This study examines the effect of mavacamten on exercise physiology using cardiopulmonary exercise testing.
Key Points
Question
What are the effects of mavacamten on peak and submaximal cardiopulmonary exercise testing (CPET) parameters?
Findings
This prespecified exploratory analysis of the EXPLORER-HCM study shows that mavacamten improved a range of CPET parameters beyond peak oxygen uptake, including measures at both peak and subpeak exercise.
Meaning
The favorable effect of mavacamten on submaximal exertional tolerance provides further insights into the beneficial impact of mavacamten.
Abstract
Importance
Mavacamten, a cardiac myosin inhibitor, improved peak oxygen uptake (pVO2) in patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM) in the EXPLORER-HCM study. However, the full extent of mavacamten’s effects on exercise performance remains unclear.
Objective
To investigate the effect of mavacamten on exercise physiology using cardiopulmonary exercise testing (CPET).
Design, Setting, and Participants
Exploratory analyses of the data from the EXPLORER-HCM study, a randomized, double-blind, placebo-controlled, phase 3 trial that was conducted in 68 cardiovascular centers in 13 countries. In total, 251 patients with symptomatic obstructive HCM were enrolled.
Interventions
Patients were randomly assigned in a 1:1 ratio to mavacamten or placebo.
Main Outcomes and Measures
The following prespecified exploratory cardiovascular and performance parameters were assessed with a standardized treadmill or bicycle ergometer test protocol at baseline and week 30: carbon dioxide output (VCO2), minute ventilation (VE), peak VE/VCO2 ratio, ventilatory efficiency (VE/VCO2 slope), peak respiratory exchange ratio (RER), peak circulatory power, ventilatory power, ventilatory threshold, peak metabolic equivalents (METs), peak exercise time, partial pressure of end-tidal carbon dioxide (PETCO2), and VO2/workload slope.
Results
Two hundred fifty-one patients were enrolled. The mean (SD) age was 58.5 (11.9) years and 59% of patients were male. There were significant improvements with mavacamten vs placebo in the following peak-exercise CPET parameters: peak VE/VCO2 ratio (least squares [LS] mean difference, −2.2; 95% CI, −3.05 to −1.26; P < .001), peak METs (LS mean difference, 0.4; 95% CI, 0.17-0.60; P < .001), peak circulatory power (LS mean difference, 372.9 mL/kg/min × mm Hg; 95% CI, 153.12-592.61; P = .001), and peak PETCO2 (LS mean difference, 2.0 mm Hg; 95% CI, 1.12-2.79; P < .001). Mavacamten also improved peak exercise time compared with placebo (LS mean difference, 0.7 minutes; 95% CI, 0.13-1.24; P = .02). There was a significant improvement in nonpeak-exercise CPET parameters, such as VE/VCO2 slope (LS mean difference, −2.6; 95% CI, −3.58 to −1.52; P < .001) and ventilatory power (LS mean difference, 0.6 mm Hg; 95% CI, 0.29-0.90; P < .001) favoring mavacamten vs placebo.
Conclusions and Relevance
Mavacamten improved a range of CPET parameters beyond pVO2, indicating consistent and broad benefits on maximal exercise capacity. Although improvements in peak-exercise CPET parameters are clinically meaningful, the favorable effects of mavacamten on submaximal exertional tolerance provide further insights into the beneficial impact of mavacamten in patients with obstructive HCM.
Trial Registration
ClinicalTrials.gov Identifier: NCT03470545
Introduction
Hypertrophic cardiomyopathy (HCM) is a complex disease that is caused by excessive cardiac myosin-actin cross-bridging with core pathophysiologic features that include left ventricular hypertrophy, hypercontractility, and multifactorial diastolic dysfunction.1,2,3,4,5 Consequently, HCM can be a debilitating disease resulting in impaired functionality and quality of life.6 The most common symptoms of HCM are dyspnea, fatigue, palpitations, chest pain, lightheadedness, and syncope.7 The burden of disease experienced by patients includes limitations to physical activity and daily activities, negative effects on emotional state, and adverse effects on work productivity.7
Cardiopulmonary exercise testing (CPET) is a useful, noninvasive tool to assess functional capacity and understand the underlying mechanisms of exercise intolerance. Given that most patients with HCM do not exercise or exert themselves to peak exercise capacity, the ability of CPET to assess both peak and submaximal exercise responses makes it a unique and powerful indicator of what a patient can physically achieve in their daily activities.8,9 Furthermore, CPET is a valuable method to estimate prognosis in patients with HCM.10 Indeed, peak oxygen uptake (pVO2), and ventilatory efficiency (minute ventilation [VE]/carbon dioxide output [VCO2] slope) have been independently associated with overall mortality, death due to heart failure, sudden cardiac death, heart transplant, and functional deterioration.11,12,13,14,15 Furthermore, circulatory power has been shown to be an independent predictor of death or hospitalization due to heart failure, heart transplant, and progression to New York Heart Association (NYHA) functional class III or IV.15
Mavacamten is a first-in-class, small-molecule, selective inhibitor of cardiac myosin that targets the underlying pathophysiology of HCM.16,17 In the EXPLORER-HCM study (NCT03470545), treatment with mavacamten led to significant and clinically meaningful improvements in symptoms, functional capacity, left ventricular outflow tract (LVOT) gradients, biomarkers, and key aspects of quality of life in patients with symptomatic obstructive HCM.18 More specifically, mavacamten was associated with a significant improvement in pVO2 at week 30 compared with placebo.
The objective of this prespecified exploratory analysis of EXPLORER-HCM was to evaluate additional maximal and submaximal parameters from CPET, beyond pVO2, to examine the effects of mavacamten on exercise physiology in patients with obstructive HCM, and to expand our understanding of this disease.
