This systematic review and meta-analysis identifies sleep abnormalities across clinical high risk for psychosis, early psychosis, and chronic psychosis.
Key Points
Question
Do sleep abnormalities differ in occurrence and severity in clinical high risk for psychosis (CHR-P), early psychosis (EP), and chronic psychosis (CP)?
Findings
In this systematic review and meta-analysis including 5135 patients from 21 studies, sleep disturbance prevalence was 50% and was comparable across psychosis stages. Comparing 1575 patients and 977 controls revealed poor self-reported sleep quality throughout stages; individuals with CP had more arousal vs CHR-P and reduced spindle duration vs EP.
Meaning
These findings suggest that sleep disturbances are highly prevalent throughout psychosis stages and that CHR-P, EP, and CP show common and distinct self-reported and objective sleep alterations, thus representing clinical targets and research domains for psychosis.
Abstract
Importance
Abnormal sleep is frequent in psychosis; however, sleep abnormalities in different stages (ie, clinical high risk for psychosis [CHR-P], early psychosis [EP], and chronic psychosis [CP]) have not been characterized.
Objective
To identify sleep abnormalities across psychosis stages.
Data Sources
Web of Science and PubMed were searched between inception and June 15, 2022. Studies written in English were included.
Study Selection
Sleep disturbance prevalence studies and case-control studies reporting sleep quality, sleep architecture, or sleep electroencephalography oscillations in CHR-P, EP, or CP.
Data Extraction and Synthesis
This systematic review and meta-analysis followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. Stage-specific and pooled random-effects meta-analyses were conducted, along with the assessment of heterogeneity, study quality, and meta-regressions (clinical stage, sex, age, medication status, and psychotic symptoms).
Main Outcomes and Measures
Sleep disturbance prevalence, self-reported sleep quality, sleep architecture (total sleep time, sleep latency, sleep efficiency, nonrapid eye movement, rapid eye movement stages, and number of arousals), and sleep electroencephalography oscillations (spindle density, amplitude, and duration, and slow wave density).
Results
Fifty-nine studies with up to 6710 patients (n = 5135 for prevalence) and 977 controls were included. Sleep disturbance prevalence in pooled cases was 50% (95% CI, 40%-61%) and it was similar in each psychosis stage. Sleep quality was worse in pooled cases vs controls (standardized mean difference [SMD], 1.00 [95% CI, 0.70-1.30]). Sleep architecture alterations included higher sleep onset latency (SMD [95% CI]: pooled cases, 0.96 [0.62-1.30]; EP, 0.72 [0.52-0.92]; CP, 1.36 [0.66-2.05]), higher wake after sleep onset (SMD [95% CI]: pooled cases, 0.5 [0.29-0.71]; EP, 0.62 [0.34-0.89]; CP, 0.51 [0.09-0.93]), higher number of arousals (SMD [95% CI]: pooled cases, 0.45 [0.07-0.83]; CP, 0.81 [0.30-1.32]), higher stage 1 sleep (SMD [95% CI]: pooled cases, 0.23 [0.06-0.40]; EP, 0.34 [0.15-0.53]), lower sleep efficiency (SMD [95% CI]: pooled cases, −0.75 [−0.98 to −0.52]; EP, −0.90 [−1.20 to −0.60]; CP, −0.73 [−1.14 to −0.33]), and lower rapid eye movement density (SMD [95% CI]: pooled cases, 0.37 [0.14-0.60]; CP, 0.4 [0.19-0.77]). Spindle parameter deficits included density (SMD [95% CI]: pooled cases, −1.06 [−1.50 to −0.63]; EP, −0.80 [−1.22 to −0.39]; CP, −1.39 [−2.05 to −0.74]; amplitude: pooled cases, −1.08 [−1.33 to −0.82]; EP, −0.86 [−1.24 to −0.47]; CP, −1.25 [−1.58 to −0.91]; and duration: pooled cases: −1.2 [−1.69 to −0.73]; EP, −0.71 [−1.08 to −0.34]; CP, −1.74 [−2.10 to −1.38]). Individuals with CP had more frequent arousals vs CHR-P (z = 2.24, P = .02) and reduced spindle duration vs EP (z = −3.91, P < .001).
Conclusions and Relevance
In this systematic review and meta-analysis, sleep disturbances were found to be prevalent throughout the course of psychosis, and different psychosis stages showed both shared and distinct abnormalities in sleep quality, architecture, and spindles. These findings suggest that sleep should become a core clinical target and research domain from at-risk to early and chronic stages of psychosis.
Introduction
Sleep abnormalities have been observed in psychotic disorders since the dawn of psychiatric literature.1 Sleep disturbances, such as insomnia, are commonly reported by individuals with chronic psychosis (CP)2 and are associated with subsequent relapse.3 Altered sleep often precedes a psychotic episode in early psychosis (EP),4 and disrupted sleep contributes to predicting transition to psychosis in youth at clinical high risk for psychosis (CHR-P).5 Thus, sleep abnormalities not only co-occur with psychotic symptoms but are also implicated in the development, manifestation, and recurrence of psychosis.6
Sleep disturbance prevalence, which is usually assessed with self-reported questionnaires (eg, the Pittsburgh Sleep Quality Index [PSQI])7 is approximately 25% in the general population.7,8 Several studies have reported higher sleep disturbance prevalence in psychosis, although rates vary substantially (21%-100%)9,10,11 and have thus far never been meta-analyzed in different psychosis stages.
Altered sleep patterns across psychosis stages can also be examined in case-control comparisons. Several case-control studies have used subjective sleep assessments (eg, PSQI), which are inexpensive and easy to implement in large clinical cohorts and have reported worse sleep quality in CHR-P,12 EP,13 and CP14 vs healthy comparison groups. Other sleep studies have used actigraphy, electroencephalography (EEG), and polysomnography to objectively quantify altered sleep characteristics in psychosis. Traditionally, these studies have focused on sleep architecture. Meta-analyses of sleep architecture findings from actigraphy15 and polysomnography/EEG studies16,17,18 revealed shorter total sleep time and longer sleep onset latency and wake after sleep onset in CP. Polysomnography/EEG studies also showed decreased deep nonrapid eye movement sleep and reduced latency and duration of rapid eye movement (REM) sleep in these patients.18 Furthermore, shorter total sleep time and larger wake after sleep onset were reported in individuals with EP and CHR-P,19 suggesting that altered sleep architecture is an early feature of psychosis.
