This cohort study performs a post hoc analysis of data for US and Australian participants in the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial to determine the association between level of high-density lipoprotein cholesterol and increased fracture risk in healthy older adults.
Key Points
Question
Is level of high-density lipoprotein cholesterol (HDL-C) associated with an increased fracture risk in healthy older adults?
Findings
In this cohort study of 16 262 community-dwelling healthy participants 70 years and older, 1659 participants experienced at least 1 fracture over a median follow-up of 4 years. In the fully adjusted model, each 1-SD increment in HDL-C level was associated with a 14% higher risk of fractures, and when HDL-C level was analyzed in categories, individuals in the highest quintile had a 33% higher risk of fractures than those in the lowest quintile.
Meaning
Findings from this study indicate that higher HDL-C levels may be associated with an increased risk of fractures.
Abstract
Importance
Increased levels of high-density lipoprotein cholesterol (HDL-C) have been associated with osteoporosis. Preclinical studies have reported that HDL-C reduces bone mineral density by reducing osteoblast number and function. However, the clinical significance of these findings is unclear.
Objective
To determine whether higher HDL-C levels are predictive of an increased fracture risk in healthy older adults.
Design, Setting, and Participants
This cohort study is a post hoc analysis of data from the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial and the ASPREE-Fracture substudy. ASPREE was a double-blind, randomized, placebo-controlled primary prevention trial of aspirin that recruited participants between 2010 and 2014. These comprised community-based older adults (16 703 Australians aged ≥70 years, 2411 US participants ≥65 years) without evident cardiovascular disease, dementia, physical disability, and life-limiting chronic illness. The ASPREE-Fracture substudy collected data on fractures reported postrandomization from Australian participants. Cox regression was used to calculate hazard ratio (HR) and 95% CI. Data analysis for this study was performed from April to August 2022.
Exposure
Plasma HDL-C.
Main Outcomes and Measures
Fractures included were confirmed by medical imaging and included both traumatic and minimal trauma fractures. Fractures were adjudicated by an expert review panel.
Results
Of the 16 262 participants who had a plasma HDL-C measurement at baseline (8945 female participants [55%] and 7319 male [45%]), 1659 experienced at least 1 fracture over a median (IQR) of 4.0 years (0.02-7.0 years). In a fully adjusted model, each 1-SD increment in HDL-C level was associated with a 14% higher risk of fractures (HR, 1.14; 95% CI, 1.08-1.20). The results remained similar when these analyses were stratified by sex. Sensitivity and stratified analyses demonstrated that these associations persisted when the analyses were repeated to include only (1) minimal trauma fractures, (2) participants not taking osteoporosis medications, (3) participants who were never smokers and reported that they did not drink alcohol, and (4) participants who walked outside for less than 30 minutes per day and reported no participation in moderate/vigorous physical activity and to examine only (5) statin use. No association was observed between non–HDL-C levels and fractures.
Conclusions and Relevance
This study suggests that higher levels of HDL-C are associated with an increased fracture risk. This association was independent of common risk factors for fractures.
Introduction
Fractures are common, and the rate is increasing with the rapidly growing global shift toward an aging society. Despite the availability of antiosteoporosis medications, minimal trauma fractures affect 25% of men and 44% of women 60 years and older.1 A recent meta-analysis that included 12 cross-sectional and case-control studies reported that the level of high-density lipoprotein cholesterol (HDL-C) was elevated in patients with osteoporosis.2 Preclinical studies have shown HDL-C reduces bone mineral density (BMD) by stimulating molecular mechanisms that reduce osteoblast number and function.3 Given these findings, it would be anticipated that a high HDL-C level could be associated with an increased risk of fractures. Two previous studies of this relationship have yielded inconclusive results.4,5 Thus, the question of any association between plasma HDL-C level and fracture risk in community-based older individuals remains unanswered.
Methods
Using data from the Aspirin in Reducing Events in the Elderly (ASPREE) study (which enrolled 16 703 Australians aged ≥70 years and 2411 US participants ≥65 years) and the ASPREE-Fracture substudy (Australians only), we examined the associations between plasma HDL-C level and incident fractures. Between March 2010 and December 2014, ASPREE recruited community-dwelling older adults with no evident cardiovascular disease, dementia, physical disability, or chronic illness expected to limit survival to less than 5 years. Participants were randomized to receive aspirin, 100 mg daily, or matching placebo.6 Ethics approval was obtained from institutional ethics review committees in Australia and the United States. Participants provided informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
During in-person annual visits, anthropometric and laboratory measurements were recorded, and data regarding medical comorbidities, lifestyle and sociodemographic factors, prescription medications, and other related health variables were collected. Fasting lipid levels, including HDL-C and non–HDL-C, were measured by a local pathologist.
