Key Points
Question
Can prediction of posthospitalization suicides be improved by adding information from clinical notes and public records to a standard machine learning model?
Findings
In this prognostic study including 448 788 hospitalizations, performance of a machine learning model to predict suicides after Veteran Health Administration psychiatric hospital discharges improved significantly when including predictors extracted from clinical notes with natural language processing and from a social determinants of health public records database. The resulting model had positive net benefit for plausible decision thresholds over risk horizons between 3 and 12 months.
Meaning
In this study, clinical notes and public records improved machine learning model prediction of suicides after psychiatric hospital discharges.
This prognostic study evaluates whether machine learning model prediction for postdischarge suicide could be improved by adding information extracted from clinical notes and public records.
Abstract
Importance
The months after psychiatric hospital discharge are a time of high risk for suicide. Intensive postdischarge case management, although potentially effective in suicide prevention, is likely to be cost-effective only if targeted at high-risk patients. A previously developed machine learning (ML) model showed that postdischarge suicides can be predicted from electronic health records and geospatial data, but it is unknown if prediction could be improved by adding additional information.
Objective
To determine whether model prediction could be improved by adding information extracted from clinical notes and public records.
Design, Setting, and Participants
Models were trained to predict suicides in the 12 months after Veterans Health Administration (VHA) short-term (less than 365 days) psychiatric hospitalizations between the beginning of 2010 and September 1, 2012 (299 050 hospitalizations, with 916 hospitalizations followed within 12 months by suicides) and tested in the hospitalizations from September 2, 2012, to December 31, 2013 (149 738 hospitalizations, with 393 hospitalizations followed within 12 months by suicides). Validation focused on net benefit across a range of plausible decision thresholds. Predictor importance was assessed with Shapley additive explanations (SHAP) values. Data were analyzed from January to August 2022.
Main Outcomes and Measures
Suicides were defined by the National Death Index. Base model predictors included VHA electronic health records and patient residential data. The expanded predictors came from natural language processing (NLP) of clinical notes and a social determinants of health (SDOH) public records database.
Results
The model included 448 788 unique hospitalizations. Net benefit over risk horizons between 3 and 12 months was generally highest for the model that included both NLP and SDOH predictors (area under the receiver operating characteristic curve range, 0.747-0.780; area under the precision recall curve relative to the suicide rate range, 3.87-5.75). NLP and SDOH predictors also had the highest predictor class-level SHAP values (proportional SHAP = 64.0% and 49.3%, respectively), although the single highest positive variable-level SHAP value was for a count of medications classified by the US Food and Drug Administration as increasing suicide risk prescribed the year before hospitalization (proportional SHAP = 15.0%).
Conclusions and Relevance
In this study, clinical notes and public records were found to improve ML model prediction of suicide after psychiatric hospitalization. The model had positive net benefit over 3-month to 12-month risk horizons for plausible decision thresholds. Although caution is needed in inferring causality based on predictor importance, several key predictors have potential intervention implications that should be investigated in future studies.
Introduction
Suicide is the 10th leading cause of death in the US.1 The highest concentration is among the 1% of the population with a past-year psychiatric hospitalization,2 who account for 14% of all suicides.3 Intensive case management might reduce these postdischarge suicides4,5,6 but would be cost-effective only if targeted at high-risk patients. A machine learning (ML) model based on electronic health records and geospatial data was developed for this purpose in the Veterans Health Administration (VHA).7 Based on the strength of that model, an experimental trial was funded for suicide prevention with intensive postdischarge case management of high-risk patients.8 However, it was decided that further study was needed before implementing the trial to determine if model accuracy could be improved by adding 2 types of predictors found to be important in other suicide prediction models: (1) predictors extracted from clinical notes using natural language processing (NLP) methods9 and (2) indicators of patient-level social determinants of health (SDOH) extracted from public records.10 Results of this investigation are presented here along with an analysis of the net benefit (NB) of the expanded model across a range of plausible decision thresholds.
Methods
Sample
The models were trained in the 299 050 short-term (less than 365 days) VHA psychiatric hospitalizations that occurred between January 1, 2010, and September 1, 2012 (916 hospitalizations followed within 12 months by a suicide) and tested in a prospective validation sample of the 149 738 short-term VHA psychiatric hospitalizations that occurred between September 2, 2012, and December 31, 2013 (393 hospitalizations followed within 12 months by a suicide). The primary outcome, suicide within 12 months postdischarge, was identified in the National Death Index (NDI).11 The study protocol was approved by the Research Ethics Committees of the Veterans Administration Center of Excellence for Suicide Prevention and Harvard Medical School with a waiver of informed consent based on data being deidentified. The study followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline12 for reporting analyses designed to develop and validate predictive models.
Data Sources
The predictors in the original model came from 3 sources7: (1) the VHA Corporate Data Warehouse (CDW)13 (eTable 1 in Supplement 1); (2) the Veterans Administration (VA) Suicide Prevention Applications Network for reporting of suicidal behaviors14; and (3) a geospatial SDOH database assembled from diverse government sources (eTable 2 in Supplement 1) about plausible predictors of suicide15 in the patient’s residential neighborhood (ie, block group and census tracts), county, and state. Two new databases were added for the expanded models: (4) a consolidated free text file of VHA clinical notes for all inpatient and outpatient visits in the 12 months before and including hospitalization16; and (5) the LexisNexis SDOH database of public records (eTable 3 in Supplement 1) as of the month before hospitalization.17 Demographic information, including race and ethnicity information, were taken from the VHA VistA Patient file stored in the CDW. Both self-reported and observer-reported race and ethnicity are recorded in this file. Self-reports were taken when available, and observer reports were only taken when self-reports were missing. Data were missing for no more than 3% of records and only in the geospatial SDOH database and LexisNexis SDOH database. In most of these cases, records were nonmissing in earlier records or, in the case of the geospatial SDOH database, in contiguous areas, allowing nearest-neighbor imputations. Remaining missing values and inconsistencies were reconciled using rational imputations and median value imputations.
Predictors
Four broad predictor classes, each somewhat expanding the predictors in the earlier model, included: (1) psychopathological risk factors (diagnoses, treatments, suicidality), including information about these variables during the hospitalization; (2) physical disorders and treatments and counts of prescribed medications classified by the US Food and Drug Administration (FDA) as increasing suicide risk (eTable 4 in Supplement 1); (3) facility-level quality indicators (eg, inpatient staff turnover rate) over the 12 months prior to the date of the hospitalization; and (4) indicators of SDOH at both the patient level (from International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]/ICD-10-CM codes and sociodemographic information) and the geospatial level as of the month before the hospitalization. These variables were selected based on review of earlier research on predictors of suicide after psychiatric hospital discharge18,19,20,21 and in more general patient samples.22,23,24 Details about constructs and assessments are presented in the eMethods in Supplement 1. A total of 7902 predictors were extracted from these databases. An additional 1837 predictors were extracted from clinical notes using NLP methods (eMethods in Supplement 1). The LexisNexis SDOH database included an additional 442 predictors. Categorical predictors in all databases were 1-hot encoded as 0-1 dummy variables. Ordinal, interval, and ratio variables were standardized to a mean of 0 and variance of 1, with values more than 3 SDs above or below mean truncated to 3 or −3 to minimize the effects of outliers.
