This cohort study develops and validates a surgical quality score by examining the association between surgical quality metric adherence and the overall survival and recurrence-free survival among US veterans diagnosed with early-stage non–small cell lung cancer.
Key Points
Question
What is the association between surgical quality metric adherence and overall survival and recurrence-free survival among US veterans with early-stage non–small cell lung cancer?
Findings
In this cohort study of 9628 veterans, adherence to intraoperative quality metrics was found to be associated with improved overall survival and recurrence-free survival. These findings were validated in a cohort of 107 674 nonveteran patients using data from the National Cancer Database.
Meaning
The findings of this study suggest that efforts to improve adherence to surgical quality metrics may improve patient outcomes following curative-intent resection of early-stage lung cancer.
Abstract
Importance
Surgical resection remains the preferred treatment for functionally fit patients diagnosed with early-stage non–small cell lung cancer (NSCLC). Process-based intraoperative quality metrics (QMs) are important for optimizing long-term outcomes following curative-intent resection.
Objective
To develop a practical surgical quality score for patients diagnosed with clinical stage I NSCLC who received definitive surgical treatment.
Design, Setting, and Participants
This retrospective cohort study used a uniquely compiled data set of US veterans diagnosed with clinical stage I NSCLC who received definitive surgical treatment from October 2006 through September 2016. The data were analyzed from April 1 to September 1, 2022. Based on contemporary treatment guidelines, 5 surgical QMs were defined: timely surgery, minimally invasive approach, anatomic resection, adequate lymph node sampling, and negative surgical margin. The study developed a surgical quality score reflecting the association between these QMs and overall survival (OS), which was further validated in a cohort of patients using data from the National Cancer Database (NCDB). The study also examined the association between the surgical quality score and recurrence-free survival (RFS).
Exposures
Surgical treatment of early-stage NSCLC.
Main Outcomes and Measures
Overall survival and RFS.
Results
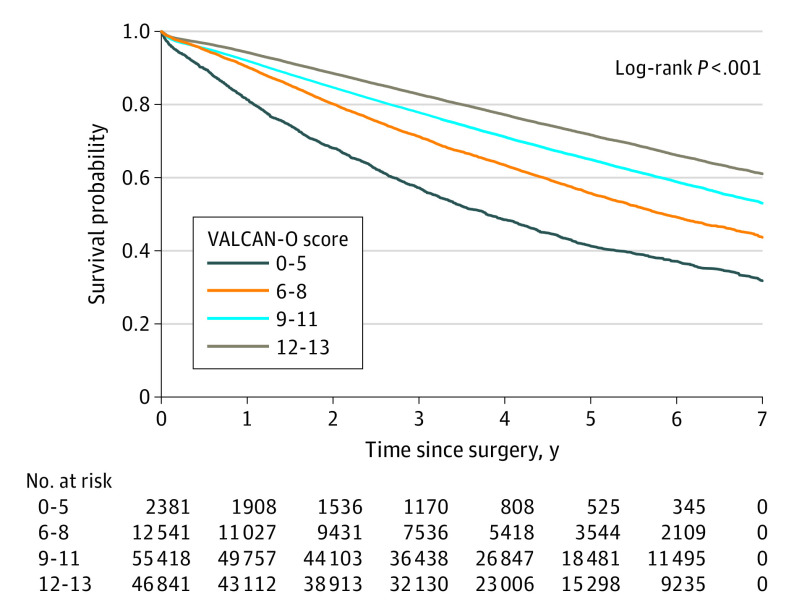
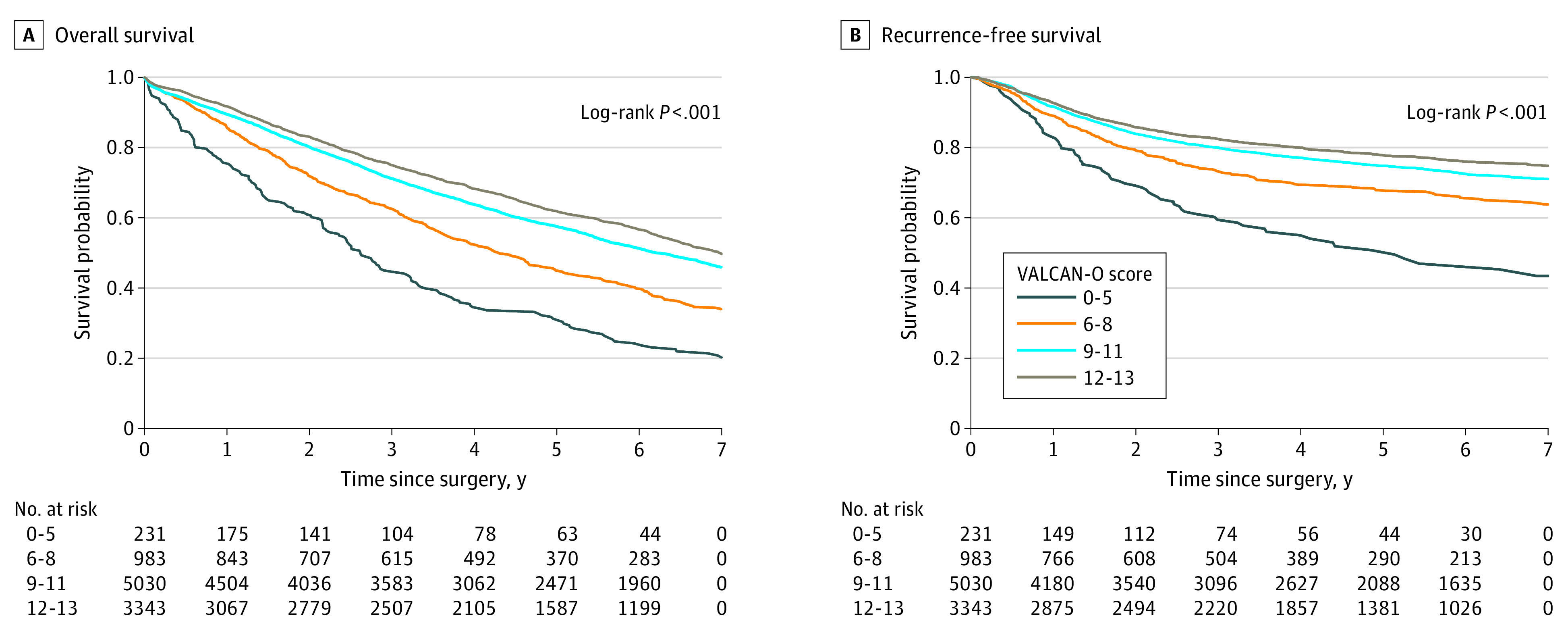
The study included 9628 veterans who underwent surgical treatment between 2006 and 2016. The cohort consisted of 1446 patients who had a mean (SD) age of 67.6 (7.9) years and included 9278 males (96.4%) and 350 females (3.6%). Among the cohort, 5627 individuals (58.4%) identified as being smokers at the time of surgical treatment. The QMs were met as follows: timely surgery (6633 [68.9%]), minimally invasive approach (3986 [41.4%]), lobectomy (6843 [71.1%]) or segmentectomy (532 [5.5%]), adequate lymph node sampling (3278 [34.0%]), and negative surgical margin (9312 [96.7%]). The median (IQR) follow-up time was 6.2 (2.5-11.4) years. An integer-based score (termed the Veterans Affairs Lung Cancer Operative quality [VALCAN-O] score) from 0 (no QMs met) to 13 (all QMs met) was constructed, with higher scores reflecting progressively better risk-adjusted OS. The median (IQR) OS differed substantially between the score categories (score of 0-5 points, 2.6 [1.0-5.7] years of OS; 6-8 points, 4.3 [1.7-8.6] years; 9-11 points, 6.3 [2.6-11.4] years; and 12-13 points, 7.0 [3.0-12.5] years; P < .001). In addition, risk-adjusted RFS improved in a stepwise manner between the score categories (6-8 vs 0-5 points, multivariable-adjusted hazard ratio [aHR], 0.62; 95% CI, 0.48-0.79; P < .001; 12-13 vs 0-5 points, aHR, 0.39; 95% CI, 0.31-0.49; P < .001). In the validation cohort, which included 107 674 nonveteran patients, the score remained associated with OS.
Conclusions and Relevance
The findings of this study suggest that adherence to intraoperative QMs may be associated with improved OS and RFS. Efforts to improve adherence to surgical QMs may improve patient outcomes following curative-intent resection of early-stage lung cancer.
Introduction
