Key Points
Question
What is the association between mid-life carotid atherosclerosis and late-life hearing loss?
Findings
This cross-sectional analysis of data from 3594 participants in the Atherosclerosis Risk in Communities study found that an additional 0.1 mm higher mean carotid intima-media thickness (cIMT) was associated with 0.59 decibels higher 4-frequency pure tone average (PTA). Presence of carotid plaque was also associated with higher 4-frequency PTA; however, the imprecision of the estimate precludes making definitive conclusions.
Meaning
The findings of this cross-sectional analysis suggest that carotid atherosclerosis in midlife—measured as the mean cIMT during 9 years—was associated with worse hearing later in life; prevention and control during middle age may positively affect the hearing health of older adults.
Abstract
Objective
To describe the association between midlife carotid atherosclerosis and late-life hearing loss among participants in the Atherosclerosis Risk in Communities (ARIC) study.
Design, Setting, and Participants
For this cross-sectional study and temporal analysis of a cohort within the ongoing ARIC prospective cohort study, participants were recruited from 4 communities in the US. The analysis evaluated information on mean carotid intima-media thickness (cIMT), from visit 1 (1987-1989) to visit 4 (1994-1996), carotid plaque presence at visit 4, and audiometric data from visit 6 (2016-2017). The cIMT measures were calculated from ultrasonography recordings by trained readers at the ARIC Ultrasound Reading Center. At each visit, cIMT was computed as the average of 3 segments: the distal common carotid, the carotid artery bifurcation, and the proximal internal carotid arteries. Presence of carotid plaque was determined based on an abnormal wall thickness, shape, or wall texture. Audiometric 4-frequency pure tone average (PTA) was measured and calculated for the better-hearing ear and modeled as a continuous variable. Linear regression estimated the association between cIMT and carotid plaque with hearing, adjusting for age, sex, race and study center, education level, body mass index (calculated as weight in kilograms divided by height in meters squared), smoking status, hypertension, cholesterol levels, diabetes, and exposure to occupational noise. Missing data (exposure and covariates) were imputed with multiple imputation by chained equations. Data analyses were performed from April 6 to July 13, 2022.
Main Outcomes and Measures
Hearing loss assessed using 4-frequency (0.5, 1.0, 2.0, and 4.0 kilohertz) PTA for both ears and carotid plaque at visit 4 and mean cIMT from visit 1 to visit 4.
Results
Among a total of 3594 participants (mean [SD] age at visit 4, 59.4 [4.6] years; 2146 [59.7%] female; 819 [22.8%] Black and 2775 [77.2%] White individuals), fully adjusted models indicated that an additional 0.1 mm higher mean cIMT was associated with 0.59 dB (95% CI, 0.17 to 1.02 dB) higher PTA. Compared with participants without carotid plaque, plaque presence was associated with 0.63 dB (95% CI, −0.57 to 1.84 dB) higher PTA.
Conclusion and Relevance
The findings of this cross-sectional study with temporal analyses of a cohort with the ongoing ARIC study found that subclinical atherosclerosis in midlife was associated with worse hearing in older adulthood. Prevention and control of carotid atherosclerosis during middle age may positively affect the hearing health of older adults.
This cross-sectional analysis of an ongoing prospective cohort study describes the association between midlife carotid atherosclerosis and late-life hearing loss in the Atherosclerosis Risk in Communities study.
Introduction
The prevalence of hearing loss is expected to increase with our aging population.1 Hearing loss causes both reduced hearing sensitivity and impaired speech understanding, and has been associated with depression,2 social isolation,3 and even dementia.4,5 Therefore, strategies to prevent or delay the onset or progression of hearing loss are highly warranted.
Age-related hearing loss has been attributed to the degeneration of the cochlear sensory-neural structures and the stria vascularis. Given the high amount of vascularization of these parts of the inner ear, some epidemiologic studies have found cardiovascular risk factors, including coronary heart disease, stroke, and high triglyceride levels, to be associated with higher prevalence of hearing loss and worse hearing thresholds.6,7,8,9 However, to date, few population-based studies have assessed the association between carotid atherosclerosis (measured by intima-media thickness [IMT] and plaque score) and hearing loss. Two of these studies, 1 from the US10 and 1 from Germany,11 found that IMT was associated with a higher incidence and prevalence of hearing loss measured via audiometric data and self-report. Also, a study from the Netherlands12 found that atherosclerosis (IMT and number of carotid plaques) was associated with hearing loss; however, in this particular study, the association was evident almost exclusively with hearing loss in the right ear.
To our knowledge, the present study is among the first studies to report differences between right and left ear in hearing thresholds associated with atherosclerosis; as a result, replication in other populations is warranted. We aimed to estimate the association between midlife carotid atherosclerosis measured with hearing loss later in life in the community-based Atherosclerosis Risk in Communities (ARIC) study. We hypothesized that carotid atherosclerosis (mean carotid IMT [cIMT] and plaque presence) is associated with overall higher pure tone audiometric thresholds, and that owing to potential right ear blood flow disadvantages, as observed in animal models,13 this association may differ by ear side. We also investigated if the association between carotid atherosclerosis and hearing thresholds varied for different hearing frequencies. Moreover, because prevalence of hearing loss varies between females and males, we also investigated whether any association differed by sex.