Methods
Study Design
The details of the EXPLORER-HCM trial design and primary results have been reported elsewhere.18,19 The trial protocol and statistical analysis plan are available in Supplement 2 and Supplement 3, respectively. In brief, EXPLORER-HCM was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Patients were randomized 1:1 to receive mavacamten (starting dose, 5 mg) or placebo for 30 weeks. Study drug (mavacamten or matching placebo, which were identical in appearance) was labeled with a unique identifying number that was assigned to a patient through the interactive response system. The principal investigator, site staff, and participants were masked. Randomization was stratified by current β-blocker use (yes or no), NYHA functional class (II or III), ergometer type (treadmill or bicycle), and consent for cardiovascular magnetic resonance imaging substudy (yes or no). Key inclusion criteria included being 18 years of age or older, having a diagnosis of obstructive HCM (unexplained left ventricular hypertrophy with maximal left ventricular wall thickness of 15 mm or more [or 13 mm or more with a family history of HCM]), having at least 1 peak LVOT gradient of 50 mm Hg or higher at rest or with provocation (Valsalva maneuver or after exercise), having a Valsalva LVOT gradient of at least 30 mm Hg, having a left ventricular ejection fraction of at least 55%, and being able to perform upright CPET with a respiratory exchange ratio (RER) of 1.0 or more or between 0.91 and 1.0 if peak exercise was achieved, as assessed by the core laboratory. Key exclusion criteria included a history of syncope or sustained ventricular tachyarrhythmia with exercise in the 6 months before screening, atrial fibrillation present at screening, and treatment or planned treatment with disopyramide, ranolazine, or a combination of β-blockers and nondihydropyridine calcium channel blockers.
Outcomes and Data Collection
CPET was conducted using a standardized treadmill or bicycle ergometer test protocol at screening and at week 30 prior to dosing with the study drug. The same modality (treadmill or upright bicycle) and the same time of day (as much as possible) were used for all CPET assessments conducted for a given patient. The treadmill protocol was a modified Naughton protocol and the cycle ergometer protocol was an incremental protocol with increases in work rate of 25 watts every 2 minutes. Site technicians were trained and certified for the protocol and followed a detailed CPET manual. Supine testing using a recumbent bicycle was not allowed and symptom-limited exercise tests were performed after a 4-hour fast. In addition to pVO2, which was a component of the composite primary end point and a prespecified secondary efficacy end point, the following prespecified exploratory cardiovascular and performance parameters were assessed: VCO2, minute ventilation (VE), peak VE/VCO2 ratio, VE/VCO2 slope (determined from rest to peak exercise), peak RER, peak circulatory power (pVO2 × peak systolic blood pressure), ventilatory power (peak systolic blood pressure/VE/VCO2 slope), peak metabolic equivalents (METs), partial pressure of end-tidal carbon dioxide (PETCO2), peak exercise time, ventilatory threshold, and VO2/workload slope. Per protocol, participants receiving β-blockers or calcium channel blockers should have been receiving the same dose at screening and at the end-of-treatment CPET, whenever possible, and these medications were administered prior to CPET. CPET data were analyzed centrally by the core laboratory (Cardiovascular and Metabolic Disease Research Institute, Mountain View, California). Reference values for some key CPET parameters are presented in eTable 1 in Supplement 1.
Statistical Analysis
The end points were summarized by treatment group and visit using descriptive statistics in the intention-to-treat population, defined as all randomized patients, regardless of whether they received the study drug. The estimates of treatment-group differences of the change from baseline to week 30 and the 95% CIs based on normal approximation were calculated. Between-group comparisons were based on analysis of covariance and all statistical tests were conducted at a 2-sided significance α level of .05. Treatment group (mavacamten vs placebo), baseline value of the corresponding end point of interest, and the 3 stratification factors (β-blocker use, NYHA class, and ergometer type) were treated as fixed effects. Least squares (LS) mean differences of the CPET parameters were tested separately and the P values generated from exploratory analyses were not adjusted for multiplicity and are considered to be nominal (descriptive). Owing to the low rate of discontinuation, missing data were not imputed. Post hoc correlations (pVO2 vs N-terminal pro-brain natriuretic peptide [NT-proBNP], VE/VCO2 slope vs NT-proBNP, and VE/VCO2 slope vs Kansas City Cardiomyopathy Clinical Summary Score [KCCQ-CSS]) were analyzed in the intention-to-treat population using changes in mean values from baseline to week 30 for the relevant outcomes. Nominal P values were calculated for the test of correlations. SAS version 9.4 (SAS Institute) was used for statistical analyses.
Results
Patient Population
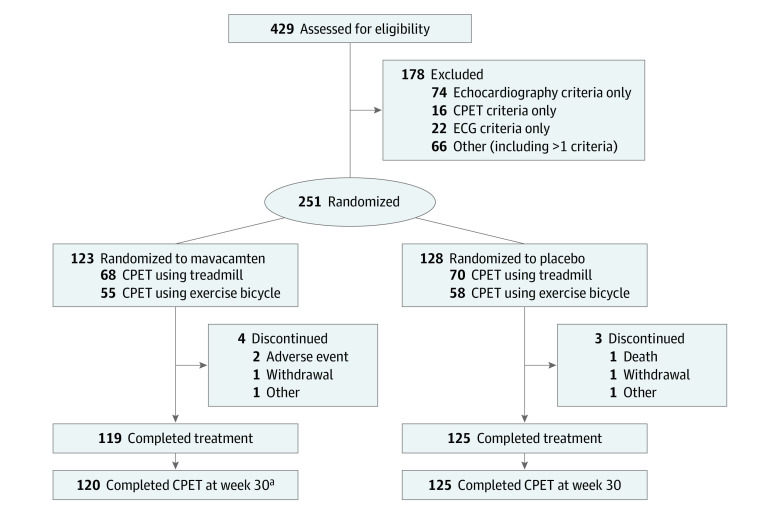
Patient demographics and baseline characteristics in the EXPLORER-HCM study have been described in detail previously and are presented in Table 1.18,19 In summary, from May 30, 2018, to July 12, 2019, 429 patients were screened and 251 were randomized to receive mavacamten (n = 123) or placebo (n = 128) (Figure 1). The mean (SD) age of the patient population was 58.5 (11.9) years and 59% of patients were male. Most patients (183 of 251 ) had NYHA class II symptoms at baseline (73%) and most were receiving background HCM therapy (β-blockers: 94 of 123 patients for mavacamten [76%] and 95 of 128 patients for placebo [74%]; calcium channel blockers: 25 of 123 patients for mavacamten [20%] and 17 of 128 patients for placebo [13%]; Table 1). In both treatment arms, 45% of patients performed CPET using an exercise bicycle and 55% using a treadmill (Figure 1). Baseline CPET parameter values were similar for both treatment groups (Table 1) and consistent with values reported previously for patients with HCM.10,20 Heart rate and systolic blood pressure at rest and at peak exercise are summarized in eTable 2 in Supplement 1.