Data on incident fractures occurring postrandomization were collected during the annual visits and at telephone contacts every 6 months. Each reported fracture was confirmed by clinical notes, discharge summaries, and medical imaging reports and classified by cause and site (eg, vertebral, hip or nonhip, nonvertebral) by an expert review committee consisting of expert clinicians and research personnel.7
Cox regression was used to calculate the hazard ratios (HRs) and 95% CI from the time of randomization to the first fracture occurring during the ASPREE follow-up period. Initially, plasma HDL-C and non-HDL-C values were treated as continuous variables. Thereafter, they were analyzed according to quintiles (Q) with Q1 being the lowest 20%; Q2 through Q4, the middle 60%; and Q5, the highest 20%. The lowest quintile (Q1) of HDL-C level was used as the reference. Various analytical models were adjusted for 1 or more of age, sex, physical activity, alcohol use, frailty status (determined via modified Fried frailty phenotype, which defines frailty as the presence of weakness, slowness, exhaustion, low physical activity, and weight loss), education, body mass index (BMI), smoking status, aspirin, diabetes, chronic kidney disease (estimated glomerular filtration rate <60 mL/min), and use of lipid-lowering medications or antiosteoporosis medications. The associations between HDL-C level and fractures were examined using HR curve (spline curve) to determine whether there was evidence of a nonlinear association, treating an HDL-C level of 58 mg/dL as the reference. (To convert to millimoles per liter, multiply by 0.0259.)
The associations between non–HDL-C levels and fractures were examined. Analyses examining the associations between plasma HDL-C level and fractures were repeated for participants (1) with minimal trauma fractures, (2) not taking osteoporosis medications, (3) who were never smokers and reported they did not drink alcohol currently, and (4) who walked outside less than 30 minutes a day and reported no participation in moderate/vigorous physical activity and to examine (5) statin use. We also repeated the analyses after stratification by sex because female participants had higher rates of fractures than male participants. Statistical significance was defined as a 2-sided P < .05. Stata MP version 17 (StataCorp) was used for analysis, which was performed from April to August 2022.
Results
A total of 16 262 participants with plasma HDL-C measurements at baseline were included; 8945 participants (55%) were female, and 7319 were male (45%). Of these 1659 individuals (10.2%) sustained at least 1 fracture. There were 711 minimal trauma fractures (eg, falls from standing height) and 948 other trauma fractures (mainly falls on stairs, ladders, stools, etc) over a median (IQR) of 4.0 years (0.02-7.0 years). Participants in the highest quintile of HDL-C values had a lower BMI, high prevalence of current/former smoking, current alcohol use, 12 years or longer of school, greater physical activity, and higher use of antiosteoporosis medication. They had less chronic kidney disease, diabetes, prefrailty/frailty, or treatment with lipid-lowering medications at baseline (Table 1). Similar prevalence of these covariates was observed for both male and female individuals (eTable 1 in Supplement 1). Higher rates of fractures occurred in the highest quintile of HDL-C level (Table 1).
Table 1. General Characteristics of Participants Overall and by Quintiles of HDL Cholesterol Level.