Methods
This cross-sectional study with temporal analyses was reviewed and approved by the institutional review board of each participating center in 4 US communities (Washington county, Maryland; Forsyth county, North Carolina; Jackson, Mississippi; and Minneapolis, Minnesota). Written informed consent was obtained from all participants at each visit, and the study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study Population
The ARIC study is an ongoing population-based study with 15 792 participants. The study has been conducted in the 4 US communities since 1987. Study participants were seen at follow-up visits every 3 years from visit 1 (1987-1989) to visit 4 (1996-1998).14 Hearing was measured at visit 6 in 2016 or 2017.
Measures for atherosclerosis were assessed during clinic visits 1 through 4, and audiometric hearing assessment was offered to all participants at clinic visit 6. The analytic sample included only participants who attended visit 6 and had complete audiometric data (3628 participants). We excluded participants who did not have complete data for cIMT and/or plaque for visit 1 through 4 (27 individuals); and, owing to small numbers, those who self-reported being of a race other than Black or White or who reported being Black and from Washington County or Minneapolis (7 individuals).
Hearing Measures
Hearing loss was assessed using pure tone air conduction audiometry; a detailed description of the study protocol can be found elsewhere.15 A 4-frequency (0.5, 1.0, 2.0, and 4.0 kilohertz [kHz]) pure tone average (PTA) was calculated for each ear, with higher scores indicating worse hearing. The study analysis used the PTA for the left and right ears and for the better-hearing ear. We defined high (4.0 and 8.0 kHz), medium (1.0 and 2.0 kHz), and low (0.25 and 0.50 kHz) frequency PTAs, and also calculated these for the better-hearing ear, and for the left and right ears. All PTAs were modeled as continuous variables.
Carotid Atherosclerosis Measures
To approximate carotid atherosclerosis, we used measures of the presence of carotid plaque and cIMT from visit 1 to visit 4 using ultrasonography images recorded on a videotape. The cIMT was measured centrally by trained readers at the ARIC Ultrasound Reading Center and was assessed in 3 segments: the distal common carotid, the carotid artery bifurcation, and the proximal internal carotid arteries; measurements of the far wall were attempted at each of these segments. At each visit, cIMT was computed as an imputed mean of the measurements across these 3 segments of both the right and left sides, where any missing measures were imputed from the nonmissing measures.16 Mean cIMT (scaled to 0.1 mm units) was computed as the mean of all available cIMT measures from visit 1 to visit 4. Participants were categorized in tertiles based on the statistical distribution of the mean cIMT.
In addition, trained readers adjudicated the presence of a carotid plaque at visit 4 when 2 of the following 3 criteria were met: (1) an abnormal wall thickness defined as cIMT greater than 1.5 mm; (2) an abnormal shape, either protrusion into the lumen or loss of alignment with adjacent arterial wall boundary; and (3) an abnormal wall texture seen as brighter echoes rather than adjacent boundaries.
Additional Covariates
Baseline covariates included sex, level of education (less than high school, high school or equivalent, or greater than high school), and race−study center (these variables were modeled jointly because of the close link between race and study center). From visit 4 (the last visit at which carotid ultrasonography images were acquired), we included, age (years), body mass index (BMI; weight in kg/height in m2), smoking status (never, former, or current smoker), hypertension diagnosis (systolic blood pressure ≥140 mm Hg; diastolic blood pressure ≥90 mm Hg; or participant self-reported taking medications for lowering blood pressure), cholesterol levels (high-density lipoprotein [HDL] and low-density lipoprotein [LDL]), lipid-lowering medication use, and diabetes status (fasting blood glucose level, ≥126 mg/dL; nonfasting level, ≥200 mg/dL; participant self-reported physician-diagnosed diabetes, or self-reported diabetes medication use). Finally, exposure to occupational noise (measured as a binary variable of self-reported exposure to very loud noise at work) was measured at visit 6 along with hearing assessments.
Other variables used for the multiple imputation model were stroke prevalence, based on (1) baseline diagnostic computer algorithm reporting a stroke on the left or right carotid artery or on the vertebrobasilar system and (2) the closed event years of ARIC surveillance data; and congestive heart disease prevalence (electrocardiogram data, participant self-reported physician-diagnosed “heart attack,” coronary bypass, or coronary angioplasty). As potential mediators of the association of cIMT with hearing loss, these variables were not included in the primary analysis.