Table 1. Demographics and Baseline Characteristicsa.
| Demographics and baseline characteristics | No. (%) | |
|---|---|---|
| Mavacamten (n = 123) | Placebo (n = 128) | |
| Age, mean (SD), y | 58.5 (12.2) | 58.5 (11.8) |
| Sex | ||
| Male | 66 (54) | 83 (65) |
| Female | 57 (46) | 45 (35) |
| Background HCM therapy | ||
| β-Blocker | 94 (76) | 95 (74) |
| Calcium channel blocker | 25 (20) | 17 (13) |
| Neither | 4 (3) | 16 (13) |
| Heart rate, mean (SD), bpm | 63 (10.1) | 62 (10.6) |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 128.4 (16.2) | 128.4 (14.6) |
| Diastolic | 75.5 (10.8) | 76.1 (9.9) |
| NYHA functional class | ||
| II | 88 (72) | 95 (74) |
| III | 35 (28) | 33 (26) |
| Peak-exercise CPET parameters, mean (SD) | ||
| Peak VO2, mL/kg/min | 18.9 (4.9) | 19.9 (4.9) |
| Peak VE/VCO2 ratio | 35.4 (5.2) | 34.2 (5.5) |
| Peak METs | 5.4 (1.4) | 5.7 (1.4) |
| Peak circulatory power, mL/kg/min × mm Hg | 3087.1 (1165.2) | 3284.8 (1173.3) |
| Peak exercise time, min | 10.1 (4.0) | 10.5 (4.2) |
| Peak RER | 1.1 (0.1) | 1.1 (0.1) |
| Peak PETCO2, mm Hg | 33.7 (4.5) | 34.7 (5.1) |
| Nonpeak-exercise CPET parameters, mean (SD) | ||
| VE/VCO2 slope | 33.6 (6.2) | 32.4 (6.2) |
| Ventilatory power, mm Hg | 4.9 (1.4) | 5.2 (1.5) |
| Ventilatory threshold (ie, anaerobic threshold), mL/kg/min | 11.5 (2.3) | 11.5 (2.6) |
| PETCO2 at rest, mm Hg | 32.5 (4.2) | 33.0 (4.3) |
| VO2/workload slope | 0.75 (0.25) | 0.76 (0.26) |
| Biomarkers, geometric mean (CV%), ng/L | ||
| NT-proBNP | 777 (136) | 616 (108) |
| High-sensitivity cardiac troponin I | 12.5 (208) | 12.5 (373) |
| Echocardiographic parameters, mean (SD) | ||
| LVOT gradient, mm Hg | ||
| Rest | 52 (29) | 51 (32) |
| Valsalva | 72 (32) | 74 (32) |
| Postexercise | 86 (34) | 84 (36) |
Abbreviations: bpm, beats per minute; CPET, cardiopulmonary exercise testing; CV, coefficient of variation; HCM, hypertrophic cardiomyopathy; LVOT, left ventricular outflow tract; MET, metabolic equivalent; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PETCO2, partial pressure of end-tidal carbon dioxide; RER, respiratory exchange ratio; VCO2, carbon dioxide output; VE, minute ventilation; VO2, oxygen uptake.
β-blocker use and NYHA function classification are based on information captured on electronic case report forms. The heart rate and SBP data presented here are from vital signs as opposed to the data presented in eTable 2 in Supplement 1, which present data measured during the CPET assessment.
Figure 1. CONSORT Diagram.
All randomized patients received at least 1 dose of study drug and were included in the efficacy analyses. Two patients who discontinued for other reasons (mavacamten, n = 1; placebo, n = 1) did not complete the week-30 visits within the window owing to scheduling issues. CPET indicates cardiopulmonary exercise testing; ECG, electrocardiogram.
aOne patient discontinued treatment, but performed the CPET assessment at week 30.
CPET Parameters
Peak-Exercise CPET Parameters
Compared with placebo, mavacamten improved peak VE/VCO2 ratio (LS mean difference, −2.2; 95% CI, −3.05 to −1.26; P < .001), peak METs (LS mean difference, 0.4; 95% CI, 0.17-0.60; P < .001), peak circulatory power (LS mean difference, 372.9 mL/kg/min × mm Hg; 95% CI, 153.12-592.61 mL/kg/min × mm Hg; P = .001), peak exercise time (LS mean difference, 0.7 minutes; 95% CI, 0.13-1.24 minutes; P = .02), and peak PETCO2 (LS mean difference, 2.0 mm Hg; 95% CI, 1.12-2.79 mm Hg; P < .001) (Figure 2, Table 2). There was no significant difference between treatment groups in peak RER (LS mean difference, 0.02; 95% CI, −0.003 to 0.040; P = .09).
Figure 2. Treatment Difference of the Change From Baseline to Week 30 Between Mavacamten and Placebo in Peak-Exercise Cardiopulmonary Exercise Testing (CPET) Parameters.
