This prognostic study uses a machine learning predictive model of survival after hepatocellular carcinoma recurrence to allocate patients to their best potential treatment: reoperative hepatectomy or thermoablation, chemoembolization, or sorafenib.
Key Points
Question
How can the best potential oncological treatment be identified for recurrent hepatocellular carcinoma after surgery among curative approaches, chemoembolization, and sorafenib?
Findings
In this prognostic study of 701 patients with recurrent hepatocellular carcinoma, a machine learning model was created to predict the survival after recurrence, and its area under the receiver operating characteristic curve was 78.5% at 5 years after recurrence; the model’s area under the receiver operating characteristic curve reached 76.8% in another Italian cohort and 70.9% in a Japanese cohort. According to the model, most patients would have benefited from reoperative hepatectomy or thermoablation.
Meaning
The herein presented algorithm should help in allocating patients with recurrent hepatocellular carcinoma to the best potential treatment according to their specific characteristics in a treatment hierarchy fashion.
Abstract
Importance
Clear indications on how to select retreatments for recurrent hepatocellular carcinoma (HCC) are still lacking.
Objective
To create a machine learning predictive model of survival after HCC recurrence to allocate patients to their best potential treatment.
Design, Setting, and Participants
Real-life data were obtained from an Italian registry of hepatocellular carcinoma between January 2008 and December 2019 after a median (IQR) follow-up of 27 (12-51) months. External validation was made on data derived by another Italian cohort and a Japanese cohort. Patients who experienced a recurrent HCC after a first surgical approach were included. Patients were profiled, and factors predicting survival after recurrence under different treatments that acted also as treatment effect modifiers were assessed. The model was then fitted individually to identify the best potential treatment. Analysis took place between January and April 2021.
Exposures
Patients were enrolled if treated by reoperative hepatectomy or thermoablation, chemoembolization, or sorafenib.
Main Outcomes and Measures
Survival after recurrence was the end point.
Results
A total of 701 patients with recurrent HCC were enrolled (mean [SD] age, 71 [9] years; 151 [21.5%] female). Of those, 293 patients (41.8%) received reoperative hepatectomy or thermoablation, 188 (26.8%) received sorafenib, and 220 (31.4%) received chemoembolization. Treatment, age, cirrhosis, number, size, and lobar localization of the recurrent nodules, extrahepatic spread, and time to recurrence were all treatment effect modifiers and survival after recurrence predictors. The area under the receiver operating characteristic curve of the predictive model was 78.5% (95% CI, 71.7%-85.3%) at 5 years after recurrence. According to the model, 611 patients (87.2%) would have benefited from reoperative hepatectomy or thermoablation, 37 (5.2%) from sorafenib, and 53 (7.6%) from chemoembolization in terms of potential survival after recurrence. Compared with patients for which the best potential treatment was reoperative hepatectomy or thermoablation, sorafenib and chemoembolization would be the best potential treatment for older patients (median [IQR] age, 78.5 [75.2-83.4] years, 77.02 [73.89-80.46] years, and 71.59 [64.76-76.06] years for sorafenib, chemoembolization, and reoperative hepatectomy or thermoablation, respectively), with a lower median (IQR) number of multiple recurrent nodules (1.00 [1.00-2.00] for sorafenib, 1.00 [1.00-2.00] for chemoembolization, and 2.00 [1.00-3.00] for reoperative hepatectomy or thermoablation). Extrahepatic recurrence was observed in 43.2% (n = 16) for sorafenib as the best potential treatment vs 14.6% (n = 89) for reoperative hepatectomy or thermoablation as the best potential treatment and 0% for chemoembolization as the best potential treatment. Those profiles were used to constitute a patient-tailored algorithm for the best potential treatment allocation.
Conclusions and Relevance
The herein presented algorithm should help in allocating patients with recurrent HCC to the best potential treatment according to their specific characteristics in a treatment hierarchy fashion.
Introduction
Although hepatocellular carcinoma (HCC) treatment relies on surgery, this is affected by a high rate of recurrence up to 60% at 5 years.1 Several therapies have been investigated extensively in the literature to improve survival after recurrence (SAR); however, no clear indications are available on which treatment should be chosen according to the recurrent tumor presentation. Few guidelines2,3 provide an indication for those events, suggesting that the treatment should be selected according to the stage. However, this approach may be reductionist: it subclassifies patients’ risk across a few risk profiles, suggesting only 1 treatment per stage. Moreover, this approach has been noted as being far from a real scenario in the era of multidisciplinary approaches.4
Recently, the introduction of machine learning algorithms in medicine drastically changed the potential to develop highly accurate prediction models5,6: those techniques changed our way of learning from data, identifying several profiles with efficient tailoring. The decision-making in oncology is the sum of the oncological knowledge and the physicians’ experience: the first is the result of several years of research, while the second is intrinsically connected to the physician’s skills, previous experiences, availability of services, but also the patient’s will. While the last item cannot be predicted, the oncological benefit could be automatically evaluated, providing substantial support to make evidence-based medical decisions. Thus, to our knowledge, this study is the first national attempt to identify the best potential treatment among reoperative hepatectomy and thermoablation, chemoembolization, or sorafenib in predicting SAR by a machine learning and patient-tailored approach. Therefore, we trained and externally validated a potential outcome prediction algorithm for treatment selection and developed a web-based application for forecasting.
Methods
Study Overview, Patient Selection, and Study Design
This is a secondary analysis of previously collected registry data prospectively enrolled by a national Italian register on hepatocellular carcinoma, the HE.RC.O.LE.S. (Hepatocarcinoma Recurrence on the Liver Study; NCT04053231) group, which includes participation by 30 surgical centers. The ethical committee of the coordinating center (Monza e Brianza Ethical Committee) approved the study protocol on December 21, 2018. Because of the retrospective and observational nature of the present study, the committee evaluated that written consent was not mandatory. Results are reported according to principles of Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.7 All consecutive adult patients (age ≥18 years) with a radiological and/or histologically proven HCC who underwent surgery between January 2008 and December 2019 were evaluated. Inclusion criteria for this study were (1) patients who underwent surgery for the first diagnosis of HCC without previous therapies, (2) patients who experienced a recurrence after surgery during the follow-up period, and (3) a recurrence treated by reoperative hepatectomy or thermoablation, transarterial chemoembolization, or sorafenib. Exclusion criteria were (1) a recurrence episode not following the absolute first treatment of the HCC, (2) missing data at follow-up, and (3) recurrence treatments other than those described in the inclusion criteria (included salvage liver transplant or being on a waiting list; using other tyrosin-kinase inhibitors or any type of immunotherapy). Selected patients were then divided into those who were treated for their recurrence by reoperative hepatectomy or thermoablation, those who were submitted to sorafenib, and those who were submitted to chemoembolization.