More recently, several studies investigated sleep-specific EEG oscillations, including spindles, in individuals with psychosis. Deficits in spindle parameters (ie, density, amplitude, and duration) were established in CP20 and EP.21,22 Furthermore, a 2022 meta-analysis reported reduced spindle density in psychotic disorders that yielded large effect sizes and was associated with disease progression.23
Therefore, systematically investigating the occurrence and severity of sleep abnormalities in CHR-P, EP, and CP may help differentiate sleep dysfunctions associated with chronicity and long-term medication exposure (ie, observed only/primarily in CP) from those implicated in the manifestation of full-blown psychosis (ie, occurring first in EP) and from sleep alterations related to vulnerability to psychosis (ie, present since CHR-P).24 This meta-analysis assessed, for the first time to our knowledge, the prevalence of sleep disturbances, along with subjective and objective sleep alterations throughout the course of psychosis, including CHR-P, EP, and CP.
Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.25 The protocol was registered in PROSPERO (CRD42021240503).
Inclusion and Exclusion Criteria
For inclusion, studies had to be published between inception and June 15, 2022, and written in English. Diagnosis of stages of psychosis was established using a recognized clinical assessment tool (see the eMethods in Supplement 1 for clinical stages definition). Studies needed to provide measures of the prevalence of sleep disturbances in individuals at different psychosis stages and/or quantification of sleep characteristics in these individuals, assessed with polysomnography, EEG, actigraphy, or self-reports. Inclusion and exclusion criteria are explained in greater detail in the eMethods in Supplement 1.
Search Strategy
One author (J.B.) performed the literature search on the Web of Science and PubMed from inception until June 15, 2022. The description of study search terms is provided in the eMethods in Supplement 1. A manual search of the references of included articles and of relevant prior reviews/meta-analyses were also performed. The eMethods in Supplement 1 contain details on study selection and data extraction.
Methodological Quality Appraisal
Quality appraisal was assessed using the Agency for Healthcare Research and Quality26 methodology checklist for cross-sectional/prevalence studies. For additional details, see the eMethods in Supplement 1.
Statistical Analysis
Sleep disturbance prevalence was evaluated in 3 distinct analyses: (1) a pooled cases analysis of sleep disturbance prevalence aggregating all psychosis stages; (2) a stage-specific case analysis of sleep disturbance; and (3) a moderator analysis comparing clinical stages with one another. We also performed a secondary analysis in a subgroup of studies assessing insomnia. Sleep disturbance prevalence effect sizes were analyzed as logit transformed values, quantifying the log odds of sleep disturbance.
Sleep architecture and oscillatory alterations were evaluated in 3 different analyses: (1) a pooled case-control comparison of each sleep variable aggregating all psychosis stages; (2) a stage-specific case-control analysis of sleep abnormalities; and (3) a moderator analysis comparing clinical stages with one another. Sleep architecture and sleep oscillatory parameters were analyzed as standardized mean differences (SMDs) across groups using the Hedges g statistic.27 For all hypothesis testing, we used 2-sided tests with statistical significance at the P < .05 level.
A random-effects linear regression model was fitted for each sleep parameter, and calculated effect sizes were weighted according to inverse variance to account for the variability of each study.28 Meta-analysis models were estimated using restricted maximum likelihood estimation using the rma function in the R metafor package in R software version 4.1.0 (R Foundation) (method = PLO for prevalence analyses; method = SMD for case-control comparisons).
The recovery of missing or partial data from studies and the assessment of study heterogeneity using funnel plots, Cochran Q statistic,29 I2 statistic,30 and Egger tests31 are further discussed in the eMethods in Supplement 1.
Moderator analyses were conducted to assess the influence of clinical stage (ie, CHR-P, EP, and CP), age (ie, mean age across the study), sex (ie, proportion of male individuals in the study), antipsychotic medications (ie, proportion of each study sample taking antipsychotics), and positive and negative symptoms severity on sleep parameters using linear mixed-effect meta-analysis models. For each sleep parameter, we regressed the differences between patient and control groups from the available sample on each moderator variable. For prevalence, we regressed the log odds of sleep disturbances on each moderator variable. To further investigate the interactions of age, sex, and medication with staging, we applied linear mixed-effect models regressing sleep parameter differences on age, sex, medication, and positive and negative symptom severity moderator variables for each stage separately. A threshold of P < .017 was used to establish statistical significance after correcting for multiple comparison for 3 explanatory variables (ie, sex, age, and proportion medicated) using Bonferroni correction. We also examined the effect sizes of psychotic symptoms (ie, Positive and Negative Syndrome Scale), and a Bonferroni-corrected P value threshold of .025 was used to establish statistical significance.
Results
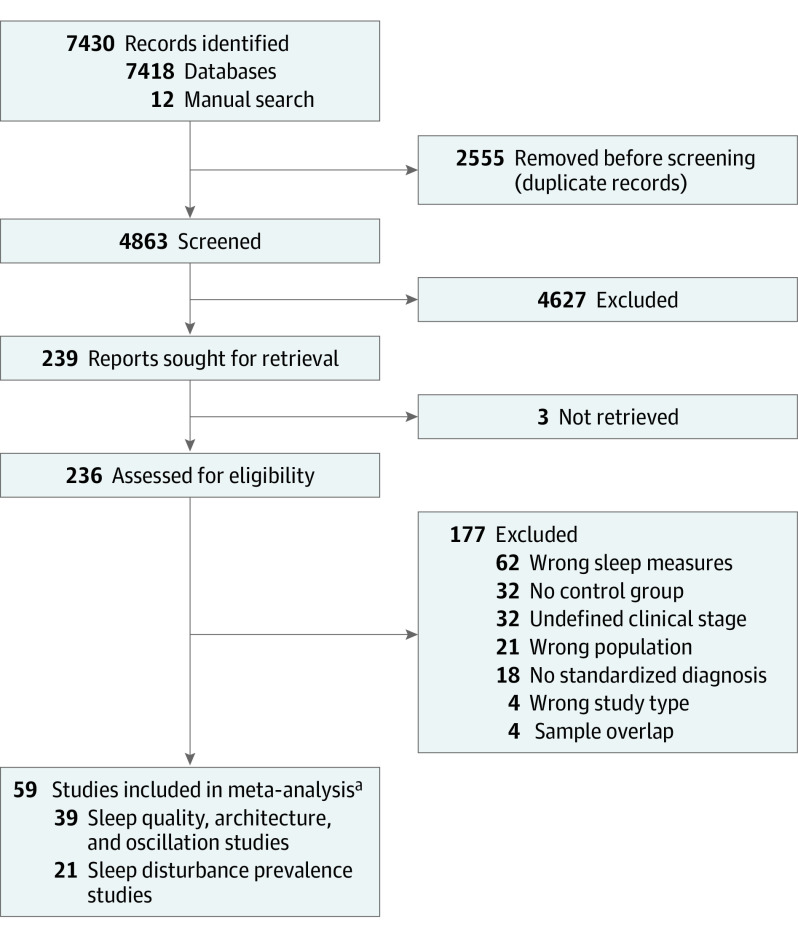
The initial search yielded 7418 records (Figure 1). After removing duplicates, 4863 publications were screened, resulting in 236 studies considered for full-text review. Twelve additional articles were identified through reference checking. After a full-text review, 59 articles were included, with 21 studies assessing sleep disturbance prevalence in 5135 patients (eTable 1 in Supplement 1) and 39 studies measuring sleep alterations subjectively (eg, sleep quality) and/or objectively (eg, sleep architecture and sleep oscillatory measures) in 1575 patients and 977 controls (eTable 2 in Supplement 1).