Lung cancer remains the leading cause of cancer-related mortality in the United States.1 For functionally fit patients diagnosed with early-stage disease, the preferred treatment is surgical resection.2 Inherent to the modern treatment of lung cancer is the delivery of high-quality, evidence-based care.3 Specifically in the setting of surgical resection, most contemporary lung cancer treatment guidelines recommend several modifiable, process-based surgical quality metrics (QMs) that should be met for all patients diagnosed with early-stage non–small cell lung cancer (NSCLC).4,5,6 These metrics include timely surgery,7,8,9 receipt of anatomic resection,10,11 a minimally invasive approach,12,13 negative surgical margin,14 and adequate lymph node sampling.15,16,17 Prior work from our group has demonstrated that adherence to these surgical QMs is associated with improved outcomes among patients diagnosed with NSCLC who received definitive surgical treatment.18 Despite this, guideline-concordant surgical care is infrequently achieved in early-stage NSCLC.19
Several barriers to the standardization of surgical quality have been described. For example, steep learning curves related to video-assisted thoracic surgery have been associated with poor adoption of minimally invasive lung cancer resection20,21; similarly, various clinician- and patient-specific factors have been associated with inadequate nodal sampling.22 Collectively, these barriers highlight the challenge of disseminating and implementing evidence-based, guideline-concordant practice standards within thoracic surgery.23
The Veterans Health Administration (VHA) is the largest integrated health care system in the US.24 Although the VHA serves a unique patient population,25,26 patterns of lung cancer care (including rates of adherence to intraoperative QMs) and outcomes are similar between veterans and the general US population.10 Standardization of lung cancer treatment quality within the VHA may therefore have a disproportionate impact on early-stage NSCLC outcomes among veterans and provide a road map for implementation in the general US population.
In this study, we sought to develop a pragmatic surgical quality score (termed the Veterans Affairs Lung Cancer Operative quality [VALCAN-O] score) based on the association between previously proposed process-based, intraoperative, modifiable QMs and overall survival (OS). We also examined the association between this score and recurrence-free survival (RFS). We then validated this score using a cohort of patients from the National Cancer Database (NCDB).
Methods
Study Population
In this retrospective cohort study, a unique cohort of veterans diagnosed with early-stage NSCLC who underwent definitive surgical treatment through the VHA from October 2006 through September 2016 was compiled. The cohort was assembled using the VHA Informatics and Computing Infrastructure, which contains several clinical and administrative data sets within the Corporate Data Warehouse, such as Oncology Raw and Veterans Affairs Surgical Quality Improvement Program.27 Veterans diagnosed with NSCLC were identified using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes. Surgical treatments were confirmed using either the International Classification of Diseases, Ninth Revision (ICD-9) or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) procedure or Current Procedural Terminology codes. Inclusion criteria were all adults diagnosed with clinical stage I (≤5 cm, node-negative) NSCLC who underwent surgery (American Joint Committee on Cancer, seventh edition).28 We excluded patients who received neoadjuvant therapy, those who presented with recurrent disease from a previously diagnosed cancer, patients with unavailable date of diagnosis, and those who were missing any of the prespecified QMs. Race was reported as Black, White, or other (ie, any code other than Black or White) as coded in the Corporate Data Warehouse and defined according to the American College of Surgery Facility Oncology Registry Data Standards. Race was included to assess potential factors associated with surgical quality. A dedicated team of researchers performed manual medical record review—augmented with natural language processing techniques—across 24 months to ensure minimal missingness of data, especially in relation to the surgical QMs. The study was approved by the St Louis VHA’s Research and Development Committee and institutional review board, which also waived the requirement for informed consent given the deidentified analysis.
