This survey study analyzes data from objective assessments of visual function for older US adults to determine the current prevalence of impairment in distance and near visual acuity and contrast sensitivity.
Key Points
Question
What is the current prevalence of vision impairment (VI) among older US adults?
Findings
In this survey study, based on objective assessments of visual function with habitual correction in the 2021 nationally representative National Health and Aging Trends Study, 27.8% of US adults 71 years and older had VI. Distance and near visual acuity and contrast sensitivity impairment were present in 10.3%, 22.3%, and 10.0%, respectively; a higher prevalence of VI was associated with older age, less education and income, non-White race, and Hispanic ethnicity.
Meaning
More than one-quarter of US adults 71 years and older had VI; there were differences in visual function based on socioeconomic and demographic factors.
Abstract
Importance
Existing estimates of the prevalence of vision impairment (VI) in the United States are based on self-reported survey data or measures of visual function that are at least 14 years old. Older adults are at high risk for VI and blindness. There is a need for up-to-date, objectively measured, national epidemiological estimates.
Objective
To present updated national epidemiological estimates of VI and blindness in older US adults based on objective visual function testing.
Design, Setting, and Participants
This survey study presents a secondary data analysis of the 2021 National Health and Aging Trends Study (NHATS), a population-based, nationally representative panel study of Medicare beneficiaries 65 years and older. NHATS includes community-dwelling older adults or their proxies who complete in-person interviews; annual follow-up interviews are conducted regardless of residential status. Round 11 NHATS data were collected from June to November 2021, and data were analyzed in August 2022.
Interventions
In 2021, NHATS incorporated tablet-based tests of distance and near visual acuity and contrast sensitivity with habitual correction.
Main Outcomes and Measures
National prevalence of impairment in presenting distance visual acuity (>0.30 logMAR, Snellen equivalent worse than 20/40), presenting near visual acuity (>0.30 logMAR, Snellen equivalent worse than 20/40), and contrast sensitivity (>1 SD below the sample mean). Prevalence estimates stratified by age and socioeconomic and demographic data were calculated.
Results
In the 2021 round 11 NHATS sample, there were 3817 respondents. After excluding respondents who did not complete the sample person interview (n = 429) and those with missing vision data (n = 362), there were 3026 participants. Of these, 29.5% (95% CI, 27.3%-31.8%) were 71 to 74 years old, and 55.2% (95% CI, 52.8%-57.6%) were female respondents. The prevalence of VI in US adults 71 years and older was 27.8% (95% CI, 25.5%-30.1%). Distance and near visual acuity and contrast sensitivity impairments were prevalent in 10.3% (95% CI, 8.9%-11.7%), 22.3% (95% CI, 20.3%-24.3%), and 10.0% (95% CI, 8.5%-11.4%), respectively. Older age, less education, and lower income were associated with all types of VI. A higher prevalence of near visual acuity and contrast sensitivity impairments was associated with non-White race and Hispanic ethnicity.
Conclusions and Relevance
More than 1 in 4 US adults 71 years and older had VI in 2021, higher than prior estimates. Differences in the prevalence of VI by socioeconomic and demographic factors were observed. These data could inform public health planning.
Introduction
Vision impairment (VI) disproportionately affects older adults. Approximately 60% of those with VI or blindness in the United States are 65 years and older, totaling at least 8 million individuals in prior studies.1,2,3,4 The number of people with VI and blindness in the United States is projected to double from 2015 to 2050, primarily from growth of the older population.3,5 Poor vision is associated with numerous adverse outcomes in later life, including depression,6 dementia,7 motor vehicle crashes,8 and even mortality.9 In 2017, the costs of caring for older adults with VI and blindness in the United States was $134.2 billion.10
Existing estimates of the prevalence of VI in the United States are based on measures of best-corrected visual function that are now at least 14 years old and self-reported survey data.11 The last time visual function was objectively measured (eg, visual acuity) in a nationally representative sample was in the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2008.12 NHANES excluded individuals living in institutional settings and contained a relatively small sample of older adults, although these groups are at disproportionately high risk for VI. Thus, there is a critical need for up-to-date, objectively measured, national epidemiological estimates of VI prevalence among older adults.
In 2021, the National Health and Aging Trends Study (NHATS), a nationally representative panel study of Medicare beneficiaries 65 years and older, began including objective measures of presenting visual function in its annual protocol.13 Using data from the 2021 NHATS survey, we present the first national epidemiological estimates of VI and blindness in the United States based on visual function testing in nearly one and a half decades.
Methods
NHATS is a nationally representative panel study of Medicare beneficiaries 65 years and older in the United States that has been ongoing since 2011. A total of 7609 community- and residential care–dwelling older adults or their proxies completed in-person interviews in round 1. Annual follow-up interviews have been conducted with participants regardless of residential status. Replenishment of the sample was undertaken in 2015. Race and ethnicity data are self-reported by respondents upon enrollment in the study. Multiple categories are allowed, and respondents are asked to select a primary race in these cases. NHATS oversamples older and Black individuals. NHATS was approved by the Johns Hopkins University institutional review board; all participants provided informed consent. This analysis was deemed not regulated because it consisted of secondary analysis of deidentified publicly available data.