The primary study end point was SAR. The main aim was to assess the treatment of choice for HCC recurrence after surgery (among reoperative hepatectomy or thermoablation, chemoembolization, or sorafenib), which can lead to the best SAR according to different patient profiles.
Data of patients who had recurrence after a surgical treatment for HCC enrolled in another Italian national register on liver cancer (Italian Liver Cancer [ITA.LI.CA] study) were used as an external validation cohort. Another external validation cohort was obtained by the participation at the study of the surgeons attending the hepato-biliary-pancreatic surgery division of the University of Tokyo in Japan.
Definitions and Follow-up Protocol
Variables descriptions are provided in eMethods 1 in Supplement 1. The indication for reoperative hepatectomy or thermoablation, sorafenib, and chemoembolization was assessed by multidisciplinary meetings on a patient-by-patient basis and involved surgeons, hepatologists, oncologists, radiologists, interventional radiologists, and infectivologists as the sum of different evaluations about the underlying liver function, the tumor burden, and the comorbidities, creating tailored treatments for each individual case.
All patients were followed up using local protocols8 every 3 months for the first 2 years and then every 6 months. SAR was defined as the time interval between the date of the assigned treatment for recurrence and any cause of death. Time to recurrence was defined as the time interval in months from the first surgery to the date of recurrence. In case of no event, patients were censored at the last available visit. Patient surveillance was closed at the end of March 2019.
Statistical Analysis
The problem of missing values was tackled using multiple imputation by chained equations method. The selection of features that play a role as prognostic factors for SAR but also as treatment modifiers was performed by fitting a Cox model with least absolute shrinkage and selection operator (LASSO) penalization.9 Ten-fold crossvalidation was used to choose the optimal value of the shrinkage parameter lambda.10 All features that were retained by this procedure were used in the subsequent development of the prediction model.
The prediction model was built as a standard Cox model with all second-order interactions of treatment with the features selected by LASSO. The predictive performance of the model was evaluated using receiver operating characteristic (ROC) methodology (with bootstrap-validated area under the ROC curve index) and calibration plots (with Brier score).11,12 The same procedures were adopted to perform an external validation of the model performance on data of patients with recurrence after surgery taken form the ITA.LI.CA and the Japanese cohorts.
For each patient, the model estimates were used to predict the potential SAR at 3 and 5 years under each treatment. Subsequently, the potentially optimal treatment within patients was determined as the one leading to the highest predicted SAR.
Considering the possible combinations of the 7 features included, the model was able to deal with potentially infinite different patients' risk profiles, and consequently it was built up as an R Shiny web application to let users calculate the potential SAR in time under each treatment for every profile of interest.
To simulate how the algorithm works in a clinical scenario, we also refitted the same prediction model described before but using variables’ dichotomization to reduce the number of potential risk profiles obtainable in case of continuous variables. Thus, we only considered the 5 features showing the largest impact as treatment effect modifiers (age <75 or ≥75 years, cirrhosis: yes/no, number of recurrent nodules: 1 or >1, single/bilobar recurrence, and intra-/extrahepatic recurrence) and we created 25 = 32 risk profiles from all possible combinations of the levels of these features (the remaining 2 features were fixed at size <50 mm and time to recurrence of 15 months). Potential SAR prediction at 3 and 5 years under each treatment was predicted for all these profiles.
When the difference in SAR between 2 treatments was less than 5%, both treatments were labeled as optimal. A tree diagram based on these simulations was drawn. The detailed description of statistical methods is provided as eMethods 2 in Supplement 1. Significance was considered when P < .05. All the analyses were carried out using R version 4.0.3 (R Foundation). Analysis took place between January and April 2021.
Results
During the study period, 2699 patients were enrolled in HE.RC.O.LE.S. Of these, 1235 (47.5%) experienced a recurrence after the first surgical treatment. Data on patients excluded were reported in eMethods 3 in Supplement 1. Finally, 701 patients (56.76%) were enrolled and further analyzed. For 293 patients (41.8%), recurrence was treated with reoperative hepatectomy or thermoablation, while 188 (26.8%) underwent sorafenib and 220 (31.4%) underwent chemoembolization. A flowchart is available in eFigure 1 in Supplement 1. The baseline characteristics among groups are summarized in Table 1 (overall mean [SD] age, 71 [9] years; 151 [21.5%] female). SAR rates at 5 years from the first surgery in the 3 treatment groups for recurrence were 61.9% (95% CI, 53.6%-70.3%) for reoperative hepatectomy or thermoablation, 34.5% (95% CI, 23.8%-45.3%) for chemoembolization, and 32.3% (95% CI, 22.0%-42.7%) for sorafenib. Median SAR was not reached for reoperative hepatectomy or thermoablation and was 42.8 (95% CI, 32.9-50) months for chemoembolization and 17.9 (95% CI, 13.2-24) months for sorafenib. Median disease-free survival after the recurrence was 37 (95% CI, 31-56), 21.3 (95% CI, 16.9-27.6), and 15 (95% CI, 12.3-19) months for reoperative hepatectomy or thermoablation, chemoembolization, and sorafenib, respectively.
Table 1. Comparison of Features Among Groups of Treatment Actually Received and Groups of Best Potential Treatments.
