow-up, obtained by blinded assessors. The SPPB assesses global physical function in older adults across 3 components: balance, gait speed, and strength. Each component is scored from 0 to 4, and the total SPPB score can range from 0 to 12, with lower scores indicating worse physical function. The established clinically meaningful minimum change threshold for SPPB is 0.5 points and 1 point represents a substantial change.18,19,20,21 Other outcomes of interest at 3 months were 6MWD, QOL as assessed by the Kansas City Cardiomyopathy Questionnaire, and the visual analog scale (VAS) from the European Quality of Life assessment (EuroQoL VAS; range, 0 to 100, with higher score indicating better QOL), the burden of depressive symptoms as assessed by the Geriatric Depression Scale-15 score (range, 0 to 15, with higher score indicating worse depressive symptoms), and cognitive function via the Montreal Cognitive Assessment (MoCA; range, 0 to 30, with higher score indicating better cognition). Six-month clinical outcomes of interest included all-cause rehospitalization rate and combined all-cause rehospitalization or death rates. The protocol for assessing functional status outcomes and adjudication of the clinical outcomes has been published previously.11,13
Statistical Analysis
Baseline participant characteristics were reported as means with SDs or medians with IQRs for continuous variables and counts and frequencies for categorical variables. Participant characteristics were compared across study groups (prefrail vs frail) using t test for continuous variables and χ2 tests for categorical variables in the overall cohort and within each treatment arm. Effects of the study intervention on 3-month outcomes between frail and prefrail groups were analyzed using analysis of covariance, adjusting for baseline measure, clinical site, age, sex, and ejection fraction (EF) category, as in prior REHAB-HF studies.22,23 Least-square (LS) means were reported as estimates of the intervention effects. The effect sizes for the 3-month outcomes were reported as the between-group difference in the LS means. While SPPB score is an ordinal variable with 13 categories (range, 0 to 12), it was treated as a continuous variable per the prespecified analytical approach for the main trial and subgroup analysis.11,22 This approach is consistent with prior literature24,25,26 and the existing statistical expert recommendations for ordinal variables with many categories as a continuous variable for regression analysis when it meets the normality assumption.27 The regression diagnostic tests using residuals confirmed the normality assumptions for SPPB. Six-month clinical outcomes for the 2 study arms stratified by baseline frailty status were also presented as LS means of rate (counts per patient) for outcomes of all-cause rehospitalizations and combined all-cause rehospitalizations and death. The effect of intervention on 6-month clinical outcomes that were counts or days based were assessed using Poisson regression adjusting for clinical site, age, sex, and EF category and reported as rate ratios with 95% CIs. Consistent with the previous approach, the outcome of all-cause rehospitalization was also adjusted for the baseline SPPB score.11,22
The association of change in frailty (from baseline to 3-month follow-up) and clinical outcomes at 6 months was assessed using similar regression models described above. Adverse events occurring before the 3-month assessment could contribute to worsening in the follow-up frailty burden and confound the association between change in frailty and downstream outcome. Accordingly, participants with rehospitalization events (142 of 337 [42.1%]) occurring before follow-up frailty assessment were excluded from the 6-month clinical outcome analysis. Separate models were constructed for each outcome and each study arm with adjustments for age, sex, clinical site, EF category, and randomization. Two-tailed P values less than .05 were used to determine statistical significance for overall comparisons. The frailty category and treatment arm interaction were considered significant if P < .10. SAS Enterprise Guide version 7.1 (SAS Institute) was used for all statistical analyses.
Results
Baseline Characteristics by Frailty Status
The REHAB-HF trial included 349 participants randomized between September 2014 to September 2019, of which 12 (3.5%) were identified as nonfrail (no Fried criteria), 145 (41.5%) as prefrail (1 to 2 Fried criteria), and 192 (55.0%) as frail (3 or more Fried criteria). Because of the small number of nonfrail participants, the present analysis included only frail and prefrail participants (eFigure 2 in Supplement 3). The baseline characteristics of frail vs prefrail participants in the overall cohort and within each treatment arm are shown in Table 1 and eTable 1 in Supplement 3. Of 337 included participants, 181 (53.7%) were female, 167 (49.6%) were Black, and the mean (SD) age was 72 (8) years. In the overall cohort, compared with prefrail participants, frail participants had a higher burden of comorbidities, higher rates of cognitive impairment and depressive symptoms, lower baseline physical function, and worse QOL measures. There were no significant differences between the frail and prefrail participants in HF severity based on New York Heart Association class, EF, natriuretic peptide levels, and length of hospital stay. Medical therapy was similar among the prefrail vs frail participants (eTable 1 in Supplement 3). A similar pattern of differences in the burden of overall comorbidities, measures of HF severity, measures of functional impairment, and QOL was observed among frail vs prefrail participants within each treatment arm (Table 1).
