Key Points
Questions
Are patient risk factors and tumor characteristics associated with poor treatment outcomes in patients with primary cutaneous squamous cell carcinoma (cSCC), and which treatment modalities minimize poor outcomes?
Findings
In this systematic review and meta-analysis of 129 studies with more than 125 000 patients with cSCC, several patient risk factors, tumor characteristics, and treatment modalities were associated with poor outcomes. The highest risks for local recurrence and disease-specific death were associated with tumor invasion beyond subcutaneous fat, and the highest risk of metastasis was associated with perineural invasion.
Meaning
The findings of this meta-analysis demonstrate the prognostic value of different risk factors and effectiveness of treatment modalities; as such, these findings can guide prognostication, workup, and treatment of cSCC.
Abstract
Importance
Primary cutaneous squamous cell carcinoma is usually curable; however, a subset of patients develops poor outcomes, including local recurrence, nodal metastasis, distant metastasis, and disease-specific death.
Objectives
To evaluate all evidence-based reports of patient risk factors and tumor characteristics associated with poor outcomes in primary cutaneous squamous cell carcinoma and to identify treatment modalities that minimize poor outcomes.
Data Sources
PubMed, Embase, and SCOPUS databases were searched for studies of the topic in humans, published in the English language, from database inception through February 8, 2022.
Study Selection
Two authors independently screened the identified articles and included those that were original research with a sample size of 10 patients or more and that assessed risk factors and/or treatment modalities associated with poor outcomes among patients with primary cutaneous squamous cell carcinoma.
Data Extraction and Synthesis
Data extraction was performed by a single author, per international guidelines. The search terms, study objectives, and protocol methods were defined before study initiation. A total of 310 studies were included for full-text assessment. Owing to heterogeneity of the included studies, a random-effects model was used. Data analyses were performed from May 25 to September 15, 2022.
Main Outcomes and Measures
For studies of risk factors, risk ratios and incidence proportions; and for treatment studies, incidence proportions.
Results
In all, 129 studies and a total of 137 449 patients with primary cutaneous squamous cell carcinoma and 126 553 tumors were included in the meta-analysis. Several patient risk factors and tumor characteristics were associated with local recurrence, nodal metastasis, distant metastasis, disease-specific death, and all-cause death were identified. Among all factors reported by more than 1 study, the highest risks for local recurrence and disease-specific death were associated with tumor invasion beyond subcutaneous fat (risk ratio, 9.1 [95% CI, 2.8-29.2] and 10.4 [95% CI, 3.0- 36.3], respectively), and the highest risk of any metastasis was associated with perineural invasion (risk ratio, 5.0; 95% CI, 2.3-11.1). Patients who received Mohs micrographic surgery had the lowest incidence of nearly all poor outcomes; however, in some results, the 95% CIs overlapped with those of other treatment modalities.
Conclusions and Relevance
This meta-analysis identified the prognostic value of several risk factors and the effectiveness of the available treatment modalities. These findings carry important implications for the prognostication, workup, treatment, and follow-up of patients with primary cutaneous squamous cell carcinoma.
Trial Registration
PROSPERO Identifier: CRD42022311250
This systematic review and meta-analysis of 129 studies evaluates the predictors and treatment outcomes for primary cutaneous squamous cell carcinoma among a total of 137 449 patients.
Introduction
Cutaneous squamous cell carcinoma (cSCC) is the second most common malignant neoplasm in the US, with an estimated annual incidence of 1.1 million to 1.8 million per year.1,2,3,4 Although cSCC usually carries an excellent prognosis, a subset of patients develops poor outcomes, including local recurrence (LR), nodal metastasis (NM), distant metastasis (DM), and disease-specific death (DSD). We performed a systematic review and meta-analysis of all electronically available studies of cSCC and reviewed the associations of risk factors and treatments with poor outcomes to identify those with higher risk.
Methods
This systematic review and meta-analysis study was exempt from review, and informed consent was not required because the study used only previously published publicly available reports. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines. The protocol was registered with PROSPERO, and a few amendments were made to the analysis methodology.
Data Sources and Strategy
An electronic literature search was performed on February 8, 2022, using PubMed, Embase, and SCOPUS databases. Articles published from database inception through February 8, 2022, were included in the search. Boolean search terms were cutaneous [AND] squamous cell carcinoma [AND] treatment type (various) [OR] risk factors [AND] recurrence [OR] metastasis [OR] mortality [OR] disease-specific death.
Eligibility Criteria
Study selection was performed using the following inclusion criteria: (1) study type was a randomized clinical trial, case-series report, case-control report, or cohort study; (2) the study results included at least 1 histopathologic finding, tumor morphologic feature, or comorbid medical risk factor for cSCC or the use of a single curative-intent treatment modality; and (3) the study’s outcome of interest was either an LR, NM, DM, DSD, and/or all-cause death or any combination of these outcomes. A study was excluded if it (1) was not published in English; (2) included nonhuman species; (3) was a review article, abstract, errata, poster presentation, conference proceeding, or textbook chapter; (4) included pediatric patients; (5) had a sample size smaller than 10 patients with cSCC; (6) had data on cSCC that could not be extracted from other data; (7) had cSCC data that could not be extracted SCC in situ, noncutaneous SCC, or anogenital SCC data; (8) included patients with genetic disorders predisposing to cSCC; (9) had an outcome of interest present in all patients at study initiation; (10) had no assessment of risk factors or single curative-intent treatment; and/or (11) had no assessment of the outcome of interest.
Study Selection and Data Extraction
Titles and abstracts were assessed on these criteria by 2 independent blinded reviewers (G.A.Z., M.L.S.). If the title or abstract did not include enough information to apply exclusion criteria, the full text was assessed. Reviewers compared results, and discrepancies were resolved by third author arbitration (J.A.C.). The full text of remaining articles was reviewed in detail to determine eligibility based on the inclusion criteria. Data abstraction was performed by an author (G.A.Z.). In cases of study duplicity, the more recent and complete studies were selected for inclusion. Risk of bias was evaluated for each study by one of the authors (A.N.P.) using the Newcastle−Ottawa Scale for non-randomized studies5 and the Cochrane Collaboration tool6 for randomized studies.
Statistical Analysis
Meta-analysis of risk factor data was performed through 2 strategies based on the format of the reported data—random effects analysis of risk ratio (RR) data (when it was possible to extract or calculate hazard or odds ratios) and random effects analysis of incidence data (when it was possible to calculate incidence proportions). Random effects analysis was performed using Comprehensive Meta-Analysis, version 3, Biostat). Whenever possible, the RRs and 95% CIs were reported for each risk factor per outcome measure. The I2 statistic was used to quantify heterogeneity; a value exceeding 50% was considered to be substantial heterogeneity. Forest plots were created for all associations of risk factors and outcomes using Excel, version 16.61.1 (Microsoft). Treatment data were analyzed by random effects analysis for incidence proportions for each treatment modality per outcome.
Results
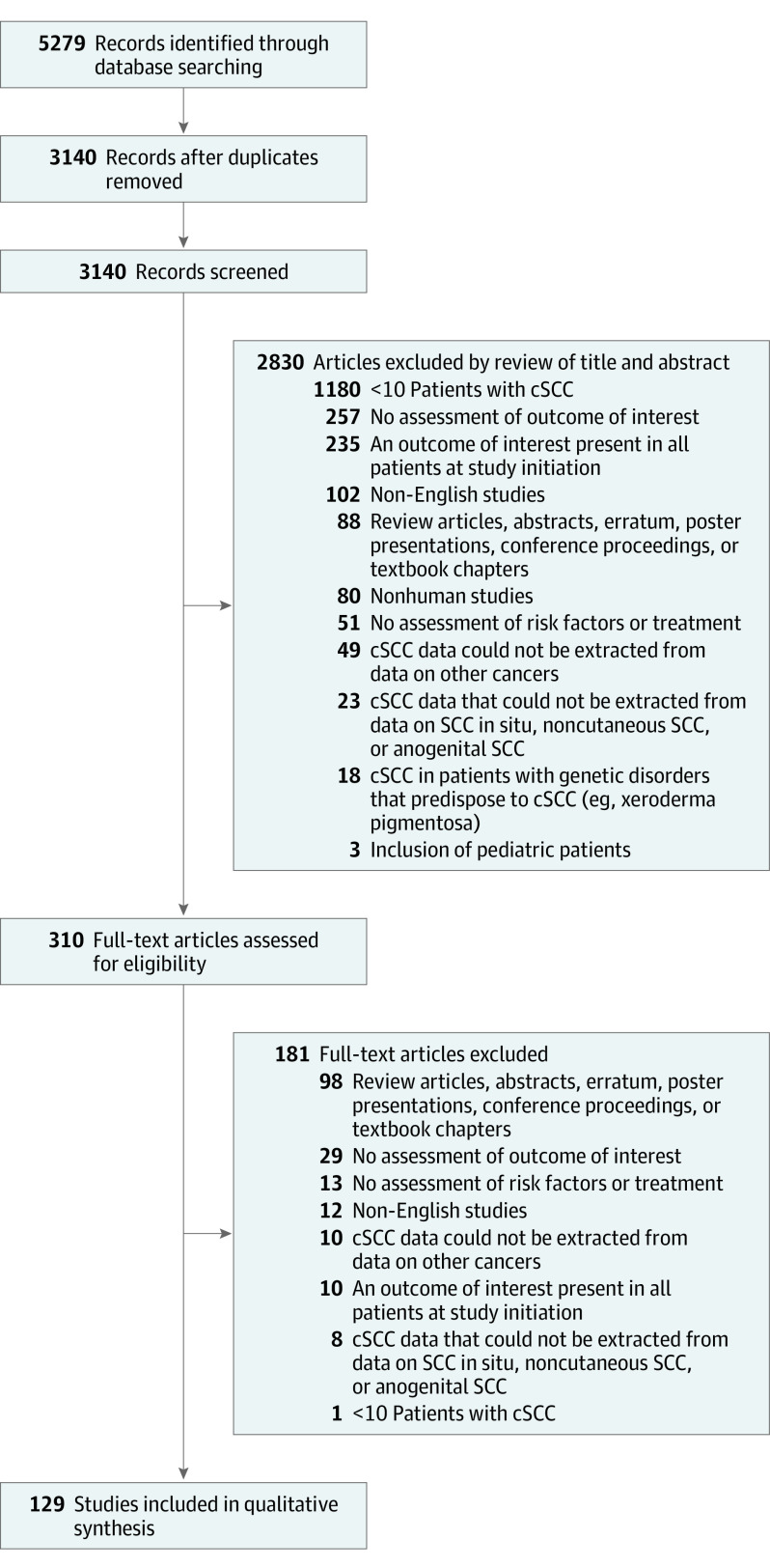
The initial search identified 5279 articles. After removal of duplicates, the titles and abstracts of 3140 articles were reviewed. After inclusion criteria were applied, the full text of 310 articles were assessed. Of these, 129 studies7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135 were included in final analysis (Figure 1). Study characteristics, including risk of bias, are described in eTable 1 in the Supplement. Of these 129 studies, 114 studies7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121 assessed risk factors, with a total of 137 449 patients and 126 553 tumors. Of these, 87 studies8,10,11,13,14,15,16,17,18,19,21,23,25,27,30,31,33,34,35,37,38,39,40,41,43,44,45,47,48,50,52,53,55,56,57,58,59,60,61,62,63,64,66,69,70,71,72,73,74,75,77,78,79,80,81,83,84,85,86,87,88,90,91,92,93,94,96,97,98,100,101,102,104,106,107,108,109,110,111,112,113,114,115,118,119,120,121 with 122 484 tumors were included in the random effects analysis risk ratio, and 62 studies7,8,10,12,13,15,17,18,19,20,22,23,25,26,27,28,29,30,31,32,36,37,38,40,42,46,47,48,50,53,54,55,56,59,63,65,67,68,70,71,73,74,76,79,80,81,82,83,84,85,89,90,92,93,94,95,98,99,101,103,114,116 with 116 958 tumors were included in analysis of incidence data. Treatment modality was assessed by 28 studies8,10,27,34,35,40,41,53,59,80,90,96,97,101,122,123,124,125,126,127,128,129,130,131,132,133,134,135 with 10 967 patients and 6118 cSCC tumors.