| Features | Treatment received | Best potential treatmenta | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | P value | No. (%) | P value | |||||
| Reoperative hepatectomy or thermoablation (n = 293) | Sorafenib (n = 188) | Chemoembolization (n = 220) | Reoperative hepatectomy or thermoablation (611 [87.2%]) | Sorafenib (37 [5.2%]) | Chemoembolization (53 [7.6%]) | |||
| Actual treatment | NA | .01 | ||||||
| Reoperative hepatectomy or thermoablation | NA | NA | NA | 247 (40.4) | 16 (43.2) | 30 (56.6) | ||
| Sorafenib | NA | NA | NA | 171 (28.0) | 13 (35.1) | 4 (7.5) | ||
| Chemoembolization | NA | NA | NA | 193 (31.6) | 8 (21.6) | 19 (35.8) | ||
| Age at recurrence, median (IQR), y | 72.90 (66.93-76.59) | 71.26 (63.32-76.97) | 72.82 (66.56-77.16) | .10 | 71.59 (64.76-76.06) | 78.52 (75.28-83.38) | 77.02 (73.89-80.46) | <.001 |
| Female | 65 (22.2) | 45 (23.9) | 41 (18.6) | .41 | 124 (20.3) | 13 (35.1) | 14 (26.4) | .07 |
| Male | 228 (77.8) | 143 (76.1) | 179 (81.4) | 487 (79.7) | 24 (64.9) | 39 (73.6) | ||
| Cirrhosis | 194 (67.4) | 123 (65.8) | 140 (63.6) | .68 | 412 (67.4) | 31 (83.8) | 18 (34.0) | <.001 |
| Child-Pugh class B | 11 (6.0) | 7 (5.7) | 10 (7.4) | .84 | NA | NA | NA | NA |
| No. of recurrence nodules | ||||||||
| >1 | 70 (24.5) | 120 (74.1) | 123 (67.2) | <.001 | 340 (55.6) | 18 (48.6) | 24 (45.3) | .27 |
| Median (IQR) | 1 (1-1) | 2 (1-5) | 2 (1-4) | <.001 | 2.00 (1.00-3.00) | 1.00 (1.00-2.00) | 1.00 (1.00-2.00) | .03 |
| Bilobar recurrence | 31 (13.2) | 49 (49.5) | 68 (42.2) | <.001 | 165 (27.0) | 21 (56.8) | 36 (67.9) | <.001 |
| Size, cm | ||||||||
| ≥5 | 21 (7.9) | 36 (23.4) | 26 (15.0) | <.001 | 95 (15.5) | 3 (8.1) | 2 (3.8) | .03 |
| Median (IQR) | 2 (1.5-2.5) | 2.5 (1.5-4.4) | 2 (1.5-3.4) | <.001 | 2.00 (1.50-3.30) | 2.40 (1.60-3.30) | 1.50 (1.00-2.00) | <.001 |
| MVI | 98 (39.2) | 78 (47.0) | 75 (41.2) | .28 | 258 (42.2) | 19 (51.4) | 19 (35.8) | .34 |
| Extrahepatic recurrence | 28 (9.6) | 61 (33.9) | 11 (5.2) | <.001 | 89 (14.6) | 16 (43.2) | 0 | <.001 |
| TTR, median (IQR), months | 17.87 (7.93-34.46) | 10.46 (5.05-30.08) | 13.26 (6.79-23.34) | .001 | 15.11 (6.57-28.98) | 6.79 (2.43-17.64) | 21.90 (10.98-41.41) | <.001 |
Abbreviations: MVI, microvascular invasion; NA, not applicable; SAR, survival after recurrence; TTR, time to recurrence (from first surgery).
The treatment leading to the highest SAR for each patient.
A prediction model for SAR, treatment effect modifiers’ identification, and a combination created the prediction algorithm. The association of SAR with the features selected by LASSO was investigated using univariate Cox models (eTable 1 in Supplement 1). The output of the application of LASSO penalization to the Cox model for the selection of predictive features is shown in eFigure 2 and eTable 2 in Supplement 1. Variables selected were treatment, age, cirrhosis, number and size of the recurrent nodules, bilobar presentation, extrahepatic spread, and time to recurrence. Treatment was associated with SAR, showing that patients treated with sorafenib (hazard ratio [HR], 3.68 [95% CI, 2.66-5.08]) or chemoembolization (HR, 1.99 [95% CI, 1.43-2.78]) were at a higher risk of death than patients receiving reoperative hepatectomy or thermoablation. Overall, the features showing the greatest prognostic association with outcomes were number of recurrent nodules more than 1 (HR, 2.15 [95% CI, 1.64-2.83]), size of the biggest recurrent nodule of 50 mm or more (HR, 2.89 [95% CI, 2.14-3.90]), and extrahepatic recurrence (HR, 2.95 [95% CI, 2.19-3.97]). Moreover, to explore the role of these features as treatment effect modifiers, the comparison of the association of treatments with SAR was evaluated in the subgroups defined by each feature (Figure 1). Sorafenib showed a worse performance with respect to reoperative hepatectomy or thermoablation in people younger than 75 years (HR, 5.03 [95% CI, 3.63-7.54]) than in those older than 75 years (HR, 2.01 [95% CI, 1.16-3.46]); a similar trend is shown also by chemoembolization vs no chemoembolization (HR, 2.23 [95% CI, 1.47-3.39] vs HR, 1.62 [95% CI, 0.94-2.82]).
Figure 1. Comparison of the Association of Treatments With Survival After Recurrence Evaluated Using Cox Univariate Models in the Subgroups Defined by Each Feature Selected by Least Absolute Shrinkage and Selection Operator Penalization.