Quality Metrics and Covariates
Based on contemporary treatment guidelines and previously validated literature, including some of our prior work, we defined 5 process-based QMs that should be met in all patients diagnosed with early-stage NSCLC: timely surgery (defined as surgery within 12 weeks of radiographic suspicion, as previously described by our group),7,8,9 anatomic resection (via lobectomy or segmentectomy),10,11,29 minimally invasive approach,12,13 negative margin,14 and adequate lymph node sampling.15,16 Timely surgery has been previously associated with worse cancer-specific outcomes.7 Adequate nodal sampling was defined as sampling at least 10 lymph nodes30; however, several sampling levels were assessed in the model (0, 1-4, 5-9, and ≥10 nodes) to avoid dichotomization of a continuous variable. Of note, while several sampling guidelines currently exist for early-stage NSCLC, we chose a count-based standard, as these were prevailing recommendations during the study period.31 Additional patient-, treatment-, and tumor-related covariates were also extracted, as described previously by our group.7,10,25,26
Outcomes
Our primary outcome was OS, defined as the time between date of surgery and death from any cause. Overall survival was assessed using the VHA Vital Status Files32 and was censored at the end of the study follow-up period (May 1, 2020). We also examined the association between the developed score and RFS. Recurrence-free survival was defined as the time between the date of surgery and cancer recurrence and was characterized using a combination of clinical documentation and billing codes suggestive of recurrence, as described previously in the VHA.25,33 For this study, RFS was censored at the date of the last follow-up or death (which was treated as a competing event in multivariable models).
Score Development
To develop the surgical quality score, a multivariable Cox proportional hazards regression model was constructed to examine the association between OS and surgical QMs. The model also controlled for pertinent patient-, treatment-, and tumor-related variables (including patient age, sex, race, smoking status, body mass index, comorbidity burden, number of prescriptions, hospital volume, tumor location, tumor size, histology, and pathologic stage) in addition to the 5 QMs. Beta coefficients for the QMs were fixed from this model to develop the integer-based VALCAN-O score (0-13 points), with higher scores representing “higher-quality” (ie, guideline-concordant) operations.34 To provide clinical context, patients were further subdivided into risk categories based on the estimated probability of survival at 5 years (ie, score of 12-13: approximately 60% 5-year survival; score of 9-11: approximately 50%-60%; score of 6-8: approximately 40%-50%; and score of 0-5: approximately 40%). Because certain QMs (ie, sublobar resection) may be associated with pulmonary function, we also assessed the VALCAN-O score in subgroup analyses of patients with normal and impaired pulmonary function (forced expiratory volume percentage predicted, ≥80%, 50%-79%, or <50%) with available data.
Statistical Analysis
The data were analyzed from April 1, 2022, to September 1, 2022. Cohort descriptive statistics were presented as mean (SD) for continuous variables and absolute numbers (proportions) for categorical variables. Patients diagnosed with pathologic stage IV disease were excluded from OS and RFS analyses (including score development). Kaplan-Meier curves were used to display OS and RFS. Given the highly curated nature of this data set, missing data were minimal and were displayed using unknown categories. Multivariable, proportional, cause-specific hazard-competing risk regression was used to examine the association between VALCAN-O score and RFS, with recurrence as the event and death as the competing event.35 The 2-sided P < .05 was considered statistically significant. All analyses were performed in SAS, version 9.3 (SAS Institute Inc).
To further apply our findings to current practice trends, we extended our cohort of veterans with early-stage NSCLC using the same methods previously described in this manuscript to include data from 2017 to 2019 (validation cohort 1). We used this extended cohort to describe longitudinal trends in the VALCAN-O score within the VHA from 2006 to 2019 (prior to the COVID-19 pandemic).
In addition, to validate our findings in a nonveteran population, we obtained a similar cohort of patients with early-stage NSCLC from the NCDB (2010-2016; minimally invasive approach data were unavailable prior to 2010). We applied the same inclusion and exclusion criteria to this cohort (validation cohort 2). Since the NCDB defines dates of diagnosis based on clinical criteria,7 we defined timely surgery as occurring within 8 weeks of the clinical diagnosis based on prior studies from the NCDB.8,9
Results
Study Cohort and Quality Metrics
The study included 9628 veterans undergoing surgical treatment for early-stage NSCLC from October 2006 through September 2016. Demographic and tumor-related factors are shown in Table 1. The mean (SD) age of the patients was 67.6 (7.9) years. The cohort included 9278 males (96.4%) and 350 females (3.6%), and all of them identified as being of the following races and ethnicities: Black (1446 [15.0%]), White (7961 [82.7%]), other (130 [1.4%]), or unknown (91 [1.0%]). Among the patients, 5627 (58.4%) were currently smoking at the time of surgery. Most of the tumors were adenocarcinomas (5136 [53.3%]) and exhibited higher-grade features (tumor grades II-IV, 7868 [81.7%]).
Table 1. Characteristics of the Veterans Health Administration Study Population.