Figure 1. Search Methodology and Results of Literature Search on Risk Factors Associated With Poor Outcome in Primary Cutaneous Squamous Cell Carcinoma (cSCC).
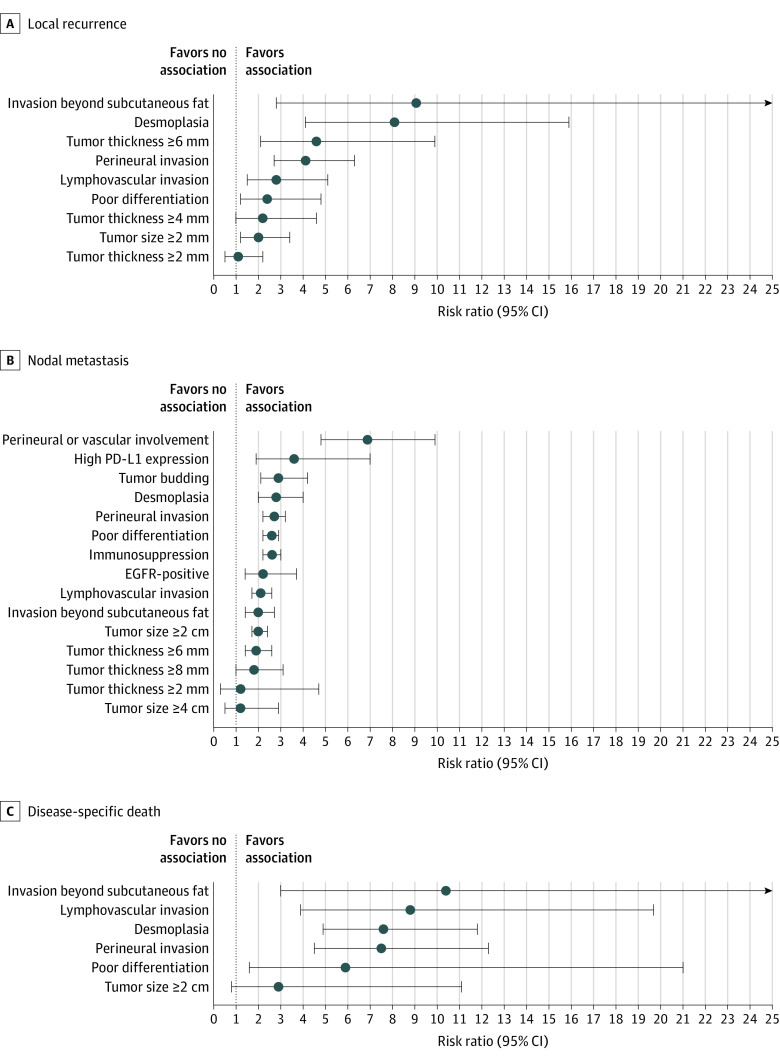
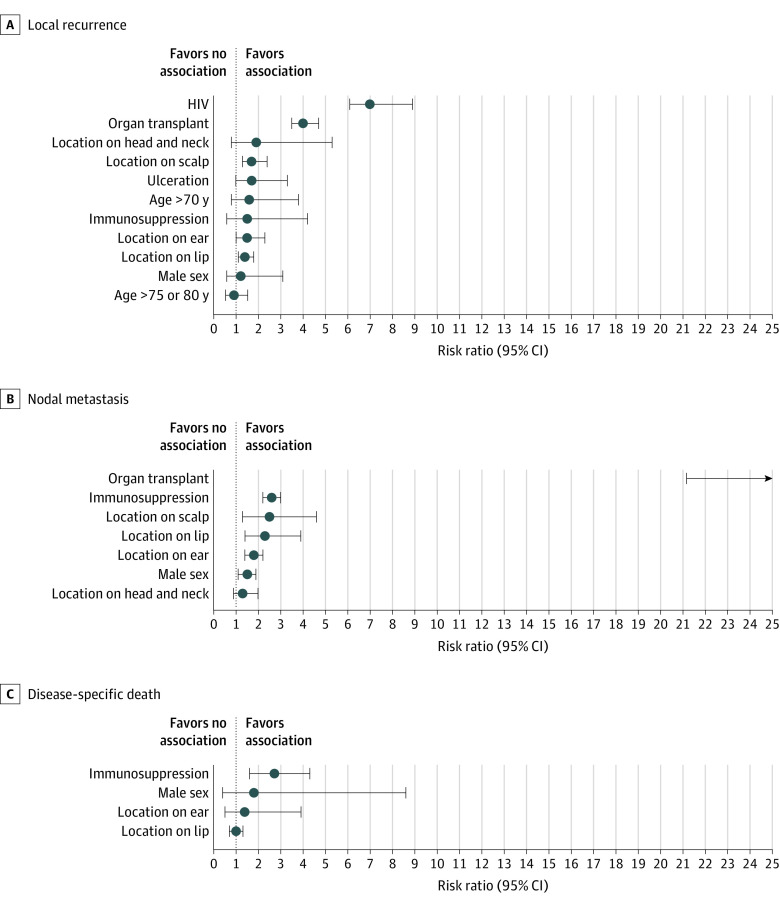
For the analysis of RR, analyzed histopathologic and clinical risk factors and associations with LR, NM, and DSD are outlined in Tables 1 and 2, respectively. Associations for histopathologic and clinical risk factors and the outcomes described by more than 1 study are outlined in Figures 2 and 3, respectively. All associations with DM, any metastasis (AM), or combined outcomes are outlined in eTable 2 in the Supplement, and those described in more than 1 study are shown in the eFigure in the Supplement.
Table 1. Results of Random Effects Analysis of Risk Ratio for Each Histopathologic Risk Factor for Local Recurrence, Nodal Metastasis, and Disease-Specific Death.