Vision Tests in NHATS
In 2019, NHATS piloted a protocol for measuring objective visual function in participants’ homes, including distance and near visual acuity and contrast sensitivity, using a tablet-based platform.13 Pilot participants were more likely to have objectively measured VI than self-reported vision difficulty. The pilot demonstrated that objective visual function measurements could be incorporated into an interviewer-administered protocol in participants’ homes.13 In a clinical evaluation study, there were narrow limits of agreement between tablet-based and gold-standard vision tests, and agreement was not dependent on participants’ level of vision.14 In 2021, NHATS began testing distance visual acuity, near visual acuity, and contrast sensitivity using tablet-based tests (Ridgevue Publishing) in participants’ homes. All participants who were eligible for a sample person interview were eligible for visual function testing.15
We adopted World Health Organization (WHO) definitions of VI based on binocular testing of presenting vision with habitual correction (eg, glasses or contact lenses), if available.16 In NHATS, all tests assessed binocular presenting vision to obtain a free-living measure of visual function corresponding to participants’ day-to-day experience. Visual acuity was measured using letters displayed on a tablet. The tablet was placed 59 inches from the respondent for distance visual acuity and contrast sensitivity testing; it was held at the participant’s preferred reading distance for near visual acuity. For distance and near visual acuity testing, respondents were shown 5 letters per screen. They were asked to read the letters aloud; letters became smaller with each subsequent screen. Visual acuity testing was discontinued once the respondent read fewer than 3 of 5 letters correct on a given screen or when they reached the screen with the smallest letters. For contrast sensitivity, respondents were shown 2 letters per screen, and the letters had a lower contrast on each subsequent screen. Contrast sensitivity testing was discontinued when the respondent read no letters correctly on a given screen or when they reached the screen with the lowest contrast. For all tests, the number of letters read correctly was recorded; a logMAR was calculated for measures of visual acuity, and log contrast (logCS) was calculated to measure contrast sensitivity. Near acuity was calculated as logMAR = (0.02 × [55 − ∑ letters read correctly]) + log10 40/x, where x is the preferred reading distance in centimeters.
Variables
Socioeconomic and demographic variables included age in 5-year intervals (71-74 years for the youngest group), sex (male or female), race and ethnicity (Hispanic, non-Hispanic Black, non-Hispanic White, and other non-Hispanic), educational attainment (<high school degree, high school degree, some college, and ≥college degree), and family income (in quartiles of $0-<$21 000, $21 000-<$40 000, $40 000-<$75 000, and ≥$75 000 based on 2021 NHATS income distribution). For the 30.0% of respondents missing income data, information was carried forward from round 9 (the last assessment of income). Because of panel replenishment in 2015, all participants in the 2021 NHATS survey were 71 years and older.
Distance VI was defined using WHO definitions16 (mild VI: >0.30-0.48 logMAR, Snellen equivalent <20/40-20/60; moderate VI: >0.48-1.0 logMAR, Snellen equivalent <20/60-20/200; severe VI: >1.0-1.3 logMAR, Snellen equivalent <20/200-20/400; blind: logMAR >1.3, Snellen equivalent approximately <20/400). Due to small counts, severe VI and blindness categories were collapsed. The definition of near VI followed the WHO definition of acuity worse than N6, which corresponds to approximately greater than 0.3 logMAR or Snellen equivalent less than 20/40.16 There is no widely accepted definition of contrast sensitivity impairment. Prior studies have used a cutoff of less than 1.55 logCS to define contrast sensitivity impairment17 because this was 2 SD below the mean in a normative sample.18 We present results using the threshold of less than 1.55 logCS, as well as a cutoff of more than 1 SD below the NHATS sample mean.
Analysis
Weights have been developed for each round of the NHATS survey to adjust for differential probabilities of selection and nonresponse in order to make nationally representative parameter estimates.15 All weighted analyses accounted for the NHATS complex survey design, including sample strata, units, and weights. The full sample final analytical weights for round 11 provided by NHATS were applied.
We excluded participants missing data for any of the vision tests, as very few had missing data for only a single test. Unweighted raw counts and percentages as well as weighted percentages and their 95% CIs were generated for socioeconomic and demographic characteristics. For each of the 3 vision measures, we generated for the overall sample and by each categorical sociodemographic variable (1) the weighted logMAR or logCS mean and SD, (2) the weighted prevalence of VI and their 95% CIs, and (3) differences between socioeconomic and demographic strata using second-order Rao-Scott χ2 tests. This approach was also used to compare the unweighted sample characteristics among the final analytic sample and those who had incomplete vision data. To estimate the number of US adults 71 years and older with VI, weighted prevalence data was standardized to the July 1, 2020, US census data, excluding Alaska, Hawaii, and Puerto Rico because NHATS participants are recruited from the lower 48 contiguous states. To evaluate the association between VI and socioeconomic and demographic variables, we used multivariable logistic regression to model each measure of VI. Each model was adjusted for age group, sex, race and ethnicity, education, and income.
Round 11 NHATS data were collected from June to November 2021; data were analyzed in August 2022. All analyses were conducted with SAS version 9.4 (SAS Institute). All tests were 2-sided. For all analyses, P values were not corrected for multiple comparisons. We followed American Association for Public Opinion Research (AAPOR) reporting guidelines.