| Characteristic | Study cohort, No. (%) (N = 9628) |
|---|---|
| Demographic variables | |
| Age, mean (SD), y | 67.6 (7.9) |
| Sex | |
| Male | 9278 (96.4) |
| Female | 350 (3.6) |
| Racea | |
| Black | 1446 (15.0) |
| White | 7961 (82.7) |
| Other | 130 (1.4) |
| Unknown | 91 (0.9) |
| Smoking status (at time of surgical treatment) | |
| Current | 5627 (58.4) |
| Former | 3868 (40.2) |
| Never | 133 (1.4) |
| BMI | |
| <18.5 | 797 (8.3) |
| 18.5-24.9 | 3086 (32.0) |
| 25-29.9 | 3281 (34.1) |
| 30-34.9 | 1757 (18.2) |
| ≥35 | 707 (7.3) |
| Charlson Comorbidity Index score, median (IQR) | 7 (5-8) |
| No. of unique prescriptions, median (IQR) | 13 (8-18) |
| Distance from hospital, miles | |
| <10 | 2105 (21.9) |
| 10-50 | 3878 (40.3) |
| ≥50 | 3645 (37.9) |
| Area Deprivation Index | |
| Q1 (least deprived) | 2334 (24.2) |
| Q2 | 2427 (25.2) |
| Q3 | 2494 (25.9) |
| Q4 (most deprived) | 2339 (24.3) |
| Unknown | 34 (0.4) |
| Tumor-related variables | |
| Histology | |
| Adenocarcinoma | 5136 (53.3) |
| Squamous cell carcinoma | 3258 (33.8) |
| Other | 1234 (12.8) |
| Grade | |
| I | 1186 (12.3) |
| II | 4781 (49.7) |
| III | 2959 (30.7) |
| IV | 128 (2.3) |
| Unknown | 574 (6.0) |
| Location | |
| Right upper lobe | 3492 (36.3) |
| Right middle lobe | 535 (5.6) |
| Right lower lobe | 1518 (15.8) |
| Left upper lobe | 2639 (27.4) |
| Left lower lobe | 1329 (13.8) |
| Unknown | 115 (1.2) |
| Tumor size, mm | |
| ≤10 | 852 (8.8) |
| 11-20 | 3786 (39.3) |
| 21-30 | 2620 (27.2) |
| 31-40 | 1451 (15.1) |
| >40 | 711 (7.4) |
| Unknown | 208 (2.2) |
| Pathologic stage | |
| I | 8385 (87.1) |
| II | 794 (8.2) |
| III | 408 (4.2) |
| IV | 41 (0.4) |
| Surgical quality metrics | |
| Timely surgery | |
| ≤12 wk | 6633 (68.9) |
| >12 wk | 2995 (31.1) |
| Incision | |
| Thoracotomy | 5642 (58.6) |
| Minimally invasive | 3986 (41.4) |
| Resection | |
| Lobectomy | 6843 (71.1) |
| Wedge | 2101 (21.8) |
| Segmentectomy | 532 (5.5) |
| Pneumonectomy | 152 (1.6) |
| Nodal sampling adequacy, No. of LNs | |
| 0 | 1000 (10.4) |
| 1-4 | 2362 (24.5) |
| 5-9 | 2988 (31.0) |
| ≥10 | 3278 (34.0) |
| Surgical marginb | |
| R0 | 9312 (96.7) |
| R1+ | 316 (3.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LNs, lymph nodes.
SI conversion factor: To convert miles to kilometers, multiply by 1.6.
As coded in the Veterans Health Administration Corporate Data Warehouse. Other includes any code other than Black or White in the American College of Surgery Facility Oncology Registry Data Standards manual.
For the surgical margin, R0 indicates negative margin and R1+ indicates positive margin.
In terms of QMs, most patients received timely surgery (6633 [68.9%]) and underwent lobectomies (6843 [71.1%]) or wedge resections (2101 [21.8%]), while a smaller number of people underwent segmentectomy (532 [5.5%]). The most common surgical approach was thoracotomy (5642 [58.6%]) followed by minimally invasive surgery (3986 [41.4%]). Adequate nodal sampling (defined as ≥10 lymph nodes) was achieved in 3278 patients (34.0%). Finally, most of the operations attained negative surgical margins (9312 [96.7%]).
Score Development
To develop the score, we examined the association between the specified surgical QMs and OS. The median (IQR) follow-up period was 6.2 (2.5-11.4) years. An integer-based score (VALCAN-O score) from 0 (no QMs met) to 13 (all QMs met) was constructed, with higher scores reflecting progressively improved risk-adjusted OS (Table 2; eTable 1 in the Supplement). The score distribution is shown in eFigure 1 in the Supplement.
Table 2. Development of the VALCAN-O Score.
| Variablea | aHR (95% CI) | β | P value | VALCAN-O points |
|---|---|---|---|---|
| Delayed surgery | ||||
| >12 wk | 1 [Reference] | 0 | ||
| ≤12 wk | 0.89 (0.84 to 0.94) | −0.12 | <.001 | 1 |
| Surgical approach | ||||
| Open | 1 [Reference] | 0 | ||
| Minimally invasive | 0.92 (0.87 to 0.97) | −0.09 | .003 | 1 |
| Extent of resection | ||||
| Wedge | 1 [Reference] | 0 | ||
| Lobectomy | 0.83 (0.77 to 0.89) | −0.19 | <.001 | 2 |
| Segmentectomy | 0.82 (0.72 to 0.94) | −0.19 | .004 | 2 |
| Pneumonectomyb | 1.08 (0.88 to 1.34) | 0.08 | .47 | 0 |
| Nodal sampling adequacy, LNs | ||||
| 0 | 1 [Reference] | 0 | ||
| 1-4 | 0.88 (0.89 to 0.96) | −0.13 | .008 | 1 |
| 5-9 | 0.82 (0.74 to 0.90) | −0.20 | <.001 | 2 |
| ≥10 | 0.78 (0.70 to 0.86) | −0.25 | <.001 | 3 |
| Surgical marginc | ||||
| R1+ | 1 [Reference] | 0 | ||
| R0 | 0.58 (0.51 to 0.67) | −0.54 | <.001 | 6 |
Abbreviations: aHR, adjusted hazard ratio; LNs, lymph nodes; VALCAN-O, Veterans Affairs Lung Cancer Operative quality.
Model controlling for displayed covariates in addition to age, sex, race, body mass index, smoking status, Charlson Comorbidity Index score, number of unique prescriptions, hospital volume, location of tumor, histology, pathologic stage, and tumor size. The full model is available in eTable 1 in the Supplement.
Pneumonectomy was given a score of 0 to prevent negative scores in the model.
For the surgical margin, R0 indicates negative margin and R1+ indicates positive margin.