| Risk factor | Risk ratio (95% CI) | P value | Studies, No. | I2 statisic, % |
|---|---|---|---|---|
| Local recurrence | ||||
| Invasion beyond subcutaneous fat | 9.1 (2.8-29.2) | <.001 | 5 | 0 |
| Desmoplasia | 8.1 (4.1-15.9) | <.001 | 3 | 17 |
| Tumor thickness ≥6 mm | 4.6 (2.1-9.9) | <.001 | 3 | 1 |
| Perineural invasion | 4.1 (2.7-6.3) | <.001 | 12 | 0 |
| Lymphovascular invasion | 2.8 (1.5-5.1) | <.001 | 6 | 0 |
| Poor differentiation | 2.4 (1.2-4.8) | .02 | 12 | 0 |
| Tumor thickness ≥8 mm | 2.2 (0.6-7.8) | .23 | 1 | 0 |
| Tumor thickness ≥4 mm | 2.2 (1.0-4.6) | .05 | 2 | 0 |
| Tumor size ≥2 cm | 2.0 (1.2-3.4) | .01 | 10 | 0 |
| Tumor size ≥5 cm | 1.6 (0.4-7.5) | .52 | 1 | 0 |
| Tumor thickness ≥2 mm | 1.1 (0.5-2.2) | .85 | 2 | 0 |
| Nodal metastasis | ||||
| Tumor thickness ≥5 mm | 18.7 (2.5-139) | .004 | 1 | 0 |
| Tumor thickness ≥4 mm | 7.2 (2.1-24.3) | .002 | 1 | 0 |
| Perineural/vascular involvement | 6.9 (4.8-9.9) | <.001 | 3 | 12 |
| Cytokeratin 19 expression | 5.0 (0.7-35.8) | .11 | 1 | 0 |
| High PD-L1 expression | 3.6 (1.9-7.0) | .001 | 2 | 0 |
| LLT-1 expression | 3.4 (1.4-8.3) | .007 | 1 | 0 |
| Tumor budding | 2.9 (2.1-4.2) | <.001 | 3 | 50 |
| Desmoplasia | 2.8 (2.0-4.0) | <.001 | 2 | 0 |
| Perineural invasion | 2.7 (2.2-3.2) | <.001 | 19 | 0 |
| Poor differentiation | 2.6 (2.2-2.9) | <.001 | 18 | 29 |
| Membrane E-cadherin | 2.2 (1.7-3.0) | <.001 | 1 | 0 |
| EGFR+ | 2.2 (1.4-3.7) | .002 | 2 | 0 |
| Lymphovascular invasion | 2.1 (1.7-2.6) | <.001 | 11 | 0 |
| Cortactin | 2.0 (1.1-3.9) | .03 | 1 | 0 |
| Tumor size ≥2 cm | 2.0 (1.7-2.4) | <.001 | 13 | 0 |
| Invasion beyond subcutaneous fat | 2.0 (1.4-2.7) | <.001 | 8 | 0 |
| Tumor thickness ≥6 mm | 1.9 (1.4-2.6) | <.001 | 5 | 28 |
| Tumor thickness ≥8 mm | 1.8 (1.0-3.1) | .05 | 2 | 0 |
| Cytoplasm E-Cadherin | 1.3 (0.7-2.3) | .39 | 1 | 0 |
| ERBB3+ | 1.3 (0.7-2.4) | .44 | 1 | 0 |
| Tumor size ≥4 cm | 1.2 (0.5-2.9) | .71 | 2 | 0 |
| Tumor thickness ≥2 mm | 1.2 (0.3-4.7) | .82 | 2 | 0 |
| Tumor thickness ≥3 mm | 1.2 (0.1-9.9) | .88 | 1 | 0 |
| FAK expression | 1.0 (0.6-1.9) | .95 | 1 | 0 |
| Tumor size ≥5 cm | 1.0 (0.6-1.9) | .95 | 1 | 0 |
| ERBB4+ | 0.8 (0.4-1.6) | .58 | 1 | 0 |
| Podoplanin | 0.8 (0.5-1.3) | .38 | 1 | 0 |
| Disease-specific death | ||||
| Invasion beyond subcutaneous fat | 10.4 (3.0-36.3) | <.001 | 5 | 0 |
| Tumor size ≥5 cm | 8.9 (4.7-17.0) | <.001 | 1 | 0 |
| Lymphovascular invasion | 8.8 (3.9-19.7) | <.001 | 3 | 0 |
| Desmoplasia | 7.6 (4.9-11.8) | <.001 | 2 | 1 |
| Perineural invasion | 7.5 (4.5-12.3) | <.001 | 7 | 2 |
| Tumor thickness ≥6 mm | 6.5 (3.4-12.3) | <.001 | 1 | 0 |
| LLT-1 expression | 6.2 (1.8-21.3) | .004 | 1 | 0 |
| Poor differentiation | 5.9 (1.6-21.0) | .007 | 8 | 6 |
| Tumor size ≥2 cm | 2.9 (0.8-11.1) | .12 | 6 | 0 |
| Podoplanin | 2.8 (1.0-8.0) | .05 | 1 | 0 |
| FAK expression | 1.6 (0.8-3.2) | .23 | 1 | 0 |
| Cortactin | 1.0 (0.5-1.9) | .98 | 1 | 0 |
Abbreviations: EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase; LLT-1, lectin-like transcript 1 expression; PD-L1, programmed death ligand-1.
Table 2. Results of Random Effects Analysis: Risk Ratio for Each Clinical Risk Factor for Local Recurrence, Nodal Metastasis, and Disease-Specific Death.
| Risk factor | Risk ratio (95% CI) | P value | Studies, No. | I2statistic, % |
|---|---|---|---|---|
| Local recurrence | ||||
| HIV | 7.0 (2.1-23.1) | .001 | 2 | 0 |
| Organ transplantation | 4.0 (2.2-7.0) | <.001 | 4 | 5 |
| Chronic lymphocytic leukemia | 3.8 (1.5-9.8) | .005 | 1 | 0 |
| Age >65 y | 2.3 (0.5-9.9) | .26 | 1 | 0 |
| Tumor on ear or lip | 2.0 (0.6-6.8) | .26 | 1 | 0 |
| Tumor on head or neck | 1.9 (0.9-3.9) | .09 | 5 | 0 |
| Ulceration | 1.7 (1.2-2.4) | .001 | 4 | 0 |
| Tumor on scalp | 1.7 (0.8-3.6) | .21 | 3 | 0 |
| Age >70 y | 1.6 (0.5-5.0) | .46 | 2 | 0 |
| Tumor on ear | 1.5 (0.8-3.1) | .23 | 6 | 0 |
| Immunosuppression | 1.5 (1.1-2.2) | .03 | 14 | 0 |
| Growth rate ≥4 mm/mo | 1.4 (0.5-4.1) | .50 | 1 | 0 |
| Tumor on lip | 1.4 (0.6-3.6) | .45 | 4 | 0 |
| Male sex | 1.2 (0.9-1.6) | .13 | 10 | 0 |
| Age >60 y | 1.0 (0.4-2.9) | .96 | 1 | 0 |
| Age >75 y | 0.9 (0.5-1.5) | .91 | 3 | 9 |
| Nodal metastasis | ||||
| Organ transplant | 38.6 (21.2-70.3) | <.001 | 2 | 0 |
| Chronic lymphocytic leukemia | 4.5 (2.1-9.5) | <.001 | 1 | 0 |
| Hypothyroidism | 4.0 (2.8-5.7) | <.001 | 1 | 0 |
| Age >65 y | 3.9 (1.7-8.9) | .001 | 1 | 0 |
| Growth rate ≥4 mm/mo | 3.2 (1.1-9.7) | .04 | 1 | 0 |
| Tumor on ear or lip | 2.9 (1.4-5.9) | .003 | 1 | 0 |
| HIV | 2.8 (0.8-10.3) | .11 | 1 | 0 |
| Age >70 y | 2.7 (1.4-5.0) | .003 | 1 | 0 |
| Immunosuppression | 2.6 (2.2-3.0) | <.001 | 8 | 17 |
| Tumor on scalp | 2.5 (1.3-4.6) | .004 | 4 | 0 |
| Tumor on lip | 2.3 (1.4-3.9) | .002 | 6 | 5 |
| Hematologic malignant tumor | 2.1 (0.4-11.3) | .38 | 1 | 0 |
| Diabetes | 1.8 (0.3-12.1) | .53 | 1 | 0 |
| Tumor on ear | 1.8 (1.4-2.2) | <.001 | 9 | 12 |
| Male sex | 1.5 (1.1-1.9) | .002 | 8 | 3 |
| Tumor on head or neck | 1.3 (0.9-2.0) | .14 | 4 | 0 |
| Age >75 y | 1.3 (0.3-5.9) | .76 | 2 | 0 |
| Smoking history | 1.1 (0.7-1.7) | .75 | 1 | 0 |
| Disease-specific death | ||||
| Tumor on head or neck | 4.4 (1.6-12.2) | .005 | 1 | 0 |
| Organ transplant | 4.1 (0.7-24.2) | .12 | 1 | 0 |
| Immunosuppression | 2.7 (1.6-4.5) | <.001 | 9 | 36 |
| Ulceration | 2.2 (1.2-4.1) | .01 | 1 | 0 |
| Male sex | 1.8 (0.4-8.6) | .45 | 3 | 0 |
| Tumor on ear | 1.4 (0.5-3.9) | .58 | 2 | 8 |
| Tumor on lip | 1.0 (0.7-1.3) | .94 | 2 | 0 |
| Tumor on ear or lip | 0.9 (0.2-3.8) | .90 | 1 | 0 |
| Age >65 y | 0.5 (0.2-1.1) | .10 | 1 | 0 |
Figure 2. Forest Plots of Results of Random Effects Analysis for Pairs of Histopathologic Risk Factors and Local Recurrence, Nodal Metastasis, and Disease-specific Death Identified in More Than 1 Study.
Circles denote the composite risk ratio; whiskers, the 95% CIs. EGFR indicates epidermal growth factor receptor, and PD-L1, programmed death ligand-1.
Figure 3. Forest Plots of Results of Random Effects Analysis for Pairs of Clinical Risk Factors and Local Recurrence, Nodal Metastasis, and Disease-specific Death Identified in More Than 1 Study.
Circles denote the composite risk ratio; whiskers, the 95% CIs. For nodal metastasis organ transplant (panel B), the risk ratio is approximately 39.
Several notable tumor factors were associated with poor outcomes. Tumors on the lip, ear, and scalp were associated with a significantly increased risk of NM (lip RR, 2.3 [95% CI, 1.4-3.9]; ear RR, 1.8 [95% CI, 1.4-2.2]; scalp RR, 2.5 [95% CI, 1.3-4.6]) and AM (lip RR, 1.8 [95% CI, 1.4-2.4]; ear RR, 2.8 [95% CI, 1.2-3.2]). Tumors with a diameter of 2 cm or more had a significantly increased risk of LR (RR, 2.0; 95% CI, 1.2-3.4), NM (RR, 2.0; 95% CI, 1.7-2.4), AM (RR, 2.9; 95% CI, 1.6-5.1), and all-cause death (RR, 1.8; 95% CI, 1.3-2.6). Tumors with invasion beyond subcutaneous fat were associated with a statistically increased risk of LR (RR, 9.1; 95% CI, 2.8-29.2), NM (RR, 2.0; 95% CI, 1.4-2.7), AM (RR, 4.0; 95% CI, 2.4-6.5), and DSD (RR, 10.4; 95% CI, 3.0-36.3). Ulcerated tumors were associated with increased risks of LR (RR, 1.7; 95% CI, 1.2-2.4) and DSD (RR, 2.2; 95% CI, 1.2-4.1). Poor differentiation was associated with LR (RR, 2.4; 95% CI, 1.2-4.8), NM (RR, 2.6; 95% CI, 2.2-2.9), DM (RR, 3.9; 95% CI, 1.9-8.2), AM (RR, 2.9; 95% CI, 1.6-5.1), DSD (RR, 5.9; 95% CI, 1.6-21.0), all-cause death (RR, 1.8; 95% CI, 1.1-2.4), and locoregional (RR, 2.6; 95% CI, 1.7-4.0). Desmoplastic stroma was associated with a statistically increased risk of LR (RR, 8.1; 95% CI, 4.1-15.9), NM (RR, 2.8; 95% CI, 2.0-4.0), DM (RR, 17.3; 95% CI, 4.4-68.4), and DSD (RR, 7.6; 95% CI, 4.9-11.8). Tumor budding was associated with a statistically increased risk of NM (RR, 2.9; 95% CI, 2.1-4.2). Perineural invasion was associated with statically significant risks of LR (RR, 4.1; 95% CI, 2.7-6.3), NM (RR, 2.7; 95% CI, 2.2-3.2), DM (RR, 4.8; 95% CI, 2.2-10.4), AM (RR, 5.0; 95% CI, 2.3-11.1), DSD (RR, 7.5; 95% CI, 4.5-12.3), all-cause death (RR, 1.8; 95% CI, 1.4-2.3), and had the highest RR among all risk factors for DM and AM identified in more than 1 study. Lymphovascular invasion had similarly elevated RRs, with statistically significant increased risks of LR (RR, 2.8; 95% CI, 1.5-5.1), NM (RR, 2.1; 95% CI, 1.7-2.6), DM (RR, 9.3; 95% CI, 1.3-66.0), AM (RR, 3.2; 95% CI, 2.1-5.0), DSD (RR, 8.8; 95% CI, 3.9-19.7), and approached statistical significance for all-cause death (RR, 1.9; 95% CI, 1.0-3.6; P = .07).