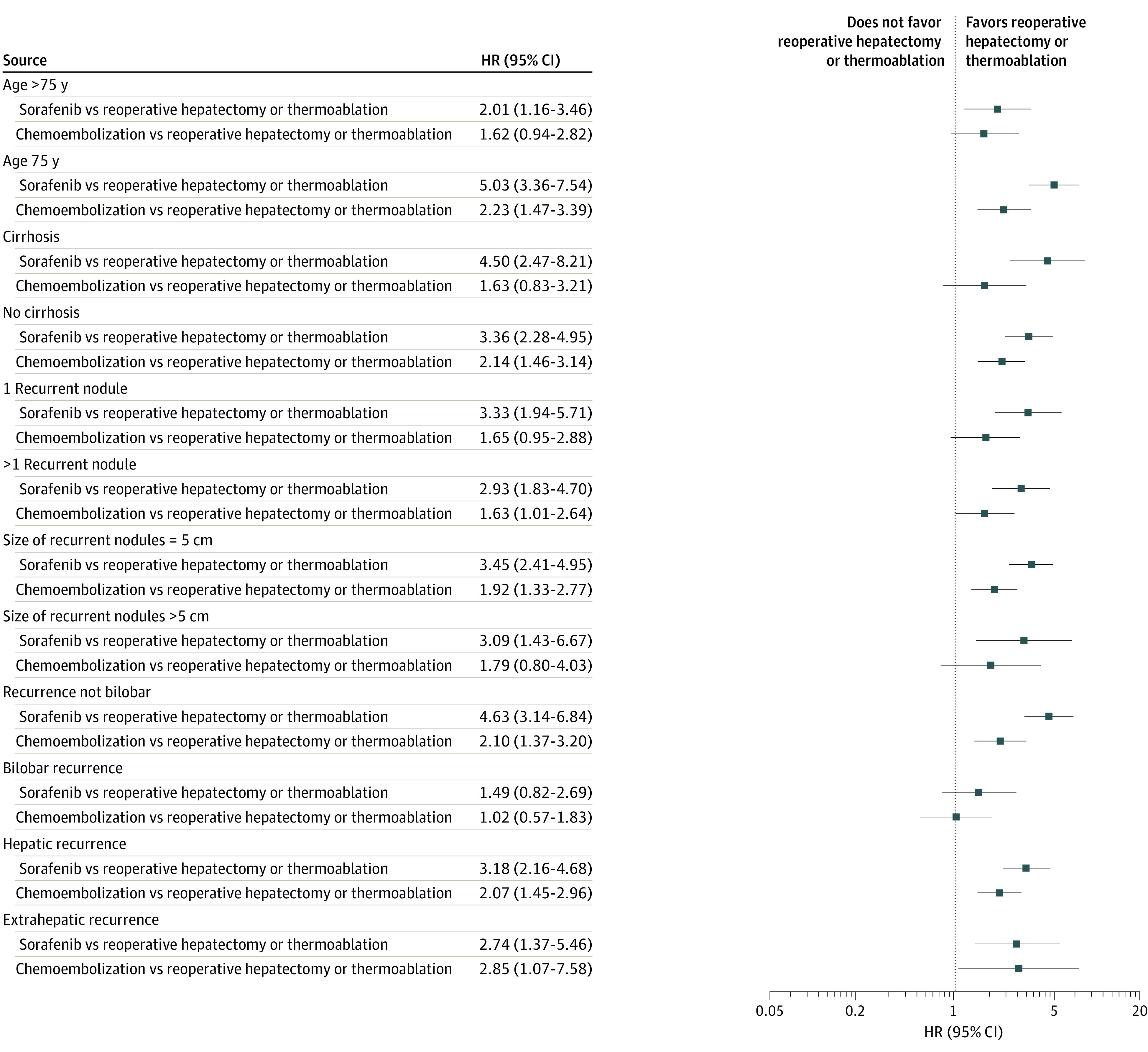
HR indicates hazard ratio.
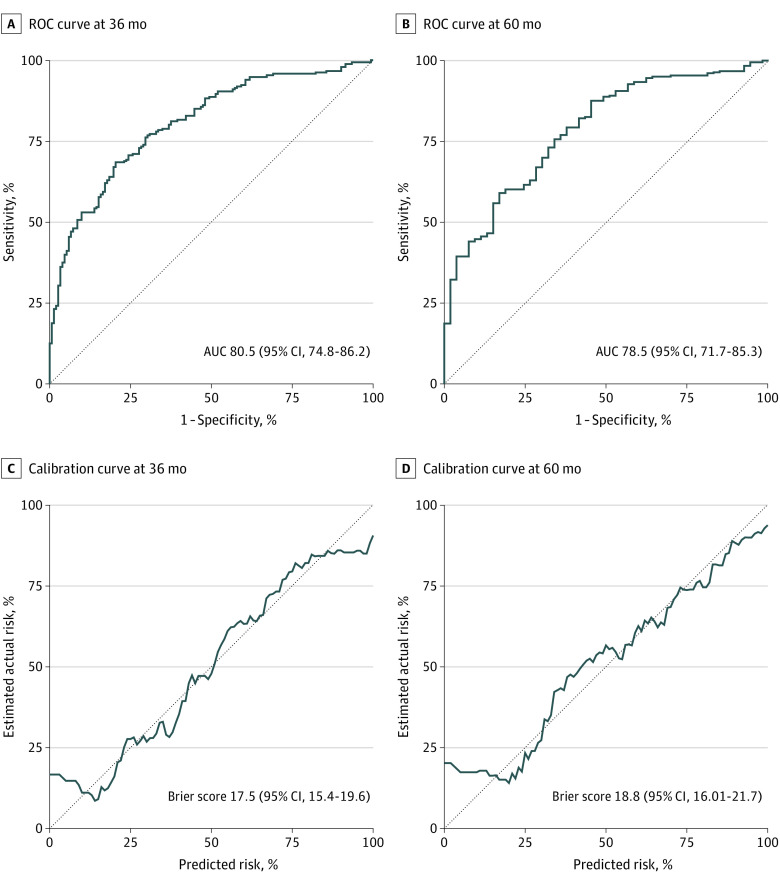
The coefficients (ie, logarithm of the HR) of the multivariable Cox model used to estimate the potential SAR under each treatment are shown in Table 2. The number of recurrent nodules and size included more than 1 coefficient because they were modeled flexibly using fractional polynomials. Thus, the model included all second-order interactions of treatment with each feature. The area under the ROC curve of the model’s ROC was high (80.5% [95% CI, 74.8%-86.2%] at 36 months and 78.5% [95% CI, 71.7%-85.3%] at 60 months) and remained satisfactory even after correcting for overoptimism using bootstrap resampling (74.3% [95% CI, 69.9%-79.5%] at 36 months and 72.1% [95% CI, 64.9%-79.1%] at 60 months; Figure 2A and B). A good level of calibration was reached as observed and the predicted SAR were in strong agreement (Figure 2C and D). The performance of the model was also tested on 2 external validation sets, the ITA.LI.CA cohort and the cohort from Tokyo University, including patients with recurrence after surgery. The model provided again a good discrimination level: in the ITA.LI.CA cohort, area under the ROC curve was 71.4% (95% CI, 64.6%-78.3%) at 36 months and 76.8% (95% CI, 69.1%-84.5%) at 60 months (eFigure 3 in Supplement 1) and in the cohort from Tokyo University, area under the ROC curve was 69.7% (95% CI, 63.8%-75.6%) at 36 months and 70.9% (95% CI, 64.3%-77.4%) at 60 months (eFigure 4 in Supplement 1). A description of the characteristics of patients of the 2 validation sets is shown in eTable 3 (ITA.LI.CA. cohort) and eTable 4 (Tokyo University) in Supplement 1, while a comparison among these cohorts and the derivation set is available in eTables 5 and 6 in Supplement 1.