Results
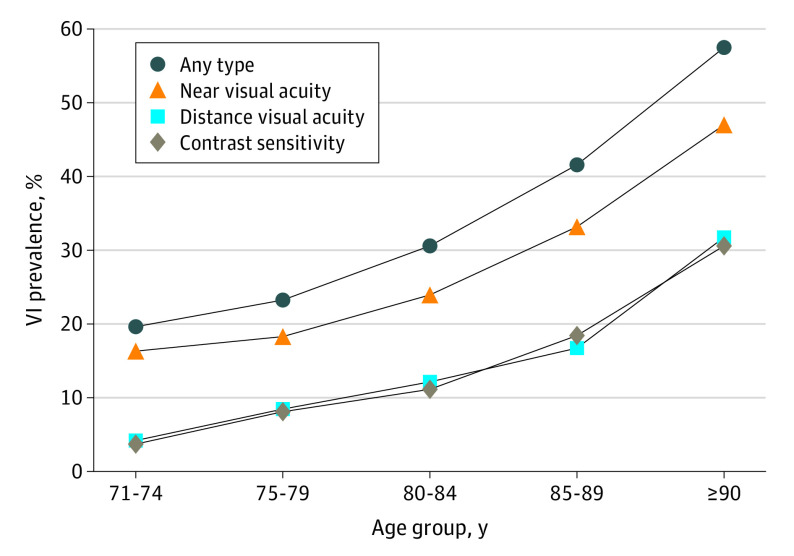
There were 3817 respondents in the 2021 NHATS sample. After excluding respondents who did not complete the sample person interview (n = 429) and those with missing vision data (n = 362), there were 3026 participants included in this analysis. Characteristics of the individuals who were included in and excluded from the analytic sample are presented in eTable 1 in Supplement 1. Weighted sample characteristics are included in Table 1; 29.5% were aged 71 to 74 years (95% CI, 27.3%-31.8%), 55.2% (95% CI, 52.8%-57.6%) were female respondents, and 79.8% (95% CI, 77.2%-82.4%) were non-Hispanic White respondents. We estimate that more than 1 in 4 adults 71 years and older in the United States has a VI (27.8%; 95% CI, 25.5%-30.1%). The Figure illustrates the prevalence of any VI, distance and near VI, and contrast sensitivity impairment by age.
Table 1. Weighted Sample Characteristics for 3026 Participants With Complete Vision Data in Round 11 of the National Health and Aging Trends Study.
| Characteristic | Raw No. (%) | Weighted percentage (95% CI) |
|---|---|---|
| Age groups, y | ||
| 71-74 | 420 (13.9) | 29.5 (27.3-31.8) |
| 75-79 | 900 (29.7) | 32.7 (30.3-35.2) |
| 80-84 | 768 (25.4) | 20.0 (18.5-21.5) |
| ≥85 | 938 (31.0) | 17.7 (16.5-18.9) |
| Sex (self-reported) | ||
| Male | 1287 (42.5) | 44.8 (42.4-47.2) |
| Female | 1739 (57.5) | 55.2 (52.8-57.6) |
| Race and ethnicitya | ||
| Black, non-Hispanic | 597 (19.7) | 7.7 (6.6-8.9) |
| Hispanic | 136 (4.5) | 6.7 (4.8-8.6) |
| White, non-Hispanic | 2191 (72.4) | 79.8 (77.2-82.4) |
| Other non-Hispanic | 68 (2.3) | 3.3 (2.1-4.5) |
| Education | ||
| Less than high school graduate | 457 (15.1) | 12.3 (10.6-14.0) |
| High school graduate | 766 (25.3) | 23.8 (21.2-26.3) |
| Some college, no degree | 660 (21.8) | 22.7 (20.6-24.8) |
| College graduate or more | 1111 (36.7) | 39.2 (35.7-42.7) |
| Income, $ | ||
| 0 to <21 000 | 743 (24.6) | 19.9 (17.9-22.0) |
| 21 000 to <40 000 | 711 (23.5) | 22.0 (19.7-24.3) |
| 40 000 to <75 000 | 764 (25.3) | 27.2 (24.8-29.6) |
| ≥75 000 | 807 (26.6) | 30.9 (27.1-34.7) |
Race and ethnicity data are self-reported by respondents upon enrollment. Multiple categories are allowed, and respondents are asked to select a primary race in these cases. The other non-Hispanic category includes reported races and ethnicities that are not Black, Hispanic, or White.
Figure. Prevalence of Vision Impairment (VI) by Age Group.
The prevalence of distance VI in US adults 71 years and older was 10.3% (95% CI, 8.9%-11.7%), corresponding to 2 934 788 individuals (95% CI, 2 495 659-3 373 917). The mean (SD) distance visual acuity was 0.13 logMAR (0.005) (Snellen equivalent approximately 20/27). The prevalence of distance VI was associated with older age (4.2% [95% CI, 2.3%-6.1%] among those aged 71-74 years, 8.4% [95% CI, 6.5%-10.3%] 75-79 years, 12.1% [95% CI, 9.3%-14.9%] 80-84 years, and 21.8% [95% CI, 17.6%-26.1%] ≥85 years; P < .001), lower education (18.2% [95% CI, 13.7%-22.6%] less than high school graduate, 10.9% [95% CI, 8.2%-13.6%] high school graduate, 9.0% [95% CI, 6.6%-11.5%] some college, and 8.5% [95% CI, 6.4%-10.6%] college degree; P < .001), and lower income (16.0% [95% CI, 13.2%-18.8%] 0 to <$21 000, 11.1% [95% CI, 8.6%-13.7%] $21 000 to <$40 000, 8.5% [95% CI, 6.2%-10.8%] $40 000 to <$75 000, and 7.5% [95% CI, 5.1%-9.8%] ≥$75 000; P < .001), but not race and ethnicity (16.5% [95% CI, 13.0%-20.0%] among Black non-Hispanic respondents, 12.0% [95% CI, 5.5%-18.5%] among Hispanic respondents, 9.6% [95% CI, 8.1%-11.5%] among White non-Hispanic respondents, and 13.2% [95% CI, 3.5%-22.8%] among other non-Hispanic respondents; P = .10) or sex (10.3% [95% CI, 8.2%-12.4%] among male respondents and 10.2% [95% CI, 8.2%-12.3%] among female respondents; P = .95).
