Abstract
Given the emerging difficulties with malaria drug resistance and vector control, as well as the persistent lack of an effective vaccine, new malaria vaccine development strategies are needed. We used a novel methodology to synthesize and fully characterize multiple antigen peptide (MAP) conjugates containing protective epitopes from Plasmodium falciparum and evaluated their immunogenicity in four different strains of mice. A di-epitope MAP (T3-T1) containing two T-cell epitopes of liver stage antigen-1 (LSA-1), a di-epitope MAP containing T-cell epitopes from LSA-1 and from merozoite surface protein-1, and a tri-epitope MAP (T3-CS-T1) containing T3-T1 and a potent B-cell epitope from the circumsporozoite protein central repeat region were tested in this study. Mice of all four strains produced peptide-specific antibodies; however, the magnitude of the humoral response indicated strong genetic restriction between the different strains of mice. Anti-MAP antibodies recognized stage-specific proteins on the malaria parasites in an immunofluorescence assay. In addition, serum from hybrid BALB/cJ × A/J CAF1 mice that had been immunized with the tri-epitope MAP T3-CS-T1 successfully inhibited the malaria sporozoite invasion of hepatoma cells in vitro. Spleen cells from immunized mice also showed a genetically restricted cellular immune response when stimulated with the immunogen in vitro. This study indicates that well-characterized MAPs combining solid-phase synthesis and conjugation chemistries are potent immunogens and that this approach can be utilized for the development of subunit vaccines.
Malaria continues to be a major cause of mortality and morbidity in tropical areas of Africa, Asia, South America, and the South Pacific, causing an estimated 300 to 400 million new cases and more than 1.1 million deaths annually (51). The emergence of parasites that are resistant to multiple drugs and of mosquitoes that are insecticide resistant has exacerbated the problem. While a number of vaccine candidates have made it into clinical trials, few have shown great promise (20, 27, 35, 39, 42, 45, 49). These factors emphasize the need for the continued development of new malaria vaccine strategies to improve public health in areas of malaria endemicity and for visitors and short-term residents of those areas.
The life cycle of the malaria parasite is complex; the stages in humans are morphologically and antigenically distinct and immunity tends to be stage specific (10, 22). This stage-specific gene expression actually presents an opportunity to target antigens in several stages as potential vaccine candidates. The circumsporozoite (CS) protein (5) on the surface of early sporozoites, liver-stage antigen-1 (LSA-1) (17, 23, 52), expressed when sporozoites invade liver cells, and merozoite surface protein-1 (MSP-1) (21), expressed by late liver- and blood-stage parasites, are among the handful of antigens that have been shown to have stage-specific activity to target different developmental stages of the parasite and potentially lead to better protection.
Crude antigen or attenuated malaria vaccines would be hard to produce, given the difficulty and hazards associated with mass production of parasites, the potential presence of adventitious agents, and the possibility of side effects due to incomplete attenuation. Synthetic polypeptides as vaccine antigens provide a safer alternative to these conventional vaccine approaches. Peptide vaccines can be even more effective by focusing the host immune response on epitopes known to play a role in protective immunity and have been shown to elicit better cell-mediated immunity and to induce specific antibody responses (18, 30, 34, 38), although constructs containing linear B-cell epitopes from malaria antigens have not always met with their expected success (2, 9, 19). Both antibody-dependent and -independent T-cell-mediated protective immune mechanisms are operative at different stages of the parasite life cycle (4, 10), so the ideal vaccine should combine epitopes identified as strong inducers of immunity.
Over the past several years, considerable progress has been made toward the development and structural design of complex polypeptides to be used as antigens. Multiple antigen peptide (MAP) conjugates provide a means to include different stage-specific peptides on one molecule, resulting in a multiepitope, multistage vaccine molecule that can potentially lead to better protection. MAPs (11, 46) offer a very attractive alternative to the conventional linear peptide approach based on a small immunologically inert core molecule of radial branching lysine residues onto which a number of peptide antigens can be anchored. This results in a large macromolecule with a unique three-dimensional configuration which has a high molar ratio of peptide antigen to core molecule and does not require a carrier protein for elicitation of the immune response. The MAP system has already been shown to be valuable in immunological studies of vaccine development in malaria and other systems (7, 28, 33, 36, 47).
The construction of multiepitope malaria vaccines of defined composition has been challenging, with technical difficulties in both the synthesis and purification of product. Use of classical solid-phase synthesis methodologies in making the traditional MAP presents difficulties that often result in a highly heterogeneous product (36). In many cases it becomes impractical to obtain a reasonable amount of well-characterized product to be evaluated as a potential vaccine candidate. We recently overcame these difficulties and have successfully synthesized a novel multiple peptide conjugate containing immunogenic epitopes from two Plasmodium falciparum surface proteins (CS protein and MSP-1) and from LSA-1 that can be used to produce well-defined subunit vaccines in high yield (3). A solid-phase peptide core was synthesized on a branched lysine base and then purified by high-performance liquid chromatography. The resultant molecule was then reacted with purified holoacetyl peptides to form the MAPs in liquid phase, with molecular masses of 10 to 13 kDa.
Protective P. falciparum epitopes identified through immunoepidemiologic studies in humans (8, 24, 41, 44) and in mice (28, 36) were combined in di- or tri-epitope conformations into three MAP constructs, and their immunogenicity was evaluated in mice of diverse genetic backgrounds. Incorporation of immunodominant T-cell epitopes from LSA-1 or MSP-1 and a B-cell epitope from the CS protein may provide better protection in a primary infection or in boosting of a natural infection. In this report we describe (i) the antibody responses in one outbred, one F1 hybrid, and two inbred strains of mice that were immunized with MAPs formulated in Freund's adjuvant to study the potential for genetic restriction; (ii) the reactivities of the MAP-elicited antibodies with various stages of P. falciparum; and (iii) the in vitro inhibitory activity of the MAP-elicited antibodies from mice immunized with a MAP molecule containing CS protein repeat sequences.
MATERIALS AND METHODS
Mice and immunization.
