Key Points
Question
Can 24-hour rest-activity characteristics from wrist-wearable devices predict adverse posttraumatic neuropsychiatric symptoms following traumatic stress exposure?
Findings
In this cohort study including 2021 participants observed over time after traumatic stress exposure, reduced 24-hour activity variance based on wrist accelerometry identified individuals with greater pain severity. Changes in several rest-activity measures were associated with changes in pain, sleep, and anxiety over time, and simple thresholds for these biomarkers identified individuals with good recovery for pain, sleep, and anxiety with high predictive value.
Meaning
These findings suggest that wrist-activity biomarkers may have utility as screening tools for adverse pain, sleep, and anxiety symptom outcomes after trauma exposure.
This cohort study evaluates whether wrist-wearable devices can provide useful biomarkers for recovery after traumatic stress exposure.
Abstract
Importance
Adverse posttraumatic neuropsychiatric sequelae after traumatic stress exposure are common and have higher incidence among socioeconomically disadvantaged populations. Pain, depression, avoidance of trauma reminders, reexperiencing trauma, anxiety, hyperarousal, sleep disruption, and nightmares have been reported. Wrist-wearable devices with accelerometers capable of assessing 24-hour rest-activity characteristics are prevalent and may have utility in measuring these outcomes.
Objective
To evaluate whether wrist-wearable devices can provide useful biomarkers for recovery after traumatic stress exposure.
Design, Setting, and Participants
Data were analyzed from a diverse cohort of individuals seen in the emergency department after experiencing a traumatic stress exposure, as part of the Advancing Understanding of Recovery After Trauma (AURORA) study. Participants recruited from 27 emergency departments wore wrist-wearable devices for 8 weeks, beginning in the emergency department, and completed serial assessments of neuropsychiatric symptoms. A total of 19 019 patients were screened. Of these, 3040 patients met study criteria, provided informed consent, and completed baseline assessments. A total of 2021 provided data from wrist-wearable devices, completed the 8-week assessment, and were included in this analysis. The data were randomly divided into 2 equal parts (n = 1010) for biomarker identification and validation. Data were collected from September 2017 to January 2020, and data were analyzed from May 2020 to November 2022.
Exposures
Participants were recruited for the study after experiencing a traumatic stress exposure (most commonly motor vehicle collision).
Main Outcomes and Measures
Rest-activity characteristics were derived and validated from wrist-wearable devices associated with specific self-reported symptom domains at a point in time and changes in symptom severity over time.
Results
Of 2021 included patients, 1257 (62.2%) were female, and the mean (SD) age was 35.8 (13.0) years. Eight wrist-wearable device biomarkers for symptoms of adverse posttraumatic neuropsychiatric sequelae exceeded significance thresholds in the derivation cohort. One of these, reduced 24-hour activity variance, was associated with greater pain severity (r = −0.14; 95% CI, −0.20 to −0.07). Changes in 6 rest-activity measures were associated with changes in pain over time, and changes in the number of transitions between sleep and wake over time were associated with changes in pain, sleep, and anxiety. Simple cutoffs for these biomarkers identified individuals with good recovery for pain (positive predictive value [PPV], 0.85; 95% CI, 0.82-0.88), sleep (PPV, 0.63; 95% CI, 0.59-0.67, and anxiety (PPV, 0.76; 95% CI, 0.72-0.80) with high predictive value.
Conclusions and Relevance
These findings suggest that wrist-wearable device biomarkers may have utility as screening tools for pain, sleep, and anxiety symptom outcomes after trauma exposure in high-risk populations.
Introduction
Up to 90% of individuals experience at least 1 traumatic event.1 While most individuals recover, adverse posttraumatic neuropsychiatric sequelae (APNS) are common and produce morbidity.2 Common APNS symptoms include pain and other somatic symptoms, depression, avoidance of trauma reminders, trauma reexperiencing, anxiety, hyperarousal, sleep disruption, and nightmares. These symptoms are associated with negative consequences, including emotional distress, functional impairments,3,4 and reduced quality of life.5,6
More than 1 in 5 individuals in the US use a wrist-wearable device capable of accelerometry.7 Wrist-wearable devices can assess 24-hour rest, wake, and activity pattern characteristics that have been associated with alterations in pain, fatigue, and mood8,9,10 and might influence clinical and psychological outcomes. For example, sleep quality and daytime activity have been found to influence pain, posttraumatic stress, and other symptoms.11,12,13,14,15,16,17,18,19 To our knowledge, no large study has used wrist-wearable devices to examine neuropsychiatric sequelae following traumatic stress exposure.
In this study, we used longitudinal wrist-wearable data obtained from a socioeconomically disadvantaged adult population presenting to emergency departments (EDs) after traumatic stress exposure. We obtained serial assessments of 10 common neuropsychiatric symptom domains to identify 24-hour rest, wake, and activity characteristics associated with APNS symptom outcomes. We sought to derive and validate rest-activity characteristics associated with specific APNS symptoms, and changes in rest-activity characteristics associated with changes in APNS symptoms over time. We hypothesized that such characteristics could be identified.
Methods
Study Overview and Sample Characteristics
Data were obtained from the Advancing Understanding of Recovery After Trauma (AURORA) study. The AURORA study collected a combination of prospective data from a diverse sample of trauma survivors recruited from EDs in the early aftermath of trauma. The full AURORA study methodology has been published elsewhere,2 and we have followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.20 The AURORA study protocol was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. Qualifying traumatic events included motor vehicle collision, physical assault, sexual assault, fall more than 10 ft, and mass casualty incidents. AURORA study enrollment began in September 2017; participants who had completed the 8-week period of follow-up assessment by January 2020 were included in this analysis. Individuals were eligible if they presented to one of 27 EDs within the AURORA study network within 72 hours of the trauma, were aged 18 to 65 years, and were able to speak and read English. Individuals were excluded if they had a solid organ injury Grade of 1 or greater per the American Association for the Surgery of Trauma, had significant hemorrhage, required operative intervention, or were likely to be admitted for more than 72 hours. A total of 3040 patients met all these criteria, provided informed consent, and completed baseline ED assessments. Of these, 2021 provided watch data, completed the 8-week assessment after ED enrollment, and were included in this analysis.