Table 1. Baseline Characteristics of Study Participants Across the Prefrail and Frail Strata Within Each Treatment Arm.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Rehabilitation intervention | Attention control | |||||
| Prefrail | Frail | P value | Prefrail | Frail | P value | |
| Total, No. | 77 | 92 | NA | 68 | 100 | NA |
| Age, mean (SD), y | 71.9 (8.1) | 73.9 (8.5) | .13 | 71.4 (7.1) | 73.1 (8.1) | .16 |
| Sex | ||||||
| Female | 35 (45) | 49 (53) | .31 | 37 (54) | 60 (60) | .47 |
| Male | 42 (54) | 43 (47) | 31 (46) | 40 (40) | ||
| Race and ethnicity | ||||||
| American Indian or Alaska Native | 1 (1) | 3 (3) | .03 | 0 | 3 (3) | .80 |
| Asian | 1 (1) | 1 (1) | 0 | 2 (2) | ||
| Black | 42 (55) | 31 (34) | 33 (49) | 44 (44) | ||
| Hispanic | 1 (1) | 2 (2) | 1 (1) | 3 (3) | ||
| Multiracial | 0 | 6 (7) | 2 (3) | 3 (3) | ||
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 0 | ||
| White | 33 (43) | 51 (55) | 33 (49) | 48 (48) | ||
| Body mass index, mean (SD)a | 32.1 (7.5) | 33.5 (8.9) | .27 | 33.0 (7.3) | 33.3 (9.9) | .81 |
| Ejection fraction ≥45% | 37 (48) | 53 (58) | .21 | 35 (51) | 56 (56) | .56 |
| NYHA class | ||||||
| II | 16 (21) | 15 (16) | .75 | 12 (18) | 19 (19) | .58 |
| III | 43 (56) | 54 (59) | 38 (56) | 48 (48) | ||
| IV | 18 (23) | 23 (25) | 18 (26) | 33 (33) | ||
| BNP, median (IQR), pg/mL | 685 (383-1045) | 622 (462-1157) | .57 | 497 (276-739) | 631 (259-1350) | .52 |
| NT-proBNP, median (IQR), pg/mL | 3761 (2094-6507) | 3131 (1492-10 654) | .65 | 2180 (1425-4415) | 3248 (1395-5358) | .64 |
| Length of stay, median (IQR), d | 4 (3-6) | 5 (3-7) | .45 | 5 (3-7) | 5 (3-7) | .24 |
| Comorbidities | ||||||
| Total comorbidities, mean (SD) | 5.0 (1.6) | 5.7 (2.4) | .05 | 4.7 (1.7) | 5.3 (1.9) | .03 |
| Hypertension | 71 (93) | 82 (89) | .50 | 63 (93) | 93 (93) | .93 |
| Myocardial infarction | 17 (22) | 12 (13) | .12 | 9 (13) | 22 (22) | .15 |
| Coronary revascularization | 25 (32) | 27 (29) | .66 | 15 (22) | 32 (32) | .16 |
| Atrial fibrillation | 31 (40) | 53 (58) | .03 | 33 (49) | 52 (52) | .66 |
| Diabetes | 44 (57) | 54 (59) | .84 | 32 (47) | 47 (47) | .99 |
| Hyperlipidemia | 51 (66) | 54 (59) | .31 | 47 (69) | 71 (71) | .79 |
| Chronic obstructive pulmonary disease | 18 (23) | 36 (39) | .03 | 12 (18) | 29 (29) | .09 |
| Chronic kidney disease | 24 (31) | 35 (38) | .35 | 15 (22) | 40 (40) | .02 |
| Stroke | 11 (14) | 14 (15) | .87 | 12 (18) | 14 (14) | .52 |
| Peripheral vascular disease | 9 (12) | 18 (20) | .16 | 4 (6) | 9 (9) | .46 |
| Cancer | 13 (17) | 26 (28) | .08 | 11 (16) | 22 (22) | .35 |
| Geriatric conditions | ||||||
| Urinary incontinence, No./total No. (%)b | 4/61 (7) | 14/79 (18) | .04 | 7/57 (12) | 13/79 (16) | .50 |
| Fall in previous 3 mo, No./total No. (%)b | 10/61 (16) | 13/79 (16) | .99 | 8/58 (14) | 12/79 (15) | .82 |
| MoCA score <26 | 56 (73) | 78 (85) | .05 | 52 (76) | 80 (80) | .58 |
| GDS-15 score ≥5 | 34 (44) | 40 (43) | .93 | 16 (24) | 60 (60) | <.001 |
| Functional status/QOL measures at baseline, mean (SD) | ||||||
| SPPB score | 6.9 (2.7) | 5.0 (2.5) | <.001 | 7.1 (2.5) | 5.2 (2.3) | <.001 |
| Balance score | 2.8 (1.6) | 2.3 (1.3) | .01 | 2.9 (1.2) | 2.4 (1.3) | .01 |
| 4-m Walk score | 2.8 (1.0) | 1.8 (0.9) | <.001 | 2.7 (1.1) | 2.0 (0.9) | <.001 |
| Chair rise score | 1.3 (1.2) | 0.9 (1.1) | .01 | 1.5 (1.3) | 0.9 (1.0) | <.001 |
| 6-min Walk distance, m | 220 (104) | 159 (83) | <.001 | 231 (106) | 158 (94) | <.001 |
| KCCQ overall score | 43 (21) | 36 (19) | .02 | 50 (21) | 35 (18) | <.001 |
| EuroQol VAS | 62 (19) | 54 (24) | .02 | 65 (19) | 53 (21) | <.001 |
Abbreviations: BNP, brain natriuretic peptide; EuroQol VAS, European Quality of Life Assessment visual analog scale; GDS-15, Geriatric Depression Scale-15; KCCQ, Kansas City Cardiomyopathy Questionnaire; MoCA, Montreal Cognitive Assessment Score; NA, not applicable; NT-proBNP, N-terminal pro–brain natriuretic peptide; NYHA, New York Heart Association; SPPB, Short Physical Performance Battery.
Calculated as weight in kilograms divided by height in meters squared.
Data were not collected for the full sample due to these characteristics being added later in the trial.
