Abstract
Coxiella burnetii is an obligate intracellular bacterium that resides in an acidified phagolysosome and has a remarkable ability to persist in the extracellular environment. C. burnetii has evolved a developmental cycle that includes at least two morphologic forms, designated large cell variants (LCV) and small cell variants (SCV). Based on differential protein expression, distinct ultrastructures, and different metabolic activities, we speculated that LCV and SCV are similar to typical logarithmic- and stationary-phase growth stages. We hypothesized that the alternate sigma factor, RpoS, a global regulator of genes expressed under stationary-phase, starvation, and stress conditions in many bacteria, regulates differential expression in life cycle variants of C. burnetii. To test this hypothesis, we cloned and characterized the major sigma factor, encoded by an rpoD homologue, and the stress response sigma factor, encoded by an rpoS homologue. The rpoS gene was cloned by complementation of an Escherichia coli rpoS null mutant containing an RpoS-dependent lacZ fusion (osmY::lacZ). Expression of C. burnetii rpoS was regulated by growth phase in E. coli (induced upon entry into stationary phase). A glutathione S-transferase–RpoS fusion protein was used to develop polyclonal antiserum against C. burnetii RpoS. Western blot analysis detected abundant RpoS in LCV but not in SCV. These results suggest that LCV and SCV are not comparable to logarithmic and stationary phases of growth and may represent a novel adaptation for survival in both the phagolysosome and the extracellular environment.
Coxiella burnetii is an obligate intracellular bacterium that has developed a unique strategy to permit multiplication and survival in the phagolysosome of eukaryotic host cells. The life cycle of C. burnetii is incompletely characterized, but it has at least two morphologically and physiologically distinct participants, large-cell variants (LCV) and small-cell variants (SCV) (7, 14, 28, 31, 38). These two cell populations can be purified to near homogeneity by equilibrium centrifugation in 32% cesium chloride (14, 50). LCV appear to be similar to typical gram-negative bacteria, as they appear during exponential phase of growth, with a clearly distinguishable outer membrane, periplasmic space, cytoplasmic membrane, and diffuse nucleoid, attaining lengths exceeding 1 μm. In contrast, SCV are 0.2 to 0.5 μm in diameter, with electron-dense, condensed chromatin and condensed cytoplasm. SCV are resistant to osmotic shock, oxidative stress, heat shock, sonication, and pressure, unlike the more fragile LCV (1, 2, 13, 30). Differences in resistance to breakage by osmotic and pressure stress were employed to suggest that LCV have greater metabolic activity than SCV based on their ability to transport and evolve labeled carbon dioxide from [14C]glucose and [14C]glutamate when incubated in axenic media (30). These two cell variants have also been shown to differentially express several proteins. The histone-like protein Hq-1 (14), and a small (∼4.5-kDa) basic peptide, ScvA (R. A. Heinzen, R. A., D. Howe, L. P. Mallavia, and T. Hackstadt, presented at the 11th Sesqui-Annual Meeting of the American Society for Rickettsiology and Rickettsial Diseases, St. Simons Island, Georgia, 1994), were detected only in SCV. Elongation factor Tu (EF-Tu) was detected only in LCV, while EF-Ts (45) and the major outer membrane protein P1 (29) were both dramatically upregulated in LCV.
These observations were the basis for recently proposed models of C. burnetii development (15, 40). In these models, we speculated that LCV and SCV function like logarithmic-phase and stationary-phase bacteria, respectively (40). In Escherichia coli, the transition to an altered physiological state is mediated by a global regulator of gene expression, ςs (or RpoS), encoded by the rpoS gene. RpoS is a sigma subunit that confers promoter specific transcriptional initiation by RNA polymerase to genes that are expressed during stationary phase. Although associated with the onset of stationary phase, RpoS is also upregulated in response to various stress conditions. RpoS is present at very low levels in exponentially growing cells, but in response to various stress and other conditions (acid stress, oxidative stress, osmotic stress, heat shock, cold shock, nutrient starvation, near-UV light, stringent response, and density sensing) it is strongly upregulated and activates over 60 genes, resulting in multistress resistance and other observed morphologic and physiological alterations (20, 23, 27). Based on our model, we predicted that SCV express an RpoS that regulates protein expression specific for that stage. To test this hypothesis, we identified rpoS and rpoD (as a control for constitutively expressed sigma factor) and evaluated their expression by LCV and SCV. Identification of a prototypic rpoS gene in C. burnetii is intriguing, since recent genomic studies with two obligate intracellular pathogens, Rickettsia prowazekii (3) and Chlamydia trachomatis (21), indicated that the genomes of these organisms do not encode such a sigma factor. Our studies demonstrate that, in contrast to the prediction of our model, SCV do not contain significant RpoS while LCV express abundant RpoS, suggesting that LCV and SCV life cycle variants may not be the functional equivalent forms of logarithmic- and stationary-phase bacteria.
MATERIALS AND METHODS
Media and chemicals.
Luria-Bertani (LB) medium was purchased from Difco Laboratories (Detroit, Mich.), and M9 minimal medium was prepared according to a laboratory manual (39). Antibiotics were incorporated into media at the following concentrations to maintain plasmids in E. coli: ampicillin at 100 μg ml−1 and kanamycin at 50 μg ml−1, tetracycline at 12.5 μg ml−1, and chloramphenicol at 20 μg ml−1. 5-Bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal), isopropyl-β-d-thiogalactopyranoside (IPTG), o-nitrophenyl-β-d-galactopyranoside (ONPG), and a 30% (wt/wt) solution of hydrogen peroxide were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Bacteria and plasmids.