Table 2 also presents the prevalence of different severities of distance VI impairment. Overall, the prevalence of mild, moderate, and severe VI or blind was 5.5% (95% CI, 4.5%-6.5%), 3.9% (95% CI, 3.1%-4.7%), and 0.9% (95% CI, 0.5%-1.3%), respectively.
Table 2. Mean Distance Visual Acuity and Prevalence of Impairment Across Weighted Sample Characteristics.
| logMAR (SD) | Prevalence of distance visual acuity impairment, % (95% CI)a | |||||
|---|---|---|---|---|---|---|
| Any VI | P valueb | Mild VI | Moderate VI | Severe VI and blind | ||
| Overall | 0.13 (0.005) | 10.3 (8.9-11.7) | 5.5 (4.5-6.5) | 3.9 (3.1-4.7) | 0.9 (0.5-1.3) | |
| Age group, y | <.001 | |||||
| 71-74 | 0.08 (0.008) | 4.2 (2.3-6.1) | 2.6 (1.1-4.1) | 1.6 (0.5-2.7) | 0 | |
| 75-79 | 0.12 (0.007) | 8.4 (6.5-10.3) | 5.0 (3.5-6.4) | 2.7 (1.5-3.9) | 0.8 (0.1-1.5) | |
| 80-84 | 0.14 (0.009) | 12.1 (9.3-14.9) | 5.9 (3.7-8.1) | 5.1 (3.0-7.3) | 1.1 (0.2-2.0) | |
| ≥85 | 0.21 (0.012) | 21.8 (17.6-26.1) | 10.9 (8.2-13.6) | 8.8 (6.3-11.3) | 2.1 (0.8-3.5) | |
| Sex (self-reported) | .95 | |||||
| Male | 0.12 (0.007) | 10.3 (8.2-12.4) | 5.3 (3.7-7.0) | 3.8 (2.5-5.2) | 1.2 (0.5-1.8) | |
| Female | 0.13 (0.006) | 10.2 (8.2-12.3) | 5.6 (4.2-7.0) | 4.0 (2.8-5.2) | 0.6 (0.2-1.1) | |
| Race and ethnicityc | .10 | |||||
| Black, non-Hispanic | 0.16 (0.012) | 16.5 (13.0-20.0) | 10.0 (7.5-12.5) | 5.0 (2.9-7.1) | 1.5 (0.6-2.3) | |
| Hispanic | 0.13 (0.016) | 12.0 (5.5-18.5) | 7.9 (2.7-13.2) | 3.4 (0.1-6.7) | 0.7 (0.0-2.1) | |
| White, non-Hispanic | 0.12 (0.005) | 9.6 (8.1-11.5) | 4.9 (3.9-5.9) | 3.9 (2.9-4.9) | 0.9 (0.4-1.3) | |
| Other non-Hispanic | 0.18 (0.026) | 13.2 (3.5-22.8) | 8.1 (0.0-16.4) | 5.0 (0.0-11.2) | 0 | |
| Education | <.001 | |||||
| Less than high school graduate | 0.20 (0.013) | 18.2 (13.7-22.6) | 7.7 (5.2-10.3) | 8.8 (5.5-12.0) | 1.7 (0.0-3.6) | |
| High school graduate | 0.13 (0.010) | 10.9 (8.2-13.6) | 6.1 (4.0-8.1) | 3.9 (2.3-5.6) | 0.9 (0.2-1.6) | |
| Some college, no degree | 0.11 (0.009) | 9.0 (6.6-11.5) | 5.8 (4.0-7.7) | 2.3 (1.3-3.4) | 0.8 (0.0-1.7) | |
| College graduate or more | 0.11 (0.007) | 8.5 (6.4-10.6) | 4.5 (3.0-6.1) | 3.4 (2.1-4.6) | 0.6 (0.1-1.1) | |
| Income, $d | <.001 | |||||
| 0 to <21 000 | 0.18 (0.009) | 16.0 (13.2-18.8) | 6.4 (4.6-8.2) | 8.4 (6.0-10.9) | 1.2 (0.4-2.0) | |
| 21 000 to <40 000 | 0.15 (0008) | 11.1 (8.6-13.7) | 7.4 (5.4-9.3) | 2.8 (1.4-4.2) | 1.0 (0.1-2.0) | |
| 40 000 to <75 000 | 0.10 (0.008) | 8.5 (6.2-10.8) | 5.3 (3.3-7.2) | 2.5 (1.5-3.5) | 0.7 (0.0-1.4) | |
| ≥75 000 | 0.10 (0.008) | 7.5 (5.1-9.8) | 3.8 (2.1-5.5) | 3.1 (1.9-4.3) | 0.6 (0.0-1.1) | |
Abbreviation: VI, vision impairment.
Mild VI indicates a logMAR of >0.30 to <0.48; moderate VI, ≥0.48 to <1.0; severe VI, ≥1.0 to <1.3; blind, ≥1.3.
P values are based on second-order Rao-Scott χ2 tests.
Race and ethnicity data are self-reported by respondents upon enrollment. Multiple categories are allowed, and respondents are asked to select a primary race in these cases. The other non-Hispanic category includes reported races and ethnicities that are not Black, Hispanic, or White.
Quartiles are based on the income distribution of the sample.