Several patient factors were associated with poor outcomes. Most notably, patients with immunosuppression had a significantly increased risk of LR (RR, 1.5; 95% CI, 1.1-2.2), NM (RR, 2.6; 95% CI, 2.2-3.0), AM (RR, 1.6; 95% CI, 1.2-2.2), DSD (RR, 2.7; 95% CI, 1.6-4.5), all-cause death (RR, 2.7; 95% CI, 1.5-4.6), and locoregional recurrence (RR, 2.9; 95% CI, 2.0-4.2). When looking at causes of immunosuppression, recipients of solid organ transplantation were at a statistically increased risks of LR (RR, 4.0; 95% CI, 2.2-7.0) and NM (RR, 38.6; 95% CI, 21.2-70.3). We found statistically increased risks of LR (RR, 7.0; 95% CI, 2.1-23.1) in patients with HIV.
Random effects analyses of proportions of each risk factor outcome pairing are described in eTable 3 in the Supplement. For treatment data, random effects analysis of incidence proportion for each treatment modality per outcome is reported in eTable 4 in the Supplement. Mohs micrographic surgery (MMS) had lower incidence than standard excision for each outcome of interest; however, there was overlap of 95% CIs. The eReferences of sources supporting the eTables are available in the Supplement.
Discussion
This study aimed to summarize the literature on patient and tumor factors associated with poor outcomes, providing an update to prior work by Rowe and colleagues.136 The meta-analysis of these data quantifies the influence of established and emerging risk factors on patient outcomes. As such, these results have implications on current staging systems, workup protocols, and treatment algorithms of invasive cSCC.
Implications for Staging and Prognostication
The American Joint Committee on Cancer 8 (AJCC)137 and Brigham and Women’s Hospital (BWH)66 are the current standards in cSCC staging. Both systems include only tumor-specific risk factors; the AJCC is a consensus system based on expert opinion for staging of head and neck cSCC,137 whereas the BWH was built on data from 256 tumors.66 The findings of this meta-analysis support many of the features used by both systems but suggest others may be of limited prognostic value. We also identified additional risk factors that should be considered in patient prognostication.
Although patient factors are not included in either the AJCC or BWH guidelines, they are present in a number of historical138 and contemporary systems.137,139 Our findings support immunosuppression as a high-risk prognostic feature as defined by the National Comprehensive Cancer Network (NCCN) guidelines.140 In the present analysis, immunosuppression was a significant risk factor for every individual poor outcome assessed except DM, for which only 2 studies were identified compared with 7 to 18 studies for all other individual outcomes. Given its predictive value for LR, NM, AM, and DSD, omission of immunosuppression in patient prognostication provides an incomplete picture of risk. In addition, our findings suggest a potentially different risk profile based on causes of immunosuppression; however, the statistical analysis was limited by the number of studies. Further investigation is needed in this area to better characterize the distinct risk profiles of each subgroup.
Several tumor characteristics identified as high-risk in this meta-analysis are absent from current staging systems. Among these were histologic and architectural factors that were among the most consistently associated with poor outcomes. Namely, desmoplastic stroma and lymphovascular invasion showed an increased risk of poor outcomes on par with perineural invasion and deserve strong consideration for inclusion in future systems. In addition, poor differentiation was omitted from the most recent AJCC criteria137 given concerns of inconsistency in definition and application across the US. In pooling results from multiple different sites across the US and the world, our findings demonstrated that this risk factor was consistently highly predictive of every poor outcome assessed, despite the potential institutional differences in definition and use.
The findings of this study also suggest that the role of tumor size (diameter) in staging should be reexamined. Although the BWH attaches the same risk to all tumors of 2 cm in diameter or larger,66 the AJCC designates tumors of 2 to 4 cm as T2 and those of 4 cm or larger as T3.137 The findings of the present study indicate that the most important inflection in risk stratification occurs at the 2-cm threshold, thus supporting the BWH classification and challenging the AJCC T3 size stratification. Finally, other factors were associated with some but not all poor outcomes that warrant further research and possible subsequent consideration, including tumor budding, ulceration, and tumor location.
Perioperative Counseling
The results of this meta-analysis may also be used to inform perioperative counseling and work on cSCC. Currently, there is a lack of consensus on which tumors should receive further workup.140 Per NCCN guidelines, imaging studies are indicated for tumors that are suggestive of extensive disease, defined as deep structural involvement, perineural disease, or deep soft tissue involvement.140 Our findings suggest that consideration of imaging and more frequent follow-up examinations may also be appropriate in patients with high-risk microscopic features, such as desmoplastic stroma or poor differentiation. Moreover, closer follow-up may be indicated in patients with immunosuppression, especially those with a history of solid organ transplantation.
Treatment Modalities
In addition to addressing the association of risk factors, we explored and compared the association of treatment with poor outcomes. Across outcomes, MMS was found to have the lowest proportions of poor outcomes, although 95% CIs overlapped with those of other treatment modalities. These results support continued use of MMS as first-line therapy for high-risk SCC.
The Appropriate Use Criteria (AUC) for MMS details 270 scenarios for which MMS may be considered and provides recommendations of appropriate, uncertain, and inappropriate based on a synthesis of clinical experience and evidence review; it supports MMS for all histologically aggressive subtypes for all tumor sites and sizes.141 Other tumor factors identified, such as tumor budding and ulceration (Figure 2), require further investigation and may subsequently merit consideration for inclusion in the AUC.
In addition, our study data suggest that MMS should be appropriate for all cSCC in patients with immunosuppression. Although the current AUC designation for MMS is uncertain in smaller nonaggressive cSCC of the trunk and extremities,141 we found that immunosuppression predicts poor outcomes with consistency on par with several AUC aggressive tumor features and size greater than 2 cm.
Finally, our findings support the NCCN designation of perineural invasion greater than 0.1 mm, lymphovascular invasion, desmoplastic stroma, and poor differentiation as the highest-risk features in recommendations for adjuvant radiotherapy; each of these factors were among the most consistently high risk in our analysis.140 Notably, the NCCN recommendation and BWH data highlight large caliber (>0.1 mm) perineural invasive tumors as being of highest risk69; however, we were unable to stratify results by nerve diameter. Our findings also suggest that patients who are immunosuppressed, especially those with a history of solid organ transplantation, may require multidisciplinary discussion for the consideration of radiotherapy when presenting with other high-risk factors.
Histochemical Stains and Tests
In our review, we identified studies assessing risk of poor outcomes with 11 immunohistochemical extracellular molecule stains and 1 gene expression test. Positive stains of many extracellular molecules were associated with a significantly increased risk of poor outcomes in single studies and warrant further research. Stains for epidermal growth factor receptor and programmed death ligand-1, and the 40-GEP test deserve particular attention.
Limitations
Although this analysis included 129 studies, it is possible that pertinent studies may have been omitted given the inherent limitations of database literature searches. Furthermore, data format, study designs, and reporting of follow-up were heterogenous among studies, which may have affected the analysis. Finally, some risk factor−outcome pairs were only assessed by a single study, and we focused primarily on risk factors found by multiple studies.
Conclusions
This systematic review and meta-analysis found several patient risk factors and tumor characteristics associated with LR, NM, DM, DSD, and all-cause death. Several of these—ie, lymphovascular invasion, desmoplasia, and immunosuppression—were associated with a higher risk of poor outcomes; however, they are not currently included in the BWH or AJCC guidelines. These tumor characteristics should be considered in future staging systems, workup protocols, and treatment algorithms. Moreover, we identified several emerging risk factors, immunohistochemical stains, and a prognostic test that warrant further research. Lastly, patients receiving MMS had the lowest proportion of nearly all poor outcomes, supporting the use of MMS as the preferred treatment modality in patients with high-risk factors.