Bacterial isolates (C. burnetii, E. coli, and Legionella pneumophila) and plasmids used in this study are listed in Table 1. E. coli DH5α cultures were grown in LB medium at 37°C in a shaking water bath; E. coli XL1-MRF′ cells were infected with bacteriophage λZapII cloning vector (Stratagene, La Jolla, Calif.) and grown in top agar on NZY-agar plates. C. burnetii was grown in embryonated yolk sacs and purified as previously described (42). The rpoS gene from C. burnetii was cloned in frame into a prokaryotic glutathione S-transferase (GST) fusion expression vector in a two-step cloning strategy. Primers designated Cox-rpoS-For (5′ XhoI site) and Cox-rpoS-Rev amplified the entire 1,059-bp region of the rpoS gene from C. burnetii template DNA. This PCR product was cloned into the pCR2.1-TOPO cloning vector (Invitrogen, Carlsbad, Calif.). This plasmid, designated pR0S105, was double-digested with XhoI and EcoRI and cloned into comparably digested pGEX-4T-1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference(s) or source |
|---|---|---|
| C. burnetii | Nine Mile, phase I, RSA 493 | 42 |
| E. coli | ||
| DH5α | F− φ80d lacZΔM15Δ (lacZYA-argF) U169 deoR recA endA1 phoA relA1 | Gibco-BRL |
| BL21 | F−ompT hsdS (rB− mB−) gal, dcm plysE | Novagen |
| XL1-MRF′ | lac[F′ proAB lacIqZΔM15 Tn10] 2tetrΔ(mcrA)endA1 supE44 thi-1 recA1 gyrA96 relA1 | Stratagene |
| SOLR | F′ proAB lacIqZΔM15 Su− lac gyrA96 relA1 thi-1 endA1 λrsbcC recB recJ umuC::Tn5(kanr) uvrC | Stratagene |
| TOP10F′ | F′ [lacIq Tn10 (tetr)] lacZΔM15Δ deoR recA1 araD139 Δ(ara-leu)7679galU galK rpssL endA1 nupG mcrA | Invitrogen |
| MC4100 | F− Δ(argF-lac) U169 araD139 rpsL150 ptsF25 flbB5301 rpsR deoC relA1 | 46 |
| RO151 | MC4100 φ(csi-5::lacZ)(λplacMu55) | 49 |
| LM5003 | MC4100 rpoS359::Tn10 Tetr | 12, 17 |
| LM5004 | RO151, Kanr | 12, 49 |
| LM5005 | RO151 rpoS359::Tn10 Kanr Tetr | 12, 17 |
| Plasmids | ||
| pSKII(−) | Cloning vector, Apr | Stratagene |
| pCR2.1-TOPO | TA cloning vector, Apr | Invitrogen |
| pGEX-4T-1,2,3 | GST gene fusion vector, Apr | Amersham-Pharmacia Biotech |
| pSK 5-22 | pSK(−) with 1.8-kb EcoRI insert (rpoD) | This work |
| pR0S003 | pSK(−) with 4-kb HindIII insert (rpoS) | This work |
| pR0S104 | pCR2.1 with 953-bp L. pneumophila (partial) rpoS insert | This work |
| pR0S105 | pCR2.1 with 1,060-bp C. burnetii rpoS insert | This work |
| pR0S106 | pGEX 4T-1 with 1,060-bp rpoS insert in frame and correct orientation | This work |
| pR0S107 | pGEX 4T-2 with 1,060-bp rpoS insert | This work |
| pR0S108 | pGEX 4T-3 with 1,060-bp rpoS insert | This work |
| pLM507 | RSF1010 with 9.1-kb insert bearing L. pneumophila (complete) rpoS | 12 |
Genomic library construction.
C. burnetii genomic DNA libraries were constructed with HindIII-digested chromosomal DNA fragments ligated with HindIII-digested λZapII as described in the Stratagene λZapII cloning kit manual. Bacteriophage λZapII was mixed with E. coli strain XL1-MRF′ and incubated on NZY-agar plates to yield approximately 500 plaques per plate. Bacteriophage plaques were removed with sterile Pasteur pipettes and transferred to phage dilution SM buffer, and plasmids were excised as described in the Stratagene λZapII/EcoRI/CIAP cloning kit instruction manual (Stratagene). A plasmid bank was constructed by ligating 1.5- to 2-kb EcoRI-digested fragments with a multicopy vector, pSKII(−), that had been digested with EcoRI and treated with alkaline phosphatase (Boehringer Mannheim, Indianapolis, Ind.). The ligation mixture was transformed into competent library efficiency E. coli strain DH5α (Gibco-BRL, Gaithersburg, Md.).
Preparation of antibodies.
C. burnetii rpoS was PCR amplified and cloned into pCR2.1 cloning vector (Invitrogen). It was subsequently subcloned by in-frame ligation with pGEX-4T-1 GST fusion vector (Amersham-Pharmacia Biotech, Piscataway, N.J.), and the resultant fusion protein was overexpressed and purified as described in the manufacturer's protocol. Fusion protein GST-RpoS was subsequently used to immunize rabbits combined with the adjuvant Titermax (Sigma). Polyclonal immune rabbit serum (IRS) obtained in this manner was additionally preadsorbed against E. coli LM5005 cell lysates to eliminate any cross-reactivity to irrelevant E. coli proteins.
Catalase test.
Cultures were grown overnight in LB medium, centrifuged, resuspended in 5 ml of fresh LB medium at pH 2.0 (adjusted with HCl), and incubated at room temperature under aerobic growth conditions for 1 to 2 h (200 rpm). Various dilutions were plated overnight, and individual colonies were tested the next day for catalase activity by addition of 30% hydrogen peroxide. Colonies that evolved oxygen effervesced, which was indicative of a catalase-positive phenotype.
β-Galactosidase activity.
β-Galactosidase activity was assayed qualitatively on various M9 plates containing limiting amounts of carbon (0.04% glucose; carbon starvation) and 50 μg of X-Gal/ml. β-Galactosidase activity was assessed quantitatively for bacterial cultures as described by Miller (32). Specific activity is presented in micromoles per minute per milligram of protein. The substrate for LacZ hydrolysis in this assay was ONPG.
SDS-PAGE and immunoblot analysis.
Cells of C. burnetii purified from infected tissue culture cells or of E. coli (grown logarithmically or in stationary phase) expressing cloned C. burnetii proteins were resuspended in sample buffer (4% sodium dodecyl sulfate [SDS], 10% β-mercaptomethanol, 20% glycerol, and 0.25 M Tris, pH 8) and boiled for 10 min, and the solubilized protein was separated by SDS–12% polyacrylamide gel electrophoresis (PAGE). Bacterial densities were determined using a spectrophotometer. After electrophoresis, proteins were directly electroblotted onto nitrocellulose transfer membranes (Micron Separations Inc., Westboro, Mass.) as previously described (47). Nitrocellulose membranes used for immunoblotting were blocked for 1 h with 10% nonfat powdered milk and 0.2% Tween-20 in Tris-buffered saline, pH 7.4. Blots were then incubated with rabbit antiserum to C. burnetii RpoS or E. coli RpoS (ς38) (kind gift from A. Ishihama, National Institute of Genetics, Shizuoka, Japan) followed by incubation with an anti-rabbit immunoglobulin antibody conjugated to horseradish peroxidase. The blots were developed using enhanced chemiluminescence system with luminol substrate (Amersham-Pharmacia Biotech).
Functional complementation.
The C. burnetii HindIII recombinant library in pSKII(−) was introduced into an E. coli rpoS null strain designated LM5005 (12) (Table 1) by transformation. Transformants were plated on M9 plates with appropriate antibiotics and X-Gal and contained either 0.4% or 0.04% glucose as a carbon source. β-Galactosidase activity was then assessed visually by the ability of individual colonies to form a dark blue colony on indicator plates under carbon starvation conditions. Plasmids from these putative positive colonies were isolated and additionally verified by Southern blotting for hybridization against L. pneumophila rpoS. Those that hybridized were subjected to dideoxynucleotide sequencing.
Isolation of chromosomal and plasmid DNAs.
C. burnetii Nine Mile phase I chromosomal DNA was extracted using a thermolysin-SDS procedure (41). Plasmid minipreps were prepared by the alkaline lysis procedure using a plasmid purification kits (Qiagen, Valencia, Calif.).
Separation of LCV and SCV.
LCV and SCV were separated essentially as described previously (14, 50). Nine Mile phase I bacteria were purified from infected yolk sacs and then resuspended in 32% cesium chloride. The resulting C. burnetii-CsCl suspension was centrifuged at 27,000 rpm overnight, and the separated upper (SCV) and lower (LCV) bands were removed and pelleted by centrifugation. Both forms were resuspended in sucrose phosphate (0.25 M sucrose, 53.9 mM Na2HPO4, 12.8 mM KH2PO4, 72.6 mM NaCl) buffer and stored at −80°C until use.