Female 6- to 8-week-old inbred B10.BR (Jackson Laboratory, Bar Harbor, Maine), C57BL/10 (Jackson Laboratory), outbred Cr:sw (Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, Md.), and hybrid BALB/cJ × A/J CAF1 (Jackson Laboratory) mice were used in this study. MAP vaccines were dissolved in normal saline at a 0.5-mg/ml concentration. Groups of eight mice were immunized subcutaneously with 25 μg of a di- or tri-epitope MAP construct three times at 4-week intervals. Mice received the first injection of the MAP in an emulsion with complete Freund's adjuvant (Life Technologies/Gibco, Grand Island, N.Y.) and subsequent boosters with MAP in an emulsion with incomplete Freund's adjuvant. Control groups of mice received a similar amount of adjuvant in saline or a randomized MAP. Mice were bled from the retro-orbital sinus at different times during the course of immunization. Serum samples from eight mice per group were pooled and used in all serological assays. All the experimental animals were housed, fed, and used in accordance with guidelines set forth in the National Institutes of Health manual “Guide for the Care and Use of Laboratory Animals.” The Center for Biologics Evaluation and Research Animal Care and Use Committee approved the animal study protocol.
Peptides.
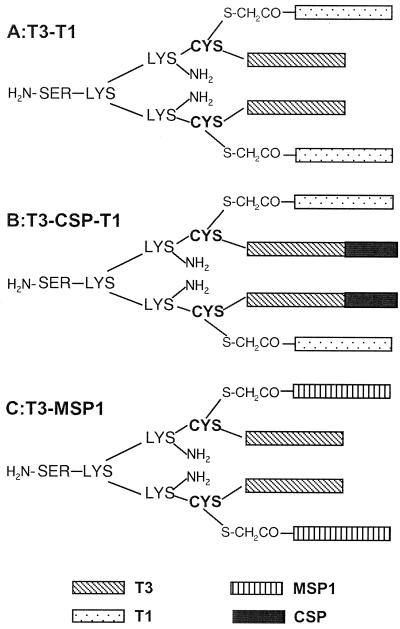
Schematic structures of the MAPs used in the study are given in Fig. 1. All of the MAPs were synthesized and then fully characterized using several methods, including matrix-assisted laser desorption ionization–time of flight mass spectroscopy and amino acid analysis, and were found to be chemically well defined as described previously (3). Briefly, the MAPs were synthesized as follows. (i) A di-epitope MAP (T3-T1) containing the T1 and T3 T-cell epitopes from LSA-1 (8) was synthesized. The di-epitope MAPs contain the T3 peptide sequence incorporated on two arms of a tetrameric core forming a base peptide molecule. The T1 epitope used in this study has known human and putative murine HLA class I binding motifs (12, 44) and is reported as a potential T-cell epitope by amphipathicity analysis (24). (ii) A di-epitope construct containing the T3 peptide sequence from LSA-1 and an N-terminal peptide (amino acids 20 to 39) of MSP-1 (T3-MSP1) which was reported to generate a proliferative response and to stimulate the secretion of gamma interferon (IFN-γ) in patients with a history of malaria was also synthesized. The T1 or MSP-1 peptide sequences are attached to the SH group of the cysteine residue of the T3 core molecule by a haloacetyl linkage, forming tetrameric T3-T1 (11,144 kDa) and T3-MSP1 (10,711 kDa) MAP molecules, respectively. (iii) Finally, a tri-epitope MAP (T3-CS-T1) containing T-cell epitopes (T1 and T3) from LSA-1 in combination with the B-cell epitope (three NANP tetrapeptide repeats) of the CS protein was produced. The tri-epitope T3-CS-T1 MAP (12,737 kDa) was designed in the same manner with an additional set of three NANP repeat sequences extended on the ends of the branches containing the T3 sequences. A control MAP (randomized; 12,737 kDa) was generated by synthesizing a di-epitope MAP containing randomized sequences of either the T1 or the T3-CS peptides.
FIG. 1.
MAP conjugates T3-T1 (A), T3-CS-T1 (B), and T3-MSP1 (C). MAPs are synthesized as a base pair of peptides on the lysine-cysteine core and are conjugated with a second pair of peptides on the alpha sulfur.
Antibody assays. (i) Peptide-specific total IgG analysis.
Antibody levels in sera from mice immunized with peptides and from control mice were assayed by enzyme-linked immunosorbent assays (ELISA) using the appropriate MAPs as capture antigens. Briefly, 50 μl of a 0.5-μg/ml solution of MAP in phosphate-buffered saline (PBS) (pH 7.4) was used to coat the wells of flat-bottom Immulon II ELISA plates (Dynatech Laboratories, Chantilly, Va.) overnight at 4°C. The wells were blocked by incubation with a 5% solution of bovine serum albumin in PBS-Tween (0.05%) at 37°C. Fifty microliters of twofold serial dilutions of the test sera or a control serum from adjuvant-vaccinated mice in 5% bovine serum albumin–PBS-Tween was added to the wells and incubated for 2 h at 37°C. The wells were washed six times with washing buffer (PBS containing 0.05% Tween 20) before incubating for 1 h at 37°C with 50 μl of a 1:500 dilution of horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin G (IgG) (Amersham, Piscataway, N.J.) and developed with an ABTS [2,2′-azinobis(3-ethylbenzthiazoline sulfonic acid)] peroxidase substrate system (Kirkegaard and Perry Laboratories, Gaithersburg, Md.). The plates were read at 405 nm using an ELISA plate reader (VERSAmax; Molecular Devices, Sunnyvale, Calif.), subtracting plate blanks as appropriate. The ELISA endpoint titers were determined as the highest dilution at which the absorbance was equal to or greater than twice the A405 value in the control wells.
(ii) Subtyping of IgG.