Self-reported Data Collection and Preparation
Sociodemographic characteristics, including race and ethnicity, were assessed via survey items.2 Following the baseline ED visit, participants completed a rotating battery of smartphone-based questionnaires consisting of 10 common APNS symptom domains: pain,21,22 depressive symptoms,23,24,25,26 sleep discontinuity,27 nightmares,28,29,30 somatic symptoms,21,31 difficulty with concentration, thinking, or fatigue,32,33,34,35 avoidance of trauma reminders, trauma reexperiencing, anxiety,36,37 and hyperarousal.38,39,40,41 As detailed elsewhere,2 these questions were chosen to capture symptoms of the most common APNS syndromes of pain, postconcussive syndrome, posttraumatic stress disorder, and depression. Each survey item was administered at 6 time points within the first 8 weeks posttrauma using the Mindstrong Discovery application (eTable 1 in Supplement 1). These survey items were used as indicator variables to develop measurement models for each APNS symptom domain, and factor scores for each symptom were computed for each participant at each time point. Joint measurement models including all 6 time points within the first 8 weeks after trauma exposure were developed to define each symptom domain. As noted elsewhere,2 8 weeks was chosen as the time frame for assessment because of critical changes in the first few days and weeks after trauma exposure that signal transition from either persistent APNS symptoms or recovery.22,42,43 Temporal correlations of these indicator variables were introduced to improve model fit if the temporal autocorrelations were not fully explained by the joint measurement model. Model fit indices (eg, comparative fit index, Tucker-Lewis index, standardized root mean square residual) were used to evaluate the fit of each measurement model.44
Wrist-Wearable Data Collection and Preparation
Participants were instructed to wear the research watch (Verily Life Sciences45) at least 21 hours a day throughout the 8-week study period. In addition to this project, the Verily study watch is currently being used to examine rest-activity biomarkers in other large-scale cohort health studies.46,47 Participants were sent home with the watch, a charging dock, and a connectivity hub with 4G LTE for data upload. The device collected continuous 3-axis accelerometry data at 30 Hz. Prior to feature extraction, days with missing data percentage larger than 20% were excluded, as well as days in which the participant was categorized as having no activity (wrist-wearable device not worn). Accelerometer data were then converted to activity counts.48 Briefly, z-axis accelerometry data were filtered using a 0.25-11 Hz bandpass to eliminate the gravitational artifacts and very slow movements.49 Afterwards, the maximum absolute values inside 1-second windows were taken and summed for each 30-second epoch.48 Sleep/wake activity patterns were then calculated from each nonoverlapping 30-second accelerometry epoch using the Cole-Kripke algorithm.50 Cosinor rhythmometry features were used to capture each participant’s circadian rhythm.51 Specifically, a cosine model was fit to the data: Y(t) = M + K cos (2πt/τ + φ), where M is the mesor, K is the amplitude, and φ is the acrophase. The mesor describes the baseline activity of the participant for the day, and the amplitude indicates the difference in daytime and nighttime activities. This algorithm was applied to 24-hour segments of data, and the number of transitions between sleep and wake and percentage of the 24-hour period scored as sleep were calculated. Means and SDs of activity counts for each 24-hour period were also calculated. The most active 10 hours and least active 5 hours, indicating the average daily activity in the wake period and the nighttime activity, respectively, were also derived using the movement data.52 See eTable 2 in Supplement 1 for rest-activity descriptive statistics.
Rest-Activity Biomarker Derivation, Validation, and Evaluation
For each self-report survey, mean 24-hour wrist-wearable device rest-activity characteristics from the day prior to and the day of self-reported symptom data collection were used as candidate rest-activity biomarkers for corresponding self-reported symptoms. Given this, participants without self-report survey data for a symptom and/or no watch data within the 2 days prior to the survey were excluded from analysis for that symptom. We endeavored to identify rest-activity characteristics associated with the severity of specific APNS symptoms either at a point in time between participants or over time within participants. These 2 types of biomarkers were indicated by cross-sectional and longitudinal associations, respectively, based on repeated measures. Biomarkers with cross-sectional (between-participant) associations helped differentiate participants with respect to APNS symptoms at a point in time. Biomarkers with longitudinal (within-participant) associations helped differentiate the change of the APNS symptoms within participants over time. We used a bivariate linear mixed model to evaluate the correlation for each pair of rest-activity features and symptoms and partition it into between-participant and within-participant correlations based on repeated measures. More specifically, between-participant and within-participant correlations were derived from the between-participant and within-participant covariance matrix, respectively.53
Statistical Analysis
Following initial quality check of rest-activity variables, the data were randomly divided into 2 equal parts (n = 1010) for biomarker identification and validation. The same identification and validation data set was used for all analyses. P values were calculated using the z test. False-discovery rate P value of .05 was used to define biomarkers that passed identification; these biomarkers were then evaluated in the validation data set. A Bonferroni-corrected 2-tailed P value of .05 was used to identify biomarkers that passed the validation step. To explore the potential utility of validated biomarkers to identify change in self-reported symptoms over time, simple cutoffs were used, where symptom worsening or improvement was defined as the symptom outcome measure and biomarker increase or decrease was used as the predictor. Each of these values was generated by subtracting the last symptom or biomarker value (obtained in the final 8-week assessment) from the first value (obtained in week 1). We then assessed the sensitivity, specificity, and positive predictive value (ie, probability of correctly identifying a case) of increasing or decreasing biomarker value for increasing or decreasing symptom score. Because several biomarkers were identified for pain, we constructed a composite biomarker for this outcome comprised of individual biomarkers that were not strongly correlated with each other (eFigure 1 in Supplement 1). To create the composite biomarker, we built a linear mixed model, with pain as the dependent variable and individual biomarkers as predictors, and used the predicted pain score from this linear mixed model as the composite biomarker. Missing data are common in large-scale, longitudinal naturalistic samples; to help explore the potential influence of missing data, we evaluated the association between watch data and self-reported data missingness. All correlations were weak (less than 0.1), suggesting that completion rate did not bias main outcome associations, and thus missing data were considered missing at random (eTable 3 in Supplement 1).