PCR amplification.
All PCRs were carried out in a DNA thermocycler (Biometra, Tampa, Fla.) using a GenAmp kit (Perkin Elmer, Branchburg, N.J.). One-hundred-microliter reactions were carried out with Taq DNA polymerase (Perkin Elmer). Degenerate primers were purchased from Genosys Biotechnologies Inc. (The Woodlands, Tex.). Primers were designated as indicated below and used at a final concentration of 0.5 μmol per 100-μl reaction volume. The amplification procedure consisted of 30 cycles of 1 min at 95°C, 1 min at 45°C, and 1 min at 72°C. PCR products were separated in a 1% agarose gel and purified using a Geneclean kit (Bio 101, Vista, Calif.). Desired PCR products were subsequently cloned into PCR cloning vector pCR2.1-TOPO-TA (Invitrogen). Primers were as follows. For L. pneumophila rpoS cloning, primers LprpoS-F1 (5′TGAGCCAGATGATGAATCTCTG3′) and LprpoS-B13 (5′TGTGTTAGTCCAACCCGCTC3′) were used. For C. burnetii rpoD cloning, degenerate primers were based upon the conserved regions 2.4 and 4.2 of rpoD genes: rpo2.4.1 (5′ACNTAYGCNACNTGGTG G3′), rpo2.4.2 (5′GCNATHATGAAYCARAC3′), rpo4.2.1 (5′CCYTCNACYTGDATYTG3′), rpoD2.2 (5′GCNAARAARTAYACNAA3′), and rpoD4.2 (5′YTGYTTNCCNACYTCYTC3′). For C. burnetii RpoS-GST fusion construction, a clone of the entire CbrpoS gene was cloned by PCR into pGEX-T4 using primers Cox-rpoS-For (5′ATGAAAACAAAAACCAC3′) and Cox-rpoS-Rev (5′CTCGAGTCAATCTTCCACTTCTTC3′) (the underlined sequence is a XhoI restriction site). International Union of Biochemistry group codes are used to designate redundancies: R = A + G; Y = C + T; H = A + T + C; D = G + A + T; N = A + G + C + T.
Southern blotting.
Genomic DNA from C. burnetii was digested with restriction enzyme according to the manufacturer's protocol (Boehringer Mannheim). DNAs were then electrophoresed through 0.8% agarose gels and transferred to a nitrocellulose membrane (39). Labeling of a DNA probe with [α-32P]dCTP was carried out using a Decaprime II random DNA labeling kit (Ambion, Austin, Tex.). Blots were incubated with the radiolabeled probe overnight at 65°C and then washed four times at high stringency for 30 min each at 65°C with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% SDS. Blots were analyzed for hybridization patterns using a PhosphorImager and appropriate software (model SF; Molecular Dynamics, Sunnyvale, Calif.).
DNA sequence analysis.
All DNAs were sequenced at Gene Technologies Laboratories, Biology Department, at Texas A&M University. Sequence homologies were compared using MacVector and BLAST programs. The Baylor College of Medicine search launcher program was used to predict putative promoter regions (http://searchlauncher.bcm.tmc.edu).
Nucleotide sequence accession numbers.
GenBank accession numbers for C. burnetii rpoS and rpoD are AF 244357 and AF273254, respectively.
RESULTS
Cloning of C. burnetii rpoD homologue.
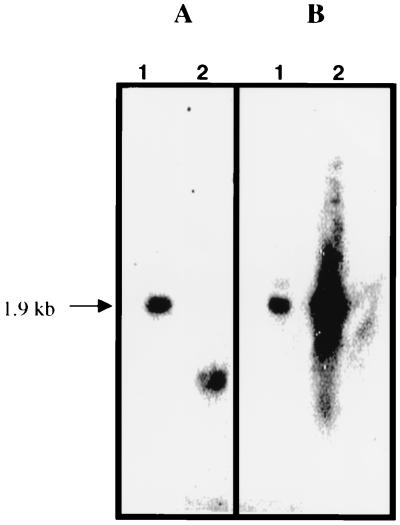
There is a high degree of conservation among sigma factors. Alignment of sigma factors from bacteria has revealed regions of strongest conservation (24). Degenerate oligonucleotide primers based on these conserved regions (rpoD2.2 and rpoD4.2) were able to PCR amplify a 553-bp internal region of the rpoD gene from C. burnetii, as determined by sequence analysis and comparison with other homologues. This internal region was used as a probe to screen a C. burnetii gene bank to identify a clone encompassing the entire rpoD gene. Restriction mapping by Southern hybridization of the 553-bp internal rpoD probe with C. burnetii chromosomal digests localized the gene to an approximately 1.9-kb EcoRI fragment (Fig. 1A, lane 1). L. pneumophila rpoD was PCR amplified as a control (based upon 16S RNA sequence, C. burnetii has been placed in the order “Legionellales” [10]), and this product also hybridized to a 1.9-kb EcoRI fragment of C. burnetii chromosomal DNA (Fig. 1B, lane 1). A size-restricted plasmid bank was subsequently prepared by ligating 1.5- to 2.0-kb EcoRI digested C. burnetii chromosomal DNA fragments to pSKII(−). The PCR amplified internal rpoD region was used as a probe to screen this plasmid bank, and a single clone was identified via colony lift hybridization. The presence of C. burnetii rpoD was confirmed by DNA sequencing and BLAST analysis. The C. burnetii RpoD homologue predicts an ∼70-kDa peptide (GenBank accession number AF273254) and showed 61% identity and 74% similarity to E. coli RpoD.
FIG. 1.
Detection of C. burnetii rpoD by Southern blotting. The PCR-amplified 558-bp internal rpoD gene region from C. burnetii was used as a probe to hybridize with C. burnetii chromosomal DNA digests. (A) Probe hybridized with EcoRI digest (1.9-kb EcoRI fragment hybridized) (lane 1) and a 558-bp C. burnetii rpoD amplicon (lane 2). (B) L. pneumophila PCR-amplified rpoD was used as the probe and hybridized with C. burnetii EcoRI digest (1.9-kb EcoRI fragment hybridized) (lane 1) and a 2.0-kb L. pneumophila rpoD amplicon (lane 2).
Cloning of C. burnetii rpoS homologue.