To further aid with data visualization and to provide a clinical perspective, the patients were divided into risk categories based on the VALCAN-O score. As shown in Figure 1, the median (IQR) OS differed substantially between score categories (0-5 points, 2.6 [1.0-5.7] years; 6-8 points, 4.3 [1.7-8.6] years; 9-11 points, 6.3 [2.6-11.4] years; and 12-13 points, 7.0 [3.0-12.5] years; P < .001). Additionally, risk-adjusted RFS improved in a stepwise manner between the score categories (6-8 vs 0-5 points, multivariable-adjusted hazard ratio [aHR], 0.62; 95% CI, 0.48-0.79; P < .001; 12-13 vs 0-5 points, aHR, 0.39; 95% CI, 0.31-0.49; P < .001) (Figure 1 and eTable 2 in the Supplement). To further test the reliability of this score in various subgroups of patients, we performed several subanalyses based on the degree of pulmonary impairment, surgical year, and final pathologic stage. The VALCAN-O score remained associated with improved OS and RFS in these analyses (eFigure 2 in the Supplement).
Figure 1. Kaplan-Meier Curves Showing the Association Between Veterans Affairs Lung Cancer Operative Quality (VALCAN-O) Score and Overall Survival and Recurrence-Free Survival in a Veterans Health Administration Cohort.
Validation Cohort 1: Temporal Geographic Trends in VHA
We next assessed trends in surgical quality in the VHA from 2006 to 2019. We displayed this as the proportion of operations that met the highest-quality VALCAN-O score category (VALCAN-O score ≥12). As shown in Figure 2, VALCAN-O scores improved substantially over the study period; however, there was substantial regional variation. For example, the proportion of patients receiving highest-quality operations (VALCAN-O score ≥12) in Veterans Integrated Services Network (VISN) 19 increased from 32.5% (2006-2009) to 67.2% (2017-2019). Conversely, the proportion of patients receiving highest-quality operations in VISN 15 remained relatively stagnant throughout the study period (26.7% in 2006-2009 vs 29.2% in 2017-2019).
Figure 2. Geospatial Trends in Veterans Affairs Lung Cancer Operative Quality (VALCAN-O) Score According to Veterans Health Administration Administrative Region, 2006-2019.
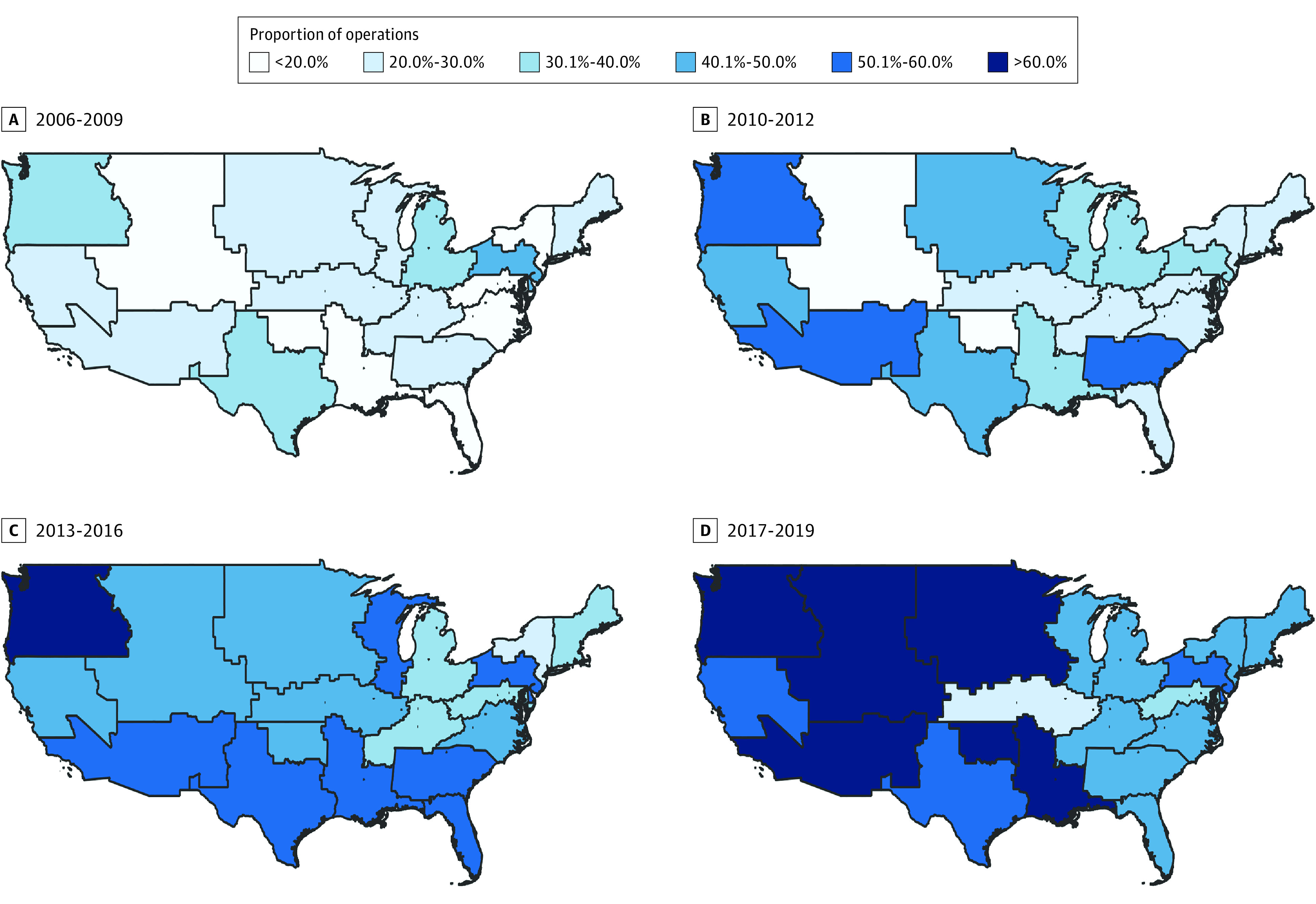
The images represent the proportion of operations in each Veterans Integrated Services Network (VISN) region obtaining a VALCAN-O score of 12 points or higher (ie, highest-quality operation), with darker blue representing a higher proportion. Alaska (VISN 20), Hawaii (VISN 21), Puerto Rico (VISN 8), and other US territories were omitted for ease of viewing.