Statistical Analysis
We described differences in participant characteristics by statistical tertiles of mean cIMT. To investigate the association of cIMT and presence of carotid plaque with hearing loss (as measured by better ear PTA), we used multivariable linear regression models. The associations were evaluated using a building model approach. Model 1 explored the unadjusted association of mean cIMT and plaque presence with hearing loss. In model 2, we included demographic covariates such as age, and linear and quadratic terms to account for nonlinear age effects, sex, race and center, and level of education. Model 3 included all covariates from model 2 and adjusted for BMI, smoking, presence of hypertension, diabetes, HDL and LDL cholesterol, and use of cholesterol-lowering medication. We used a similar building model approach for the association of carotid atherosclerosis among different PTA frequencies (low, medium, and high), and for hearing loss in the right and left ears separately. Finally, because prevalence of hearing loss varies between females and males, we estimated (1) the fully adjusted model stratified by sex, and (2) the fully adjusted model including 2 interaction terms, one between sex and mean cIMT, and the other between sex and plaque presence.
Missing cIMT data for visits 1 to 4 (visit 1, n = 182; visit 2, 149; visit 3, 1481; and visit 4, 1622), presence of carotid plaque at visit 4 (n = 1580), and other missing covariate data, were imputed using MICE (multiple imputations by chained equations)17,18 with 20 sets of imputations and 20 iterations for the burn-in period. The imputation model included all covariates in the third fully adjusted model in addition to stroke and CHD prevalence. We checked for convergence of our imputations with trace plots. Main models were estimated for the imputed and the available case.
Statistical tests were 2-tailed and P values < .05 were considered statistically significant. Data analyses were performed from April 6 to July 13, 2022, using Stata/SE, version 17.0 (StataCorp).
Results
The final analytic cohort comprised 3594 participants (mean [SD] age at visit 4, 59.4 [4.6] years; 2146 [59.7%] female; 1448 [40.3%] male; 819 [22.8%] Black and 2775 [77.2%] White individuals). The range of mean cIMT was 0.46 to 0.69 mm for the first tertile, 0.69 to 0.8 mm for the second, and 0.8 to 1.46 mm for the thirds. At visit 4, when compared with participants in the first tertile (mean age, 59.1 years) and second tertile (60.7 years) of mean cIMT, participants in the highest tertile were older (62.1 years). A total of 72.9% of participants in the highest tertile of mean cIMT were female, compared with 54.7% of participants in the second, and 38.4% in the first tertiles (absolute values for all patient characteristics are available in Table 1). A higher proportion of participants in the highest (58.8%) mean cIMT tertile group had a carotid plaque compared with older adults in the lowest (12.4%) and second (26.8%) tertiles. Participants in the first (better ear 4-frequency PTA, 30.8 decibels hearing level [dB HL]) and second (33.5 dB HL) tertiles of the mean cIMT group had better hearing (lower PTA) than participants in the highest tertile group (37.4 dB HL; Table 1).
Table 1. ARIC Study Participant Characteristics at Visit 4 (1994-1996), by Tertiles of Mean cIMTa.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Total | Mean cIMT from visit 1 to visit 4 | |||
| First tertile | Second tertile | Third tertile | ||
| Participants, No. | 3594 | 1626 | 1262 | 706 |
| Range of mean cIMT, 0.1 mm | 4.6-14.6 | 4.6-6.9 | 6.9-8.0 | 8.0-14.6 |
| Audiometric data for visit 6, mean (SD), PTA | ||||
| Better ear | 33.0 (13.8) | 30.8 (13.0) | 33.5 (13.6) | 37.4 (14.5) |
| Right ear | 36.0 (15.0) | 33.9 (14.5) | 36.3 (14.8) | 40.2 (15.6) |
| Left ear | 36.3 (15.2) | 33.9 (14.5) | 36.8 (14.8) | 41.0 (16.0) |
| Low frequencyb | 24.3 (11.7) | 23.2 (10.9) | 24.5 (11.8) | 26.2 (12.9) |
| Mid frequency | 29.4 (14.9) | 27.4 (13.9) | 29.8 (15.0) | 33.6 (16.1) |
| High frequencyc | 52.3 (17.7) | 49.2 (17.6) | 53.5 (17.1) | 57.5 (17.3) |
| Occupational noise | 860.0 (24.4) | 326.0 (20.4) | 324.0 (26.3) | 210.0 (30.4) |
| Covariates and atherosclerosis data for visit 4 | ||||
| Age, mean (SD), y | 60.3 (4.6) | 59.1 (4.1) | 60.7 (4.7) | 62.1 (5.0) |
| Female sex | 2146 (59.7) | 1185 (72.9) | 690 (54.7) | 271 (38.4) |