| Characteristic | No. (%) | P value | |||
|---|---|---|---|---|---|
| Overall | Q1 | Q2-Q4 | Q5 | ||
| No. of participants | 16 264 | 4524 | 9080 | 2660 | |
| Participants with ≥1 fracture | 1659 (10) | 412 (9) | 907 (10) | 340 (13) | <.001 |
| Types of fracture | <.001 | ||||
| Minimal trauma fracture | 711 (4) | 167 (4) | 401 (4) | 143 (5) | |
| Other trauma fracture | 948 (6) | 245 (5) | 506 (6) | 197 (7) | |
| Age, mean (SD), y | 75 (4) | 75 (4) | 75 (4) | 76 (5) | <.001 |
| Sex | |||||
| Female | 8945 (55) | 2548 (56) | 4892 (54) | 1505 (57) | .006 |
| Male | 7319 (45) | 1976 (44) | 4188 (46) | 1155 (43) | |
| Low activitya | 1044 (6) | 382 (8) | 544 (6) | 118 (4) | <.001 |
| BMI, mean (SD)b | 28 (5) | 30 (5) | 28 (4) | 26 (4) | <.001 |
| Waist circumference, mean (SD), cm | 97 (13) | 101 (12) | 97 (12) | 92 (12) | <.001 |
| Current/former smoking | 7208 (44) | 1924 (43) | 3983 (44) | 1301 (49) | <.001 |
| Current alcohol use | 12 835 (79) | 3210 (71) | 7317 (81) | 2308 (87) | <.001 |
| Education, years of school | <.001 | ||||
| <12 y | 9955 (61) | 2960 (65) | 5479 (60) | 1516 (57) | |
| ≥12 y | 6308 (39) | 1564 (35) | 3601 (40) | 1143 (43) | |
| Hypertension | 12 196 (75) | 3602 (80) | 6674 (74) | 1920 (72) | <.001 |
| Chronic kidney disease (eGFR <60 mL/min) | 2873 (18) | 1029 (23) | 1487 (17) | 357 (14) | <.001 |
| Diabetes | 1588 (10) | 762 (17) | 690 (8) | 136 (5) | <.001 |
| Prefrail/frailc | 6229 (38) | 1836 (41) | 3399 (37) | 994 (37) | .001 |
| Taking trial medication | 8109 (50) | 2248 (50) | 4494 (50) | 1367 (51) | .22 |
| Antiosteoporosis medications | 1096 (7) | 245 (5) | 606 (7) | 245 (9) | <.001 |
| Cholesterol, mean (SD), mg/dL | |||||
| Total | 204 (38) | 192 (39) | 206 (36) | 219 (35) | <.001 |
| HDL | 61 (18) | 44 (7) | 62 (10) | 89 (15) | |
| Non-HDL | 143 (36) | 148 (38) | 144 (35) | 130 (34) | <.001 |
| Lipid-lowering medications | 5368 (35) | 1729 (39) | 2844 (33) | 795 (32) | <.001 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; Q, quintile.
SI conversion factor: To convert total, HDL, or non-HDL cholesterol to mmol/L, multiply by 0.0259.
Defined as walking outside for less than 30 minutes per day and reporting no participation in moderate/vigorous physical activity.
Calculated as weight in kilograms divided by height in meters squared.
Determined via modified Fried frailty phenotype, which defines frailty as the presence of weakness, slowness, exhaustion, low physical activity, and weight loss.
The associations between HDL-C level and fractures are presented in Table 2. In the fully adjusted model, 1-SD increment in HDL-C level was associated with a 14% higher risk of fracture during the follow-up period (HR, 1.14; 95% CI, 1.08-1.20). When HDL-C was analyzed in quintiles, compared with participants in Q1, participants in Q5 had a 33% higher risk of fracture (HR, 1.33; 95% CI, 1.14-1.54). The associations between HDL-C level and fractures were linear for both male and female participants (Figure).
Table 2. Association Between Baseline HDL-C Level and at Least 1 Fracture Occurring After Recruitment.