Statistical Analysis
Analysis was carried out January to August 2022. The SuperLearner (SL) stacked generalization ML method25 was used to pool results across an ensemble of classifiers (eTable 5 in Supplement 1) to generate optimally weighted composite predictions.26 Four SL models were trained: (1) the base model, including only predictors from the first 3 databases, and 3 models with additional predictors beyond those in the base model, including (2) the NLP model, (3) the LexisNexis SDOH model, and (4) the combined model, which included the important predictors from the base, NLP, and LexisNexis SDOH models (eMethods in Supplement 1). A lasso model (ie, sparse penalized logistic regression),27 estimated as a simple benchmark for the more complex stacked generalization models, included the same predictors as the base SL model.
The models were trained to predict suicides over a 12-month risk horizon but were also used in the validation sample to predict suicides over shorter risk horizons based on a prior finding that models predicting postdischarge suicides over a 12-month risk horizon outperform models developed over shorter risk horizons when applied at those shorter horizons.7 This occurred because the larger number of suicides over 12 months increased statistical power to detect meaningful associations that were comparable over shorter horizons. Metalearner weights for the learners used to estimate the SuperLearner prediction of the combined model are presented in eTable 9 of Supplement 1. A 2-sided t test was used to determine the significance of the 12-month suicide rate between the training and validation samples. One-sided χ2 tests were used to determine if AUCs were significantly greater than 0.5. All significance tests were evaluated at α = .05.
Model Interpretation
Model Performance
Model performance focused on NB, a widely used summary measure of clinical model performance.28 NB describes the relative benefits (true-positives) vs costs (false-positives) of intervening at a given decision threshold to determine how high predicted suicide risk must be to define a patient as warranting intervention. NB at decision threshold p is defined as the ratio of the observed number of true-positives above that threshold to a discounted number of false-positives above the threshold, with discounting defined by the p/q break-even point implied when the decision threshold is set at p, where q = 1 − p. Importantly, NB can be highest for a given model at one decision threshold but not others, making the decision threshold of interest explicit when comparing models. Absence of consensus on the minimum risk needed to justify implementing an aggressive suicide prevention intervention makes it impossible to select a single decision threshold for the NB analysis. Presenting results across a plausible range of thresholds is recommended in cases where such uncertainty exists.29,30 We considered decision thresholds between roughly half the 12-month suicide rate and 2-fold that rate.
To facilitate comparison across models, NB was divided by the observed suicide rate for the horizon to define standardized NB (SNB), which has an upper bound of 1.0 for each horizon. Although standardized NB usefully combines information about discrimination and calibration, the 2 model performance metrics typically considered in evaluating prediction models, thereby addressing the uncertainty that otherwise arises when one model has better discrimination and another better calibration, we also report comparative model discrimination and calibration. Discrimination was assessed by area under the receiver operating characteristic curve (AUROC) and area under the precision recall curve (AUPRC). Given the low prevalence of suicide, AUPRC was expressed relative to the suicide rate, which is the expected value of AUPRC under a null model. Calibration was assessed with the integrated calibration index (ICI),31 the weighted mean absolute value difference between observed and predicted probabilities of suicide, expressed relative to the suicide rate.
Although model performance was evaluated in the 30% prospective validation sample, discrimination and calibration were also calculated using 10-fold cross-validation in the total sample to provide a valid comparison with the approach used in most prior ML suicide prediction studies. Estimates based on 10-fold cross-validation are typically a good deal higher than estimates based on prospective validation.32 Given that some patients had multiple hospitalizations, the cross-validation folds were constructed at the patient level so that all hospitalizations for a specific patient were in the same fold.
Predictor Importance
Predictor importance was examined using the model-agnostic kernel Shapley additive explanations (SHAP) method.33 This method estimates the effect of changing a predictor from its observed score to the sample mean averaged across all logically possible permutations of other predictors. The mean of this SHAP value for a given predictor across all hospitalizations is 0. However, the mean absolute SHAP value provides useful information about the average importance of the predictor. A bee swarm plot of individual-level observed predictor scores by SHAP values shows the dominant direction of association. Proportional mean absolute SHAP values were calculated by dividing mean absolute SHAP values of classes and important predictors within classes by the mean absolute SHAP value of the entire model (eMethods in Supplement 1).
SAS version 9.4 (SAS Institute) was used for data cleaning, imputation, management, estimating suicide prevalence, and calculating AUROC, AUPRC, ICI, and standardized NB. R version 4.0.5 (The R Foundation) was used for NLP feature extraction and to estimate SL models and SHAP values.
Results
Sociodemographic and Military Career Variable Distributions
Most training and validation sample patients were male (280 901 of 299 050 [93.9%] and 139 630 of 149 738 [93.2%], respectively), with a median (IQR) age of 55 (16) and 55 (18) years. Most patients were non-Hispanic White (training sample, 187 745 [62.8%]; validation sample, 92 067 [61.5%]), followed by non-Hispanic Black (training sample, 72 315 [24.2%]; validation sample, 36 205 [24.2%]), Hispanic (training sample, 23 002 [7.7%]; validation sample, 11 687 [7.8%]), and other race (including American Indian and Alaska Native, Asian, Native Hawaiian and Other Pacific Islander races, responses of do not know and any other race, and missing data; training sample, 15 987 [5.3%]; validation sample, 9780 [6.5%]) (eTable 6 in Supplement 1). Most served in the Vietnam War era (training sample, 120 365 of 299 050 [40.2%]; validation sample, 53 790 of 149 738 [35.9%]).
Outcome Distribution
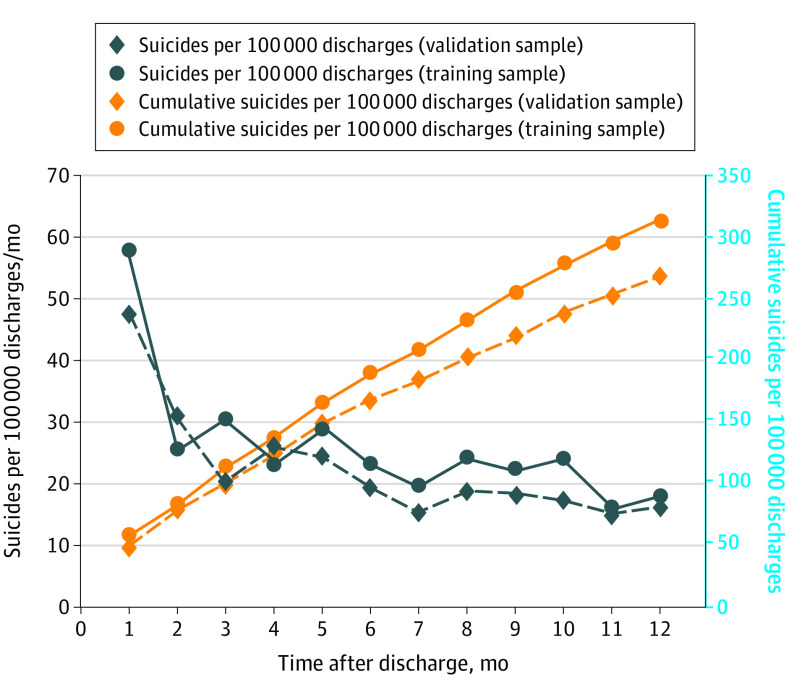
The 448 788 short-term VHA psychiatric hospitalizations from 2010 to 2013 involved 227 801 patients, 835 of whom died by suicide within 12 months of discharge from 1309 psychiatric hospitalizations. The 12-month suicide rate at the level of the hospitalization was significantly higher in the training sample (n = 916; mean [SE] rate, 306.3 [10.1] per 100 000 hospitalizations) than the validation sample (n = 393; mean [SE] rate, 262.5 [13.2] per 100 000 hospitalizations; t = −2.6; P = .008). The mean (SE) suicide rate decreased with time since discharge in both the training and validation samples, from highs of 1081.0 (137.3) per 100 000 hospitalization-years and 1149.2 (200.0) per 100 000 hospitalization-years, respectively, in the first week after discharge to lows of 245.9 (13.0) per 100 000 hospitalization-years and 198.3 (16.5) per 100 000 hospitalization-years 7 to 12 months after discharge (training sample, χ211 = 145.0; validation sample, χ211 = 61.8; P < .001) (Figure 1; eTable 7 in Supplement 1). The reasons for these decreases are unclear but illustrate the importance of using a prospective validation sample rather than a random 30% subsample selected across all study years to avoid overestimating prospective model prediction accuracy.