Treatment Effect Modification by Baseline Frailty Status
SPPB data at 3 months were available in 292 participants, including 163 frail participants (84.8%) and 129 prefrail participants (88.9%) (eFigure 2 in Supplement 3). Among the 143 intervention arm participants alive at 3 months, the mean (SD) number of intervention sessions attended was similar among frail participants (22.3 [12.4] sessions) and prefrail participants (23.6 [13.1] sessions). At 6 months, 124 of 154 living participants in the intervention arm (80.5%) reported adherence to maintenance exercise.
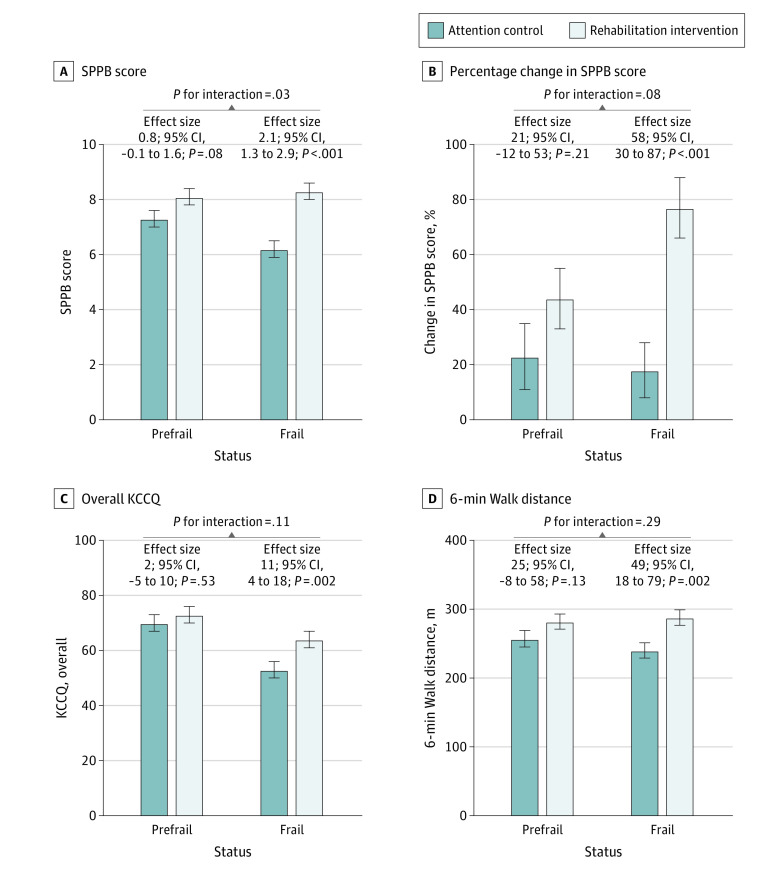
The unadjusted baseline and follow-up measures of SPPB for the intervention vs control arms for the prefrail and frail groups are shown in Figure 1. In the frail group, a substantial decline in SPPB (decrease of 1 point or more) on follow-up was observed among 30 of 86 attention control participants (35%) vs 9 of 77 intervention arm participants (12%; P = .002). In contrast, in the prefrail group, the proportion of participants with 1-point or greater decline in SPPB was comparable among the intervention vs attention control arms (11 of 66 [17%] vs 12 of 63 [19%], respectively; P for interaction of frailty status × treatment arm = .04). The primary prespecified comparison of 3-month outcomes between intervention vs control arms across the prefrail and frail groups, which are adjusted for baseline values, is shown in Figure 2. A significant interaction was observed between baseline frailty status and the treatment arm for the primary trial end point of the overall SPPB score. The effect size of the intervention on SPPB score was 2.6-fold higher among frail participants (2.1; 95% CI, 1.3-2.9) vs prefrail participants (0.8; 95% CI, −0.1 to 1.6; P for interaction = .03) (Figure 2A). We also examined this comparison as relative percentage increases in SPPB, which confirmed the primary outcome analysis. The relative improvement in SPPB with physical rehabilitation intervention (vs attention control) was significantly greater among frail participants (77% [95% CI, 56-98] vs 18% [95% CI, −1 to 38] increase from baseline to follow-up; 58% [95% CI, 30-87] greater increase in the intervention vs control arm; P < .001). In contrast, among prefrail participants, the relative increase in SPPB in the intervention vs usual care arm was modest and not significantly different (44% [95% CI, 21-66] vs 23% [95% CI, 0-46] increase from baseline to follow-up; 21% [95% CI, −12 to 53] greater increase in the intervention vs control arm; P = .21; P for interaction = .08) (Figure 2B).
Figure 1. Short Physical Performance Battery Distribution Among Study Participants at Baseline and Follow-up Stratified by Frailty Status and Treatment Arm.