| Analysis | HDL-C (continuous), HR (95% CI) | HDL-C quintiles, HR (95% CI) | ||
|---|---|---|---|---|
| Q1 | Q2-Q4 | Q5 | ||
| All participants (n = 16 264) | ||||
| IR per 1000 person-years (95% CI) | 26 (24-27) | 23 (21-25) | 25 (23-27) | 32 (29-36) |
| Model 1a | 1.15 (1.10-1.21) | 1 [Reference] | 1.10 (0.98-1.24) | 1.39 (1.20-1.60) |
| Model 2b | 1.15 (1.10-1.21) | 1 [Reference] | 1.07 (0.98-1.23) | 1.37 (1.19-1.58) |
| Model 3c | 1.15 (1.10-1.21) | 1 [Reference] | 1.10 (0.98-1.23) | 1.37 (1.19-1.59) |
| Model 4d | 1.14 (1.08-1.20) | 1 [Reference] | 1.09 (0.97-1.23) | 1.33 (1.14-1.54) |
| Analyses stratified by sexe | ||||
| Male (n = 7319) | ||||
| IR per 1000 person-years (95% CI) | 15 (14-17) | 14 (11-16) | 15 (13-17) | 21 (17-26) |
| Model 1a | 1.20 (1.09-1.33) | 1 [Reference] | 1.07 (0.85-1.34) | 1.48 (1.12-1.95) |
| Model 2b | 1.21 (1.10-1.34) | 1 [Reference] | 1.08 (0.86-1.36) | 1.51 (1.14-2.00) |
| Model 3c | 1.21 (1.10-1.34) | 1 [Reference] | 1.08 (0.86-1.36) | 1.52 (1.14-2.00) |
| Model 4d | 1.21 (1.09-1.35) | 1 [Reference] | 1.06 (0.83-1.35) | 1.45 (1.07-1.95) |
| Female (n = 8945) | ||||
| IR per 1000 person-years (95% CI) | 34 (32-36) | 30 (27-34) | 34 (32-37) | 42 (37-47) |
| Model 1a | 1.14 (1.08-1.20) | 1 [Reference] | 1.12 (0.98-1.28) | 1.35 (1.14-1.60) |
| Model 2b | 1.13 (1.07-1.20) | 1 [Reference] | 1.10 (0.96-1.26) | 1.33 (1.12-1.57) |
| Model 3c | 1.13 (1.07-1.19) | 1 [Reference] | 1.11 (0.96-1.27) | 1.33 (1.12-1.57) |
| Model 4d | 1.12 (1.06-1.19) | 1 [Reference] | 1.11 (0.96-1.28) | 1.29 (1.08-1.55) |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; IR, incidence rate.
Model 1 was adjusted for age and sex.
Model 2 was adjusted for age, sex, physical activity, and alcohol use.
Model 3 was adjusted for age, sex, physical activity, alcohol use, and prefrailty/frailty status.
Model 4 was adjusted for age, sex, physical activity, alcohol use, prefrailty/frailty status, education, body mass index, smoking status, aspirin use, diabetes, chronic kidney disease, use of lipid-lowering medication, and use of antiosteoporosis medications.
Not adjusted for sex.
Figure. Association Between Levels of High-Density Lipoprotein (HDL) Cholesterol and Fractures.
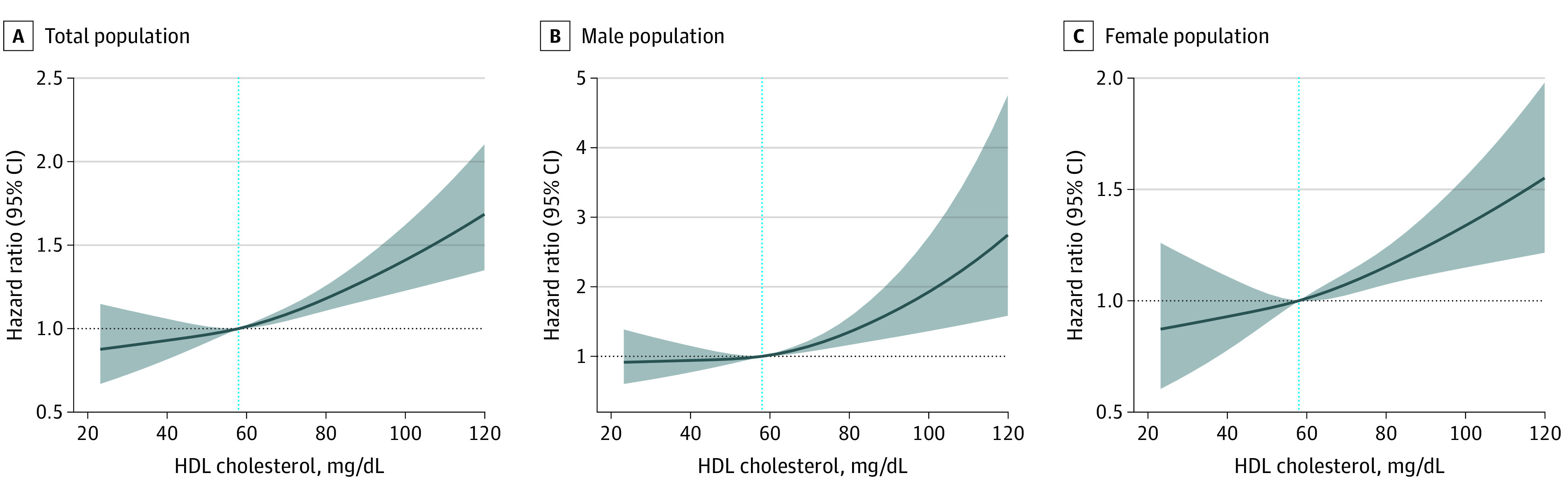
The dotted blue line indicates the reference value of 58 mg/dL. To convert HDL cholesterol to millimoles per liter, multiply by 0.0259.