Table 2. Coefficients and Standard Errors of the Cox Prediction Modela.
| Factor | Estimated coefficient (SE) |
|---|---|
| Treatment sorafenib vs reoperative hepatectomy or thermoablation | 6.59 (1.91) |
| Treatment chemoembolization vs reoperative hepatectomy or thermoablation | 3.65 (2.03) |
| Female (age) | 0.04 (0.02) |
| Cirrhosis: yes vs no | 0.15 (0.29) |
| Female (No. of recurrence nodules): 1 | −0.04 (0.65) |
| Female (No. of recurrence nodules): 2 | 7.80 (5.69) |
| Female (size recurrence nodules): 1 | 0.32 (0.17) |
| Female (size recurrence nodules): 2 | 0.85 (0.38) |
| Bilobar recurrence: yes vs no | 0.63 (0.39) |
| Extrahepatic recurrence: yes vs no | 1.02 (0.35) |
| Female (time to recurrence) | 0.68 (0.24) |
| Sorafenib × female (age) | −0.05 (0.02) |
| Chemoembolization × female (age) | −0.02 (0.02) |
| Sorafenib × cirrhosis | −0.30 (0.37) |
| Chemoembolization × cirrhosis | 0.31 (0.40) |
| Sorafenib × female (No. of recurrence nodules): 1 | −1.27 (0.89) |
| Chemoembolization × female (No. of recurrence nodules): 1 | −0.86 (0.85) |
| Sorafenib × female (No. of recurrence nodules): 2 | −4.89 (6.51) |
| Chemoembolization × female (No. of recurrence nodules): 2 | −5.47 (6.68) |
| Female (No. of recurrence nodules): 1 × female (No. of recurrence nodules): 2 | −0.15 (0.13) |
| Sorafenib × female (size recurrence nodules): 1 | 0.14 (0.12) |
| Chemoembolization × female (size recurrence nodules): 1 | −0.06 (0.17) |
| Sorafenib × female (size recurrence nodules): 2 | 0.45 (0.39) |
| Chemoembolization × female (size recurrence nodules): 2 | 0.09 (0.61) |
| Female (size recurrence nodules): 1 × female (size recurrence nodules): 2 | 0.08 (0.04) |
| Sorafenib × bilobar recurrence | −0.88 (0.51) |
| Chemoembolization × bilobar recurrence | −0.87 (0.47) |
| Sorafenib × extrahepatic recurrence | −0.62 (0.42) |
| Chemoembolization × extrahepatic recurrence | 0.90 (0.57) |
| Sorafenib × female (time to recurrence) | −0.38 (0.31) |
| Chemoembolization × female (time to recurrence) | 0.16 (0.33) |
All second-order interactions of the features with treatment are included.
Figure 2. Performance of the Algorithm in Predicting Survival After Recurrence.
AUC indicates area under the receiver operating characteristic curve; ROC, receiver operating characteristic.
For each patient, we estimated the potential SAR predicted by the model at 36 and 60 months under the 3 treatments and compared the distributions (eFigure 5 in Supplement 1). According to our model, the potential SAR would tend to be higher if all were treated with a curative approach (median [IQR] at 60 months: 0.63 [0.48-0.75]) and lower if all were treated with sorafenib (0.31 [0.20-0.47]) or chemoembolization (0.44 [0.19-0.61]).
Characteristics of the Patients After Identification of Their Best Potential Treatments
According to our model, patients were subgrouped again according to the treatment that may guarantee the potential best SAR individually. This stratification was used to understand the profiles of the patients who may benefit the more from 1 of the 3 treatments, according to our model. Reoperative hepatectomy or thermoablation was the best potential treatment for 611 patients (87.2%), while it was sorafenib for 37 (5.2%) and chemoembolization for 53 (7.6%). However, in the observed cohort only 40.4% (n = 247) of those in whom reoperative hepatectomy or thermoablation was the best potential treatment were actually treated by curative approaches; 35.1% (n = 13) were actually treated by sorafenib in which sorafenib was considered the best potential treatment by our model. In the case of chemoembolization, 35.8% (n = 19) of those who may benefit more from that approach were treated accordingly.
The characteristics of the best potential treatment cohorts are reported in Table 1, while a comparison among best potential treatment and the treatment actually received are summarized in eTable 7 in Supplement 1. Briefly, patients for whom the best potential treatment was reoperative hepatectomy or thermoablation were more frequently younger (median [IQR] age, 71.6 [64.6-76.1] years), often had cirrhosis (412 [67.4%]), frequently had larger tumors (95 individuals [15.5%] with size >5 cm), and occasionally presented with a concomitant extrahepatic spread (89 [14.6%]). Older patients (median [IQR] age, 78.5 [75.3-83.4] years), had cirrhosis (31 [83.8%]), frequently with a bilobar disease (21 [56.8%]), and with a high rate of extrahepatic spread (16 [43.2%]) were the best candidates for sorafenib. Finally, patients for whom chemoembolization was the best potential treatment had a similar median age with the sorafenib group (median [IQR] age, 77.0 [73.9-80.5] years), had a low incidence of cirrhosis (18 [34.0%]), but never had extrahepatic involvement. Median (IQR) time to recurrence was 15.11 (6.6-28.9), 6.79 (2.4-17.6), and 21.9 (10.9-41.4) months for reoperative hepatectomy or thermoablation, sorafenib, and chemoembolization, respectively, as the best potential treatment. Those risk profiles were depicted in an alluvial plot in eFigure 6 in Supplement 1.
The prediction model with the best treatment identification was also embedded in an online application, which is freely available.13 Users must select the value of each feature to create a certain patient profile and check the potential SAR under each treatment predicted by the model (eFigure 7 in Supplement 1).