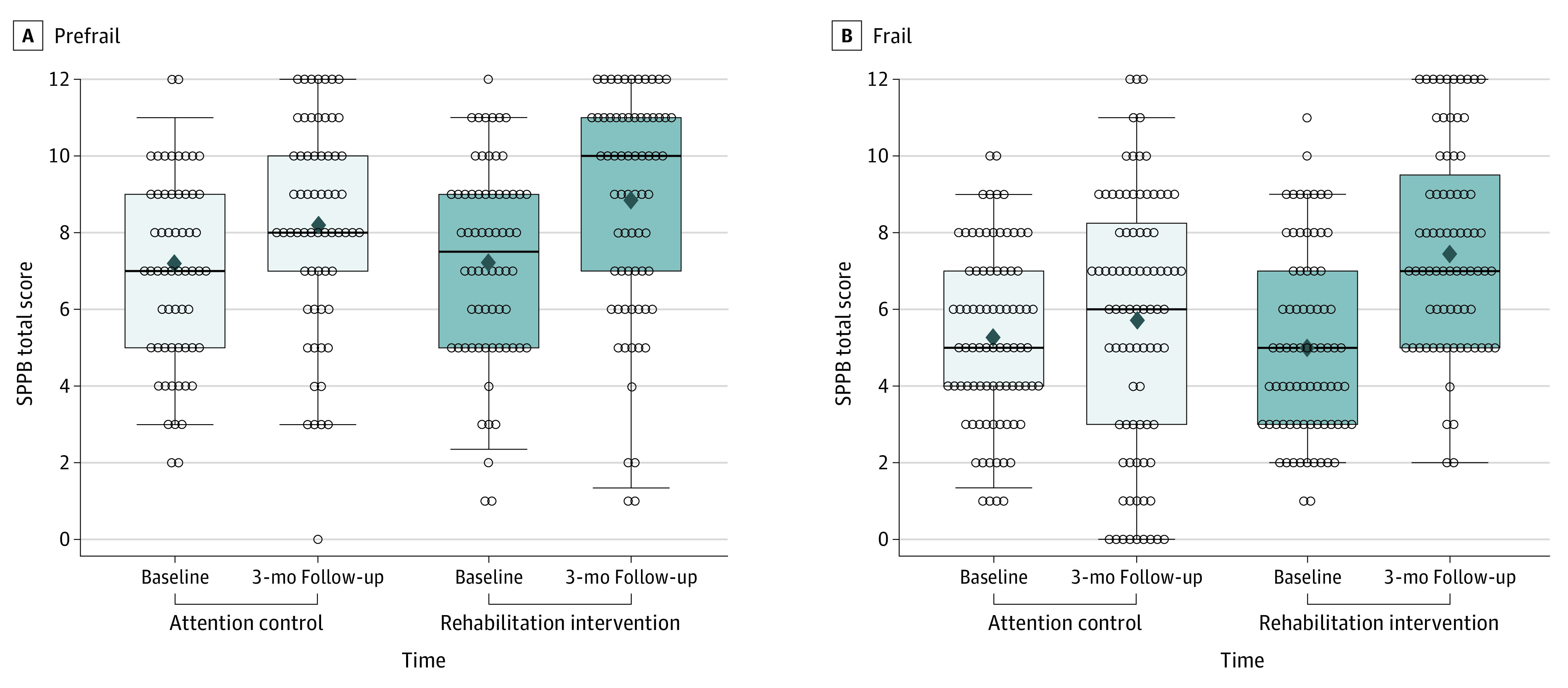
Distribution of Short Physical Performance Battery (SPPB) scores at baseline and follow-up among prefrail (A) and frail (B) participants randomized to physical rehabilitation intervention and attention control groups. The midline indicates the median; diamond, mean; box, IQR; and whiskers, 95% CIs.
Figure 2. Adjusted Analysis Comparing 3-Month Functional Outcomes in the Intervention vs Attention Control Arm Stratified by Baseline Frailty Status.
Adjusted measures of Short Physical Performance Battery (SPPB) score at 3-month follow-up, relative changes in SPPB from baseline to 3-month follow-up, and adjusted measures of Kansas City Cardiomyopathy Questionnaire (KCCQ) score and 6-minute walk distance at 3-month follow-up among intervention and attention control group participants stratified by frail and prefrail status at baseline. Follow-up data are presented as least-squares means with SEs adjusted for baseline value, clinical site, age, sex, and ejection fraction category. Effect size represents the difference in least-squares means between the intervention and attention control groups. P for interaction (prefrail vs frail × intervention arm) was significant for SPPB score and percentage change in SPPB score but was nonsignificant for all other outcomes. Error bars indicate 95% CIs.
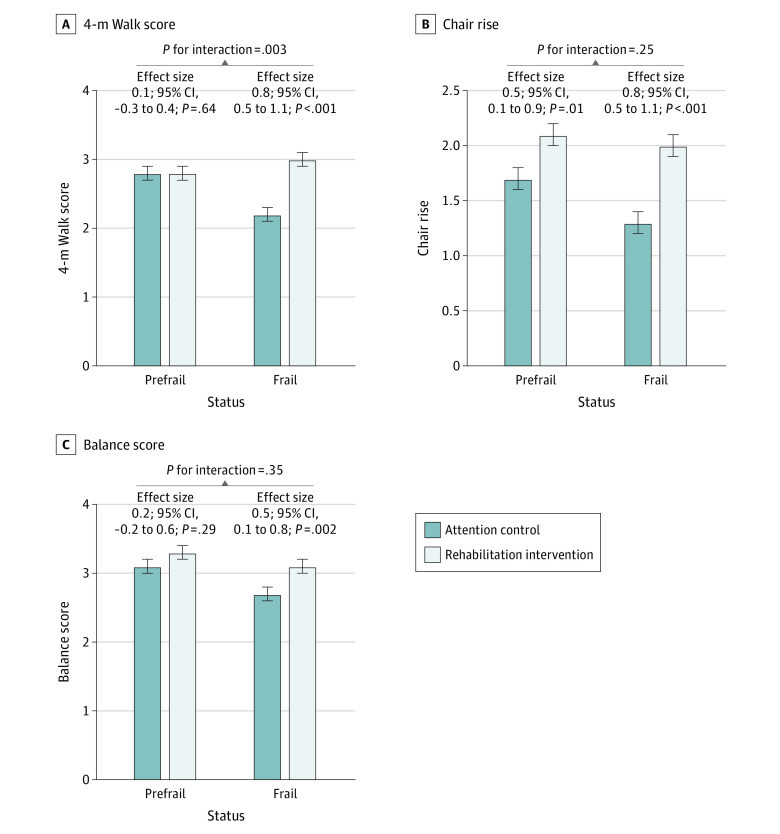
Trends consistently favored a larger intervention effect size with significant improvement among frail vs prefrail participants in 6MWD, QOL (Kansas City Cardiomyopathy Questionnaire overall score and EuroQol VAS), depressive symptoms (Geriatric Depression Scale-15), and all SPPB components (Figure 2; Figure 3; eFigure 3 in Supplement 3). Among these, the interaction was significant only for the gait speed score (frail effect size, 0.8; 95% CI, 0.5-1.1; prefrail effect size, 0.1; 95% CI, −0.3 to 0.4; P for interaction = .003). A significant treatment interaction by frailty status was also noted for the MoCA score. The intervention was associated with a nonsignificant improvement in MoCA performance among frail participants and a nonsignificant decrease in performance among prefrail participants (frail effect size, 0.7; 95% CI, −0.4 to 1.8; prefrail effect size, −1.2; 95% CI, −2.5 to 0; P for interaction = .02) (eFigure 3 in Supplement 3).