Figure 1. Monthly Suicide Hazard Rates and Cumulative Incidence Rates Over the 12 Months After Psychiatric Hospital Discharge in the Training Sample (January 1, 2010, to September 1, 2012) and Prospective Validation Sample (September 2, 2012, to December 31, 2013).
Model Performance
Discrimination and Calibration
Validation sample AUROC was in the range traditionally considered acceptable in all models other than the lasso model (AUROC range, 0.721-0.784) and poor in the lasso model (AUROC range, 0.642-0.668).34 AUROC was generally highest in the combined model (range, 0.747-0.780) (Table 1). AUPRC relative to the suicide rate was 3-fold to 6-fold higher for a random classifier in all models other than the lasso model and 1.5-fold to 2-fold higher in the lasso model. Model discrimination was substantially higher when based on 10-fold cross-validation in the total sample (AUROC range, 0.824-0.883; AUPRC relative to the suicide rate range, 49.5-81.6) (eTable 8 in Supplement 1). Model calibration was best for the lasso model (ICI relative to the suicide rate range, 0.215-0.269) (Table 1).
Table 1. Estimated Area Under the Receiver Operating Characteristic Curve (AUROC) and Area Under the Precision Recall Curve (AUPRC) Relative to the Suicide Rate of the 5 Models Over a Range of Risk Horizons in the Prospective Validation Sample.
| Risk horizon | Estimate (SE) | ||||
|---|---|---|---|---|---|
| Lasso model | Base model | NLP model | SDOH model | Combined model | |
| AUROC | |||||
| 1 wk | 0.642 (0.271) | 0.770 (0.274) | 0.721 (0.297) | 0.775 (0.266) | 0.777 (0.259) |
| 1 mo | 0.655 (0.146) | 0.784 (0.132)a | 0.756 (0.144)a | 0.781 (0.132)a | 0.780 (0.142)a |
| 3 mo | 0.657 (0.097) | 0.763 (0.087)a | 0.730 (0.097)a | 0.756 (0.092)a | 0.774 (0.089)a |
| 6 mo | 0.668 (0.073)a | 0.744 (0.070)a | 0.726 (0.072)a | 0.743 (0.071)a | 0.757 (0.071)a |
| 12 mo | 0.665 (0.059)a | 0.728 (0.054)a | 0.723 (0.057)a | 0.726 (0.057)a | 0.747 (0.057)a |
| AUPRC relative to the suicide rate | |||||
| 1 wk | 1.556 (0.082)a | 3.926 (0.393)a | 3.489 (0.380)a | 4.038 (0.415)a | 4.955 (0.807)a |
| 1 mo | 1.675 (0.077)a | 5.026 (0.426)a | 3.757 (0.224)a | 4.996 (0.385)a | 4.342 (0.306)a |
| 3 mo | 1.756 (0.050)a | 3.671 (0.144)a | 3.285 (0.123)a | 3.676 (0.143)a | 3.837 (0.130)a |
| 6 mo | 1.796 (0.032)a | 3.750 (0.146)a | 3.688 (0.435)a | 6.398 (0.680)a | 4.170 (0.242)a |
| 12 mo | 1.859 (0.028)a | 3.281 (0.121)a | 3.189 (0.201)a | 5.342 (0.339)a | 4.897 (0.293)a |
| ICI relative to the suicide rateb | |||||
| Logistic | 0.269 | 0.712 | 0.587 | 0.902 | 0.600 |
| Isotonic | 0.215 | 0.866 | 0.431 | 1.401 | 0.629 |
Abbreviations: ICI, integrated calibration index; NLP, natural language processing; SDOH, social determinants of health.
Significantly higher than expected by chance at P < .05; 1-sided test when compared with a null model.
ICI relative to the suicide rate was estimated only for the 12-month models, as the predicted values from those models were used to estimate prevalence over all shorter risk horizons.
Standardized NB
The proportion of hospitalizations screened in at the optimal standardized NB decreased as the decision threshold increased and as the risk horizon decreased, from highs of 29.9% to 35.2% (capturing 69.2% to 71.4% of true suicides) at the lowest threshold to lows of 0.7% to 4.4% (capturing 6.8% to 23.7% of true suicides) at the highest threshold. Although the base model consistently had the highest standardized NB at the lowest decision threshold (Table 2), the combined model had the highest standardized NB at all higher decision thresholds for 6-month and 12-month risk horizons. Less consistency occurred for the 3-month horizon. All models had negative standardized NB for horizons less than 3 months (results not reported), implying that the number of true-positives was so small relative to the number of false-positives that the benefit of detecting and intervening to prevent suicides was outweighed by the cost of intervening with more than p/q false-positives per suicide.
Table 2. Estimated Net Benefit (NB) of the 5 Models Over a Range of Decision Thresholds and Risk Horizons in the Prospective Validation Sample.