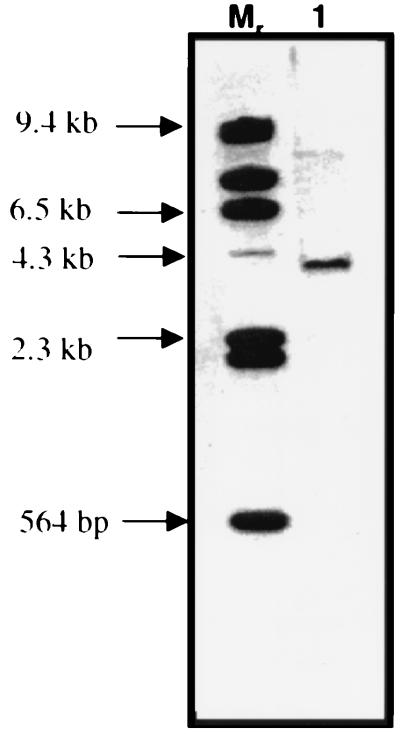
Attempts to clone a putative rpoS homologue of C. burnetii by PCR amplification with degenerate oligonucleotide primers were unsuccessful. To determine if C. burnetii encodes an rpoS stationary-phase sigma factor, the L. pneumophila rpoS gene was PCR amplified using specific primers. The L. pneumophila rpoS sequence was generously provided by personal communication from H. A. Shuman, Columbia University (12). This PCR fragment was cloned and then used as a probe against C. burnetii chromosomal DNA digested with HindIII in a Southern blot under low-stringency conditions. Using this approach, a putative rpoS homologue was localized to a 4-kb HindIII fragment (Fig. 2, lane 1). Screening a λZapII/HindIII bacteriophage library using the L. pneumophila rpoS as probe yielded no rpoS-bearing clones. As an alternate approach, we opted to attempt complementation of an E. coli rpoS null mutant (LM5005) with a C. burnetii genomic library. Strain LM5005, reconstructed from an original clone reported by Weichart and colleagues (49), was obtained from H. A. Shuman (12). This strain contains an rpoS-dependent lacZ fusion reporter (csi5::lacZ) to test for complementation of the rpoS null phenotype with rpoS genes from other bacterial genomes. A C. burnetii HindIII genomic library in the plasmid vector pSKII(−) was constructed and transformed into LM5005 and control strains LM5003 and LM5004 (Table 1). Putative complemented mutants were evaluated qualitatively for RpoS-induced β-galactosidase activity (due to increased transcription of csi5::lacZ) by identifying dark blue colonies under carbon starvation conditions. pLM507, a plasmid bearing L. pneumophila rpoS (12), was transformed into LM5005 to serve as positive control and, as predicted, developed dark blue colonies on M9 indicator plates with 0.04% glucose. This was in contrast to the light blue colonies observed when LM5005/pLM507 is grown with 0.4% glucose (nonstarving conditions). When LM5005 was transformed with the C. burnetii gene bank, putative rpoS-complemented mutants were indistinguishable from uncomplemented mutants, possibly due to the limitation of this qualitative approach for assessing LacZ activity.
FIG. 2.
Detection of C. burnetii rpoS by Southern blotting. L. pneumophila rpoS was PCR amplified and used as a probe to hybridize with C. burnetii chromosomal digest. Lane 1, HindIII digest (approximately 4-kb HindIII fragment hybridized). Mr, molecular weight marker (32P-labeled HindIII-digested lambda DNA).
Catalase activity.
We then added a strategy to enrich for rpoS-complemented E. coli. This involved incubation of transformants containing putative rpoS clones (after overnight recovery) in LB medium adjusted to pH 2.0 (37). Only cells complemented with rpoS homologues were predicted to survive the incubation in low-pH medium due to a requirement for an RpoS-dependent acid tolerance phenotype (43). Clones were subjected to two 45-min exposures to LB medium adjusted to pH 2.0 to enrich for clones bearing C. burnetii rpoS. Surviving clones were then screened indirectly for restored or complementing RpoS activity by assessing expression of RpoS-regulated catalase-dependent conversion of H2O2 to O2 and H2O by adding 30% hydrogen peroxide. Positive colonies evolved oxygen and hydrogen gas. This enrichment yielded 12 putative rpoS clones that demonstrated both catalase activity and acid tolerance. These clones were further analyzed by Southern blotting to determine whether the cloned inserts hybridized with the L. pneumophila rpoS, thereby identifying putative C. burnetii rpoS homologues (data not shown). Plasmids from five of the twelve putative clones hybridized to the probe, and one of these was selected for nucleotide sequencing.
Demonstration of β-galactosidase activity.
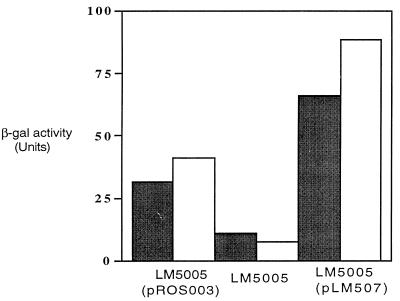
The csi5 locus is induced during carbon starvation conditions (49). Therefore, strains LM5005, LM5004, and LM5003 (Table 1) were cultured in minimal M9 medium supplemented with both low (0.04%; carbon starvation) and high (0.4%; carbon source replete) glucose concentrations. To confirm complementation by C. burnetii RpoS, β-galactosidase activity was assessed quantitatively (32) (Fig. 3). The putative complemented strain containing C. burnetii rpoS(pR0S003) showed approximately a fivefold increase in β-galactosidase activity in comparison to an uncomplemented rpoS null mutant (LM5005) under inducing conditions. As a positive control, L. pneumophila rpoS, on pLM507 (H. A. Shuman [12]), was also transformed into LM5005 and demonstrated >10-fold activity under carbon starvation.
FIG. 3.
C. burnetii RpoS functionally complements E. coli RpoS. Strains were grown overnight and then subcultured to late log phase in minimal media supplemented with 0.4% (grey bars) or 0.04% (white bars) glucose prior to assay; β-galactosidase (β-gal) activity is expressed as Miller units. The complemented strain containing C. burnetii rpoS (LM5005/pR0S003) showed an approximately fivefold increase in activity in comparison to the uncomplemented rpoS null mutant (LM5005) under carbon starvation conditions. L. pneumophila rpoS, on pLM507, transformed into LM5005 demonstrated >10-fold activity.
DNA sequence analysis.
DNA sequence was obtained, and subsequent BLAST comparison of the National Center for Biotechnology Information database indicated that the C. burnetii putative rpoS (GenBank accession number AF 244357) was closely related to other rpoS loci. As in L. pneumophila and E. coli, homologues of the surE and nlpD genes were found directly upstream of rpoS (Fig. 4). However, in E. coli, surE and nlpD are separated by pcm, which encodes protein carboxyl methyl transferase. The promoter driving rpoS transcription is located within the open reading frame (ORF) for nlpD, designated prpoSP1, in E. coli and is believed to be utilized in a growth phase-dependent manner (22). This is in addition to two nlpD promoters that contribute to a low-level expression of rpoS in a growth phase-independent manner. The nucleotide sequence of Coxiella rpoS revealed potential −10 and −35 regions of the putative ς70 RNA polymerase-dependent promoters predicted by the Baylor College of Medicine search launcher program (data not shown). The C. burnetii RpoS deduced amino acid sequence was 47% identical and 56% similar to that of E. coli RpoS (35) and 50% identical and 67% similar to that of L. pneumophila RpoS (12) by ClustalW alignment (Fig. 5). L. pneumophila RpoS showed 59.5% identity and 78.4% similarity to that of E. coli.
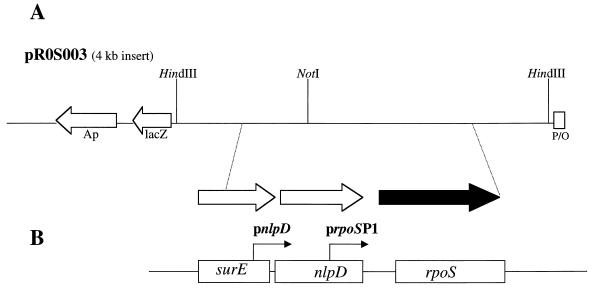
FIG. 4.