| Male sex | 1448 (40.3) | 441 (27.1) | 572 (45.3) | 435 (61.6) |
| Black race/ethnicity | 819 (22.8) | 326 (20.0) | 339 (26.9) | 154 (21.8) |
| White race/ethnicity | 2775 (77.2) | 1300 (80.0) | 923 (73.1) | 552 (78.2) |
| Education level | ||||
| <High school | 428 (11.9) | 165 (10.2) | 161 (12.8) | 102 (14.4) |
| High school/equivalent | 1480 (41.2) | 683 (42.0) | 516 (41.0) | 281 (39.8) |
| ≥Some college | 1682 (46.9) | 777 (47.8) | 582 (46.2) | 323 (45.8) |
| BMI, mean (SD) | 28.6 (5.3) | 28.0 (5.2) | 29.2 (5.3) | 29.2 (5.1) |
| Smoking status | ||||
| Never | 1517 (43.0) | 757 (47.3) | 531 (43.0) | 229 (33.0) |
| Former | 1662 (47.1) | 672 (42.0) | 592 (47.9) | 398 (57.4) |
| Current | 350 (9.9) | 172 (10.7) | 112 (9.1) | 66 (9.5) |
| Hypertension | 1297 (37.2) | 434 (27.6) | 518 (42.4) | 345 (49.9) |
| Cholesterol | ||||
| HDL, mean (SD), mg/dL | 51.4 (16.1) | 54.5 (16.8) | 50.0 (15.4) | 46.7 (14.0) |
| LDL, mean (SD), mg/dL | 122.1 (32.6) | 118.7 (32.8) | 123.7 (31.9) | 127.0 (32.3) |
| Cholesterol lowering drug | 383 (11.2) | 116 (7.5) | 140 (11.7) | 127 (19.0) |
| Diabetes | 338 (9.9) | 120 (7.7) | 119 (9.9) | 99 (14.8) |
| cIMT for visits 1-4, mean (SD) | 7.2 (1.3) | 6.2 (0.4) | 7.4 (0.3) | 9.1 (1.1) |
| Plaque presence at visit 4 | 563 (28.0) | 104 (12.4) | 194 (26.8) | 265 (58.8) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities study; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); cIMT, carotid intima-media thickness; HDL, high-density lipoprotein; kHz, kilohertz; LDL, low-density lipoprotein; PTA, pure tone average.
Missing covariates imputed in the analyses: education level, n = 4; BMI, 169; smoking status, 65; hypertension, 110; HDL cholesterol, 186; LDL cholesterol, 229; cholesterol lowering medication, 171; occupational noise, 70; diabetes, 172; and plaque presence, n = 1580. The summary statistics for the covariates correspond to the available case.
Incomplete low frequencies data (0.25 and 0.50 kHz), 4 participants.
Incomplete high frequencies data (4.0 and 8.0 kHz), 351 participants.
In the imputed model (Table 2), an additional 0.1 mm mean cIMT was associated with poorer hearing in the better ear’s 4-frequency PTA by 1.97 dB HL (95% CI, 1.55 to 2.40). After full adjustment, the estimated association was attenuated (0.59 dB HL per 0.1 mm; 95% CI, 0.17 to 1.02). Additionally, we found that carotid plaque presence was associated with 0.63 dB HL (95% CI, −0.57 to 1.84) higher PTA; however, the width of the confidence interval precluded making definitive conclusions regarding the magnitude of the association. When stratifying by ear, in the imputed fully adjusted models for the left ear, an additional 0.1 mm mean cIMT was associated with 0.76 dB HL (95% CI, 0.31 to 1.25) higher PTA. For the right ear, the estimated association was 0.42 dB HL (95% CI, −0.06 to 0.90). More details are available in Table 3 and eTables 1 and 2 in Supplement 1.
Table 2. Linear Regression Estimates for the Association Between Carotid Atherosclerosis and Better Ear 4-Frequency PTA Threshold Among ARIC Study Participants (n = 3594)a.
| Characteristic | β (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Mean cIMT, 0.1 mm | 1.97 (1.55 to 2.40) | 0.67 (0.26 to 1.08) | 0.59 (0.17 to 1.02) |
| Plaque presence | 1.65 (0.35 to 2.94) | 0.54 (−0.67 to 1.75) | 0.63 (−0.57 to 1.84) |
| Female sex | NA | −5.57 (−6.45 to −4.69) | −4.19 (−5.21 to −3.17) |
| Education level | |||
| <High school | NA | 0 [Reference] | 0 [Reference] |
| High school/equivalent | NA | −2.68 (−4.05 to −1.30) | −2.28 (−3.65 to −0.91) |
| ≥Some college | NA | −4.72 (−6.08 to −3.35) | −3.83 (−5.21 to −2.45) |
| BMI | NA | NA | 0.05 (−0.04 to 0.14) |
| Smoking status | |||
| Never | NA | NA | 0 [Reference] |
| Former | NA | NA | 0.45 (−0.42 to 1.33) |
| Current | NA | NA | −0.31 (−1.77 to 1.15) |
| Hypertension | NA | NA | −0.13 (−1.05 to 0.78) |
| Cholesterol | |||
| HDL cholesterol (mg/dL) | NA | NA | −0.01 (−0.04 to 0.02) |
| LDL cholesterol (mg/dL) | NA | NA | −0.01 (−0.02 to 0.01) |
| Cholesterol lowering drug | NA | NA | −0.19 (−1.52 to 1.14) |
| Diabetes | NA | NA | 0.22 (−1.18 to 1.61) |
| Occupational noise | NA | NA | 3.83 (2.81 to 4.85) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities study; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); cIMT, carotid intima-media thickness; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NA, not applicable; PTA, pure tone average.