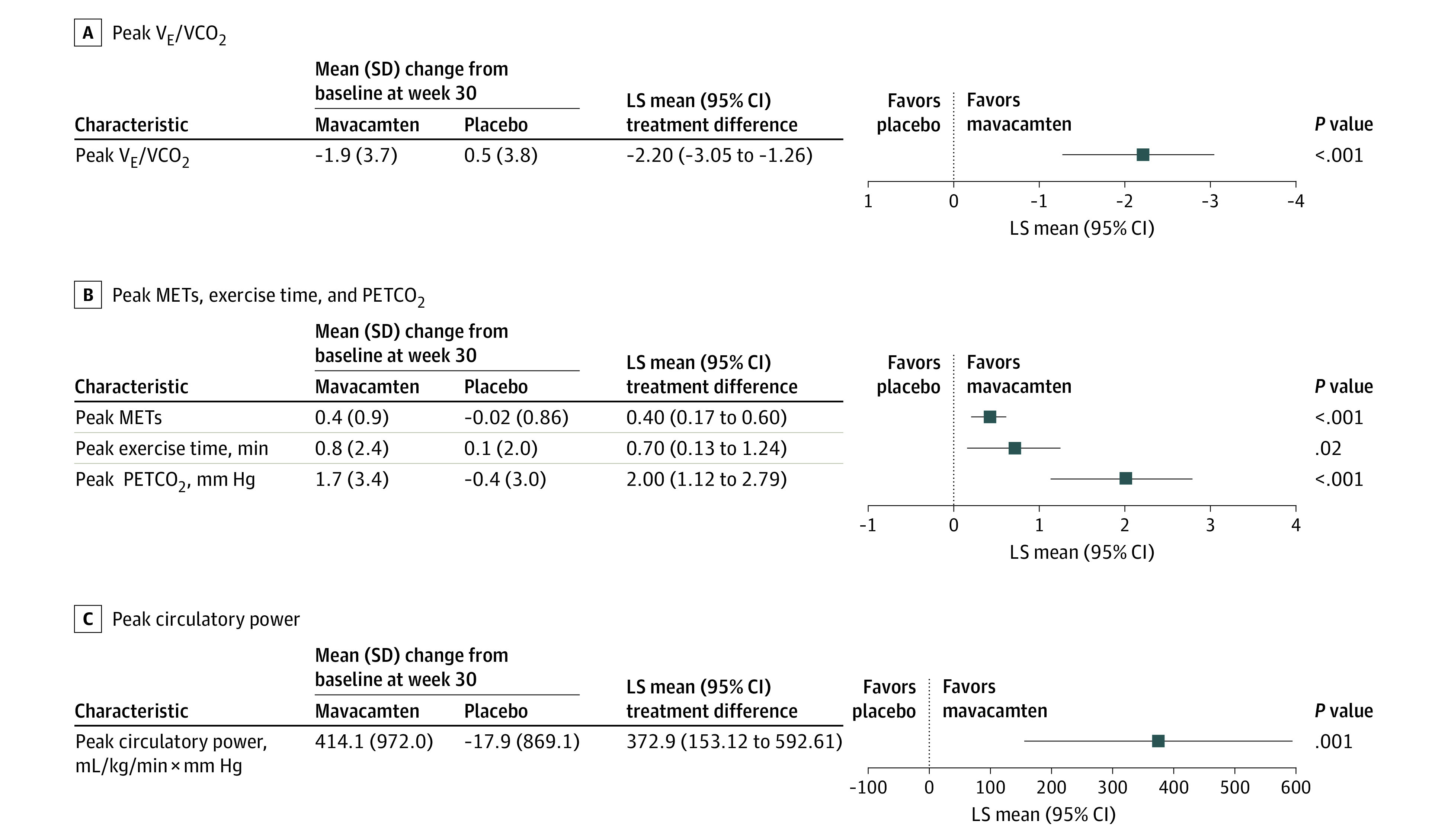
LS indicates least squares; MET, metabolic equivalent; PETCO2, partial pressure of end-tidal carbon dioxide; VCO2, carbon dioxide output; VE, minute ventilation.
Table 2. CPET Parameters at Baseline, at Week 30, and Change From Baseline to Week 30.
| Mavacamten | Placebo | Adjusted difference in difference (95% CI)a | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) [No.] | Absolute difference (SD) [No.]b | Mean (SD) [No.] | Absolute difference (SD) [No.]b | |||||
| Baseline (n = 123) | Week 30 | Baseline (n = 128) | Week 30 | |||||
| Peak-exercise CPET parameters | ||||||||
| Peak VE/VCO2 ratio | 35.4 (5.2) | 33.4 (4.8) [120] | −1.9 (3.7) [120] | 34.2 (5.5) | 34.7 (5.9) [125] | 0.5 (3.8) [125] | −2.2 (−3.05 to −1.26) | <.001 |
| Peak METs | 5.4 (1.4) | 5.8 (1.5) [120] | 0.4 (0.9) [120] | 5.7 (1.4) | 5.7 (1.5) [125] | −0.02 (0.86) [125] | 0.4 (0.17-0.60) | <.001 |
| Peak circulatory power, mL/kg/min × mm Hg | 3087.1 (1165.2) | 3505.9 (1214.4) [119] | 414.1 (972.0) [119] | 3284.8 (1173.3) | 3300.2 (1208.4) [124] | −17.9 (869.1) [121] | 372.9 (153.12-592.61) | .001 |
| Peak exercise time, min | 10.1 (4.0) | 10.9 (4.5) [120] | 0.8 (2.4) [120] | 10.5 (4.2) | 10.6 (4.3) [125] | 0.1 (2.0) [125] | 0.7 (0.13-1.24) | .02 |
| Peak RER | 1.1 (0.1) | 1.1 (0.1) [120] | 0.0 (0.1) [120] | 1.1 (0.1) | 1.1 (0.1) [125] | 0.0 (0.1) [125] | 0.02 (−0.003 to 0.040) | .09 |
| Peak PETCO2, mm Hg | 33.7 (4.5) | 35.5 (4.8) [110] | 1.7 (3.4) [106] | 34.7 (5.1) | 34.4 (5.2) [113] | −0.4 (3.0) [109] | 2.0 (1.12-2.79) | <.001 |
| Nonpeak-exercise CPET parameters | ||||||||
| VE/VCO2 slope | 33.6 (6.2) | 31.1 (5.0) [120] | −2.4 (4.6) [120] | 32.4 (6.2) | 32.9 (7.0) [125] | 0.4 (4.1) [125] | −2.6 (−3.58 to −1.52) | <.001 |
| Ventilatory power, mm Hg | 4.9 (1.4) | 5.6 (1.3) [119] | 0.7 (1.4) [119] | 5.2 (1.5) | 5.3 (1.7) [124] | −0.03 (1.23) [121] | 0.6 (0.29-0.90) | <.001 |
| Ventilatory threshold (ie, anaerobic threshold), mL/kg/min | 11.5 (2.3) | 12.2 (2.7) [111] | 0.7 (2.5) [106] | 11.5 (2.6) | 11.6 (2.6) [119] | 0.1 (2.6) [116] | 0.6 (−0.03 to 1.17) | .06 |
| PETCO2 at rest, mm Hg | 32.5 (4.2) | 33.2 (4.2) [109] | 0.6 (3.0) [105] | 33.0 (4.3) | 32.7 (3.7) [113] | −0.3 (3.2) [108] | 0.8 (0.07-1.53) | .03 |
| VO2/workload slope | 0.75 (0.25) | 0.79 (0.24) [118] | 0.04 (0.17) [116] | 0.76 (0.26) | 0.76 (0.25) [123] | −0.02 (0.20) [123] | 0.04 (0.002-0.086) | .04 |
Abbreviations: CPET, cardiopulmonary exercise testing; MET, metabolic equivalent; PETCO2, partial pressure of end-tidal carbon dioxide; RER, respiratory exchange ratio; VCO2, carbon dioxide output; VE, minute ventilation; VO2, oxygen uptake.