Analyses were conducted using R version 4.0.1 (The R Foundation) and SAS version 9.4 (SAS Institute). For additional information on study methods, see the eMethods in Supplement 1. For data flow pathways for self-report and watch data, see eFigure 2 in Supplement 1. For sample R code for data processing, see the eAppendix in Supplement 1.
Results
Sociodemographic and Clinical Characteristics
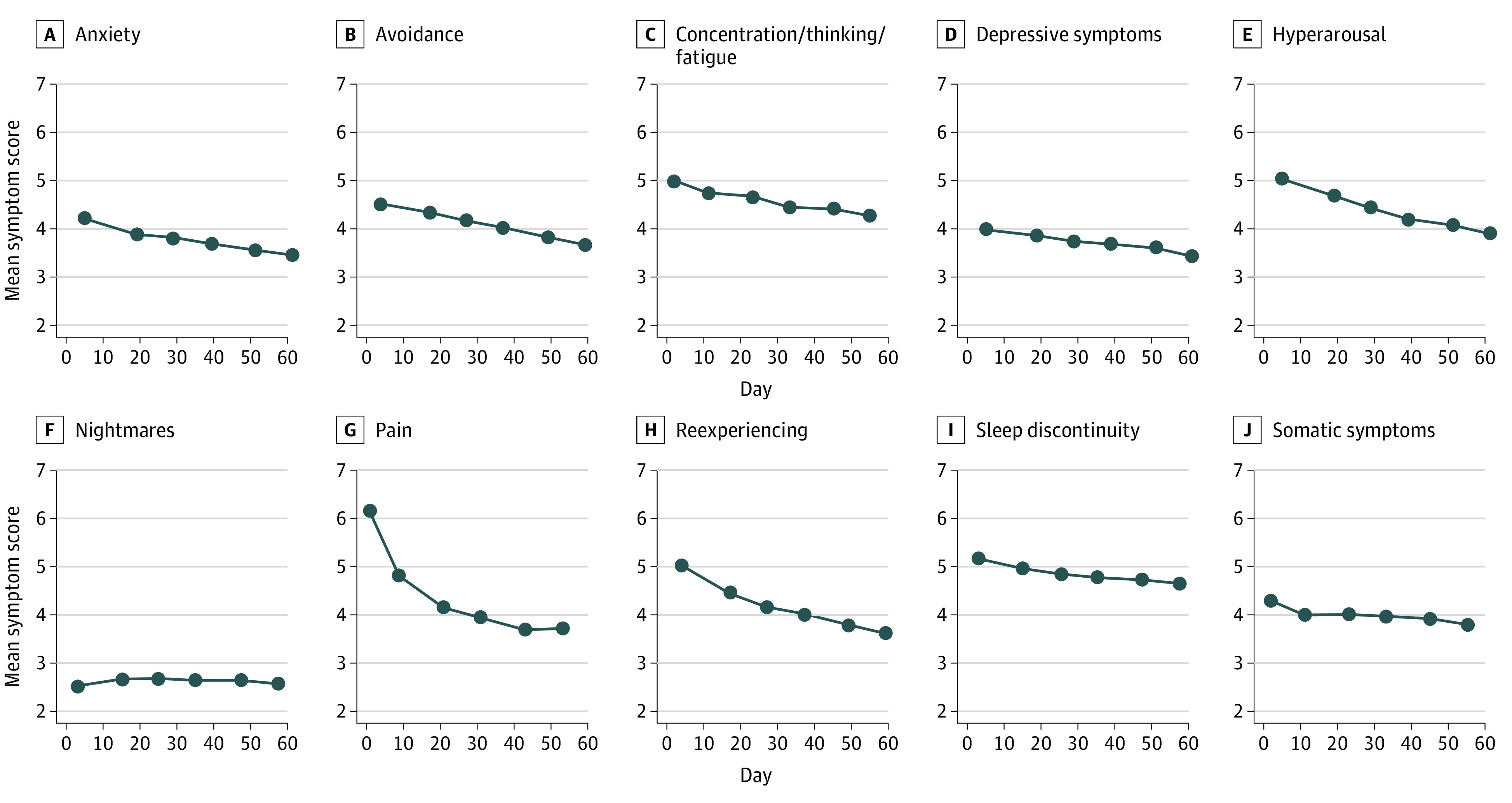
Of 2021 included patients, 1257 (62.2%) were female, and the mean (SD) age was 35.8 (13.0) years (Table 1). A total of 1014 (50.2%) were Black, 227 (11.2%) were Hispanic, 694 (34.3%) were White, and 77 (3.8%) were another race (including American Indian or Alaska Native, Asian, and Native Hawaiian or Other Pacific Islander). A total of 1595 (79.3%) did not have a college degree, and 1160 (64.2%) earned $35 000 per year or less. A total of 1519 patients (75.2%) had experienced motor vehicle collision as the traumatic event leading to study inclusion. Median symptom scores indicated a substantial burden of APNS symptoms during the weeks after trauma (Figure). For most symptoms, median severity exhibited gradual modest improvement over the study period. Pain scores demonstrated an initial steep decline in the first week after trauma, followed by gradual modest improvement.
Table 1. Demographic Information.
| Characteristic | No. (%) |
|---|---|
| Total, No. | 2021 |
| Sex | |
| Female | 1257 (62.2) |
| Male | 764 (37.8) |
| Race and ethnicitya | |
| Black | 1014 (50.2) |
| Hispanic | 227 (11.2) |
| White | 694 (34.3) |
| Other race | 77 (3.8) |
| Age, mean (SD), y | 35.8 (13.0) |
| Highest grade completed in formal education | |
| <High school | 242 (12.0) |
| High school graduate | 518 (25.6) |
| Some college | 835 (41.3) |
| College graduate | 419 (20.7) |
| Income, $ | |
| >35 000 | 647 (32.0) |
| 19 000-35 000 | 557 (27.6) |
| <19 000 | 603 (29.8) |
| Marital status | |
| Married or cohabitating | 811 (40.1) |
| Separated, divorced, widowed, or not cohabitating | 284 (14.1) |
| Annulled or never married | 915 (45.3) |
| Trauma type | |
| Motor vehicle collision | 1519 (75.2) |
| Physical assault | 195 (9.6) |
| Sexual assault | 13 (0.6) |
| Other | 294 (14.6) |
Race and ethnicity data were collected via survey items. The other race category includes American Indian or Alaska Native, Asian, and Native Hawaiian or Other Pacific Islander races.