Validation Cohort 2: NCDB Cohort
We then sought to validate our findings in a cohort of patients from the NCDB who had been diagnosed with early-stage NSCLC and were treated at non-VHA hospitals. This cohort included 107 674 patients. Additional demographic information is given in eTable 3 in the Supplement. The QMs were met as follows: timely surgery (85 142 patients [79.1%]), minimally invasive approach (42 199 [39.2%]), lobectomy (78 195 [72.6%]), adequate nodal sampling (37 885 [35.2%]), and negative surgical margin (104 778 [97.3%]). Similar to the VHA cohort, the median (IQR) OS differed substantially between score categories (0-5 points, 3.8 [1.4-8.4] years; 6-8 points, 5.9 [2.6-9.2] years; 9-11 points, 7.6 [3.4 to not reached] years; and 12-13 points, 8.7 [4.4 to not reached] years; P < .001) (Figure 3).
Figure 3. Kaplan-Meier Curves Showing the Association Between Veterans Affairs Lung Cancer Operative Quality (VALCAN-O) Score and Overall Survival in the National Cancer Database Cohort.
Discussion
Using a uniquely compiled data set from the VHA, we developed a practical, easy-to-calculate surgical quality score—the VALCAN-O score—for patients diagnosed with resectable early-stage NSCLC. The score reflects the risk-adjusted association between 5 previously validated, modifiable surgical QMs (ie, timely surgery, anatomic resection, surgical approach, adequate nodal sampling, and negative surgical margin) and long-term OS. We also found that the score was associated with RFS. While the score was developed in a cohort of nearly 10 000 veterans, which represents an admittedly unique patient population, we validated the score in a cohort of more than 100 000 US adults from the NCDB. Our data suggest that efforts to standardize and optimize surgical quality may have disproportionate repercussions for patients diagnosed with early-stage NSCLC who are receiving curative-intent surgical treatment.
Most contemporary treatment guidelines converge in recommending several process-based surgical QMs that should be routinely met in patients diagnosed with early-stage NSCLC. These include timely surgery, anatomic resection, minimally invasive surgical approach, adequate nodal sampling, and negative surgical margin, when possible.4,5,6 Although these QMs are widely considered guideline-concordant standards of care, it is striking to note the relatively poor adherence to these measures within both the VHA and civilian hospitals (NCDB). For example, we found that only 34.0% (35.2% NCDB) of the patients in either cohort received adequate nodal sampling; similarly, only 41.4% (39.2% NCDB) of the patients received minimally invasive resections and 21.8% (21.3% NCDB) of the patients received nonanatomic wedge resections. Although we found that adherence to these QMs did improve over time within the VHA, we still identified wide variability even in a modern cohort of patients through 2019. Our findings clearly indicate that further efforts are needed to improve the dissemination and implementation of guideline-concordant practices in thoracic surgery to improve lung cancer outcomes in both VHA and non-VHA settings.23
Efforts to improve surgical quality, such as through the optimization of the VALCAN-O components, may be particularly fruitful within a closed practice environment like the VHA. The VHA has recently invested substantial resources into dissemination and implementation efforts, including through the creation of institutes like the Center for Evaluation and Implementation Resources.36 The VHA has already demonstrated significant success in standardizing lung cancer care beyond what is observed in the general population. For example, the VHA has rapidly implemented robust lung cancer screening programs for veterans37; conversely, screening rates among the general US adult population are much lower.38 Similarly, efforts to standardize surgical quality for early-stage lung cancer may therefore be highly beneficial and readily adoptable within the VHA.
It is also important to emphasize that the VHA provides veterans with a quality of care that is comparable to or better than that received at civilian hospitals. We found that rates of adherence to QMs were similar in the VHA and the NCDB. This is an important observation, as the VHA is often perceived as delivering “low-quality” or inferior care to veterans. However, our findings demonstrate that veterans receive guideline-concordant care at rates similar to those for the general population. More important, the VHA treats a patient population with a much greater comorbidity burden. Therefore, while unadjusted survival appears to be worse among veterans, prior work by our group has demonstrated that after appropriate risk-adjustment, short- and long-term outcomes are similar (if not superior) in veterans compared with the general population.10 It is important to emphasize this finding given ongoing policy endeavors that may fundamentally alter the paradigm of care being delivered through the VHA.39 Our study again suggests that future policies should focus on improving this unique system that is already performing well.40 Nonetheless, both the VHA and NCDB data sets suggest that QM adherence is variable, presenting an opportunity for significant quality improvement efforts in both practice settings.
Last, it is important to reflect on the varying definitions of surgical quality.41 Traditionally, the surgical literature has focused on minimizing short-term postoperative complications and mortality as a metric of high-quality care. For example, the Society of Thoracic Surgeons Star Rating System grades hospitals based on their risk-adjusted rates of 30-day morbidity and mortality.42 Although important, those events happen infrequently after early-stage lung cancer operations.43 Furthermore, they miss a likely more important goal of curative-intent lung cancer resection: achieving long-term survival. Additionally, the diametrically opposed association between certain QMs (eg, sublobar resection, adequate nodal sampling) and short- vs long-term outcomes is noteworthy. For example, while sublobar resections are associated with a significantly lower risk of postoperative morbidity and mortality, this comes at the cost of worse long-term cancer-specific outcomes, including higher rates of cancer recurrence.11,29 Therefore, thoracic surgeons must continue to adopt a less myopic view of surgical quality that focuses on long-term outcomes, such as that which the VALCAN-O score offers.