The adjusted difference in difference corresponds to the LS mean treatment difference.
The absolute difference corresponds to the change from baseline to week 30.
Nonpeak-Exercise CPET Parameters
Mavacamten was associated with a significant improvement in VE/VCO2 slope compared with placebo (LS mean difference, −2.6; 95% CI, −3.58 to −1.52; P < .001). There was a significant increase in ventilatory power (LS mean difference, 0.6 mm Hg; 95% CI, 0.29-0.90 mm Hg; P < .001) and in VO2/workload slope (LS mean difference, 0.04; 95% CI, 0.002 to 0.09; P = .04), favoring mavacamten over placebo. Mavacamten also improved PETCO2 at rest (LS mean difference: 0.8 mm Hg; 95% CI, 0.07-1.53 mm Hg; P = .03) compared with placebo. However, although there was no significant difference in VO2 at the ventilatory threshold (LS mean difference, 0.6 mL/kg/min; 95% CI, −0.03 to 1.17 mL/kg/min; P = .06) between mavacamten and placebo in exploratory analyses that were not adjusted for multiplicity, a numerical trend toward improvement was observed (Figure 3; Table 2).
Figure 3. Treatment Difference of the Change From Baseline to Week 30 Between Mavacamten and Placebo in Submaximal Exercise Cardiopulmonary Exercise Testing (CPET) Parameters.
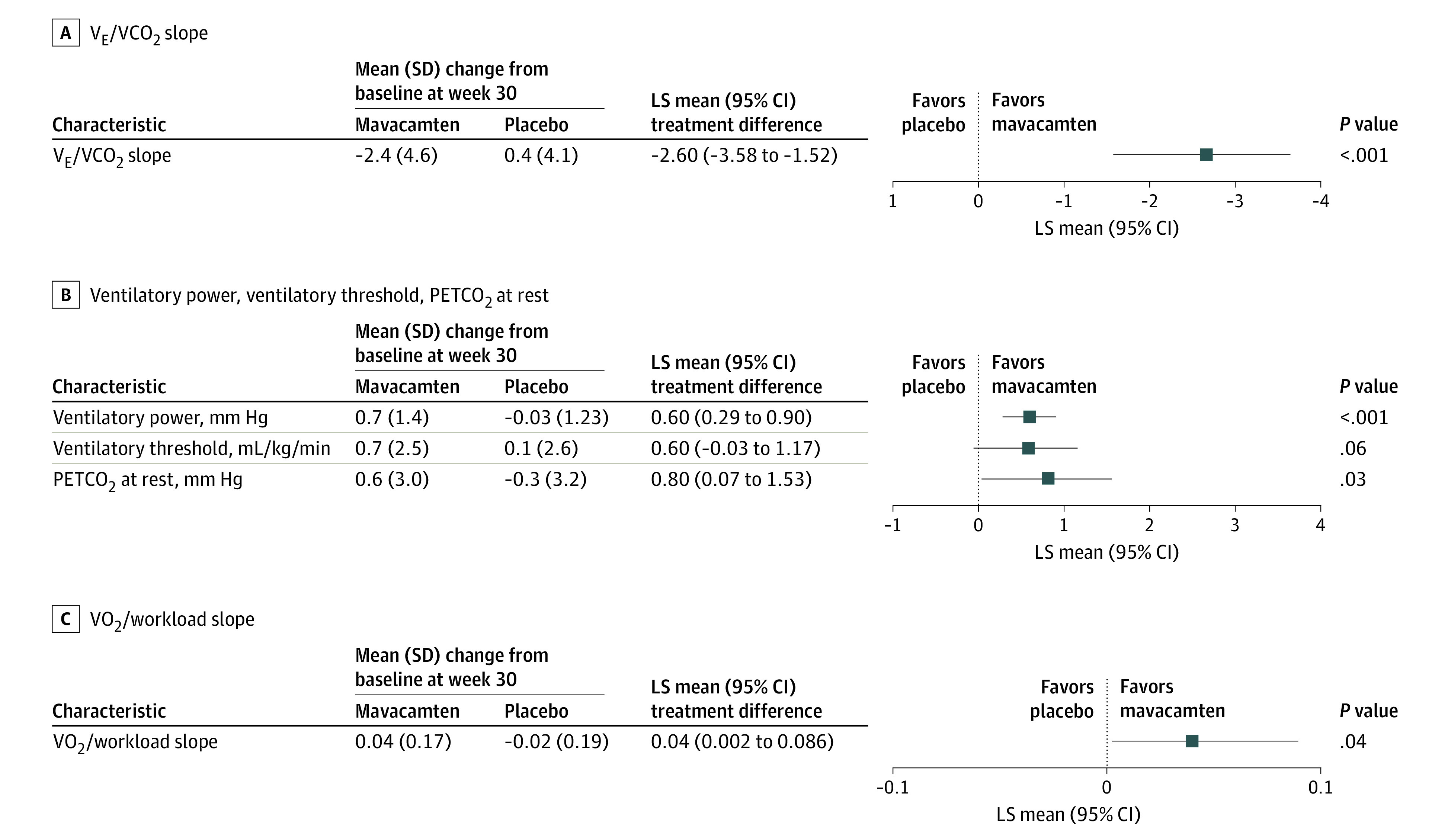
LS indicates least squares; PETCO2, partial pressure of end-tidal carbon dioxide; VCO2, carbon dioxide output; VE, minute ventilation; VO2, oxygen uptake.