| Decision thresholda | % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 12 mo | 6 mo | 3 mo | |||||||
| Total sample | True-positives | Standardized NB | Total sample | True-positives | Standardized NB | Total sample | True-positives | Standardized NB | |
| 150/100 000 | |||||||||
| Treat all patients | 100 | 100 | 42.9 | 100 | 100 | 8.3 | 100 | 100 | −52.9 |
| Lasso model | 60.5b | 83.0b | 48.4b | 60.5b | 84.9b | 29.5b | 60.5b | 82.3b | −10.1b |
| Base model | 35.2 | 69.2 | 49.2 | 35.2 | 72.2 | 40.1 | 29.9b | 71.4b | 25.9b |
| NLP model | 30.4 | 61.8 | 44.5 | 30.4 | 61.2 | 33.4 | 30.4 | 62.6 | 16.2 |
| SDOH model | 100b | 100b | 42.9b | 23.8 | 56.7 | 35.0 | 23.8 | 60.5 | 24.2 |
| Combined model | 100b | 100b | 42.9b | 17.6 | 51.8 | 35.8 | 17.6 | 52.4 | 25.5 |
| 200/100 000 | |||||||||
| Treat all patients | 100 | 100 | 23.8 | 100 | 100 | −22.3 | 100 | 100 | −103.9 |
| Lasso model | 60.6 | 83.0 | 36.8 | 36.2b | 56.3b | 12.2b | 36.2b | 54.4b | −19.3b |
| Base model | 29.4 | 61.8 | 39.5 | 29.4 | 65.3 | 29.4 | 17.9b | 49.0b | 12.5b |
| NLP model | 30.4 | 61.8 | 38.8 | 30.4 | 61.2 | 24.2 | 30.4 | 62.6 | 0.7 |
| SDOH model | 23.0 | 53.4 | 36.0 | 23.0 | 56.3 | 28.3 | 23.0 | 59.9 | 13.0 |
| Combined model | 40.7b | 75.6b | 44.7b | 17.6 | 51.8 | 30.4 | 17.6 | 52.4 | 16.6 |
| 300/100 000 | |||||||||
| Treat all patients | 100 | 100 | −14.3 | 100 | 100 | −83.6 | 100 | 100 | −206.2 |
| Lasso model | 15.8b | 35.1b | 17.1b | 15.4 | 33.9 | 5.6 | 15.4 | 32.0 | −15.3 |
| Base model | 10.1 | 30.0 | 18.5 | 10.1 | 34.3 | 15.8 | 10.1 | 35.4 | 4.5 |
| NLP model | 17.3 | 42.5 | 22.8 | 6.7b | 28.2b | 15.9b | 6.7b | 29.9b | 9.4b |
| SDOH model | 18.9 | 46.3 | 24.8 | 18.9 | 49.4 | 14.9 | 1.1b | 10.2b | 6.8b |
| Combined model | 12.1 | 40.2 | 26.5 | 12.1 | 41.6 | 19.6 | 4.8b | 25.2b | 10.5b |
| 400/100 000 | |||||||||
| Treat all patients | 100 | 100 | −52.6 | 100 | 100 | −145.1 | 100 | 100 | −308.7 |
| Lasso model | 7.4b | 18.6b | 7.3b | 7.4b | 17.1b | −1.0b | 7.4b | 17.7b | −12.6b |
| Base model | 10.1 | 30.0 | 14.7 | 10.1 | 34.3 | 9.6 | 6.1b | 23.1b | −1.6b |
| NLP model | 17.3 | 42.5 | 16.2 | 1.0b | 7.3b | 4.9b | 1.0b | 7.5b | 3.4b |
| SDOH model | 11.1 | 32.1 | 15.2 | 11.1 | 36.7 | 9.6 | 0.8b | 8.2b | 4.8b |
| Combined model | 12.1 | 40.2 | 21.9 | 12.1 | 41.6 | 12.2 | 1.9b | 12.2b | 4.4b |
| 500/100 000 | |||||||||
| Treat all patients | 100 | 100 | −91.0 | 100 | 100 | −206.6 | 100 | 100 | −411.4 |
| Lasso model | 4.7b | 12.2b | 3.3b | 3.7 | 8.6 | −2.8 | 3.7 | 8.8 | −10.1 |
| Base model | 9.9 | 29.5 | 10.8 | 4.3b | 18.4b | 5.2b | 4.3b | 19.0b | −2.9b |
| NLP model | 11.6 | 33.1 | 11.0 | 0.5b | 3.3b | 1.8b | 0.5b | 3.4b | 1.0b |
| SDOH model | 11.1 | 32.1 | 10.9 | 0.7b | 6.9b | 4.7b | 0.7b | 6.8b | 3.1b |
| Combined model | 4.4 | 22.9 | 14.6 | 4.4 | 23.7 | 10.3 | 1.3b | 8.2b | 1.6b |
Abbreviations: NLP, natural language processing; SDOH, social determinants of health.
For each decision threshold, the assumption is that the decision to implement a preventive intervention would be made only with patients who had a predicted risk above the threshold. See the eMethods in Supplement 1 for a discussion.
Logistic calibration. All others were isotonic calibration.
Predictor Importance
Significant cross-validated univariable associations (area under the curve greater than 0.50) with 12-month suicide in the training sample were found for 1666 base model predictors (21.1%), 442 NLP predictors (24.1%), and 29 LexisNexis SDOH predictors (6.6%) (Table 3). These predictors overlapped substantially, resulting in 80% of total model proportional SHAP being captured by the 100 most important predictors in the base model and 110 most important predictors in the NLP model. Only those most important predictors and the significant LexisNexis SDOH predictors (191 after excluding duplicates across models) were used to estimate the combined model.
Table 3. Predictors Used in the Analysis.
| Predictor | Distribution of potential predictors | Proportion and distribution of statistically significant predictors | Proportion and distribution of combined model predictors | |||||
|---|---|---|---|---|---|---|---|---|
| No. | Distribution, %a | No. | Proportion, %b | Distribution, %a | No. | Proportion, %b | Distribution, %a | |
| Total | 10 181 | 100 | 2137 | 21.0 | 100 | 191 | 8.9 | 100 |
| Psychopathological risk factors | ||||||||
| Diagnoses | 3364 | 33.0 | 649 | 19.3 | 30.4 | 12 | 1.8 | 6.3 |
| Treatments | 224 | 2.2 | 80 | 35.7 | 3.7 | 11 | 13.8 | 5.8 |
| Suicidality | 46 | 0.5 | 38 | 82.6 | 1.8 | 4 | 10.5 | 2.1 |
| Total | 3634 | 35.7 | 767 | 21.1 | 35.9 | 27 | 3.5 | 14.1 |
| Physical disorders | ||||||||
| Diagnoses | 3716 | 36.5 | 666 | 17.9 | 31.2 | 22 | 3.3 | 11.5 |
| Treatments | 230 | 2.3 | 96 | 41.7 | 4.5 | 7 | 7.3 | 3.7 |
| FDA medications increasing suicide risk | 28 | 0.3 | 21 | 75.0 | 1.0 | 3 | 14.3 | 1.6 |
| Total | 3974 | 39.0 | 783 | 19.7 | 36.6 | 32 | 4.1 | 16.8 |
| Facility-level quality indicators | ||||||||
| Total | 6 | 0.1 | 6 | 100 | 0.3 | 4 | 66.7 | 2.1 |
| SDOHc | ||||||||
| Geospatial indicators | 90 | 0.9 | 53 | 58.9 | 2.5 | 33 | 62.3 | 17.3 |
| LexisNexis public records | 442 | 4.3 | 29 | 6.6 | 1.4 | 29 | 100 | 15.2 |
| Patient-level factors (ICD-9-CM/ICD-10-CM codes) | 174 | 1.7 | 40 | 23.0 | 1.9 | 6 | 15.0 | 3.1 |
| Sociodemographic characteristics | 24 | 0.2 | 17 | 70.8 | 0.8 | 9 | 52.9 | 4.7 |
| Total | 733 | 7.2 | 142 | 19.4 | 6.6 | 80 | 56.3 | 41.9 |
| NLP term/topic frequency | ||||||||
| Terms | 1687 | 16.6 | 344 | 20.4 | 16.1 | 24 | 7.0 | 12.6 |
| Topics | 150 | 1.5 | 98 | 100 | 4.6 | 27 | 100 | 14.1 |
| Total | 1837 | 18.0 | 442 | 24.1 | 20.7 | 51 | 11.5 | 26.7 |
Abbreviations: FDA, US Food and Drug Administration; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; NLP, natural language processing; SDOH, social determinants of health.