Strengths and Limitations
This study is strengthened by the highly unique VHA data set, which was assembled over a period of more than 24 months by a dedicated team of VHA-based researchers who queried electronic health records, creating this relatively homogeneous cohort of patients receiving definitive surgical treatment. In addition, the data set includes outcomes data like recurrence, which is infrequently available through other national, multi-institutional databases. Conversely, this study also had some limitations. First, the VHA treats a unique patient population that consists disproportionately of males with a heavy comorbidity burden. Despite this, previous work by our group has shown that patterns of care and outcomes are similar between veteran and nonveteran populations.10 In addition, we validated this score in a large cohort of patients with early-stage NSCLC from the general population. Therefore, efforts to standardize and improve surgical quality may improve early-stage lung cancer outcomes in both the VHA and civilian systems. Second, it is important to acknowledge that these QMs cannot be met in all situations. For example, a certain subset of operations will require a thoracotomy for anatomic reasons13 or may necessitate a sublobar resection due to diminished lung function. However, our data indicated that even sicker patients with compromised pulmonary function benefit from better adherence to these surgical QMs. Third, cause-specific death, a potentially more appropriate end point, was unavailable. Fourth, we assessed lymph node sampling adequacy using a count-based approach. This was the prevailing recommendation during the study period and also the only available data within the NCDB. However, future studies will need to assess how adherence to other guidelines (eg, station-based standards) may affect outcomes.
Conclusions
The findings of this cohort study suggest that the VALCAN-O score is a practical, easy-to-calculate metric of surgical quality in early-stage NSCLC. Although adherence to quality measures has improved over time, wide variability in guideline-concordant care still exists in the US. Efforts to standardize and optimize long-term cancer-specific outcomes are critical for patients diagnosed with resectable lung cancer who are receiving curative-intent treatment. Future policy efforts should focus on more widespread implementation of these metrics.
eTable 1. Multivariable Cox Proportional Hazards Model for Overall Survival
eFigure 1. VALCAN-O Score Distribution
eTable 2. Multivariable Competing Risk Model for Recurrence-free Survival
eFigure 2. Relationship Between VALCAN-O and Overall Survival and Recurrence-Free Survival in Subgroups Based on Pulmonary Function (A, B), Surgical Year (C, D), and Final Pathologic Stage (E, F)
eTable 3. Validation Cohort (NCDB)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Puri V, Crabtree TD, Bell JM, et al. Treatment outcomes in stage I lung cancer: a comparison of surgery and stereotactic body radiation therapy. J Thorac Oncol. 2015;10(12):1776-1784. doi: 10.1097/JTO.0000000000000680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson J, Semenkovich T, Puri V. Oncologic quality indicators in thoracic surgery. Thorac Surg Clin. 2017;27(3):227-244. doi: 10.1016/j.thorsurg.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network . Non–small Cell Lung Cancer, Version 6. 2020. NCCN Guidelines in Oncology. Accessed January 9, 2020. https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf
- 5.Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5)(suppl):7S-37S. doi: 10.1378/chest.12-2377 [DOI] [PubMed] [Google Scholar]
- 6.Postmus PE, Kerr KM, Oudkerk M, et al. ; ESMO Guidelines Committee . Early and locally advanced non–small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv1-iv21. doi: 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 7.Heiden BT, Eaton DB Jr, Engelhardt KE, et al. Analysis of delayed surgical treatment and oncologic outcomes in clinical stage I non–small cell lung cancer. JAMA Netw Open. 2021;4(5):e2111613. doi: 10.1001/jamanetworkopen.2021.11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samson P, Patel A, Garrett T, et al. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage I non–small cell lung cancer. Ann Thorac Surg. 2015;99(6):1906-1912. doi: 10.1016/j.athoracsur.2015.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayne NR, Elser HC, Darling AJ, et al. Estimating the impact of extended delay to surgery for stage I non–small-cell lung cancer on survival. Ann Surg. 2021;273(5):850-857. doi: 10.1097/SLA.0000000000004811 [DOI] [PubMed] [Google Scholar]
- 10.Heiden BT, Eaton DBJ Jr, Chang SH, et al. Comparison between veteran and non-veteran populations with clinical stage I non–small cell lung cancer undergoing surgery. Ann Surg. Published online May 11, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian M, McMurry T, Meyers BF, Puri V, Kozower BD. Long-term results for clinical stage IA lung cancer: comparing lobectomy and sublobar resection. Ann Thorac Surg. 2018;106(2):375-381. doi: 10.1016/j.athoracsur.2018.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim E, Batchelor TJP, Dunning J, et al. Video-assisted thoracoscopic or open lobectomy in early-stage lung cancer. NEJM Evid. Published online January 18, 2022. doi: 10.1056/EVIDoa2100016 [DOI] [PubMed] [Google Scholar]
- 13.Heiden BT, Mitchell JD, Rome E, et al. Cost-effectiveness analysis of robotic-assisted lobectomy for non–small cell lung cancer. Ann Thorac Surg. 2021;114(1):265-272. doi: 10.1016/j.athoracsur.2021.06.090 [DOI] [PubMed] [Google Scholar]
- 14.Osarogiagbon RU, D’Amico TA. Improving lung cancer outcomes by improving the quality of surgical care. Transl Lung Cancer Res. 2015;4(4):424-431. doi: 10.3978/j.issn.2218-6751.2015.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smeltzer MP, Faris NR, Ray MA, Osarogiagbon RU. Association of pathologic nodal staging quality with survival among patients with non–small cell lung cancer after resection with curative intent. JAMA Oncol. 2018;4(1):80-87. doi: 10.1001/jamaoncol.2017.2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osarogiagbon RU, Allen JW, Farooq A, Wu JT. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol. 2012;7(2):390-396. doi: 10.1097/JTO.0b013e31823e5e2d [DOI] [PubMed] [Google Scholar]