Figure 3. Adjusted Analysis Comparing Components of Short Physical Performance Battery (SPPB) at 3-Month Follow-up in the Intervention vs Attention Control Arm Stratified by Baseline Frailty Status.
Adjusted measures of different components of SPPB score at 3-month follow-up among intervention and attention control group participants stratified by frail and prefrail status at baseline. Follow-up data are presented as least-squares means with SEs adjusted for baseline value, clinical site, age, sex, and ejection fraction category. Effect size represents the difference in least-squares means between the intervention and attention control groups. P for interaction (prefrail vs frail × intervention arm) was significant for 4-m walk score but was nonsignificant for all other outcomes. Error bars indicate 95% CIs.
A total of 9 prefrail participants (7 in the intervention arm and 2 in the control arm) and 28 frail participants (14 in each treatment arm) died during the follow-up, with no significant difference between the treatment arms across frailty strata. Although the effect of the intervention on clinical events at 6-month follow-up was not significantly different in frail vs prefrail participants, there was a nonsignificant reduction in the trial’s secondary outcome of combined all-cause rehospitalization and death (Table 2).
Table 2. Measures of 6-Month Clinical Outcomes on Follow-up in the Intervention and Attention Control Groups by Frailty Status at Baseline.
| Measure | LS means (95% CI)a | ||||||
|---|---|---|---|---|---|---|---|
| Prefrail | Frail | P value for interactionb | |||||
| Physical rehabilitation intervention | Attention control | Effect size | Physical rehabilitation intervention | Attention control | Effect size | ||
| All-cause rehospitalization | 1.19 (0.94-1.52) | 1.09 (0.83-1.42) | 1.10 (0.79-1.53) | 1.29 (1.06-1.58) | 1.57 (1.31-1.88) | 0.82 (0.64-1.06) | .17 |
| Deaths | 0.08 (0.03-0.18) | 0.03 (0.01-0.11) | 2.92 (0.60-14.1) | 0.13 (0.07-0.25) | 0.14 (0.08-0.25) | 0.96 (0.45-2.03) | .19 |
| All-cause rehospitalization and death | 1.23 (0.98-1.55) | 1.06 (0.82-1.38) | 1.16 (0.84-1.60) | 1.51 (1.25-1.82) | 1.78 (1.51-2.11) | 0.85 (0.67-1.07) | .13 |
Abbreviation: LS, least-squares.
Clinical event data presented as LS means of rate (counts per patient) adjusted for clinical site, age, sex, and ejection fraction category. All-cause rehospitalization at 6 months also adjusted for baseline Short Physical Performance Battery score. Clinical event effect sizes of rates are shown as rate ratios.
P value interaction for baseline frailty status × intervention.
Improvement in Different Components of Frailty With Physical Rehabilitation Intervention
In the intervention vs control group, there were no significant baseline differences in the prevalence of the different Fried frailty criteria (eFigure 4 in Supplement 3). At 3 months, the mean (SD) number of modified Fried frailty criteria was significantly lower in the intervention arm (baseline, 2.4 [1.0]; 3-month follow-up, 1.4 [1.0]) vs control arm (baseline, 2.5 [1.0]; 3-month follow-up, 1.7 [1.0]; P = .04). The intervention arm had a significantly lower proportion of participants who met the slowness and exhaustion criteria than the control arm (eFigure 4 in Supplement 3).
Association Between Change in Frailty and Outcomes
Among the intervention participants, a 1-unit decrease in frailty criteria at 3-month follow-up was significantly associated with lower all-cause hospitalization rates (risk ratio per 1-unit decrease in modified Fried score, 0.65; 95% CI, 0.52-0.80; P < .001) and combined all-cause rehospitalizations and death (risk ratio per 1-unit decrease in modified Fried score, 0.62; 95% CI, 0.50-0.76; P < .001) at 6 months. A similar association pattern between frailty improvement and risk of adverse clinical outcomes was also noted among usual care participants (eTable 2 in Supplement 3).