Correlation Between CPET Parameters and Other Variables
Additionally, the study team sought to determine the association between change in CPET measures and markers of disease severity, including NT-proBNP (a biomarker of cardiac wall stress) and KCCQ-CSS (a patient-reported outcome symptom score).18,21 It should be noted that reductions in NT-proBNP levels were statistically significantly greater with mavacamten than with placebo.18 In the mavacamten group, there was a negative correlation between change in pVO2 and change in log2 NT-proBNP at week 30 (Pearson correlation coefficient, −0.30; P = .002), indicating that improvements in pVO2 were associated with a decrease in NT-proBNP levels (eTable 3 in Supplement 1). Change in pVO2 and change in log2 NT-proBNP levels at week 30 were not correlated in the placebo group (Pearson correlation coefficient, 0.064; P = .48). In the mavacamten group, there was a positive correlation between improvements in VE/VCO2 slope and reduction in NT-proBNP levels (Pearson correlation coefficient, 0.27; P = .003) (eTable 3 in Supplement 1). There were no significant correlations between improvement in VE/VCO2 slope and improvement in symptoms, as measured by the KCCQ-CSS, in either treatment group (eTable 3 in Supplement 1).
Discussion
Mavacamten was previously reported to improve pVO2 in patients with obstructive HCM.18 The present results indicate that treatment with mavacamten was also able to improve the exercise capacity of patients on a range of CPET parameters, including peak-exercise independent parameters, allowing us to deepen our understanding of the full extent of mavacamten effects on exercise tolerance. The novelty of our findings resides in the fine characterization of the exercise limitation observed in patients with obstructive HCM. Because CPET parameters represent reproducible clinical surrogates for functional capacity, our findings suggest that mavacamten may potentially be associated with improvements in the ability of patients to perform physical activities.
Several factors have been identified as potential determinants of the exercise limitation in patients with HCM, including left ventricular diastolic and systolic dysfunction, LVOT obstruction, chronotropic incompetence, ventilation-to-perfusion mismatch, abnormal peripheral oxygen utilization, and the inability to increase stroke volume.8,22,23,24,25,26,27,28,29 Invasive hemodynamic studies have confirmed left ventricular diastolic dysfunction as a cause of the inability to increase stroke volume, which seems to be exacerbated by LVOT obstruction.25,28,30,31,32 The improvements in exercise capacity associated with mavacamten may be mediated by its beneficial effects on measures of diastolic function via an improved ability to increase stroke volume; however, further investigation is needed to confirm this hypothesis.33,34,35 Furthermore, the reductions associated with mavacamten in peak VE/VCO2 ratio and in VE/VCO2 slope are indicative of improved ventilatory efficiency at peak and submaximal exercise, respectively. This may be due to a reduction in exercise-induced diastolic pressures and partial correction of the ventilation-to-perfusion mismatch exacerbated during exercise observed in patients with HCM.13,36 Moreover, in patients with HCM, impaired ventilatory efficiency has been significantly associated with a composite end point of death, heart transplant, and implantation of a ventricular assist device.12 Indeed, while VE/VCO2 slope of 30 or less might be considered normal, values more than 32 were shown to be powerful predictors of progression to heart failure.10 Consequently, the reduction in mean VE/VCO2 slope from 33.6 to 31.1 associated with mavacamten is clinically meaningful. Additionally, a modest improvement was observed in peak PETCO2 with mavacamten compared with placebo though not to the normal range (eTable 1 in Supplement 1). These associations suggest that mavacamten not only improved symptoms and functional capacity, but also has the potential to improve clinical outcomes.
Given that most patients with HCM do not routinely exercise to peak exercise capacity, submaximal exercise CPET parameters may reflect what patients can typically physically achieve better than peak exercise parameters. In this context, the improvements in VE/VCO2 slope, in ventilatory power, in VO2/workload slope, and in PETCO2 at rest may better reflect the improvement in submaximal cardiopulmonary efficiency that in turn translates to symptomatic benefits that patients are experiencing after treatment with mavacamten. These parameters could be examined by clinicians when performing CPET in patients with HCM.
Although no significant association between VE/VCO2 slope and patient-reported symptoms (KCCQ-CSS) was observed in the present study, Nassif et al37 reported a significant correlation between pVO2 and KCCQ-CSS in patients enrolled in the EXPLORER-HCM study. Furthermore, mavacamten has been associated with improvements in symptoms and health status in patients with obstructive HCM, as measured by NYHA functional classification and by the KCCQ, respectively.18,21 These findings, together with the correlation observed between CPET parameters (pVO2 and VE/VCO2 slope) and measures of the neurohormonal effect of disease (NT-proBNP levels) are evidence of the beneficial effect of mavacamten on the patients’ daily lives.
Our findings suggest that the impaired exercise performance observed in patients with obstructive HCM is reversible with relief of the LVOT pressure gradient, which can be achieved following treatment with mavacamten. Indeed, a number of clinically meaningful CPET parameters consistently improved with mavacamten, and these benefits correlated with improvement in a biomarker of cardiac wall stress. Nevertheless, it seems that symptomatic improvement indicated by the change in KCCQ-CSS is more striking than that seen with CPET, suggesting that the pathophysiology of symptoms is not wholly captured by this testing. Thus, the benefits of mavacamten seem to extend beyond improvement in CPET parameters and fully understanding this represents an unmet need, and may help unravel further the pathophysiology of this complex disease. These considerations would be critical for the design of future trials in patients with obstructive or nonobstructive HCM.