- 17.Heiden BT, Eaton DB, Chang SH, et al. Assessment of updated Commission on Cancer guidelines for intraoperative lymph node sampling in early-stage NSCLC. J Thorac Oncol. 2022;S1556-0864(22):1551-1559. doi: 10.1016/j.jtho.2022.08.009 [DOI] [PubMed] [Google Scholar]
- 18.Samson P, Crabtree T, Broderick S, et al. Quality measures in clinical stage I non–small cell lung cancer: improved performance is associated with improved survival. Ann Thorac Surg. 2017;103(1):303-311. doi: 10.1016/j.athoracsur.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kehl KL, Zahrieh D, Yang P, et al. Rates of guideline-concordant surgery and adjuvant chemotherapy among patients with early-stage lung cancer in the US ALCHEMIST Study (Alliance A151216). JAMA Oncol. 2022;8(5):717-728. doi: 10.1001/jamaoncol.2022.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheel PJ III, Crabtree TD, Bell JM, et al. Does surgeon experience affect outcomes in pathologic stage I lung cancer? J Thorac Cardiovasc Surg. 2015;149(4):998-1004.e1. doi: 10.1016/j.jtcvs.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian MP, Liu J, Chapman WC, et al. Utilization trends, outcomes, and cost in minimally invasive lobectomy. Ann Thorac Surg. 2019;108(6):1648-1655. doi: 10.1016/j.athoracsur.2019.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg. 2010;89(6):1730-1735. doi: 10.1016/j.athoracsur.2010.02.094 [DOI] [PubMed] [Google Scholar]
- 23.Heiden BT, Tetteh E, Robbins KJ, et al. Dissemination and implementation science in cardiothoracic surgery: a review and case study. Ann Thorac Surg. 2021;114(2):373-382. doi: 10.1016/j.athoracsur.2021.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blosnich JR, Dichter ME, Gurewich D, et al. Health services research and social determinants of health in the nation’s largest integrated health care system: steps and leaps in the Veterans Health Administration. Mil Med. 2020;185(9-10):e1353-e1356. doi: 10.1093/milmed/usaa067 [DOI] [PubMed] [Google Scholar]
- 25.Heiden BT, Eaton DB Jr, Chang SH, et al. The impact of persistent smoking after surgery on long-term outcomes after stage I non–small cell lung cancer resection. Chest. 2022;161(6):1687-1696. doi: 10.1016/j.chest.2021.12.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heiden BT, Eaton DB Jr, Chang SH, et al. Assessment of duration of smoking cessation prior to surgical treatment of non–small cell lung cancer. Ann Surg. Published online November 18, 2021. doi: 10.1097/SLA.0000000000005312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massarweh NN, Kaji AH, Itani KMF. Practical guide to surgical data sets: Veterans Affairs Surgical Quality Improvement Program (VASQIP). JAMA Surg. 2018;153(8):768-769. doi: 10.1001/jamasurg.2018.0504 [DOI] [PubMed] [Google Scholar]
- 28.Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012;4(4):128-134. doi: 10.4329/wjr.v4.i4.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saji H, Okada M, Tsuboi M, et al. ; West Japan Oncology Group and Japan Clinical Oncology Group . Segmentectomy versus lobectomy in small-sized peripheral non–small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399(10335):1607-1617. doi: 10.1016/S0140-6736(21)02333-3 [DOI] [PubMed] [Google Scholar]
- 30.Nissen AP, Vreeland TJ, Teshome M, et al. American College of Surgeons Commission on Cancer standard for curative-intent pulmonary resection. Ann Thorac Surg. 2022;113(1):5-8. doi: 10.1016/j.athoracsur.2021.05.051 [DOI] [PubMed] [Google Scholar]
- 31.Odell DD, Feinglass J, Engelhardt K, et al. Evaluation of adherence to the Commission on Cancer lung cancer quality measures. J Thorac Cardiovasc Surg. 2019;157(3):1219-1235. doi: 10.1016/j.jtcvs.2018.09.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarlov E, Lee TA, Weichle TW, et al. Reduced overall and event-free survival among colon cancer patients using dual system care. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2231-2241. doi: 10.1158/1055-9965.EPI-12-0548 [DOI] [PubMed] [Google Scholar]
- 34.Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23(10):1631-1660. doi: 10.1002/sim.1742 [DOI] [PubMed] [Google Scholar]
- 35.Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66(6):648-653. doi: 10.1016/j.jclinepi.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 36.US Department of Veterans Affairs. Implementation and evaluation resources. Accessed April 14, 2022. https://www.queri.research.va.gov/ceir/
- 37.Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406. doi: 10.1001/jamainternmed.2016.9022 [DOI] [PubMed] [Google Scholar]
- 38.Heiden BT, Engelhardt KE, Cao C, et al. Prevalence of cigarette and e-cigarette use among U.S. adults eligible for lung cancer screening based on updated USPSTF guidelines. Cancer Epidemiol. 2022;76:102079. doi: 10.1016/j.canep.2021.102079 [DOI] [PubMed] [Google Scholar]
- 39.Massarweh NN, Itani KMF, Morris MS. The VA MISSION Act and the future of veterans’ access to quality health care. JAMA. 2020;324(4):343-344. doi: 10.1001/jama.2020.4505 [DOI] [PubMed] [Google Scholar]
- 40.Heiden BT, Eaton DB Jr, Chang SH, et al. Racial disparities in the surgical treatment of clinical stage I non–small cell lung cancer among veterans. Chest. 2022;162(4):920-929. doi: 10.1016/j.chest.2022.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenbaum L. Reassessing quality assessment—the flawed system for fixing a flawed system. N Engl J Med. 2022;386(17):1663-1667. doi: 10.1056/NEJMms2200976 [DOI] [PubMed] [Google Scholar]
- 42.Explanation of star ratings. STS Public Reporting. Accessed April 14, 2022. https://publicreporting.sts.org/gtsd-exp
- 43.Heiden BT, Kozower BD. Keeping a safe distance from surgical volume standards. J Clin Oncol. 2022;40(10):1033-1035. doi: 10.1200/JCO.21.02875 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Multivariable Cox Proportional Hazards Model for Overall Survival
eFigure 1. VALCAN-O Score Distribution
eTable 2. Multivariable Competing Risk Model for Recurrence-free Survival
eFigure 2. Relationship Between VALCAN-O and Overall Survival and Recurrence-Free Survival in Subgroups Based on Pulmonary Function (A, B), Surgical Year (C, D), and Final Pathologic Stage (E, F)
eTable 3. Validation Cohort (NCDB)