Discussion
This prespecified secondary analysis of frailty among older patients with ADHF participating in the REHAB-HF trial had several key findings. Frail participants had a significant 2.6-fold greater improvement in physical function than prefrail participants in response to the early, transitional, tailored, progressive, multidomain physical rehabilitation intervention. Frail participants also significantly improved important secondary outcomes with the intervention, including health-related QOL and symptoms of depression. Participants receiving the intervention had more substantial improvement in key frailty criteria, specifically slowness and exhaustion vs attention control. Finally, a decrease in evidence of frailty on follow-up was significantly associated with a lower risk of adverse clinical outcomes following hospital discharge. Our study findings support older adults hospitalized with ADHF who have a higher frailty burden experienced greater benefits from the innovative REHAB-HF intervention.
To our knowledge, the present study is the first to examine the influence of frailty on the response to a multidomain physical rehabilitation intervention in a large cohort of older participants with ADHF. The frailty syndrome can render frail patients less able to recover following acute hospitalizations and potentially less responsive to rehabilitation interventions,28 especially as frailty becomes severe,3,6,7 which was common in REHAB-HF participants.29 However, recent analyses support that in chronic HF, frail patients can achieve at least similar physical function and clinical benefits through cardiac rehabilitation as nonfrail patients.30,31 The present study significantly extends this prior literature finding that frailty is associated with substantially more robust improvements in physical function in response to a physical rehabilitation intervention among older patients with ADHF, whose impairments are more severe and widespread than typically observed in patients with chronic HF.9
Frail participants had up to 1.3-point greater net improvement in SPPB score with physical rehabilitation intervention (vs attention control) than prefrail participants and a 2.1-point improvement compared with frail participants in the attention control arm. These represent large and clinically meaningful improvements in physical function based on the established thresholds for the SPPB (0.5 points as minimally meaningful threshold; 1 point as substantial increase).18,19,20,21 Prior studies have demonstrated that a 1-unit increase in SPPB is associated with a 25% lower likelihood of incident mobility disability, highlighting the robust and large treatment benefit among frail participants with the rehabilitation intervention.32 As a result of greater absolute improvement in physical function, frail participants achieved similar levels of physical function as prefrail participants by the end of the 3-month intervention despite significantly worse baseline deficits. The intervention effect size was also consistently larger among frail (vs prefrail) patients for secondary outcomes, such as QOL and depressive symptoms, although the P value for interactions was not significant. However, the consistency of findings supports that frail patients may derive greater benefits from the innovative REHAB-HF intervention.
What explains the greater response to the REHAB-HF intervention among frail participants? The REHAB-HF intervention was designed to specifically target the physical dysfunction in frail patients with ADHF related to proposed underlying pathophysiologic mechanisms, including a high burden of systemic inflammation, sarcopenia, impaired mitochondrial function, capillary loss, and skeletal myopathy.1,33 Innovative features of the REHAB-HF intervention potentially contributing to the robust response among frail participants13 included early initiation, including during the index admission when feasible; targeting multiple physical function domains (strength, balance, mobility, and endurance); tailored dosing to individual deficits; continually challenging participants to progress based on protocol-driven performance benchmarks; and robust adherence strategies.13,14
It is also possible that greater baseline impairments in physical function among frail participants may have provided greater potential for functional improvement.12,30,34 However, the intervention was associated with greater absolute and relative improvements in the SPPB among frail vs prefrail patients. Also, the mean (SD) baseline SPPB among frail and prefrail patients was 5.1 (2.4) and 7 (2.6), respectively. With a range of 0 to 12, the SPPB allows sufficient room for improvement with intervention in both the frail and prefrail subgroups before reaching a ceiling effect. This suggests that the more robust treatment benefits among frail patients are not simply related to low baseline functional status or a ceiling effect among prefrail participants. Additionally, the intervention (vs attention control) was more effective in preventing a substantial decline in SPPB among frail participants, further supporting that targeted, multidomain physical rehabilitation interventions like that used in the REHAB-HF trial may be especially important in the vulnerable frail patient population.
In our exploratory analysis, we examined the effect of the study intervention on frailty status.11 Few studies have examined the effect of interventions on frailty status, and whether modifying the frailty phenotype also modifies frailty-associated risks is not well established.6,7,30,34 We found that participants in the intervention arm had decreased evidence of frailty in response to the intervention. The decrease in evidence of frailty was associated with a lower risk of adverse clinical events on follow-up. Specifically, a 1-unit decrease in the modified Fried frailty score was associated with a 37% lower all-cause rehospitalizations or death rate. Of note, the was a similar decrease in event risk among attention control participants, with decreased evidence of frailty likely related to hospitalization recovery and usual care therapies, which could include usual care rehabilitation services. These findings support that targeted interventions can achieve enhanced reductions in frailty burden, which may reduce the risk of adverse clinical events and improve QOL among older frail patients with HF.35
However, we must interpret these findings with caution. The REHAB-HF trial was not adequately powered to determine the effect of the intervention on clinical events. Furthermore, while the number of deaths in the study was small, we observed a nonsignificant numerically higher number of deaths among prefrail participants in the intervention vs attention control arm (7 vs 2 deaths). These findings underscore the need for additional research, including prospective clinical trials, investigating the effect of physical function interventions on clinical events among frail patients with HF. To this end, the investigators are conducting a National Institute on Aging–funded large, multicenter randomized clinical trial powered to evaluate the effects of the REHAB-HF intervention on the risk of all-cause death and hospitalization events in older patients hospitalized for ADHF with preserved EF, a preplanned subgroup that had higher rates of frailty and appeared to receive more benefit from the intervention than those with HF with reduced EF.22
Our study has several strengths. First, the study was conducted as a prespecified secondary analysis to the REHAB-HF trial and used prospectively assessed frailty definition using the criterion-standard Fried phenotype. Second, the study population was relatively unique, including older patients with ADHF and a high burden of frailty, functional impairment, and comorbidities. Third, the study enrolled participants from the community and tertiary care sites, enhancing the generalizability of the study findings. Fourth, the physical rehabilitation intervention was innovative and designed to target impairments in physical function across all key domains. Fifth, unlike prior exercise intervention studies in HF, the REHB-HF intervention was initiated during the hospitalization phase, when the functional deficits were most severe and the risk was the highest.