The prevalence of near VI in US adults aged 71 years and older was 22.3% (95% CI, 20.3%-24.3%), corresponding to 6 567 879 individuals (95% CI, 5 859 301-7 276 457). The mean (SD) near visual acuity was 0.21 logMAR (0.005) (Snellen equivalent approximately 20/32). The prevalence of near visual acuity impairment was associated with older age (16.3% [95% CI, 12.3%-20.2%] among those aged 71-74 years, 18.3% [95% CI, 15.4%-21.1%] 75-79 years, 23.9% [95% CI, 20.8%-27.1%] 80-84 years, and 37.9% [95% CI, 33.7%-42.2%] ≥85 years; P < .001), lower education (37.5% [95% CI, 31.7%-43.3%] less than high school graduate, 22.7% [95% CI, 19.0%-26.4%] high school graduate, 21.3% [95% CI, 17.4%-25.2%] some college, and 17.5% [95% CI, 14.7%-20.4%] college degree [P < .001]), income (35.5% [95% CI, 30.5%-40.5%] 0 to <$21 000, 25.5% [95% CI, 21.8%-29.2%] $21 000 to <$40 000, 18.1% [95% CI, 14.9%-21.3%] $40 000 to <$75 000, and 15.1% [95% CI, 12.0%-18.2%] ≥$75 000; P < .001), and race and ethnicity (31.7% [95% CI, 25.2%-38.2%] among Black non-Hispanic respondents, 32.0% [95% CI, 21.6%-42.4%] among Hispanic respondents, 20.4% [95% CI, 18.4%-22.3%] among White non-Hispanic respondents, and 25.6% [95% CI, 17.3%-34.0%] among other non-Hispanic respondents; P = .01), but not sex (21.8% [95% CI, 19.3%-24.4%] among male respondents and 22.7% [95% CI, 19.9%-25.4%] among female respondents; P = .66). Near visual acuity data across sample characteristics are presented in Table 3.
Table 3. Mean Near Visual Acuity and Prevalence of Impairment Across Weighted Sample Characteristics.
| logMAR (SD) | Prevalence of near visual acuity impairment, % (95% CI)a | P valueb | |
|---|---|---|---|
| Overall | 0.21 (0.005) | 22.3 (20.3-24.3) | |
| Age group, y | <.001 | ||
| 71-74 | 0.17 (0.008) | 16.3 (12.3-20.2) | |
| 75-79 | 0.20 (0.008) | 18.3 (15.4-21.1) | |
| 80-84 | 0.22 (0.008) | 23.9 (20.8-27.1) | |
| ≥85 | 0.30 (0.010) | 37.9 (33.7-42.2) | |
| Sex (self-reported) | .66 | ||
| Male | 0.21 (0.008) | 21.8 (19.3-24.4) | |
| Female | 0.21 (0.006) | 22.7 (19.9-25.4) | |
| Race and ethnicityc | .01 | ||
| Black, non-Hispanic | 0.25 (0.013) | 31.7 (25.2-38.2) | |
| Hispanic | 0.25 (0.021) | 32.0 (21.6-42.4) | |
| White, non-Hispanic | 0.21 (0.005) | 20.4 (18.4-22.3) | |
| Other non-Hispanic | 0.25 (0.021) | 25.6 (17.3-34.0) | |
| Education | <.001 | ||
| Less than high school graduate | 0.29 (0.010) | 37.5 (31.7-43.3) | |
| High school graduate | 0.22 (0.010) | 22.7 (19.0-26.4) | |
| Some college, no degree | 0.22 (0.011) | 21.3 (17.4-25.2) | |
| College graduate or more | 0.18 (0.006) | 17.5 (14.7-20.4) | |
| Income, $d | <.001 | ||
| 0 to <21 000 | 0.27 (0.010) | 35.5 (30.5-40.5) | |
| 21 000 to <40 000 | 0.23 (0.009) | 25.5 (21.8-29.2) | |
| 40 000 to <75 000 | 0.19 (0.009) | 18.1 (14.9-21.3) | |
| ≥75 000 | 0.17 (0.006) | 15.1 (12.0-18.2) |
Defined as >0.30 logMAR, which is approximately >N6.
P values are based on second-order Rao-Scott χ2 tests.
Race and ethnicity data are self-reported by respondents upon enrollment. Multiple categories are allowed, and respondents are asked to select a primary race in these cases. The other non-Hispanic category includes reported races and ethnicities that are not Black, Hispanic, or White.
Quartiles are based on the income distribution of the sample.
The prevalence of contrast sensitivity impairment (defined as >1 SD below the NHATS sample mean) was 10.0% (95% CI, 8.5%-11.4%), corresponding to 2 838 575 (2 408 950-3 268 200) US adults 71 years and older. The mean (SD) contrast sensitivity was 1.69 log CS (0.007). The prevalence of contrast sensitivity impairment was associated with older age (3.7% [95% CI, 1.8%-5.6%] for 71-74 years, 8.1% [95% CI, 5.4%-10.8%] 75-79 years, 11.1% [95% CI, 8.6%-13.6%] 80-84 years, and 22.6% [95% CI, 18.9%-26.3%] ≥85 years; P < .001), lower education (20.0% [95% CI, 15.4%-24.6%] less than high school graduate, 11.2% [95% CI, 8.6%-13.8%] high school graduate, 7.9% [95% CI, 5.1%-10.7%] some college, and 7.4% [95% CI, 5.6%-9.2%] college degree; P < .001), income (19.1% [95% CI, 15.6%-22.7%] among 0 to <$21 000, 10.2% [95% CI, 7.5%-12.9%] $21 000 to <$40 000, 7.0% [95% CI, 4.8%-9.3%] $40 000 to <$75 000, and 6.4% [95% CI, 4.7%-8.2%] ≥$75 000; P < .001), and race and ethnicity (14.4% [95% CI, 11.8%-17.0%] among Black non-Hispanic respondents, 17.5% [95% CI, 9.6%-25.3%] among Hispanic respondents, 9.1% [95% CI, 7.6%-10.5%] among White non-Hispanic respondents, and 9.6% [95% CI, 0.0%-19.4%] among other non-Hispanic respondents; P = .04), but not sex (10.7% [95% CI, 8.4%-12.9%] among male respondents and 9.4% [95% CI, 7.5%-11.3%] among female respondents; P = .39). Contrast sensitivity data across sample characteristics are shown in Table 4.