Per the building model approach, model 1 shows the estimates from the unadjusted model. Model 2 was adjusted for age,2 sex, race and center, and education level. Model 3 includes all covariates from model 2 and was also adjusted for BMI, smoking status, presence of hypertension, diabetes, HDL and LDL cholesterol, and use of cholesterol-lowering medication. Missing covariates imputed in analyses: education, n = 4; BMI, 169; smoking status, 65; hypertension, 110; HDL cholesterol, 186; LDL cholesterol, 229; cholesterol lowering medication, 171; occupational noise, 70; diabetes, 172; plaque presence, 1580.
Table 3. Linear Regression Estimates for the Association Between Carotid Atherosclerosis and Better Ear 4-Frequency PTA Threshold Among ARIC Study Participants (n = 3594), Stratified by Right and Left Earsa.
| Panel | β (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Right ear | |||
| Mean cIMT, 0.1 mm | 1.87 (1.41 to 2.33) | 0.50 (0.04 to 0.97) | 0.42 (−0.06 to 0.90) |
| Plaque presence | 2.04 (0.71 to 3.37) | 0.91 (−0.50 to 2.31) | 1.04 (−0.41 to 2.50) |
| Left ear | |||
| Mean cIMT, 0.1 mm | 2.20 (1.75 to 2.66) | 0.82 (0.36 to 1.28) | 0.76 (0.28 to 1.25) |
| Plaque presence | 1.90 (0.69 to 3.12) | 0.61 (−0.61 to 1.83) | 0.70 (−0.54 to 1.95) |
Abbreviation: ARIC, Atherosclerosis Risk in Communities study; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); cIMT, carotid intima-media thickness; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PTA, pure tone average.
Per the building model approach, model 1 shows the estimates from the unadjusted model. Model 2 was adjusted for age,2 sex, race and center, and education level. Model 3 includes all covariates from model 2 and was also adjusted for BMI, smoking status, presence of hypertension, diabetes, HDL and LDL cholesterol, and use of cholesterol lowering medication. Missing covariates imputed in analyses: education, n = 4; BMI, 169; smoking status, 65; hypertension, 110; HDL cholesterol, 186; LDL cholesterol, 229; cholesterol lowering medication, 171; occupational noise, 70; diabetes, 172; and plaque presence, 1580.
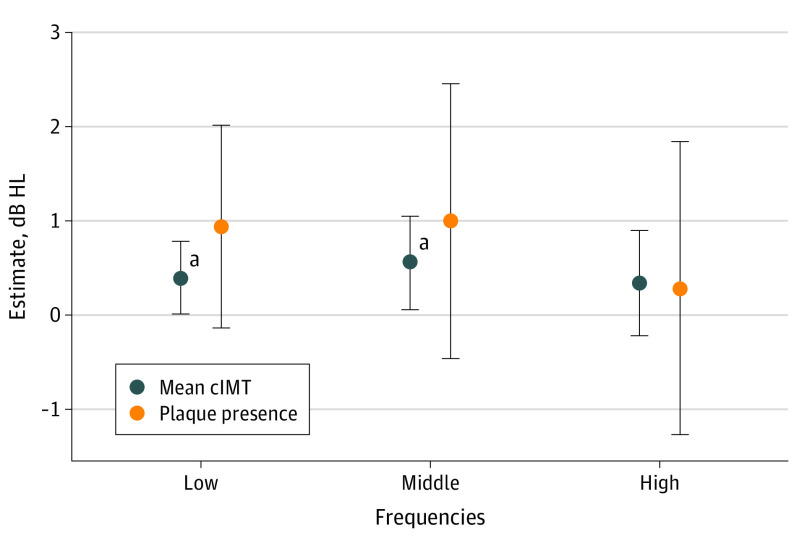
With respect to high (4.0 and 8.0 kHz), middle (1.0 and 2.0 kHz), and low (0.25 and 0.50 kHz) frequency PTAs, in the imputed model we found that cIMT was associated with higher PTA thresholds for the low and middle frequencies. In fully adjusted models, we estimated that a 0.1 mm higher mean cIMT was associated with 0.39 (95% CI, 0.01 to 0.78) dB HL higher PTA for low, 0.56 (95% CI, 0.06 to 1.05) dB HL higher PTA for the middle, and 0.34 (95% CI, −0.22 to 0.90) dB HL higher PTA for the high frequencies. We also found that plaque presence was associated with 0.94 (95% CI, −0.14 to 2.01) dB HL higher PTA for the high-frequency PTA; 1 (95% CI, −0.46 to 2.46) dB HL for the middle frequency; and 0.28 (−1.27 to 1.84) dB HL for the low frequecy (Figure and eTable 3 in Supplement 1).
Figure. Linear Regression Estimates for the Association Between Atherosclerosis at Visit 4 (1997-1999) and Hearing PTA in the Better Ear at Visit 6 (2016-2017) Among ARIC Study Participants.