Limitations
The study also has some limitations. First, although we found that improvement in frailty was associated with significantly improved clinical events, the study did not have adequate statistical power to determine whether baseline frailty significantly modified the treatment effects of the physical rehabilitation intervention on clinical outcomes. Second, the Fried phenotype focuses primarily on physical frailty. Alternative frailty models may provide different findings. Third, while the study staff performing outcome assessments were blinded to the treatment arm assignment, the study participants were not blinded. It is plausible that frail participants, who had a greater burden of physical function impairment at baseline, may have been more motivated to participate in rehabilitation exercises. However, we did not observe a meaningful difference in adherence to intervention among frail vs prefrail patients.
Conclusions
In conclusion, in this prespecified secondary analysis of the REHAB-HF trial, baseline frailty status among older patients with ADHF modified the treatment effects of an early, transitional, tailored, progressive multidomain physical rehabilitation intervention, with more robust and significant improvements in physical function among frail vs prefrail patients. These findings suggest that the REHAB-HF intervention may be effective among frail older patients with HF who have a high burden of functional impairment and, thus, are most in need of such therapies.
Trial Protocol
Statistical Analysis Plan
eTable 1. Baseline Characteristics of Study Participants Across the Treatment vs Attention Control Arms Across the Prefrail vs Frail Strata
eTable 2. Association of Decrease in Evidence of Frailty, Assessed Per 1-Unit Decrease in Modified Fried Frailty Criteria, From Baseline to 3-Month Follow-up With Risk of Adverse Clinical Events at 6-Month Follow-up
eFigure 1. CONSORT Diagram for the Primary Trial
eFigure 2. CONSORT diagram for the present analysis
eFigure 3. Adjusted Measures of EuroQol Score, MoCA Score, and Global Depression Scale Score at 3-Month Follow-up
eFigure 4. Proportion of Participants Meeting Key Components of Fried Criteria at Baseline and 3-Month Follow-up Among Rehabilitation Intervention and Attention Control Arms
Data Sharing Statement
References
- 1.Pandey A, Kitzman D, Reeves G. Frailty is intertwined with heart failure: mechanisms, prevalence, prognosis, assessment, and management. JACC Heart Fail. 2019;7(12):1001-1011. doi: 10.1016/j.jchf.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murad K, Kitzman DW. Frailty and multiple comorbidities in the elderly patient with heart failure: implications for management. Heart Fail Rev. 2012;17(4-5):581-588. doi: 10.1007/s10741-011-9258-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347(14):1068-1074. doi: 10.1056/NEJMoa020423 [DOI] [PubMed] [Google Scholar]
- 4.O’Connor CM, Whellan DJ, Lee KL, et al. ; HF-ACTION Investigators . Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439-1450. doi: 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor RS, Long L, Mordi IR, et al. Exercise-based rehabilitation for heart failure: Cochrane systematic review, meta-analysis, and trial sequential analysis. JACC Heart Fail. 2019;7(8):691-705. doi: 10.1016/j.jchf.2019.04.023 [DOI] [PubMed] [Google Scholar]
- 6.Brown RT, Covinsky KE. Frailty as an outcome in geriatrics research: not ready for prime time? Ann Intern Med. 2018;168(5):361-362. doi: 10.7326/M17-3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trombetti A, Hars M, Hsu FC, et al. ; LIFE Study Investigators . Effect of physical activity on frailty: secondary analysis of a randomized controlled trial. Ann Intern Med. 2018;168(5):309-316. doi: 10.7326/M16-2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidán MT, Blaya-Novakova V, Sánchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18(7):869-875. doi: 10.1002/ejhf.518 [DOI] [PubMed] [Google Scholar]
- 9.Reeves GR, Whellan DJ, Patel MJ, et al. Comparison of frequency of frailty and severely impaired physical function in patients ≥60 years hospitalized with acute decompensated heart failure versus chronic stable heart failure with reduced and preserved left ventricular ejection fraction. Am J Cardiol. 2016;117(12):1953-1958. doi: 10.1016/j.amjcard.2016.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey A, Keshvani N, Zhong L, et al. Temporal trends and factors associated with cardiac rehabilitation participation among Medicare beneficiaries with heart failure. JACC Heart Fail. 2021;9(7):471-481. doi: 10.1016/j.jchf.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 11.Kitzman DW, Whellan DJ, Duncan P, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385(3):203-216. doi: 10.1056/NEJMoa2026141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey A, Kitzman D, Whellan DJ, et al. Frailty among older decompensated heart failure patients: prevalence, association with patient-centered outcomes, and efficient detection methods. JACC Heart Fail. 2019;7(12):1079-1088. doi: 10.1016/j.jchf.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeves GR, Whellan DJ, Duncan P, et al. ; REHAB-HF Trial Investigators . Rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial: design and rationale. Am Heart J. 2017;185:130-139. doi: 10.1016/j.ahj.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pastva AM, Duncan PW, Reeves GR, et al. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial. Contemp Clin Trials. 2018;64:118-127. doi: 10.1016/j.cct.