ELISA plates were coated with the peptides as described earlier, washed, and blocked, followed by incubation of 50 μl of serially diluted mouse serum samples in duplicates for 1 h at 37°C. The plates were washed and incubated with biotinylated rat anti-mouse IgG subtypes, namely IgG1, IgG2a, IgG2b (Zymed Laboratories, San Francisco, Calif.), and IgG3 (Biosource International, Camarillo, Calif.). Following incubation and washings, the plates were incubated with streptavidin peroxidase (Amersham) and subsequently developed using the ABTS reagent as described above.
Cellular immune responses. (i) Splenic T-cell proliferation assays.
Twelve weeks after the primary immunization (4 weeks after the second boost) mice were sacrificed, spleens were aseptically removed, and single-cell suspensions were made in RPMI 1640 (Life Technologies/Gibco) containing 40 μg of gentamicin/ml. The lymphocytes were separated by layering the cell suspension on Lymphoprep (Nycomed Pharma, Oslo, Norway) and centrifuging at 800 × g. The lymphocytes were washed twice with RPMI 1640 and resuspended in RPMI containing 10% fetal calf serum and 40 μg of gentamicin/ml (complete medium). The cells were cultured in flat-bottom 96-well plates at a concentration of 2 × 105 cells per well in complete medium. Appropriate peptides were incubated with the seeded cells at a final concentration of 5 μg/ml in quadruplicate. As a positive control a nonspecific mitogen, concanavalin A (Sigma, St. Louis, Mo.), was added at the same concentration. After 5 days at 37°C, 1 μCi of [3H]thymidine (Amersham) was added to each well for the last 18 h of culture. The cells were then harvested and the [3H]thymidine incorporation was determined by scintillation counting. The results were calculated and expressed as a stimulation index, taking the total counts per minute from cells in the presence of peptide divided by the counts per minute from cells grown in medium alone.
(ii) IFN-γ measurement.
Supernatants were collected from in vitro cultures growing in the presence or absence of the stimulating peptide or concanavalin A. The amount of secreted IFN-γ was determined in 5-day spleen cell culture supernatants (obtained as described above) by two-site sandwich ELISA using a rat (monoclonal) anti-mouse IFN-γ antibody (Biosource International) as a capturing antibody and biotinylated rat anti-mouse IFN-γ monoclonal antibody (Pharmingen) as a detection antibody. The assay was completed by further reacting the bound antibody with streptavidin peroxidase, and the color was developed using the ABTS reagent as described above. The concentration of IFN-γ was calculated from standard curves obtained with known concentrations of mouse IFN-γ (Pharmingen) and expressed as picograms per milliliter of the supernatant.
Assays of antibody function. (i) IFA.
An immunofluorescence assay (IFA) was performed as described previously (6) using the sera obtained at 4 weeks after the third immunization. Briefly, P. falciparum (3D7) parasites representing sporozoites, liver-stage parasites, or parasitized red blood cells (obtained from in vitro cultures) were air dried onto multispot microscope slides. Serial dilutions of sera were added to each well. After incubation and washing, fluorescein-conjugated anti-mouse IgG in a counterstain was added. The slides were examined and IFA titers were reported as the highest serum dilution that gave a positive reaction compared to normal controls.
(ii) Inhibition of sporozoite invasion.
In vitro antibody-mediated inhibition of sporozoite invasion is the best correlate of in vivo immunity in murine models and was performed as described previously (25). Sporozoites were mixed with dilutions of test or control sera and added to wells containing fresh human hepatoma (HepG2-A16) cells and then were allowed to incubate for 3 h. The cultures were washed, fixed, and stained with a P. falciparum-specific monoclonal antibody (NFS1) and a peroxidase-labeled secondary antibody. The positive control (NFS1) is an anti-CS monoclonal antibody and routinely gives a >90% inhibition. All of the serum dilutions were done at 1:100 and the monoclonal antibody concentration was 100 μg/ml. Inhibition was scored as the percent decrease compared to control sera.
RESULTS
We synthesized well-characterized MAPs by novel methods utilizing both solid-phase and solution chemistry (3). In this study we assessed their antibody and cellular immune responses in mice. The study was also designed to evaluate whether the responses to these MAPs are genetically restricted. Mice from four different genetic backgrounds (B10.BR [H-2k], C57BL/10 [H-2b], CAF1 [hybrid BALB/cJ × A/J] [H-2d/a], and outbred Cr:sw) were immunized with 25 μg of a MAP emulsified in Freund's complete or incomplete adjuvant. The configuration of the MAPs is shown in Fig. 1. Control groups were vaccinated with a randomized MAP or adjuvant alone.
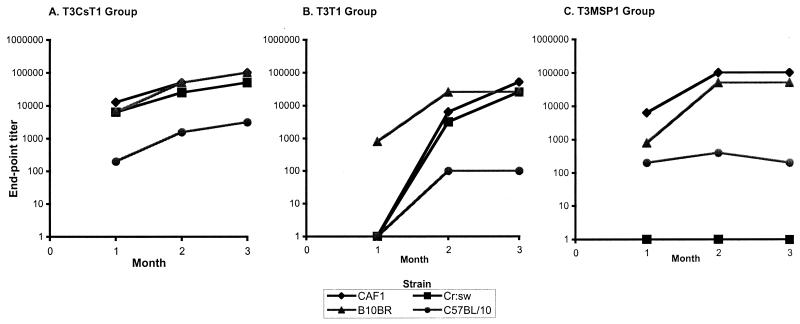
Antibodies to the tri-epitope MAP (T3-CS-T1) appeared in all groups as early as 4 weeks after primary immunization (Fig. 2). There was a consistent rise in IgG titer over the course of the study, with a peak of >1:100,000 in the CAF1 and inbred B10.BR mice and of >1:50,000 in outbred Swiss mice by 12 weeks (Fig. 2A). However, in the C57BL/10 mice, levels of peptide-specific antibodies were lower compared to the other three strains at all the time points during the course of the study. The di-epitope T3-T1 MAP showed a delayed antibody response after immunization. T3-T1 peptide-specific IgG titers were very low for all the groups (except B10.BR mice) at 4 weeks, and antibody titers increased significantly by 12 weeks (Fig. 2B). Similar to the T3-CS-T1 MAP-immunized mice, B10.BR, CAF1, and Cr:sw mice produced high-titer antibodies (>1:25,000) to the T3-T1 MAP compared to the C57BL/10 mice (1:100) at 12 weeks, suggesting that H-2b mice are low responders to all three malarial epitopes incorporated in the MAP constructs. The response to T3-MSP1 was also genetically restricted (Fig. 2C). CAF1 and B10.BR mice were high responders and C57BL/10 mice showed a very low response, while the Cr:sw mice failed to generate antipeptide antibodies following three subcutaneous immunizations with the T3-MSP1 construct.