Figure. Trajectory of Median Symptom Scores Over the First 8 Weeks After Trauma.
Derivation and Validation of Cross-Sectional Biomarkers
We first sought to identify cross-sectional, point-in-time rest-activity biomarkers that identified individuals experiencing high APNS symptoms at a point in time. Eight wrist-wearable device biomarkers for 5 APNS symptoms exceeded significance thresholds in the derivation cohort (Table 2). One of these biomarkers, reduced daily activity variance, passed the validation step for cross-sectional association with increased pain (r = −0.14; 95% CI, −0.20 to −0.07; adjusted P < .008) (Table 2).
Table 2. Wrist-Wearable Rest-Activity Biomarkers Associated With the Severity of Specific Adverse Posttraumatic Neuropsychiatric Symptoms at a Point in Timea.
| Construct | Variable | Correlation (95% CI) | P value | Adjusted P value |
|---|---|---|---|---|
| Pain | Daily activity variance | −0.14 (−0.20 to −0.07) | <.001 | <.001 |
| Somatic symptoms | Percentage of the 24-h period scored as wake | −0.09 (−0.13 to −0.01) | .02 | .21 |
| No. of transitions between sleep and wake | −0.07 (−0.10 to 0.03) | .25 | >.99 | |
| Nightmares | Percentage of the 24-h period scored as wake | −0.06 (−0.11 to 0.02) | .16 | >.99 |
| Depressive symptoms | Percentage of the 24-h period scored as wake | −0.05 (−0.12 to 0.01) | .12 | >.99 |
| Pain | Percentage of the 24-h period scored as wake | −0.04 (−0.11 to 0.02) | .18 | >.99 |
| Hyperarousal | Percentage of the 24-h period scored as wake | −0.03 (−0.09 to 0.05) | .57 | >.99 |
| Nightmares | No. of transitions between sleep and wake | −0.03 (−0.08 to 0.05) | .64 | >.99 |
Results are based on the validation set (50% of the overall sample; n = 1010); 95% CIs and P values were calculated with R package psych.
Derivation and Validation of Individual State Biomarkers
We next sought to identify changes in rest-activity biomarkers associated with changes in specific APNS symptoms during the initial 8 weeks after trauma (state biomarkers). A total of 13 APNS symptom biomarkers passed the initial derivation step, and 9 APNS symptom biomarkers passed validation (Table 3). Among these 9 validated state biomarkers, 7 were associated with changes in pain symptom severity, 1 was associated with changes in sleep quality, and 1 was associated with changes in anxiety. Increased maximum daily activity over time, increased average activity of the individual’s most active 10 hours, increased average daily activity, increased baseline activity, and increased variation in daily activity were all associated with reduced pain over time. Reduction in the number of sleep/wake transitions was associated with reductions in self-reported pain, sleep disturbance, and anxiety over time (Table 3).
Table 3. Changes in Wrist-Wearable Rest-Activity Biomarkers Associated With Changes in Symptom Domain Outcomes Over Timea.
| Construct | Variable | Correlation (95% CI) | P value | Adjusted P value |
|---|---|---|---|---|
| Pain | Maximum daily activity | −0.15 (−0.18 to −0.12) | <.001 | <.001 |
| Average activity of most active 10 h | −0.14 (−0.17 to −0.11) | <.001 | <.001 | |
| Average daily activity | −0.13 (−0.16 to −0.10) | <.001 | <.001 | |
| Baseline activityb | −0.13 (−0.16 to −0.09) | <.001 | <.001 | |
| SD of daily activity | −0.12 (−0.15 to −0.09) | <.001 | <.001 | |
| Peak activity timing | −0.07 (−0.11 to −0.04) | <.001 | <.001 | |
| Percentage of the 24-h period scored as wake | −0.05 (−0.09 to −0.02) | .001 | .02 | |
| Depressive symptoms | Maximum daily activity | −0.04 (−0.07 to 0) | .04 | .64 |
| Percentage of the 24-h period scored as wake | −0.02 (−0.05 to 0.01) | .25 | >.99 | |
| Pain | No. of transitions between sleep and wake | 0.13 (0.10 to 0.16) | <.001 | <.001 |
| Sleep discontinuity | No. of transitions between sleep and wake | 0.10 (0.06 to 0.13) | <.001 | <.001 |
| Anxiety | No. of transitions between sleep and wake | 0.06 (0.03 to 0.09) | <.001 | .007 |
| Depressive symptoms | No. of transitions between sleep and wake | 0.05 (0.02 to 0.09) | .002 | .03 |
| Somatic symptoms | No. of transitions between sleep and wake | 0.04 (0.01 to 0.08) | .003 | .06 |
Results are based on the validation set (50% of the overall sample; n = 1010).
Mesor from the circadian rhythm cosine model; 95% CIs and P values were calculated with R package psych.