Coefficients correspond to the imputed fully adjusted model. Hearing levels defined as low-frequency (0.25 and 0.5 kHz; n = 3590), mid-frequency (1.0 and 2.0 kHz; n = 3594), and high-frequency (4.0 and 8.0 kHz; n = 3243) PTA. ARIC refers to the Atherosclerosis Risk in Communities study; cIMT, carotid intima media thickness; db HL, decibel hearing level; kHz, kilohertz; and PTA, pure tone average.
aNull hypothesis not within the 95% CI.
In analyses examining potential variations in the association between carotid atherosclerosis and hearing by sex, we found no evidence of a statistically significant difference between estimates. In both the stratified analysis and the analysis including interaction terms, the confidence intervals for cIMT and plaque presence between males and females overlapped (Table 4; eTable 4 in Supplement 1).
Table 4. Linear Regression Estimates for the Association Between Carotid Atherosclerosis and Better Ear 4-Frequency PTA Threshold Among ARIC Study Participants, by Sexa.
| Panel | β (95% CI) | |
|---|---|---|
| Female | Male | |
| Carotid atherosclerosis and PTA | ||
| Participants, No. | 2146 | 1448 |
| Mean cIMT, 0.1 mm | 0.51 (−0.06 to 1.08) | 0.67 (0.03 to 1.31) |
| Plaque presence | 0.99 (−0.72 to 2.71) | 0.09 (−1.72 to 1.90) |
| Carotid atherosclerosis and PTA with interaction terms between mean cIMT and plaque presence and female sex (n = 3594) | ||
| Mean cIMT | 0.62 (0.05 to 1.19) | 0.57 (−0.01 to 1.15) |
| Plaque presence | 0.70 (−0.99 to 2.39) | 0.54 (−1.21 to 2.30) |
Abbreviations: ARIC, Atherosclerosis Risk in Communities study; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); cIMT, carotid intima-media thickness; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PTA, pure tone average.
All models were adjusted for age,2 sex, race and center, and education level, BMI, smoking status, presence of hypertension, diabetes, HDL and LDL cholesterol, and use of cholesterol lowering medication. Missing covariates imputed in analyses: education, 4; BMI, 169; smoking status, 65; hypertension, 110; HDL cholesterol, 186; LDL cholesterol, 229; cholesterol lowering medication, 171; occupational noise, 70; diabetes, 172; plaque presence, 1580.
Discussion
In this analytic cohort of 3594 participants, subclinical carotid atherosclerosis (1994-1996) was associated with greater audiometric hearing loss measured 22 years later (2016-2017). Specifically, we found that cIMT was associated with poorer hearing (higher hearing thresholds). In contrast, we found a 0.63 dB HL (95% CI, −0.57 to 1.84) difference in PTA between individuals with and without carotid plaque presence. Given the width of the confidence interval, no definitive conclusion can be made regarding the magnitude of the relationship. However, inspection of the upper bound suggests a clinically meaningful association not compatible with our data.
We recognize that our estimates suggest a modest association between cIMT and hearing loss (0.59 dB HL per 0.1 mm). At a clinical level, a 5 or 10 dB change in a single frequency is considered clinically significant,19,20 and although there are broad categories used by the World Health Organization (normal, mild, moderate, severe),21 perceived difficulty from hearing loss may vary from person to person within a single category. Moreover, we recognize that PTAs alone are not a perfect correlate to hearing health-related quality of life because hearing is a multifactorial process that depends not only on auditory function, but also on its interaction with other processes such as cognition. Therefore, instead of focusing on clinical significance, it may be more appropriate to focus on the direction of the observed association. In addition, the cumulative differences experienced by an individual may have important implications for hearing health at the population level. Moreover, the estimated PTA difference associated with a 0.1 mm increase in cIMT is equivalent to the hearing loss experienced by an average older adult in a period of 2 years in the low frequencies.22
Our main findings are consistent with those from previous work. In the Study of Health in Pomerania,11 among a sample of 2619 German individuals (mean age, 61.3 years; 49.2% female), researchers found that IMT was associated with higher odds of self-reported hearing loss (odds ratio, 1.80 per 1 mm; 95% CI, 1.01-3.19) after adjusting for age, sex, education, income, smoking status, waist circumference, diabetes, and noise exposure at home and at work.
Similarly, among 1984 participants (age range, 21-79 years at baseline; 57.4% female) of the Beaver Dam Offspring Study10 in the US, researchers found that IMT was associated with a higher 5-year incidence of hearing impairment (PTA, ≥25 dB HL) in models adjusting for demographic information, exercise, cardiovascular disease, hypertension, diabetes, cholesterol, BMI, alcohol consumption, and noise exposure at work (relative risk, 1.12; 95% CI, 1.03-1.23). These researchers also found, in fully adjusted models, that plaque presence per site was modestly associated with incidence of hearing loss (relative risk, 1.14; 95% CI, 0.98-1.32).