Table 4. Mean Contrast Sensitivity and Prevalence of Impairment Across Weighted Sample Characteristics.
| logCS (SD) | Prevalence of contrast sensitivity impairment, % (95% CI) | |||
|---|---|---|---|---|
| <1.55 logCS | P valuea | >1 SD below meanb | ||
| Overall | 1.69 (0.007) | 21.7 (19.5-23.9) | 10.0 (8.5-11.4) | |
| Age groups, y | <.001 | |||
| 71-74 | 1.75 (0.010) | 14.1 (10.2-18.1) | 3.7 (1.8-5.6) | |
| 75-79 | 1.70 (0.012) | 17.8 (14.3-21.3) | 8.1 (5.4-10.8) | |
| 80-84 | 1.67 (0.008) | 22.9 (19.6-26.2) | 11.1 (8.6-13.6) | |
| ≥85 | 1.55 (0.013) | 40.2 (35.6-44.8) | 22.6 (18.9-26.3) | |
| Sex (self-reported) | .39 | |||
| Male | 1.68 (0.009) | 21.2 (18.2-24.3) | 10.7 (8.4-12.9) | |
| Female | 1.69 (0.009) | 22.1 (19.0-25.2) | 9.4 (7.5-11.3) | |
| Race and ethnicityc | .04 | |||
| Black, non-Hispanic | 1.64 (0.013) | 28.1 (23.8-32.3) | 14.4 (11.8-17.0) | |
| Hispanic | 1.62 (0.027) | 31.3 (22.0-40.5) | 17.5 (9.6-25.3) | |
| White, non-Hispanic | 1.70 (0.006) | 20.0 (17.8-22.2) | 9.1 (7.6-10.5) | |
| Other non-Hispanic | 1.61 (0.039) | 30.1 (16.5-43.8) | 9.6 (0.0-19.4) | |
| Education | <.001 | |||
| Less than high school graduate | 1.58 (0.018) | 34.6 (29.1-40.1) | 20.0 (15.4-24.6) | |
| High school graduate | 1.67 (0.010) | 22.0 (18.2-25.7) | 11.2 (8.6-13.8) | |
| Some college, no degree | 1.71 (0.012) | 20.0 (16.1-24.0) | 7.9 (5.1-10.7) | |
| College graduate or more | 1.71 (0.009) | 18.7 (15.6-21.9) | 7.4 (5.6-9.2) | |
| Income, $d | <.001 | |||
| 0 to <21 000 | 1.58 (0.014) | 36.1 (32.1-40.1) | 19.1 (15.6-22.7) | |
| 21 000 to <40 000 | 1.66 (0.011) | 24.4 (19.8-29.0) | 10.2 (7.5-12.9) | |
| 40 000 to <75 000 | 1.73 (0.010) | 15.9 (12.7-19.1) | 7.0 (4.8-9.3) | |
| ≥75 000 | 1.73 (0.008) | 15.5 (12.3-18.7) | 6.4 (4.7-8.2) | |
Abbreviations: logCS, log contrast sensitivity; VI, vision impairment.
P values are based on second-order Rao-Scott χ2 tests.
Based on distribution of contrast sensitivity in the National Health and Aging Trends Study sample. The sample mean (SD) was 1.65 (0.27), and impairment was defined as logCS <1.37.
Race and ethnicity data are self-reported by respondents upon enrollment. Multiple categories are allowed, and respondents are asked to select a primary race in these cases. The other non-Hispanic category includes reported races and ethnicities that are not Black, Hispanic, or White.
Quartiles are based on the income distribution of the sample.
As an exploratory analysis, we constructed multivariable linear models to estimate the association of demographic and socioeconomic variables with visual function. The results of this analysis are presented in the eResults and eTable 3 in Supplement 1.
Discussion
Using data from the 2021 nationally representative NHATS, we estimated that more than one-quarter of US adults 71 years and older had a VI. At least 10% of older US adults had distance visual acuity and contrast sensitivity impairment, and more than 20% had near VI. All types of VI were associated with older age, less education, and lower income. Near VI and contrast sensitivity impairments were also associated with race and ethnicity other than non-Hispanic White.
The last study that provided objective measurements of visual function in a national US sample was NHANES from 1999 to 2008. Using 1999-2006 NHANES data, Swenor et al19 reported an increasing prevalence of distance VI with age, from 3.4% at 70 to 79 years to 15.9% at 80 years and older. In the current study, the prevalence of distance VI was somewhat higher, increasing from 6.4% at 71 to 79 years to 16.7% at 80 years and older (eTable 2 in Supplement 1). This could be due to differences in the visual acuity tests in NHANES and NHATS. In NHANES, an autorefractor containing built-in visual acuity charts was used to measure best-corrected visual acuity,1 while NHATS used tablet-based measures of presenting visual acuity that had strong agreement with clinical gold standard tests.14 In a recent meta-analysis integrating NHANES and self-reported data from various sources, Flaxman et al11 estimated the prevalence of best-corrected distance VI in adults 85 years and older was 20.7%, very close to the 21.8% estimate from NHATS. We build on prior studies by providing contemporary estimates of 3 types of VI in a large nationally representative sample of US adults 71 years and older.