Figure 1. PRISMA Workflow of Study Selection.
aOf note, 1 study32 was included in both the prevalence and sleep architecture analyses.
Prevalence of Sleep Disturbances and Insomnia
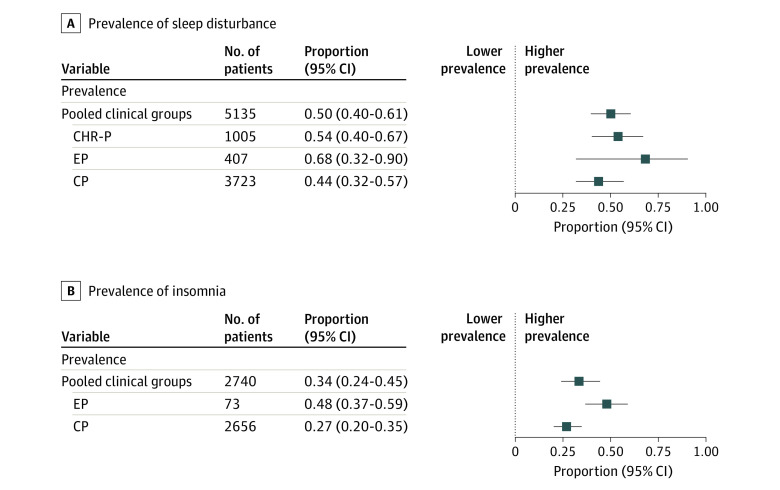
The pooled (ie, combined CHR-P, EP, and CP) prevalence of sleep disturbances was 50% across clinical stages (95% CI, 40%-61%; Q = 611.28; df = 20; Figure 2A). Stage-specific analyses yielded a sleep disturbance prevalence of 54% in CHR-P (95% CI, 40%-67%; Q = 13.66; df = 3), 68% in EP (95% CI, 32%-90%; Q = 21.2; df = 3), and 44% in CP (95% CI, 32%-57%; Q = 432.73; df = 12; Figure 2A); eFigure 1 in Supplement 1 shows forest plots of individual studies. Furthermore, prevalence of insomnia as the primary sleep disturbance was 34% (95% CI, 24%-45%) of pooled cases, 48% (95% CI, 37%-59%) of EP, and 27% (95% CI, 20%-35%) of CP (Figure 2B; see eFigure 2 in Supplement 1 for individual studies forest plot). Moderator analysis yielded no sleep disturbance or insomnia differences between clinical stages (eTable 3 in Supplement 1).
Figure 2. Forest Plots of Prevalence of Sleep Disturbance and Prevalence of Insomnia .
Logit transformation was applied for analysis and the final pooled logit was back transformed to proportions for ease of interpretation of the forest plots. CHR-P indicates clinical high risk for psychosis; CP, chronic psychosis; EP, early psychosis.
SMDs in Sleep Quality
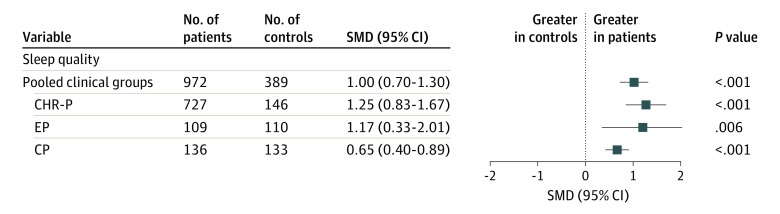
Sleep quality was assessed comparing total PSQI scores between clinical and control groups. Results indicated a significant SMD in pooled cases vs controls (SMD, 1.00 [95% CI, 0.70-1.30]; P < .001). Each clinical group showed poorer sleep quality compared with controls (SMD [95% CI]: CHR-P vs control, 1.25 [0.83-1.67]; P < .001; EP vs control, 1.17 [0.33-2.01]; P = .006; CP vs control, 0.65 [0.4-0.89]; P < .001; Figure 3; see eFigure 3 in Supplement 1 for forest plot of individual studies). Moderator analysis revealed no PSQI scores differences between different clinical stages (eTable 3 in Supplement 1).
Figure 3. Summary of Standardized Mean Differences (SMDs) in Sleep Quality as Measured by Total Pittsburgh Sleep Quality Index.
CHR-P indicates clinical high risk for psychosis; CP, chronic psychosis; EP, early psychosis.
SMDs in Sleep Architecture
Pooled cases had higher effect sizes for sleep onset latency (SMD, 0.96 [95% CI, 0.62-1.30]; P < .001), wake after sleep onset (SMD, 0.50 [95% CI, 0.29-0.71]; P < .001), number of arousals (SMD, 0.45 [95% CI, 0.07-0.83]; P = .019), stage 1 nonrapid eye movement sleep (SMD, 0.23 [95% CI, 0.06-0.40]; P = .008), and REM density (SMD, 0.37 [95% CI, 0.14-0.60]; P = .002) vs controls. Conversely, effect sizes were lower in pooled cases vs control groups for sleep efficiency (SMD, −0.75 [95% CI, −0.98 to −0.52]; P < .001) and slow wave sleep (SMD, −0.24 [95% CI, −0.44 to −0.03]; P = .023). Furthermore, total sleep time, stage 2 sleep, and REM latency did not differ between groups (Figure 4; eFigures 4-13 in Supplement 1 contain forest plots of studies for each sleep architecture variable).
Figure 4. Summary of Standardized Mean Differences (SMDs) for Sleep Architecture Parameters .