In the Rotterdam Study12 from the Netherlands, the authors found that among 3724 participants (mean age, 65.5 years; 55.4% female) atherosclerosis was associated with hearing loss. After adjusting for age, sex, education, BMI, blood pressure, smoking status, diabetes, and use of blood pressure and lipid lowering medication, 1 mm of additional maximum IMT (the near or far wall of the common carotid artery) was associated with 2.09 (95% CI, 0.08-4.10) dB higher PTA. Moreover, participants whose number of plaques classified them in the third quartile had 1.06 (95% CI, 0.01-2.08) dB higher PTA than those in the first quartile; and compared with the first quartile, those in the fourth quartile also had higher PTA (1.55 dB; 95% CI, 0.49-2.60 dB).
Compared with the findings of the Rotterdam study,12 our estimates with respect to cIMT were approximately 3 times larger (0.59 vs 0.21 dB HL per 0.1 mm in the Rotterdam study, assuming linearity of the association). Unfortunately, the width of the confidence interval prevents us from making definitive conclusions regarding the true association between carotid plaque presence and hearing loss. Some of these discrepancies may be associated with the demographic composition of the study sample, the study design (cross-sectional vs cross temporal), or differences in our exposure variable (average vs maximum thickness for cIMT, and operationalization of carotid plaque burden (ie, yes or no in ARIC vs quartiles for number of carotid plaques in Rotterdam). In the case of plaque burden, the differences between findings may suggest that the association of carotid plaque on hearing loss may be dose dependent.
Previous animal studies23,24 showed the cochlear blood flow of the left ear was higher compared with the right ear. Therefore, it had been hypothesized that this lower blood flow, potentially aggravated by the presence of atherosclerosis, may explain the association between mean cIMT and PTA for the right ear, as described in the Rotterdam study.12 Our findings suggest an association between mean cIMT and both ears’ PTAs, albeit more robust for the left ear. These finding may suggest a more generalized association between atherosclerosis and hearing loss through the entire vascular system.
Consistent with our findings, which showed that cIMT was associated with hearing loss among the low (0.25 and 0.50 kHz) and middle (1.00 and 2.00 kHz) frequencies, previous research found that among 1672 participants in the Framingham Offspring Study 25 (mean age, 59 years; 57.6% female), the prevalence of low-frequency hearing loss (also known as strial pattern hearing loss) was higher among participants in the highest quartile of cIMT. However, unlike our study’s analyses, after adjusting for age, sex, and other cardiovascular risk factors, the estimates of the Framingham study prevented definite conclusions from being drawn regarding the association between cIMT and low frequency hearing loss. These findings suggest some affect around the mid- or apical-end section of the cochlea where the stria vascularis is narrower.26 Poor blood supply to this area via the carotid artery could be a potential pathway between carotid atherosclerosis and hearing loss as previously suggested.7,27
Limitations
Given the time between when atherosclerosis was assessed and when audiometric data were collected (approximately 22 years), a study limitation is that our analytic sample included only participants who had survived and who were willing and able to attend visit 6 after all those years. Therefore, these findings may underestimate the association between carotid atherosclerosis because of the informative attrition. Additionally, audiometric data were measured only once, so we were not able to observe whether study participants were already experiencing hearing loss at baseline or if atherosclerosis was associated with the development of hearing loss over time.
Conclusions
This cross-sectional study with temporal analysis of a cohort from the ARIC study found that the presence of carotid atherosclerosis, as measured by mean cIMT over approximately 9 years in middle adulthood, was associated with worse hearing in older adulthood. In the analyses, this association remained robust after adjusting for other hearing loss risk factors. This study contributes to the growing literature on the association between carotid atherosclerosis and hearing loss, particularly among older adults. Prevention and control of carotid atherosclerosis during middle age may positively affect the hearing health of older adults.