Schematic representation of the organization of ORFs on pR0S003. (A) rpoS (filled arrow) and two additional ORFs identified on a 4-kb HindIII restriction fragment cloned from C. burnetii; (B) three similar genes found on the chromosome of E. coli determined from BLAST searches of the GenBank database. pnlpD and prpoSP1 are the major promoters involved in transcription of the nlpD and rpoS genes, respectively, in E. coli. Arrows indicate direction of transcription.
FIG. 5.
ClustalW alignment of C. burnetii, E. coli, and L. pneumophila RpoS. The core amino acid (aa) (lysine) of the putative turnover element is indicated by the arrow. N-terminal lysine residues are underlined.
DsrA RNA was recently proposed to be an antiantisense RNA that regulates translation of rpoS message by freeing the translation initiation region from the cis-acting antisense RpoS mRNA (25). A region of the DsrA corresponding to the first stem-loop showed complementarity to a region in the rpoS ORF from C. burnetii. A “turnover element” in E. coli RpoS was shown to interact with the response regulator RssB, which facilitates subsequent ClpXP (protease)-mediated degradation of RpoS (6, 34, 44). This amino acid motif was found to be conserved in the C. burnetii RpoS sequence (Fig. 5). The C. burnetii rpoS gene encoded a predicted peptide sequence that was 24 amino acids longer than E. coli RpoS, with a molecular mass of 40 kDa (compared to 38 kDa) and a highly basic pI of 9.6 (compared to 4.6 for E. coli RpoS). This difference in pI is due to an extended N-terminal region that is highly lysine rich (Fig. 5) (overall lysine content of C. burnetii RpoS is 11.6%).
Regulation of C. burnetii RpoS levels in E. coli.
Polyclonal antibodies against E. coli ς38 and ς70 were obtained from A. Ishihama (National Institute of Genetics) (20) to evaluate expression of recombinant C. burnetii RpoS and RpoD in E. coli. The RpoS-specific antibody did cross-react on Western blots with an approximately 40-kDa antigen in the complemented mutant (LM5005/pR0S003). In contrast, there was no reactivity observed with the uncomplemented mutant (LM5005/pSKII(−)) or with the L. pneumophila rpoS complemented mutant (LM5005/ pLM507) (Fig. 6). The sequence analysis suggested that C. burnetii RpoS may be susceptible to the transcriptional and posttranscriptional regulatory mechanisms described for E. coli RpoS. To examine C. burnetii RpoS levels in E. coli, culture lysates prepared from mutant strains cultured for different time periods to represent stages of growth from early log to stationary phase were compared (adjusted to an optical density of 600 nm of 0.3) with E. coli wild-type strains. Western blot analysis with an anti-E. coli ς38 polyclonal indicated that a stable C. burnetii RpoS was expressed at 8 h of growth (Fig. 7), and in overnight cell culture lysates (data not shown), i.e., at the onset of stationary phase, a pattern similar to the ς38 induction pattern was observed in wild-type E. coli culture lysates and was also comparable to the regulation seen in L. pneumophila log- and stationary-phase cultures (12, 20).
FIG. 6.
Western blot analysis of E. coli cell lysates with anti-E. coli RpoS polyclonal IRS showing immunoreactivity of an RpoS-complemented mutant. . Lane 1, wild-type E. coli DH5α expressed 38-kDa RpoS; lane 2, LM5005 (E. coli rpoS null mutant); lane 3, LM5005 complemented with L. pneumophila rpoS(pLM507); lane 4, LM5005 transformed with pBluescript SK(−) cloning vector (negative control) (no anti-RpoS reactive antigen of the appropriate size was expressed); lane 5, LM5005 complemented with C. burnetii rpoS(pR0S003), expressing a 40-kDa RpoS.
FIG. 7.
Regulation of C. burnetii RpoS in an E. coli rpoS null strain. A Western blot was probed with anti-E. coli RpoS antibody, showing the relative amounts of C. burnetii RpoS expressed by log-phase and stationary-phase cultures of LM5005/pR0S003. Each lane contains comparable amounts of bacteria grown for 1 to 8 h (early log to stationary phase).
Examination of RpoS level in C. burnetii lysates.
Polyclonal antibody against E. coli ς70 cross-reacted with a 70-kDa antigen in C. burnetii cell lysates (data not shown). The polyclonal antibody against E. coli ς38 cross-reacted with a 40-kDa antigen in C. burnetii cell lysates, but in an inconsistent manner. To obtain a more sensitive and reliable C. burnetii-specific antibody, the rpoS gene from C. burnetii was cloned in frame into a prokaryotic GST fusion expression vector in a two-step cloning strategy. To verify construction and expression of the GST-RpoS fusion, E. coli BL21(DE3) containing this plasmid (pR0S106) and suitable negative controls (alternative out-of-frame clones, pR0S107 and pR0S108) were IPTG induced, and culture lysates were separated by SDS-PAGE, Western blotted, and probed with an anti-GST antibody (data not shown). This analysis confirmed that a fusion protein of the predicted size (∼70 kDa) was being expressed by pR0S106 and not by the negative controls that expressed GST alone (30 kDa).
GST-RpoS was purified, and IRS was developed. This anti-GST-CbRpoS IRS was preadsorbed with LM5005 and purified GST to remove cross-reactive antibodies raised against irrelevant E. coli proteins and the GST component of the fusion protein used to immunize the rabbit. To confirm that the serum did react with the RpoS component of the GST-RpoS immunizing antigen, this fusion was cleaved with a proteolytic enzyme, thrombin, which specifically digests at the junction of the fusion. Western analysis confirmed the reactivity against the 40-kDa RpoS component (Fig. 8), detecting a 40-kDa antigen in C. burnetii propagated in and purified from either C. burnetii-infected embryonated hens' eggs or J774 mouse macrophages (Fig. 9). This confirmed the expression of RpoS by C. burnetii in both culture systems and provided a reliable and sensitive antibody for comparing expression by life cycle variants.
FIG. 8.
Western blotting to confirm reactivity to the RpoS component of the GST fusion product. (A) Western blot probed with anti-C. burnetii RpoS serum and reacted with undigested GST-RpoS (lane 1) or thrombin-digested GST-RpoS (lane 2); (B) Western blot probed with anti-RpoS antibody and reacted with undigested GST-RpoS (lane 1) or GST-RpoS digested with thrombin for 2 h (lane 2) or overnight (lane 3).
FIG. 9.
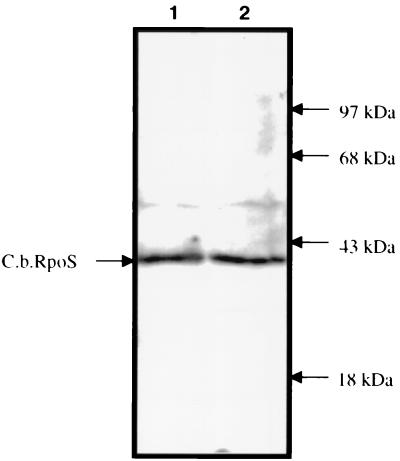
Western blot analysis of C. burnetii cell lysates probed with anti-CbRpoS IRS. Anti-RpoS IRS reacted with a 40-kDa antigen in C. burnetii Nine Mile phase I cell lysates purified from C. burnetii-infected yolk sacs (lane 1) or C. burnetii-infected J774 mouse macrophages (lane 2).