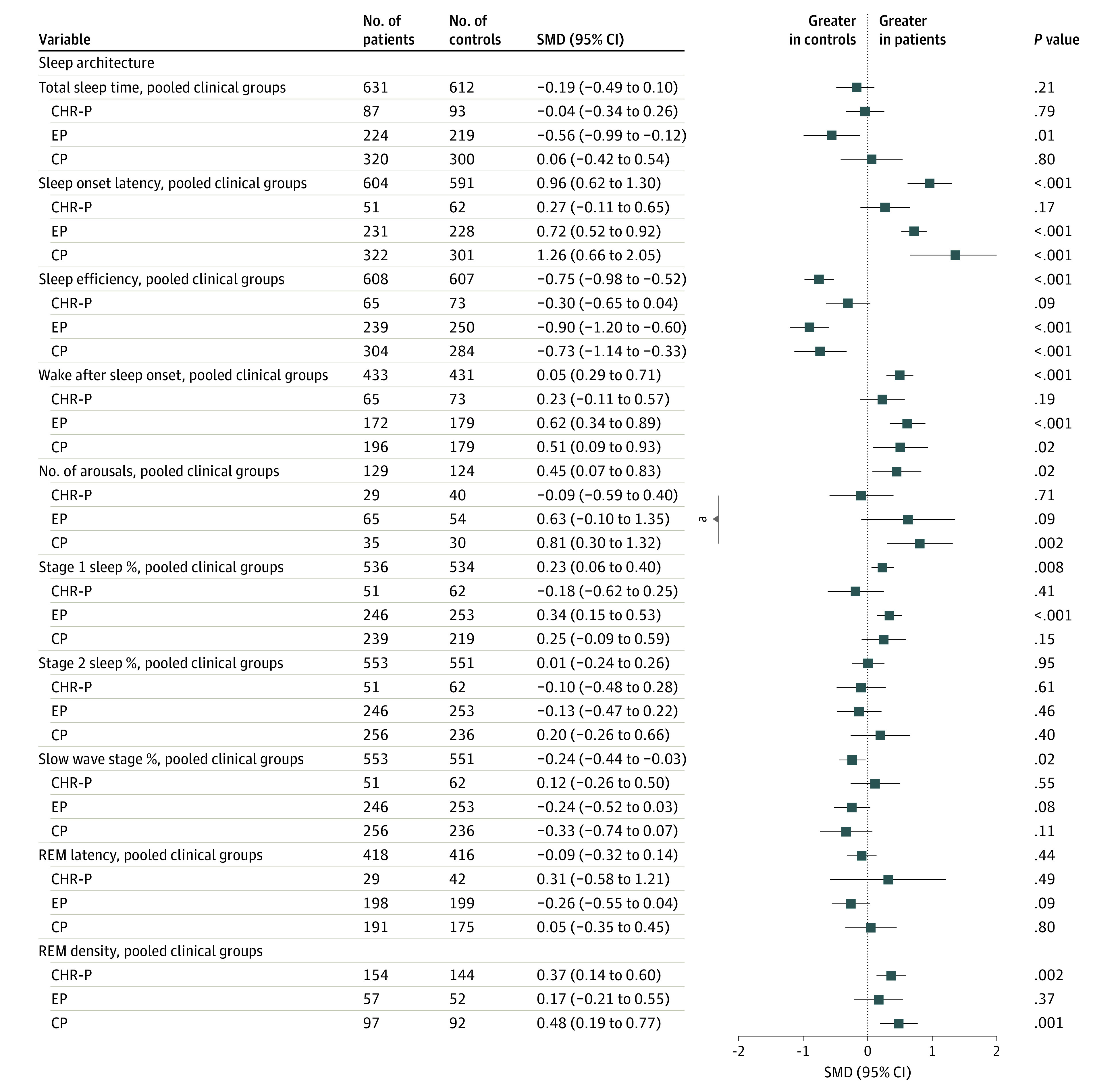
CHR-P indicates clinical high risk for psychosis; CP, chronic psychosis; EP, early psychosis; REM, rapid eye movement.
aSignificant (P < .05) effect sizes between 2 subgroups are indicated.
Stage-specific case-control comparisons revealed no sleep architecture differences in CHR-P vs controls. EP had higher sleep onset latency (SMD, 0.72 [95% CI, 0.52-0.92]; P < .001), wake after sleep onset (SMD, 0.62 [95% CI, 0.34-0.89]; P < .001), and stage 1 sleep (SMD, 0.34; [95% CI, 0.15-0.53]; P < .001), along with lower total sleep time (SMD, −0.56 [95% CI, −0.99 to −0.12]; P = .012) and sleep efficiency (SMD, −0.90 [95% CI, −1.20 to −0.60]; P < .001) compared with controls. CP showed higher sleep onset latency (SMD, 1.36 [95% CI, 0.66-2.05]; P < .001) and wake after sleep onset (SMD, 0.51 [95% CI, 0.09-0.93]; P = .018), combined with lower sleep efficiency (SMD, −0.73 [95% CI, −1.14 to −0.33]; P < .001) vs controls. CP also showed more arousals (SMD, 0.81 [95% CI, 0.30-1.32]; P = .002) and REM density (SMD, 0.48 [95% CI, 0.19-0.77]; P = .001) compared with controls. Moderator analysis revealed more frequent arousals in CP compared with CHR-P (z = 2.24, P = .02; eTable 3 in Supplement 1).
SMDs in Spindle and Slow Wave Parameters
Pooled cases showed lower spindle density (SMD, −1.06 [95% CI, −1.50 to −0.63]; P < .001), spindle amplitude (SMD = −1.08 [95% CI, −1.33 to −0.82]; P < .001), and spindle duration (SMD, −1.21 [95% CI, −1.69 to −0.73]; P < .001; Figure 5) compared with controls. Stage-specific case-control comparisons revealed that spindle parameters were lower in both EC and CP relative to controls (Figure 5; see eFigures 14-16 in Supplement 1 for forest plots of individual studies). Furthermore, moderator analysis showed no differences between EP and CP in spindle density or amplitude (eTable 3 in Supplement 1) but lower spindle duration in CP compared with EP (z = −3.91, P < .001).
Figure 5. Summary of Standardized Mean Differences (SMDs) for Sleep Spindle Parameters and Slow-Wave Density .