FIG. 2.
Antibody titers to each MAP in mice of different genetic backgrounds. Antibody levels in serum were determined by ELISA. Titers were determined based on the highest dilution of the sample that generated an optical density greater than twice that seen in the adjuvant control group. The T3-CS-T1 (A), T3-T1 (B), and T3-MSP1 (C) vaccination groups are shown.
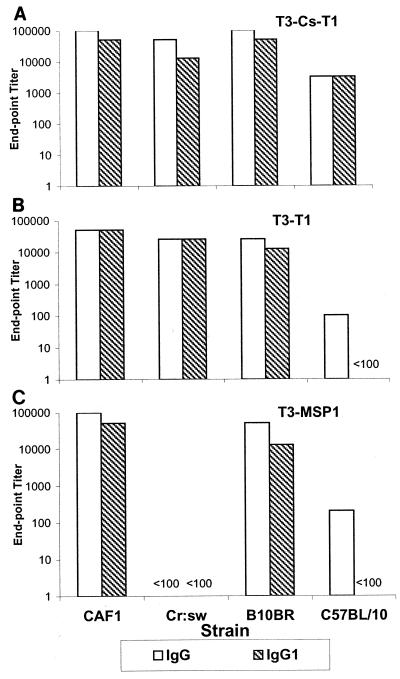
Since the isotype of an antibody may be a significant determinant of the protective nature of the immune response (29), we also carried out an analysis of IgG subclasses. Four weeks after the last immunization, antibodies in all the groups of mice that responded were predominantly of the IgG1 subtype (Fig. 3). Furthermore, negligible levels of IgG2a, IgG2b, and IgG3 were obtained in all strains tested (data not shown).
FIG. 3.
Antibody isotype analysis. Sera obtained at 4 weeks after the third immunization were tested for IgG1 by ELISA for each MAP. The T3-CS-T1 (A), T3-T1 (B), and T3-MSP1 (C) vaccination groups are shown.
The MAP molecules contained epitopes from different stages of parasites, so the determination of the antibody response to antigens from various stages of the parasite life cycle is an important aspect of the evaluation of the immunogenicity of these vaccines. IFA analyses on sporozoites and liver-stage and blood-stage parasites were carried out using the relevant sera from T3-CS-T1- and T3-MSP1-immunized mice. As shown in Table 1, sera obtained from the hybrid CAF1 and outbred Cr:sw mice immunized with the tri-epitope T3-CS-T1 MAP reacted with both sporozoites and liver-stage parasites. The same two strains of mice immunized with T3-MSP1 developed antibodies that recognized the liver-stage parasites. These, plus the sera from the inbred C57/BL10 mice, recognized blood-stage parasites. Apart from the last IFA, sera from the inbred mice failed to recognize the fixed parasites, despite high antipeptide antibody titers by ELISA in the B10.BR group. Control sera were negative for all stages of parasites at a 1:100 dilution.
TABLE 1.
Reciprocal IFA titer by vaccination group
| Mouse strain | Reciprocal IFA titer with:
|
|||
|---|---|---|---|---|
| T3-CS-T1
|
T3-MSP1
|
|||
| Sporozoites | Liver stage | Liver stage | Blood stage | |
| CAF1 | 6,400 | 4,000 | 4,000 | 3,200 |
| Cr:sw | 200 | 1,000 | 800 | 400 |
| B10.BR | Negative | Negative | Negative | Negative |
| C57BL/10 | Negative | Negative | Negative | 800 |
An important and sensitive measure of functional antibody specificity is the ability of serum to inhibit sporozoite invasion of hepatoma cells in vitro. Sera from the T3-CS-T1-immunized CAF1 and Cr:sw mice were tested for their inhibitory capacity compared to control sera. The CAF1 serum, which also had the highest ELISA and IFA titers, was able to inhibit the invasion of 79% of the sporozoites into human hepatoma cells, which suggests protection (43). On the other hand, the Cr:sw serum IFA titer was 32-fold lower and it was only able to inhibit the invasion of 51% of the sporozoites (Table 2). These results suggest that the generation of biologically active antibodies to the T3-CS-T1 MAP is under the control of immune response genes.
TABLE 2.