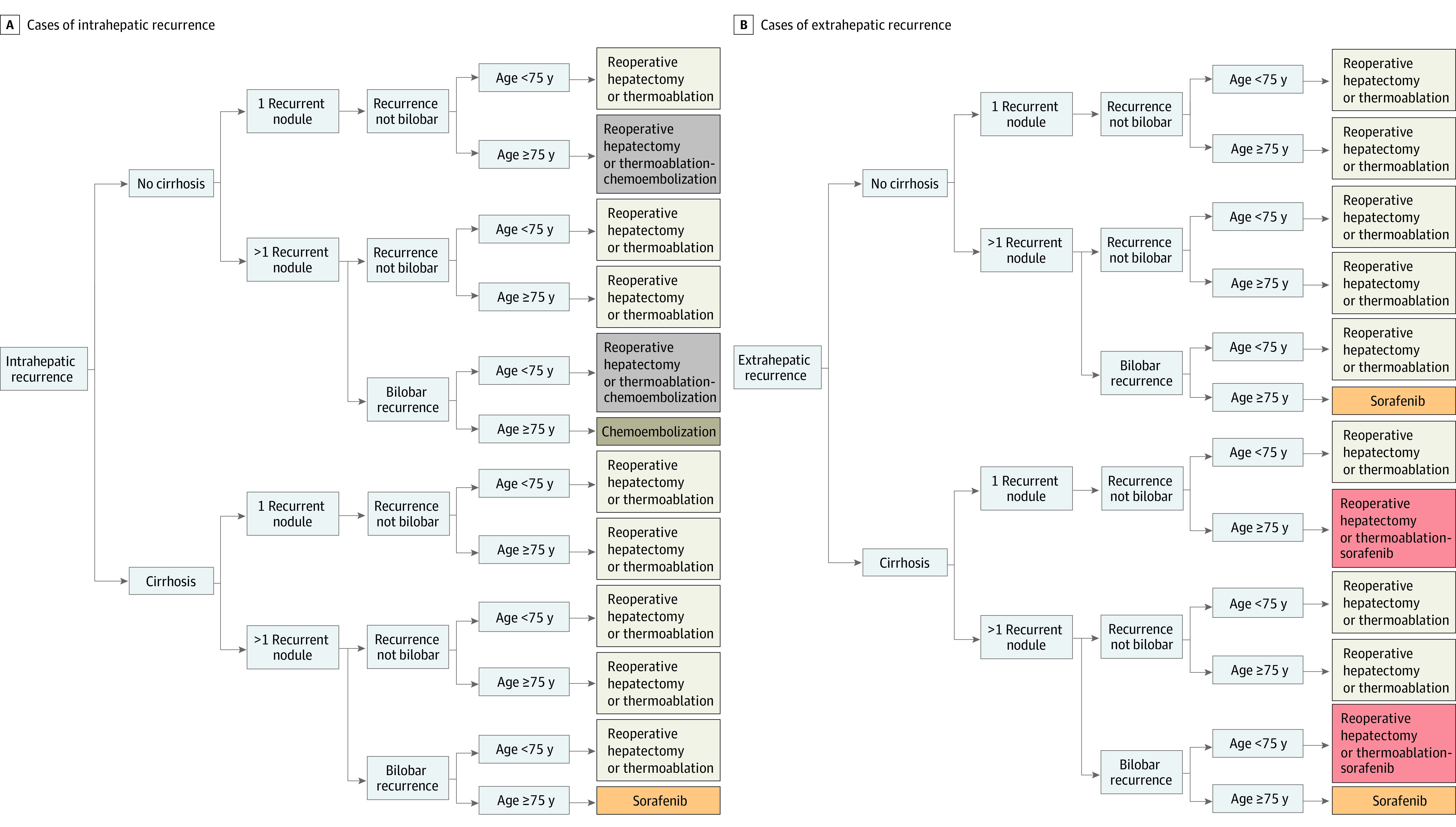
To graphically simulate how the algorithm thinks, age, size, and time to recurrence were dichotomized and predicted SAR curves for 32 risk profiles, according to the combination of 5 varying features (age, cirrhosis, number of nodules, recurrence localization, and bilobar recurrence) were then evaluated, after fixing 2 features (size of recurrent nodule, <5 cm; time to recurrence, 15 months). The curves were reported in eFigure 8 in Supplement 1 (intrahepatic recurrence) and in eFigure 9 in Supplement 1 (extrahepatic recurrence). This model’s approximation was used to build up graphical decision trees, which are available in Figure 3A (SAR at 60 months in cases of intrahepatic recurrence), Figure 3B (extrahepatic recurrence), and eFigures 10 and 11 in Supplement 1.
Figure 3. Decision Trees Generated for Simulation.
Discussion
The recognition of the best potential SAR allowed the identification that up to 87% of patients who were treated with surgery for HCC and then experienced a recurrence may find an advantage from a repetitive curative strategy. However, according to our data, almost 40% received a curative approach for their recurrence. Chemoembolization or sorafenib were frequently chosen, but they were the best potential treatment in less than 7% and 5% of participants, respectively. In other words, according to our prediction model, it seems that a large number of the cohort did not receive the best potential treatment according to the underlying characteristics. Our results, together with the model prediction accuracy, were confirmed also in cohorts different from ours, as another large Italian cohort, but also in an Eastern series as the one derived from the University of Tokyo. This suggests the urgent need for the implementation of a standardized, evidence-based treatment allocation protocol, because it seems that there is still a tendency to consider relapse after surgery as a failure of the curative intent. Although the superiority of repeated curative therapies is not surprising,14,15,16,17 different approaches could be available in the daily practice,18,19,20 as a result of the availability of multidisciplinary strategies, the high tumor heterogeneity, and the frequent coexistence of cirrhosis, which render clinical decisions more challenging.16,21 Deciding among them may be intricate: our algorithm provides a way to simulate different strategies according to the patient’s oncological characteristics. The classical stage-driven algorithms, per the Barcelona Clinic Liver Cancer,22 have already been criticized for their rigidity in allocating patients at their first diagnosis,4,23 and their role in the allocation of patients in cases of relapse has not even been recommended by the associations that have supported this system. Our methodological approach allowed us to create a highly patient-tailored algorithm, which accounted for the heterogeneity of the cases as the concomitant sum of several elements averaged by their impact on survival.
The variables selected for our model were all risk factors for mortality after recurrence, but they were also features that may change the treatment allocation. Some of them are well described as determinants of the prognosis, as in the case of a recurrent number of nodules and their sizes.14,15,16,17 The localization of the recurrence, intra- or extrahepatic, plays a very important role not only in affecting the long-term outcomes, but also in determining the best potential treatment. The extrahepatic spread was long considered, in the stage-driven algorithms, an absolute contraindication for curative intent.24 However, different authors recently demonstrated that, in selected cases, there may also be a space for a cure in this advanced setting25: our results confirmed and take into account this indication.
The time to recurrence has been largely highlighted as a biological surrogate factor: an early relapse has been correlated with an intrahepatic metastatization, while a late recurrence was considered the expression of a de novo origin driven by the underlying liver damage, which is not fixed during the tumor resection.16,26 Furthermore, the underlying liver function (here represented by the presence of cirrhosis) at the initial liver resection is strongly associated with the implementation of repeat curative therapies.16,27 Thus, while the role of age in treatment allocation is clinically evident, its role in affecting survival has recently been discussed.28,29 However, our model did not provide the tumor-specific risk of death, but the overall SAR, which is inevitably connected to aging, as evident in case of simulations among patients older than 80 years, where the survival tends to be similar and low among treatments.