eTable 1. Evaluation of cohort studies – risk factor (level 4 evidence)
eTable 2. Results of random effects analysis: risk ratio for each risk factor and distant metastasis, any metastasis, locoregional recurrence, and combined outcomes
eTable 3. Incidence proportion for each risk factor modality per outcome across all studies
eTable 4. Incidence proportion for each treatment modality per outcome across all studies
eFigure. Forest plots of results of random effects analysis: risk ratio for each risk factor-and distant metastasis, any metastasis, and all-cause death identified in more than 1 study
eReferences
References
- 1.Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156(11):1192-1198. doi: 10.1001/jamadermatol.2020.2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151(10):1081-1086. doi: 10.1001/jamadermatol.2015.1187 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society . Key Statistics for Basal and Squamous Cell Skin Cancers. 2022. Accessed June 12, 2022. https://www.cancer.org/cancer/basal-and-squamous-cell-skin-cancer/about/key-statistics.html
- 5.Deeks JJ, Dinnes J, D’Amico R, et al. ; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group . Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii-x, 1-173. doi: 10.3310/hta7270 [DOI] [PubMed] [Google Scholar]
- 6.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam M, Brown RN, Silber DH, et al. ; Cardiac Transplant Research Database Group . Increased incidence and mortality associated with skin cancers after cardiac transplant. Am J Transplant. 2011;11(7):1488-1497. doi: 10.1111/j.1600-6143.2011.03598.x [DOI] [PubMed] [Google Scholar]
- 8.Baker NJ, Webb AA, Macpherson D. Surgical management of cutaneous squamous cell carcinoma of the head and neck. Br J Oral Maxillofac Surg. 2001;39(2):87-90. doi: 10.1054/bjom.2000.0584 [DOI] [PubMed] [Google Scholar]
- 9.Breuninger H, Brantsch K, Eigentler T, Häfner HM. Comparison and evaluation of the current staging of cutaneous carcinomas. J Dtsch Dermatol Ges. 2012;10(8):579-586. doi: 10.1111/j.1610-0387.2012.07896.x [DOI] [PubMed] [Google Scholar]
- 10.Brinkman JN, Hajder E, van der Holt B, Den Bakker MA, Hovius SER, Mureau MAM. The effect of differentiation grade of cutaneous squamous cell carcinoma on excision margins, local recurrence, metastasis, and patient survival: a retrospective follow-up study. Ann Plast Surg. 2015;75(3):323-326. doi: 10.1097/SAP.0000000000000110 [DOI] [PubMed] [Google Scholar]
- 11.Brougham NDLS, Dennett ER, Cameron R, Tan ST. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012;106(7):811-815. doi: 10.1002/jso.23155 [DOI] [PubMed] [Google Scholar]
- 12.Conde-Ferreirós A, Corchete LA, Jaka A, et al. Patterns of incidental perineural invasion and prognosis in cutaneous squamous cell carcinoma: a multicenter, retrospective cohort study. J Am Acad Dermatol. 2021;84(6):1708-1712. doi: 10.1016/j.jaad.2020.08.017 [DOI] [PubMed] [Google Scholar]
- 13.Demirdover C, Geyik A, Vayvada H, Yilmaz M. The accuracy of sentinel lymph node positivity in patients with cutaneous squamous cell carcinoma. Acta Med Mediter. 2020;36(6):3345-3349. [Google Scholar]
- 14.Dimonitsas E, Champsas G, Kakagia D, et al. Tracking the risk factors associated with high-risk cSCC: a 10-year, Two-Institution, Greek study. J BUON. 2021;26(3):1148-1158. [PubMed] [Google Scholar]
- 15.Dormand EL, Ridha H, Vesely MJ. Long-term outcome of squamous cell carcinoma of the upper and lower limbs. J Plast Reconstr Aesthet Surg. 2010;63(10):1705-1711. doi: 10.1016/j.bjps.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Duran J, Morgan FC, Karia PS, Schmults CD. An evaluation of high-stage cutaneous squamous cell carcinoma outcomes by sex. Br J Dermatol. 2017;177(4):1131-1133. doi: 10.1111/bjd.15208 [DOI] [PubMed] [Google Scholar]
- 17.Eigentler TK, Leiter U, Häfner HM, Garbe C, Röcken M, Breuninger H. Survival of patients with cutaneous squamous cell carcinoma: results of a prospective cohort study. J Invest Dermatol. 2017;137(11):2309-2315. doi: 10.1016/j.jid.2017.06.025 [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto M, Yamamoto Y, Matsuzaki I, et al. Tumor budding is an independent risk factor for lymph node metastasis in cutaneous squamous cell carcinoma: a single center retrospective study. J Cutan Pathol. 2016;43(9):766-771. doi: 10.1111/cup.12740 [DOI] [PubMed] [Google Scholar]
- 19.Genders RE, Osinga JAJ, Tromp EE, O’Rourke P, Bouwes Bavinck JN, Plasmeijer EI. Metastasis risk of cutaneous squamous cell carcinoma in organ transplant recipients and immunocompetent patients. Acta Derm Venereol. 2018;98(6):551-555. doi: 10.2340/00015555-2901 [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez JL, Cunningham K, Silverman R, Madan E, Nguyen BM. Comparison of the American Joint Committee on Cancer seventh edition and Brigham and Women’s Hospital cutaneous squamous cell carcinoma tumor staging in immunosuppressed patients. Dermatol Surg. 2017;43(6):784-791. doi: 10.1097/DSS.0000000000001038 [DOI] [PubMed] [Google Scholar]
- 21.Jansen P, Petri M, Merz SF, et al. The prognostic value of sentinel lymph nodes on distant metastasis-free survival in patients with high-risk squamous cell carcinoma. Eur J Cancer. 2019;111:107-115. doi: 10.1016/j.ejca.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 22.Kovatch KJ, Smith JD, Birkeland AC, et al. Institutional experience of treatment and outcomes for cutaneous periauricular squamous cell carcinoma. OTO Open. 2019;3(3):X19875077. doi: 10.1177/2473974X19875077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manyam B, Garsa AA, Chin RI, et al. A multi-institutional comparison of outcomes of immunocompromised and immunocompetent patients treated with surgery and radiation therapy for cutaneous squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2016;94(4):948. doi: 10.1016/j.ijrobp.2015.12.286 [DOI] [Google Scholar]
- 24.Manyam BV, Gastman B, Zhang AY, et al. Inferior outcomes in immunosuppressed patients with high-risk cutaneous squamous cell carcinoma of the head and neck treated with surgery and radiation therapy. J Am Acad Dermatol. 2015;73(2):221-227. doi: 10.1016/j.jaad.2015.04.037 [DOI] [PubMed] [Google Scholar]
- 25.Vasconcelos L, Melo JC, Miot HA, Marques ME, Abbade LPF. Invasive head and neck cutaneous squamous cell carcinoma: clinical and histopathological characteristics, frequency of local recurrence and metastasis. An Bras Dermatol. 2014;89(4):562-568. doi: 10.1590/abd1806-4841.20142810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ong CS, Keogh AM, Kossard S, Macdonald PS, Spratt PM. Skin cancer in Australian heart transplant recipients. J Am Acad Dermatol. 1999;40(1):27-34. doi: 10.1016/S0190-9622(99)70525-6 [DOI] [PubMed] [Google Scholar]
- 27.Roozeboom MH, Lohman BGPM, Westers-Attema A, et al. Clinical and histological prognostic factors for local recurrence and metastasis of cutaneous squamous cell carcinoma: analysis of a defined population. Ned Tijdschr Dermatol Venereol. 2012;22(1):50. [DOI] [PubMed] [Google Scholar]
- 28.Ross AS, Whalen FM, Elenitsas R, Xu X, Troxel AB, Schmults CD. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35(12):1859-1866. doi: 10.1111/j.1524-4725.2009.01354.x [DOI] [PubMed] [Google Scholar]
- 29.Salmon PJM, Hussain W, Geisse JK, Grekin RC, Mortimer NJ. Sclerosing squamous cell carcinoma of the skin, an underemphasized locally aggressive variant: a 20-year experience. Dermatol Surg. 2011;37(5):664-670. doi: 10.1111/j.1524-4725.2010.01850.x [DOI] [PubMed] [Google Scholar]
- 30.Seddon A, Hock B, Miller A, et al. Cutaneous squamous cell carcinomas with markers of increased metastatic risk are associated with elevated numbers of neutrophils and/or granulocytic myeloid derived suppressor cells. J Dermatol Sci. 2016;83(2):124-130. doi: 10.1016/j.jdermsci.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 31.Shaw JHF. Predictors of outcome for cutaneous squamous cell cancer. N Z Med J. 2015;128(1411):13-19. [PubMed] [Google Scholar]
- 32.Stevenson ML, Criscito MC, Wilken R, et al. Use of adjuvant radiotherapy in the treatment of high-risk cutaneous squamous cell carcinoma with perineural invasion. JAMA Dermatol. 2020;156(8):918-921. doi: 10.1001/jamadermatol.2020.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tam S, Yao CMKL, Amit M, et al. Association of immunosuppression with outcomes of patients with cutaneous squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg. 2020;146(2):128-135. doi: 10.1001/jamaoto.2019.3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terra JB, Gaster MB, Halmos GB, et al. Local control of 151 head and neck cutaneous squamous cell carcinoma after radiotherapy: a retrospective study on efficacy and prognostic factors. Clin Otolaryngol. 2017;42(4):851-855. doi: 10.1111/coa.12707 [DOI] [PubMed] [Google Scholar]
- 35.Thiem DGE, Scharr K, Pabst AM, Saka B, Kämmerer PW. Facial cutaneous squamous cell carcinoma-microscopic safety margins and their impact on developing local recurrences. J Craniomaxillofac Surg. 2020;48(1):49-55. doi: 10.1016/j.jcms.2019.11.022 [DOI] [PubMed] [Google Scholar]
- 36.Tomaszewski JM, Gavriel H, Link E, Boodhun S, Sizeland A, Corry J. Aggressive behavior of cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. Laryngoscope. 2014;124(9):2043-2048. doi: 10.1002/lary.24586 [DOI] [PubMed] [Google Scholar]