Inhibition of sporozoite invasion of HepG2-A16 hepatoma cells by serum from mice immunized with T3-CS-T1 MAP
| Group/immunogen | No. of sporozoites/well (mean ± SD) | Inhibition (%) |
|---|---|---|
| Media control | 466 ± 39 | |
| Positive control | 45 ± 7 | 90 |
| CAF1/randomized | 433 ± 80 | |
| CAF1/T3-CS-T1 | 92 ± 14 | 79 |
| Cr:sw/randomized | 390 ± 21 | |
| Cr:sw/T3-CS-T1 | 193 ± 34 | 51 |
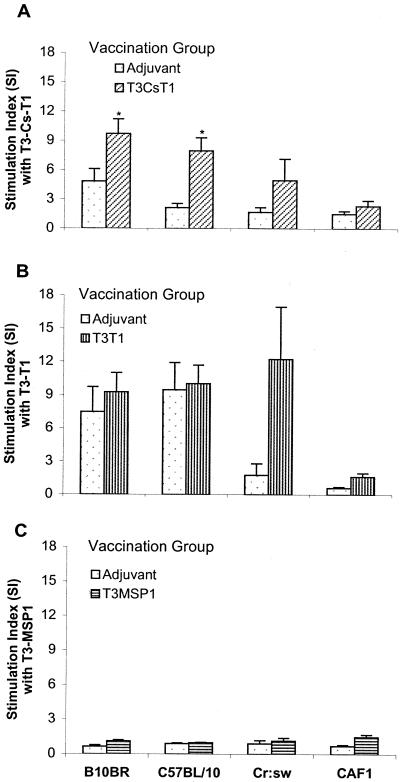
The four different strains of mice were immunized with each peptide individually to study the MAP-specific splenic T-cell proliferative responses to the homologous peptide. Cells from B10.BR, C57BL/10, and Cr:sw mice immunized with the T3-CS-T1 MAP or the T3-T1 MAP proliferated in the presence of the cognate peptide, while cells from CAF1 mice failed to respond (Fig. 4). The stimulation index for T3-CS-T1-immunized mice was significantly higher (P < 0.05) than that for adjuvant control cells when stimulated in vitro with the homologous peptide. When the T3-T1 peptide was used as an immunogen, the inbred strains B10.BR and C57BL/10 also showed a proliferative response, but this was complicated by a positive response in the adjuvant control-immunized mice. This suggests that the T3-T1 peptide may have nonspecifically stimulated the cells from the inbred mice. Spleen cells from the T3-T1-vaccinated outbred Cr:sw mice showed proliferation that was significantly higher than that of the control group. No significant T-cell proliferative responses could be detected in the mice immunized with the T3-MSP1 peptide.
FIG. 4.
Analysis of T-cell proliferation induced by MAP constructs. Mice of different genetic backgrounds (six to eight per group) were immunized with the T3-CS-T1 (A), T3-T1 (B), or T3-MSP1 (C) MAP. Control groups of mice received adjuvant alone. At the end of the immunization protocol spleen cells were restimulated in vitro with the respective MAP at a concentration of 5 μg/ml. The results are shown as means ± standard errors of the means. ∗, significant difference (Student's t test; P < 0.05) compared to adjuvant control group.
IFN-γ levels were analyzed in the culture supernatants of spleen cells stimulated with cognate immunogen. No significant IFN-γ production was found in the peptide-stimulated cultures compared to media control wells from any of the vaccination groups (data not shown).
DISCUSSION
Several malaria protein T- and B-cell epitopes have been identified by epidemiological means as well as by studies in animal models (26, 37). Immunization with synthetic MAPs provides a unique strategy for generating an immune response against multiple stages of the Plasmodium life cycle. The immune response of the sporozoite-immunized host is likely to be multifactorial with respect to effector mechanisms and target antigens. The combined effect of antibody and cellular immunity, specifically targeted to sporozoite and exo-erythrocytic antigens, may contribute to high levels of protection that develop in sporozoite-immunized hosts (10, 36). The development of malaria vaccines to mimic this multifactorial response has stimulated great interest in identifying and testing the antigenic epitopes from sporozoites as well as from liver-stage parasites. A study of the response to the P. falciparum antigen LSA-1 during natural infection reported from the Worsera area of Papua New Guinea, where malaria is endemic, showed that T-cell memory response to the T3 and T1 epitopes is common in adults. A correlation between an absence of blood-stage parasites and LSA-1-induced IFN-γ production was also found by stimulation of peripheral blood mononuclear cells and subsequent cytokine analysis by ELISA (8). Epidemiological studies showed that antibody to the peptides corresponding to the N- and C-terminal peptides and the 17-amino-acid central repeat region of LSA-1 could be detected in sera from 40 to 50% of the subjects screened in the Papua New Guinea study.
There has been a growing interest in producing macromolecular synthetic vaccines containing multiple T- and B-cell epitopes of defined composition. Studies have shown that MAPs are better immunogens than linear peptides and that they are able to induce protective immunity against malaria in rodents (47, 50). A conventional stepwise solid-phase synthetic approach has been used for traditional MAP synthesis that simply incorporates a new amino acid on each of four or eight arms of a lysine core (47). While these MAPs are immunogenic, they are highly heterogeneous as a result of accumulated errors during synthesis (11, 36), making purification and characterization extremely difficult. This severely limits the use of such molecules in human vaccine studies due to safety concerns and reproducibility in the manufacturing process. Using a manufacturing technique that allows complete characterization, a necessary step in large-scale drug production, and rapid assessment of alternative antigens, we synthesized three constructs containing immunogenic epitopes from two of the pre-erythrocytic antigens, CS protein and LSA-1, and from the blood-stage antigen MSP-1. The MAPs used in the present study are different from the MAP developed by Tam and others (47) in several ways. In our MAP synthesis, cysteines were used as peptide-anchoring residues on a basic lysine core molecule. Two different peptide epitopes could be incorporated on a MAP four-core molecule, while Tam and colleagues used the same peptide on all arms of the lysine core. The MAPs used in this study were fully characterized by matrix-assisted laser desorption ionization–time of flight mass spectroscopy, amino acid analysis, size-exclusion chromatography, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (3).
The murine reaction to subunit malaria vaccines has been shown to be genetically restricted and dependent on the fine details of major histocompatibility complex haplotype and amino acid sequence of the peptide epitope (1, 48). The present study shows that the well-characterized MAPs can be immunogenic in mice of different genetic backgrounds. Both the di-epitope T3-T1 and tri-epitope T3-CS-T1 MAPs, when formulated with Freund's adjuvant, were immunogenic. Immunization resulted in significant and almost comparable antibody titers in B10.BR (H-2k), CAF1 (H-2d/a), and outbred Cr:sw mice, while the C57BL/10 (H-2b) mice were poor responders to both immunogens. Genetically restricted antibody responses were also seen when the T3-MSP1 MAP was used as an immunogen. This construct induced high-titer antibodies only in inbred H-2k and hybrid H-2d/a mice. Interestingly, the outbred mice failed to generate antibodies even after boosting with the immunogen. This suggests that the H-2b and Cr:sw mice may only respond strongly to the T1 epitope in the context of these MAP vaccines, and the H-2b mice are generally low responders to all three malarial epitopes studied. In this context, it is important to mention that in previous studies H-2b mice were also found to be nonresponsive to the same CS protein (15) and MSP-1 (40) epitope sequences that were used in this study. No published report of murine genetically restricted immunogenicity for the T3 epitope is available.