It is of interest that all of those factors were not under the control of the clinicians and were dependent on the underlying liver condition and the tumor biology. In this sense, the treatment choice is the only factor in which the physicians have the opportunity to influence the outcome. The era of multidisciplinary meetings has improved the ability to modify this factor appropriately: a novel algorithm, such as the one here proposed, which is found on the ability of machine learning approaches to greatly increase the prediction power,30 may become a smart tool with which to simulate various scenarios under different treatments for the same patient. In other words, it systematically directs the choice toward the potentially most effective option on an individual basis. Our model provides a simulator to standardize the potential survival benefit according to some bio-oncological features; however, it cannot account for the feasibility of one treatment. The latter is a sum of factors as comorbidities, frailty considerations, patient will, but also surgeon’s experience. Those elements cannot be foreseen or synthesized by a machine, and automatization in oncological decision-making should be considered as a chimera. Our model, in fact, is thought to help multidisciplinary meetings in making oncological simulations, as a support and not as a replacement of the physicians’ professionalism and experience. Moreover, the parameters considered in the model reflected the tumor burden and the patients’ conditions without considering factors that could diverge in different populations, like the etiology: this can permit a worldwide use of the model.
Limitations
The limits of the present study may be different. Although a large sample size, the most extreme cases are rare in our model (eg, young patients or extremely high number of nodules) and the forecasting ability in those settings may be poor. Although the underlying liver damage was a prognosis and treatment effect modifier, the severity of cirrhosis was not significant; this may be due to the good remnant liver functions of a cohort of patients that had been relatively recently treated by surgery. Special consideration is mandatory to discuss the absence of salvage liver transplant as a choice in our model. Although the well-known survival benefit of salvage liver transplant for recurrent HCC,31 in the HE.RC.O.LE.S. database that approach has been chosen in only 48 patients (1.7%). The lack of data avoided any solid application in our analysis. However, the database depicts a 10-year real scenario among several hospitals (including those with a transplant program), suggesting that, at least in Italy, salvage liver transplant is still a rare indication. Nevertheless, the implementation of that treatment represents the room of growth for our model in the near future.
Conclusions
The herein presented algorithm should help in allocating patients with recurrent HCC to the best potential treatment according to their specific oncologic characteristics in a treatment hierarchy fashion.
eMethods 1. Variable definitions
eMethods 2. Statistical analysis in detail
eMethods 3. Patients excluded
eFigure 1. Flow-chart depicting the enrolment process from the original dataset
eFigure 2. Lasso model for variable selection
eFigure 3. Time-dependent ROC curves to validate the predictive model for SAR at (A) 3 and (B) 5 years in the external ITALICA cohort
eFigure 4. Time-dependent ROC curves to validate the predictive model for SAR at (A) 3 and (B) 5 years in the external Tokyo University Hospital cohort
eFigure 5. Box-plot comparison of the distribution of potential SAR under the three considered treatments after application of the algorithm at A) 36 months and B) 60 months
eFigure 6. Alluvial plot showing the features composition of each BPT group
eFigure 7. A snapshot from the web-app available at https://recurrence.hercolesgroup.eu is provided
eFigure 8. Predicted SAR according to all profiles and under each treatment
eFigure 9. Predicted SAR according to all profiles and under each treatment
eFigure 10. Algorithm based on SAR at 36 months for intrahepatic recurrence
eFigure 11. Algorithm based on SAR at 36 months for extrahepatic recurrence
eTable 1. Univariate Cox model to evaluate the association of features with SAR
eTable 2. Variables considered for the selection based on the Cox model with LASSO penalization
eTable 3. Description of patients of the ITALICA validation set
eTable 4. Description of patients of the Tokyo University Hospital validation set
eTable 5. Comparison of the characteristics of patients among treatments in the ITALICA (left part) and Tokyo University Hospital (right part) validation sets
eTable 6. Comparison of derivation set with ITALICA and Tokyo University Hospital validation sets
eTable 7. Comparison of features among groups of treatment actually received (on the right), and among groups of Best Potential Treatments (i.e. the treatment leading to the highest SAR for each patient; on the left)
Nonauthor collaborators. HE.RC.O.LE.S. Group
References
- 1.Famularo S, Donadon M, Cipriani F, et al. ; HE.RC.O.LE.S. Group . Hepatocellular carcinoma surgical and oncological trends in a national multicentric population: the HERCOLES experience. Updates Surg. 2020;72(2):399-411. doi: 10.1007/s13304-020-00733-6 [DOI] [PubMed] [Google Scholar]
- 2.Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49(10):1109-1113. doi: 10.1111/hepr.13411 [DOI] [PubMed] [Google Scholar]
- 3.Wen T, Jin C, Facciorusso A, et al. ; MDT of West China Hospital* . Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg Nutr. 2018;7(5):353-371. doi: 10.21037/hbsn.2018.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257(5):929-937. doi: 10.1097/SLA.0b013e31828329b8 [DOI] [PubMed] [Google Scholar]
- 5.Bzdok D, Altman N, Krzywinski M. Statistics versus machine learning. Nat Methods. 2018;15(4):233-234. doi: 10.1038/nmeth.4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319(13):1317-1318. doi: 10.1001/jama.2017.18391 [DOI] [PubMed] [Google Scholar]
- 7.Collins GS, Reitsma JB, Altman DG, Moons KGM; members of the TRIPOD group . Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Eur Urol. 2015;67(6):1142-1151. doi: 10.1016/j.eururo.2014.11.025 [DOI] [PubMed] [Google Scholar]
- 8.Famularo S, Di Sandro S, Giani A, et al. Recurrence patterns after anatomic or parenchyma-sparing liver resection for hepatocarcinoma in a western population of cirrhotic patients. Ann Surg Oncol. 2018;25(13):3974-3981. doi: 10.1245/s10434-018-6730-0 [DOI] [PubMed] [Google Scholar]
- 9.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385-395. doi: [DOI] [PubMed] [Google Scholar]