We found differences in the prevalence of VI based on socioeconomic and demographic characteristics. The prevalence of both near visual acuity and contrast sensitivity impairment was greater among non-Hispanic Black and Hispanic individuals compared with non-Hispanic White individuals. Additionally, lower education and income were associated with all types of VI. When socioeconomic and demographic characteristics were incorporated into multivariable linear regression models, all measures of visual function were worse with increasing age, lower education, and lower income; additionally, male sex was associated with worse contrast sensitivity. However, race and ethnicity were not significantly associated with any measure of visual function. This suggests that the observed differences between racial and ethnic groups may be driven by socioeconomic factors like education and income. Because the NHATS sample was last replenished in 2015, it may also be underpowered to detect differences between racial and ethnic groups in the multivariable model, an issue that may be resolved with ongoing sample replenishment and oversampling of Black and Hispanic respondents. These findings build on the emerging work that has been published on social determinants of vision health1,20,21 and substantiate the need for additional research to disentangle individual- and community-level factors that shape vision health outcomes and access to eye care.
Of note, most near VI can be treated with reading glasses, which are generally inexpensive and widely available at pharmacies, grocery stores, and discount stores in the United States. Similarly, a large fraction of distance VI is due to uncorrected or undercorrected refractive error and could also be treated with glasses.1,2,22,23 However, Medicare, the dominant insurer of older adults in the United States, does not provide an eyeglass benefit except after cataract surgery.24 Although NHATS does not contain data to estimate the proportion of VI that could be corrected with eyeglasses, existing data suggest that a majority of individuals with distance visual acuity impairment in the United States might achieve good vision with a pair of eyeglasses.1
Because NHATS contains data on adults who transitioned to institutional settings since their baseline interview, oversamples ages 85 years and older, and is focused on the health of older adults, it is a rich source of data to study these groups that are at high risk of VI and for whom there are few existing data. Understanding the epidemiology of VI and blindness in this population is critical because adults 85 years and older are the fastest growing age group in the United States5 and may also be at high risk for such downstream sequelae of VI as injurious falls,25 depression,6 cognitive decline,26 and early mortality.9 We found that the prevalence of all 3 measures of VI increased sharply among individuals 85 years and older, among whom the prevalence of distance VI and contrast sensitivity impairment were more than 20% and near VI was greater than 37%. The complex interplay between visual function and brain health may influence these results, especially among the oldest participants. While the influence of vision on brain health appears to be stronger than the influence of brain health on vision,27 there is evidence that brain health (eg, cognitive decline) may influence vision testing and/or visual function.28 Given the high prevalence of VI in older adults, designing interventions to maximize visual function in this population is critical. Future studies may evaluate optimizing vision with interventions like eyeglasses and cataract surgery to prevent or slow the onset of nonocular sequelae of poor vision, such as injurious falls and cognitive decline.
Strengths and Limitations
Strengths of this study include the use of a nationally representative data set providing the first objectively measured population data on VI and blindness among older US adults since 2008. Objective visual function tests are the gold standard for estimating VI. The comparatively large sample size of adults 85 years and older in NHATS allows for estimates of VI in this fast-growing segment of the population. This study is limited by use of cross-sectional data; as such, the incidence of VI and blindness cannot be estimated, nor can vision trends over time. Only presenting visual acuity (as opposed to best-corrected) was measured. Missing data were more common among respondents who were older, female, members of racial and ethnic groups other than non-Hispanic White, and less educated and those who had lower incomes. This may bias our prevalence estimates downward because some of these characteristics were associated with a higher prevalence of VI. Additionally, 2021 NHATS did not include people younger than 71 years, so the prevalence of VI and blindness cannot be estimated for younger adults. However, NHATS will undergo sample replenishment in 2022 and will then provide visual function data on a larger cohort of adults 65 years and older with oversampling of Black individuals, members of the oldest age groups, and, for the first time, Hispanic individuals.
Conclusions
This study found more than one-quarter of US adults 71 years and older were visually impaired. Visual impairment is more prevalent among those who are older, Hispanic, non-White, and less educated and have lower incomes. The up-to-date data presented in this study are vital for informing surveillance of vision health in the United States and may enable public health programs to target individuals at highest risk of poor vision.