Limitations
The results presented here should be interpreted in the context of several limitations. These analyses are exploratory in nature and should be viewed as hypothesis generating. However, it should be noted that most CPET parameters consistently improved with treatment and that no specific parameter was weighed over another, although some are likely correlated, the clinical interpretation of each parameter is conducted separately. Furthermore, the analytical strategy used here was aligned with the EXPLORER-HCM trial. The single–time point CPET measurement after treatment is also a limitation, as is the variability of symptom status in HCM. Another limitation is the lack of direct measures of daily living or energy expenditure. While multiple mechanisms of exercise impairment were assessed, the extent to which they overlap cannot be entirely accounted for. Additionally, the type of ergometer used can affect the measures of the CPET parameters; however, this potential issue was overcome because randomization was stratified by type of ergometer.
Conclusions
In conclusion, mavacamten therapy improved a range of CPET parameters beyond pVO2, indicating consistent and broad benefits on maximal exercise capacity. The favorable effects of mavacamten on submaximal exertional tolerance provide further insights into the beneficial effect of mavacamten in patients with obstructive HCM.
eTable 1. Normal and abnormal values for key CPET parameters
eTable 2. Resting and peak heart rate and systolic blood pressure measured during CPET at Baseline, at Week 30 and Change from Baseline to Week 30
eTable 3. Change in pVO2 and Change in VE/VCO2 Slope Versus Change in Log2 NT-proBNP at Week 30, and Change in VE/VCO2 Slope Versus Change in KCCQ-CSS at Week 30
Protocol amendment 5 PCL.
SAP2.0.
Data sharing statement.
References
- 1.Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655-668. doi: 10.1056/NEJMra1710575 [DOI] [PubMed] [Google Scholar]
- 2.Robert-Paganin J, Auguin D, Houdusse A. Hypertrophic cardiomyopathy disease results from disparate impairments of cardiac myosin function and auto-inhibition. Nat Commun. 2018;9(1):4019. doi: 10.1038/s41467-018-06191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(7):749-770. doi: 10.1161/CIRCRESAHA.117.311059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Velden J, Tocchetti CG, Varricchi G, et al. Metabolic changes in hypertrophic cardiomyopathies: scientific update from the Working Group of Myocardial Function of the European Society of Cardiology. Cardiovasc Res. 2018;114(10):1273-1280. doi: 10.1093/cvr/cvy147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heitner SB, Jacoby D, Lester SJ, et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med. 2019;170(11):741-748. doi: 10.7326/M18-3016 [DOI] [PubMed] [Google Scholar]
- 6.Borsari W, Davis L, Meiers E, Salberg L, Barbara M. Living with hypertrophic cardiomyopathy: the patient’s perspective. Future Cardiol. 2021;18(1):43-50. doi: 10.2217/fca-2021-0091 [DOI] [PubMed] [Google Scholar]
- 7.Zaiser E, Sehnert AJ, Duenas A, Saberi S, Brookes E, Reaney M. Patient experiences with hypertrophic cardiomyopathy: a conceptual model of symptoms and impacts on quality of life. J Patient Rep Outcomes. 2020;4(1):102. doi: 10.1186/s41687-020-00269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S, Elliott P, Whyte G, et al. Utility of cardiopulmonary exercise in the assessment of clinical determinants of functional capacity in hypertrophic cardiomyopathy. Am J Cardiol. 2000;86(2):162-168. doi: 10.1016/S0002-9149(00)00854-7 [DOI] [PubMed] [Google Scholar]
- 9.Mezzani A. Cardiopulmonary exercise testing: basics of methodology and measurements. Ann Am Thorac Soc. 2017;14(suppl 1):S3-S11. doi: 10.1513/AnnalsATS.201612-997FR [DOI] [PubMed] [Google Scholar]
- 10.Magrì D, Santolamazza C. Cardiopulmonary exercise test in hypertrophic cardiomyopathy. Ann Am Thorac Soc. 2017;14(suppl 1):S102-S109. doi: 10.1513/AnnalsATS.201611-884FR [DOI] [PubMed] [Google Scholar]
- 11.Masri A, Pierson LM, Smedira NG, et al. Predictors of long-term outcomes in patients with hypertrophic cardiomyopathy undergoing cardiopulmonary stress testing and echocardiography. Am Heart J. 2015;169(5):684-692.e1. doi: 10.1016/j.ahj.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 12.Finocchiaro G, Haddad F, Knowles JW, et al. Cardiopulmonary responses and prognosis in hypertrophic cardiomyopathy: a potential role for comprehensive noninvasive hemodynamic assessment. JACC Heart Fail. 2015;3(5):408-418. doi: 10.1016/j.jchf.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 13.Coats CJ, Rantell K, Bartnik A, et al. Cardiopulmonary exercise testing and prognosis in hypertrophic cardiomyopathy. Circ Heart Fail. 2015;8(6):1022-1031. doi: 10.1161/CIRCHEARTFAILURE.114.002248 [DOI] [PubMed] [Google Scholar]
- 14.Magrì D, Limongelli G, Re F, et al. Cardiopulmonary exercise test and sudden cardiac death risk in hypertrophic cardiomyopathy. Heart. 2016;102(8):602-609. doi: 10.1136/heartjnl-2015-308453 [DOI] [PubMed] [Google Scholar]
- 15.Magrì D, Re F, Limongelli G, et al. Heart failure progression in hypertrophic cardiomyopathy—possible insights from cardiopulmonary exercise testing. Circ J. 2016;80(10):2204-2211. doi: 10.1253/circj.CJ-16-0432 [DOI] [PubMed] [Google Scholar]