Entries in the distribution columns represent the contribution of predictors in the row heading to the total in the column. The percentage estimates in each distribution column sum to 100%.
Entries in the proportion column represent the proportion of variation in the prior column for the same row that continue to exist in the current column. For example, the 649 significant psychiatric diagnoses in the first row and second column represent 19.3% of all the 3364 psychiatric diagnoses included in the initial potential predictor set, and the 12 psychiatric diagnoses in the final predictor set represent 1.8% of those 649 significant predictors.
Three of the NLP variables in the predictor set for the Combined model are included in this total as well as in the NLP total. One is the term trauma. The other 2 are topics in which the prominent terms are either homeless/shelter/homelessness/lack of housing or divorce/stressors.
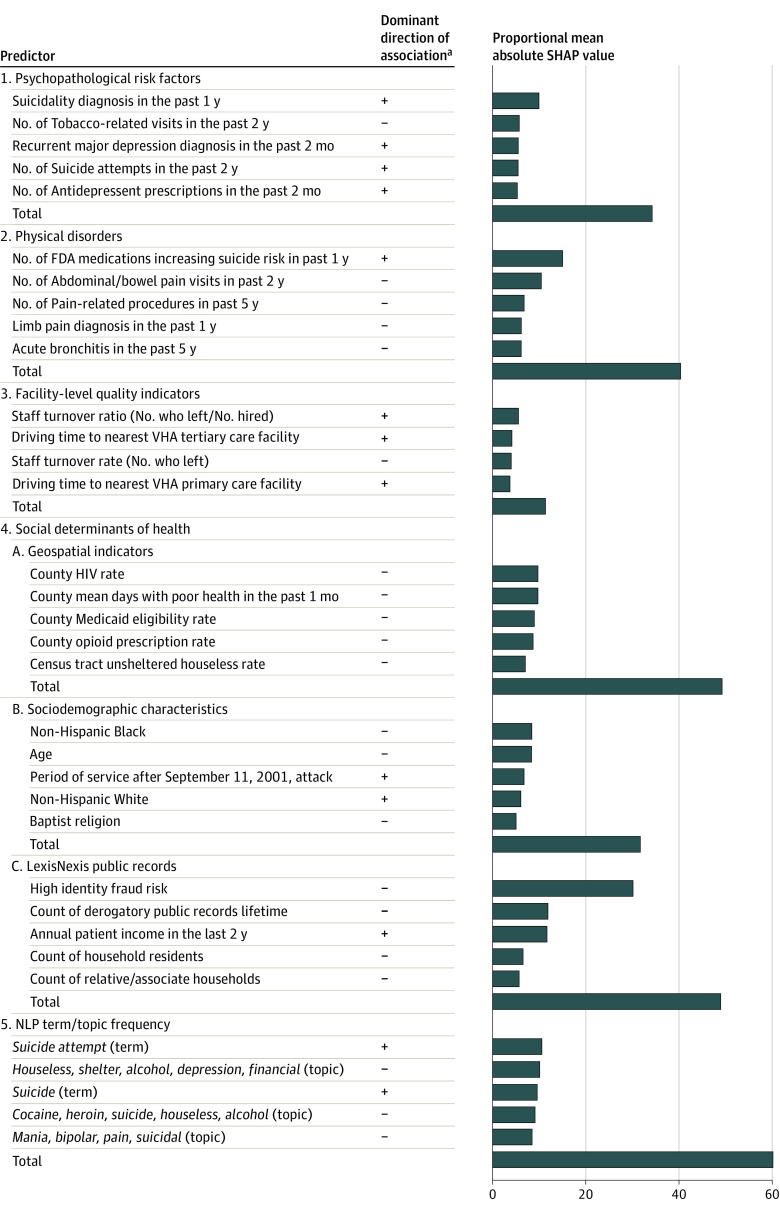
Class-level proportional SHAP for these important predictors in the combined model were highest for the NLP (64.0%) and SDOH (49.3%) predictors and lowest for facility-level quality indicators (11.4%) (Figure 2). These proportional SHAP values sum to more than 100% because most patients had some characteristics associated with increased suicide risk and others associated with decreased suicide risk. The key NLP predictors (ie, the 5 highest proportional SHAP values) included counts of the terms suicide attempt and suicide, both positively associated with suicide risk, and counts of several topic clusters (2 of them including the word suicide), all negatively associated with suicide risk. Component proportional SHAP for SDOH was higher for LexisNexis (proportional SHAP = 48.9%), geospatial (proportional SHAP = 48.4%), and sociodemographic (proportional SHAP = 31.7%) variables than patient-level ICD-9-CM/ICD-10-CM codes (proportional SHAP = 8.4%). The key LexisNexis predictors included 4 variables negatively associated with suicide risk (high identity fraud risk, derogatory public records, number of household residents, and number of relative/associate households on record) and 1 positively associated (patient income). Recent income changes were considered but were not key predictors. The key geospatial predictors, although involving disadvantage either at the county or census tract levels, all had negative associations with suicide risk. The key sociodemographics included being Non-Hispanic White and most recently in service after the September 11, 2001, attack (both positively associated with suicide risk) and age, being Non-Hispanic Black, and being Baptist (all negatively associated with suicide risk).
Figure 2. Dominant Direction of Association With Suicide and Proportional Mean Absolute Shapley Additive Explanations (SHAP) Values of Key Predictors of the Combined Model in the Prospective Validation Samplea,b.
Number of US Food and Drug Administration (FDA) indicates the total number of different medications prescribed with suicide as an adverse reaction in Boxed Warning, Warnings and Precautions, or Adverse Reactions sections of the FDA drug labeling document in the 1 year before hospitalization. Term indicates a count of the number of times a specific 1-, 2-, or 3-word string was mentioned in clinical notes over the 12 months prior to and during the hospitalization. Topic indicates a weighted (by distance of the term profile from the centroid of the topic) count of the number of times a series of terms defined by a multivariate term profile based on a Latent Dirichlet Allocation was mentioned in clinical notes over the 12 months prior to and during the hospitalization. NLP indicates Natural Language Processing; VHA, Veterans Health Administration.
aSHAP values for a specific predictor can differ for patients having the same score on the predictor because of interactions with other predictors, leading to variation in the sign of the association between the predictor and the outcome. The dominant direction of association recorded in the figure was based on visual inspection of the bee swarm plot in the eFigure in Supplement 1.
bKey predictors are defined as the 5 predictors in each class with the highest mean absolute SHAP values. See eTables 1-4 in Supplement 1 for a more detailed description of the predictor variables.
Interestingly, physical disorders had larger proportional SHAP values (40.3%) than psychopathological risk factors (34.2%). However, by far the single most important predictor in the physical disorder class was a count of medications classified by the FDA as increasing suicide risk prescribed in the year before hospitalization. Four key psychopathological predictors were positively associated with suicide risk: suicidality diagnosed in the past year, number of suicide attempts in the past 2 years, recent diagnoses of recurrent major depression, and recent antidepressant prescriptions. The fifth key psychopathological predictor was negatively associated with suicide risk: number of visits for tobacco-related disorders in the past 2 years. Facility-level quality indicators had the lowest effect (proportional SHAP = 11.4%), with the key predictors including past year staff turnover rate and ratio and driving time to the nearest VHA primary and tertiary care facilities.