- 37.Vinicius LV, Scapulatempo C, Perpetuo NM, et al. Prognostic and risk factors in patients with locally advanced cutaneous squamous cell carcinoma of the trunk and extremities. J Skin Cancer. 2011;2011:420796. doi: 10.1155/2011/420796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yung AE, Que MS, Lo S, et al. Validation of the American Joint Committee on Cancer Staging in squamous cell carcinoma of the vermilion lip. Ann Surg Oncol. 2021;28(6):3092-3099. doi: 10.1245/s10434-020-09431-4 [DOI] [PubMed] [Google Scholar]
- 39.Amaral T, Osewold M, Presser D, Meiwes A, Garbe C, Leiter U. Advanced cutaneous squamous cell carcinoma: real world data of patient profiles and treatment patterns. J Eur Acad Dermatol Venereol. 2019;33(S8)(suppl 8):44-51. doi: 10.1111/jdv.15845 [DOI] [PubMed] [Google Scholar]
- 40.Arbab M, Margalit DN, Tishler RB, et al. Outcomes following radiation for cutaneous squamous cell carcinoma of the head and neck: associations between immune suppression and recurrence. Head Neck. 2019;41(7):2111-2115. doi: 10.1002/hed.25663 [DOI] [PubMed] [Google Scholar]
- 41.Arron ST, Wysong A, Hall MA, et al. Gene expression profiling for metastatic risk in head and neck cutaneous squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2022;7(1):135-144. doi: 10.1002/lio2.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blechman AB, Carucci JA, Stevenson ML. Stratification of poor outcomes for cutaneous squamous cell carcinoma in immunosuppressed patients using the American Joint Committee on Cancer eighth edition and Brigham and Women’s Hospital Staging Systems. Dermatol Surg. 2019;45(9):1117-1124. doi: 10.1097/DSS.0000000000001774 [DOI] [PubMed] [Google Scholar]
- 43.Bourlidou E, Vahtsevanos K, Kyrgidis A, et al. Risk factors for local recurrence of basal cell carcinoma and cutaneous squamous cell carcinoma of the middle third of the face: a 15-year retrospective analysis based on a single centre. Eur J Dermatol. 2019;29(5):490-499. doi: 10.1684/ejd.2019.3643 [DOI] [PubMed] [Google Scholar]
- 44.Bovill ES, Banwell PE. Re-excision of incompletely excised cutaneous squamous cell carcinoma: histological findings influence prognosis. J Plast Reconstr Aesthet Surg. 2012;65(10):1390-1395. doi: 10.1016/j.bjps.2012.04.031 [DOI] [PubMed] [Google Scholar]
- 45.Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713-720. doi: 10.1016/S1470-2045(08)70178-5 [DOI] [PubMed] [Google Scholar]
- 46.Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester Epidemiology Project population-based study in Minnesota. J Am Acad Dermatol. 2015;72(2):302-309. doi: 10.1016/j.jaad.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cañueto J, Cardeñoso E, García JL, et al. Epidermal growth factor receptor expression is associated with poor outcome in cutaneous squamous cell carcinoma. Br J Dermatol. 2017;176(5):1279-1287. doi: 10.1111/bjd.14936 [DOI] [PubMed] [Google Scholar]
- 48.Cañueto J, Martín-Vallejo J, Cardeñoso-Álvarez E, Fernández-López E, Pérez-Losada J, Román-Curto C. Rapid growth rate is associated with poor prognosis in cutaneous squamous cell carcinoma. Clin Exp Dermatol. 2018;43(8):876-882. doi: 10.1111/ced.13570 [DOI] [PubMed] [Google Scholar]
- 49.Carter JB, Johnson MM, Chua TL, Karia PS, Schmults CD. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149(1):35-41. doi: 10.1001/jamadermatol.2013.746 [DOI] [PubMed] [Google Scholar]
- 50.Chabrillac E, Lusque A, Cavallier Z, et al. Cutaneous squamous cell carcinoma tumour size is associated with sentinel lymph node metastasis in a cohort of 69 patients. Acta Derm Venereol. 2019;99(13):1241-1245. doi: 10.2340/00015555-3293 [DOI] [PubMed] [Google Scholar]
- 51.Conde-Ferreirós A, Corchete LA, Puebla-Tornero L, et al. Definition of prognostic subgroups in the T3 stage of the eighth edition of the American Joint Committee on Cancer staging system for cutaneous squamous cell carcinoma: tentative T3 stage subclassification. J Am Acad Dermatol. 2021;85(5):1168-1177. doi: 10.1016/j.jaad.2020.03.088 [DOI] [PubMed] [Google Scholar]
- 52.Durham AB, Lowe L, Malloy KM, et al. Sentinel lymph node biopsy for cutaneous squamous cell carcinoma on the head and neck. JAMA Otolaryngol Head Neck Surg. 2016;142(12):1171-1176. doi: 10.1001/jamaoto.2016.1927 [DOI] [PubMed] [Google Scholar]
- 53.Dzubow LM, Rigel DS, Robins P. Risk factors for local recurrence of primary cutaneous squamous cell carcinomas. Treatment by microscopically controlled excision. Arch Dermatol. 1982;118(11):900-902. doi: 10.1001/archderm.1982.01650230028021 [DOI] [PubMed] [Google Scholar]
- 54.Erkan S, Savundra JM, Wood B, Acharya AN, Rajan GP. Clinical perineural invasion of the trigeminal and facial nerves in cutaneous head and neck squamous cell carcinoma: outcomes and prognostic implications of multimodality and salvage treatment. Head Neck. 2017;39(7):1280-1286. doi: 10.1002/hed.24607 [DOI] [PubMed] [Google Scholar]
- 55.Estall V, Allen A, Webb A, Bressel M, McCormack C, Spillane J. Outcomes following management of squamous cell carcinoma of the scalp: a retrospective series of 235 patients treated at the Peter MacCallum Cancer Centre. Australas J Dermatol. 2017;58(4):e207-e215. doi: 10.1111/ajd.12520 [DOI] [PubMed] [Google Scholar]
- 56.Farberg AS, Hall MA, Douglas L, et al. Integrating the 40-gene expression profile (40-GEP) test into management of high-risk cutaneous squamous cell carcinoma. J Clin Aesthet Dermatol. 2020;12(5)(suppl):S18. [Google Scholar]
- 57.Gore SM, Shaw D, Martin RC, et al. Prospective study of sentinel node biopsy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2016;38(suppl 1):E884-E889. doi: 10.1002/hed.24120 [DOI] [PubMed] [Google Scholar]
- 58.Grover P, Flukes S, Jacques A, et al. Clinicopathological characteristics and clinical morbidity in high-risk head and neck cutaneous squamous cell carcinoma patients in Western Australia. Intern Med J. 2022;52(6):944-951. doi: 10.1111/imj.15630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta N, Weitzman RE, Murad F, et al. Identifying Brigham and Women’s Hospital stage T2a cutaneous squamous cell carcinomas at risk of poor outcomes. J Am Acad Dermatol. 2022;86(6):1301-1308. doi: 10.1016/j.jaad.2021.11.046 [DOI] [PubMed] [Google Scholar]
- 60.Haisma MS, Plaat BEC, Bijl HP, et al. Multivariate analysis of potential risk factors for lymph node metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J Am Acad Dermatol. 2016;75(4):722-730. doi: 10.1016/j.jaad.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 61.Harris BN, Bayoumi A, Rao S, Moore MG, Farwell DG, Bewley AF. Factors associated with recurrence and regional adenopathy for head and neck cutaneous squamous cell carcinoma. Otolaryngol Head Neck Surg. 2017;156(5):863-869. doi: 10.1177/0194599817697053 [DOI] [PubMed] [Google Scholar]
- 62.Harris BN, Pipkorn P, Nguyen KNB, et al. Association of adjuvant radiation therapy with survival in patients with advanced cutaneous squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg. 2019;145(2):153-158. doi: 10.1001/jamaoto.2018.3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haug K, Breuninger H, Metzler G, et al. Prognostic impact of perineural invasion in cutaneous squamous cell carcinoma: results of a prospective study of 1,399 tumors. J Invest Dermatol. 2020;140(10):1968-1975. doi: 10.1016/j.jid.2020.01.035 [DOI] [PubMed] [Google Scholar]
- 64.Hausauer AK, Maurer T, Leslie KS, Parvataneni R, Stuart SE, Chren MM. Recurrence after treatment of cutaneous basal cell and squamous cell carcinomas in patients infected with human immunodeficiency virus. JAMA Dermatol. 2013;149(2):239-241. doi: 10.1001/2013.jamadermatol.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jackson JE, Dickie GJ, Wiltshire KL, et al. Radiotherapy for perineural invasion in cutaneous head and neck carcinomas: toward a risk-adapted treatment approach. Head Neck. 2009;31(5):604-610. doi: 10.1002/hed.20991 [DOI] [PubMed] [Google Scholar]
- 66.Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402-410. doi: 10.1001/jamadermatol.2013.2456 [DOI] [PubMed] [Google Scholar]
- 67.Jenkins G, Smith AB, Kanatas AN, Houghton DR, Telfer MR. Anatomical restrictions in the surgical excision of scalp squamous cell carcinomas: does this affect local recurrence and regional nodal metastases? Int J Oral Maxillofac Surg. 2014;43(2):142-146. doi: 10.1016/j.ijom.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 68.Kadakia S, Ducic Y, Marra D, et al. Cutaneous squamous cell carcinoma of the scalp in the immunocompromised patient: review of 53 cases. Oral Maxillofac Surg. 2016;20(2):171-175. doi: 10.1007/s10006-016-0545-6 [DOI] [PubMed] [Google Scholar]
- 69.Kamiya S, Kato J, Kamiya T, et al. Association between PD-L1 expression and lymph node metastasis in cutaneous squamous cell carcinoma. Asia Pac J Clin Oncol. 2020;16(2):e108-e112. doi: 10.1111/ajco.13102 [DOI] [PubMed] [Google Scholar]
- 70.Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):327-334. doi: 10.1200/JCO.2012.48.5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato J, Hida T, Sugita S, et al. Cytokeratin 19 expression is a risk factor for metastasis in cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2018;32(7):e299-e301. doi: 10.1111/jdv.14845 [DOI] [PubMed] [Google Scholar]