No association between non–HDL-C values and fractures was observed (eTable 2 in Supplement 1). Results remained similar when these analyses were stratified by sex. In sensitivity analyses and stratified analyses (based on statin use) examining the associations between HDL-C level and fractures, the observed results persisted (eTables 3 and 4 in Supplement 1).
Discussion
The principal finding of the study was that higher levels of HDL-C predicted an increased fracture risk over the subsequent 4 years in healthy older adults. This increase in fracture risk appeared to be independent of traditional risk factors for fractures, including age, sex, physical activity, alcohol use, frailty status, education, BMI, smoking status, diabetes, chronic kidney disease, and use of lipid-lowering or antiosteoporosis medications. These results persisted in sensitivity analyses in restricted subgroups of interest and in stratified analyses. Overall, these findings suggest that high HDL-C level adds to traditional risk factors for fracture.
Our results contrast with the findings of the Study of Women’s Health Across the Nation (SWAN), which found no association in younger women undergoing a transition through menopause.4 The Cardiovascular Health Study (CHS), which relied on record linkage with Medicare claims, reported an increased risk of hip fracture in women.5 Our study is based on stronger methodology and is generalizable to the broad population of healthy older individuals.
The findings add to growing evidence of unfavorable effects linked to high HDL-C levels. As well as an increased fracture risk, other recent studies have reported that moderate (60-80 mg/dL) and high (>80 mg/dL) levels of HDL-C are associated with adverse cardiovascular outcomes in high-risk populations.7,8 In the presence of a systematic inflammatory state such as that occurring with aging, HDL-C has been reported to enhance inflammation, which might underpin a range of chronic diseases in an older age group.9 This association is in keeping with the lack of cardiovascular or other benefits generally seen in all participants in clinical trials of agents that increase HDL-C levels, although none of these studies reported fracture risk.
Mechanisms whereby a raised HDL-C level might increase fracture risk are unclear. A prime consideration would be confounding by factors such as increased physical activity, which could increase HDL-C but also increase fracture risk. However, the fact that the fracture risk was relatively unchanged when we excluded individuals who performed moderate/vigorous physical activity reduces the likelihood of this explanation. A previously reported association between high HDL-C levels and low BMD and osteoporosis10 and a Mendelian randomization study showing a genome-wide association between high HDL-C and low BMD11 could suggest a pathophysiological explanation. A recent analysis of genome-wide association studies reported HDL-C level but not triglyceride level as a causal risk factor for decreased heel and lumber spine BMD.12 Preclinical studies have shown HDL-C reduces BMD by stimulating molecular mechanisms that reduce osteoblast number.13
Strength and Limitations
Our study extends previous studies with its focus on healthy community-dwelling adults 70 years and older in whom fractures are relatively common, coupled with intensive and rigorous data collection and expert adjudication of fractures by radiology reports and radiographs. It has the usual constraints of an observational study, although the internal consistency of the findings among key subgroups provides support for their validity.
Conclusions
This cohort study provides robust evidence that higher levels of HDL-C are associated with incident fractures in both male and female individuals, independent of conventional risk factors. There have been 2 large-scale randomized trials of medications that increase HDL-C.14,15 In neither of these were fracture rates reported. These findings highlight another potential concern with high HDL-C levels and another likely adverse effect of the drugs that substantially increases plasma HDL-C levels. Further research is needed to determine the pathophysiological explanation for these findings.