Discussion
The objective of this study was to evaluate the effects of adding predictors from clinical notes extracted using NLP and from public records to a previously developed ML model to improve model performance before using the model in an experimental intensive postdischarge suicide prevention intervention. Results showed clearly that both types of predictors improved model prediction accuracy. We also carried out an analysis that documented a positive NB of the preferred model for plausible decision thresholds over risk horizons between 3 and 12 months. Although numerous prior studies used ML methods to predict suicides from electronic health records,35,36,37 suicides after hospital discharges were only seldom the focus,38 making it difficult to draw direct comparisons between our results and those of prior studies.
Our finding of positive NB speaks directly to the criticism that suicide prediction models are not actionable given the rarity of suicides among high-risk patients.39,40,41,42,43 As we7,44 and others45,46 have noted elsewhere, this criticism is misplaced because it focuses on the rarity of the outcome, whereas a more appropriate focus is NB. The positive NB we found is broadly consistent with findings in other areas of medicine that interventions for relatively rare but severe outcomes are actionable when the benefits of preventing the outcome far exceed the costs of intervention. For example, statins are recommended for adults aged 40 to 75 years with mildly elevated total cholesterol levels even though prevalence of extreme cardiovascular events over a 12-month risk horizon at the intervention decision threshold specified for statin use is no higher than prevalence of suicide at the decision thresholds over the same risk horizons that we considered here.47 Furthermore, postdischarge interventions, such as case management for patients at high suicide risk, have other benefits besides suicide prevention.48
The fact that NB was negative over short risk horizons, in comparison, is broadly consistent with previous research on imminent risk of suicidal behaviors.49 Importantly, though, this negative NB was not because our model did not predict imminent risk. Quite the contrary: AUROC was 0.777 for 1-week suicides. The negative NB instead reflected the high number of false-positives over short risk horizons relative to the number of true-positives. For example, a 1-week risk model with 75% sensitivity at 95% specificity, which would be considered excellent based on conventional criteria,34 would result in roughly 6000 false-positives for every true-positive, given the low 1-week prevalence of postdischarge suicides. No plausible cost-effectiveness scenario would justify implementing an intensive case management program if the only goal was to prevent suicides over such a short horizon.29 Fortunately, though, the model to predict 12-month suicides was also optimal for predicting imminent suicide risk, making it unnecessary to focus on separate segments of the patient population for short-term and longer-term prevention. This discovery is a valuable contribution to the field in that it both documents that prediction of imminent risk can be improved by using a longer risk horizon and shows that the cost-effectiveness of intervening with patients at high imminent risk could be increased to the extent that the intervention might reduce suicides over a much longer risk period.
Our finding that NLP and LexisNexis SDOH variables improve model prediction accuracy is broadly consistent with prior studies of NLP and SDOH variables predicting suicide,9,50,51,52,53 although to our knowledge, no prior studies investigated these associations among postdischarge psychiatric inpatients. Four results about these predictors are especially noteworthy in terms of potential clinical implications.
First, although the importance of SDOH predictors supports the recent emphasis on targeting SDOH for suicide prevention,54,55,56 caution is needed in interpreting these associations unequivocally as being causal given that modifiable SDOH predictors could also be noncausal risk markers.57 Second, the low proportional SHAP of psychopathological variables is most plausible interpretated as due to constrained variance in that all inpatients have serious psychiatric problems. This might also help explain the third noteworthy result: that many predictors typically considered risk factors for suicide had significant negative associations with suicide among recently discharged psychiatric inpatients (eg, pain-related physical disorders; NLP topics involving homelessness, substance use disorder, and bipolar disorder; public records involving fraud risk and derogatory public records), whereas income was positively associated with suicide risk. It is noteworthy that these predictors have the same signs in their zero-order associations with suicide among recent inpatients, whereas these zero-order associations are generally the opposite sign in the larger VHA population. At least 2 possibilities exist for this pattern: (1) the clinical severity threshold might be lower for hospitalizing patients with these disadvantaged characteristics than other patients due to these disadvantaged characteristics themselves increasing probability of hospitalization, resulting in lower suicide risk among hospitalized patients with these characteristics and/or (2) postdischarge interventions are more intensive for patients with these disadvantaged characteristics than other patients. Both possibilities warrant further study given their implications for changing clinical practice guidelines. Fourth, the highest positive variable-level proportional SHAP was for a count of medications prescribed to the patient in the prior year classified by the FDA as increasing suicide risk. It was impossible to disaggregate this association to specific medications given that each medication was prescribed only rarely, but it is noteworthy that other potential predictors that did not have strong proportional SHAP values were counts of prescribed antidepressants and other types of psychotropic medications. Further disaggregation of these medications might be possible in the total VHA patient population, though, and should be carried out in future studies.
Limitations
This study has limitations. First, absence of consensus on the minimum level of risk needed to justify implementing an aggressive suicide prevention intervention made it impossible to select a single decision threshold for the NB analysis. Presenting results across a plausible range of thresholds, as we did here, is recommended in cases where such uncertainty exists.30 Second, generalization beyond the VHA is likely to be low because of the unique characteristics of veterans, the comparatively high access to high-quality care enjoyed by VHA patients, and the fact that some administrative predictors in our model are unavailable in health systems other than the VHA. Third, prospective generalization within the VHA over time is also uncertain, as the model was trained and validated during years well before the COVID-19 pandemic and the changes in health care associated with the pandemic. We will be able to investigate this issue by applying the model to the training sample of our trial.
Conclusions
Within the context of these limitations, we found that improvements could be made to a standard ML model for postdischarge suicides by adding predictors extracted from clinical notes and public records. The expanded model had positive NB at plausible decision thresholds for risk horizons of 3 to 12 months. Several results regarding important predictors might have clinical implications, but further research is needed on these possibilities before such implications would be actionable.
eMethods.
eTable 1. Baseline Administrative Predictors
eTable 2. Social Determinants of Health: Geospatial Indicators
eTable 3. Social Determinants of Health: LexisNexis Predictors
eTable 4. Medications Classified by FDA as Increasing Risk of Suicide
eTable 5. Classifiers Used in the Super Learner Ensemble
eTable 6. Distributions of Sociodemographic and Military Career Characteristics
eTable 7. Prevalence of Suicide After Psychiatric Hospital Discharge in the Total, Training, and Prospective Validation Samples
eTable 8. Ten-Fold Cross-Validated AUC-ROC and AUC-PR of the Combined Model Over a Range of Risk Horizons in the Total Sample
eTable 9. Metalearner Weights for the Combined Model Estimated in the 10% Metalearner Weight Training Sample
eFigure. Bee swarm plot
eReferences.