Utility of Example State Biomarkers
The potential utility of state biomarkers as screening tools for posttrauma outcomes was then assessed, using cutoff scores (Table 4). Worsening and improvement in self-reported symptoms was defined as symptom severity in the eighth week minus symptom severity in the first week greater than 0 and less than 0, respectively. Similarly, cutoff scores for change in rest-activity characteristics were defined based on positive vs negative change in rest-activity score at these 2 time points. We obtained high positive predictive values for symptom improvement and high negative predictive values for symptom worsening, suggesting rest-activity measures derived from wrist-wearable devices may have utility as initial screening measures for APNS. In addition, a composite biomarker for pain was developed using a linear mixed model based on the multiple derived and validated rest-activity pain biomarkers. When multiple predictive biomarkers for pain were combined, positive and negative predictive values were similar (Table 4). For additional results, including state biomarkers for subgroups of participants, see eTables 4 and 5 in Supplement 1
Table 4. Prediction of Symptom Trajectory Using Biomarker Trajectorya.
| Outcome | Sample with outcome, No. (%) | Feature variable | Sensitivity | Specificity | PPVb | NPVb | Accuracy |
|---|---|---|---|---|---|---|---|
| Worseningc | |||||||
| Pain | 139 (17) | Maximum daily activity | 0.44 | 0.63 | 0.20 | 0.84 | 0.60 |
| Pain | 139 (17) | Average activity of most active 10 h | 0.42 | 0.67 | 0.21 | 0.85 | 0.63 |
| Pain | 139 (17) | No. of transitions between sleep and wake | 0.38 | 0.60 | 0.17 | 0.82 | 0.56 |
| Pain | 139 (17) | Average daily activity | 0.44 | 0.67 | 0.22 | 0.85 | 0.63 |
| Pain | 139 (17) | Baseline activityc,d | 0.42 | 0.67 | 0.21 | 0.85 | 0.63 |
| Pain | 139 (17) | SD of daily activity | 0.40 | 0.67 | 0.20 | 0.84 | 0.62 |
| Pain | 139 (17) | Peak activity timing | 0.42 | 0.58 | 0.18 | 0.83 | 0.56 |
| Sleep | 315 (39) | No. of transitions between sleep and wake | 0.37 | 0.69 | 0.43 | 0.63 | 0.56 |
| Anxiety | 197 (24) | No. of transitions between sleep and wake | 0.36 | 0.66 | 0.25 | 0.76 | 0.58 |
| Pain | 139 (17) | Composite biomarker | 0.42 | 0.68 | 0.21 | 0.85 | 0.64 |
| Improvementc | |||||||
| Pain | 660 (83) | Maximum daily activity | 0.63 | 0.44 | 0.84 | 0.20 | 0.60 |
| Pain | 660 (83) | Average activity of most active 10 h | 0.67 | 0.42 | 0.85 | 0.21 | 0.63 |
| Pain | 660 (83) | No. of transitions between sleep and wake | 0.60 | 0.38 | 0.82 | 0.17 | 0.56 |
| Pain | 660 (83) | Average daily activity | 0.67 | 0.44 | 0.85 | 0.22 | 0.63 |
| Pain | 660 (83) | Baseline activity | 0.67 | 0.42 | 0.85 | 0.21 | 0.63 |
| Pain | 660 (83) | Standard deviation of daily activity | 0.67 | 0.40 | 0.84 | 0.20 | 0.62 |
| Pain | 660 (83) | Peak activity timing | 0.58 | 0.42 | 0.83 | 0.18 | 0.56 |
| Sleep | 500 (61) | No. of transitions between sleep and wake | 0.69 | 0.37 | 0.63 | 0.43 | 0.56 |
| Anxiety | 612 (76) | No. of transitions between sleep and wake | 0.66 | 0.36 | 0.76 | 0.25 | 0.58 |
| Pain | 660 (83) | Composite biomarker | 0.68 | 0.42 | 0.85 | 0.21 | 0.64 |
Results are based on the validation set (50% of the overall sample; n = 1010).
Positive predictive value indicates the probability of correctly identifying that an individual fits the category, while negative predictive value indicates the probability of correctly identifying that an individual does not fit the category.
Worsening and improvement in self-report symptoms was defined as symptom severity in week 8 minus symptom severity in week 1 greater than 0 and less than 0, respectively. Similarly, cutoff scores for change in rest-activity characteristics were defined based on positive vs negative change in rest-activity score at these 2 time points. Prediction was made based on the change in rest-activity characteristic and its correlation with the symptom. For example, if the rest-activity characteristic was positively correlated with the symptom and it increased between week 1 and 8, we would predict the symptom was worsening.
Mesor from the circadian rhythm cosine model.
Discussion
Most individuals experience traumatic events, and many individuals who come to the ED for care after traumatic stress struggle with 1 or more persistent APNS. This is particularly true for individuals from socioeconomically disadvantaged populations.54 Wrist-wearable devices with accelerometry are common, and 24-hour rest-activity characteristics obtained from wearable devices might identify those who will recover from trauma in high-risk populations. We derived and validated cross-sectional and longitudinal associations between 24-hour activity patterns and APNS symptoms during the 8 weeks following a traumatic event, a high-risk period during which individuals transition to symptom recovery or persistence. To our knowledge, this study is the first to examine such associations. We identified 1 cross-sectional rest-activity biomarker of an APNS symptom: reduced daily activity variance was a biomarker for increased pain. In addition, we identified 9 rest-activity biomarkers that changed with APNS symptoms over time. Six activity-related biomarkers changed with pain: increased maximum daily activity over time, increased average activity of the individual’s most active 10 hours, increased average daily activity, increased baseline activity, increased variation in daily activity, and increased peak activity. We created a composite biomarker comprised of these measures, which did not outperform the individual measures. One sleep-related biomarker was associated with changes in pain, sleep, and anxiety symptoms over time. Simple biomarker or symptom change cutoffs suggest that these biomarkers might have utility as initial screening tools to identify individuals with potential good recovery in these domains who might not need further evaluation. In clinical practice, they could serve as ancillary data to help patients and physicians identify whether symptoms are improving or worsening after trauma. Notably, the magnitude of associations between individual rest-activity biomarkers and APNS outcomes were small, and no single biomarker achieved both high positive and negative predictive value for APNS symptom change. Given this, these biomarkers would likely have the most utility if used to augment other measures, such as self-report. Additionally, it should be noted that these biomarkers performed similarly to other objective measures commonly used in clinical practice.55,56
Across the 10 APNS symptom domains evaluated, rest-activity biomarkers demonstrated the strongest associations with pain. For example, pain was the only cross-sectional (ie, between-participant( biomarker that passed both the derivation and validation assessments. Specifically, individuals with more severe pain at a point in time demonstrated diminished daily activity variance, indicating less movement during the day and greater sleep disruption during the night. This finding is consistent with prior research suggesting pain conditions are associated with blunted rest-activity rhythms.8,9,10 Pain also showed negative within-participant correlations of daily activity over time, indicating that as pain increased over time for an individual, daily activity and variance of daily activity decreased. Participants with worsening pain over the study period also showed increasing number of transitions between sleep and wake. These findings are consistent with other studies that have found temporal relationships between pain severity, activity, and sleep quality in the immediate aftermath of traumatic events.57,58
In addition to pain, we also observed temporal associations in trauma survivors between changes in rest-activity measures and self-reported anxiety and sleep quality. Worsening sleep continuity (as measured by sleep/wake transitions) over the 8-week study period was associated with worsening self-reported difficulties with anxiety and sleep quality, while improving sleep continuity was associated with improvements in self-reported anxiety and sleep quality. This finding makes sense in the context of research suggesting sleep problems and anxiety symptoms are related to each other bidirectionally15 and that sleep problems prior to29,59 or in the immediate aftermath of traumatic events is a vulnerability factor for other difficulties, such as posttraumatic stress disorder and depression.60,61 In this study, improving sleep consolidation was associated with improving self-reported anxiety symptoms over the study period, suggesting that interventions to improve sleep consolidation may be helpful to implement soon after trauma to improve other long-term mental health outcomes. Brief behavioral interventions for sleep62,63 can be delivered by telehealth, which may be particularly practical to implement for patients presenting to EDs.