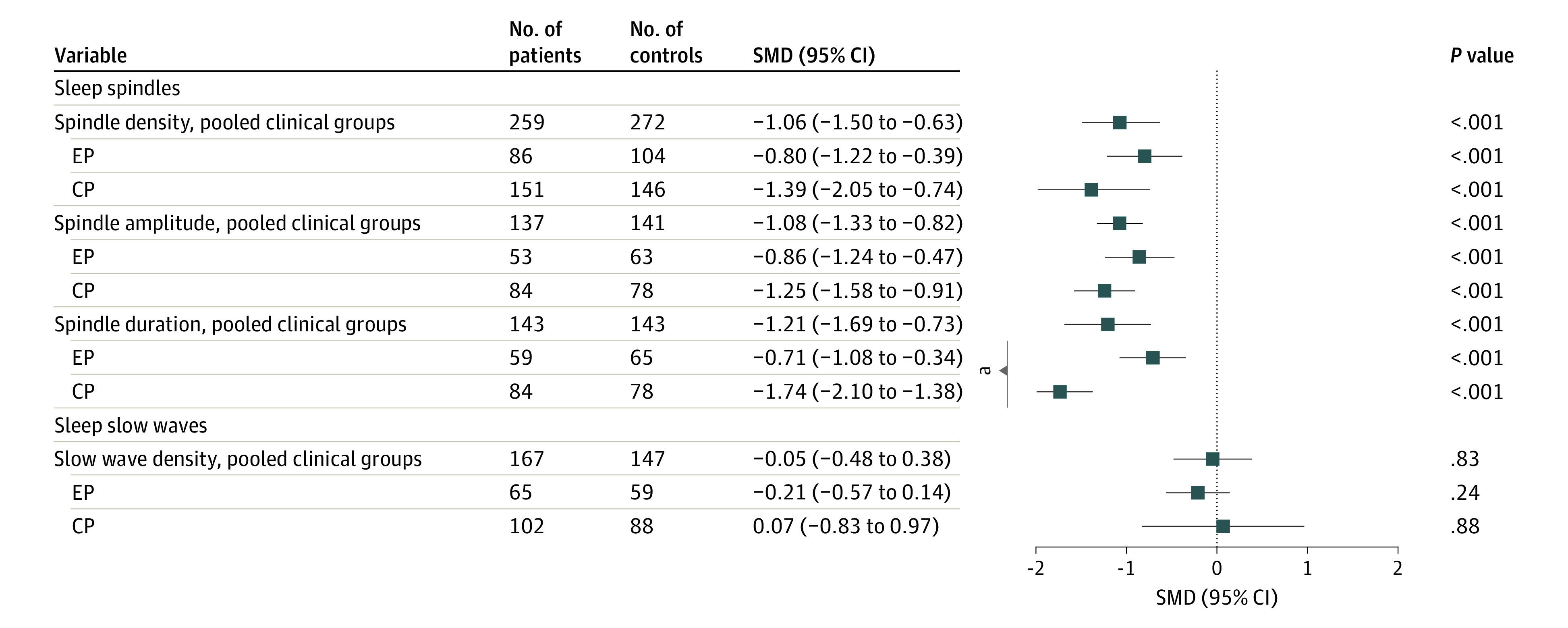
CP indicates chronic psychosis; EP, early psychosis.
aSignificant (P < .05) effect sizes between 2 subgroups are indicated.
Finally, slow wave density was not altered in patient groups relative to controls (Figure 5; eFigure 17 in Supplement 1 contains a forest plot of individual studies for slow wave density).
Supplement 1 contain results for meta-regressions accounting for medication, age, sex, and positive and negative symptoms (eResults and eTables 4-8 in Supplement 1), study heterogeneity and publication bias (eResults, eFigure 18 in Supplement 1), study quality appraisal (eTables 9-10 in Supplement 1), and the PRISMA checklist (eTable 11 in Supplement 1).25
Discussion
This meta-analysis investigated sleep abnormalities across clinical stages of psychosis and identified both uniformly present and stage-specific sleep disruptions.
Sleep Disturbance Prevalence Is Consistently High Throughout Psychosis Stages
Sleep disturbance prevalence has been commonly found to be higher in psychosis compared with the general population,7,8 although prior studies reported a variable incidence (21%-100%).9,10,11 Here, we established that sleep disturbances were present in 50% of pooled clinical cases, with similar prevalence in different psychosis stages, including at-risk individuals. This suggests that sleep disturbances are not only present throughout the course of psychosis, including before the manifestation of a psychotic episode, but are also consistently high in each psychosis stage, thus representing a critical issue that should be addressed in these individuals.
Sleep Quality Is Poor Throughout Stages of Psychosis
Case-control comparisons of self-reported sleep quality indicated poorer subjective sleep quality in pooled cases and in each clinical stage. Therefore, in addition to sleep disturbances being common, the intensity of perceived sleep distress is also more severe throughout the course of psychosis, including CHR-P, corroborating prior meta-analyses of sleep quality in CHR-P.9,19 Notably, in CHR-P, poorer sleep quality leads to worse negative symptoms33 and contributes to predicting transition to psychosis.5 Together, these findings expose the need to address subjective sleep complaints throughout the course of psychosis, even in the at-risk stage. Therefore, it would be important for primary care and mental health professionals to systematically screen for sleep disturbances and to promote sleep hygiene practices (eg, abstaining from caffeine, nicotine, and alcohol near bedtime, avoiding napping, and maintaining regular sleep and rise times and exposure to daylight) in prodromal individuals.
Shared and Distinct Sleep Architecture Alterations Are Present in EP and CP but Not in CHR-P
Consistent with prior meta-analyses,15,16,17,18 case-control comparisons of sleep architecture revealed increased sleep onset latency, wake after sleep onset, number of arousals, stage 1 sleep and REM density, along with lower sleep efficiency and slow wave sleep in pooled clinical cases.
Prior work furthermore suggested the presence of specific sleep alterations in early stages of psychosis.10,19 However, comparisons from at-risk to chronic stages had thus far not been performed. Here, stage-specific case-control comparisons showed that sleep architecture abnormalities were absent in CHR-P and driven by EP and CP stages. Altered sleep characteristics shared among EP and CP included increased sleep onset latency, increased wake after sleep onset, and reduced sleep efficiency. These findings are consistent with insomnia, as well as other disturbances including circadian phase delay, which is supported by recent studies reporting an association between evening chronotype in at-risk34 and full-blown psychosis.35 Together, these results suggest that difficulties in initiating and maintaining sleep first occur in full-blown psychosis and remain relatively stable throughout the course of the disorder, as none of the measures worsened in CP vs EP.
EP was also associated with a reduction in total sleep time and a higher percentage of stage 1 sleep, a pattern that was not observed when comparing other clinical stages to their respective control groups. A plausible interpretation of these findings is that individuals in the early course of psychosis experience considerable sleep loss and overall shallower sleep, a pattern that is furthermore corroborated by the higher rates of insomnia in EP found in this study. Sleep disruptions in these individuals are also likely involved in psychotic symptomatology, where psychotic experiences worsen sleep and sleep exacerbates psychotic symptoms.6,36 From a treatment perspective, early-course patients may therefore benefit from routine insomnia screening and targeted sleep interventions, including cognitive behavioral therapy for insomnia, which is effective in ameliorating difficulties in initiating and maintaining sleep.37,38
CP was the only clinical group with more arousals and increased REM density compared with controls. Higher REM density has been associated with increased suicidality in patients with psychosis,39 and pharmacological reviews indicated that antipsychotic medications can enhance REM density, although effect sizes vary between antipsychotic compounds.11,40 Weight gain is a frequent adverse reaction to long-term antipsychotic treatment41 and has been associated with sleep apnea in schizophrenia.42 Brief awakenings can help restore airflow in such conditions, which may account for the increased frequency of arousals in CP. Moderator analyses further indicated that individuals with CP had more arousals compared with CHR-P and that the number of arousals was significantly affected by medication (P = .001), above and beyond disease effects (z = −3.01 for medication vs z = 2.37 for pooled cases vs controls). Altogether, these findings indicate that the effects of antipsychotic medications on sleep should be closely monitored, especially in CP, and proper medication adjustments (eg, decrease medication doses, switch to a different compound) should be considered based on their impact on these sleep patterns.