Data Sharing Statement
References
- 1.Heron M. Deaths: leading causes for 2016. Accessed July 26, 2022. https://www.cdc.gov/nchs/data/nvsr/nvsr67/nvsr67_06.pdf
- 2.Ahmedani BK, Simon GE, Stewart C, et al. Health care contacts in the year before suicide death. J Gen Intern Med. 2014;29(6):870-877. doi: 10.1007/s11606-014-2767-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung DT, Ryan CJ, Hadzi-Pavlovic D, Singh SP, Stanton C, Large MM. Suicide rates after discharge from psychiatric facilities: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(7):694-702. doi: 10.1001/jamapsychiatry.2017.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matarazzo BB, Farro SA, Billera M, Forster JE, Kemp JE, Brenner LA. Connecting veterans at risk for suicide to care through the HOME program. Suicide Life Threat Behav. 2017;47(6):709-717. doi: 10.1111/sltb.12334 [DOI] [PubMed] [Google Scholar]
- 5.Miller IW, Gaudiano BA, Weinstock LM. The coping long term with active suicide program: description and pilot data. Suicide Life Threat Behav. 2016;46(6):752-761. doi: 10.1111/sltb.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley B, Brown GK, Brenner LA, et al. Comparison of the safety planning intervention with follow-up vs usual care of suicidal patients treated in the emergency department. JAMA Psychiatry. 2018;75(9):894-900. doi: 10.1001/jamapsychiatry.2018.1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler RC, Bauer MS, Bishop TM, et al. Using administrative data to predict suicide after psychiatric hospitalization in the Veterans Health Administration system. Front Psychiatry. 2020;11:390. doi: 10.3389/fpsyt.2020.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veterans Coordinated Community Care (3C) Study (3C). ClinicalTrials.gov identifier: NCT05272176. Updated October 31, 2022. Accessed August 2, 2022. https://www.clinicaltrials.gov/ct2/show/NCT05272176?term=3C&draw=2&rank=2
- 9.Cohen J, Wright-Berryman J, Rohlfs L, Trocinski D, Daniel L, Klatt TW. Integration and validation of a natural language processing machine learning suicide risk prediction model based on open-ended interview language in the emergency department. Front Digit Health. 2022;4:818705. doi: 10.3389/fdgth.2022.818705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blosnich JR, Montgomery AE, Dichter ME, et al. Social determinants and military veterans’ suicide ideation and attempt: a cross-sectional analysis of electronic health record data. J Gen Intern Med. 2020;35(6):1759-1767. doi: 10.1007/s11606-019-05447-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Centers for Disease Control and Prevention . National Death Index. Accessed March 2, 2022. https://www.cdc.gov/nchs/ndi/index.htm
- 12.Collins GS, Reitsma JB, Altman DG, Moons KG; TRIPOD Group . Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Circulation. 2015;131(2):211-219. doi: 10.1161/CIRCULATIONAHA.114.014508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Department of Veterans Affairs . Corporate Data Warehouse (CDW). Accessed March 1, 2022. https://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm
- 14.Hoffmire C, Stephens B, Morley S, Thompson C, Kemp J, Bossarte RM. VA suicide prevention applications network: a national health care system-based suicide event tracking system. Public Health Rep. 2016;131(6):816-821. doi: 10.1177/0033354916670133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong MS, Steers WN, Hoggatt KJ, Ziaeian B, Washington DL. Relationship of neighborhood social determinants of health on racial/ethnic mortality disparities in US veterans—mediation and moderating effects. Health Serv Res. 2020;55(suppl 2):851-862. doi: 10.1111/1475-6773.13547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VA Health Services Research and Development Service; Internet Archive . Consortium for Healthcare Informatics Research (CHIR). Accessed February 22, 2022. https://web.archive.org/web/20221028115247/https:/www.hsrd.research.va.gov/for_researchers/chir.cfm
- 17.LexisNexis Risk Solutions Group . Social determinants of health. Accessed March 2, 2022. https://risk.lexisnexis.com/healthcare/social-determinants-of-health
- 18.Bickley H, Hunt IM, Windfuhr K, Shaw J, Appleby L, Kapur N. Suicide within two weeks of discharge from psychiatric inpatient care: a case-control study. Psychiatr Serv. 2013;64(7):653-659. doi: 10.1176/appi.ps.201200026 [DOI] [PubMed] [Google Scholar]
- 19.Large M, Sharma S, Cannon E, Ryan C, Nielssen O. Risk factors for suicide within a year of discharge from psychiatric hospital: a systematic meta-analysis. Aust N Z J Psychiatry. 2011;45(8):619-628. doi: 10.3109/00048674.2011.590465 [DOI] [PubMed] [Google Scholar]
- 20.Park S, Choi JW, Kyoung Yi K, Hong JP. Suicide mortality and risk factors in the 12 months after discharge from psychiatric inpatient care in Korea: 1989-2006. Psychiatry Res. 2013;208(2):145-150. doi: 10.1016/j.psychres.2012.09.039 [DOI] [PubMed] [Google Scholar]
- 21.Troister T, Links PS, Cutcliffe J. Review of predictors of suicide within 1 year of discharge from a psychiatric hospital. Curr Psychiatry Rep. 2008;10(1):60-65. doi: 10.1007/s11920-008-0011-8 [DOI] [PubMed] [Google Scholar]
- 22.Bachmann S. Epidemiology of suicide and the psychiatric perspective. Int J Environ Res Public Health. 2018;15(7):1425. doi: 10.3390/ijerph15071425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klonsky ED, May AM, Saffer BY. Suicide, suicide attempts, and suicidal ideation. Annu Rev Clin Psychol. 2016;12:307-330. doi: 10.1146/annurev-clinpsy-021815-093204 [DOI] [PubMed] [Google Scholar]
- 24.O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1(1):73-85. doi: 10.1016/S2215-0366(14)70222-6 [DOI] [PubMed] [Google Scholar]
- 25.van der Laan MJ, Rose S. Targeted Learning: Casual Inference for Observational and Experimental Data. Springer; 2011. [Google Scholar]
- 26.Polley E, LeDell E, Kennedy C, Lendle S, van der Laan M. SuperLearner: super learner prediction. Version 2.0-24. Accessed February 18, 2022. https://cran.r-project.org/web/packages/SuperLearner/index.html
- 27.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1-22. doi: 10.18637/jss.v033.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res. 2019;3:18. doi: 10.1186/s41512-019-0064-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross EL, Zuromski KL, Reis BY, Nock MK, Kessler RC, Smoller JW. Accuracy requirements for cost-effective suicide risk prediction among primary care patients in the US. JAMA Psychiatry. 2021;78(6):642-650. doi: 10.1001/jamapsychiatry.2021.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wynants L, van Smeden M, McLernon DJ, Timmerman D, Steyerberg EW, Van Calster B; Topic Group ‘Evaluating Diagnostic Tests and Prediction Models’ of the STRATOS Initiative . Three myths about risk thresholds for prediction models. BMC Med. 2019;17(1):192. doi: 10.1186/s12916-019-1425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC, Steyerberg EW. The Integrated Calibration Index (ICI) and related metrics for quantifying the calibration of logistic regression models. Stat Med. 2019;38(21):4051-4065. doi: 10.1002/sim.8281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varma S, Simon R. Bias in error estimation when using cross-validation for model selection. BMC Bioinformatics. 2006;7:91. doi: 10.1186/1471-2105-7-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundberg SM, Lee SI. A unified approach to interpreting model predictions. Accessed August 4, 2022. https://dl.acm.org/doi/10.5555/3295222.3295230