Limitations
Limitations should be considered when interpreting our study results. All trauma survivors enrolled presented to the ED for evaluation; the generalizability of study findings to patients who do not present to the ED is not known. In addition, most were survivors of motor vehicle collisions, and the generalizability of study findings to other types of trauma is also unknown. Additionally, analyses required concurrent accelerometry and self-reported data. While missing data were not correlated with any of our specific outcome measures, there could be other potential missing data patterns we could not account for. We applied the sleep-wake detection algorithm to 24-hour data instead of a participant-identified sleep period, raising the possibility that sedentary segments could be confused as sleep. However, passive monitoring with accelerometers without concurrent sleep diaries is naturalistic and performed in other large-scale studies.64 Study evaluation was limited to the 8 weeks immediately following a traumatic event. Future studies should examine wrist-wearable biomarker associations with APNS symptoms and symptom changes over longer time durations. Finally, existing users of wearable technology are generally more health conscious, wealthier, insured, and have higher education and access to wireless technologies,7,46 which differed from the population of this study. While our study more closely resembles the population likely to present to the ED after experiencing trauma, there may be challenges implementing wearable-based assessment and intervention tools in this group in clinical practice. However, given this, we found no specific patterns regarding missing data for wearable devices vs self-report, suggesting that our results are at least internally valid.
Conclusions
Wrist-wearable devices are common and frequently use built-in accelerometers to detect 24-hour activity patterns.65 Thus, a proportion of the population may be using devices capable of yielding useful information to help screen for trauma survivors with high pain levels at a point in time and/or poor pain, sleep, and/or anxiety recovery over time. In the future, such biomarkers might be useful to identify trauma survivors who merit further evaluation for adverse trauma outcomes, particularly in vulnerable populations. Such biomarkers might also be useful to help clinicians and patients evaluate their responses to treatment interventions for pain, sleep, or anxiety and to help patients understand how their activity, rest, and sleep affect their health.
eMethods. Statistical Analyses
eTable 1. Latent Constructs Indicator Variable Questions From Smartphone-Based Follow-up Surveys
eTable 2. Descriptive Statistics of Rest-Activity Features
eTable 3. Correlation of Main Outcomes With Completion Rate of Main Tasks
eTable 4. State Activity Biomarkers for Subgroup of Participants Starting With Moderate or Severe Pain
eTable 5. State Activity Biomarkers Stratified by Sex
eFigure 1. Histogram and Correlation of Wrist Wearable Features
eFigure 2. Data Flow Pathways for Self-Report and Watch Data
eAppendix. Sample Analysis Code
Data Sharing Statement
References
- 1.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537-547. doi: 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLean SA, Ressler K, Koenen KC, et al. The AURORA study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol Psychiatry. 2020;25(2):283-296. doi: 10.1038/s41380-019-0581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Momartin S, Silove D, Manicavasagar V, Steel Z. Comorbidity of PTSD and depression: associations with trauma exposure, symptom severity and functional impairment in Bosnian refugees resettled in Australia. J Affect Disord. 2004;80(2-3):231-238. doi: 10.1016/S0165-0327(03)00131-9 [DOI] [PubMed] [Google Scholar]
- 4.Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;164(4):394-400. doi: 10.1001/archinte.164.4.394 [DOI] [PubMed] [Google Scholar]
- 5.Clapp JD, Beck GJ, Palyo SA, Grant DM. An examination of the synergy of pain and PTSD on quality of life: additive or multiplicative effects? Pain. 2008;138(2):301-309. doi: 10.1016/j.pain.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gormsen L, Rosenberg R, Bach FW, Jensen TS. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur J Pain. 2010;14(2):127.e1-127.e8. doi: 10.1016/j.ejpain.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekaran R, Katthula V, Moustakas E. Patterns of use and key predictors for the use of wearable health care devices by US adults: insights from a national survey. J Med internet Res. 2020;22(10):e22443. doi: 10.2196/22443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson AC, Palermo TM. Physical activity and function in adolescents with chronic pain: a controlled study using actigraphy. J Pain. 2012;13(2):121-130. doi: 10.1016/j.jpain.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korszun A, Young EA, Engleberg NC, Brucksch CB, Greden JF, Crofford LA. Use of actigraphy for monitoring sleep and activity levels in patients with fibromyalgia and depression. J Psychosom Res. 2002;52(6):439-443. doi: 10.1016/S0022-3999(01)00237-9 [DOI] [PubMed] [Google Scholar]
- 10.Neikrug AB, Donaldson G, Iacob E, Williams SL, Hamilton CA, Okifuji A. Activity rhythms and clinical correlates in fibromyalgia. Pain. 2017;158(8):1417-1429. doi: 10.1097/j.pain.0000000000000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lautenbacher S, Kundermann B, Krieg J-C. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357-369. doi: 10.1016/j.smrv.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 12.McLean SA, Clauw DJ, Abelson JL, Liberzon I. The development of persistent pain and psychological morbidity after motor vehicle collision: integrating the potential role of stress response systems into a biopsychosocial model. Psychosom Med. 2005;67(5):783-790. doi: 10.1097/01.psy.0000181276.49204.bb [DOI] [PubMed] [Google Scholar]