- 10.Simon N, Friedman J, Hastie T, Tibshirani R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J Stat Softw. 2011;39(5):1-13. doi: 10.18637/jss.v039.i05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerds TA, Schumacher M. Efron-type measures of prediction error for survival analysis. Biometrics. 2007;63(4):1283-1287. doi: 10.1111/j.1541-0420.2007.00832.x [DOI] [PubMed] [Google Scholar]
- 12.Gerds TA, Schumacher M. Consistent estimation of the expected Brier score in general survival models with right-censored event times. Biom J. 2006;48(6):1029-1040. doi: 10.1002/bimj.200610301 [DOI] [PubMed] [Google Scholar]
- 13.What is the best potential treatment for recurrent HCC? HERCOLES Algorithm. Accessed November 21, 2022. http://recurrence.hercolesgroup.eu/
- 14.Famularo S, Donadon M, Cipriani F, et al. ; HE.RC.O.LE.S. Group . Curative versus palliative treatments for recurrent hepatocellular carcinoma: a multicentric weighted comparison. HPB (Oxford). 2021;23(6):889-898. doi: 10.1016/j.hpb.2020.10.007 [DOI] [PubMed] [Google Scholar]
- 15.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947-955. doi: 10.1097/SLA.0000000000000710 [DOI] [PubMed] [Google Scholar]
- 16.Yoh T, Seo S, Taura K, et al. Surgery for recurrent hepatocellular carcinoma: achieving long-term survival. Ann Surg. 2021;273(4):792-799. doi: 10.1097/SLA.0000000000003358 [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Cho EH, Kim SB, Kim R. Prognosis after intrahepatic recurrence in the patients who underwent curative resection for hepatocellular carcinoma. Ann Hepatobiliary Pancreat Surg. 2020;24(4):431-436. doi: 10.14701/ahbps.2020.24.4.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gbolahan OB, Schacht MA, Beckley EW, LaRoche TP, O’Neil BH, Pyko M. Locoregional and systemic therapy for hepatocellular carcinoma. J Gastrointest Oncol. 2017;8(2):215-228. doi: 10.21037/jgo.2017.03.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poon RTP, Fan ST, O’Suilleabhain CB, Wong J. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg. 2002;195(3):311-318. doi: 10.1016/s1072-7515(02)01226-7 [DOI] [PubMed] [Google Scholar]
- 20.Nagano H, Obi S, Hatano E, et al. Multicenter, randomized, controlled trial of S-1 monotherapy versus S-1 and interferon-α combination therapy for hepatocellular carcinoma with extrahepatic metastases. Hepatol Res. 2018;48(9):717-726. doi: 10.1111/hepr.13067 [DOI] [PubMed] [Google Scholar]
- 21.Fields TD, Philips P, Scoggins CR, et al. Multi-disciplinary concurrent management of recurrent hepatocellular therapy is superior to sequential therapy. World J Surg. 2017;41(5):1331-1339. doi: 10.1007/s00268-016-3844-z [DOI] [PubMed] [Google Scholar]
- 22.European Association for the Study of the Liver . Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 23.Vitale A, Farinati F, Pawlik TM, et al. The concept of therapeutic hierarchy for patients with hepatocellular carcinoma: a multicenter cohort study. Liver Int. 2019;39(8):1478-1489. doi: 10.1111/liv.14154 [DOI] [PubMed] [Google Scholar]
- 24.Vilgrain V, Pereira H, Assenat E, et al. ; SARAH Trial Group. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624-1636. doi: 10.1016/S1470-2045(17)30683-6 [DOI] [PubMed] [Google Scholar]
- 25.Chua TC, Morris DL. Exploring the role of resection of extrahepatic metastases from hepatocellular carcinoma. Surg Oncol. 2012;21(2):95-101. doi: 10.1016/j.suronc.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Li C, Wen T, et al. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol. 2015;27(8):933-940. doi: 10.1097/MEG.0000000000000383 [DOI] [PubMed] [Google Scholar]
- 27.Roayaie S, Bassi D, Tarchi P, Labow D, Schwartz M. Second hepatic resection for recurrent hepatocellular cancer: a Western experience. J Hepatol. 2011;55(2):346-350. doi: 10.1016/j.jhep.2010.11.026 [DOI] [PubMed] [Google Scholar]
- 28.Famularo S, Di Sandro S, Giani A, et al. The impact of age and ageing on hepatocarcinoma surgery: short- and long-term outcomes in a multicentre propensity-matched cohort. Liver Int. 2019;39(5):894-904. doi: 10.1111/liv.14075 [DOI] [PubMed] [Google Scholar]
- 29.Kaibori M, Yoshii K, Yokota I, et al. ; Liver Cancer Study Group of Japan . Impact of advanced age on survival in patients undergoing resection of hepatocellular carcinoma: report of a Japanese nationwide survey. Ann Surg. 2019;269(4):692-699. doi: 10.1097/SLA.0000000000002526 [DOI] [PubMed] [Google Scholar]
- 30.Chen JH, Asch SM. Machine learning and prediction in medicine: beyond the peak of inflated expectations. N Engl J Med. 2017;376(26):2507-2509. doi: 10.1056/NEJMp1702071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kostakis ID, Machairas N, Prodromidou A, et al. Comparison between salvage liver transplantation and repeat liver resection for recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Transplant Proc. 2019;51(2):433-436. doi: 10.1016/j.transproceed.2019.01.072 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Variable definitions
eMethods 2. Statistical analysis in detail
eMethods 3. Patients excluded
eFigure 1. Flow-chart depicting the enrolment process from the original dataset
eFigure 2. Lasso model for variable selection
eFigure 3. Time-dependent ROC curves to validate the predictive model for SAR at (A) 3 and (B) 5 years in the external ITALICA cohort
eFigure 4. Time-dependent ROC curves to validate the predictive model for SAR at (A) 3 and (B) 5 years in the external Tokyo University Hospital cohort
eFigure 5. Box-plot comparison of the distribution of potential SAR under the three considered treatments after application of the algorithm at A) 36 months and B) 60 months
eFigure 6. Alluvial plot showing the features composition of each BPT group
eFigure 7. A snapshot from the web-app available at https://recurrence.hercolesgroup.eu is provided
eFigure 8. Predicted SAR according to all profiles and under each treatment
eFigure 9. Predicted SAR according to all profiles and under each treatment
eFigure 10. Algorithm based on SAR at 36 months for intrahepatic recurrence
eFigure 11. Algorithm based on SAR at 36 months for extrahepatic recurrence
eTable 1. Univariate Cox model to evaluate the association of features with SAR
eTable 2. Variables considered for the selection based on the Cox model with LASSO penalization
eTable 3. Description of patients of the ITALICA validation set
eTable 4. Description of patients of the Tokyo University Hospital validation set
eTable 5. Comparison of the characteristics of patients among treatments in the ITALICA (left part) and Tokyo University Hospital (right part) validation sets
eTable 6. Comparison of derivation set with ITALICA and Tokyo University Hospital validation sets
eTable 7. Comparison of features among groups of treatment actually received (on the right), and among groups of Best Potential Treatments (i.e. the treatment leading to the highest SAR for each patient; on the left)
Nonauthor collaborators. HE.RC.O.LE.S. Group