- 34.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed. Wiley; 2013. doi: 10.1002/9781118548387 [DOI] [Google Scholar]
- 35.Grendas LN, Chiapella L, Rodante DE, Daray FM. Comparison of traditional model-based statistical methods with machine learning for the prediction of suicide behaviour. J Psychiatr Res. 2021;145:85-91. doi: 10.1016/j.jpsychires.2021.11.029 [DOI] [PubMed] [Google Scholar]
- 36.Kirtley OJ, van Mens K, Hoogendoorn M, Kapur N, de Beurs D. Translating promise into practice: a review of machine learning in suicide research and prevention. Lancet Psychiatry. 2022;9(3):243-252. doi: 10.1016/S2215-0366(21)00254-6 [DOI] [PubMed] [Google Scholar]
- 37.Lejeune A, Le Glaz A, Perron PA, et al. Artificial intelligence and suicide prevention: a systematic review. Eur Psychiatry. 2022;65(1):1-22. doi: 10.1192/j.eurpsy.2022.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang T, Rosellini AJ, Horváth-Puhó E, et al. Using machine learning to predict suicide in the 30 days after discharge from psychiatric hospital in Denmark. Br J Psychiatry. 2021;219(2):440-447. doi: 10.1192/bjp.2021.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolton JM. Suicide risk assessment in the emergency department: out of the darkness. Depress Anxiety. 2015;32(2):73-75. doi: 10.1002/da.22320 [DOI] [PubMed] [Google Scholar]
- 40.Hoge CW. Suicide reduction and research efforts in service members and veterans-sobering realities. JAMA Psychiatry. 2019;76(5):464-466. doi: 10.1001/jamapsychiatry.2018.4564 [DOI] [PubMed] [Google Scholar]
- 41.Mulder R, Newton-Howes G, Coid JW. The futility of risk prediction in psychiatry. Br J Psychiatry. 2016;209(4):271-272. doi: 10.1192/bjp.bp.116.184960 [DOI] [PubMed] [Google Scholar]
- 42.Owens D, Kelley R. Predictive properties of risk assessment instruments following self-harm. Br J Psychiatry. 2017;210(6):384-386. doi: 10.1192/bjp.bp.116.196253 [DOI] [PubMed] [Google Scholar]
- 43.Large M, Myles N, Myles H, et al. Suicide risk assessment among psychiatric inpatients: a systematic review and meta-analysis of high-risk categories. Psychol Med. 2018;48(7):1119-1127. doi: 10.1017/S0033291717002537 [DOI] [PubMed] [Google Scholar]
- 44.Kessler RC. Clinical epidemiological research on suicide-related behaviors—where we are and where we need to go. JAMA Psychiatry. 2019;76(8):777-778. doi: 10.1001/jamapsychiatry.2019.1238 [DOI] [PubMed] [Google Scholar]
- 45.Simon GE, Shortreed SM, Coley RY. Positive predictive values and potential success of suicide prediction models. JAMA Psychiatry. 2019;76(8):868-869. doi: 10.1001/jamapsychiatry.2019.1516 [DOI] [PubMed] [Google Scholar]
- 46.Kennedy CJ, Mark DG, Huang J, van der Laan MJ, Hubbard AE, Reed ME. Development of an ensemble machine learning prognostic model to predict 60-day risk of major adverse cardiac events in adults with chest pain. medRxiv. Preprint posted online March 13, 2021. doi: 10.1101/2021.03.08.21252615 [DOI]
- 47.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 48.McCarthy JF, Cooper SA, Dent KR, et al. Evaluation of the recovery engagement and coordination for health-veterans enhanced treatment suicide risk modeling clinical program in the Veterans Health Administration. JAMA Netw Open. 2021;4(10):e2129900. doi: 10.1001/jamanetworkopen.2021.29900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribeiro JD, Huang X, Fox KR, Walsh CG, Linthicum KP. Predicting imminent suicidal thoughts and nonfatal attempts: the role of complexity. Clin Psychol Sci. 2019;7(5):941-957. doi: 10.1177/2167702619838464 [DOI] [Google Scholar]
- 50.Levis M, Leonard Westgate C, Gui J, Watts BV, Shiner B. Natural language processing of clinical mental health notes may add predictive value to existing suicide risk models. Psychol Med. 2021;51(8):1382-1391. doi: 10.1017/S0033291720000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levis M, Levy J, Dufort V, Gobbel GT, Watts BV, Shiner B. Leveraging unstructured electronic medical record notes to derive population-specific suicide risk models. Psychiatry Res. 2022;315:114703. doi: 10.1016/j.psychres.2022.114703 [DOI] [PubMed] [Google Scholar]
- 52.Alemi F, Avramovic S, Renshaw KD, Kanchi R, Schwartz M. Relative accuracy of social and medical determinants of suicide in electronic health records. Health Serv Res. 2020;55(suppl 2):833-840. doi: 10.1111/1475-6773.13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blosnich JR, Montgomery AE, Taylor LD, Dichter ME. Adverse social factors and all-cause mortality among male and female patients receiving care in the Veterans Health Administration. Prev Med. 2020;141:106272. doi: 10.1016/j.ypmed.2020.106272 [DOI] [PubMed] [Google Scholar]
- 54.Blanco C, Wall MM, Olfson M. A population-level approach to suicide prevention. JAMA. 2021;325(23):2339-2340. doi: 10.1001/jama.2021.6678 [DOI] [PubMed] [Google Scholar]
- 55.Iskander JK, Crosby AE. Implementing the national suicide prevention strategy: time for action to flatten the curve. Prev Med. 2021;152(pt 1):106734. doi: 10.1016/j.ypmed.2021.106734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Academies of Sciences, Engineering, and Medicine; Division of Behavioral and Social Sciences and Education; Board on Behavioral, Cognitive, and Sensory Sciences ; Yoder L, eds. Community Interventions to Prevent Veteran Suicide: The Role of Social Determinants: Proceedings of a Virtual Symposium. National Academies Press; 2022. [PubMed] [Google Scholar]
- 57.Kraemer HC, Kazdin AE, Offord DR, Kessler RC, Jensen PS, Kupfer DJ. Coming to terms with the terms of risk. Arch Gen Psychiatry. 1997;54(4):337-343. doi: 10.1001/archpsyc.1997.01830160065009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Baseline Administrative Predictors
eTable 2. Social Determinants of Health: Geospatial Indicators
eTable 3. Social Determinants of Health: LexisNexis Predictors
eTable 4. Medications Classified by FDA as Increasing Risk of Suicide
eTable 5. Classifiers Used in the Super Learner Ensemble
eTable 6. Distributions of Sociodemographic and Military Career Characteristics
eTable 7. Prevalence of Suicide After Psychiatric Hospital Discharge in the Total, Training, and Prospective Validation Samples
eTable 8. Ten-Fold Cross-Validated AUC-ROC and AUC-PR of the Combined Model Over a Range of Risk Horizons in the Total Sample
eTable 9. Metalearner Weights for the Combined Model Estimated in the 10% Metalearner Weight Training Sample
eFigure. Bee swarm plot
eReferences.
Data Sharing Statement