Differential expression of RpoS.
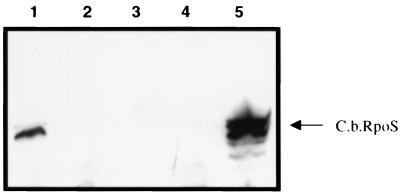
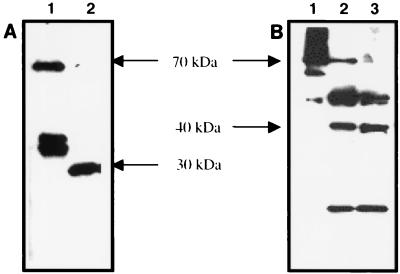
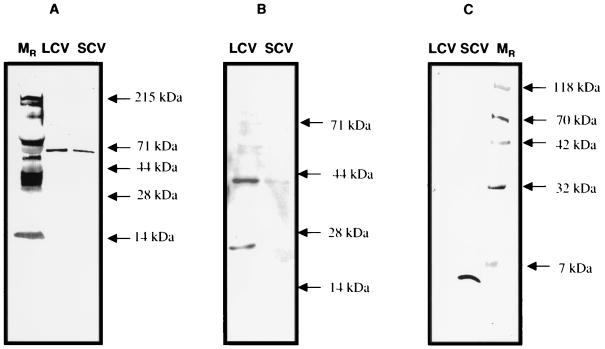
To determine differential expression of RpoS, C. burnetii was purified from infected J774 mouse macrophages. SCV and LCV were then separated by 32% cesium chloride isopycnic gradient centrifugation. Whole-cell lysates of each form were then separated by SDS-PAGE and subsequently analyzed by immunoblot using serum specific for C. burnetii RpoS (anti-GST-CbRpoS serum). The amounts of total protein of LCV and SCV applied to each lane were comparable, and several other antigens were detected by specific antiserum as controls, including ScvA (SCV specific) (16) (Fig. 10C), Com-1 (not differentially expressed) (data not shown), and RpoD (predicted to be not differentially expressed) (Fig. 10A). RpoS level was found to be dramatically upregulated in the LCV and barely detectable in the SCV (Fig. 10B). This result strongly suggested that LCV and SCV are not representative of bacterial forms in logarithmic and stationary phases of growth.
FIG. 10.
Differential expression by LCV and SCV. LCV and SCV were separated by CsCl gradient centrifugation, and equal amounts of total protein were separated by SDS-PAGE and immunoblotted. Blots were probed with anti-RpoD antibody, which detected an approximately 70-kDa antigen in both LCV and SCV (A); with anti-RpoS antibody, which detected a 40-kDa antigen in LCV (B); or with anti-ScvA, which detected 5-kDa ScvA in the SCV (C).
DISCUSSION
LCV and SCV differentially express proteins that appear specific for a unique role for each form in a developmental life cycle of C. burnetii. A description of these differentially expressed proteins should provide evidence for a more accurate model of this life cycle. A reverse genetic approach was used to identify two LCV-specific or upregulated translation factors, EF-Tu and EF-Ts (45). A comparison of DNA-binding proteins demonstrated that the histone-like protein Hq-1 was SCV specific (14). These findings led to the hypothesis that LCV and SCV are similar to log- and stationary-phase growth forms, respectively (15, 40). Earlier comparisons of metabolic activity and morphology are also consistent with this hypothesis (28, 30). To test this hypothesis, we identified and characterized a homologue of the stationary-phase transcription factor, RpoS. Western blot comparison of SCV and LCV demonstrated that SCV do not contain significant amount of RpoS, while abundant RpoS was detected in LCV.
We initially attempted to PCR amplify sigma factor homologues of C. burnetii by comparing alignment of sigma factors from other bacteria and designing degenerate primers from regions of strong conservation. An internal region of a C. burnetii rpoD homologue was cloned by PCR amplification with degenerate primers (24). Cloning of an rpoS homologue based on degenerate oligonucleotide PCR was not successful. As an alternate strategy, the L. pneumophila (the phylogenetically closest pathogen to C. burnetii) rpoS gene was PCR amplified and shown to hybridize with a 4-kb HindIII fragment of C. burnetii chromosomal DNA, suggesting that C. burnetii may possess an rpoS. An L. pneumophila rpoS homologue has recently been cloned by complementation of an E. coli rpoS null mutation in a strain that has an rpoS-dependent csi5::lacZ fusion (12). We obtained this rpoS null strain and adopted a similar strategy to attempt cloning of a C. burnetii rpoS gene. One potential problem in the application of this strategy was that partial complementation by C. burnetii RpoS could render β-galactosidase activity due to restored RpoS function indistinguishable from background β-galactosidase activity in a qualitative assay. To overcome this problem, an enrichment step was added where transformed mutants able to survive acid stress were selected and then screened for RpoS-dependent catalase activity. LM5005 (the E. coli rpoS null strain), when complemented with L. pneumophila rpoS(pLM507) (Table 1), did exhibit a distinctive dark blue color, in contrast to an uncomplemented mutant, under carbon starvation conditions. This observation was consistent with results from experiments examining β-galactosidase activity quantitatively. The specific activity of the L. pneumophila rpoS-complemented mutant showed a 10-fold induction, compared with only a 5-fold induction in the C. burnetii rpoS-complemented mutant. We speculate that the stronger functional complementation by L. pneumophila rpoS relative to C. burnetii may be due to (i) closer sequence similarity between L. pneumophila and E. coli RpoS (60% identity and 78% similarity) compared with C. burnetii RpoS (only 47% identity and 56% similarity with E. coli RpoS) or (ii) the C. burnetii RpoS lysine-rich N terminus.
Our current model of sigma factor regulation comes from studies performed with several organisms, including E. coli and Salmonella spp. These studies demonstrated that the expression of sigma factor (ςs) protein encoded by rpoS was extremely low in rapid exponential growth but increased markedly upon entry into stationary phase and was required for the induction of more than 30 genes (20, 27). A recent study suggested that sigma factors compete for a limiting amount of RNA polymerase during stationary phase (9). An extensive and complex set of mechanisms for regulating expression of RpoS levels under different conditions exist (5, 18, 25, 26, 33, 36, 48, 51). C. burnetii RpoS levels increased in response to entry into stationary phase in the E. coli null mutant, indicating susceptibility to E. coli regulatory mechanisms and indirectly suggesting that similar pathways in C. burnetii for regulating intracellular RpoS levels exist. The major level of regulation in E. coli appears to be posttranscriptional. Some stationary-phase-induced genes also require RpoD for their induction. Sequence analysis of mRNA control elements in C. burnetii rpoS suggests that it may possess some of the same regulatory signals as in E. coli. In E. coli, DsrA RNA regulates the translation of RpoS message by an antiantisense mechanism (25). The sequence of the first stem-loop of DsrA RNA is complementary to the upstream leader of rpoS mRNA and helps to free the translation initiation region from the cis-acting antisense RNA, thereby allowing translation to occur. A region of the DsrA RNA corresponding to the first stem-loop shows complementarity to a region in the rpoS ORF from C. burnetii (data not shown). RpoS proteolysis after translation is also important for maintaining very low levels in exponentially growing bacteria. This regulated degradation mediated by ClpXP protease is facilitated by a response regulator, RssB. RssB interacts with a turnover element recently characterized around a crucial amino acid, lysine-173 (6). This proteolysis-promoting motif was found to be conserved in the predicted amino acid sequence for C. burnetii RpoS.