Sleep Spindles, but Not Slow Waves, Are Severely Altered in EP and CP
Meta-analyses of sleep oscillations revealed no alteration in slow wave parameters in clinical cases vs controls. In contrast, decreased spindle density, spindle amplitude, and duration were observed in pooled cases vs controls. Stage-specific analyses further indicated that these deficits were present in both EP and CP and yielded some of the largest effect sizes in case-control comparisons (z range, −4.93 to −8.31). Of note, spindle measures could not be assessed in CHR-P, as only 1 study reported spindle measures in this group.43 Moderator analyses further indicated that patients with CP showed a more pronounced reduction of spindle duration compared with EP. Worsening of spindle deficits in chronic stages of psychosis were also reported by a recent meta-analysis on sleep spindles,23 although the clinical groups (schizophrenia, first-episode psychosis, and familial risk) and spindle measure (spindle density) only partially overlapped with our study. Furthermore, our moderator analyses revealed considerably larger effect sizes in spindle measures for case-control comparisons (z range, −4.93 to −8.31) relative to the effect sizes for the proportion medicated (z range, −1.14 to −2.13), and prior studies have consistently shown an absence of correlation between antipsychotic medication and spindle deficits in chronic patients.44,45 Together, these findings indicate that spindle deficits are unlikely to be related to antipsychotic medications and may represent a neurophysiological biomarker that could be used to monitor the course of psychotic disorders. Furthermore, given increasing evidence for an association between spindle deficits and clinical and cognitive dysfunction in individuals with psychosis,24,46 spindles may represent a promising target for novel treatment interventions. In this context, noninvasive brain stimulation has shown promise to restore sleep oscillations, including spindles.47
Limitations
This meta-analysis has some limitations. First, while included studies were selected based on comparable sleep assessment tools, substantial variability in across-study methodology remained. This was most pronounced in sleep disturbance prevalence studies, as sleep disorders were assessed with established diagnostic tools (eg, DSM criteria for insomnia) in only 1 study.48 Similarly, across-study methods used to measure sleep oscillations varied considerably (eg, manual vs automated spindle detection, 2-256 electrodes). Notwithstanding this methodological variability, we reported robust, consistent findings, especially regarding spindle deficits in clinical vs control groups. Second, a few of the included studies were rated as poor (n = 2), and several were rated as weak (n = 26). However, the quality of most of these studies was good (n = 27) or excellent (n = 5). Third, some analyses had limited statistical power. Specifically, pooled sample sizes of clinical groups included larger samples in CHR-P and CP individuals in prevalence studies, with relatively few prevalence studies in EP. Conversely, for sleep quality and architecture studies, CHR-P sample sizes were smaller compared with EP and CP, indicating that objectively measured sleep is understudied in at-risk populations. The same applied to sleep spindles, which were reported in only 1 study in CHR-P.43 Fourth, due to insufficient data availability, spindles were not stratified into fast and slow spindles, although some evidence suggests that distinct alterations in these 2 types of spindles may occur in psychosis (eDiscussion in Supplement 1).49 Similarly, while acute psychosis is likely associated with specific sleep alterations,50,51 insufficient data were available to incorporate this factor in the current meta-analysis. Fifth, the sleep assessments presented here were based on cross-sectional data rather than on longitudinal evaluations. Nonetheless, this meta-analysis represents the most comprehensive effort to date to delineate sleep abnormalities along the course of psychosis, from at-risk to chronic stages.
Conclusions
This systematic review and meta-analysis found that sleep disturbances were highly prevalent throughout the course of psychosis and that different stages of psychosis showed both shared and distinct abnormalities in sleep quality, sleep architecture, and sleep spindle parameters. Altogether, these findings indicate several prospective research directions. To begin with, future studies should use standardized, validated tools to report the prevalence of well-established sleep disorders, as these are common in psychosis but have been rarely assessed in specific clinical stages of psychosis. Moreover, longitudinal sleep studies following at-risk populations through illness stages are necessary to further characterize the interplay between sleep abnormalities and psychosis. To achieve this, research efforts should move beyond conventionally assessed sleep measures and evaluate different sleep patterns using emerging mobile technologies to assess sleep in the home environment.3 Future work is also needed to further delineate sleep alterations specific to acute and remitted psychosis, as well as the impact of psychotic symptoms severity. Additionally, future studies should better understand the role of antipsychotic medications and different medication types throughout different stages of psychosis. Finally, given the pervasive spindle alterations in EP and CP, an important future direction involves examining spindle properties from CHR-P52 to CP stages to determine whether spindle alterations may represent risk/susceptibility, monitoring, and/or prognostic biomarkers for psychosis. In doing so, studies should differentiate between fast and slow spindles to accurately delineate psychosis-related sleep alterations. Findings from these studies may help establish sleep as a core clinical target and research domain from prodromal to early and chronic stages of psychosis.
eMethods.
eTable 1. Study characteristics for papers included in the sleep disturbance prevalence analysis
eTable 2. Study characteristics for papers included in the case-control comparisons of sleep quality, sleep architecture and sleep oscillations
eTable 3. Sleep parameters differences in effect size (p-value) across different clinical stages of psychosis
eTable 4. Meta-regressions of sex, age, and proportion of sample on antipsychotic medication
eTable 5. Meta-regressions of sex, age, and proportion of sample on antipsychotic medication in CHR-P
eTable 6. Meta-regressions of sex, age, and proportion of sample on antipsychotic medication in EP
eTable 7. Meta-regressions of sex, age, and proportion of sample on antipsychotic medication in CP
eTable 8. Meta-regressions of psychotic symptoms
eTable 9. AHRQ based study quality for sleep disturbance prevalence studies (N=21)
eTable 10. AHRQ based study quality for sleep architecture studies (N=39)
eTable 11. PRISMA 2020 Checklist
eFigure 1. Sleep disturbance prevalence forest plot of individual studies