eTable 1. General characteristics of participants stratified by sex: overall, and by quintiles of HDL-C
eTable 2. Association between baseline non-HDL-C (mg/dL) and at least one fracture occurring after recruitment (HR, 95% CI)
eTable 3. Association between baseline HDL-C (mg/dL) and at least one fracture occurring after recruitment (HR, 95% CI)
eTable 4. Association between baseline HDL-C (mg/dL) and at least one fracture occurring after recruitment based on statin use status (HR, 95% CI)
Data sharing statement
References
- 1.Frost SA, Kelly A, Gaudin J, et al. Establishing baseline absolute risk of subsequent fracture among adults presenting to hospital with a minimal-trauma-fracture. BMC Musculoskelet Disord. 2020;21(1):133. doi: 10.1186/s12891-020-3161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao H, Li Y, Zhang M, Qi L, Tang Y. Blood lipid levels in patients with osteopenia and osteoporosis: a systematic review and meta-analysis. J Bone Miner Metab. 2021;39(3):510-520. doi: 10.1007/s00774-020-01189-9 [DOI] [PubMed] [Google Scholar]
- 3.Papachristou NI, Blair HC, Kypreos KE, Papachristou DJ. High-density lipoprotein (HDL) metabolism and bone mass. J Endocrinol. 2017;233(2):R95-R107. doi: 10.1530/JOE-16-0657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang PY, Gold EB, Cauley JA, et al. Triglyceride levels and fracture risk in midlife women: Study of Women’s Health Across the Nation (SWAN). J Clin Endocrinol Metab. 2016;101(9):3297-3305. doi: 10.1210/jc.2016-1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barzilay JI, Buzkova P, Kuller LH, et al. The association of lipids and lipoproteins with hip fracture risk: the Cardiovascular Health Study. Am J Med. 2022;135(9):1101-1108.e1. doi: 10.1016/j.amjmed.2022.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNeil JJ, Woods RL, Nelson MR, et al. ; ASPREE Investigator Group . Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) Study. J Gerontol A Biol Sci Med Sci. 2017;72(11):1586-1593. doi: 10.1093/gerona/glw342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38(32):2478-2486. doi: 10.1093/eurheartj/ehx163 [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Dhindsa D, Almuwaqqat Z, et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiol. 2022;7(7):672-680. doi: 10.1001/jamacardio.2022.0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Márquez AB, Nazir S, van der Vorst EPC. High-density lipoprotein modifications: a pathological consequence or cause of disease progression? Biomedicines. 2020;8(12):549. doi: 10.3390/biomedicines8120549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisnyansky M, Kapelushnik N, Ben-Bassat A, et al. Reduced activity of geranylgeranyl diphosphate synthase mutant is involved in bisphosphonate-induced atypical fractures. Mol Pharmacol. 2018;94(6):1391-1400. doi: 10.1124/mol.118.113670 [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Shao Z, Gao Y, Yu X, Huang S, Zeng P. Are blood lipids risk factors for fracture? integrative evidence from instrumental variable causal inference and mediation analysis using genetic data. Bone. 2020;131:115174. doi: 10.1016/j.bone.2019.115174 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Greenbaum J, Shen H, et al. Detecting causal relationship between metabolic traits and osteoporosis using multivariable Mendelian randomization. Osteoporos Int. 2021;32(4):715-725. doi: 10.1007/s00198-020-05640-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair HC, Kalyvioti E, Papachristou NI, et al. Apolipoprotein A-1 regulates osteoblast and lipoblast precursor cells in mice. Lab Invest. 2016;96(7):763-772. doi: 10.1038/labinvest.2016.51 [DOI] [PubMed] [Google Scholar]
- 14.Bowman L, Hopewell JC, Chen F, et al. ; HPS3/TIMI55–REVEAL Collaborative Group . Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377(13):1217-1227. doi: 10.1056/NEJMoa1706444 [DOI] [PubMed] [Google Scholar]
- 15.Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. ; ACCELERATE Investigators . Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376(20):1933-1942. doi: 10.1056/NEJMoa1609581 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. General characteristics of participants stratified by sex: overall, and by quintiles of HDL-C
eTable 2. Association between baseline non-HDL-C (mg/dL) and at least one fracture occurring after recruitment (HR, 95% CI)
eTable 3. Association between baseline HDL-C (mg/dL) and at least one fracture occurring after recruitment (HR, 95% CI)
eTable 4. Association between baseline HDL-C (mg/dL) and at least one fracture occurring after recruitment based on statin use status (HR, 95% CI)
Data sharing statement