eTable 1. Comparison of unweighted sample characteristics of analytic sample and excluded participants
eTable 2. Weighted prevalence of vision impairment for ages 71-79 and ages 80+
eTable 3. Multivariable linear regression models predicting distance and near visual acuity and contrast sensitivity as a function of socioeconomic and demographic characteristics
eResults
Data sharing statement
References
- 1.Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the United States. JAMA. 2006;295(18):2158-2163. doi: 10.1001/jama.295.18.2158 [DOI] [PubMed] [Google Scholar]
- 2.Bourne R, Steinmetz JD, Flaxman S, et al. ; GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study . Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e130-e143. doi: 10.1016/S2214-109X(20)30425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varma R, Vajaranant TS, Burkemper B, et al. Visual impairment and blindness in adults in the United States: demographic and geographic variations from 2015 to 2050. JAMA Ophthalmol. 2016;134(7):802-809. doi: 10.1001/jamaophthalmol.2016.1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . Vision and Eye Health Surveillance System. Published June 17, 2021. Accessed July 15, 2022. https://www.cdc.gov/visionhealth/vehss/index.html
- 5.Population Reference Bureau. Mather M, Jacobsen LA, Ard KMP. Population Bulletin. Accessed December 2, 2023. http://www.prb.org
- 6.Killeen OJ, Xiang X, Powell D, et al. Longitudinal associations of self-reported visual, hearing, and dual sensory difficulties with symptoms of depression among older adults in the United States. Front Neurosci. 2022;16:786244. doi: 10.3389/fnins.2022.786244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers MAM, Langa KM. Untreated poor vision: a contributing factor to late-life dementia. Am J Epidemiol. 2010;171(6):728-735. doi: 10.1093/aje/kwp453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piyasena P, Olvera-Herrera VO, Chan VF, et al. Vision impairment and traffic safety outcomes in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Glob Health. 2021;9(10):e1411-e1422. doi: 10.1016/S2214-109X(21)00303-X [DOI] [PubMed] [Google Scholar]
- 9.Ehrlich JR, Ramke J, Macleod D, et al. Association between vision impairment and mortality: a systematic review and meta-analysis. Lancet Glob Health. 2021;9(4):e418-e430. doi: 10.1016/S2214-109X(20)30549-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rein DB, Wittenborn JS, Zhang P, et al. The economic burden of vision loss and blindness in the United States. Ophthalmology. 2022;129(4):369-378. doi: 10.1016/j.ophtha.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 11.Flaxman AD, Wittenborn JS, Robalik T, et al. ; Vision and Eye Health Surveillance System study group . Prevalence of visual acuity loss or blindness in the US: a bayesian meta-analysis. JAMA Ophthalmol. 2021;139(7):717-723. doi: 10.1001/jamaophthalmol.2021.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, National Center for Health Statistics . National Health and Nutrition Examination Survey (NHANES) home page. Published June 23, 2022. Accessed July 15, 2022. https://www.cdc.gov/nchs/nhanes/index.htm
- 13.Hu M, Freedman VA, Ehrlich JR, Reed NS, Billington C, Kasper JD. Collecting objective measures of visual and auditory function in a national in-home survey of older adults. J Surv Stat Methodol. 2021;9(2):309-334. doi: 10.1093/jssam/smaa044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varadaraj V, Assi L, Gajwani P, et al. Evaluation of tablet-based tests of visual acuity and contrast sensitivity in older adults. Ophthalmic Epidemiol. 2021;28(4):293-300. doi: 10.1080/09286586.2020.1846758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Health and Aging Trends Study (NHATS) . Accessed August 1, 2022. https://www.nhats.org/researcher/nhats
- 16.ICD-11 for Mortality and Morbidity Statistics . 9D90 Vision impairment including blindness. Accessed August 1, 2022. https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1103667651
- 17.Varadaraj V, Munoz B, Simonsick EM, Swenor BK. Vision impairment and participation in cognitively stimulating activities: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2021;76(5):835-841. doi: 10.1093/gerona/glaa184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mäntyjärvi M, Laitinen T. Normal values for the Pelli-Robson contrast sensitivity test. J Cataract Refract Surg. 2001;27(2):261-266. doi: 10.1016/S0886-3350(00)00562-9 [DOI] [PubMed] [Google Scholar]
- 19.Swenor BK, Ramulu PY, Willis JR, Friedman D, Lin FR. The prevalence of concurrent hearing and vision impairment in the United States. JAMA Intern Med. 2013;173(4):312-313. doi: 10.1001/jamainternmed.2013.1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on Public Health Approaches to Reduce Vision Impairment and Promote Eye Health ; Welp A, Woodbury RB, McCoy MA, Teutsch SM, eds. Making Eye Health a Population Health Imperative: Vision for Tomorrow. National Academies Press; 2016. https://www.ncbi.nlm.nih.gov/books/NBK385157/ [PubMed] [Google Scholar]
- 21.Besagar S, Yonekawa Y, Sridhar J, et al. Association of socioeconomic, demographic, and health care access disparities with severe visual impairment in the US. JAMA Ophthalmol. Published online November 3, 2022. doi: 10.1001/jamaophthalmol.2022.4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanNewkirk MR, Weih L, McCarty CA, Taylor HR. Cause-specific prevalence of bilateral visual impairment in Victoria, Australia: the Visual Impairment Project. Ophthalmology. 2001;108(5):960-967. doi: 10.1016/S0161-6420(01)00554-1 [DOI] [PubMed] [Google Scholar]
- 23.Muñoz B, West SK, Rodriguez J, et al. Blindness, visual impairment and the problem of uncorrected refractive error in a Mexican-American population: Proyecto VER. Invest Ophthalmol Vis Sci. 2002;43(3):608-614. [PubMed] [Google Scholar]
- 24.Medicare.gov . Eyeglasses coverage. Accessed August 11, 2022. https://www.medicare.gov/coverage/eyeglasses-contact-lenses
- 25.Ehrlich JR, Hassan SE, Stagg BC. Prevalence of falls and fall-related outcomes in older adults with self-reported vision impairment. J Am Geriatr Soc. 2019;67(2):239-245. doi: 10.1111/jgs.15628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varadaraj V, Munoz B, Deal JA, et al. Association of vision impairment with cognitive decline across multiple domains in older adults. JAMA Netw Open. 2021;4(7):e2117416. doi: 10.1001/jamanetworkopen.2021.17416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng DD, Swenor BK, Christ SL, West SK, Lam BL, Lee DJ. Longitudinal associations between visual impairment and cognitive functioning: the Salisbury Eye Evaluation Study. JAMA Ophthalmol. 2018;136(9):989-995. doi: 10.1001/jamaophthalmol.2018.2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vu TA, Fenwick EK, Gan ATL, et al. The bidirectional relationship between vision and cognition: a systematic review and meta-analysis. Ophthalmology. 2021;128(7):981-992. doi: 10.1016/j.ophtha.2020.12.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Comparison of unweighted sample characteristics of analytic sample and excluded participants
eTable 2. Weighted prevalence of vision impairment for ages 71-79 and ages 80+
eTable 3. Multivariable linear regression models predicting distance and near visual acuity and contrast sensitivity as a function of socioeconomic and demographic characteristics
eResults
Data sharing statement