Synthetic vaccines may not always properly mimic the conformation of the epitopes within the whole parasite protein. This is of particular relevance as it can affect (i) protective properties of the immune response and (ii) the ability of the parasite to boost the response in a vaccinated host upon natural infection. When the T3-CS-T1 MAP was used as an immunogen, IFA titers against the sporozoites as well as liver-stage parasites varied greatly among the responder strains, being highest for CAF1, lower for the outbred mice, and negligible in both the inbred strains of mice. A similar trend in IFA titers was seen when T3-MSP1 was used as an immunogen (CAF1 > Cr:sw > B10.BR). Interestingly, relatively low IFA titers against T3-MSP1 were present in sera from Cr:sw mice despite the lack of demonstrable antibodies against this peptide by ELISA.
We found that an LSA-1-based MAP combined with CS protein sequences in a tri-epitope conjugate (i.e., T3-CS-T1) stimulated significant proliferative responses in the inbred (B10.BR and C57BL/10) mice, a moderate response in the outbred (Cr:sw) mice, and no response in the hybrid (CAF1) strains of mice. In contrast, the T3-T1 construct induced specific proliferative responses in outbred mice alone when stimulated with the MAP in vitro compared to control cells treated in a similar fashion. The lack of correlation between antibody titers and proliferation responses observed here could be due to the cryptic nature of these epitopes in CAF1 mice, a phenomenon observed in previous studies (14, 16). IFN-γ is the most potent cytokine to affect P. falciparum liver schizogony in vitro (31) and in primate and rodent models (13, 32). In our study, antigen-specific IFN-γ secretion was negligible for all the groups. Combined with the preponderance of IgG1, these results suggest that the response was primarily Th2 in nature.
One important test of vaccine efficacy is whether elicited antibodies can inhibit invasion, growth, and/or development of parasites. Since we were testing a MAP construct (T3-CS-T1) that had the NANP repeat sequence from the CS protein molecule, we asked whether the antibody responses in this group were adequate by an in vitro assay of protection. Only CAF1 sera gave a significant inhibition of the invasion of sporozoites into hepatoma cells. This compares favorably with the inhibition seen with other immunization systems (43) and correlates with the high antibody and IFA titers that were generated by T3-CS-T1 in these mice.
The immunogenicity of a MAP vaccine is likely to be due in part to the complexity of its structure and the presence of several epitopes covalently bound in one molecule, allowing cross-linking of surface receptors on immune cells. The relative arrangement of epitopes may also be a crucial factor in determining the immune responses generated when these are used as vaccine molecules (1). The complexity of MAP synthesis and characterization has always been regarded as an obstacle to developing a well-characterized product that can be tested in humans (28). To that end, we have successfully synthesized different MAP constructs containing two or three different immunogenic epitopes from P. falciparum and have completely characterized the products. The present study shows that these MAP constructs are indeed immunogenic in mice. Like most other subunit (recombinant or peptide based) malaria vaccine constructs, the MAPs reported here also generate responses that are genetically restricted (10). The current study should be extended to test the immunogenicity of these constructs with different adjuvants and new combinations with other protective epitopes in MAP formulations synthesized using the techniques herein described.
ACKNOWLEDGMENT
M.B.J. was supported by a postdoctoral fellowship from the Oak Ridge Institute for Science and Education.
REFERENCES
- 1.Ahlborg N, Nardin E H, Perlmann P, Berzins K, Andersson R. Immunogenicity of chimeric multiple antigen peptides based on Plasmodium falciparum antigens: impact of epitope orientation. Vaccine. 1998;16:38–44. doi: 10.1016/s0264-410x(97)00155-2. [DOI] [PubMed] [Google Scholar]
- 2.Ballou W R, Hoffman S L, Sherwood J A, Hollingdale M R, Neva F A, Hockmeyer W T, Gordon D M, Schneider I, Wirtz R A, Young J F, et al. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987;i:1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- 3.Boykins R A, Joshi M, Syin C, Dhawan S, Nakhasi H. Synthesis and construction of a novel multiple peptide conjugate system: strategy for a subunit vaccine design. Peptides. 2000;21:9–17. doi: 10.1016/s0196-9781(99)00172-2. [DOI] [PubMed] [Google Scholar]
- 4.Brown G V. Progress in the development of malaria vaccines: context and constraints. Parassitologia. 1999;41:429–432. [PubMed] [Google Scholar]
- 5.Cerami C, Kwakye-Berko F, Nussenzweig V. Binding of malarial circumsporozoite protein to sulfatides [Gal(3-SO4)beta 1-Cer] and cholesterol-3-sulfate and its dependence on disulfide bond formation between cysteines in region II. Mol Biochem Parasitol. 1992;54:1–12. doi: 10.1016/0166-6851(92)90089-3. [DOI] [PubMed] [Google Scholar]
- 6.Charoenvit Y, Leef M F, Yuan L F, Sedegah M, Beaudoin R L. Characterization of Plasmodium yoelii monoclonal antibodies directed against stage-specific sporozoite antigens. Infect Immun. 1987;55:604–608. doi: 10.1128/iai.55.3.604-608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christodoulides M, Heckels J E. Immunization with a multiple antigen peptide containing defined B- and T-cell epitopes: production of bactericidal antibodies against group B Neisseria meningitidis. Microbiology. 1994;140:2951–2960. doi: 10.1099/13500872-140-11-2951. [DOI] [PubMed] [Google Scholar]