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson MB, Gilbert ON, Duncan PW, et al. Intervention adherence in REHAB-HF: predictors and relationship with physical function, quality of life, and clinical events. J Am Heart Assoc. 2022;11(11):e024246. doi: 10.1161/JAHA.121.024246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 17.McNallan SM, Chamberlain AM, Gerber Y, et al. Measuring frailty in heart failure: a community perspective. Am Heart J. 2013;166(4):768-774. doi: 10.1016/j.ahj.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 19.Soubra R, Chkeir A, Novella JL. A systematic review of thirty-one assessment tests to evaluate mobility in older adults. Biomed Res Int. 2019;2019:1354362. doi: 10.1155/2019/1354362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13(6):538-544. doi: 10.1007/s12603-009-0104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perera S, Studenski S, Newman A, et al. ; Health ABC Study . Are estimates of meaningful decline in mobility performance consistent among clinically important subgroups? (Health ABC study). J Gerontol A Biol Sci Med Sci. 2014;69(10):1260-1268. doi: 10.1093/gerona/glu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mentz RJ, Whellan DJ, Reeves GR, et al. Rehabilitation intervention in older patients with acute heart failure with preserved versus reduced ejection fraction. JACC Heart Fail. 2021;9(10):747-757. doi: 10.1016/j.jchf.2021.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray EM, Whellan DJ, Chen H, et al. Physical rehabilitation in older patients hospitalized with acute heart failure and diabetes: insights from REHAB-HF. Am J Med. 2022;135(1):82-90. doi: 10.1016/j.amjmed.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornsby WE, Sareini MA, Golbus JR, et al. Lower extremity function is independently associated with hospitalization burden in heart failure with preserved ejection fraction. J Card Fail. 2019;25(1):2-9. doi: 10.1016/j.cardfail.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pahor M, Blair SN, Espeland M, et al. ; LIFE Study Investigators . Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157-1165. doi: 10.1093/gerona/61.11.1157 [DOI] [PubMed] [Google Scholar]
- 26.Segar MW, Singh S, Goyal P, et al. Prefrailty, impairment in physical function, and risk of incident heart failure among older adults. J Am Geriatr Soc. 2021;69(9):2486-2497. doi: 10.1111/jgs.17218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robitzsch A. Why ordinal variables can (almost) always be treated as continuous variables: clarifying assumptions of robust continuous and ordinal factor analysis estimation methods. Frontiers Educ. Published online October 8, 2020. doi: 10.3389/feduc.2020.589965 [DOI] [Google Scholar]
- 28.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. doi: 10.1056/NEJMp1212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warraich HJ, Kitzman DW, Whellan DJ, et al. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults ≥60 years with acute decompensated heart failure with preserved versus reduced ejection fraction. Circ Heart Fail. 2018;11(11):e005254. doi: 10.1161/CIRCHEARTFAILURE.118.005254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mudge AM, Pelecanos A, Adsett JA. Frailty implications for exercise participation and outcomes in patients with heart failure. J Am Geriatr Soc. 2021;69(9):2476-2485. doi: 10.1111/jgs.17145 [DOI] [PubMed] [Google Scholar]
- 31.Pandey A, Segar MW, Singh S, et al. Frailty status modifies the efficacy of exercise training among patients with chronic heart failure and reduced ejection fraction: an analysis from the HF-ACTION trial. Circulation. 2022;146(2):80-90. doi: 10.1161/CIRCULATIONAHA.122.059983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasunilashorn S, Coppin AK, Patel KV, et al. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64(2):223-229. doi: 10.1093/gerona/gln022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandey A, Shah SJ, Butler J, et al. Exercise intolerance in older adults with heart failure with preserved ejection fraction: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;78(11):1166-1187. doi: 10.1016/j.jacc.2021.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kehler DS, Giacomantonio N, Firth W, Blanchard CM, Rockwood K, Theou O. Association between cardiac rehabilitation and frailty. Can J Cardiol. 2020;36(4):482-489. doi: 10.1016/j.cjca.2019.08.032 [DOI] [PubMed] [Google Scholar]
- 35.Billingsley H, Rodriguez-Miguelez P, Del Buono MG, Abbate A, Lavie CJ, Carbone S. Lifestyle interventions with a focus on nutritional strategies to increase cardiorespiratory fitness in chronic obstructive pulmonary disease, heart failure, obesity, sarcopenia, and frailty. Nutrients. 2019;11(12):E2849. doi: 10.3390/nu11122849 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Baseline Characteristics of Study Participants Across the Treatment vs Attention Control Arms Across the Prefrail vs Frail Strata
eTable 2. Association of Decrease in Evidence of Frailty, Assessed Per 1-Unit Decrease in Modified Fried Frailty Criteria, From Baseline to 3-Month Follow-up With Risk of Adverse Clinical Events at 6-Month Follow-up
eFigure 1. CONSORT Diagram for the Primary Trial
eFigure 2. CONSORT diagram for the present analysis
eFigure 3. Adjusted Measures of EuroQol Score, MoCA Score, and Global Depression Scale Score at 3-Month Follow-up
eFigure 4. Proportion of Participants Meeting Key Components of Fried Criteria at Baseline and 3-Month Follow-up Among Rehabilitation Intervention and Attention Control Arms
Data Sharing Statement