eFigure 2. Insomnia prevalence forest plot of individual studies
eFigure 3. Total PSQI forest plot of individual studies
eFigure 4. Total sleep time forest plot of individual studies
eFigure 5. Sleep onset latency forest plot of individual studies
eFigure 6. Sleep efficiency forest plot of individual studies
eFigure 7. Wake after sleep onset forest plot of individual studies
eFigure 8. Number of arousals forest plot of individual studies
eFigure 9. Stage 1 sleep % forest plot of individual studies
eFigure 10. Stage 2 sleep % forest plot of individual studies
eFigure 11. Slow wave stage % forest plot of individual studies
eFigure 12. Rapid eye movement latency forest plot of individual studies
eFigure 13. Rapid eye movement density forest plot of individual studies
eFigure 14. Sleep spindle density forest plot of individual studies
eFigure 15. Sleep spindle amplitude forest plot of individual studies
eFigure 16. Sleep spindle duration forest plot of individual studies
eFigure 17. Slow wave density forest plot of individual studies
eFigure 18. Funnel plots for each sleep parameter considered in the meta-analysis
eResults
eDiscussion
eReferences
Data Sharing Statement
References
- 1.Kraeplin E. Dementia Praecox and Paraphenia. Robert E. Krieger Publishing Co; 1919. [Google Scholar]
- 2.Hou CL, Li Y, Cai MY, et al. Prevalence of insomnia and clinical and quality of life correlates in Chinese patients with schizophrenia treated in primary care. Perspect Psychiatr Care. 2017;53(2):80-86. doi: 10.1111/ppc.12139 [DOI] [PubMed] [Google Scholar]
- 3.Meyer N, Joyce DW, Karr C, et al. The temporal dynamics of sleep disturbance and psychopathology in psychosis: a digital sampling study. Psychol Med. 2021;52(13):1-10. doi: 10.1017/S0033291720004857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353-370. doi: 10.1093/schbul/22.2.353 [DOI] [PubMed] [Google Scholar]
- 5.Ruhrmann S, Schultze-Lutter F, Salokangas RKR, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241-251. doi: 10.1001/archgenpsychiatry.2009.206 [DOI] [PubMed] [Google Scholar]
- 6.Waite F, Sheaves B, Isham L, Reeve S, Freeman D. Sleep and schizophrenia: from epiphenomenon to treatable causal target. Schizophr Res. 2020;221:44-56. doi: 10.1016/j.schres.2019.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 8.Tang J, Liao Y, Kelly BC, et al. Gender and regional differences in sleep quality and insomnia: a general population-based study in Hunan province of China. Sci Rep. 2017;7(1):43690. doi: 10.1038/srep43690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke L, Chisholm K, Cappuccio FP, et al. Sleep disturbances and the at risk mental state: a systematic review and meta-analysis. Schizophr Res. 2021;227:81-91. doi: 10.1016/j.schres.2020.06.027 [DOI] [PubMed] [Google Scholar]
- 10.Davies G, Haddock G, Yung AR, Mulligan LD, Kyle SD. A systematic review of the nature and correlates of sleep disturbance in early psychosis. Sleep Med Rev. 2017;31:25-38. doi: 10.1016/j.smrv.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 11.Cohrs S. Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS Drugs. 2008;22(11):939-962. doi: 10.2165/00023210-200822110-00004 [DOI] [PubMed] [Google Scholar]
- 12.Zaks N, Velikonja T, Parvaz MA, et al. Sleep disturbance in individuals at clinical high risk for psychosis. Schizophr Bull. 2022;48(1):111-121. doi: 10.1093/schbul/sbab104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasidharan A, Kumar S, Nair AK, et al. Further evidences for sleep instability and impaired spindle-delta dynamics in schizophrenia: a whole-night polysomnography study with neuroloop-gain and sleep-cycle analysis. Sleep Med. 2017;38:1-13. doi: 10.1016/j.sleep.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 14.Sahbaz C, Özer OF, Kurtulmus A, Kırpınar I, Sahin F, Guloksuz S. Evidence for an association of serum melatonin concentrations with recognition and circadian preferences in patients with schizophrenia. Metab Brain Dis. 2019;34(3):865-874. doi: 10.1007/s11011-019-00395-3 [DOI] [PubMed] [Google Scholar]
- 15.Meyer N, Faulkner SM, McCutcheon RA, Pillinger T, Dijk DJ, MacCabe JH. Sleep and circadian rhythm disturbance in remitted schizophrenia and bipolar disorder: a systematic review and meta-analysis. Schizophr Bull. 2020;46(5):1126-1143. doi: 10.1093/schbul/sbaa024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967. doi: 10.1093/oxfordjournals.schbul.a007145 [DOI] [PubMed] [Google Scholar]
- 17.Baglioni C, Nanovska S, Regen W, et al. Sleep and mental disorders: a meta-analysis of polysomnographic research. Psychol Bull. 2016;142(9):969-990. doi: 10.1037/bul0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan MS, Chung KF, Yung KP, Yeung WF. Sleep in schizophrenia: a systematic review and meta-analysis of polysomnographic findings in case-control studies. Sleep Med Rev. 2017;32:69-84. doi: 10.1016/j.smrv.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 19.Dondé C, Jaffiol A, Khouri C, et al. Sleep disturbances in early clinical stages of psychotic and bipolar disorders: a meta-analysis. Aust N Z J Psychiatry. 2022;56(9):1068-1079. doi: 10.1177/00048674211068395 [DOI] [PubMed] [Google Scholar]
- 20.Castelnovo A, Graziano B, Ferrarelli F, D’Agostino A. Sleep spindles and slow waves in schizophrenia and related disorders: main findings, challenges and future perspectives. Eur J Neurosci. 2018;48(8):2738-2758. doi: 10.1111/ejn.13815 [DOI] [PubMed] [Google Scholar]
- 21.Manoach DS, Demanuele C, Wamsley EJ, et al. Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Front Hum Neurosci. 2014;8:762. doi: 10.3389/fnhum.2014.00762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaskie RE, Graziano B, Ferrarelli F. Topographic deficits in sleep spindle density and duration point to frontal thalamo-cortical dysfunctions in first-episode psychosis. J Psychiatr Res. 2019;113:39-44. doi: 10.1016/j.jpsychires.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 23.Lai M, Hegde R, Kelly S, et al. Investigating sleep spindle density and schizophrenia: a meta-analysis. Psychiatry Res. 2022;307:114265. doi: 10.1016/j.psychres.2021.114265 [DOI] [PubMed] [Google Scholar]
- 24.Ferrarelli F. Sleep abnormalities in schizophrenia: state of the art and next steps. Am J Psychiatry. 2021;178(10):903-913. doi: 10.1176/appi.ajp.2020.20070968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostom A, Dubé C, Cranney A, et al. Celia Disease. Appendix D: quality assessment forms. Agency for Healthcare Research and Quality; 2004. Accessed June 11, 2022. https://www.ncbi.nlm.nih.gov/books/NBK35156/
- 27.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107-128. doi: 10.3102/10769986006002107 [DOI] [Google Scholar]
- 28.Lee CH, Cook S, Lee JS, Han B. Comparison of two meta-analysis methods: inverse-variance-weighted average and weighted sum of z-scores. Genomics Inform. 2016;14(4):173-180. doi: 10.5808/GI.2016.14.4.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. doi: 10.1002/9781119536604 [DOI] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fekih-Romdhane F, Nefzi H, Sassi H, Cherif W, Cheour M. Sleep in first-episode schizophrenia patients, their unaffected siblings and healthy controls: a comparison. Early Interv Psychiatry. 2021;15(5):1167-1178. Published online October 9, 2020. doi: 10.1111/eip.13058 [DOI] [PubMed] [Google Scholar]