A unique aspect of the C. burnetii RpoS was its highly basic pI of 9.6 (in contrast to 4.6 for E. coli RpoS). C. burnetii RpoD also has an unusually high pI. Observations in our laboratory of protein profiles of C. burnetii cell lysates separated on two-dimensional gels show a predominance of proteins with high pI (K. Kiss, personal communication). We speculate that because of the low pH (∼4.8) of the environment in which C. burnetii resides, many of the cytoplasmic proteins have been adapted to provide a proton “sink” for buffering protons that passively enter the cell. Hackstadt showed that the cytoplasm of C. burnetii was neutral and a significant proton motive force was maintained (11).
Contrary to our expectations, C. burnetii RpoS expression was upregulated by the LCV rather than the SCV, suggesting that this alternate sigma factor may have no role in regulating the transition from LCV to SCV cell type or in regulating expression of SCV-specific genes. It is possible that an additional alternate sigma factor could control SCV-specific genes. Western blot analysis with antibody against several sigma factors reacted only with RpoD, RpoS, a potential 54-kDa sigma factor (not cloned), and a 28-kDa sigma factor (RpoH) (J. Seshu, personal communication), but other sigma factors may remain undetected.
What is the function of the C. burnetii RpoS? Genomic studies with R. prowazekii and Chlamydia spp. demonstrated that these obligate organisms do not carry typical rpoS genes and have lost extensive coding capacity through gene deletion and mutation for functions common to extracellular bacteria. C. burnetii has adapted to thrive in a unique intracellular niche distinct from other intracellular bacteria. RpoS roles have been known to vary among organisms. L. pneumophila RpoS was recently shown to not be required for stationary-phase-dependent resistance to stress (12). These data suggested that L. pneumophila RpoS regulates genes required for survival in a protozoan host, quite different from what has been reported for E. coli RpoS. In Salmonella, KatF (same as RpoS) mutants had significantly reduced virulence in mice (8), and KatF is implicated in the initial invasion and colonization of the gut. Yersinia enterocolitica RpoS is required for the expression of a heat-stable enterotoxin yet has no role in promoting virulence in mice (4, 19). Vibrio cholerae rpoS mutants are stress sensitive and show reduced expression of hemagglutinin and protease, but the mutation has no effect on the ability of V. cholerae to colonize mice (52). Perhaps the C. burnetii RpoS plays a role in mediating transition from SCV to LCV (instead of LCV to SCV) in response to signals perceived in the phagolysosomal compartment, although this seems counterintuitive considering the apparent resemblance between these forms and log- and stationary-phase organisms. Alternatively, this sigma factor may solely regulate genes involved in surviving stresses in metabolically active LCV. Identification and confirmation of RpoS-regulated genes from C. burnetii using an E. coli strain expressing C. burnetii RpoS would provide insight into the role of inducible genes in intracellular survival.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI37744 from the National Institute of Allergy and Infectious Diseases.
We thank Howard A. Shuman (Columbia University) for his generous gift of L. pneumophila rpoS sequence and strains, Akira Ishihama (National Institute of Genetics) for E. coli anti-Rpo antibodies, and Larry Harris-Haller (Gene Technology Labs, Texas A&M University) for sequence analysis. We thank Jon Skare for critical review of the manuscript.
REFERENCES
- 1.Amano K, Williams J C. Sensitivity of Coxiella burnetii peptidoglycan to lysosome hydrolysis and correlation of sacculus rigidity with peptidoglycan-associated proteins. J Bacteriol. 1984;160:989–993. doi: 10.1128/jb.160.3.989-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano K, Williams J C, McCaul T E, Peacock M G. Biochemical and immunological properties of Coxiella burnetii cell wall and peptidoglycan-protein complex fractions. J Bacteriol. 1984;160:982–988. doi: 10.1128/jb.160.3.982-988.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson A, Zomorodipour A, Andersson J. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1999;247:713–719. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 4.Badger J, Miller V I. Role of RpoS in survival of Yersinia enterocolitica to a variety of environmental stresses. J Bacteriol. 1995;177:5370–5373. doi: 10.1128/jb.177.18.5370-5373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth M, Marshall C, Muffler A, Fischer S, Hengge-Aronis R. Role for the histone-like protein H-NS in growth phase-dependent and osmotic regulation of ςs and many ςs-dependent genes in Escherichia coli. J Bacteriol. 1995;177:3455–3464. doi: 10.1128/jb.177.12.3455-3464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker G, Klauck E, Hengge-Aronis R. Regulation of RpoS proteolysis in Escherichia coli: the response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proc Natl Acad Sci USA. 1999;96:6439–6444. doi: 10.1073/pnas.96.11.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis G E, Cox H R. A filter-passing infectious agent isolated from ticks. I. Isolation from Dermacentor andersonii, reactions in animals, and filtration. Public Health Rep. 1938;53:2259. [Google Scholar]
- 8.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. The alternate ς factor KatF (RpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farewell A, Kvint K, Nystrom T. Negative regulation by RpoS: a case of sigma factor competition. Mol Microbiol. 1998;29:1039–1051. doi: 10.1046/j.1365-2958.1998.00990.x. [DOI] [PubMed] [Google Scholar]
- 10.Garrity, G. M. (ed.). Bergey's manual of systematic bacteriology, 2nd ed., vol. 2, in press. Springer-Verlag, New York, N.Y.