- 13.Smagula SF, Krafty RT, Thayer JF, Buysse DJ, Hall MH. Rest-activity rhythm profiles associated with manic-hypomanic and depressive symptoms. J Psychiatr Res. 2018;102:238-244. doi: 10.1016/j.jpsychires.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luik AI, Zuurbier LA, Direk N, Hofman A, Van Someren EJ, Tiemeier H. 24-Hour activity rhythm and sleep disturbances in depression and anxiety: a population-based study of middle-aged and older persons. Depress Anxiety. 2015;32(9):684-692. doi: 10.1002/da.22355 [DOI] [PubMed] [Google Scholar]
- 15.Richards A, Kanady JC, Neylan TC. Sleep disturbance in PTSD and other anxiety-related disorders: an updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms. Neuropsychopharmacology. 2020;45(1):55-73. doi: 10.1038/s41386-019-0486-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4(4):CD011279. doi: 10.1002/14651858.CD011279.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pigeon WR, Moynihan J, Matteson-Rusby S, et al. Comparative effectiveness of CBT interventions for co-morbid chronic pain & insomnia: a pilot study. Behav Res Ther. 2012;50(11):685-689. doi: 10.1016/j.brat.2012.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme M-È. Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clin Psychol Rev. 2011;31(4):638-652. doi: 10.1016/j.cpr.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 19.Anderson E, Shivakumar G. Effects of exercise and physical activity on anxiety. Front Psychiatry. 2013;4:27. doi: 10.3389/fpsyt.2013.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163-W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1 [DOI] [PubMed] [Google Scholar]
- 21.Moldofsky H, Rothman L, Kleinman R, Rhind SG, Richardson JD. Disturbed EEG sleep, paranoid cognition and somatic symptoms identify veterans with post-traumatic stress disorder. BJPsych Open. 2016;2(6):359-365. doi: 10.1192/bjpo.bp.116.003483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulirsch JC, Weaver MA, Bortsov AV, et al. No man is an island: living in a disadvantaged neighborhood influences chronic pain development after motor vehicle collision. Pain. 2014;155(10):2116-2123. doi: 10.1016/j.pain.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7(3):254-275. doi: 10.1038/sj.mp.4001032 [DOI] [PubMed] [Google Scholar]
- 24.Doerig N, Krieger T, Altenstein D, et al. Amygdala response to self-critical stimuli and symptom improvement in psychotherapy for depression. Br J Psychiatry. 2016;208(2):175-181. doi: 10.1192/bjp.bp.114.149971 [DOI] [PubMed] [Google Scholar]
- 25.Gollan JK, Buchanan A, Connolly M, et al. Differences in the neural correlates of affective responses in depressed and healthy women. Psychiatry Res. 2015;234(3):336-345. doi: 10.1016/j.pscychresns.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 26.Liu CH, Ma X, Song LP, et al. Abnormal spontaneous neural activity in the anterior insular and anterior cingulate cortices in anxious depression. Behav Brain Res. 2015;281:339-347. doi: 10.1016/j.bbr.2014.11.047 [DOI] [PubMed] [Google Scholar]
- 27.Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159(5):855-857. doi: 10.1176/appi.ajp.159.5.855 [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi I, Sledjeski EM, Spoonster E, Fallon WF Jr, Delahanty DL. Effects of early nightmares on the development of sleep disturbances in motor vehicle accident victims. J Trauma Stress. 2008;21(6):548-555. doi: 10.1002/jts.20368 [DOI] [PubMed] [Google Scholar]
- 29.Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69-74. doi: 10.1093/sleep/33.1.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Liempt S, van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety. 2013;30(5):469-474. doi: 10.1002/da.22054 [DOI] [PubMed] [Google Scholar]
- 31.McAndrew LM, Lu SE, Phillips LA, Maestro K, Quigley KS. Mutual maintenance of PTSD and physical symptoms for Veterans returning from deployment. Eur J Psychotraumatol. 2019;10(1):1608717. doi: 10.1080/20008198.2019.1608717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140. doi: 10.1037/a0038039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen H, Griffiths K, Mackinnon A, Jacomb P. A quantitative review of cognitive deficits in depression and Alzheimer-type dementia. J Int Neuropsychol Soc. 1997;3(6):631-651. doi: 10.1017/S1355617797006310 [DOI] [PubMed] [Google Scholar]
- 34.Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11(3):111-119. [PubMed] [Google Scholar]
- 35.Henry J, Crawford JR. A meta-analytic review of verbal fluency deficits in depression. J Clin Exp Neuropsychol. 2005;27(1):78-101. doi: 10.1080/138033990513654 [DOI] [PubMed] [Google Scholar]
- 36.Boffa JW, Norr AM, Raines AM, Albanese BJ, Short NA, Schmidt NB. Anxiety sensitivity prospectively predicts posttraumatic stress symptoms following a campus shooting. Behav Ther. 2016;47(3):367-376. doi: 10.1016/j.beth.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 37.Olatunji BO, Fan Q. Anxiety sensitivity and post-traumatic stress reactions: evidence for intrusions and physiological arousal as mediating and moderating mechanisms. J Anxiety Disord. 2015;34:76-85. doi: 10.1016/j.janxdis.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 38.Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133(5):725-746. doi: 10.1037/0033-2909.133.5.725 [DOI] [PubMed] [Google Scholar]
- 39.Jovanovic T, Sakoman AJ, Kozarić-Kovačić D, et al. Acute stress disorder versus chronic posttraumatic stress disorder: inhibition of fear as a function of time since trauma. Depress Anxiety. 2013;30(3):217-224. doi: 10.1002/da.21991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orr SP, Metzger LJ, Lasko NB, et al. ; Harvard/Veterans Affairs Post-traumatic Stress Disorder Twin Study Investigators . Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: association with posttraumatic stress disorder. Arch Gen Psychiatry. 2003;60(3):283-288. doi: 10.1001/archpsyc.60.3.283 [DOI] [PubMed] [Google Scholar]