- 8.Connelly M, King C L, Bucci K, Walters S, Genton B, Alpers M P, Hollingdale M, Kazura J W. T-cell immunity to peptide epitopes of liver-stage antigen 1 in an area of Papua New Guinea in which malaria is holoendemic. Infect Immun. 1997;65:5082–5087. doi: 10.1128/iai.65.12.5082-5087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolan S A, Miller L H, Wellems T E. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Investig. 1990;86:618–624. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doolan D L, Hoffman S L. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 11.Drijfhout J W, Bloemhoff W. A new synthetic functionalized antigen carrier. Int J Pept Protein Res. 1991;37:27–32. doi: 10.1111/j.1399-3011.1991.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 12.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee H G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira A, Schofield L, Enea V, Schellekens H, van der Meide P, Collins W E, Nussenzweig R S, Nussenzweig V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986;232:881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- 14.Gammon G, Shastri N, Cogswell J, Wilbur S, Sadegh-Nasseri S, Krzych U, Miller A, Sercarz E. The choice of T-cell epitopes utilized on a protein antigen depends on multiple factors distant from as well as at the determinant site. Immunol Rev. 1987;98:53–73. doi: 10.1111/j.1600-065x.1987.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 15.Good M F, Berzofsky J A, Maloy W L, Hayashi Y, Fujii N, Hockmeyer W T, Miller L H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986;164:655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Good M F, Branigan J, Smith G, Houghten R A. Peptide immunization can elicit malaria protein-specific memory helper but not proliferative T cells. Pept Res. 1990;3:110–115. [PubMed] [Google Scholar]
- 17.Guerin-Marchand C, Druilhe P, Galey B, Londono A, Patarapotikul J, Beaudoin R L, Dubeaux C, Tartar A, Mercereau-Puijalon O, Langsley G. A liver-stage-specific antigen of Plasmodium falciparum characterized by gene cloning. Nature. 1987;329:164–167. doi: 10.1038/329164a0. [DOI] [PubMed] [Google Scholar]
- 18.Herrera M A, de Plata C, Gonzalez J M, Corradin G, Herrera S. Immunogenicity of multiple antigen peptides containing Plasmodium vivax CS epitopes in BALB/c mice. Mem Inst Oswaldo Cruz. 1994;89:71–76. doi: 10.1590/s0074-02761994000600017. [DOI] [PubMed] [Google Scholar]
- 19.Herrington D A, Clyde D F, Losonsky G, Cortesia M, Murphy J R, Davis J, Baqar S, Felix A M, Heimer E P, Gillessen D, et al. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987;328:257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman S L, Doolan D L, Sedegah M, Wang R, Scheller L F, Kumar A, Weiss W R, Le T P, Klinman D M, Hobart P, Norman J A, Hedstrom R C. Toward clinical trials of DNA vaccines against malaria. Immunol Cell Biol. 1997;75:376–381. doi: 10.1038/icb.1997.59. [DOI] [PubMed] [Google Scholar]
- 21.Holder A A, Freeman R R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984;160:624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holder A A, Guevara Patino J A, Uthaipibull C, Syed S E, Ling I T, Scott-Finnigan T, Blackman M J. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia. 1999;41:409–414. [PubMed] [Google Scholar]
- 23.Hollingdale M R, Aikawa M, Atkinson C T, Ballou W R, Chen G X, Li J, Meis J F, Sina B, Wright C, Zhu J D. Non-CS pre-erythrocytic protective antigens. Immunol Lett. 1990;25:71–76. doi: 10.1016/0165-2478(90)90094-7. [DOI] [PubMed] [Google Scholar]
- 24.Hollingdale M R, McCormick C J, Heal K G, Taylor-Robinson A W, Reeve P, Boykins R, Kazura J W. Biology of malarial liver stages: implications for vaccine design. Ann Trop Med Parasitol. 1998;92:411–417. doi: 10.1080/00034989859393. [DOI] [PubMed] [Google Scholar]
- 25.Hollingdale M R, Nardin E H, Tharavanij S, Schwartz A L, Nussenzweig R S. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol. 1984;132:909–913. [PubMed] [Google Scholar]
- 26.Jones T R, Hoffman S L. Malaria vaccine development. Clin Microbiol Rev. 1994;7:303–310. doi: 10.1128/cmr.7.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaslow D C. Transmission-blocking vaccines: uses and current status of development. Int J Parasitol. 1997;27:183–189. doi: 10.1016/s0020-7519(96)00148-8. [DOI] [PubMed] [Google Scholar]
- 28.Le T P, Church L W, Corradin G, Hunter R L, Charoenvit Y, Wang R, de la Vega P, Sacci J, Ballou W R, Kolodny N, Kitov S, Glenn G M, Richards R L, Alving C R, Hoffman S L. Immunogenicity of Plasmodium falciparum circumsporozoite protein multiple antigen peptide vaccine formulated with different adjuvants. Vaccine. 1998;16:305–312. doi: 10.1016/s0264-410x(97)00165-5. [DOI] [PubMed] [Google Scholar]
- 29.Ling I T, Ogun S A, Momin P, Richards R L, Garcon N, Cohen J, Ballou W R, Holder A A. Immunization against the murine malaria parasite Plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine. 1997;15:1562–1567. doi: 10.1016/s0264-410x(97)00076-5. [DOI] [PubMed] [Google Scholar]
- 30.Marussig M, Renia L, Motard A, Miltgen F, Petour P, Chauhan V, Corradin G, Mazier D. Linear and multiple antigen peptides containing defined T and B epitopes of the Plasmodium yoelii circumsporozoite protein: antibody-mediated protection and boosting by sporozoite infection. Int Immunol. 1997;9:1817–1824. doi: 10.1093/intimm/9.12.1817. [DOI] [PubMed] [Google Scholar]