- 11.Hackstadt T. Estimation of the cytoplasmic pH of Coxiella burnetii and effect of substrate oxidation on proton motive force. J Bacteriol. 1983;154:591–597. doi: 10.1128/jb.154.2.591-597.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hales L M, Shuman H A. The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J Bacteriol. 1999;181:4879–4889. doi: 10.1128/jb.181.16.4879-4889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzen R A. Intracellular development of Coxiella burnetii. In: Anderson B, Bendinelli M, Friedman H, editors. Rickettsial infection and immunity. New York, N.Y: Plenum Press; 1997. pp. 99–129. [Google Scholar]
- 14.Heinzen R A, Hackstadt T. A developmental stage-specific histone H1 homolog of Coxiella burnetii. J Bacteriol. 1996;178:5049–5052. doi: 10.1128/jb.178.16.5049-5052.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinzen R A, Hackstadt T, Samuel J E. Developmental biology of Coxiella burnetii. Trends Microbiol Sci. 1999;7:149–154. doi: 10.1016/s0966-842x(99)01475-4. [DOI] [PubMed] [Google Scholar]
- 16.Heinzen R A, Howe D, Mallavia L P, Rockey D D, Hackstadt T. Developmentally regulated synthesis of an unusually small, basic peptide by Coxiella burnetii. Mol Microbiol. 1996;22:9–19. doi: 10.1111/j.1365-2958.1996.tb02651.x. [DOI] [PubMed] [Google Scholar]
- 17.Hengge-Aronis R. Back to log phase: ςs as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol Microbiol. 1993;21:887–893. doi: 10.1046/j.1365-2958.1996.511405.x. [DOI] [PubMed] [Google Scholar]
- 18.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1497–1512. [Google Scholar]
- 19.Iriarte M, Stainier I, Cornelis G R. The rpoS gene from Yersinia enterocolitica and its influence on expression of virulence factors. Infect Immun. 1995;63:1840–1847. doi: 10.1128/iai.63.5.1840-1847.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jishage M, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of ς70 and ς38. J Bacteriol. 1995;177:6832–6835. doi: 10.1128/jb.177.23.6832-6835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalman S, Mitchell W, Marathe R, Lammel C, Fan J, Hyman R W, Olinger L, Grimwood J, Davis R, Stephens R S. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat Genet. 1999;21:385–389. doi: 10.1038/7716. [DOI] [PubMed] [Google Scholar]
- 22.Lange R, Fischer D, Hengge-Aronis R. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the ςs subunit of RNA polymerase in Escherichia coli. J Bacteriol. 1995;177:4676–4680. doi: 10.1128/jb.177.16.4676-4680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loewen P C, Bei H, Strutinsky J, Sparling R. Regulation in the rpoS regulon of Escherichia coli. Can J Microbiol. 1998;44:707–717. doi: 10.1139/cjm-44-8-707. [DOI] [PubMed] [Google Scholar]
- 24.Lonetto M, Gribskov M, Gross C A. The ς70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc Natl Acad Sci USA. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCann M P, Fraley C D, Matin A. The putative ς factor KatF is regulated posttranscriptionally during carbon starvation. J Bacteriol. 1993;175:2143–2149. doi: 10.1128/jb.175.7.2143-2149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCann M P, Kidwell J P, Matin A. The putative ς factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J Bacteriol. 1991;173:4188–4194. doi: 10.1128/jb.173.13.4188-4194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCaul T F. The developmental cycle of Coxiella burnetii. In: Williams J C, Thompson H A, editors. Q fever: the biology of Coxiella burnetii. Boca Raton, Fla: CRC Press; 1991. pp. 223–258. [Google Scholar]
- 29.McCaul T F, Banerjee-Bhatnagar N, Williams J C. Antigenic differences between Coxiella burnetii cells revealed by postembedding immunoelectron microscopy and immunoblotting. Infect Immun. 1991;59:3243–3253. doi: 10.1128/iai.59.9.3243-3253.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCaul T F, Hackstadt T, Williams J C. Ultrastructural and biological aspects of Coxiella burnetii under physical disruptions. In: Burgdorfer W, Anacker R L, editors. Rickettsiae and rickettsial diseases. New York, N.Y: Academic Press; 1981. p. 267. [Google Scholar]
- 31.McCaul T F, Williams J C. Developmental cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiations. J Bacteriol. 1981;147:1063–1076. doi: 10.1128/jb.147.3.1063-1076.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller J H. Experiments in molecular biology. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratories; 1972. [Google Scholar]
- 33.Muffler A, Fischer D, Hengge-Aronis R. The RNA-binding protein HF-I, known as a host factor for phage Qβ RNA replication, is essential for rpoS translation in Escherichia coli. Gene Dev. 1996;10:1143–1151. doi: 10.1101/gad.10.9.1143. [DOI] [PubMed] [Google Scholar]
- 34.Muffler A, Traulsen D D, Altuvia S, Storz G, Hengge-Aronis R. The response regulator RssB controls stability of the ςs subunit of RNA polymerase in Escherichia coli. Mol Microbiol. 1996;15:1333–1339. [PMC free article] [PubMed] [Google Scholar]
- 35.Mulvey M R, Loewen P C. Nucleotide sequence of katF of Escherichia coli suggest KatF protein is a novel ς transcription factor. Nucleic Acids Res. 1989;17:9979–9991. doi: 10.1093/nar/17.23.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paesold G, Krause M. Analysis of rpoS mRNA in Salmonella dublin: identification of multiple transcripts with growth-phase-dependent variation in transcript stability. J Bacteriol. 1999;181:1264–1268. doi: 10.1128/jb.181.4.1264-1268.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prince R W, Xu Y S, Libby S J, Fang F C. Cloning and sequencing of the gene encoding the RpoS sigma factor from Salmonella typhimurium 14028s. Biochim Biophys Acta. 1994;1219:198–200. doi: 10.1016/0167-4781(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg M, Kordova N. Study of intracellular forms of Coxiella burnetii in the electron microscope. Acta Virol. 1960;4:52–61. [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Samuel J E. Development of Coxiella. In: Brun Y V, Skimkets L J, editors. Prokaryotic development. Washington, D.C.: ASM Press; 2000. pp. 427–440. [Google Scholar]
- 41.Samuel J E, Frazier M E, Kahn M L, Thomashow L S, Mallavia L P. Isolation and characterization of a plasmid from phase I Coxiella burnetii. Infect Immun. 1983;41:488–493. doi: 10.1128/iai.41.2.488-493.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuel J E, Frazier M E, Mallavia L P. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect Immun. 1985;49:775–779. doi: 10.1128/iai.49.3.775-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schellhorn H E, Audia J P, Wei L I C, Chang L. Identification of conserved, RpoS-dependent stationary-phase genes of Escherichia coli. J Bacteriol. 1998;180:6283–6291. doi: 10.1128/jb.180.23.6283-6291.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schweder T, Lee K, Lomovskaya O, Matin A. Regulation of Escherichia coli starvation sigma factor (ςs) by ClpXP protease. J Bacteriol. 1996;178:470–476. doi: 10.1128/jb.178.2.470-476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seshadri R, Hendrix L R, Samuel J E. Differential expression of translational elements by life cycle variants of Coxiella burnetii. Infect Immun. 1999;67:6026–6033. doi: 10.1128/iai.67.11.6026-6033.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silhavy T J, Berman M J, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. pp. 107–112. [Google Scholar]
- 47.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webb C, Moreno M, Wilmes-Riesenberg M, Curtiss R, III, Foster J W. Effects of DksA and ClpP protease on sigma S production and virulence in Salmonella typhimurium. Mol Microbiol. 1999;34:112–123. doi: 10.1046/j.1365-2958.1999.01581.x. [DOI] [PubMed] [Google Scholar]
- 49.Weichart D, Lange R, Henneberg N, Hengge-Aronis R. Identification and characterization of stationary phase-inducible genes in Escherichia coli. Mol Microbiol. 1993;10:407–420. [PubMed] [Google Scholar]
- 50.Wiebe M E, Burton P R, Shankel D M. Isolation and characterization of two cell types of Coxiella burnetii. J Bacteriol. 1972;110:368–377. doi: 10.1128/jb.110.1.368-377.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashino T, Ueguchi C, Mizuno T. Quantitative control of the stationary phase-specific sigma factor, ςs, in Escherichia coli: involvement of the nucleoid protein H-NS. EMBO. 1995;14:594–602. doi: 10.1002/j.1460-2075.1995.tb07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yildiz F H, Schoolnik G K. Role of rpoS in stress survival and virulence of Vibrio cholerae. J Bacteriol. 1998;18:773–784. doi: 10.1128/jb.180.4.773-784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]