- 72.Knuutila JS, Riihilä P, Kurki S, Nissinen L, Kähäri VM. Risk factors and prognosis for metastatic cutaneous squamous cell carcinoma: a cohort study. Acta Derm Venereol. 2020;100(16):adv00266. doi: 10.2340/00015555-3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Korhonen N, Ylitalo L, Luukkaala T, et al. Recurrent and metastatic cutaneous squamous cell carcinomas in a cohort of 774 patients in Finland. Acta Derm Venereol. 2020;100(8):adv00121. doi: 10.2340/00015555-3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krediet JT, Beyer M, Lenz K, et al. Sentinel lymph node biopsy and risk factors for predicting metastasis in cutaneous squamous cell carcinoma. Br J Dermatol. 2015;172(4):1029-1036. doi: 10.1111/bjd.13508 [DOI] [PubMed] [Google Scholar]
- 75.Kreppel M, Krakowezki A, Kreppel B, et al. Podoplanin expression in cutaneous head and neck squamous cell carcinoma--prognostic value and clinicopathologic implications. J Surg Oncol. 2013;107(4):376-383. doi: 10.1002/jso.23238 [DOI] [PubMed] [Google Scholar]
- 76.Kropp L, Balamucki CJ, Morris CG, et al. Mohs resection and postoperative radiotherapy for head and neck cancers with incidental perineural invasion. Am J Otolaryngol. 2013;34(5):373-377. doi: 10.1016/j.amjoto.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 77.Kyrgidis A, Tzellos TG, Kechagias N, et al. Cutaneous squamous cell carcinoma (SCC) of the head and neck: risk factors of overall and recurrence-free survival. Eur J Cancer. 2010;46(9):1563-1572. doi: 10.1016/j.ejca.2010.02.046 [DOI] [PubMed] [Google Scholar]
- 78.Leibovitch I, Huilgol SC, Selva D, Hill D, Richards S, Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. Perineural invasion. J Am Acad Dermatol. 2005;53(2):261-266. doi: 10.1016/j.jaad.2005.03.048 [DOI] [PubMed] [Google Scholar]
- 79.Leus AJG, Haisma MS, Terra JB, et al. Age-related differences in tumour characteristics and prognostic factors for disease progression in cutaneous squamous cell carcinoma of the head and neck. Acta Derm Venereol. 2022;102:adv00652. doi: 10.2340/actadv.v101.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsumoto A, Li JN, Matsumoto M, Pineider J, Nijhawan RI, Srivastava D. Factors predicting outcomes of patients with high-risk squamous cell carcinoma treated with Mohs micrographic surgery. J Am Acad Dermatol. 2021;85(3):588-595. doi: 10.1016/j.jaad.2021.01.063 [DOI] [PubMed] [Google Scholar]
- 81.Mayo E, Sharma S, Horne J, Yuen HM, Lee A, Gulati A. Squamous cell carcinoma of the pinna: which histological features could be used to predict prognosis? Br J Oral Maxillofac Surg. 2017;55(5):524-529. doi: 10.1016/j.bjoms.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 82.McLaughlin EJ, Miller L, Shin TM, et al. Rate of regional nodal metastases of cutaneous squamous cell carcinoma in the immunosuppressed patient. Am J Otolaryngol. 2017;38(3):325-328. doi: 10.1016/j.amjoto.2017.01.035 [DOI] [PubMed] [Google Scholar]
- 83.Mo J, Miller CJ, Karakousis G, Keele L, Cohen J, Krouse RS. The scalp is a high-risk site for cutaneous squamous cell carcinoma metastasis. J Am Acad Dermatol. 2021;84(6):1742-1744. doi: 10.1016/j.jaad.2020.09.035 [DOI] [PubMed] [Google Scholar]
- 84.Moore BA, Weber RS, Prieto V, et al. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115(9):1561-1567. doi: 10.1097/01.mlg.0000173202.56739.9f [DOI] [PubMed] [Google Scholar]
- 85.Mourouzis C, Boynton A, Grant J, et al. Cutaneous head and neck SCCs and risk of nodal metastasis-UK experience. J Craniomaxillofac Surg. 2009;37(8):443-447. doi: 10.1016/j.jcms.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 86.Mullen JT, Feng L, Xing Y, et al. Invasive squamous cell carcinoma of the skin: defining a high-risk group. Ann Surg Oncol. 2006;13(7):902-909. doi: 10.1245/ASO.2006.07.022 [DOI] [PubMed] [Google Scholar]
- 87.Nelson TG, Ashton RE. Low incidence of metastasis and recurrence from cutaneous squamous cell carcinoma found in a UK population: do we need to adjust our thinking on this rare but potentially fatal event? J Surg Oncol. 2017;116(6):783-788. doi: 10.1002/jso.24707 [DOI] [PubMed] [Google Scholar]
- 88.Obermeier K, Tröltzsch M, Ehrenfeld M, Smolka W. Risk factors for lymph node metastases of facial cutaneous squamous cell carcinoma. J Craniomaxillofac Surg. 2017;45(8):1138-1142. doi: 10.1016/j.jcms.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 89.Ogawa T, Kiuru M, Konia TH, Fung MA. Acantholytic squamous cell carcinoma is usually associated with hair follicles, not acantholytic actinic keratosis, and is not “high risk”: diagnosis, management, and clinical outcomes in a series of 115 cases. J Am Acad Dermatol. 2017;76(2):327-333. doi: 10.1016/j.jaad.2016.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oh Y, Kim J, Zheng Z, Kim SK, Chung KY, Roh MR. Risk factors for recurrence in cutaneous squamous cell carcinoma after Mohs micrographic surgery: a retrospective review of 237 Asian patients. J Dermatol. 2020;47(1):72-77. doi: 10.1111/1346-8138.15129 [DOI] [PubMed] [Google Scholar]
- 91.Saito Y, Fujikawa H, Takatsuka S, Abe R, Takenouchi T. Risk factors for lymph node metastasis in cutaneous squamous cell carcinoma: a long-term retrospective study of Japanese patients. Int J Clin Oncol. 2021;26(3):606-612. doi: 10.1007/s10147-020-01830-7 [DOI] [PubMed] [Google Scholar]
- 92.Sayan A, Paraneetharan S, Ilankovan V. Importance of anatomical site in the metastases of auricular cutaneous squamous cell carcinoma: an observational study. Br J Oral Maxillofac Surg. 2020;58(7):824-828. doi: 10.1016/j.bjoms.2020.04.029 [DOI] [PubMed] [Google Scholar]
- 93.Takeda A, Akimoto M, Nemoto M, Kounoike N, Uchinuma E. Preoperative risk factors of lymph node metastasis in cutaneous squamous cell carcinoma. J Plast Surg Hand Surg. 2013;47(3):204-208. doi: 10.3109/2000656X.2012.750611 [DOI] [PubMed] [Google Scholar]
- 94.Tanamal PJ, Geng CX, Nijhawan RI, et al. Oncologic outcomes in primary squamous cell carcinoma of the auricle: a retrospective cohort analysis. Eur Arch Otorhinolaryngol. 2022;279(1):335-341. doi: 10.1007/s00405-021-06763-z [DOI] [PubMed] [Google Scholar]
- 95.Thomas CJ, Wood GC, Marks VJ. Mohs micrographic surgery in the treatment of rare aggressive cutaneous tumors: the Geisinger experience. Dermatol Surg. 2007;33(3):333-339. [DOI] [PubMed] [Google Scholar]
- 96.Tschetter AJ, Campoli MR, Zitelli JA, Brodland DG. Long-term clinical outcomes of patients with invasive cutaneous squamous cell carcinoma treated with Mohs micrographic surgery: a 5-year, multicenter, prospective cohort study. J Am Acad Dermatol. 2020;82(1):139-148. doi: 10.1016/j.jaad.2019.06.1303 [DOI] [PubMed] [Google Scholar]
- 97.van Lee CB, Roorda BM, Wakkee M, et al. Recurrence rates of cutaneous squamous cell carcinoma of the head and neck after Mohs micrographic surgery vs. standard excision: a retrospective cohort study. Br J Dermatol. 2019;181(2):338-343. doi: 10.1111/bjd.17188 [DOI] [PubMed] [Google Scholar]
- 98.Venables ZC, Autier P, Nijsten T, et al. Nationwide incidence of metastatic cutaneous squamous cell carcinoma in England. JAMA Dermatol. 2019;155(3):298-306. doi: 10.1001/jamadermatol.2018.4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Warren TA, Panizza B, Porceddu SV, et al. Outcomes after surgery and postoperative radiotherapy for perineural spread of head and neck cutaneous squamous cell carcinoma. Head Neck. 2016;38(6):824-831. doi: 10.1002/hed.23982 [DOI] [PubMed] [Google Scholar]
- 100.Wu MP, Sethi RKV, Emerick KS. Sentinel lymph node biopsy for high-risk cutaneous squamous cell carcinoma of the head and neck. Laryngoscope. 2020;130(1):108-114. doi: 10.1002/lary.27881 [DOI] [PubMed] [Google Scholar]
- 101.Xiong DD, Beal BT, Varra V, et al. Outcomes in intermediate-risk squamous cell carcinomas treated with Mohs micrographic surgery compared with wide local excision. J Am Acad Dermatol. 2020;82(5):1195-1204. doi: 10.1016/j.jaad.2019.12.049 [DOI] [PubMed] [Google Scholar]
- 102.Xu MJ, Lazar AA, Garsa AA, et al. Major prognostic factors for recurrence and survival independent of the American Joint Committee on Cancer eighth edition staging system in patients with cutaneous squamous cell carcinoma treated with multimodality therapy. Head Neck. 2018;40(7):1406-1414. doi: 10.1002/hed.25114 [DOI] [PubMed] [Google Scholar]
- 103.Warren TA, Whiteman DC, Porceddu SV, Panizza BJ. Insight into the epidemiology of cutaneous squamous cell carcinoma with perineural spread. Head Neck. 2016;38(9):1416-1420. doi: 10.1002/hed.24453 [DOI] [PubMed] [Google Scholar]
- 104.Agar NJM, Kirton C, Patel RS, Martin RCW, Angelo N, Emanuel PO. Predicting lymph node metastases in cutaneous squamous cell carcinoma: use of a morphological scoring system. N Z Med J. 2015;128(1411):59-67. [PubMed] [Google Scholar]
- 105.Fujimoto M, Yamamoto Y, Takai T, et al. Tumor budding is an objective high-risk factor associated with metastasis and poor clinical prognosis in cutaneous squamous cell carcinoma sized <4 cm. Am J Surg Pathol. 2019;43(7):975-983. doi: 10.1097/PAS.0000000000001284 [DOI] [PubMed] [Google Scholar]
- 106.García-Pedrero JM, Martínez-Camblor P, Diaz-Coto S, et al. Tumor programmed cell death ligand 1 expression correlates with nodal metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J Am Acad Dermatol. 2017;77(3):527-533. doi: 10.1016/j.jaad.2017.05.047 [DOI] [PubMed] [Google Scholar]