- 31.Mellouk S, Green S J, Nacy C A, Hoffman S L. IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an l-arginine-dependent effector mechanism. J Immunol. 1991;146:3971–3976. [PubMed] [Google Scholar]
- 32.Mellouk S, Maheshwari R K, Rhodes-Feuillette A, Beaudoin R L, Berbiguier N, Matile H, Miltgen F, Landau I, Pied S, Chigot J P, et al. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J Immunol. 1987;139:4192–4195. [PubMed] [Google Scholar]
- 33.Moreno C A, Rodriguez R, Oliveira G A, Ferreira V, Nussenzweig R S, Moya Castro Z R, Calvo-Calle J M, Nardin E. Preclinical evaluation of a synthetic Plasmodium falciparum MAP malaria vaccine in Aotus monkeys and mice. Vaccine. 1999;18:89–99. doi: 10.1016/s0264-410x(99)00184-x. [DOI] [PubMed] [Google Scholar]
- 34.Nardelli B, Tam J P. The MAP system. A flexible and unambiguous vaccine design of branched peptides. Pharmacol Biotechnol. 1995;6:803–819. [PubMed] [Google Scholar]
- 35.Nardin E, Zavala F, Nussenzweig V, Nussenzweig R S. Pre-erythrocytic malaria vaccine: mechanisms of protective immunity and human vaccine trials. Parassitologia. 1999;41:397–402. [PubMed] [Google Scholar]
- 36.Nardin E H, Calvo-Calle J M, Oliveira G A, Clavijo P, Nussenzweig R, Simon R, Zeng W, Rose K. Plasmodium falciparum polyoximes: highly immunogenic synthetic vaccines constructed by chemoselective ligation of repeat B-cell epitopes and a universal T-cell epitope of CS protein. Vaccine. 1998;16:590–600. doi: 10.1016/s0264-410x(97)00238-7. [DOI] [PubMed] [Google Scholar]
- 37.Nardin E H, Nussenzweig R S. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu Rev Immunol. 1993;11:687–727. doi: 10.1146/annurev.iy.11.040193.003351. [DOI] [PubMed] [Google Scholar]
- 38.Nardin E H, Oliveira G A, Calvo-Calle J M, Nussenzweig R S. The use of multiple antigen peptides in the analysis and induction of protective immune responses against infectious diseases. Adv Immunol. 1995;60:105–149. doi: 10.1016/s0065-2776(08)60585-4. [DOI] [PubMed] [Google Scholar]
- 39.Nosten F, Luxemburger C, Kyle D E, Gordon D M, Ballou W R, Sadoff J C, Brockman A, Permpanich B, Chongsuphajaisiddhi T, Heppner D G. Phase I trial of the SPf66 malaria vaccine in a malaria-experienced population in Southeast Asia. Am J Trop Med Hyg. 1997;56:526–532. doi: 10.4269/ajtmh.1997.56.526. [DOI] [PubMed] [Google Scholar]
- 40.Parra M, Hui G, Johnson A H, Berzofsky J A, Roberts T, Quakyi I A, Taylor D W. Characterization of conserved T- and B-cell epitopes in Plasmodium falciparum major merozoite surface protein 1. Infect Immun. 2000;68:2685–2691. doi: 10.1128/iai.68.5.2685-2691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quakyi I A, Currier J, Fell A, Taylor D W, Roberts T, Houghten R A, England R D, Berzofsky J A, Miller L H, Good M F. Analysis of human T cell clones specific for conserved peptide sequences within malaria proteins. Paucity of clones responsive to intact parasites. J Immunol. 1994;153:2082–2092. [PubMed] [Google Scholar]
- 42.Roggero M A, Weilenmann C, Bonelo A, Audran R, Renggli J, Spertini F, Corradin G, Lopez J A. Plasmodium falciparum CS C-terminal fragment: preclinical evaluation and phase I clinical studies. Parassitologia. 1999;41:421–424. [PubMed] [Google Scholar]
- 43.Shi Y P, Hasnain S E, Sacci J B, Holloway B P, Fujioka H, Kumar N, Wohlhueter R, Hoffman S L, Collins W E, Lal A A. Immunogenicity and in vitro protective efficacy of a recombinant multistage Plasmodium falciparum candidate vaccine. Proc Natl Acad Sci USA. 1999;96:1615–1620. doi: 10.1073/pnas.96.4.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sidney J, Grey H M, Southwood S, Celis E, Wentworth P A, del Guercio M F, Kubo R T, Chesnut R W, Sette A. Definition of an HLA-A3-like supermotif demonstrates the overlapping peptide-binding repertoires of common HLA molecules. Hum Immunol. 1996;45:79–93. doi: 10.1016/0198-8859(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 45.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 46.Tam J P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tam J P, Clavijo P, Lu Y A, Nussenzweig V, Nussenzweig R, Zavala F. Incorporation of T and B epitopes of the circumsporozoite protein in a chemically defined synthetic vaccine against malaria. J Exp Med. 1990;171:299–306. doi: 10.1084/jem.171.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian J H, Miller L H, Kaslow D C, Ahlers J, Good M F, Alling D W, Berzofsky J A, Kumar S. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J Immunol. 1996;157:1176–1183. [PubMed] [Google Scholar]
- 49.Tine J A, Lanar D E, Smith D M, Wellde B T, Schultheiss P, Ware L A, Kauffman E B, Wirtz R A, De Taisne C, Hui G S, Chang S P, Church P, Hollingdale M R, Kaslow D C, Hoffman S, Guito K P, Ballou W R, Sadoff J C, Paoletti E. NYVAC-Pf7: a poxvirus-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. Infect Immun. 1996;64:3833–3844. doi: 10.1128/iai.64.9.3833-3844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang R, Charoenvit Y, Corradin G, Porrozzi R, Hunter R L, Glenn G, Alving C R, Church P, Hoffman S L. Induction of protective polyclonal antibodies by immunization with a Plasmodium yoelii circumsporozoite protein multiple antigen peptide vaccine J. Immunol. 1995;154:2784–2793. . (Erratum, 155:1637.) [PubMed] [Google Scholar]
- 51.WHO. 2000, posting date. Overcoming Antimicrobial Resistance: World Health Report on Infectious Diseases 2000. World Health Organization, Geneva, Switzerland. [Online.] http://www.who.int.
- 52.Zhu J, Hollingdale M R. Structure of Plasmodium falciparum liver stage antigen-1. Mol Biochem Parasitol. 1991;48:223–226. doi: 10.1016/0166-6851(91)90117-o. [DOI] [PubMed] [Google Scholar]