eTable 1. Linear Regression Estimates for the Association between Carotid Atherosclerosis and better-ear 4-frequency PTA threshold in the ARIC Study Available Case
eTable 2. Linear Regression Estimates for the Association between Carotid Atherosclerosis, and better-ear 4-frequency PTA threshold stratified by right and left ear in the ARIC Study
eTable 3. Linear Regression Estimates for the Association between Atherosclerosis and Hearing in the ARIC Study
eTable 4. Linear Regression Estimates for the Association between Carotid Atherosclerosis and better-ear 4-frequency PTA threshold by sex in the ARIC Study
Data Sharing Statement
References
- 1.Lin FR. Hearing loss in older adults: who’s listening? JAMA. 2012;307(11):1147-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosh S, Carriere I, Daien V, et al. ; Sense-Cog Consortium . The relationship between hearing loss in older adults and depression over 12 years: findings from the Three-City prospective cohort study. Int J Geriatr Psychiatry. 2018;33(12):1654-1661. [DOI] [PubMed] [Google Scholar]
- 3.Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg. 2014;150(3):378-384. [DOI] [PubMed] [Google Scholar]
- 4.Deal JA, Betz J, Yaffe K, et al. Hearing impairment and incident dementia and cognitive decline in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2017;72(5):703-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernabei R, Bonuccelli U, Maggi S, et al. ; Workshop on Hearing Loss and Cognitive Decline in Older Adults . Hearing loss and cognitive decline in older adults: questions and answers. Aging Clin Exp Res. 2014;26(6):567-573. [DOI] [PubMed] [Google Scholar]
- 6.Gates GA, Mills JH. Presbycusis. Lancet. 2005;366(9491):1111-1120. [DOI] [PubMed] [Google Scholar]
- 7.Gates GA, Cobb JL, D’Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg. 1993;119(2):156-161. [DOI] [PubMed] [Google Scholar]
- 8.Hull RH, Kerschen SR. The influence of cardiovascular health on peripheral and central auditory function in adults: a research review. Am J Audiol. 2010;19(1):9-16. [DOI] [PubMed] [Google Scholar]
- 9.Helzner EP, Patel AS, Pratt S, et al. Hearing sensitivity in older adults: associations with cardiovascular risk factors in the health, aging and body composition study. J Am Geriatr Soc. 2011;59(6):972-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer ME, Schubert CR, Nondahl DM, et al. Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis. 2015;238(2):344-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John U, Baumeister SE, Kessler C, Völzke H. Associations of carotid intima-media thickness, tobacco smoking and overweight with hearing disorder in a general population sample. Atherosclerosis. 2007;195(1):e144-e149. [DOI] [PubMed] [Google Scholar]
- 12.Croll PH, Bos D, Vernooij MW, et al. Carotid atherosclerosis is associated with poorer hearing in older adults. J Am Med Dir Assoc. 2019;20(12):1617-1622.e1. [DOI] [PubMed] [Google Scholar]
- 13.Prazma J, Carrasco VN, Butler B, Waters G, Anderson T, Pillsbury HC. Cochlear microcirculation in young and old gerbils. Arch Otolaryngol Head Neck Surg. 1990;116(8):932-936. [DOI] [PubMed] [Google Scholar]
- 14.Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk in Communities) Study: JACC focus seminar 3/8. J Am Coll Cardiol. 2021;77(23):2939-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed NS, Lin F, Meinke D, Themann C, Goman A. Hearing Manual of Operations (Visit 6). 2016. Accessed December 8, 2022. https://jhucochlearcenter.org/themes/business_responsive_theme/images/ARIC_HEARING_MOP_v6.12_pdf.pdf
- 16.Pursnani S, Diener-West M, Sharrett AR. The effect of aging on the association between coronary heart disease risk factors and carotid intima media thickness: an analysis of the Atherosclerosis Risk in Communities (ARIC) cohort. Atherosclerosis. 2014;233(2):441-446. [DOI] [PubMed] [Google Scholar]
- 17.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3-15. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Simonsick EM, Ferrucci L, Lin FR. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38(1):25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow AHC, Cai T, McPherson B, Yang F. Otitis media with effusion in children: cross-frequency correlation in pure tone audiometry. PLoS One. 2019;14(8):e0221405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . World report on hearing. Published online 2021. Accessed July 11, 2022. https://www.who.int/publications/i/item/world-report-on-hearing
- 22.Rigters SC, van der Schroeff MP, Papageorgiou G, Baatenburg de Jong RJ, Goedegebure A. Progression of hearing loss in the aging population: repeated auditory measurements in the Rotterdam Study. Audiol Neurootol. 2018;23(5):290-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carraro M, Harrison RV. Degeneration of stria vascularis in age-related hearing loss; a corrosion cast study in a mouse model. Acta Otolaryngol. 2016;136(4):385-390. [DOI] [PubMed] [Google Scholar]
- 24.Brown JN, Miller JM, Nuttall AL. Age-related changes in cochlear vascular conductance in mice. Hear Res. 1995;86(1-2):189-194. [DOI] [PubMed] [Google Scholar]
- 25.Tyagi S, Friedland DR, Rein L, et al. Abnormal hearing patterns are not associated with endothelium-dependent vasodilation and carotid intima-media thickness: The Framingham Heart Study. Vasc Med. 2021;26(6):595-601. [DOI] [PubMed] [Google Scholar]
- 26.Shi X. Physiopathology of the cochlear microcirculation. Hear Res. 2011;282(1-2):10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedland DR, Cederberg C, Tarima S. Audiometric pattern as a predictor of cardiovascular status: development of a model for assessment of risk. Laryngoscope. 2009;119(3):473-486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Linear Regression Estimates for the Association between Carotid Atherosclerosis and better-ear 4-frequency PTA threshold in the ARIC Study Available Case
eTable 2. Linear Regression Estimates for the Association between Carotid Atherosclerosis, and better-ear 4-frequency PTA threshold stratified by right and left ear in the ARIC Study
eTable 3. Linear Regression Estimates for the Association between Atherosclerosis and Hearing in the ARIC Study
eTable 4. Linear Regression Estimates for the Association between Carotid Atherosclerosis and better-ear 4-frequency PTA threshold by sex in the ARIC Study
Data Sharing Statement