- 41.Shalev AY, Peri T, Brandes D, Freedman S, Orr SP, Pitman RK. Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. Am J Psychiatry. 2000;157(2):255-261. doi: 10.1176/appi.ajp.157.2.255 [DOI] [PubMed] [Google Scholar]
- 42.Sterling M, Hendrikz J, Kenardy J. Similar factors predict disability and posttraumatic stress disorder trajectories after whiplash injury. Pain. 2011;152(6):1272-1278. doi: 10.1016/j.pain.2011.01.056 [DOI] [PubMed] [Google Scholar]
- 43.Hu J, Bortsov AV, Ballina L, et al. Chronic widespread pain after motor vehicle collision typically occurs through immediate development and nonrecovery: results of an emergency department-based cohort study. Pain. 2016;157(2):438-444. doi: 10.1097/j.pain.0000000000000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu L, Bentler PM. Cutoff criterion for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1-55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- 45.Alphabet I. Developing a sensor-based wearable for continuous monitoring. Accessed August 26, 2022. https://verily.com/solutions/study-watch/
- 46.Smuck M, Odonkor CA, Wilt JK, Schmidt N, Swiernik MA. The emerging clinical role of wearables: factors for successful implementation in healthcare. NPJ Digit Med. 2021;4(1):45. doi: 10.1038/s41746-021-00418-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arges K, Assimes T, Bajaj V, et al. The Project Baseline Health Study: a step towards a broader mission to map human health. NPJ Digit Med. 2020;3(1):84. doi: 10.1038/s41746-020-0290-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borazio M, Berlin E, Kücükyildiz N, Scholl P, Van Laerhoven K. Towards benchmarked sleep detection with wrist-worn sensing units. Paper presented at: 2014 IEEE International Conference on Healthcare Informatics; September 15-17, 2014; Verona, Italy. [Google Scholar]
- 49.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342-392. doi: 10.1093/sleep/26.3.342 [DOI] [PubMed] [Google Scholar]
- 50.Kosmadopoulos A, Sargent C, Darwent D, Zhou X, Roach GD. Alternatives to polysomnography (PSG): a validation of wrist actigraphy and a partial-PSG system. Behav Res Methods. 2014;46(4):1032-1041. doi: 10.3758/s13428-013-0438-7 [DOI] [PubMed] [Google Scholar]
- 51.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11(1):16. doi: 10.1186/1742-4682-11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505-518. doi: 10.3109/07420529908998724 [DOI] [PubMed] [Google Scholar]
- 53.Huang Q, Railkar R. PROC MIXED: calculate correlation coefficients in the presence of repeated measurements. Accessed February 19, 2020. https://www.pharmasug.org/proceedings/2019/ST/PharmaSUG-2019-ST-321.pdf
- 54.Chiu KB, deRoon-Cassini TA, Brasel KJ. Factors identifying risk for psychological distress in the civilian trauma population. Acad Emerg Med. 2011;18(11):1156-1160. doi: 10.1111/j.1553-2712.2011.01206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wootton D, Feldman C. The diagnosis of pneumonia requires a chest radiograph (x-ray)—yes, no or sometimes? Pneumonia (Nathan). 2014;5(1)(suppl 1):1-7. doi: 10.15172/pneu.2014.5/464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leman P. Validity of urinalysis and microscopy for detecting urinary tract infection in the emergency department. Eur J Emerg Med. 2002;9(2):141-147. doi: 10.1097/00063110-200206000-00008 [DOI] [PubMed] [Google Scholar]
- 57.Borchgrevink GE, Kaasa A, McDonagh D, Stiles TC, Haraldseth O, Lereim I. Acute treatment of whiplash neck sprain injuries. a randomized trial of treatment during the first 14 days after a car accident. Spine (Phila Pa 1976). 1998;23(1):25-31. doi: 10.1097/00007632-199801010-00006 [DOI] [PubMed] [Google Scholar]
- 58.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539-1552. doi: 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neylan TC, Kessler RC, Ressler KJ, et al. Prior sleep problems and adverse post-traumatic neuropsychiatric sequelae of motor vehicle collision in the AURORA study. Sleep. 2021;44(3):zsaa200. doi: 10.1093/sleep/zsaa200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Babson KA, Feldner MT. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord. 2010;24(1):1-15. doi: 10.1016/j.janxdis.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169-184. doi: 10.1016/j.smrv.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 62.Troxel WM, Germain A, Buysse DJ. Clinical management of insomnia with brief behavioral treatment (BBTI). Behav Sleep Med. 2012;10(4):266-279. doi: 10.1080/15402002.2011.607200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maguen S, Gloria R, Huggins J, et al. Brief behavioral treatment for insomnia improves psychosocial functioning in veterans: results from a randomized controlled trial. Sleep. 2021;44(3):zsaa205. doi: 10.1093/sleep/zsaa205 [DOI] [PubMed] [Google Scholar]
- 64.Doherty A, Jackson D, Hammerla N, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. PLoS One. 2017;12(2):e0169649. doi: 10.1371/journal.pone.0169649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baron KG, Duffecy J, Berendsen MA, Cheung Mason I, Lattie EG, Manalo NC. Feeling validated yet? a scoping review of the use of consumer-targeted wearable and mobile technology to measure and improve sleep. Sleep Med Rev. 2018;40:151-159. doi: 10.1016/j.smrv.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Statistical Analyses
eTable 1. Latent Constructs Indicator Variable Questions From Smartphone-Based Follow-up Surveys
eTable 2. Descriptive Statistics of Rest-Activity Features
eTable 3. Correlation of Main Outcomes With Completion Rate of Main Tasks
eTable 4. State Activity Biomarkers for Subgroup of Participants Starting With Moderate or Severe Pain
eTable 5. State Activity Biomarkers Stratified by Sex
eFigure 1. Histogram and Correlation of Wrist Wearable Features
eFigure 2. Data Flow Pathways for Self-Report and Watch Data
eAppendix. Sample Analysis Code
Data Sharing Statement