- 107.Gonzalez-Guerrero M, Martínez-Camblor P, Vivanco B, et al. The adverse prognostic effect of tumor budding on the evolution of cutaneous head and neck squamous cell carcinoma. J Am Acad Dermatol. 2017;76(6):1139-1145. doi: 10.1016/j.jaad.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 108.Hernández-Ruiz E, Hernández-Muñoz I, Masferrer E, et al. A myxoid fibrotic reaction pattern is associated with metastatic risk in cutaneous squamous cell carcinoma. Acta Derm Venereol. 2019;99(1):89-94. [DOI] [PubMed] [Google Scholar]
- 109.Karayannopoulou G, Panteris E, Kanitakis J. Tumour budding is an independent predictive factor of cutaneous squamous-cell carcinoma aggressiveness. Anticancer Res. 2020;40(5):2695-2699. doi: 10.21873/anticanres.14240 [DOI] [PubMed] [Google Scholar]
- 110.Khandelwal AR, Ma X, Egan P, et al. Biomarker and pathologic predictors of cutaneous squamous cell carcinoma aggressiveness. Otolaryngol Head Neck Surg. 2016;155(2):281-288. doi: 10.1177/0194599816641913 [DOI] [PubMed] [Google Scholar]
- 111.Lobl M, Feinstein S, Lauer S, Sutton A, Wysong A. recurrence status, perineural invasion, and hypothyroidism are associated with lymph node metastasis in cutaneous squamous cell carcinoma: a case-control study. Dermatol Surg. 2022;48(4):381-386. doi: 10.1097/DSS.0000000000003396 [DOI] [PubMed] [Google Scholar]
- 112.Munguía-Calzada P, Fernández-Vega I, Martínez-Camblor P, et al. Correlation of focal adhesion kinase expression with nodal metastasis in patients with head and neck cutaneous squamous cell carcinoma. Head Neck. 2019;41(5):1290-1296. doi: 10.1002/hed.25556 [DOI] [PubMed] [Google Scholar]
- 113.Cañueto J, Burguillo J, Moyano-Bueno D, et al. Comparing the eighth and the seventh editions of the American Joint Committee on Cancer staging system and the Brigham and Women’s Hospital alternative staging system for cutaneous squamous cell carcinoma: implications for clinical practice. J Am Acad Dermatol. 2019;80(1):106-113.e2. doi: 10.1016/j.jaad.2018.06.060 [DOI] [PubMed] [Google Scholar]
- 114.Cheng JY, Li FY, Ko CJ, Colegio OR. Cutaneous squamous cell carcinomas in solid organ transplant recipients compared with immunocompetent patients. JAMA Dermatol. 2018;154(1):60-66. doi: 10.1001/jamadermatol.2017.4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gonzalez JL, Cunningham K, Silverman R, Madan E, Nguyen BM. Case-control study of tumor stage-dependent outcomes for cutaneous squamous cell carcinoma in immunosuppressed and immunocompetent patients. Dermatol Surg. 2019;45(12):1467-1476. doi: 10.1097/DSS.0000000000001930 [DOI] [PubMed] [Google Scholar]
- 116.Kanitakis J, Karayannopoulou G, Roux A, Euvrard S. Histopathologic features predictive of aggressiveness of post-transplant cutaneous squamous-cell carcinomas. Anticancer Res. 2015;35(4):2305-2308. [PubMed] [Google Scholar]
- 117.Karayannopoulou G, Euvrard S, Kanitakis J. Tumour budding correlates with aggressiveness of cutaneous squamous-cell carcinoma. Anticancer Res. 2016;36(9):4781-4785. doi: 10.21873/anticanres.11036 [DOI] [PubMed] [Google Scholar]
- 118.Krediet JT, Kanitakis J, Bob A, et al. Prognostic value of the area and density of lymphatic vessels in cutaneous squamous cell carcinoma. J Dtsch Dermatol Ges. 2016;14(11):1114-1121. doi: 10.1111/ddg.12880 [DOI] [PubMed] [Google Scholar]
- 119.Peat B, Insull P, Ayers R. Risk stratification for metastasis from cutaneous squamous cell carcinoma of the head and neck. ANZ J Surg. 2012;82(4):230-233. doi: 10.1111/j.1445-2197.2011.05994.x [DOI] [PubMed] [Google Scholar]
- 120.Santos-Juanes J, Fernández-Vega I, Lorenzo-Herrero S, et al. Lectin-like transcript 1 (LLT1) expression is associated with nodal metastasis in patients with head and neck cutaneous squamous cell carcinoma. Arch Dermatol Res. 2019;311(5):369-376. doi: 10.1007/s00403-019-01916-x [DOI] [PubMed] [Google Scholar]
- 121.Sepehripour S, Dawood O, Hatter S, et al. An assessment of histological margins and recurrence of completely excised cutaneous SCC. J Plast Reconstr Aesthet Surg. 2020;73(5):899-903. doi: 10.1016/j.bjps.2019.09.022 [DOI] [PubMed] [Google Scholar]
- 122.Cognetta AB, Howard BM, Heaton HP, Stoddard ER, Hong HG, Green WH. Superficial x-ray in the treatment of basal and squamous cell carcinomas: a viable option in select patients. J Am Acad Dermatol. 2012;67(6):1235-1241. doi: 10.1016/j.jaad.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 123.Hernández-Machin B, Borrego L, Gil-García M, Hernández BH. Office-based radiation therapy for cutaneous carcinoma: evaluation of 710 treatments. Int J Dermatol. 2007;46(5):453-459. doi: 10.1111/j.1365-4632.2006.03108.x [DOI] [PubMed] [Google Scholar]
- 124.Kim SK, Barker CA. Outcomes of radiation therapy for advanced T3/T4 nonmelanoma cutaneous squamous cell and basal cell carcinoma. Br J Dermatol. 2018;178(1):e30-e32. doi: 10.1111/bjd.15728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Košec A, Svetina L, Lukšić I. Significance of clinical stage, extent of surgery and outcome in cutaneous squamous cell carcinoma of the head and neck. Int J Oral Maxillofac Surg. 2013;42(1):82-88. doi: 10.1016/j.ijom.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 126.Leibovitch I, Huilgol SC, Selva D, Hill D, Richards S, Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53(2):253-260. doi: 10.1016/j.jaad.2005.02.059 [DOI] [PubMed] [Google Scholar]
- 127.Choi SH, Kim KH, Song KH. Effect of methyl aminolevulinate photodynamic therapy with and without ablative fractional laser treatment in patients with microinvasive squamous cell carcinoma: a randomized clinical trial. JAMA Dermatol. 2017;153(3):289-295. doi: 10.1001/jamadermatol.2016.4463 [DOI] [PubMed] [Google Scholar]
- 128.Ruiz ES, Koyfman SA, Que SKT, Kass J, Schmults CD. Evaluation of the utility of localized adjuvant radiation for node-negative primary cutaneous squamous cell carcinoma with clear histologic margins. J Am Acad Dermatol. 2020;82(2):420-429. doi: 10.1016/j.jaad.2019.07.048 [DOI] [PubMed] [Google Scholar]
- 129.Diana L, Ramelyte E, Dummer R, Imhof L. Radiotherapy in periocular cutaneous malignancies: a retrospective study. Journal of the Dermatology Nurses' Association Conference: 24th World Congress of Dermatology Milan Italy. 2020;12(2). [Google Scholar]
- 130.Hutting KH, Bos PG, Kibbelaar RE, Veeger NJGM, Marck KW, Mouës CM. Effective excision of cutaneous squamous cell carcinoma of the face using analysis of intra-operative frozen sections from the whole specimen. J Surg Oncol. 2018;117(3):473-478. doi: 10.1002/jso.24870 [DOI] [PubMed] [Google Scholar]
- 131.Stanciu A, Florica CE, Zota A, Tebeica T, Leventer M, Bobirca F. Surgical outcomes of more than 1300 cases of Mohs micrographic surgeries from a private Mohs clinic in Romania. Chirurgia (Bucur). 2020;115(1):69-79. doi: 10.21614/chirurgia.115.1.69 [DOI] [PubMed] [Google Scholar]
- 132.Fernanda Sachse M, Labareda J, Lima Duque JM, Cirne de Castro JL. Cryosurgery of malignant cutaneous tumours: six years’ experience. Skin Cancer. 1988;3(2):103-110. [Google Scholar]
- 133.Marrazzo G, Zitelli JA, Brodland D. Clinical outcomes in high-risk squamous cell carcinoma patients treated with Mohs micrographic surgery alone. J Am Acad Dermatol. 2019;80(3):633-638. doi: 10.1016/j.jaad.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 134.Pereira MF, Morgado MA. Cryosurgery of malignant cutaneous tumours: ten years experience. Skin Cancer. 1994;9(4):179-185. [Google Scholar]
- 135.Turner RJ, Leonard N, Malcolm AJ, Lawrence CM, Dahl MG. A retrospective study of outcome of Mohs’ micrographic surgery for cutaneous squamous cell carcinoma using formalin fixed sections. Br J Dermatol. 2000;142(4):752-757. doi: 10.1046/j.1365-2133.2000.03422.x [DOI] [PubMed] [Google Scholar]
- 136.Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976-990. doi: 10.1016/0190-9622(92)70144-5 [DOI] [PubMed] [Google Scholar]
- 137.Amin MB, Edge SB, Frederick L, Greene FL, eds. The AJCC Cancer Staging Manual, 8th edition. Springer; 2017. doi: 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 138.Beahrs OH, Henson DE, Hutter RVP, Myers MH, eds. Manual for Staging of Cancer, 3rd edition. JB Lippincott; 1988. doi: 10.1097/00000421-198812000-00027 [DOI] [Google Scholar]
- 139.Krown SE, Testa MA, Huang J; AIDS Clinical Trials Group Oncology Committee . AIDS-related Kaposi’s sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. J Clin Oncol. 1997;15(9):3085-3092. doi: 10.1200/JCO.1997.15.9.3085 [DOI] [PubMed] [Google Scholar]
- 140.National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology: Squamous Cell Skin Cancer. 2022. Accessed November 7, 2022. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1465
- 141.Connolly SM, Baker DR, Coldiron BM, et al. ; Ad Hoc Task Force; Ratings Panel . 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550. doi: 10.1016/j.jaad.2012.06.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Evaluation of cohort studies – risk factor (level 4 evidence)
eTable 2. Results of random effects analysis: risk ratio for each risk factor and distant metastasis, any metastasis, locoregional recurrence, and combined outcomes
eTable 3. Incidence proportion for each risk factor modality per outcome across all studies
eTable 4. Incidence proportion for each treatment modality per outcome across all studies
eFigure. Forest plots of results of random effects analysis: risk ratio for each risk factor-and distant metastasis, any metastasis, and all-cause death identified in more than 1 study
eReferences