- 33.Lunsford-Avery JR, Orr JM, Gupta T, et al. Sleep dysfunction and thalamic abnormalities in adolescents at ultra high-risk for psychosis. Schizophr Res. 2013;151(1-3):148-153. doi: 10.1016/j.schres.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lunsford-Avery JR, Pelletier-Baldelli A, Korenic SA, et al. Eveningness chronotype preference among individuals at clinical high risk for psychosis. Schizophr Res. 2021;236:3-8. doi: 10.1016/j.schres.2021.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linke M, Jankowski KS. Chronotype in individuals with schizophrenia: a meta-analysis. Schizophr Res. 2021;235:74-79. doi: 10.1016/j.schres.2021.07.020 [DOI] [PubMed] [Google Scholar]
- 36.Chiu VW, Ree M, Janca A, Waters F. Sleep in schizophrenia: exploring subjective experiences of sleep problems, and implications for treatment. Psychiatr Q. 2016;87(4):633-648. doi: 10.1007/s11126-015-9415-x [DOI] [PubMed] [Google Scholar]
- 37.Chiu VW, Ree M, Janca A, Iyyalol R, Dragovic M, Waters F. Sleep profiles and CBT-I response in schizophrenia and related psychoses. Psychiatry Res. 2018;268:279-287. doi: 10.1016/j.psychres.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 38.Hwang DK, Nam M, Lee YG. The effect of cognitive behavioral therapy for insomnia in schizophrenia patients with sleep disturbance: a non-randomized, assessor-blind trial. Psychiatry Res. 2019;274:182-188. doi: 10.1016/j.psychres.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 39.Keshavan MS, Reynolds CF, Montrose D, Miewald J, Downs C, Sabo EM. Sleep and suicidality in psychotic patients. Acta Psychiatr Scand. 1994;89(2):122-125. doi: 10.1111/j.1600-0447.1994.tb01498.x [DOI] [PubMed] [Google Scholar]
- 40.Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. doi: 10.1016/j.smrv.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 41.Casey DE, Haupt DW, Newcomer JW, et al. Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. J Clin Psychiatry. 2004;65 suppl 7:4-18. [PubMed] [Google Scholar]
- 42.Myles H, Myles N, Antic NA, et al. Obstructive sleep apnea and schizophrenia: a systematic review to inform clinical practice. Schizophr Res. 2016;170(1):222-225. doi: 10.1016/j.schres.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 43.Purple RJ, Cosgrave J, Vyazovskiy V, Foster RG, Porcheret K, Wulff K. Sleep-related memory consolidation in the psychosis spectrum phenotype. Neurobiol Learn Mem. 2020;174:107273. doi: 10.1016/j.nlm.2020.107273 [DOI] [PubMed] [Google Scholar]
- 44.Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483-492. doi: 10.1176/ajp.2007.164.3.483 [DOI] [PubMed] [Google Scholar]
- 45.Markovic A, Buckley A, Driver DI, et al. Sleep spindle activity in childhood onset schizophrenia: diminished and associated with clinical symptoms. Schizophr Res. 2020;223:327-336. doi: 10.1016/j.schres.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manoach DS, Stickgold R. Abnormal sleep spindles, memory consolidation, and schizophrenia. Annu Rev Clin Psychol. 2019;15(1):451-479. doi: 10.1146/annurev-clinpsy-050718-095754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Fröhlich F. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr Biol. 2016;26(16):2127-2136. doi: 10.1016/j.cub.2016.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang YS, Guilleminault C, Chen CH, Lai PC, Hwang FM. Narcolepsy-cataplexy and schizophrenia in adolescents. Sleep Med. 2014;15(1):15-22. doi: 10.1016/j.sleep.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 49.Schilling C, Schlipf M, Spietzack S, et al. Fast sleep spindle reduction in schizophrenia and healthy first-degree relatives: association with impaired cognitive function and potential intermediate phenotype. Eur Arch Psychiatry Clin Neurosci. 2017;267(3):213-224. doi: 10.1007/s00406-016-0725-2 [DOI] [PubMed] [Google Scholar]
- 50.Kaskie RE, Gill KM, Ferrarelli F. Reduced frontal slow wave density during sleep in first-episode psychosis. Schizophr Res. 2019;206:318-324. doi: 10.1016/j.schres.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 51.Keshavan MS, Reynolds CF III, Miewald MJ, et al. Delta sleep deficits in schizophrenia: evidence from automated analyses of sleep data. Arch Gen Psychiatry. 1998;55(5):443-448. doi: 10.1001/archpsyc.55.5.443 [DOI] [PubMed] [Google Scholar]
- 52.Mayeli A, Wilson JD, Donati FL, LaGoy AD, Ferrarelli F. Sleep spindle alterations relate to working memory deficits in individuals at clinical high-risk for psychosis. Sleep. 2022;45(11):zsac193. doi: 10.1093/sleep/zsac193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Study characteristics for papers included in the sleep disturbance prevalence analysis
eTable 2. Study characteristics for papers included in the case-control comparisons of sleep quality, sleep architecture and sleep oscillations
eTable 3. Sleep parameters differences in effect size (p-value) across different clinical stages of psychosis
eTable 4. Meta-regressions of sex, age, and proportion of sample on antipsychotic medication
eTable 5. Meta-regressions of sex, age, and proportion of sample on antipsychotic medication in CHR-P
eTable 6. Meta-regressions of sex, age, and proportion of sample on antipsychotic medication in EP
eTable 7. Meta-regressions of sex, age, and proportion of sample on antipsychotic medication in CP
eTable 8. Meta-regressions of psychotic symptoms
eTable 9. AHRQ based study quality for sleep disturbance prevalence studies (N=21)
eTable 10. AHRQ based study quality for sleep architecture studies (N=39)
eTable 11. PRISMA 2020 Checklist
eFigure 1. Sleep disturbance prevalence forest plot of individual studies
eFigure 2. Insomnia prevalence forest plot of individual studies
eFigure 3. Total PSQI forest plot of individual studies
eFigure 4. Total sleep time forest plot of individual studies
eFigure 5. Sleep onset latency forest plot of individual studies
eFigure 6. Sleep efficiency forest plot of individual studies
eFigure 7. Wake after sleep onset forest plot of individual studies
eFigure 8. Number of arousals forest plot of individual studies
eFigure 9. Stage 1 sleep % forest plot of individual studies
eFigure 10. Stage 2 sleep % forest plot of individual studies
eFigure 11. Slow wave stage % forest plot of individual studies
eFigure 12. Rapid eye movement latency forest plot of individual studies
eFigure 13. Rapid eye movement density forest plot of individual studies
eFigure 14. Sleep spindle density forest plot of individual studies
eFigure 15. Sleep spindle amplitude forest plot of individual studies
eFigure 16. Sleep spindle duration forest plot of individual studies
eFigure 17. Slow wave density forest plot of individual studies
eFigure 18. Funnel plots for each sleep parameter considered in the meta-analysis
eResults
eDiscussion
eReferences
Data Sharing Statement