- 16.Green EM, Wakimoto H, Anderson RL, et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351(6273):617-621. doi: 10.1126/science.aad3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson RL, Trivedi DV, Sarkar SS, et al. Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A. 2018;115(35):E8143-E8152. doi: 10.1073/pnas.1809540115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivotto I, Oreziak A, Barriales-Villa R, et al. ; EXPLORER-HCM study investigators . Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396(10253):759-769. doi: 10.1016/S0140-6736(20)31792-X [DOI] [PubMed] [Google Scholar]
- 19.Ho CY, Olivotto I, Jacoby D, et al. Study design and rationale of EXPLORER-HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy. Circ Heart Fail. 2020;13(6):e006853. doi: 10.1161/CIRCHEARTFAILURE.120.006853 [DOI] [PubMed] [Google Scholar]
- 20.Bayonas-Ruiz A, Muñoz-Franco FM, Ferrer V, et al. Cardiopulmonary exercise test in patients with hypertrophic cardiomyopathy: a systematic review and meta-analysis. J Clin Med. 2021;10(11):2312. doi: 10.3390/jcm10112312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spertus JA, Fine JT, Elliott P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10293):2467-2475. doi: 10.1016/S0140-6736(21)00763-7 [DOI] [PubMed] [Google Scholar]
- 22.Patel V, Critoph CH, Elliott PM. Mechanisms and medical management of exercise intolerance in hypertrophic cardiomyopathy. Curr Pharm Des. 2015;21(4):466-472. doi: 10.2174/138161282104141204142436 [DOI] [PubMed] [Google Scholar]
- 23.Hung RK, Al-Mallah MH, Whelton SP, et al. Effect of beta-blocker therapy, maximal heart rate, and exercise capacity during stress testing on long-term survival (from The Henry Ford Exercise Testing Project). Am J Cardiol. 2016;118(11):1751-1757. doi: 10.1016/j.amjcard.2016.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Baak MA. Beta-adrenoceptor blockade and exercise. an update. Sports Med. 1988;5(4):209-225. doi: 10.2165/00007256-198805040-00002 [DOI] [PubMed] [Google Scholar]
- 25.Lele SS, Thomson HL, Seo H, Belenkie I, McKenna WJ, Frenneaux MP. Exercise capacity in hypertrophic cardiomyopathy. role of stroke volume limitation, heart rate, and diastolic filling characteristics. Circulation. 1995;92(10):2886-2894. doi: 10.1161/01.CIR.92.10.2886 [DOI] [PubMed] [Google Scholar]
- 26.Jones S, Elliott PM, Sharma S, McKenna WJ, Whipp BJ. Cardiopulmonary responses to exercise in patients with hypertrophic cardiomyopathy. Heart. 1998;80(1):60-67. doi: 10.1136/hrt.80.1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dybro AM, Rasmussen TB, Nielsen RR, Andersen MJ, Jensen MK, Poulsen SH. Randomized trial of metoprolol in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2021;78(25):2505-2517. doi: 10.1016/j.jacc.2021.07.065 [DOI] [PubMed] [Google Scholar]
- 28.Dybro AM, Rasmussen TB, Nielsen RR, et al. Effects of metoprolol on exercise hemodynamics in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2022;79(16):1565-1575. doi: 10.1016/j.jacc.2022.02.024 [DOI] [PubMed] [Google Scholar]
- 29.Palau P, Seller J, Domínguez E, et al. Effect of β-blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. 2021;78(21):2042-2056. doi: 10.1016/j.jacc.2021.08.073 [DOI] [PubMed] [Google Scholar]
- 30.Sugrue DD, McKenna WJ, Dickie S, et al. Relation between left ventricular gradient and relative stroke volume ejected in early and late systole in hypertrophic cardiomyopathy. Assessment with radionuclide cineangiography. Br Heart J. 1984;52(6):602-609. doi: 10.1136/hrt.52.6.602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruzyłło W, Chojnowska L, Demkow M, et al. Left ventricular outflow tract gradient decrease with non-surgical myocardial reduction improves exercise capacity in patients with hypertrophic obstructive cardiomyopathy. Eur Heart J. 2000;21(9):770-777. doi: 10.1053/euhj.1999.1905 [DOI] [PubMed] [Google Scholar]
- 32.Critoph CH, Patel V, Mist B, Elliott PM. Cardiac output response and peripheral oxygen extraction during exercise among symptomatic hypertrophic cardiomyopathy patients with and without left ventricular outflow tract obstruction. Heart. 2014;100(8):639-646. doi: 10.1136/heartjnl-2013-304914 [DOI] [PubMed] [Google Scholar]
- 33.Ho CY, Mealiffe ME, Bach RG, et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2020;75(21):2649-2660. doi: 10.1016/j.jacc.2020.03.064 [DOI] [PubMed] [Google Scholar]
- 34.Hegde SM, Lester SJ, Solomon SD, et al. Effect of mavacamten on echocardiographic features in symptomatic patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2021;78(25):2518-2532. doi: 10.1016/j.jacc.2021.09.1381 [DOI] [PubMed] [Google Scholar]
- 35.Cremer PC, Geske JB, Owens A, et al. Myosin inhibition and left ventricular diastolic function in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy: insights from the VALOR-HCM study. Circ Cardiovasc Imaging. Published online November 6, 2022. doi: 10.1161/CIRCIMAGING.122.014986 [DOI] [PubMed] [Google Scholar]
- 36.Arena R, Owens DS, Arevalo J, et al. Ventilatory efficiency and resting hemodynamics in hypertrophic cardiomyopathy. Med Sci Sports Exerc. 2008;40(5):799-805. doi: 10.1249/MSS.0b013e31816459a1 [DOI] [PubMed] [Google Scholar]
- 37.Nassif M, Fine JT, Dolan C, et al. Validation of the Kansas City Cardiomyopathy Questionnaire in symptomatic obstructive hypertrophic cardiomyopathy. JACC Heart Fail. 2022;10(8):531-539. doi: 10.1016/j.jchf.2022.03.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Normal and abnormal values for key CPET parameters
eTable 2. Resting and peak heart rate and systolic blood pressure measured during CPET at Baseline, at Week 30 and Change from Baseline to Week 30
eTable 3. Change in pVO2 and Change in VE/VCO2 Slope Versus Change in Log2 NT-proBNP at Week 30, and Change in VE/VCO2 Slope Versus Change in KCCQ-CSS at Week 30
Protocol amendment 5 PCL.
SAP2.0.
Data sharing statement.